Abstract
The goal of cancer immunotherapy is the generation of an effective, stable, and self-renewing antitumor T-cell population. One such approach involves the use of high-affinity cancer-specific T-cell receptors in gene-therapy protocols. Here, we present the generation of functional tumor-specific human T cells in vivo from genetically modified human hematopoietic stem cells (hHSC) using a human/mouse chimera model. Transduced hHSC expressing an HLA-A*0201–restricted melanoma-specific T-cell receptor were introduced into humanized mice, resulting in the generation of a sizeable melanoma-specific naïve CD8+ T-cell population. Following tumor challenge, these transgenic CD8+ T cells, in the absence of additional manipulation, limited and cleared human melanoma tumors in vivo. Furthermore, the genetically enhanced T cells underwent proper thymic selection, because we did not observe any responses against non–HLA-matched tumors, and no killing of any kind occurred in the absence of a human thymus. Finally, the transduced hHSC established long-term bone marrow engraftment. These studies present a potential therapeutic approach and an important tool to understand better and to optimize the human immune response to melanoma and, potentially, to other types of cancer.
Keywords: stem cell therapy, hematopoietic progenitors, T-cell engineering, tumor immunotherapy, BLT mice
Immune-based therapies, including the adoptive transfer of tumor-specific autologous T cells, have been used to target melanoma and other cancers (1–6). These autologous T cells have been generated either by ex vivo manipulation of antigen-specific T cells with cytokines or by genetically engineering T cells to exhibit strong antitumor responses (5–16). Recent clinical studies have used chimeric antigen receptors to modify T cells genetically to target and deplete leukemia cells (5, 6). Alternatively, Morgan et al. (17) transduced autologous T cells ex vivo with a vector expressing a natural T-cell receptor (TCR) specific for the melanoma-associated antigen recognized by T-cells 1 [MART-1(26-35)] epitope and reintroduced them into patients, resulting in tumor regression in two of the 15 subjects (17). This very promising approach has some drawbacks, however. The introduction of a TCR into peripheral T cells may lead to the generation of autoreactive clones because of transgenic TCR chain mispairing with endogenous TCR chains (18) that do not undergo proper negative selection (19). Also, T cells require extensive manipulation before transduction and thus may lose some of their potency (4) and ability to become long-term memory cells (20). Finally, peripheral blood T cells can provide strong immune responses but generally are not long lived and may not support a lasting therapy (9, 21). Some of these limitations could be circumvented by the use of genetically modified human hematopoietic stem cells (hHSC) to generate mature and functional antigen-specific T cells. Studies using ovalbumin-specific TCR-transduced murine hematopoietic progenitors resulted in functional ovalbumin-specific murine T cells in vivo (22, 23). Immune responses were elicited after vaccination with an OVA-specific epitope; however, this was not a disease model, so the ability to ameliorate disease could not be assessed. Another group transduced mouse progenitors with an HLA-DR4–restricted TCR specific for melanoma (24). The resulting murine transgenic T cells were functional against murine tumor cell lines transduced with HLA-DR4, but it is unclear how this approach would work in human cells and on human tumors.
The use of human progenitors to generate human transgenic T cells has been restricted mainly to in vitro studies. Antigen-specific human T cells have been developed in vitro using OP9 stromal cells expressing the human Notch ligand Delta-like 1 (25, 26). In this case, because of the lack of a functional thymus, these cells did not undergo proper T-cell selection; the lack of selection could lead to the development of autoreactive clones.
Recently our group used severe combined immunodeficient (SCID)-hu mice bearing human thymus/liver (thy/liv) implants (27–29) to generate HIV-specific cytotoxic T lymphocytes (CTLs) from transduced hHSC (30). This approach resulted in the generation of HIV-specific transgenic T cells, but the lack of peripheral reconstitution in this model prevented the assessment of in vivo T-cell functionality. In the studies presented here, we use a modified version of the bone marrow/liver/thymus (BLT) humanized mouse model (31, 32), which contains a human thymus, allows long-term peripheral immune reconstitution, and is a very effective model to test transgenic T-cell functionality in vivo (31, 32). In this model, we transplanted human hematopoietic progenitors transduced with an HLA-A*0201–restricted, melanoma-specific TCR. After transplantation, we observed high levels of reconstitution with transgenic CTLs. The mice were challenged with HLA-A*0201–matched and nonmatched human melanoma tumors and were monitored for tumor reduction and/or clearance. The majority of the mice possessing TCR-transgenic cells cleared the HLA-A*0201+–matched tumors. Phenotypic analysis of the MART-specific CTLs following tumor challenge revealed the presence of both central and effector memory CD8 T cells. Finally, transduced human bone marrow cells were detected 4 mo after implantation, suggesting long-term bone marrow reconstitution in treated mice. Our results demonstrate that genetically engineering autologous human hematopoietic progenitors with disease-specific TCRs can lead to the generation of long-lasting and functional cytotoxic T cells that could provide an effective therapy against chronic conditions such as cancer.
Results
Generation of MART-1–Specific Transgenic CTLs.
