Abstract
Genetic damage through mutations and genome rearrangements has been hypothesized to contribute to aging. The specific mechanisms responsible for age-induced increases in mutation and chromosome rearrangement frequencies and a potential causative role for DNA damage in aging are under active investigation. Retrotransposons are mobile genetic elements that cause insertion mutations and contribute to genome rearrangements through nonallelic recombination events in humans and other organisms. We have investigated the role of endogenous Ty1 retrotransposons in aging-associated increases in genome instability using the Saccharomyces cerevisiae chronological aging model. We show that age-induced increases in loss of heterozygosity and chromosome loss events are consistently diminished by mutations or treatments that reduce Ty1 retrotransposition. Ty1 mobility is elevated in very old yeast populations, and new retromobility events are often associated with chromosome rearrangements. These results reveal a correlation between retrotransposition and genome instability during yeast aging. Retrotransposition may contribute to genetic damage during aging in diverse organisms and provides a useful tool for studying whether genetic damage is a causative factor for aging.
Keywords: long-terminal repeat retrotransposon, mobile element, chromosomal rearrangement, lifespan
A substantial amount of research has identified associations between aging and genome instability in humans and model organisms, but a causative role for DNA damage and genome instability in normal aging has not been conclusively demonstrated. Increases in frequencies of mutations and chromosome rearrangements and in accumulation of DNA repair foci with increasing age have been detected in a variety of cells and organisms (1). Multiple human syndromes characterized by premature symptoms of aging or onset of aging-related diseases, such as Werner syndrome and Cockayne syndrome, result from deficiencies in enzymes involved in DNA replication and repair (2, 3). Elegant work in a Drosophila model has demonstrated that different DNA repair pathways are used preferentially in old and young cells to repair DNA damage (1). Continued studies of mechanisms of aging-associated genome instability and the consequences of increasing or decreasing levels of DNA damage on lifespan should help to clarify whether genome instability is an important causative factor for aging.
Retrotransposons are ubiquitous eukaryotic mobile DNA elements that can promote genome instability in a variety of ways. Retrotransposition involves transcription of an element, reverse transcription of the RNA copy into a cDNA by a reverse transcriptase encoded by the element, and integration of the cDNA into a new genomic site. Retromobility refers to integration of cDNA at new sites as well as recombination events between cDNA and preexisting genomic retrotransposon sequences. In addition to acting as insertional mutagens, retrotransposons are frequently present at the sites of chromosome rearrangements, because DNA repair processes can capture and insert cDNA into the genome at sites of damage (4–11). Additionally, retrotransposons can produce reverse transcripts of cellular mRNA that are incorporated into genomes as processed pseudogenes (12, 13). Retrotransposon insertions have caused a variety of human diseases (14), and a potential role for human retrotransposons in aging has been recently reviewed (15). It is challenging to study the activity of individual endogenous retrotransposons, manipulate their mobility, and quantitatively assess the consequences of endogenous retrotransposon mobility on genome stability in humans. However, Saccharomyces cerevisiae has proven to be an excellent model for studying endogenous retrotransposons (16), because the mobility of individual endogenous yeast Ty1 retrotransposons can be routinely quantified, and a variety of means exist to manipulate retrotransposition levels.
S. cerevisiae has also proven to be a useful model organism for studying aging through two different experimental systems (17, 18). Replicative aging is a measure of the number of times a given yeast mother cell can divide to produce daughter cells, whereas chronological aging measures the survival of yeast cells maintained in saturated cultures that are nutrient-depleted. Numerous studies have demonstrated that replicative aging and chronological aging in yeast are regulated by conserved growth-signaling and nutrient-sensing pathways that influence aging in other organisms, including humans (18, 19). Despite some observations of potential yeast-specific aging factors (18, 20), yeast aging is associated with changes in metabolic and stress response pathways, increases in oxidative stress, and increases in the accumulation of mutations and chromosome rearrangements, which are commonly associated with aging in diverse organisms (18, 19, 21–24).
We took advantage of the yeast chronological aging model to address whether retrotransposons promote age-associated genome instability. We show that mutations and treatments that decrease Ty1 retrotransposition produce corresponding reductions in age-induced loss of heterozygosity (LOH) and/or chromosome loss in aging diploid yeast populations. Ty1 retromobility was elevated in old diploid yeast populations, and cells with new retromobility events harbored chromosome rearrangements. These data are consistent with the hypothesis that retrotransposons promote genome instability as cells age.
Results
Mutations and Treatments That Reduce Ty1 Retrotransposition Reduce Levels of LOH and Chromosome Loss During Aging.
We developed a genetic system in two different strains of S. cerevisiae to quantify genome instability in chronologically aging yeast populations. Our system consisted of a hemizygous URA3 gene insertion at position 522206 on the right arm of chromosome VIII and a hemizygous TRP1 gene insertion at position 56054 on the left arm of the same copy of chromosome VIII in diploid strains (Fig. S1). Exposure of cells to medium containing 5-fluoroorotic acid (5-FOA) selects against the function of URA3 (25). Cultures were initiated at low cell densities, allowed to grow to stationary phase, and then maintained in stationary phase for the duration of the experiments. The frequency with which cells became resistant to 5-FOA was determined during the initial growth period and at regular intervals during maintenance in stationary phase to measure the frequency of LOH at different time points during aging. Resistance to 5-FOA could arise from small-scale mutations in URA3, gene-conversion recombination events between the two copies of chromosome VIII, chromosome rearrangements, or loss of the entire marked chromosome VIII. We grew 5-FOA–resistant derivatives on medium lacking tryptophan to select for the function of TRP1 to distinguish chromosome loss events, because these events would result in loss of both URA3 and TRP1 function (Fig. S1).
