Abstract
Telomerase copies its internal RNA template to synthesize telomeric DNA repeats. Unlike other polymerases, telomerase can retain its single-stranded product through multiple rounds of template dissociation and repositioning to accomplish repeat addition processivity (RAP). Tetrahymena telomerase holoenzyme RAP depends on a subunit, Teb1, with autonomous DNA-binding activity. Sequence homology and domain modeling suggest that Teb1 is a paralog of RPA70C, the largest subunit of the single-stranded DNA-binding factor replication protein (RPA), but unlike RPA, Teb1 binds DNA with high specificity for telomeric repeats. To understand the structural basis and significance of telomeric-repeat DNA recognition by Teb1, we solved crystal structures of three proposed Teb1 DNA-binding domains and defined amino acids of each domain that contribute to DNA interaction. Our studies indicate that two central Teb1 DNA-binding oligonucleotide/oligosaccharide-binding-fold domains, Teb1A and Teb1B, achieve high affinity and selectivity of telomeric-repeat recognition by principles similar to the telomere end-capping protein POT1 (protection of telomeres 1). An additional C-terminal Teb1 oligonucleotide/oligosaccharide-binding-fold domain, Teb1C, has features shared with the RPA70 C-terminal domain including a putative direct DNA-binding surface that is critical for high-RAP activity of reconstituted holoenzyme. The Teb1C zinc ribbon motif does not contribute to DNA binding but is nonetheless required for high-RAP activity, perhaps contributing to Teb1 physical association with the remainder of the holoenzyme. Our results suggest the biological model that high-affinity DNA binding by Teb1AB recruits holoenzyme to telomeres and subsequent Teb1C–DNA association traps product in a sliding-clamp-like manner that does not require high-affinity DNA binding for high stability of enzyme-product association.
Eukaryotic genome stability requires proteins that bind at chromosome ends by direct recognition of telomeric-repeat DNA, including protein(s) that protect the 3′ single-stranded overhang (1). Principles that underlie the telomeric-repeat ssDNA-binding specificity of chromosome end-capping proteins have been illuminated through a combination of structure determination and structure-based functional analyses (2, 3). Although the telomeric ssDNA-binding proteins characterized to date all recognize G-rich repeat sequences, the structural determinants of interaction specificity can vary, such that end-binding complexes from different organisms have unique dependencies on a precise length or register of repeat sequence or in the requirement for DNA self-recognition by intramolecular folding. One common theme in telomeric-repeat ssDNA recognition, as in ssDNA recognition in general, is the display of specificity-determining protein side chains on the surface of an oligonucleotide/oligosaccharide-binding (OB)-fold domain (4–6).
Telomeric-repeat ssDNA is also bound by telomerase, in part by base pairing with the template of telomerase RNA and in part by contacting telomerase reverse transcriptase and other subunits within a biologically functional telomerase holoenzyme (7, 8). In yeast and human cells, the telomerase-interacting proteins that contact DNA with high affinity may not be telomerase-specific. Instead, end-capping telomeric-repeat ssDNA-binding proteins have dual functionality: In S-phase, they recruit telomerase and potentially become assembled into an elongating form of telomerase holoenzyme, but throughout most of the cell cycle they insulate the 3′ overhang from processing (8). In contrast, Tetrahymena has a devoted telomerase holoenzyme subunit with telomeric-repeat ssDNA-binding activity termed Teb1, which based on overexpression and depletion phenotypes appears to compete directly with the end-capping protein Pot1a for telomere binding (9, 10). Teb1 association with the Tetrahymena telomerase catalytic core requires a telomerase-specific telomere adaptor subcomplex (TASC) and converts the limited repeat addition processivity (RAP) of the catalytic core with TASC to the high product interaction stability and high RAP of endogenously assembled holoenzyme (10, 11).
Recombinant Teb1 has subnanomolar affinity for its preferred permutation of an 18-nt telomeric-repeat ssDNA (10, 11). Curiously, even though sequence homology and domain modeling suggest that Teb1 is a paralog of the largest subunit of the general ssDNA-binding factor replication protein (RPA) (the Rpa1 or human RPA70 subunit), Teb1 does not detectably bind ssDNAs lacking Tetrahymena T2G4 telomeric repeats (10, 11). To investigate the structural basis for the high sequence specificity of Teb1 ssDNA binding, we solved crystal structures of its three proposed ssDNA-binding domains and defined domain interfaces of DNA interaction. We also tested structural requirements for Teb1 function as a telomerase processivity factor, revealing a particularly critical role for a surface of the Teb1 C-terminal OB fold that contributes weakly to the overall affinity of Teb1-DNA interaction.
Results
Characterization of Teb1 High-Affinity ssDNA-Binding Domains.