Based on our previously described (30) humanized mouse model, the SCID-hu mouse, the generation of functional transgenic CD8 T cells from modified hHSC was feasible. Nevertheless, despite the successful generation of functional anti-HIV CD8+ T cells in the human thymus, the lack of peripheral reconstitution in the SCID-hu mouse precluded assessment of in vivo efficacy.
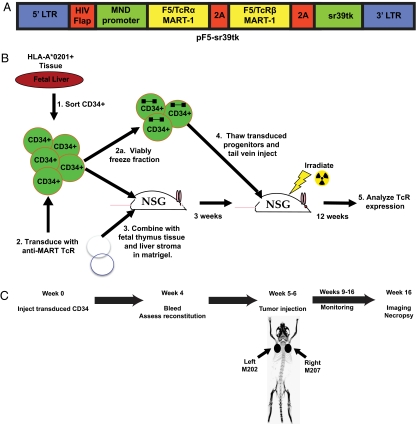
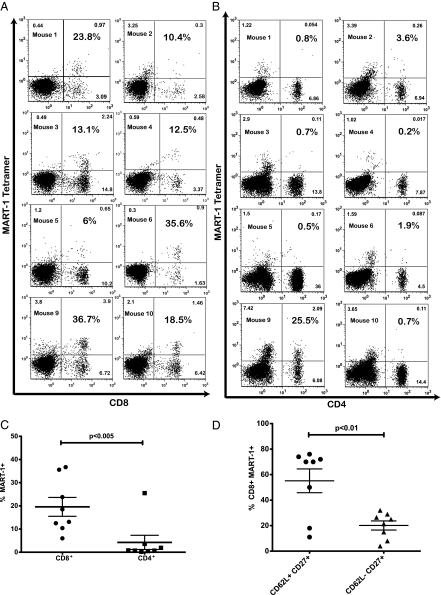
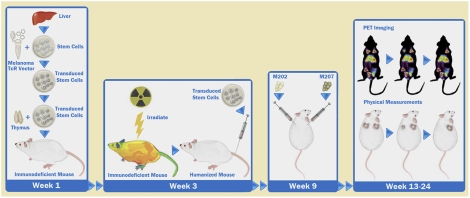
To determine the in vivo antimelanoma activity of the transgenic T cells, we used a modified version of the BLT humanized mouse model (31, 32) that allows full immune reconstitution in the periphery and in multiple lymphoid tissues and mimics human immune function (31, 32). In addition, transgenic lymphoid and myeloid lineages can be generated from genetically modified hHSC. Such a model would allow us to challenge the treated mice in vivo and properly assess antitumor activity. The presence of a human thymic implant ensures proper T-cell selection that would minimize the production of autoreactive T-cell clones. We constructed a lentiviral vector encoding the F5 MART-1 TCR and optimized herpes simplex virus 1 thymidine kinase (HSV1-tk) (sr39Tk), which can be used as a PET reporter to image T cells in vivo (Fig. 1A) (33, 34). F5 MART-1 is an improved MART-1–specific TCR displaying enhanced affinity to the antigen and was provided by the Rosenberg group from the National Cancer Institute (Bethesda, MD) (35). In this system (Fig. 1B), we reconstituted the thy/liv implant with a mix of transduced and nontransduced progenitors, followed by an i.v. injection of previously frozen autologous transduced progenitors (Fig. 1 B and C). Control mice were generated similarly, using nontransduced progenitors from the same donor. T-cell development and reconstitution were monitored by repeated blood draws (Fig. 1 B and C). In three independent experiments, 4–6 wk after progenitor injection, we successfully generated high levels of mature circulating CD8+ T cells that expressed the MART-1–specific TCR (Fig. 2 A and C). Furthermore, we rarely detected the expression of the MART-1 TCR in circulating CD4 T cells (Fig. 2 B and C), suggesting that the transduced cells underwent the proper T-cell lineage-commitment processes. Furthermore, when MART-1 TCR-transgenic hHSC were introduced into human thy/liv implants that were not HLA-A*0201 restricted, we did not detect MART-1 tetramer+ CD8 cells in the periphery, indicating that appropriate positive selection was required for generation of TCR-transgenic mature cells (Fig. S1). These results also suggest that functional TCR-transgenic T cells are not being generated in the murine thymus and are consistent with our previous results using anti-HIV TCRs (30). Finally the majority of circulating CD8+MART-1+ T cells displayed a naïve T-cell phenotype (Fig. 2D).
Fig. 1.