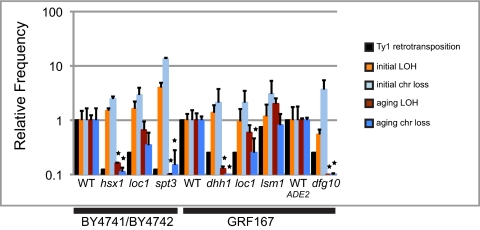
We decreased Ty1 retromobility in our strains by deleting or disrupting the DHH1, DFG10, HSX1, LOC1, LSM1, or SPT3 gene and then measured genome instability in the chronologically aging populations (Fig. 1 and Table 1). Mutation of SPT3 substantially reduces Ty1 transcription (26); mutation of HSX1 [tR(CCU)J] disrupts the normal translational frame-shifting that produces Ty1 proteins (27); mutations in DHH1 and LSM1 decrease Ty1 retromobility at posttranscriptional steps (28, 29); and the steps of retromobility influenced by mutations in DFG10 and LOC1 are under active investigation. We used a semiquantitative Ty1 integration PCR assay to confirm that the mutants tested had lower levels of Ty1 mobility (Fig. S2 and SI Results). Initial LOH and chromosome loss frequencies were similar to WT frequencies or elevated in the mutants (Fig. 1 and Table 1). As expected, LOH frequencies and chromosome loss frequencies were substantially elevated in the WT strains, with age-induced increases of 20- and 32-fold (BY4741/BY4742) or 14- and 72-fold (GRF167), respectively (Table 1). The age-associated increases in LOH and chromosome loss frequencies were diminished in all the mutant strains, except lsm1, and these differences were statistically significant for hsx1 and spt3 in the BY4741/BY4742 background and for dfg10, dhh1, and loc1 (chromosome loss only for loc1) in the GRF167 background. The similarity between lsm1 and WT strains with regard to aging-associated increases in LOH could be explained by the relatively modest decrease in retrotransposition in lsm1 mutants (29); indeed, we observed minor effects on Ty1 integration in our PCR assay (Fig. S2).
Fig. 1.
Ty1 retrotransposition levels and aging-associated increases in genome instability are frequently correlated. Relative mean (±SD) of median values for initial and aging-associated LOH and chromosome (chr) loss frequencies was calculated for the indicated WT and mutant strains using the data presented in Table 1. Relative retrotransposition for each mutant was determined using Ty1 integration PCR data, as described in SI Materials and Methods. All WT values were set to 1, and values for mutants were normalized to the corresponding WT value. A star over a bar for aging LOH or aging chr loss indicates P < 0.05 using a two-tailed t test assuming unequal variance.
Table 1.
Mutations and treatments that decrease Ty1 mobility tend to diminish age-associated increases in LOH and chromosome loss frequencies
| Genotype | Days until 50% viability | Days until 10% viability | LOH frequency, initial (×10−5) | LOH frequency, fold increase | Chromosome loss frequency, initial (×10−6) | Chromosome loss frequency, fold increase |
| WT (BY) | 12 ± 0.88 | 24 ± 1.3 | 3.0 ± 1.5 | 20 ± 4.6 | 2.1 ± 1.4 | 32 ± 21 |
| hsx1::LEU2 | 13 ± 3.8 | 35 ± 3.6* | 4.5 ± 0.40 | 3.2 ± 0.10* | 5.1 ± 0.61 | 3.6 ± 0.66* |
| loc1::KanMX | 16 ± 0.15* | 26 ± 1.3 | 4.88 ± 1.7 | 13 ± 6.1 | 6.1 ± 2.3 | 11 ± 7.9 |
| spt3::KanMX | 12 ± 0.04 | 15 ± 0.60* | 12 ± 2.8 | 1.6 ± 0.43* | 28 ± 1.3 | 4.8 ± 4.1* |
| WT + PFA | 19 ± 0.1* | 38 ± 9.3 | 5.3 ± 4.9 | 5.8 ± 0.92* | 25 ± 23 | 3.9 ± 1.4* |
| WT (BY) glu | 12 ± 0.9 | 26 ± 4.5 | 1.9 ± 0.43 | 36 ± 13 | 1.1 ± 0.58 | 98 ± 28 |
| WT eth/gly | 19 ± 0.73* | 56 ± 4.5* | 2.3 ± 0.66 | 5.1 ± 1.6* | 2.2 ± 0.91 | 9.0 ± 0.25* |
| WT (GRF) | 17 ± 4.3 | 32 ± 5.7 | 2.2 ± 0.69 | 14 ± 4.8 | 0.53 ± 0.27 | 72 ± 13 |
| dhh1::KanMX | 15 ± 2.0 | 27 ± 8.3 | 3.0 ± 1.1 | 1.8 ± 0.18* | 1.1 ± 0.87 | 2.8 ± 0.89* |
| loc1::KanMX | 26 ± 3.7* | 41 ± 8.2 | 2.1 ± 1.4 | 8.2 ± 3.1 | 1.1 ± 0.73 | 18 ± 15* |
| lsm1::KanMX | 13 ± 5.4 | 22 ± 6.4 | 2.6 ± 1.7 | 28 ± 7.2 | 1.6 ± 1.2 | 58 ± 37 |
| WT (ADE2) | 9.1 ± 0.2 | 24 ± 1.1 | 9.3 ± 7.1 | 19 ± 1.8 | 0.49 ± 0.38 | 91 ± 11 |
| dfg10::ADE2 | 19 ± 0.28* | 27 ± 0.88 | 5.1 ± 1.1 | 1.3 ± 0.04* | 1.8 ± 0.88 | 7.0 ± 2.7* |
Mean (±SD) of median values for each category from two or more independent experiments each using three to four replicates for the BY4741/BY4742 background (BY, first 7 rows) or GRF167 background (GRF, last 6 rows). WT glu or WT eth/gly refers to strains grown in medium containing 2% (wt/vol) glucose or 2% (vol/vol) ethanol plus 2% (vol/vol) glycerol, respectively. WT (ADE2) refers to ADE2 derivatives in the GRF167 background used for comparison with dfg10::ADE2 mutants. Days to 50% or 10% viability were determined from trend lines for each dataset, and LOH and chromosome loss were measured as frequencies of obtaining 5-FOAr and 5-FOAr Trp− derivatives, respectively. Initial values were obtained from the first time point or the first time point at which cfu/mL was at least 33% of the initial control cfu/mL. Fold increase was determined by dividing the peak value obtained over the course of a chronological aging experiment by the initial value. *P < 0.05 using a two-tailed t test assuming unequal variance.
