Abstract
The linear ubiquitin chain assembly complex (LUBAC) is a key nuclear factor-κB (NF-κB) pathway component that produces linear polyubiquitin chains. The HOIL-1L subunit of LUBAC has been shown to bind linear chains; however, detailed structural and functional analyses on the binding between LUBAC and linear chains have not been performed. In this study, we found that the Npl4 zinc finger (NZF) domain of HOIL-1L specifically binds linear polyubiquitin chains and determined the crystal structure of the HOIL-1L NZF domain in complex with linear diubiquitin at 1.7-Å resolution. The HOIL-1L NZF domain consists of a zinc-coordinating “NZF core” region and an additional α-helical “NZF tail” region. The HOIL-1L NZF core binds both the canonical Ile44-centered hydrophobic surface on the distal ubiquitin and a Phe4-centered hydrophobic patch on the proximal ubiquitin, representing a mechanism for the specific recognition of linear chains. The NZF tail binds the proximal ubiquitin to enhance the binding affinity. These recognition mechanisms were supported by the accompanying in vitro and in vivo structure-based mutagenesis experiments.
Keywords: crystallography, surface-plasmon resonance, inflammatory signaling
Nuclear factor-κB (NF-κB) plays essential roles to regulate gene expression during inflammatory and immune responses (1, 2). Signaling for NF-κB activation involves the synthesis of polyubiquitin (polyUb) chains linked by Lys48, Lys63, Met1, and Lys11 (referred to hereafter as K48, K63, linear, and K11 chains, respectively) (3–10). Inflammatory cytokines such as tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β) induce the formation of polyUb chains that have been postulated to recruit enzyme complexes important for NF-κB activation. For IL-1β-induced NF-κB activation, K63 chains recruit the transforming growth factor β-activated kinase 1 (TAK1) and inhibitor of NF-κB (IκB) kinase (IKK) complexes (8, 9, 11, 12). The catalytic IKKβ subunit of the IKK complex is activated by TAK1 and phosphorylates IκB, leading to its K48-linked ubiquitination and proteasomal degradation and to NF-κB activation (1, 2, 8, 9, 12–14). For TNFα-induced NF-κB activation, polyUb chains are believed to recruit kinase complexes, although the division of the roles of linear, K11, K63, and K48 chains for the recruitment remains obscure (15).
Stimulation by TNFα and IL-1β induces the formation of linear chains. In response to the stimulation, a linear Ub chain assembly complex (LUBAC) conjugates linear chains onto specific lysine residues in the regulatory subunit of IKK complex [termed IKKγ or NF-κB essential modulator (NEMO)] (i.e., K285 and K309 in human NEMO) (16). LUBAC was first identified as a heterodimeric complex consisting of HOIP and HOIL-1L. Recently, SHANK-associated RH domain interacting protein (SHARPIN) was identified as the third component of LUBAC (17–19). Loss of SHARPIN causes chronic dermatitis and an immunodeficiency in mice (17–21). SHARPIN can form a heterodimeric complex with HOIP and a heterotrimeric complex with HOIP and HOIL-1L. Both complexes can conjugate linear chains on NEMO as well as the heterodimeric complex consisting of HOIP and HOIL-1L (17–19). The conjugated linear chains are specifically recognized by NEMO, HOIL-1L, and SHARPIN and are critical for NF-κB activation.
Both HOIL-1L and SHARPIN contain a Ub-like (UBL) domain and a Npl4 zinc-finger (NZF) domain (22). The UBL domains of HOIL-1L and SHARPIN are required for the binding to HOIP to assemble LUBAC (17–19). Although the UBL domain of HOIL-1L (and presumably SHARPIN) is sufficient to support linear chain synthesis by HOIP (22), cytokine induced NF-κB activation requires the NZF domain of HOIL-1L and SHARPIN (16–18). NZF domains are typically approximately 30 residue protein–protein interaction modules that coordinate a single zinc ion with four conserved cysteine residues (23, 24). Most NZF domains found in proteins involved in Ub-related processes bind to Ub with moderate affinities (Kd values of 100 μM or higher) (23–25) via a highly conserved TF/Φ motif, which binds an Ile44-centered hydrophobic patch on Ub (23, 24). Although Ub-binding NZF domains typically bind polyUb chains without apparent specificity, the NZF domains of TAB2 and TAB3 specifically bind to K63 chains due to the presence of an additional Ub-binding surface that is conserved in TAB2 and TAB3 (26–28).