The lentiviral vector and experimental mouse model used to generate MART-1–specific CTL. (A) A schematic diagram of the lentiviral vector used. The MART-1TCR chains and the sr39tk reporter are joined by 2A self-cleaving peptides for optimal processing of the expressed polypeptide resulting in the generation of mature proteins. (B) A schematic diagram on the modified BLT model used in these studies for the generation of chimeric mice carrying MART-1–specific T cells. The thy/liv implant was reconstructed from transduced and nontransduced CD34 cells isolated from an HLA-A*0201 fetal liver. A fraction of the transduced cells is frozen and injected into the irradiated mice 3 wk later. Mice then are assayed over a period of 12 wk. (C) The timeline of the experimental protocol. Briefly, after mice were injected with transduced progenitors (step 4 in B), we allowed T-cell development and peripheral reconstitution. Tumors generally were injected 1 or 2 wk later with 5 × 106 cells per injection. Starting 1 mo after tumor injection, tumor size and T-cell presence were monitored for 6–8 wk; then mice were killed. The spleen, blood, thymic implant, bone marrow, and tumors were collected and assessed for transgenic T-cell functionality. The figure shows the sites of tumor injections. M202 is an HLA*0201+MART+ cell line that could serve as a target for the transgenic CTL, and M207 is an HLA*0201−MART+ cell line that could not. NSG, NOD scid gamma, (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ).
Fig. 2.
Reconstitution levels and phenotype of MART-1–specific T cells after injection of genetically modified hHSC. One representative experiment is shown, and each panel represents one individual mouse. Blood samples collected 4 wk after the introduction of transduced hHSC were analyzed by flow cytometric analysis for MART-1 TCR expression by tetramer, CD45, CD4, and CD8. The populations shown here are gated on CD45+ cells. Statistical significance was determined by a paired Student's t test (P < 0.05). (A) Levels of CD8 T cells expressing the MART-1 TCR. Percentages shown in bold represent the fraction of CD8 T cells that is tetramer+. (B) Expression of MART-1 TCR in CD4 T cells. Numbers shown in bold indicate the fraction of CD4 T cells expressing the MART-1 TCR. (C) A cumulative dot plot of the CD4 and CD8 T-cell populations in A and B expressing the MART-1 TCR. (D) Blood samples also were stained for CD62L and CD27 to assess the maturation state of CD8+MART-1+ T cells. The majority of cells were CD62L+, suggesting a naïve phenotype.
In Vivo Assessment of Antitumor Responses.
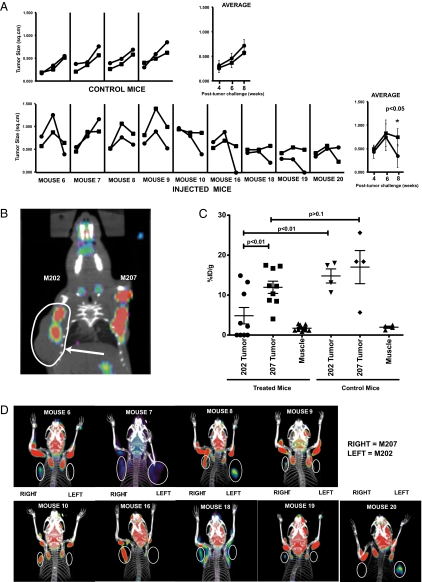
To assess the in vivo efficacy of the transgenic T cells, we challenged the mice with two types of melanoma tumors, M202, an HLA-A*0201+MART+ cell line, that could serve as a target for the transgenic CTL, and M207, an HLA-A*0201−MART+ line, that could not (Fig. 1C). Following tumor injection, we monitored the presence of tumor-specific CTLs and tumor sizes biweekly for 8 wk (Fig. 1C). Based on physical measurements, in treated chimeric animals the target M202 tumors regressed over time compared with the nontarget M207 tumors, whereas in control mice, the growth of both tumors was comparable (Fig. 3A).
Fig. 3.
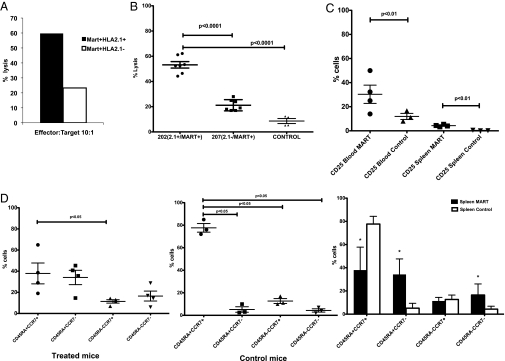
MART-1–specific CTLs limit growth of MART-1/HLA-A*0201 melanoma tumors. (A) Surface measurements of tumors from representative mice of two different experiments. The tumors were injected in the left [M202, circles (●)] and right [M207. squares (■)] shoulder. Control mice were transplanted with autologous nontransduced CD34 cells. The tumors were measured 4, 6, and 8 wk after tumor challenge. The end point at week 10 was assessed by PET imaging. (B) Shown is a representative mouse in which the M202 tumor (gray shading) is larger that the M207 tumor. However, PET imaging (red) indicates that glucose uptake is higher in the M207 tumor and that the additional tissue seen in the M202 tumor is mostly necrotic. (C) Levels of FDG uptake by the M202 and M207 tumors in control and treated mice as assessed by PET imaging. Muscle serves as a background control. Statistical significance was determined by one-way ANOVA. (D) Representative PET images from different mice. The circles were added to facilitate the identification of the M202 (Left) and M207 (Right) tumors. Statistical significance for all of the experiments (unless otherwise indicated) was determined by a paired Student‘s t test with significant values set at P < 0.05.