As a further test of the hypothesis that retromobility gives rise to aging-associated genome instability, we exposed cells to the reverse transcriptase inhibitor phosphonoformic acid (PFA), which inhibits Ty1 retromobility by reducing levels of Ty1 reverse transcripts (30). Exposure to PFA significantly diminished the age-associated increase in LOH from 20-fold to 5.8-fold and the age-associated increase in chromosome loss from 32-fold to 3.9-fold (Table 1). To identify media conditions that reduce Ty1 retromobility, we used a genetic assay to measure retromobility of a single chromosomal Ty1his3AI element. The his3AI retromobility indicator gene allows Ty1 mobility to be detected through restoration of a functional HIS3 gene during Ty1 replication (31). The BY4741/BY4742 strain was grown in different media previously shown to influence lifespan (20, 32–34). Growth in 2% glycerol (vol/vol) plus 2% (vol/vol) ethanol decreased Ty1his3AI mobility fivefold, whereas other media resulted in little or no change in mobility (<2.5-fold; Fig. S3). Reduced retrotransposition in 2% (vol/vol) glycerol plus 2% (vol/vol) ethanol medium was accompanied by significantly smaller age-associated increases in LOH (36-fold vs. 5.1-fold) and chromosome loss (98-fold vs. 9.0-fold; Table 1). Overall, these results are consistent with the idea that retrotransposition promotes genome instability during yeast aging.
It could be argued that the aging-associated increase in LOH and chromosome loss frequencies we observed in the WT populations result from improved survival of mutant cells present early in the growth of a culture, rather than true increases in the frequencies of these events in a cell population during aging. To address this issue, we compared the chronological aging of the parent strains with that of derivatives that had undergone LOH or chromosome loss events. Independent 5-FOA–resistant (Ura−) derivatives with LOH or chromosome loss events were obtained from populations aged until cells exhibited <25% viability. The Ura− isolates were mixed at equal cell densities with the corresponding Ura+ parent strains to initiate competitive chronological aging experiments. We determined the relative number of Ura+ and Ura− cells at different time points during aging. Note that the frequency of losing URA3 function in the parent strain was too low to influence the results of this assay.
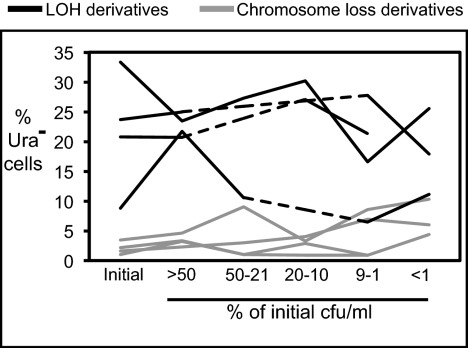
The results of four independent experiments indicate that the Ura− cells were losing viability at a rate similar to the parental cells. The median values for the initial percentage of Ura− cells present in the cell populations were variable for derivatives with LOH or chromosome loss events for each of the four experiments, reflecting different initial growth rates between the Ura− derivatives and the parent strains (Fig. 2). The percentage of Ura− cells in each experiment remained relatively constant during aging. The ratio of the percentage of Ura− cells at the final time point to the percentage of Ura− cells at the initial time point was 0.76–1.27 for derivatives with LOH events and 0.42–4.1 for derivatives with chromosome loss events. This set of results is consistent with age-dependent increases in LOH and chromosome loss frequencies resulting from new events that occur in old cells, rather than selection for cells with preexisting genetic changes.
Fig. 2.
Survival of cells with chromosome VIII LOH or chromosome loss events during chronological aging is similar to survival of parental strains. Ura− cells with chromosome VIII LOH or chromosome loss events were mixed at equal starting densities with Ura+ parental strains, and the relative fractions of Ura− cells were measured at various time points during chronological aging. Median percent Ura− cells for all time points that fell within the indicated range of the initial cfu/mL for each of four experiments performed with three independent derivatives with LOH (black lines) or chromosome loss (gray lines) events is plotted. Dotted lines indicate categories of cfu/mL for which no data points were obtained for a particular experiment.
Most LOH Events Result from Large-Scale Recombination or Deletion Events.
