Abstract
Impaired blood flow in the tumor vascular bed caused by structurally and functionally abnormal blood vessels not only hinders the delivery of chemotherapeutic agents but also aggravates tumor hypoxia, making the tumor cells further resistant to antineoplastic drugs. Therefore, normalization of tumor blood vessels may be an important approach to increase therapeutic efficacy in the treatment of cancer patients. As blood vessels are supplied by sympathetic nerves containing dopamine (DA), and DA regulates functions of normal blood vessels through its receptors present in these vessels, we investigated the effect of DA on tumor vasculature. Here we report loss of sympathetic innervation and endogenous DA in abnormal and immature tumor blood vessels in malignant colon and prostate tumor tissues. In contrast, exogenous administration of DA normalizes the morphology and improves the functions of these vessels by acting on pericytes and endothelial cells, the two major cellular components of blood vessels. DA acts through its D2 receptors present in these cells to up-regulate directly the expression of angiopoietin 1 (Ang1) in pericytes and the expression of the zinc finger transcriptional factor, Krüppel-like factor-2 (KLF2) in tumor endothelial cells. Importantly, this vessel stabilization by DA also significantly increases the concentration of anticancer drug in tumor tissues. These results show a relationship between vascular stabilization and a neurotransmitter and indicate that DA or its D2 receptor-specific agonists can be an option for the treatment of cancer and disorders in which normalization of blood vessels may have therapeutic benefits.
It now is well established that tumor blood vessels are structurally and functionally abnormal (1–4). In contrast to normal blood vessels, which are well organized and lined by quiescent adult endothelial cells and supporting mature pericytes, tumor vessels are highly disorganized and made up of active endothelial cells (1–6). Moreover, in these tumor vessels pericytes are lacking or immature pericytes attach loosely to the endothelial cells (1–3). This vascular immaturity in turn causes destabilization of vessel structure, impairment of endothelial barrier function, and decreased blood flow leading to increased hypoxia in tumor tissues (1–3). In addition, the impaired blood flow in abnormal tumor blood vessels not only compromises the delivery of anticancer drugs but also aggravates tumor hypoxia, making tumor cells more resistant to these agents (1–3). Thus, stabilization or normalization of tumor vessels can be an important approach to increase the therapeutic efficacy of anticancer drugs (1, 7).
Dopamine (DA) is a monoamine catecholamine neurotransmitter, which acts through its D1 and D2 class of receptors present in the target organs (8, 9). In addition to its conventional role in the brain, recent reports indicate that DA also controls several functions in the periphery (8, 9). DA has been shown to regulate behavior, movement, and cardiovascular, renal, endocrine, gastrointestinal, and immune functions (8–10). Normal blood vessels are supplied by sympathetic nerves, and neurotransmitters released from these nerve endings regulate many critical vascular functions (8, 11–15). Because DA is one of the catecholamine neurotransmitters in the sympathetic nerve endings and regulates important vascular functions such as vessel tone and blood pressure by acting through its receptors present in blood vessels (8, 9, 13–20), we investigated whether DA has other effects on abnormal tumor blood vessels.
Results
Loss of Sympathetic Innervation of Tumor Blood Vessels Is Associated with Abnormal Tumor Blood Vessel Morphology and Leakiness.
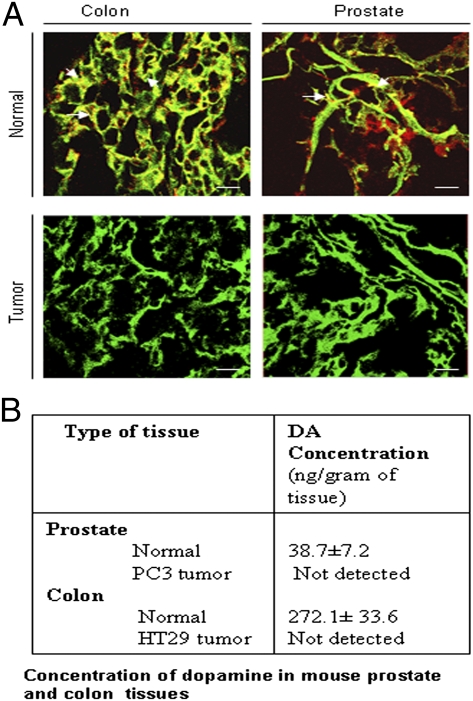
To investigate the regulatory role of DA on tumor blood vessels, we first determined the morphology and leakiness of tumor vasculature in mice bearing orthotopic human prostate (PC3) and human colon (HT29) tumors (21, 22). Blood vessels from both tumors showed aberrant architectural patterns with increased dilation and tortuosity in comparison with the normal organs. Because normal blood vessels are supplied by sympathetic nerves (11, 12), we examined the sympathetic nerve supply to blood vessels in PC3 and HT29 tumor tissues. Using confocal microscopy, we observed the absence of tyrosine hydroxylase, a well-established marker of sympathetic nerves, in tumor blood vessels visualized by injecting FITC-lectin to the tumor-bearing animals (Fig. 1A); this finding indicates the absence of sympathetic nerves in the blood vessels of these tumors (23–26). Then, to determine the effect of sympathectomy on the status of DA in these tumor tissues, we measured the concentration of DA in tumor tissues by HLPC with electrochemical detection (27). Our results indicated complete loss of DA in PC3 and HT29 tumor tissues (Fig. 1B) (28, 29).