Recently, HOIL-1L was found to specifically bind linear chains (12), but it remains unclear how HOIL-1L specifically recognizes linear chains and whether this recognition is important for NF-κB activation. We present data demonstrating that the NZF domain of HOIL-1L specifically binds linear chains and report a crystal structure of the HOIL-1L NZF domain in complex with a linear diUb chain (linear Ub2) at 1.7-Å resolution. We found that the HOIL-1L NZF domain consists of a zinc-coordinating “NZF core” and a following α-helical “NZF tail,” which is unique to HOIL-1L. In addition to recognizing the Ile44-centered hydrophobic patch of the distal Ub in a manner similar to previously reported NZF•Ub interactions, the NZF core also binds a Phe4-centered hydrophobic patch of the proximal Ub in a manner that enables the specific binding of linear chains. The NZF tail contacts the proximal Ub to enhance the binding affinity. Together with in vitro and in vivo structure-based mutagenesis experiments, our structure reveals the mechanism for specific recognition of linear chains by the HOIL-1L NZF domain and the molecular basis for this important component of NF-κB activation pathways.
Results
The NZF Domain of HOIL-1L Specifically Binds to Linear Ub2.
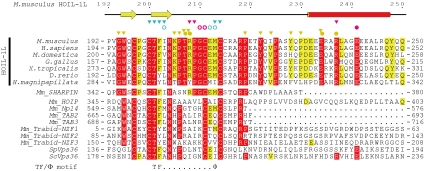
The N-terminal half of HOIL-1L contains single UBL and NZF domains (Fig. S1 and Table S1) (22). Recent structural studies examining Ub-binding NZF domains have revealed an important consensus motif TF/Φ (Φ, any hydrophobic residues) for Ub binding (23, 24, 27, 28). Amino acid sequence alignment of HOIL-1L NZF domains from several organisms indicated that the NZF domain of HOIL-1L contains a highly conserved TF/Φ motif, suggesting that the NZF domain likely interacts with Ub (Fig. S2). In addition, the sequence alignment of HOIL-1L highlights another conserved sequence following the NZF domain (corresponding to residues 221–250 of mouse HOIL-1L), suggesting that this additional conserved region could also be involved in the interaction with Ub (Fig. S2). Surface-plasmon resonance (SPR) analyses using K48-Ub2, K63-Ub2, and linear Ub2 species clearly showed that the HOIL-1L NZF domain specifically binds to linear Ub2 (Kd values of 17.2, 317, and 330 μM for linear Ub2, K48-Ub2, and K63-Ub2, respectively) and that the additional conserved region enhances the affinity between HOIL-1L and linear Ub2 6.8-fold (Table 1 and Fig. S3). Therefore, we refer to the region containing residues 192–250 as the “NZF domain,” whereas the regions composed of residues 192–220 and residues 221–250 are referred to as “NZF core” and “NZF tail,” respectively. The linkage-specific interaction of the HOIL-1L NZF domain with linear Ub2 is distinct from the linkage-dependent interaction between the TAB2/TAB3 NZF domain and K63-Ub2 and the linkage-independent interaction between the Npl4 NZF domain and Ub (Table 1 and Fig. S4). The HOIL-1L NZF domain is a unique Zn finger motif that specifically recognizes linear chains.
Table 1.
Dissociation constants determined by SPR analyses
|
KD, μM |
|||||
| MonoUb | Linear Ub2 | K48-Ub2 | K63-Ub2 | ||
| HOIL-1L | WT | 462 ± 19 | 17.2 ± 0.1 | 317 ± 19 | 330 ± 13 |
| Δtail | 390 ± 22 | 118 ± 13 | 487 ± 31 | 528 ± 28 | |
| T201A | 2,100 ± 305 | 996 ± 48 | 2,045 ± 16 | 1,972 ± 116 | |
| R208A | 564 ± 15 | 261 ± 2 | 398 ± 19 | 390 ± 21 | |
| Npl4 | 300 ± 3 | 297 ± 2 | 215 ± 4 | 336 ± 4 | |
| TAB2 | 918 ± 35 | 1,096 ± 43 | 680 ± 47 | 64.2 ± 1.0 | |
| NEMO | 152 ± 10 | 4.48 ± 0.03 | 169 ± 7 | 140 ± 3 | |
(means ± SD).