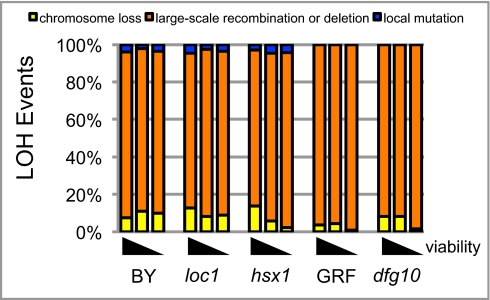
We further analyzed LOH events in two WT and three mutant strains (dfg10, hsx1, and loc1) making use of a second hemizygous gene, HIS3, located ∼15 kb telomere-distal to URA3 on the same copy of chromosome VIII so as to group LOH events into three classes (Fig. S4 and SI Materials and Methods). In all strains analyzed, most (82–99%) LOH events were associated with loss of HIS3 function but retention of TRP1 function (Fig. 3). Such events are likely the result of recombination, break-induced replication, or chromosome deletions that either replaced the marked chromosome arm sequences with the corresponding sequences of the unmarked copy of chromosome VIII or caused the loss of the genomic region containing URA3 and HIS3. Loss of both TRP1 and HIS3 functions attributable to chromosome loss was the next most frequent class of events. Local changes in the URA3 locus (retention of TRP1 and HIS3 function) were only observed in the BY4741/BY4742 background (Fig. 3). PCR analysis of these local mutations indicated that ∼10% (∼0.2–0.5% of total events) were attributable to point mutations in URA3, indicating that most local changes were gene-conversion recombination events or relatively large insertions/deletions. The percentage of events in each class was generally similar in WT and mutant strains, and also over the course of aging (Fig. 3).
Fig. 3.
LOH events primarily arise through large-scale recombination or deletion events. Percent of LOH events that arose attributable to the indicated classes of events is shown for two WT strains (BY, BY4741/BY4742 background; GRF, GRF167 ADE2 background) and for three mutants with reduced Ty1 retrotransposition (hsx1, loc1, dfg10). The three bars for each genotype show the results for LOH events that occurred in populations as viability decreased from 80% to 100%, to 40–80%, and then to less than 15%. The number of events tested for each genotype for each viability range was as follows: BY: 513, 2,208, 1,371; loc1: 596, 960, 723; hsx1: 522, 703, 487; GRF: 1,153, 94, 382; dfg10: 380, 280, 59.
Ty1 Mobility Is Not Always Correlated with Lifespan.
An influence of retrotransposition on yeast lifespan during chronological aging was supported by some but not all of the results for different mutant strains and treatment conditions. It is worth noting that the lifespan of yeast strains grown at 20 °C, the optimal temperature for Ty1 mobility (35, 36), was substantially longer than the lifespan typically observed for strains grown at 30 °C (17). Populations of hsx1, loc1, and dfg10 mutants and cells exposed to PFA or grown in 2% (vol/vol) glycerol plus 2% (vol/vol) ethanol medium showed improved survival and took longer to reach 50% or 10% viability (Table 1). The dhh1 mutants had no change in viability, and the spt3 mutants reached 10% viability more quickly than the WT strain, however, despite low levels of Ty1 mobility and aging-associated LOH and chromosome loss in these mutant strains. Analysis of the data for an influence of Ty1 on lifespan is complicated by potential gene- or treatment-specific effects on lifespan that are independent of Ty1 retromobility.
An experiment comparing the lifespan of a Saccharomyces paradoxus strain completely lacking Ty1 elements with that of derivatives harboring a single Ty1 element did not support a direct role for Ty1 retrotransposition in cell survival during chronological aging (Fig. S5 and SI Results). However, we previously found that genomic copies of Ty1 can participate in recombination events with Ty1-generated reverse transcripts to cause chromosome rearrangements in S. cerevisiae (37), and the absence of dispersed genomic copies of Ty1 in the S. paradoxus strains may limit the potential for Ty1 to cause genome rearrangements.
Ty1 Mobility Frequency Is Elevated in Old Yeast Populations and Is Associated with the Appearance of Chromosome Rearrangements.
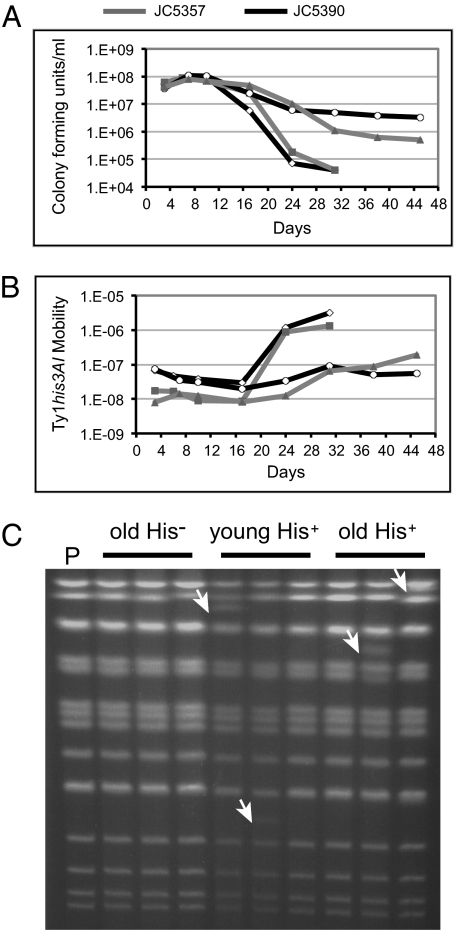
We used two diploid strains homozygous for independently integrated chromosomal Ty1his3AI elements to measure retromobility during chronological aging. The frequency of Ty1 mobility differed slightly between the two strains and remained stable or slightly decreased during the first half of these experiments (Fig. 4). Retromobility was greatly elevated at late stages of three of four experiments, reaching levels 24-, 46-, or 77-fold higher than the initial value for each experiment (Fig. 4). Substantial increases in retromobility were not observed until populations reached ∼1 × 106 cfu/mL or less. Interestingly, the populations in the one experiment in which an increase in retromobility was not observed exhibited relatively steady densities of colony-forming units that were greater than 1 × 106 over the course of the experiment. Pulsed-field gel electrophoresis identified novel chromosome bands and the absence of one or more parental chromosome bands in the electrophoretic karyotypes of His+ cells obtained from aged populations (Fig. 4C). Novel and/or missing chromosome bands were identified by pulsed-field gel electrophoresis in 6 (50%) of 12 His+ derivatives from old populations (≥24 d), in 1 (7.1%) of 14 His− derivatives from old populations, and in 4 (29%) of 14 His+ derivatives of young populations (3–7 d) from the BY4741/BY4742 background (P < 0.05 for old His+ vs. old His−, Fisher's exact test). The chromosome rearrangements are therefore specifically associated with retromobility in these populations.