Fig. 1.
Loss of sympathetic innervations in tumor blood vessels. (A) Confocal microscopic images of blood vessels perfulsed with FITC-lectin (green) and positive for tyrosine hydrolase (red), a well-established marker of sympathetic nerves (white arrows) in normal prostate and colon tissues (Upper). In contrast, tumor blood vessels show lack of tyrosine hydroxylase staining, thereby indicating loss of sympathetic nerves (Lower). (Scale bars, 50 μm.) (B) Concentration of DA in mouse colon and prostate tissues determined by HPLC with electrochemical detection. Orthotopic colon and prostate tumor tissues show complete loss of DA (≤0.01 ng/mL). Results are mean ± SE; n = 15.
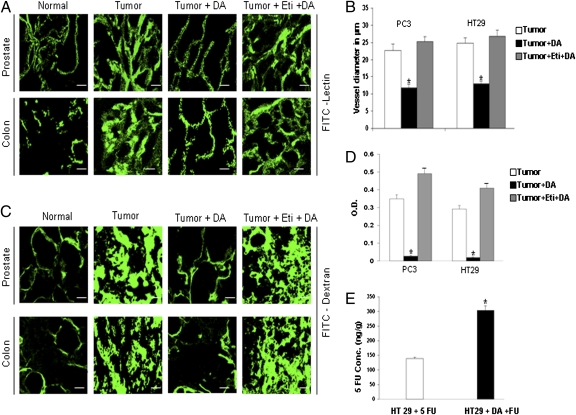
Next, the morphology of tumor blood vessels was examined by confocal microscopy after tumor-bearing animals were injected with FITC-lectin (3). The permeability or leakiness of blood vessels in these tumor tissues was determined after FITC dextran perfusion using confocal microscope (30) and also by modified miles assay (31). The tumor vascular network displayed a chaotic vascular architecture that was tortuous, dilated, and showed extensive leakiness with complete loss of hierarchy (Fig. 2 A–D). These data indicate an association between the absence of sympathetic nerves, loss of dopamine, and abnormal tumor vessels.
Fig. 2.
DA restores normal vessel morphology in PC3 and HT29 tumors. (A) Confocal images of vessels perfused with FITC-lectin in control (Tumor), DA-treated (Tumor + DA), and eticlopride + DA-treated (Tumor + Eti + DA) tumors. (B) Mean vessel diameters were quantified in control, DA-treated, and eticlopride + DA-treated tumor groups (five fields per tumor). (C) Vessels perfused with FITC-dextran in PC3 and HT29 tumors. Untreated tumor vessels exhibit dilation, tortuosity, and extensive leakiness visualized by massive extravasation of dextran. In contrast, treatment with DA markedly reduced dilation, tortuosity, and leakiness. Pretreatment with the DA D2 receptor antagonist eticlopride abolishes the effects of DA. (D) Modified Miles assay shows significantly decreased vessel leakiness in PC3 and HT29 tumors following DA treatment. This effect of DA was lost after pretreatment with eticlopride. Vascular permeability was assessed colorimetrically by the degree of extravasation of Evans blue dye from tumor vessels. (E) Nude mice bearing s.c. HT29 tumors treated with DA and then with FU show significantly increased concentration of intratumoral 5-FU 30 min after 5-FU injection. (Scale bars, 50 μm.) *P < 0.05. All error bars represent SEM; n = 15 for each experimental group.
DA Acts Through D2 Receptors to Stabilize the Morphology of Blood Vessels and Inhibit Leakiness of Tumor Vasculature.
Because abnormal tumor blood vessels were associated with the absence of sympathetic nerves and DA (Fig. 1 A and B), and as DA has been shown to regulate several important blood vessel functions (8, 9, 13–15), we investigated the effect of exogenous DA treatment on the morphology of tumor blood vessels. DA was administered i.p. to orthotopic PC3 and HT29 tumor-bearing animals at a low nontoxic dose (50 mg⋅kg−1⋅d−1, corresponding to ∼5% of the median LD50 in mice) for 7 d (32) when tumors were detected in these animals. In contrast to untreated tumor blood vessels, blood vessels of PC3 and HT29 tumors on day 8 after completion of DA treatment resembled normal vessels in regard to leakiness and distribution (Fig.2 A–D). However, this effect of DA was abrogated completely when these tumor-bearing animals were pretreated with a dopamine D2 receptor antagonist, eticlopride (10 mg/kg i.p.) (Fig. 2 A–D), thus indicating that this action of DA is mediated through D2 receptors. The other two catecholamines, epinephrine and norepinephrine, and the DA D1 receptor-specific antagonist SCH 23390 had no effect on the morphology of tumor blood vessels (Fig. S1 A and B). These results demonstrated that, among the catecholamines, only DA, by acting specifically through its D2 receptors, could normalize tumor blood vessel morphology and thus reduce leakiness of blood vessels.