Overall Structure of the NZF Domain of HOIL-1L in Complex with Linear Ub2.
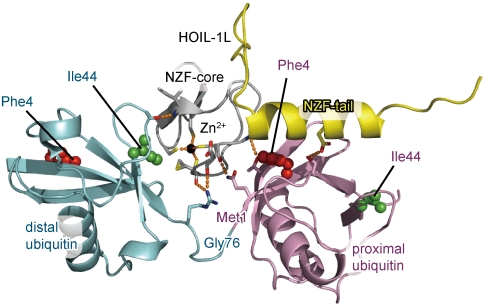
To reveal the structural basis of the specific interaction between the HOIL1-L NZF domain and linear chains, we determined crystal structures of the HOIL-1L NZF domain in complex with linear Ub2 (Fig. 1). Two distinct crystal forms diffracting up to 1.7- and 1.9-Å resolutions exhibit nearly identical stoichiometric complexes (rmsd < 0.55 Å over 199 residues excluding 17 amino acids in structurally variable regions). The HOIL-1L NZF core (residues 192–220) consists of a pair of antiparallel β-sheets and a following loop (residues 204–220), which is almost identical to those of the Npl4, TAB2, and TAB3 NZF domains (rmsd < 0.66 Å; 24 residues in total, except for the structurally variable regions). A zinc ion is coordinated by Cys197, Cys200, Cys211, and Cys214 of HOIL-1L (Fig. S5). The NZF tail of HOIL-1L consists of a connecting loop (residues 221–231) and a helix (residues 232–245). Pro219, Tyr222, Ile224, and Tyr228 on the connecting loop surround two loops containing residues 194–195 and residues 205–208 in the NZF core with extensive hydrophobic interaction, while three hydrogen bonds are formed between the NZF core and tail (Fig. S6). These intramolecular interactions appear likely to define the relative orientation between the NZF core and tail. The interactions between the NZF core and the connecting loop are crucial for the binding to linear Ub2, as shown by pull-down assays using the HOIL-1L mutants, P219A and Y222A (Fig. 2A). The covalent linkage connecting the distal and proximal Ub moieties does not contact the HOIL-1L NZF domain (Fig. 1 and Fig. S5B), as observed in several other structures of complexes between Ub receptors and Ub2 species (14, 27–29).
Fig. 1.
Crystal structure of the HOIL-1L NZF domain in complex with linear Ub2. The NZF core and tail of HOIL-1L are colored gray and yellow, respectively. The proximal and distal Ub moieties are colored pink and cyan, respectively. Phe4 and Ile44 of Ub moieties are shown as red and green spheres, respectively. A zinc ion is shown as a black sphere. Hydrogen bonds are indicated as dashed orange lines.
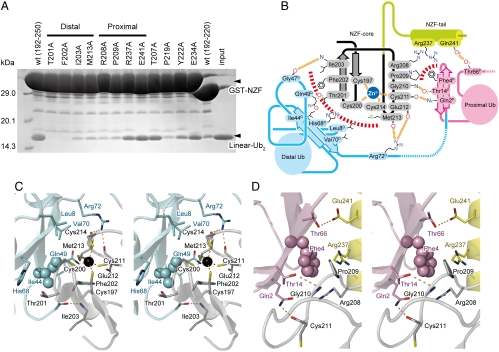
Fig. 2.
Specific recognition of the distal and proximal Ub moieties by the HOIL-1L NZF domain. (A) Pull-down assays of GST-fused HOIL-1L NZF mutants with linear Ub2. Bound proteins were analyzed by SDS-PAGE and stained with Coomassie brilliant blue. (B) Schematic representation of the binding interface between the HOIL-1L NZF domain and linear Ub2. Hydrogen bonds and hydrophobic interactions are displayed as dashed orange and red lines, respectively. The NZF core and tail in HOIL-1L are labeled by gray and yellow backgrounds, respectively. The proximal and distal Ub moieties are highlighted by pink and cyan backgrounds, respectively. (C) Stereo view of the interface between the distal Ub and the HOIL-1L NZF domain. Drawing schemes are the same as in Fig. 1. (D) Stereo view of the interface between the proximal Ub and the HOIL-1L NZF domain. Drawing schemes are the same as in Fig. 1
Distal Ub Recognition.