Fig. 4.
Ty1 mobility is elevated and associated with chromosome rearrangements in old cells. (A) Each line shows the mean cfu/mL for an independent chronological aging experiment with three replicates performed using one of the two indicated diploid strains. (B) Mean of His+ prototroph frequencies as a measure of Ty1his3AI mobility corresponding to the strains and aging experiments shown in A. (C) Intact yeast chromosomes from parental strain JC5357 (P) and three His− isolates lacking retromobility events from old populations, three His+ isolates harboring new Ty1 retromobility events from young populations, and three His+ isolates harboring new Ty1 retromobility events from old populations separated by clamped homogeneous electrical field gel electrophoresis and visualized with ethidium bromide. White arrows indicate bands not present in the parental lane.
Discussion
We have demonstrated an association between retromobility and genome instability during chronological aging of S. cerevisiae. Mutations and treatments that reduce Ty1 retrotransposition resulted in corresponding decreases in age-induced LOH and chromosome loss frequencies in diploid yeast strains from two genetic backgrounds. Ty1 could potentially play a role in formation of the LOH events we observed, because the majority of LOH events resulted from large-scale recombination/deletion events rather than point mutations. Ty1 retromobility was greatly elevated in old yeast populations and correlated with high frequencies of chromosome rearrangements. This work suggests that retrotransposition could be a useful target to modulate levels of age-induced genome instability and to examine whether genome instability plays a causative role in aging.
The correlation between Ty1 mobility and age-related genome instability established by our results is consistent with the possibility that Ty1 mobility is one of the causes of age-related genome instability. Ty1 elements could contribute to LOH and chromosome loss events by integrating into one of two heterozygous alleles, by triggering allelic or nonallelic recombination, and by causing chromosome breaks through the action of Ty1 integrase (Fig. S6). Interaction of DNA repair and recombination proteins with reverse transcripts during integration could lead to recombination or break-induced replication events that involve allelic or nonallelic genomic sites that have Ty1 sequences. The sequenced yeast genome contains three Ty1 solo LTRs and one full-length element within 60 kb of the position of the hemizygous marker we introduced into our strains (www.yeastgenome.org). Failure to repair the single-stranded DNA gaps that flank Ty1 integration events could stimulate recombination events or give rise to DNA breaks during replication, thereby activating DNA damage signaling activities that lead to break-induced replication or chromosome rearrangements. Stimulation of such events could also occur in the absence of integration if Ty1 integrase possesses a nonspecific endonuclease activity similar to what has been observed for some retroviral integrases (38, 39). Retrotransposition into replicating DNA could stimulate recombination events or impair replication fork progression. Failure to repair chromosome breaks or to complete replication could result in chromosome loss. In addition, replication stress has been linked to yeast chronological aging (34, 40), and Ty1 retromobility is activated by hydroxyurea (41), an agent that causes replication stress. Therefore, replication stress could promote aging-associated genome instability by activating Ty1.
Retrotransposons could promote age-associated genome instability in many organisms, based on similarities in the mechanism of retrotransposition and regulation through common cellular stress conditions (42). Yeast, mammalian, fungal, and plant retrotransposons can be activated by oxidative stress and/or DNA damage (10, 43–47). Oxidative stress and DNA damage are associated with aging in a variety of organisms (19). In addition, human L1 retrotransposons generate DNA breaks at frequencies greater than the frequency of retrotransposition (48). Increases in mutations and genome rearrangements caused by retrotransposon insertions, endonuclease activities of retrotransposon proteins, and interactions between DNA repair proteins and reverse transcripts could therefore be common occurrences during aging of many species.
Because we did not always observe an inverse correlation between Ty1 retromobility and survival during stationary phase for the different mutants and conditions, we cannot conclude that Ty1 activity directly reduces lifespan. However, changes in cellular processes that influence lifespan independent of retrotransposition might obscure an effect of Ty1 on lifespan. Mutations in DHH1, LOC1, LSM1, and SPT3 result in a wide variety of phenotypes, whereas mutations in DFG10 and HSX1 result in limited phenotypes beyond their roles in regulating Ty1 (www.yeastgenome.org). For instance, although Ty1 mobility is somewhat reduced in lsm1 mutants, these strains exhibit a reduced chronological lifespan (49) and elevated genome instability attributable to changes in the regulation of histone gene expression (50).