Finally, to confirm further the role of DA D2 receptors in regulating the morphology and leakiness of tumor blood vessels, we performed our experiments in DA D2 receptor- KO mice using Lewis lung carcinoma cells (LLC) (SI Results and Fig. S2) (33).
DA Improves Blood Flow and Reduces Hypoxia in Tumor Tissues.
Several reports have indicated that improper vasoregulation within abnormal tumor vessels leads to chaotic and compromised blood flow, resulting in an hypoxic tumor microenvironment (1–3). It has been shown further that correction of these structural abnormalities improves blood flow and consequently reduces hypoxia in tumor tissues (1–3). We therefore reasoned that DA, by stabilizing aberrant tumor vessels, would decrease hypoxia and increase blood flow in these tissues. Indeed, we observed hypoxia to be reduced significantly in DA-treated PC3 and HT29 tumors in comparison with untreated controls, and this reduction in hypoxic fraction within the tumor tissues was visualized by the formation of pimonidazole adducts (Fig. S3 A–C) (34). In addition, tumor blood flow in DA-treated animals, measured noninvasively by laser Doppler flowmetry (LDF), increased significantly in comparison with untreated controls (Fig. S3 D and E) (35). Since animals need to be stabilized for at least 30 min before blood flow is measured, and the process requires nearly 1 h to complete, we determined the effects of DA on tumor blood flow in animals bearing s.c. implanted PC3 and HT29 tumors, because animals bearing orthotopic tumors do not survive 1 h after surgical exposure (35). This reduction in tumor tissue hypoxia and increase in tumor blood flow was noted even on day 15 after DA treatment (Fig. S4 A–C).
DA Significantly Increases the Concentration and Hence the Efficacy of Anticancer Drug in Tumor Tissues.
Because normalization of blood flow in tumor blood vessels has been reported to increase the concentration of anticancer drugs in tumor tissues (1–3, 7), we investigated whether the DA-mediated increase in tumor blood flow could increase the concentration of 5-fluorouracil (5-FU), a drug commonly used in the treatment of colon cancer (36). Mice bearing s.c. HT29 tumor (100 mm3) were divided into two groups. One group was treated i.p. with DA (50 mg⋅kg−1⋅d−1 for 7 d), and the other group was the untreated control. On day 8, animals from both the groups were selected for further study. A single dose of 5-FU (20 mg/kg i.p.) was administered to the animals in both groups. Tumor tissues were collected 30 min after administration of 5-FU because it has been reported that the concentration of 5-FU in tumor tissues peaks at ∼30 min (37). The level of 5-FU in these tumor tissues was quantified by liquid chromatography electrospray ionization tandem mass spectrometry (38). We observed that 5-FU concentrations in tumor tissues were increased significantly (∼2.2 times) in DA-treated animals as compared with controls treated only with 5-FU (Fig. 2E). This result shows that DA treatment can significantly increase the concentration of anticancer drugs in tumor tissues.
Because treatment with DA increases 5-FU concentration in tumor tissues (Fig. 2E), we decided to determine if this increased concentration of 5-FU following DA treatment is more effective in inhibiting tumor growth than treatment with 5-FU alone. Mice bearing s.c. HT29 tumors were treated with DA (50 mg⋅kg−1⋅d−1 i.p.) for 7 d. Starting on day 8, tumor-bearing DA-treated animals were treated either with 5-FU (20 mg⋅kg−1⋅d−1 i.p.) for 5 consecutive days or remained as controls without any further treatment. In addition, DA-untreated mice bearing HT29 tumors of similar size also were divided into two additional treatment groups: vehicle and 5-FU (20 mg⋅kg−1⋅d−1 i.p.) for 5 consecutive days. The animals were killed after completion of 5-FU treatment. At the end of treatment on day 14, tumor volumes in the vehicle, 5-FU–only, and DA-only treatment groups were 538.33 ± 27.46 mm3, 310.07 ± 21.09 mm3, and 323.42 ± 26.17 mm3, respectively. Treatment with DA followed by 5-FU showed maximum inhibition of tumor growth (85.96 ± 13.45 mm3) in comparison with the groups treated with vehicle, 5-FU only, and DA only (Fig. S3F). These results confirmed that the DA-mediated increase in 5-FU concentration in tumor tissues resulted in a significant increase in the therapeutic efficacy of 5-FU that correlates well with increased blood flow and decreased hypoxia in tumor tissues following DA treatment.
DA D2 Receptors Are Present in Pericytes and Tumor Endothelial Cells.