Similar to the NZF domains of TAB2 and TAB3, the HOIL-1L NZF domain interacts with the Ile44-centered hydrophobic patch of the distal Ub, which is formed by the side chains of Leu8, Ile44, and Val70 and aliphatic portions of Gln49 and His68 side chains. This Ile44-centered patch is recognized by a hydrophobic surface of the HOIL-1L NZF domain, which is formed by the side chains of Phe202, Ile203, and Met213 and aliphatic portions of the Cys200, Thr201, and Cys214 side chains (Fig. 2 B and C). Thr201, Phe202, and Met213 of HOIL-1L constitute the consensus TF/Φ motif for the Ub-binding NZF domain, whereas Ile203 is conserved or replaced by functionally equivalent hydrophobic residues (Fig. 3). Further, the main chain NH of Ile203 and the main chain CO of Glu212 and Met213 in HOIL-1L hydrogen bond with the main chain CO of Gly47 and the Nη atom of Arg72 in the distal Ub, respectively (Fig. 2 B and C). Pull-down analyses using the HOIL-1L mutants, T201A, F202A, I203A, and M213A, which were designed to disrupt distal Ub binding, confirmed that the observed hydrophobic interactions between the HOIL-1L NZF domain and the distal Ub are critical for binding to linear Ub2 (Fig. 2A). An SPR analysis showed that the most critical mutant T201A reduced the affinity 58-fold (Table 1).
Fig. 3.
Comparison of the amino acid sequences of Ub-binding NZF domains; 100% and more than 70% identical residues among the HOIL-1L subunits from representative organisms are highlighted by red and yellow background, respectively. A residue that hydrogen bonds to the proximal Ub by the side chain is marked with a pink filled circle. Residues that hydrogen bond to the proximal and distal Ub moieties by their main chains but not side chains are marked with pink and cyan open circles, respectively. Residues that are involved in hydrophobic interactions with the proximal and distal Ub moieties are marked with pink and cyan inverted triangles, respectively. Residues involved in interactions between the NZF core and tail are marked with either yellow inverted triangles (hydrophobic interactions) or circles (hydrophilic interactions). The secondary structure of the mouse HOIL-1L NZF domain is shown above the alignment. The TF/Φ motif is shown below the alignment. Abbreviations are as follows: Mm, Mus musculus; Sp, Schizosaccharomyces pombe; Sc, Saccharomyces cerevisiae.
Proximal Ub Recognition.
In contrast to the canonical recognition of the distal Ub, the NZF domain of HOIL-1L interacts with the Phe4-centered patch of the proximal Ub, which is distinct from and does not overlap with the Ile44-centered patch discussed above. Pro209 and aliphatic portions of Arg208 and Arg237 in the NZF tail of HOIL-1L form hydrophobic interactions with the side chain of Phe4 in the proximal Ub and bury 407 Å2 (Fig. 2 B and D). These residues are highly conserved and unique to the HOIL-1L NZF domain, suggesting that HOIL-1L may be unique in its mechanism of interaction with linear chains (Fig. 3). Adjacent to this hydrophobic interface, the main-chain CO of Cys211 and NH of Gly210 in HOIL-1L hydrogen bond with the Nϵ atom of Gln2 and Oγ atom of Thr14 in the proximal Ub, respectively (Fig. 2 B and D). The Oϵ atom of Glu241 in HOIL-1L hydrogen bonds with the Oγ atom of Thr66 in the proximal Ub. The contribution of these intermolecular interactions to linear Ub2 recognition was examined by pull-down analyses using HOIL-1L mutants designed to disrupt proximal Ub binding. The most disruptive mutations were R208A and P209A in the NZF core, which exhibited drastic decreases in their binding to linear Ub2 relative to wild type (Fig. 2A). An SPR analysis showed that R208A mutation reduced affinity 15-fold (Table 1). R237A and E241A mutations in the NZF tail only modestly decrease binding (Fig. 2A). Interaction with the NZF core is thus critical for binding affinity and specificity, while the NZF tail appears to enhance binding affinity.
Linkage Specificity.