We also expected that survival during stationary phase and levels of genome instability would show an inverse correlation if the accumulation of mutations and genome rearrangements directly contributes to aging. Previous studies have found LOH increases during replicative and chronological aging in yeast (21, 22, 24). One of these studies identified a correlation between natural variation in replicative lifespan and the increase in LOH during chronological aging, although the increase in LOH during chronological aging was observed to lag slightly behind the drop in viability (22). We did not always observe a longer lifespan for strains with lower frequencies of LOH or chromosome loss. Therefore, our results are not fully consistent with yeast chronological aging resulting solely from an increase in the accumulation of deleterious genetic changes. However, cells have to survive DNA damage to exhibit an increased frequency of mutations, and preferential death of cells experiencing DNA damage and replication stress during yeast aging has been observed (34). Such preferential cell death before mutagenic repair events occur could mask a correlation between lifespan and indicators of genome instability. Future work will need to address carefully whether cell death from DNA damage before the accumulation of mutations has a substantial impact on chronological lifespan.
The frequency of retrotransposition in human populations may be greater than originally suspected, based on a few recent reports of genome-wide studies of individual human genomes. These reports identified many polymorphic retrotransposon insertions using independent methods, and a substantial fraction of the insertions could represent recent events, based on very low allele frequencies (51–53). Our results linking retrotransposition to aging-related genome instability in yeast and the growing appreciation of how dynamic these elements are in human genomes emphasize the importance of investigating the contribution of retrotransposition to aging in humans.
Materials and Methods
Yeast Strains and Media.
Yeast strains were grown using standard media. Most S. cerevisiae strains were diploids made by mating derivatives of BY4741 (MATa ura3Δ0 leu2Δ0 his3Δ1 met15Δ0) and BY4742 (MATα ura3Δ0 leu2Δ0 his3Δ1 lys2Δ0) from the S288c background or JC2980 (MATα his3Δ200 ade2::hisG ura3-167 leu2::hisG trp1::hisG) and JC4545 (MATa his3Δ200 ade2::hisG ura3-167 leu2::hisG trp1::hisG) from the GRF167 background. Ty1 mobility assays were performed using diploids JC5357 (MATa/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 LYS2/lys2Δ0 met15Δ0/MET15 ura3Δ0/ura3Δ0 Ty1his3AI/Ty1his3AI) and JC5390 (MATa::URA3/MATα ade2::hisG/ADE2 his3Δ200/his3Δ200 TRP1/trp1::hisG ura3-167/ura3-167 Ty1588:NEO/+ Ty1his3AI/Ty1his3AI) from the BY4741/BY4742 and GRF167 backgrounds, respectively. S. paradoxus strains were derivatives of DG1768 (54). Details of strain constructions are provided in SI Materials and Methods.
Chronological Aging, Viability, and LOH Assays.
Yeast strains were inoculated at densities of 5 × 103 cells/mL into 25 or 50 mL of broth in 125- or 250-mL flasks, respectively, and grown with shaking at 20 °C in synthetic complete (SC) medium-uracil plus 2% (wt/vol) glucose plus fourfold excess of histidine, leucine, lysine, methionine, and tryptophan, unless otherwise noted. We used medium lacking uracil to reduce the proliferation of cells lacking URA3 function during the initial growth to saturation. After 3–4 d of incubation, and thereafter at intervals of ∼3–7 d, aliquots of cells were removed and analyzed. Cells were diluted in water, mixed with one or two volumes of 0.4% trypan blue in PBS, and incubated for 1 h before scoring the number of live and dead cells in a population of ≥200 cells using a hemacytometer to determine viability of populations. Cells were diluted in water and spread onto rich medium to determine the number of cfu/mL. To measure LOH, cells were diluted in water or centrifuged and washed in water and spread onto SC medium containing 5-FOA. LOH frequencies were calculated by dividing the number of resistant colonies by the total number of colony forming units plated. All plates were incubated at 30 °C for up to 5 d. Chromosome loss frequencies were determined by replicating 5-FOA–resistant colonies to SC medium lacking tryptophan and dividing the number of colonies that failed to grow by the total number of colony forming units originally plated on 5-FOA medium. Initial values were those obtained after the initial 3–4 d of growth or from the first time point when cfu/mL was at least 33% of the initial WT cfu/mL. Fold increase during aging was determined as the peak value during the course of the aging experiment divided by the initial value. For competitive aging experiments, we mixed 5 × 104 cells/mL each of a Ura+ parent strain and a Ura− (5-FOAr) derivative in medium containing uracil. We determined the relative number of cells from Ura+ parent strains and 5-FOAr derivatives at different time points during aging by spreading cells onto rich medium and then replicating colonies to medium lacking uracil, on which only the parental Ura+ colonies could grow. Three independent 5-FOAr derivatives obtained from aging cultures at <25% viability were used for each of four experiments.
Pulsed-Field Gel Electrophoresis to Detect Chromosome Rearrangements.
Yeast chromosomal DNA was prepared, and clamped homogeneous electrical field gels were prepared and run essentially as described previously (37), except that gels were run at 4.5 V/cm for 51 h with switch times of 50–135 s.
Supplementary Material
Acknowledgments
We thank D. Garfinkel for providing the Ty1-less haploid S. paradoxus strain. This work was supported, in part, by Grant K99AG031911 from the National Institute on Aging (to P.H.M., Grant CA016056 from the National Cancer Institute to Roswell Park Cancer Institute) and Grant GM52072 from the National Institutes of Health (to M.J.C.).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Telomerase and Retrotransposons: Reverse Transcriptases That Shaped Genomes,” held September 29–30, 2010, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/telomerase_and_retrotransposons.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100271108/-/DCSupplemental.