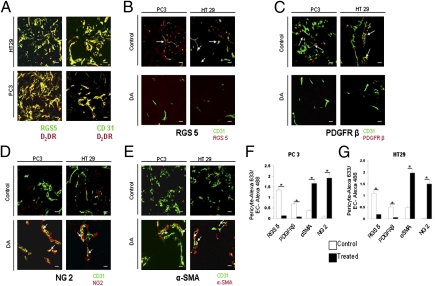
Pericytes and endothelial cells are the two major cell types that not only form blood vessels but also regulate critical vascular functions, including blood flow (1, 3, 39–44). Abnormalities in the distribution and function of these cells in blood vessels lead to the development of defective vessel phenotypes and functions (1, 3, 40–42). Acting through its D2 receptors, DA could reverse abnormal tumor vessel morphology, reduce hypoxia in tumor tissues, and improve blood flow (Fig. 2 and Fig. S3). We therefore confirmed the presence of DA D2 receptors in pericytes and tumor endothelial cells (TEC) by confocal microscopy (Fig. 3A).
Fig. 3.
DA D2 receptors are present both in pericytes and tumor endothelial cells, and DA increases the number of mature pericytes in tumor blood vessels. (A) Immunofluorescence staining for DA D2 receptors (red) in pericytes (RGS5, green) and tumor endothelial cells (CD31, green) of PC3 and HT29 tumor tissues. (B–E) Confocal images from control and DA-treated PC3 and HT29 tumor tissues labeled with anti-RGS5 (red) (B), anti-PDGFRβ (red) (C), anti-NG2 (red) (D), and anti-αSMA (red) (E). Endothelial cells stained with anti-CD31 (green) show DA D2 receptors in RGS5+ pericytes as well as in tumor endothelial cells of PC3 and HT29 tumors. For these images a 40× objective was used. (F and G) The ratio of the total area of red staining (pericyte marker) to green staining (EC = CD31) is provided (five fields per tumor). A quantitative decrease is seen in the number of RGS5+ and PDGFRβ+ pericytes (B, C, F, and G), and a significant increase is seen in the number of NG2+ and αSMA+ pericytes in tumor blood vessels after treatment with DA (D–G). (Scale bars in A, B, C and D, 20 μm.) All error bars represent SEM. *P < 0.001. n = 15 for each experimental group.
DA Treatment Promotes Mature Pericyte Coverage in Tumor Blood Vessels.
In the tumor vascular bed, the supporting pericytes that surround the endothelial cells develop multiple morphological and architectural abnormalities (1–4, 40–42) as well as altered expression of marker proteins (3). Because DA treatment was associated with the reversal of abnormal tumor vessel morphology, leakiness, and blood flow in PC3 and HT29 tumor tissues (Fig. 2 and Fig. S3), we investigated the effect of DA treatment on the pericyte coverage in tumor blood vessels. We observed that the majority of pericytes in untreated PC3 and HT29 tumors were immature pericytes expressing PDGF receptor β (PDGFRβ) and regulator of G protein signaling 5 (RGS5) (3). In contrast, mature pericytes predominantly expressing α smooth muscle actin (αSMA) or neural/glial antigen 2 (NG2) were demonstrated in the blood vessels of DA-treated PC3 and HT29 tumors (Fig. 3 B–G) (3). Similar pericyte coverage also was demonstrated in animals treated with the DA D2 receptor-specific agonist quinpirole (10 mg⋅kg−1⋅d−1 i.p.) for 7 d (Fig. S5).
Acting Through its D2 Receptors, DA Directly and Specifically Up-Regulates Angiopoietin 1 Expression in Pericytes.
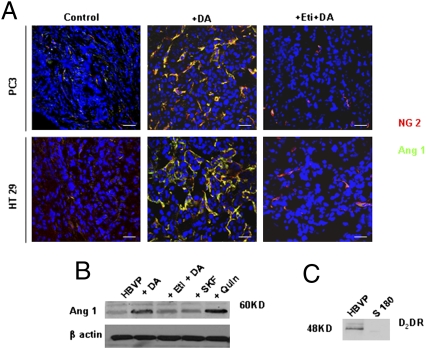
Several reports indicate the importance of angiopoietin 1 (Ang1) as a pericyte-derived vessel-stabilizing signal (39, 42–44). Because DA was shown to stabilize abnormal tumor vessels (Fig. 2 and Fig. S3), we investigated the effect of DA treatment on Ang1 expression in the pericytes of tumor tissues. Although Ang1 was not expressed in the pericytes of tumor tissues in untreated controls, we observed striking Ang1 expression in the pericytes of tumor tissues collected from animals treated with DA (Fig. 4A). In contrast, this DA-mediated up-regulation of Ang1 expression was lost when these animals were treated with the D2 receptor-specific antagonist eticlopride before treatment with DA (Fig. 4A), indicating that this action of DA is mediated specifically through its D2 receptors present in the pericytes (Fig. 4A).
Fig. 4.