Recent structural studies have shown that linkage-specific Ub receptors recognize the spacing between the distal and proximal Ub moieties when fixed in a specific conformation to discriminate the cognate Ub chains from the other noncognate chains (14, 27–31). This strategy is also applied in the specific recognition of linear chains by the HOIL-1L NZF domain. The proximal and distal Ub-binding residues in the HOIL-1L NZF domain are arranged to capture the specific conformation of linear chains by simultaneous binding to both proximal and distal Ub moieties, where the inter-Ub spacing is recognized. There is no direct contact between the HOIL-1L NZF domain and the linear linkage between the distal and proximal Ub moieties (Fig. 1 and Fig. S5B).
In the HOIL-1L-NZF•linear Ub2 complex, Met1 is nearest (approximately 12 Å) to residue Leu71 (positioned in the end of the last β-sheet) in the distal Ub (Fig. S7). With this orientation and spacing, the conjugated C-terminal tail conformation of the distal Ub appears likely to be unstrained and further stabilized by hydrogen bonds with the proximal Ub (Fig. S8). Although Met1 and Lys63 are located near one another on the Ub surface, the ϵ-amino group of Lys63 projects away from the terminal amino group of Met1 and is approximately 19 Å from Leu71 in the distal Ub in the HOIL-1L-NZF•linear Ub2 complex (Fig S7). The ϵ-amino and δ-methylene groups of Lys63 are not fixed in the HOIL-1L NZF domain and thus may swing to come closer (approximately 15 Å) to the C-terminal tail of the distal Ub. Even in such a case, however, the C-terminal tail conformation of the distal Ub that enables Gly76-Lys63 isopeptide linkage would likely require considerable deformation and lack the aforementioned hydrogen bonds with the proximal Ub, which would be energetically disadvantageous. Lys48 is located on the face opposite Met1 (Fig S7). Thus, the HOIL-1L NZF domain binds neither K63 nor K48 chains. Although the present study shows that the HOIL-1L NZF domain specifically binds to linear chains, we cannot exclude the possibility that K29- and/or K33-linked Ub chains could bind the HOIL-1L NZF domain (Fig. S7) because Lys29 and Lys33 are as close to Gly76 in the distal Ub as Met1.
NF-κB Activation Through the Interaction Between the HOIL1-L NZF Domain and Linear Chains.
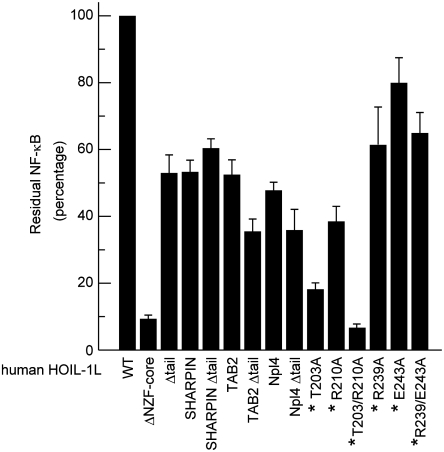
It has been shown previously that the NZF domain of HOIL-1L is essential for TNFα-induced NF-κB activation (16). To further investigate the physiological relevance of the interaction between the HOIL-1L NZF domain and linear chains for NF-κB activation, we used a dual luciferase activity assay to monitor NF-κB activity in HEK293T cells coexpressing wild-type HOIP and mutant HOIL-1L whose NZF core or domain (i.e., core plus tail) is replaced by the TAB2 or Npl4 NZF domain. As mentioned above, the TAB2 NZF domain specifically binds to K63 chains, whereas the Npl4 NZF domain binds to Ub chains of any linkage with similar affinities (Table 1 and Fig. S4). In either case, replacement of the HOIL-1L NZF core or domain reduced NF-κB activation significantly, about 2- or 2.5-fold, respectively (Fig. 4). This result indicates that the specific interaction between the HOIL-1L NZF domain and linear chains is important for NF-κB activation.
Fig. 4.
NF-κB activation by HOIL-1L mutants. Relative luciferase activities in HEK293T cells coexpressing the indicated HOIL-1L mutants (mean ± SEM, n = 3) and the wild-type HOIP are shown, where the activity in the cells coexpressing the wild-type HOIL-1L and HOIP is set to 100%. Residue numbers marked with asterisks refer to human HOIL-1L (see also the main text and Table S2).