References
- 1.Engels WR, Johnson-Schlitz D, Flores C, White L, Preston CR. A third link connecting aging with double strand break repair. Cell Cycle. 2007;6:131–135. doi: 10.4161/cc.6.2.3758. [DOI] [PubMed] [Google Scholar]
- 2.Andressoo JO, Hoeijmakers JH, Mitchell JR. Nucleotide excision repair disorders and the balance between cancer and aging. Cell Cycle. 2006;5:2886–2888. doi: 10.4161/cc.5.24.3565. [DOI] [PubMed] [Google Scholar]
- 3.Brosh RM, Jr, Bohr VA. Human premature aging, DNA repair and RecQ helicases. Nucleic Acids Res. 2007;35:7527–7544. doi: 10.1093/nar/gkm1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore JK, Haber JE. Capture of retrotransposon DNA at the sites of chromosomal double-strand breaks. Nature. 1996;383:644–646. doi: 10.1038/383644a0. [DOI] [PubMed] [Google Scholar]
- 5.Teng SC, Kim B, Gabriel A. Retrotransposon reverse-transcriptase-mediated repair of chromosomal breaks. Nature. 1996;383:641–644. doi: 10.1038/383641a0. [DOI] [PubMed] [Google Scholar]
- 6.Dunham MJ, et al. Characteristic genome rearrangements in experimental evolution of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2002;99:16144–16149. doi: 10.1073/pnas.242624799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrish TA, et al. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat Genet. 2002;31:159–165. doi: 10.1038/ng898. [DOI] [PubMed] [Google Scholar]
- 8.Umezu K, Hiraoka M, Mori M, Maki H. Structural analysis of aberrant chromosomes that occur spontaneously in diploid Saccharomyces cerevisiae: Retrotransposon Ty1 plays a crucial role in chromosomal rearrangements. Genetics. 2002;160:97–110. doi: 10.1093/genetics/160.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abeysinghe SS, Chuzhanova N, Krawczak M, Ball EV, Cooper DN. Translocation and gross deletion breakpoints in human inherited disease and cancer I: Nucleotide composition and recombination-associated motifs. Hum Mutat. 2003;22:229–244. doi: 10.1002/humu.10254. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood LD, Felix K, Janz S. Elevated presence of retrotransposons at sites of DNA double strand break repair in mouse models of metabolic oxidative stress and MYC-induced lymphoma. Mutat Res. 2004;548(1-2):117–125. doi: 10.1016/j.mrfmmm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Morrish TA, et al. Endonuclease-independent LINE-1 retrotransposition at mammalian telomeres. Nature. 2007;446:208–212. doi: 10.1038/nature05560. [DOI] [PubMed] [Google Scholar]
- 12.Derr LK, Strathern JN. A role for reverse transcripts in gene conversion. Nature. 1993;361:170–173. doi: 10.1038/361170a0. [DOI] [PubMed] [Google Scholar]
- 13.Esnault C, Maestre J, Heidmann T. Human LINE retrotransposons generate processed pseudogenes. Nat Genet. 2000;24:363–367. doi: 10.1038/74184. [DOI] [PubMed] [Google Scholar]
- 14.Belancio VP, Deininger PL, Roy-Engel AM. LINE dancing in the human genome: Transposable elements and disease. Genome Med. 2009;1:97. doi: 10.1186/gm97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Laurent G, 3rd, Hammell N, McCaffrey TA. A LINE-1 component to human aging: Do LINE elements exact a longevity cost for evolutionary advantage? Mech Ageing Dev. 2010;131:299–305. doi: 10.1016/j.mad.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesage P, Todeschini AL. Happy together: The life and times of Ty retrotransposons and their hosts. Cytogenet Genome Res. 2005;110(1-4):70–90. doi: 10.1159/000084940. [DOI] [PubMed] [Google Scholar]
- 17.Madia F, Gattazzo C, Fabrizio P, Longo VD. A simple model system for age-dependent DNA damage and cancer. Mech Ageing Dev. 2007;128:45–49. doi: 10.1016/j.mad.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464:513–519. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007;35:7545–7556. doi: 10.1093/nar/gkm1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMurray MA, Gottschling DE. An age-induced switch to a hyper-recombinational state. Science. 2003;301:1908–1911. doi: 10.1126/science.1087706. [DOI] [PubMed] [Google Scholar]
- 22.Qin H, Lu M, Goldfarb DS. Genomic instability is associated with natural life span variation in Saccharomyces cerevisiae. PLoS ONE. 2008;3:e2670. doi: 10.1371/journal.pone.0002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burhans WC, Weinberger M. Acetic acid effects on aging in budding yeast: Are they relevant to aging in higher eukaryotes? Cell Cycle. 2009;8:2300–2302. doi: 10.4161/cc.8.14.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindstrom DL, Leverich CK, Henderson KA, Gottschling DE. Replicative age induces mitotic recombination in the ribosomal RNA gene cluster of Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1002015. doi: 10.1371/journal.pgen.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 26.Winston F, Durbin KJ, Fink GR. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell. 1984;39:675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami K, et al. A rare tRNA-Arg(CCU) that regulates Ty1 element ribosomal frameshifting is essential for Ty1 retrotransposition in Saccharomyces cerevisiae. Genetics. 1993;135:309–320. doi: 10.1093/genetics/135.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Checkley MA, Nagashima K, Lockett SJ, Nyswaner KM, Garfinkel DJ. P-body components are required for Ty1 retrotransposition during assembly of retrotransposition-competent virus-like particles. Mol Cell Biol. 2010;30:382–398. doi: 10.1128/MCB.00251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutko JA, Kenny AE, Gamache ER, Curcio MJ. 5′ to 3′ mRNA decay factors colocalize with Ty1 gag and human APOBEC3G and promote Ty1 retrotransposition. J Virol. 