DA directly and specifically up-regulates Ang1 expression in pericytes. (A) Confocal images showing up-regulation of Ang1 in pericytes following DA treatment of PC3 and HT29 tumors. Pretreatment with the DA D2 receptor antagonist eticlopride abrogates this effect of DA. Western blot analysis shows the expression of Ang1 in HBVP. (Scale bars, 50 μm.) (B) Treatment with DA (1 μM) or the DA D2 receptor agonist quinpirole (1 μM) significantly up-regulates Ang1 expression in HBVP. Treatment with the DA D1 receptor agonist SKF 38393 fails to up-regulate Ang1 expression in HBVP. (C) The effect of both DA and quinpirole (Quin) is lost after pretreatment with eticlopride (1 μM). Western blot shows the presence of DA D2 receptors in HBVP but not in control S180 cells. Results shown are representative of six separate experiments.
Next, to elucidate whether DA had any direct and specific effect on Ang1 expression in pericytes, we demonstrated DA D2 receptor and Ang1 expressions in human brain vascular pericytes (HBVP) (41). Because there was loss of sympathetic nerve supply in tumor blood vessels (Fig. 1A) and as 1 μM of DA is present in the extracellular fluid surrounding neural synapses (45), we used this concentration of DA for our in vitro experiments. Our results showed significant increase of Ang1 expression in these cells when serum-starved HBVP were treated with DA (1 μM) or the DA D2 receptor-specfic agonist quinpirole (1 μM) (Fig. 4B). However, pretreatment of these cells with eticlopride (1 μM) abrogated this action of DA, thus indicating that this action of DA is mediated through its D2 receptors (Fig. 4B). This result was confirmed further, because the DA D1 receptor-specific agonist SKF 38393 also failed to increase Ang1 expression in these cells (Fig. 4B). Western Blot analysis demonstrated the presence of DA D2 receptors in HBVP (Fig. 4C). In addition, these experiments performed in serum-starved HBVP suggested that the effect of DA on Ang1 expression in pericytes is direct and is not the result of its actions on other growth factors. Furthermore, because there are reports indicating that up-regulation of Ang1 in pericytes stimulates migration of these cells (46, 47), we examined the action of DA on HBVP migration (SI Results and Fig. S6).
DA Induces Krüppel-Like Factor-2 Expression in TEC by Acting Through D2 Receptors.
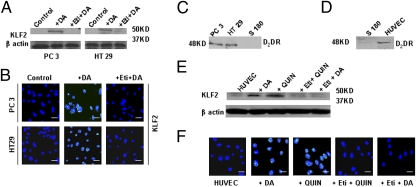
Adult endothelial cells in normal vasculature usually are in the quiescent state to maintain endothelial integrity and barrier functions of these vessels (5, 6). In contrast, endothelial cells in tumor blood vessels are active, resulting in loss of these functions (5, 6). Because DA treatment normalized tumor blood vessels, and since Krüppel-like factor-2 (KLF2), a member of Krüppel-like factor family of transcription factors, has been reported to induce quiescence in endothelial cells (48, 49), we determined the expression of this transcription factor in TEC isolated and collected from PC3 and HT29 tumor-bearing mice, using techniques we had used before (36, 50). In these experiments TEC were pooled from tumor-bearing mice. Although KLF2 was absent in TEC collected from untreated controls, significantly higher expression of KLF2 was demonstrated by Western blot and confocal microscopy in TEC collected from DA-treated animals (Fig. 5 A and B). However, this action of DA in TEC was abrogated when animals were treated with eticlopride, before treatment with DA (Fig. 5 A and B), thus indicating that this action of DA was mediated specifically through its D2 receptors. Our Western blot data further confirmed that TEC isolated from PC3 and HT29 tumor tissues express DA D2 receptors (Fig. 5C).
Fig. 5.
DA treatment up-regulates KLF2 expression in TEC. Western blot (A) and confocal images (B) show high expression of KLF2 in TEC isolated from DA-treated PC3 and HT29 tumors. TEC isolated from mice receiving treatment with the DA D2 receptor antagonist eticlopride before treatment with DA did not show this high expression of KLF2. (C) Western blot analysis confirming the presence of DA D2 receptors in TEC isolated from PC3 and HT29 tumors but not in control S180 cells. (D) Western blot shows the presence of DA D2 receptors in HUVEC. (E) Western blot analysis and (F) confocal images show up-regulation of KLF2 in HUVEC following treatment with DA and the DA D2 receptor-specific agonist quinpirole (Quin) for 6 h. However, the up-regulation of KLF2 expression was abrogated by prior treatment of HUVEC with eticlopride. (Scale bars in B and F, 50 μm.)
DA-Mediated KLF2 Expression in Endothelial Cells Is Direct and Occurs Specifically Through D2 Receptors Present in These Cells.