Tumor size measurements can be masked by tissue scarring or “dead space” resulting from tissue necrosis caused by the antitumor immune response and resulting in the appearance of false-positive tumor growth. Thus, to ensure that the tumors measured were live tissues, we used PET imaging for [18F]-fluorodeoxyglucose ([18F]FDG) uptake in the second mouse cohort to measure tumor metabolic activity by glucose uptake. To facilitate imaging, the tumors were implanted on the left (M202) and right (M207) mouse shoulder. As shown in Fig. 3B, we observed some animals in which the M202 tumor was larger than the M207 tumor by physical measurement, but PET imaging revealed a large dark spot suggesting dead tissue. When analyzed in aggregate, we saw a statistically significant decrease in tumor metabolic activity in mice receiving transduced hHSC (Fig. 3 C and D, and Movies S1, S2, S3, S4, S5, S6, S7, S8, and S9). More specifically, when comparing the M202 and M207 tumors in mice receiving transduced progenitors (treated mice), M202 tumors were targeted in seven of the nine treated mice, and four of nine mice cleared the M202 tumors (Fig. 3 C and D). The antitumor response is specific to the M202 tumor, because M202 tumor growth was unaffected in mice receiving nontransduced progenitors from the same donor (control mice) (Fig. 3C). Furthermore in the control mice, both M202 and M207 tumors exhibited high levels of glucose uptake that were not statistically significantly different (Fig. 3C). Similarly, the glucose uptake levels of the M207 (nontargeted) tumors were not significantly different between the treated and control mice (Fig. 3C). These data, in conjunction with physical measurements of the tumors (Fig. 3A), suggest that the two tumors initially grow comparably; however transgenic HLA-A*0201–restricted CTLs specifically kill HLA-A*0201+ tumors in treated animals. Furthermore, the antitumor activity is likely caused by a specific response generated by the MART-transgenic CTLs rather than by nonspecific or alloimmune activity. Together these data strongly demonstrate that the reconstituted transgenic CTLs are functional and effectively limit or clear HLA-matched tumor growth.
Detection of Tumor-Infiltrating Lymphocytes in Matched Tumors.
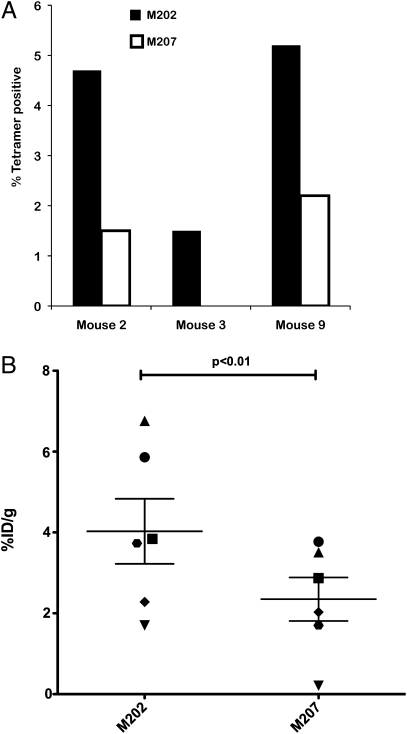
To confirm the above observations further, we examined the presence of tumor-infiltrating lymphocytes in both the HLA-matched and nonmatched tumors. To this end we used the sr39tk probe ([18F]fluoro-3hydroxymethylbutyl)guanine; [18F]FHBG) (34) to image the mice in vivo and also used flow cytometric analysis of the excised tumors to detect the presence of tumor-infiltrating T lymphocytes (TILs). At the end point of our two experiments, we excised, processed, and analyzed the tumors by flow cytometry to detect MART-1–specific TILs. We were able to detect specifically the presence of CD45 expressing MART-1+ cells in a small number of HLA-matched tumors, but these cells were absent in non–HLA-matched tumors (Fig. 4A). This infrequent event was attributed to the small number of CD45+ cells and transgenic T cells relative to the size and number of tumor cells. To increase the sensitivity of our assay, in a separate cohort of mice we used PET scanning to image the mice in vivo to detect the presence of transgenic T cells expressing the sr39tk gene. Mice were imaged 6 wk after tumor injection. At this time point tumors had not grown significantly in size, facilitating detection of TILs using PET (34). As shown in Fig. 4B, there were statistically significantly higher numbers of transduced peripheral blood cells in the M202 tumors than in the control M207 tumors. Thus, our results suggest that the TCR-transgenic CTLs specifically targeted the MHC-matched tumors, as confirmed both by the increased levels of reporter expression shown by PET imaging and flow cytometric analysis of tumor-cell suspensions.
Fig. 4.