2010;84:5052–5066. doi: 10.1128/JVI.02477-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee BS, Bi L, Garfinkel DJ, Bailis AM. Nucleotide excision repair/TFIIH helicases RAD3 and SSL2 inhibit short-sequence recombination and Ty1 retrotransposition by similar mechanisms. Mol Cell Biol. 2000;20:2436–2445. doi: 10.1128/mcb.20.7.2436-2445.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curcio MJ, Garfinkel DJ. Single-step selection for Ty1 element retrotransposition. Proc Natl Acad Sci USA. 1991;88:936–940. doi: 10.1073/pnas.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith DL, Jr, McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–662. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 33.Murakami CJ, Burtner CR, Kennedy BK, Kaeberlein M. A method for high-throughput quantitative analysis of yeast chronological life span. J Gerontol A Biol Sci Med Sci. 2008;63(2):113–121. doi: 10.1093/gerona/63.2.113. [DOI] [PubMed] [Google Scholar]
- 34.Weinberger M, et al. Growth signaling promotes chronological aging in budding yeast by inducing superoxide anions that inhibit quiescence. Aging (Albany NY) 2010;2:709–726. doi: 10.18632/aging.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paquin CE, Williamson VM. Temperature effects on the rate of ty transposition. Science. 1984;226:53–55. doi: 10.1126/science.226.4670.53. [DOI] [PubMed] [Google Scholar]
- 36.Lawler JF, Jr, Haeusser DP, Dull A, Boeke JD, Keeney JB. Ty1 defect in proteolysis at high temperature. J Virol. 2002;76:4233–4240. doi: 10.1128/JVI.76.9.4233-4240.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maxwell PH, Curcio MJ. Retrosequence formation restructures the yeast genome. Genes Dev. 2007;21:3308–3318. doi: 10.1101/gad.1604707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caumont AB, et al. Expression of functional HIV-1 integrase in the yeast Saccharomyces cerevisiae leads to the emergence of a lethal phenotype: Potential use for inhibitor screening. Curr Genet. 1996;29:503–510. doi: 10.1007/BF02426953. [DOI] [PubMed] [Google Scholar]
- 39.Katzman M, Sudol M. Nonspecific alcoholysis, a novel endonuclease activity of human immunodeficiency virus type 1 and other retroviral integrases. J Virol. 1996;70:2598–2604. doi: 10.1128/jvi.70.4.2598-2604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberger M, et al. DNA replication stress is a determinant of chronological lifespan in budding yeast. PLoS ONE. 2007;2:e748. doi: 10.1371/journal.pone.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curcio MJ, et al. S-phase checkpoint pathways stimulate the mobility of the retrovirus-like transposon Ty1. Mol Cell Biol. 2007;27:8874–8885. doi: 10.1128/MCB.01095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beauregard A, Curcio MJ, Belfort M. The take and give between retrotransposable elements and their hosts. Annu Rev Genet. 2008;42:587–617. doi: 10.1146/annurev.genet.42.110807.091549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mhiri C, et al. The promoter of the tobacco Tnt1 retrotransposon is induced by wounding and by abiotic stress. Plant Mol Biol. 1997;33:257–266. doi: 10.1023/a:1005727132202. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda K, Nakayashiki H, Takagi M, Tosa Y, Mayama S. Heat shock, copper sulfate and oxidative stress activate the retrotransposon MAGGY resident in the plant pathogenic fungus Magnaporthe grisea. Mol Genet Genomics. 2001;266:318–325. doi: 10.1007/s004380100560. [DOI] [PubMed] [Google Scholar]
- 45.Scholes DT, Kenny AE, Gamache ER, Mou Z, Curcio MJ. Activation of a LTR-retrotransposon by telomere erosion. Proc Natl Acad Sci USA. 2003;100:15736–15741. doi: 10.1073/pnas.2136609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stribinskis V, Ramos KS. Activation of human long interspersed nuclear element 1 retrotransposition by benzo(a)pyrene, an ubiquitous environmental carcinogen. Cancer Res. 2006;66:2616–2620. doi: 10.1158/0008-5472.CAN-05-3478. [DOI] [PubMed] [Google Scholar]
- 47.Stoycheva T, Pesheva M, Venkov P. The role of reactive oxygen species in the induction of Ty1 retrotransposition in Saccharomyces cerevisiae. Yeast. 2010;27:259–267. doi: 10.1002/yea.1749. [DOI] [PubMed] [Google Scholar]
- 48.Gasior SL, Wakeman TP, Xu B, Deininger PL. The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol. 2006;357:1383–1393. doi: 10.1016/j.jmb.2006.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palermo V, Cundari E, Mangiapelo E, Falcone C, Mazzoni C. Yeast lsm pro-apoptotic mutants show defects in S-phase entry and progression. Cell Cycle. 2010;9:3991–3996. doi: 10.4161/cc.9.19.13210. [DOI] [PubMed] [Google Scholar]
- 50.Herrero AB, Moreno S. Lsm1 promotes genomic stability by controlling histone mRNA decay. EMBO J. 2011;30:2008–2018. doi: 10.1038/emboj.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck CR, et al. LINE-1 retrotransposition activity in human genomes. Cell. 2010;141:1159–1170. doi: 10.1016/j.cell.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang CR, et al. Mobile interspersed repeats are major structural variants in the human genome. Cell. 2010;141:1171–1182. doi: 10.1016/j.cell.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iskow RC, et al. Natural mutagenesis of human genomes by endogenous retrotransposons. Cell. 2010;141:1253–1261. doi: 10.1016/j.cell.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garfinkel DJ, Nyswaner K, Wang J, Cho JY. Post-transcriptional cosuppression of Ty1 retrotransposition. Genetics. 2003;165:83–99. doi: 10.1093/genetics/165.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.