Further, the functional importance of the distal and proximal Ub recognition, which was revealed by the present HOIL-1L NZF•linear Ub2 complex structure, was demonstrated by 5- and 2.5-fold reduction in NF-κB activation by human HOIL-1L T203A (equivalent to mouse HOIL-1L T201A) and R210A (equivalent to mouse HOIL-1L R208 mutant) mutants, respectively (Fig. 4). A T203A/R210A double mutation in human HOIL-1L reduced activation of NF-κB 15-fold, demonstrating that the simultaneous disruption of both distal and proximal Ub binding is comparable to that observed with deletion of the entire NZF domain (Fig. 4). A mutant lacking either NZF helix or tail (i.e., the helix plus the connecting loop) reduced activation 2-fold, whereas either or both R239A and E243A mutations reduced activation moderately, 1.3- to 1.7-fold (Fig. 4). These results are consistent with the results of in vitro binding assays using SPR and pull-down analyses.
Comparison of Linear Chains Recognition by NZF and UBAN.
The domain for Ub-binding of ABINs and NEMO (UBAN) is the only other Ub-binding domain known to specifically bind linear chains (Table 1). The crystal structure of the NEMO UBAN domain in complex with linear Ub2 revealed a recognition mechanism of linear chains by NEMO UBAN domain (14). The basis for the binding to both proximal and distal Ub moieties is completely different between HOIL-1L NZF and NEMO UBAN (i.e., zinc finger versus coiled coil). Thus, the relative orientation between the distal and proximal Ub moieties in the HOIL-1L-bound state is totally different from those in its free and NEMO-bound states (Fig. S9) (14, 32). Superposition of the distal Ub moiety in the NEMO UBAN•linear Ub2 complex onto that in the HOIL-1L NZF•linear Ub2 complex shows that the proximal Ub moieties in these two complexes are related by 97° rotation (Fig. S10). In contrast, the NZF of HOIL-1L and UBAN of NEMO appear to be unique in not binding the canonical Ile44-centered hydrophobic patch of the proximal Ub and instead recognizing the Phe4-centered patch (Fig. S11) (14). The side chains of Val316 in mouse NEMO and Pro209 in mouse HOIL-1L are stacked on the aromatic ring of Phe4 in the proximal Ub. Around this hydrophobic interaction, the Oϵ atoms of Glu320 in mouse NEMO and Glu241 in mouse HOIL-1L hydrogen bond with the Oγ atom of Thr66 in the proximal Ub, while the Nη atom of Arg312 in mouse NEMO and carbonyl oxygen of Cys211 in mouse HOIL-1L hydrogen bond with the Nϵ atom of Gln2 in the proximal Ub. Recognition of the Phe4-centered patch may be a common strategy for specific binding to linear chains.
Comparison Between the HOIL-1L and TAB2 NZF Domains.
The NZF domain of HOIL-1L binds the distal and proximal Ub moieties with buried surface areas of 419 and 407 Å2, respectively, whereas that of TAB2 does the same with buried surface areas of 332 and 367 Å2, respectively. Superposition of the NZF structures of TAB2 and HOIL-1L shows that the shape of the interacting surface for the distal Ub moiety is essentially the same (Fig. 3), although we observe subtle rotational differences (approximately 14°) in the relative orientation between the distal Ub and the NZF domain among the present HOIL-1L•linear Ub2 and two reports of TAB2•K63-Ub2 complexes (Fig. S12) (27, 28). Specifically, Thr of the TF/Φ motif directly hydrogen bonds with the distal Ub in the TAB2•K63-Ub2 complex determined by our group. However, the hydrogen bond is indirect, via a bridging water molecule, in the present HOIL-1L•linear Ub2 complex and in the TAB2•K63-Ub2 complex determined by the Komander group (Fig. S13). In contrast to the distal Ub moieties, the proximal Ub moieties in the TAB2 NZF•K63-Ub2 and HOIL-1L NZF•linear Ub2 complexes are differently positioned and related by 141° rotation (Fig. S10).