Detection of TILs in the matched M202 tumor. (A) Total tumor cells were stained for CD45 and MART-1 tetramer and analyzed by flow cytometry to detect TILs. The data indicate percentage of CD45+ cells that are MART-1 TCR-positive by tetramer staining. The levels of CD45+ cells in these tumors ranged from 0.4–0.5% of total cells. Shown are individual mice from one representative experiment. (B) Mice were imaged using an sr39tk-specific probe 6 wk after tumor injection to detect infiltration of transgenic T cells into M202 and M207 tumors. Tumors from the same mouse are matched with the same symbol. Shown are six mice from one representative experiment. Statistical significance was determined by a paired Student‘s t test with significant values set at P < 0.05. %ID/g, injected dose per gram of body weight.
Transgenic CTLs Demonstrate Antitumor Activity ex Vivo.
After killing some of our mice, we looked at the functionality of the transgenic CTLs ex vivo. In our first experimental group, splenocytes from each humanized mouse were assessed for CTL activity against the M202 and M207 targets, following 5-d ex vivo stimulation. As shown in Fig. 5A, the transgenic CTLs maintained their antitumor activity following in vitro manipulation. We also wished to determine if antitumor killing activity occurred without any in vitro manipulation (Fig. 5B). To this end, we harvested splenocytes from our second cohort and performed a direct CTL killing assay without additional ex vivo stimulation. As shown in Fig. 5B, the MART-1–specific CTLs were able to kill the specific HLA-A*0201+ targets effectively. Together, these data suggest that these transgenic T cells exhibit high levels of antitumor activity following in vivo exposure to melanoma tumors without any additional manipulation ex vivo.
Fig. 5.
Ex vivo testing and the phenotype of MART-1–transgenic CTL after in vivo tumor challenge. (A) Splenocytes from mice injected with transduced CD34 were pooled and stimulated ex vivo with MART-1 peptide (1μg/mL) and irradiated feeders. Five days later the cells were used in a CTL killing assay. (B) After mouse culling, splenocytes were collected and used in a CTL assay without any ex vivo stimulation. Transgenic MART-1 CTL from mice injected with transduced progenitors specifically killed the M202 targets but did not kill the M207− controls. Cell cytotoxicities are at an effector:target ratio of 10:1. “Control” indicates splenocytes collected from mice that were injected with nontransduced hHSC and incubated with the M202 targets. Statistical significance for all of the experiments was determined by a paired Student's t test with significant values set at P < 0.05. (C) Samples from blood and spleens of killed mice were collected and used in a FACS-based phenotyping of CD45+CD8+CD3+MART-1+ T cells. Control mice were mice injected with nontransduced autologous hHSC. MART-1–expressing cells displayed higher levels of CD25. (D) From the same animals and cell population as in C, we also examined the maturation state of MART-1 cells. The bar graph below indicates the averages from each group shown in the dot plots above and the statistically significant differences between the treated mice and the controls. Asterisks indicate statistical significance. Statistical analysis of the averages (bar graphs) was determined by a Student's t test with significant values set at P < 0.05 to compare the differences between the control and treated mice. For the dot plots, statistical significance was determined by one-way ANOVA (P < 0.05) comparing the different CD8 T-cell subpopulations.
The high level of CTL activity exhibited by the MART-1–specific CTLs was supported by the phenotypic characteristics of CD45+CD3+CD8+MART-1+ cells in the spleens and blood. As shown in Fig. 5C, there were significantly higher levels of CD25-expressing cells, suggesting the presence of activated T cells, in animals receiving TCR-transduced HSC than in control animals not receiving the TCRs. In addition, in treated mice, we observed the presence of naïve (CD45RA+CCR7+), central (CD45RA−CCR7+), and effector (CD45RA−CCR7−) memory cells as well as terminally differentiated (CD45RA+CCR7−) cells in the spleen, suggesting that the tumor challenge resulted in antigen-induced CD8 T-cell maturation (Fig. 5D). In contrast, in control mice, the CD8 T-cell population was predominantly naïve. The presence of effector memory cells and terminally differentiated effectors can account for the strong antimelanoma cytotoxic activity observed ex vivo.
In addition, data compiled from our second cohort of mice showed a correlation between tumor metabolic activity and levels of reconstitution or ex vivo cytolytic activity. Mice exhibiting a higher percentage of CD8/MART-1+/TCR+ cells in spleen cleared or limited tumor growth more efficiently than did mice having less reconstitution of transgenic cells. Similarly, higher ex vivo CTL activity correlated strongly with decreased tumor growth and clearance of the tumors (Fig. S2 and Table S1).
Transduced Progenitors Stably Populated Mouse Bone Marrow.