To confirm the direct effect of DA on KLF2 expression in endothelial cells, serum-starved human umbilical vein endothelial cells (HUVEC) expressing the DA D2 receptor (Fig. 5D) were treated with DA (1 μM) or with a DA D2 receptor-specific agonist, quinpirole (1 μM). In contrast to the low levels of KLF2 expressed in untreated control HUVEC, as detected by Western blot and confocal microscopy, KLF2 expression was increased significantly 6 h after DA or quinpirole treatment in these cells (Fig. 5 E and F). However, pretreatment of these cells with eticlopride (1 μM), inhibited these effects of DA or quinpirole, thus indicating that this effect of DA occurs through its D2 receptors (Fig. 5 E and F). In addition, these experiments performed in serum-starved HUVEC indicate that the effect of DA on KLF2 expression in endothelial cells is direct and independent of its actions on growth factors. These results thus confirm that DA directly can induce KLF2 expression and quiescence in endothelial cells by acting specifically through its D2 receptors present in these cells. Since KLF2 plays a prominent role in preventing vascular leakage, and because KLF2 can inhibit hydrogen peroxide (H2O2)-induced permeability in HUVEC, we investigated the effect of DA treatment on H2O2-induced permeability in these cells (SI Results and Fig. S7) (51).
Activation of ERK5 by Stimulation of DA D2 Receptors Is Associated with Increased Expression of KLF2 in Endothelial Cells.
ERK5 is a member of the MAPK family (52–54). Because ERK5 activation is absolutely necessary for KLF2-mediated endothelial cell homeostasis, we investigated the effect of DA on ERK5 function and its correlation with DA-induced KLF2 expression in endothelial cells (SI Results and Fig. S8) (52–54).
Discussion
Blood vessels in solid tumor are structurally and functionally abnormal in all aspects, with impaired blood flow that leads to a hostile hypoxic microenvironment within the tumor (1–4). In the present investigation, an association between these structural and functional abnormalities of tumor blood vessels and the loss of sympathetic nerve supply to tumor blood vessels and subsequent depletion of neurotransmitter DA was observed in colon and prostate tumor tissues. This relationship was strengthened further, because treatment with a low, nontoxic dose of exogenous DA could normalize these tumor vessels by correcting the structural defects, increasing blood flow, and decreasing hypoxia in tumor tissues. The mechanism was attributed to DA D2 receptor-mediated direct and specific effects on both pericytes and endothelial cells in the tumor vascular bed. Thus, as a consequence of this action, the concentration and efficacy of the anticancer drug 5-FU was increased considerably in DA-treated tumor tissues.
Angiopoietin ligands (Ang1 and Ang2) and their receptor, TEK tyrosine kinase endothelial (Tie2), are critical for vessel formation and stabilization (39–44). Ang1, on binding to the Tie2 receptor, activates it by inducing dimerization, which results in phosphorylation of the kinase domain of Tie2. Ang2 also binds to Tie2 but does not induce phosphorylation of Tie2 at physiological concentrations and has been suggested to work as a naturally occurring antagonist of Ang1 (39–44). It has been reported that Ang1 produced by the pericytes imparts stabilizing signals to the endothelium that increase the higher-order structure and function of blood vessels (39–44). Furthermore Ang1-null mice die at midgestation from cardiovascular failure, and their blood vessels show reduced pericyte coverage (39). Overexpression of Ang1, on the other hand, leads to a stabilized vasculature (39–44). Also, treatment with recombinant Ang1 has been shown to rescue retinal defects caused by pericyte loss (39, 46). Here we report that DA can induce overexpression of Ang1 directly in the pericytes and also can regulate the mobilization and recruitment of these cells, in turn mediating normalization of tumor blood vessels. This action of DA is direct and independent of its action on vascular endothelial growth factor.
KLF2 is a member of Krüppel-like factor family of transcription factors, a multigene family of transcription factors with a zinc finger domain (55). KLF2 expression in endothelial cells has been shown to modulate vessel stabilization, and this transcription factor also is a critical regulator of various functions of endothelial cells, including differentiation and proliferation (48, 51, 56). Furthermore, KLF2 has been shown to induce quiescence in endothelial cells so that endothelial barrier and other normal functions of the blood vessels are maintained (48, 49, 51, 56). Our results show that DA can directly and specifically induce KLF2 expression in endothelial cells and thereby regulate endothelial permeability. This action of DA also is independent of its action on VEGF. These results indicate that this neurotransmitter can mediate remodeling of blood vessels by regulating endothelial cell quiescence, vessel stabilization, and normal vascular barrier function.
Furthermore ERK5, also known as big MAPK1, belongs to the MAPK family (52–54), and it is the most critical factor controlling the expression of KLF2 in endothelial cells (52). It differs from other MAPKs in possessing a transcriptional activation domain (52–54). In the present study, we demonstrate that DA can regulate the expression of KLF2 in endothelial cells directly and specifically through the ERK5 pathway, because silencing ERK5 in these cells abolishes the effects of DA.