The NZF domain of TAB2 contains an incomplete TF/Φ motif (i.e., TF/Q motif) and binds monoUb with a lower affinity than that of Npl4, which contains a complete TF/Φ motif (Table 1). Instead, the TAB2 NZF domain has an additional surface that interacts with the Ile44-centered patch of the proximal K63-linked Ub to enhance the affinity for K63 chains (Table 1 and Fig. S11). We therefore previously proposed that an incomplete TF/Φ motif is likely a prerequisite for linkage-specific binding to Ub chains (27). However, this should be reconsidered because the HOIL-1L NZF domain specifically binds linear chains but has the complete TF/Φ motif and binds monoUb with a similar affinity to the Npl4 NZF domain (Table 1). An additional surface for interacting with the proximal Ub is likely to be sufficient for the NZF domain to discriminate between the cognate and noncognate Ub chains by the affinity differences.
Comparison Between the HOIL-1L and SHARPIN NZF Domain.
On the basis of our understanding of the interaction between the HOIL-1L NZF domain and linear Ub2, we analyzed amino acid sequences of other NZF domains to determine whether other proteins are likely to bind linear Ub chains via similar linkage-specific NZF domains. Although the Basic Local Alignment Search Tool (BLAST) could find no other NZF domains that contain the additional NZF tail, the NZF domain of SHARPIN contains a conserved Arg-Pro sequence, which corresponds to Arg208-Pro209 of HOIL-1L (Fig. 3). This dipeptide sequence is a key determinant for binding to linear chains, as shown above. To analyze the specificity of polyUb binding by the NZF domain of SHARPIN, we used a GST pull-down assay. As shown in Fig. S4, the NZF domain of SHARPIN bound to linear Ub2 and weakly to K63-Ub2, but did not bind to K48-Ub2. This result is consistent with a coimmunoprecipitation assay using the full-length SHARPIN with linear, K48 and K63 chains (19), suggesting that the interaction between SHARPIN and Ub chains primarily relies on the NZF domain. Although the SHAPRIN NZF domain preferentially binds to linear chains, it cannot perfectly replace either HOIL-1L NZF core or domain (Fig. 4) for NF-κB activation, likely due to lower affinity to linear chains (Fig. S4).
Discussion
In this study, we have determined the crystal structure of the HOIL-1L NZF domain in complex with a linear Ub chain, illustrating the structural basis for the binding of linear linkage by this domain. SPR and pull-down experiments support the relevance of the observed physical interaction in vitro and our luciferase reporter assays provide insights into the requirements for NF-κB activation in vivo. To further investigate which signaling step requires the HOIL-1L NZF domain for NF-κB activation, we first tested a possibility that the interaction between the HOIL-1L NZF domain and linear chains is responsible for the ligase activity of LUBAC. We performed linear polyubiquitination of NEMO by LUBAC in vitro and in vivo, using a mutant LUBAC comprised of the wild-type HOIP and NZF-domain-lacking or T203A/R210A HOIL-1L mutant (Fig. S14 A and B, respectively). Both mutants can polyubiquitinate NEMO as efficiently as wild-type LUBAC both in vitro and in vivo, showing that the interaction between HOIL-1L NZF and linear chains is irrelevant to the ligase activity of LUBAC. Therefore, we favor another possibility that the HOIL-1L NZF domain recognizes linearly ubiquitinated substrates, for example, in TNF-R1 signaling complexes for NF-κB activation, although we could not conclude that the HOIL-L NZF domain plays a critical role to interact with ubiquitinated substrates in TNF-R1 signaling complexes under the stimulation of TNFα, because of the experimental difficulty in accurately monitoring the temporal-spatial stability of the binding between LUBAC and TNF-R1 signaling complexes in vivo. Signaling cascades controlling NF-κB activation involve interactions among numerous proteins and protein complexes, including a wide variety of Ub chains. Our work constitutes an integral part of ongoing efforts to understand the regulation of NF-κB signaling in molecular detail.
Methods
Detailed methods on sample preparation, crystallization, structure determination, and functional studies (SPR, pull-down and luciferase reporter assays, and in vitro and in vivo polyubiquitination assays) are provided in SI Methods. Briefly, the NZF domain of mouse HOIL-1L (residues 192–250) was overproduced in Escherichia coli and purified by anion exchange and gel-filtration columns. The purified protein was crystallized in complex with linear Ub2 in two distinct crystal forms (P43212 and P6). The complex structures were determined by molecular replacement method using the TAB2•K63-Ub2 complex and monoUb structures as search models. For SPR experiments, GST-monoUb or diUb was captured by antiGST antibody covalently immobilized on the sensor chip. Ub receptors were injected. For pull-down experiments, GST-fused Ub receptors were immobilized on glutathione beads and the bound Ub species were subjected to SDS-PAGE and CBB staining.