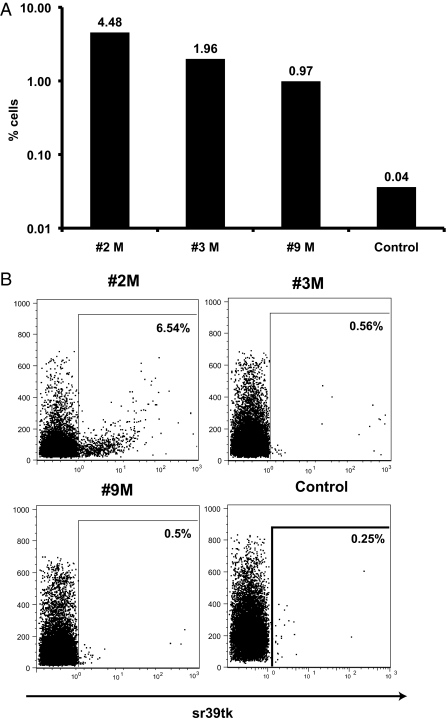
Finally, we examined the longevity of the transduced CD34 progenitors in the bone marrow. Longevity is crucial because in a clinical setting it would be beneficial for the patient to have a pool of progenitors replenishing the immune system with fresh antitumor cells. Bone marrow cells from mice injected with transduced and nontransduced progenitors were assayed for the presence of integrated vector and the expression of the sr39tk reporter. We detected presence of the lentiviral vector used to transduce the progenitor cells by quantitative real-time PCR (Fig. 6A). Because of the lack of CD3 expression on progenitor cells, TCR should not be present on the cell surface. Thus, we looked at the expression of the sr39tk reporter gene. Following stimulation of progenitor cells with stem cell factor (SCF) and megakaryocyte growth and development factor (MGDF), sr39tk was expressed in the bone marrow samples with high levels of vector present (Fig. 6B). Thus, the transduced CD34 cells stably repopulated the bone marrow of the irradiated recipient mice. Together these data suggest that this approach could result in a long-term engineering of the immune system leading to a therapeutic effect in patients with advanced melanoma.
Fig. 6.
Levels of bone marrow reconstitution. Shown is a representative experiment with three different mice. The control mouse was injected with nontransduced autologous progenitors. (A) Bone marrow cells were used in a real-time PCR assay to detect the presence of our lentiviral vector. (B) Progenitor cells were stimulated with SCF (50 ng/mL) and MGDF (100 ng/mL) and were used in a flow cytometric assay to detect expression of sr39tk.
Discussion
The data presented in this study demonstrate the generation of functional transgenic human T cells specific for melanoma from genetically modified human HSC in vivo. Unlike previous work, in this study we used a mouse/human chimera model and used only human tissues to generate and assess the feasibility and functionality of a potential gene therapy protocol. This model can be a platform for the study and development of effective therapeutic interventions against melanoma and other chronic diseases.
Although previous studies have shown that one can use genetically modified human progenitors to generate transgenic T cells, these studies were done either in vitro or in vivo without assessing in vivo functionality (25, 26, 30). A number of studies have been able to generate mature mouse T cells from mouse HSC (22–24). However, in the study by Yang et al. (22), the progenitor cells were not directed against a disease-specific antigen. A recent study engineered murine HSC to express an HLA-DR4–restricted TCR against the melanoma tyrosine-related protein 1 antigen (24). Although these studies showed the generation of functional antigen-specific murine T cells, the absence of a human thymus raises questions about appropriate negative selection for human antigens. Furthermore, the distinctions in human and murine lineage commitment and development underscore the importance of developing models that focus on genetically modified human progenitor cells (36).
In contrast to the above models, our studies establish the utility of this approach in a fully humanized system. Using the BLT model, we successfully generated high levels of naïve MART-1–specific CD8+ T cells. We did not detect any MART-1–expressing CD4+ T cells except in isolated cases. Furthermore, we noted no expression of the MART-1 TCR on mature CD8+ cells in mice that had thy/liv implants that did not express the HLA-A*0201 molecule. Thus, expression of the MART-1 TCR predominantly in CD8+ T cells can be attributed to the presence of a functional human thymus (thy/liv implant) that allowed proper T-cell selection processes to take place. Interestingly, on occasion we noted CD8−/CD4− cells expressing the MART-specific TCR (Fig. 2). These cells were CD56+; thus we believe they are natural killer T cells. Future studies will explore whether these cells or the occasional MART TCR+ CD4 T cells mentioned above are functional against melanoma target cells.
In the BLT system described here, we were able to challenge treated mice with HLA-matched and nonmatched tumors and assess the functionality of the MART-1+CD8+ cells. Using both physical measurements and imaging studies, we showed that the transgenic T cells targeted and in many cases cleared the HLA-matched M202 melanomas. The efficacy of the antitumor response was not influenced by tumor growth rates, because the M202 and M207 tumors grew at a similar rate when injected in mice transplanted with autologous but nontransduced hHSC. This result suggests that the observed antitumor response is linked directly to the MART-1–specific CD8+ T cells. Moreover, the relative lack of clearance of the control tumors relative to the HLA- A*0201–matched tumors both in vivo and ex vivo indicates that antiallogeneic responses are not responsible for the antitumor activity observed on the targeted M202 tumors. In contrast, we did note some loss of tumor mass in the control tumors (M207) in animals receiving TCR-transgenic stem cells. We believe that this limited regression is caused by bystander activation of an allogeneic response, which was initiated subsequent to cytokine expression by activation of MART-specific T cells.