It has been reported that reduced blood flow in tumor blood vessels resulting from abnormal vascular architecture not only lowers the concentration of anticancer drugs in tumor tissues but also induces hypoxia in these tissues, thereby making the tumor cells further resistant to these drugs (1–3). Therefore, in the treatment of cancer patients, normalization of tumor blood vessels may be an important approach to increase the efficacy of anticancer drugs (1, 3, 7). Our present study demonstrates that DA, by stabilizing tumor blood vessels, significantly increases tumor blood flow and decreases hypoxia in tumor tissues. In addition, we also observed that DA, by increasing tumor blood flow and reducing tumor hypoxia, improves the efficacy of anticancer drugs in tumor-bearing animals. Thus, this effect of DA is unique. In our previous study, DA was administered simultaneously with an anticancer drug. Therefore, DA-mediated increase in the efficacy of the antineoplastic drug in that study was not caused by a DA-induced normalization of tumor vessels (36). Furthermore, our findings also confirm that the other two catecholamines, epinephrine and norepinephrine, have no effect on the process of normalization of tumor blood vessels. DA acts directly and specifically through its D2 receptors present in both pericytes and endothelial cells in the tumor vascular bed to promote vascular maturation, and DA D1 receptors are not involved in this process. Most importantly, these results show improved drug delivery in tumor tissues through a newly identified, neurotransmitter-mediated mechanism.
Finally, our study not only establishes an important role for a neurotransmitter in vascular remodeling but also indicates a use for DA or its D2 receptor-specific agonists in patients with cancer and/or other disorders in which normalization of dysfunctional blood vessels may improve the therapeutic response (1–3, 7, 57).
Materials and Methods
A detailed description of the protocol is provided in SI Materials and Methods. All reagents and additional procedures used in this study, including cell and reagents, mice, tumor transplantation, treatment, blood vessel morphology and permeability assay, laser Doppler flowmetry, tissue hypoxia study, confocal microscopy, quantification of drug concentration in tumor tissues, tumor growth measurement, isolation of tumor endothelial cells, wound assay, Western blot analysis, and statistical analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Shubham Bakshi for technical support. Confocal microscopy was undertaken at the campus microscopy and imaging core facility of Ohio State University. This work was supported by National Institutes of Health Grant CA124763 (to S.B.) and by US Department of Defense Grant W81XWH-07-1-0051 (to S.B.). D.C. was supported in part by American Heart Assciation Grant 10BGIA4230012.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108696108/-/DCSupplemental.
References
- 1.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 2.Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: Novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamzah J, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 5.Engerman RL, Pfaffenbach D, Davis MD. Cell turnover of capillaries. Lab Invest. 1967;17:738–743. [PubMed] [Google Scholar]
- 6.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: Continuous labelling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 8.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 9.Rubí B, Maechler P. Minireview: New roles for peripheral dopamine on metabolic control and tumor growth: Let's seek the balance. Endocrinology. 2010;151:5570–5581. doi: 10.1210/en.2010-0745. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: An update. Brain Behav Immun. 2010;24:525–528. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuru H, Tanimitsu N, Hirai T. Role of perivascular sympathetic nerves and regional differences in the features of sympathetic innervation of the vascular system. Jpn J Pharmacol. 2002;88:9–13. doi: 10.1254/jjp.88.9. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P. Blood vessels and nerves: Common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 13.Krimer LS, Muly EC, 3rd, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, et al. SOAP II Investigators Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 15.Zeng C, Zhang M, Asico LD, Eisner GM, Jose PA. The dopaminergic system in hypertension. Clin Sci (Lond) 2007;112:583–597. doi: 10.1042/CS20070018. [DOI] [PubMed] [Google Scholar]
- 16.Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: Definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukamoto K, et al. Projections to the alimentary canal from the dopaminergic neurons in the dorsal motor nucleus of the vagus of the rat. Auton Neurosci. 2005;123:12–18. doi: 10.1016/j.autneu.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Katzung BG. In: Anatomy of the Autonomic Nervous System. Katzung BG, Masters SB, Trevor AJ, editors. New York: McGraw-Hill; 2009. p. 78. [Google Scholar]
- 19.Amenta F, Ricci A, Tayebati SK, Zaccheo D. The peripheral dopaminergic system: Morphological analysis, functional and clinical applications. Ital J Anat Embryol. 2002;107:145–167. [PubMed] [Google Scholar]
- 20.Tayebati SK, Lokhandwala MF, Amenta F. Dopamine and vascular dynamics control: Present status and future perspectives. Curr Neurovasc Res. 2011;8:246–257. doi: 10.2174/156720211796558032. [DOI] [PubMed] [Google Scholar]
- 21.Davies MA, et al. Adenoviral-mediated expression of MMAC/PTEN inhibits proliferation and metastasis of human prostate cancer cells. Clin Cancer Res. 2002;8:1904–1914. [PubMed] [Google Scholar]