Supplementary Material
Acknowledgments.
We thank C. Toyoshima for support of this research. We are grateful to M. Komada for providing us with the overexpression vectors of Ub mutants for K63-Ub2 and K48-Ub2 synthesis. We thank the beam-line staffs at NW12A and BL5A of Photon Factory (Tsukuba, Japan) and BL41XU and BL32XU of SPring8 (Hyogo, Japan) for technical help during data collection. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan to S.F., K.I., Y.S., and A. Yamagata, and by a grant from The Nakajima Foundation to S.F. This work was also partially supported by Targeted Proteins Research Program (K. I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3B08 and 3B0A).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109088108/-/DCSupplemental.
References
- 1.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Greten FR. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda F, Dikic I. Atypical ubiquitin chains: New molecular signals ‘Protein Modifications: Beyond the Usual Suspects’ review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwai K, Tokunaga F. Linear polyubiquitination: A new regulator of NF-κB activation. EMBO Rep. 2009;10:706–713. doi: 10.1038/embor.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanarek N, London N, Schueler-Furman O, Ben-Neriah Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000166. 10.1101/cshperspect.a000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dynek JN, et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 2010;29:4198–4209. doi: 10.1038/emboj.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Wertz IE, Dixit VM. Ubiquitin-mediated regulation of TNFR1 signaling. Cytokine Growth Factor Rev. 2008;19:313–324. doi: 10.1016/j.cytogfr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 10.Habelhah H. Emerging complexity of protein ubiquitination in the NF-κB pathway. Genes Cancer. 2010;1:735–747. doi: 10.1177/1947601910382900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng L, et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 12.Haas TL, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 14.Rahighi S, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell. 2009;136:1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Skaug B, Zeng W, Chen ZJ. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFα and IL-1β. Mol Cell. 2009;36:302–314. doi: 10.1016/j.molcel.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokunaga F, et al. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nat Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 17.Tokunaga F, et al. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda F, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Seymour RE, Sundberg JP. Inhibition of NF-κB signaling retards eosinophilic dermatitis in SHARPIN-deficient mice. J Invest Dermatol. 2011;131:141–149. doi: 10.1038/jid.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seymour RE, et al. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8:416–421. doi: 10.1038/sj.gene.6364403. [DOI] [PubMed] [Google Scholar]
- 22.Kirisako T, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam SL, et al. Ubiquitin interactions of NZF zinc fingers. EMBO J. 2004;23:1411–1421. doi: 10.1038/sj.emboj.7600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, et al. Structure and ubiquitin interactions of the conserved zinc finger domain of Npl4. J Biol Chem. 2003;278:20225–20234. doi: 10.1074/jbc.M300459200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer HH, Wang Y, Warren G. Direct binding of ubiquitin conjugates by the mammalian p97 adaptor complexes, p47 and Ufd1-Npl4. EMBO J. 2002;21:5645–5652. doi: 10.1093/emboj/cdf579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanayama A, et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Yoshikawa A, Yamashita M, Yamagata A, Fukai S. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by NZF domains of TAB2 and TAB3. EMBO J. 2009;28:3903–3909. doi: 10.1038/emboj.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulathu Y, Akutsu M, Bremm A, Hofmann K, Komander D. Two-sided ubiquitin binding explains specificity of the TAB2 NZF domain. Nat Struct Mol Biol. 2009;16:1328–1330. doi: 10.1038/nsmb.1731. [DOI] [PubMed] [Google Scholar]
- 29.Sato Y, et al. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by tandem UIMs of RAP80. EMBO J. 2009;28:2461–2468. doi: 10.1038/emboj.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varadan R, Assfalg M, Raasi S, Pickart C, Fushman D. Structural determinants for selective recognition of a Lys48-linked polyubiquitin chain by a UBA domain. Mol Cell. 2005;18:687–698. doi: 10.1016/j.molcel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Trempe JF, et al. Mechanism of Lys48-linked polyubiquitin chain recognition by the Mud1 UBA domain. EMBO J. 2005;24:3178–3189. doi: 10.1038/sj.emboj.7600797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komander D, et al. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.