Interestingly, we found that the transgenic CTLs maintained cytolytic activity ex vivo without the need for additional manipulation, such as cytokine and peptide stimulation, in culture. This result suggested that a sizeable fraction of the MART-1–specific T cells found in the spleens of the humanized mice maintained an effector phenotype. Indeed, we observed a diverse distribution of naïve, memory (central and effector), and terminally differentiated CD8+ T cells in spleen and blood of treated animals. Thus, the in vivo tumor challenge induced a significant immune response that led to normal antigen-driven CD8 T-cell maturation.
Our studies also establish that transgenic human HSC have long-term engraftment potential. Bone marrow samples from treated mice had substantial levels of integrated vector and expressed the reporter upon stimulation. This result is quite crucial, because the replenishment of fresh tumor-specific CTLs can help prevent tumor regression.
Engineered peripheral blood T cells have shown promise as immune-based therapies to target cancer; however, their impact is short term, and they can have potential side effects such as autoimmune reactions (9, 19). The approach described here provides an opportunity to develop a long-term therapy that uses the immune system's natural T-cell selection processes. In this study, we have demonstrated as a proof of principle the therapeutic efficacy of a stem cell-based genetically enhanced immune response against melanoma and established a viable and successful model for testing new, alternative therapeutic approaches for chronic diseases such as cancer; this model has closer relevance to the human immune system and applicability for the study of future therapies.
Methods
Construction of MART-Specific Lentiviral Vector.
The lentiviral vector pF5-sr39tk expressing the F5 TCR chains and the sr39tk reporter was constructed as previously described (37). The TCR chains and the reporter gene are separated by picornaviral-derived 2A sequences that allow the expression of a polycistronic mRNA and the generation of the three protein products (38). Lentiviral vector stocks were produced by transfection of 293FT cells with the pF5-sr39tk, pCMV-ΔR8.2-Δvpr (packaging construct), and pCMV-VSV-G (envelope) plasmids.
Isolation and Transduction of CD34 hHSC.
Fresh human fetal liver was obtained from Advanced Bioscience Resources Inc. The tissue was processed, and CD34 cells were purified as previously described (SI Methods) (30) and were transduced (multiplicity of infection = 5) using retronectin (Takara Bio, Inc.). Cells then were washed and used for the generation of the BLT mice. A fraction of the transduced cells was frozen to be used in CD34 cell injections.
Humanized BLT Mice.
Mice were generated as previously described (28, 32). Three weeks after the generation of humanized BLT mice, the mice were irradiated at 300 rad using a cobalt-60 source to remove endogenous cells. Then the frozen transduced CD34 cells were thawed and injected. Mice were bled 4 and 6 wk after injection to assess reconstitution. All animal protocols were performed under the approval of the University of California, Los Angeles (UCLA) Animal Research Committee.
Tumor Treatment and Measurement.
The tumor cell lines M202 and M207 were established from metastatic melanoma lesions under UCLA Institutional Review Board approval #02–08-067 and have been previously described (39). At the day of tumor injection, cells were harvested, split in 5 × 106 cell aliquots, and resuspended in 50 μL of medium and an equal volume of Matrigel (BD Biosciences) to improve cell engraftment. Injections were done s.c. Tumor sizes were measured by the use of a caliper every 2 wk.
PET and CT Imaging.
MicroPET/CT scans were done using the microPET Inveon scanner (Siemens Preclinical Solutions) and MicroCATII CT scanner (Siemens Preclinical Solutions). For glucose uptake, mice were fasted for 4–6 h before [18F]FDG injection. For tracer injection and imaging, mice were anesthetized with 2% isoflurane. Imaging started 60 min after an i.p. injection of 7.4 MBq (80 μCi) [18F]FDG or [18F]FHBG via tail vein. Additional details on PET and CT imaging are included in SI Methods. All image analysis was done using OsiriX (Pixmeo, Geneva, Switzerland) software.
Ex Vivo Functional Assays.
Splenocytes from treated and untreated BLT mice were cultured with irradiated (3,500 rads), peptide-coated (1 μg/mL) 174xCEM.T1 (T1) cells (a professional antigen-presenting cell line that express high levels of HLA-A*0201) or non–peptide-coated cells in the presence of 20 IU/mL of IL-2. MART-1 (ELAGIGILTV) peptide was purchased from Invitrogen. Five days later the effectors were used in a CTL killing assay against the M202 and M207 targets. In some experiments the effectors were used without stimulation. The CTL killing assay was carried out using the LIVE/DEAD cell-mediated cytotoxicity kit (Invitrogen) (SI Methods).
Flow Cytometry.
Cells were phenotypically analyzed using fluorochrome-conjugated monoclonal antibodies specific for human CD3 (BD Biosciences), CD4, CD8, CD25, CD27, CD45, CD45RA, CD45 RO, CD56, CD57, CD62L and CCR7 (Beckman Coulter). Tetramer-expressing cells were identified using MHC Class I tetramer containing the MART-1 (26–35) peptide conjugated to allophycocyanin or phycoerythrin (PE) (Beckman Coulter). sr39tk was detected using a thymidine kinase 1 antibody (EPR3193) (Abcam) and PE-conjugated goat anti-rabbit (Beckman Coulter). Cells were processed by a Coulter FC500 instrument and results were analyzed FlowJo (TreeStar, Inc.) software.