- 22.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- 23.Burt AD, et al. Localization of adrenergic and neuropeptide tyrosine-containing nerves in the mammalian liver. Hepatology. 1989;9:839–845. doi: 10.1002/hep.1840090608. [DOI] [PubMed] [Google Scholar]
- 24.Miller LE, Jüsten HP, Schölmerich J, Straub RH. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J. 2000;14:2097–2107. doi: 10.1096/fj.99-1082com. [DOI] [PubMed] [Google Scholar]
- 25.Liang CS, Yatani A, Himura Y, Kashiki M, Stevens SY. Desipramine attenuates loss of cardiac sympathetic neurotransmitters produced by congestive heart failure and NE infusion. Am J Physiol Heart Circ Physiol. 2002;282:H363–H371. doi: 10.1152/ajpheart.00853.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kanazawa H, et al. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest. 2010;120:408–421. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldrup E, Richter EA. DOPA, dopamine, and DOPAC concentrations in the rat gastrointestinal tract decrease during fasting. Am J Physiol Endocrinol Metab. 2000;279:E815–E822. doi: 10.1152/ajpendo.2000.279.4.E815. [DOI] [PubMed] [Google Scholar]
- 28.Wagner J, Palfreyman M, Zraika M. Determination of dopa, dopamine, dopac, epinephrine, norepinephrine, alpha-monofluoromethyldopa and alpha-difluoromethyldopa in various tissues of mice and rats using reversed-phase ion-pair liquid chromatography with electrochemical detection. J Chromatogr A. 1979;164:41–54. doi: 10.1016/s0378-4347(00)81570-4. [DOI] [PubMed] [Google Scholar]
- 29.Walker JK, Gainetdinov RR, Mangel AW, Caron MG, Shetzline MA. Mice lacking the dopamine transporter display altered regulation of distal colonic motility. Am J Physiol Gastrointest Liver Physiol. 2000;279:G311–G318. doi: 10.1152/ajpgi.2000.279.2.G311. [DOI] [PubMed] [Google Scholar]
- 30.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Satchi-Fainaro R, et al. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005;7:251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Zaroslinski JF, et al. The pharmacology and subacute toxicology of dopamine. Proc R Soc Med. 1977;70(Suppl 2):2–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Berdel WE, et al. Influence of 1-beta-D-arabinofuranosylcytosine conjugates of lipids on the growth and metastasis of Lewis lung carcinoma. Cancer Res. 1988;48:826–829. [PubMed] [Google Scholar]
- 34.Samoszuk MK, Walter J, Mechetner E. Improved immunohistochemical method for detecting hypoxia gradients in mouse tissues and tumors. J Histochem Cytochem. 2004;52:837–839. doi: 10.1369/jhc.4B6248.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ley CD, Horsman MR, Kristjansen PE. Early effects of combretastatin-A4 disodium phosphate on tumor perfusion and interstitial fluid pressure. Neoplasia. 2007;9:108–112. doi: 10.1593/neo.06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502–2510. doi: 10.1158/1078-0432.CCR-07-1778. [DOI] [PubMed] [Google Scholar]
- 37.Adams ER, Leffert JJ, Craig DJ, Spector T, Pizzorno G. In vivo effect of 5-ethynyluracil on 5-fluorouracil metabolism determined by 19F nuclear magnetic resonance spectroscopy. Cancer Res. 1999;59:122–127. [PubMed] [Google Scholar]
- 38.Remaud G, Boisdron-Celle M, Morel A, Gamelin A. Sensitive MS/MS-liquid chromatography assay for simultaneous determination of tegafur, 5-fluorouracil and 5-fluorodihydrouracil in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;824:153–160. doi: 10.1016/j.jchromb.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 40.Morikawa S, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: Cellular interactions and contributions to malignancy. Cancer Res. 2005;65:9741–9750. doi: 10.1158/0008-5472.CAN-04-4337. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 43.Fiedler U, Augustin HG. Angiopoietins: A link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 45.Ewing AG, Bigelow JC, Wightman RM. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science. 1983;221:169–171. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- 46.Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2163–2171. doi: 10.1167/iovs.07-1206. [DOI] [PubMed] [Google Scholar]
- 47.Iurlaro M, et al. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–3643. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- 48.Dekker RJ, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 49.Boon RA, et al. KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol. 2007;27:532–539. doi: 10.1161/01.ATV.0000256466.65450.ce. [DOI] [PubMed] [Google Scholar]
- 50.Chakroborty D, et al. Depleted dopamine in gastric cancer tissues: Dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–4356. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- 51.Lin Z, et al. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sohn SJ, Li D, Lee LK, Winoto A. Transcriptional regulation of tissue-specific genes by the ERK5 mitogen-activated protein kinase. Mol Cell Biol. 2005;25:8553–8566. doi: 10.1128/MCB.25.19.8553-8566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts OL, Holmes K, Müller J, Cross DA, Cross MJ. ERK5 and the regulation of endothelial cell function. Biochem Soc Trans. 2009;37:1254–1259. doi: 10.1042/BST0371254. [DOI] [PubMed] [Google Scholar]
- 54.Nigro P, et al. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood. 2010;116:1971–1979. doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson KP, Kern CB, Crable SC, Lingrel JB. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Krüppel-like factor: Identification of a new multigene family. Mol Cell Biol. 1995;15:5957–5965. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuo CT, et al. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain RK, Finn AV, Kolodgie FD, Gold HK, Virmani R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: A potential strategy for plaque stabilization. Nat Clin Pract Cardiovasc Med. 2007;4:491–502. doi: 10.1038/ncpcardio0979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.