Real-Time PCR Analysis.
DNA from 106 bone marrow cells was extracted and used in a quantitative real-time PCR to assess levels of integrated vector (SI Methods).
Statistics.
Statistical analysis tests were performed as indicated in the article and figure legends. Data were analyzed with paired or unpaired Student's t test or one-way ANOVA and Bonferroni multiple post hoc comparisons. All statistical analyses were performed with GraphPad Prism v5.0 (GraphPad Software). Values with P < 0.05 were considered significant.
Additional Methods.
More detailed methods are given in SI Methods.
Supplementary Material
Acknowledgments
We thank Salehi Khosrowdad, Alvin Welch, Bernard Levin, and Larry Pang for their technical assistance and the University of California, Los Angeles (UCLA) AIDS Institute/UCLA Center for AIDS Research Mouse/Human Chimera Core and its director Dr. Scott Kitchen for the development and housing of BLT mice. This work was funded in part by National Institutes of Health (NIH) Grant P50 CA086306; California Institute for Regenerative Medicine (CIRM) Grants RC1-00149-1 (to J.A.Z.) and RS1-00203-1 (to Z.G.); CIRM New Faculty Award RN2-00902-1 and support from the California Institute of Technology-UCLA Joint Center for Translational Medicine (to A.R.); UCLA Center for AIDS Research NIH/National Institute of Allergy and Infectious Diseases Grant AI028697, and by UCLA AIDS Institute, and the CIRM Tools and Technology Award RT1-01126 (to C.G.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 20287.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115050108/-/DCSupplemental.
References
- 1.Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–342. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 2.Ochsenbein AF. Principles of tumor immunosurveillance and implications for immunotherapy. Cancer Gene Ther. 2002;9:1043–1055. doi: 10.1038/sj.cgt.7700540. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: Mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 4.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 5.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95) doi: 10.1126/scitranslmed.3002842. 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobisse S, et al. Reprogramming T lymphocytes for melanoma adoptive immunotherapy by T-cell receptor gene transfer with lentiviral vectors. Cancer Res. 2009;69:9385–9394. doi: 10.1158/0008-5472.CAN-09-0494. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: Engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 12.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 13.Sadelain M, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 15.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan RA, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendle GM, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16(5):561. doi: 10.1038/nm.2128. 565–570. [DOI] [PubMed] [Google Scholar]
- 19.Willemsen RA, et al. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Ther. 2000;7:1369–1377. doi: 10.1038/sj.gt.3301253. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee C, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: Direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Qin XF, Baltimore D, Van Parijs L. Generation of functional antigen-specific T cells in defined genetic backgrounds by retrovirus-mediated expression of TCR cDNAs in hematopoietic precursor cells. Proc Natl Acad Sci USA. 2002;99:6204–6209. doi: 10.1073/pnas.092154599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha SP, et al. Transplantation of mouse HSCs genetically modified to express a CD4-restricted TCR results in long-term immunity that destroys tumors and initiates spontaneous autoimmunity. J Clin Invest. 2010;120:4273–4288. doi: 10.1172/JCI43274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67:2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Lent AU, et al. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. J Immunol. 2007;179:4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- 27.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bristol GC, Gao LY, Zack JA. Preparation and maintenance of SCID-hu mice for HIV research. Methods. 1997;12:343–347. doi: 10.1006/meth.1997.0488. [DOI] [PubMed] [Google Scholar]
- 29.McCune JM, et al. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 30.Kitchen SG, et al. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PLoS ONE. 2009;4:e8208. doi: 10.1371/journal.pone.0008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melkus MW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu S, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115:1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaghoubi SS, et al. Imaging progress of herpes simplex virus type 1 thymidine kinase suicide gene therapy in living subjects with positron emission tomography. Cancer Gene Ther. 2005;12:329–339. doi: 10.1038/sj.cgt.7700795. [DOI] [PubMed] [Google Scholar]
- 34.Shu CJ, et al. Quantitative PET reporter gene imaging of CD8+ T cells specific for a melanoma-expressed self-antigen. Int Immunol. 2009;21:155–165. doi: 10.1093/intimm/dxn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson LA, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payne KJ, Crooks GM. Immune-cell lineage commitment: Translation from mice to humans. Immunity. 2007;26:674–677. doi: 10.1016/j.immuni.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Koya RC, et al. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proc Natl Acad Sci USA. 2010;107:14286–14291. doi: 10.1073/pnas.1008300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Felipe P, Martín V, Cortés ML, Ryan M, Izquierdo M. Use of the 2A sequence from foot-and-mouth disease virus in the generation of retroviral vectors for gene therapy. Gene Ther. 1999;6:198–208. doi: 10.1038/sj.gt.3300811. [DOI] [PubMed] [Google Scholar]
- 39.Søndergaard JN, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl Med. 2010;8:39. doi: 10.1186/1479-5876-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]