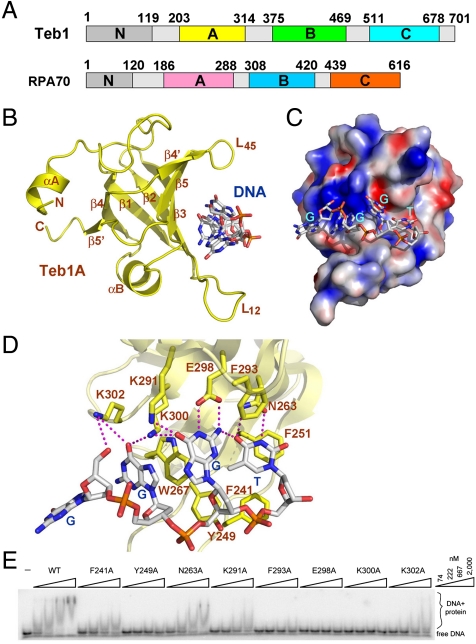
Tetrahymena Teb1 is a telomerase-specific protein with a domain organization similar to RPA70 (10). For simplicity, the predicted OB folds of Teb1 are designated as Teb1N, Teb1A, Teb1B, and Teb1C in order from the N to C terminus according to the RPA70 convention (Fig. 1A). Teb1A and Teb1B were previously shown to bind specifically to the G-rich strand of Tetrahymena telomeric repeats (11). To further assess the ssDNA-binding activity of Teb1AB (Teb1 residues 187–504), we tested its binding to a panel of ssDNAs by electrophoretic mobility shift assay (EMSA). Efficient binding to Teb1AB required a ssDNA of 10 nt or longer including the core telomeric-repeat sequence GGGTTGGGGT (tT10). Removal of a single nucleotide from either end of tT10 greatly weakened protein–DNA interaction (Fig. S1). This Teb1AB requirement for a 10-nt length and specific sequence of telomeric-repeat ssDNA notably parallels the requirements for telomeric-repeat ssDNA recognition by the tandem OB-fold domains of human POT1 (protection of telomeres 1) (12).
Fig. 1.
Crystal structure of Teb1A and its interaction with ssDNA. (A) Domain organization of Tetrahymena Teb1 and human RPA70. In Teb1/RPA70, the four OB folds from the N to C terminus are colored in gray/gray, yellow/purple, green/blue, and cyan/orange, respectively. (B) Ribbon diagram of the Teb1A–ssDNA complex from the Teb1AΔB–ssDNA structure. The ssDNA is in stick model and colored as carbon, white; oxygen, red; nitrogen, blue; and phosphorus, orange. The secondary structure elements of the protein are labeled. Only the 5′ four nucleotides in tT10 (G1G2G3T4) are visible in the electron density map. (C) Electrostatic potential surface representation of Teb1A. The orientation of the complex is rotated by 90° about a vertical axis relative to the complex in B. (D) Interactions between Teb1A and G1G2G3T4 with the side chains of Teb1A and the ssDNA in stick representation. The Teb1A–ssDNA intermolecular hydrogen bonds are shown as dashed magenta lines. (E) DNA-binding impact of Teb1A amino acid substitutions assayed by EMSA. Teb1A single-domain proteins were used in threefold concentration steps over the range of 74–2,000 nM with 10 pM 5′-GGGTTGGGGTTG-3′.
To understand how Teb1AB recognizes telomeric ssDNA, we first determined the crystal structures of Teb1A and Teb1B individually using single-wavelength anomalous dispersion (SAD) and refined to resolutions of 1.8 and 2.3 Å, respectively (Table S1). Next, we engineered a Teb1AB mutant (Teb1AΔB) with a deletion of the loop (residues 315–374) between Teb1A and Teb1B. We reconstituted and crystallized the complex of Teb1AΔB with tT10 and determined the complex structure at a resolution of 2.6 Å by molecular replacement (Table S1). Four nucleotides at the tT10 5′ end that bind to Teb1A were highly ordered in the crystals (Fig. S2). The individually solved structures of Teb1A and Teb1B are not altered in the Teb1AΔB–tT10 complex. The final model contains Teb1 residues 203–314 (Teb1A) and 375–467 (Teb1B) and was refined to a crystallographic residual of 22.03% (Rfree = 26.25%) (Table S1).
The Teb1A-ssDNA Interface.
As predicted, the structure of Teb1A reveals that it adopts an OB fold consisting of a highly curved five-stranded antiparallel β-barrel (Fig. 1B). In addition to the β-barrel, Teb1A contains an N-terminal extended loop covering one end of the β-barrel (Fig. 1B). The other end of the β-barrel is closed by a short helix between strands β3 and β4. The ordered region of ssDNA G1G2G3T4 (Fig. 1C and Fig. S2) binds into a concave basic groove of Teb1A formed by one side of the β-barrel and two protruding loops, L12 (between strands β1 and β2) and L45 (between strands β4 and β5).
The ssDNA adopts an irregular, extended conformation with its backbone exposed to solvent and its bases buried in a solvent-excluded contact area (Fig. 1C). The overall configuration of the ssDNA is defined by two sets of stacking interactions between the bases of the ssDNA and aromatic residues of Teb1A: The base of G2 stacks against W267, and the base of T4 packs onto the phenol ring of F251 (Fig. 1D). The side chains of both F251 and W267 are not only important for ssDNA binding but are also essential for the stability of Teb1A folding, because attempts to purify the F251A and W267A amino acid substitution variants of Teb1A only yielded insoluble products. G3 does not stack with any residue of Teb1A. Instead, the base of G3 together with that of T4 and the phenol ring of F293 form a hydrophobic cluster that appears to stabilize the Teb1A–ssDNA interaction (Fig. 1D). Consistent with this conclusion, alanine substitution of F293 led to a complete loss of Teb1A–ssDNA binding (Fig. 1E). In addition to the bases, the backbone of the ssDNA also contributes to Teb1A binding. The deoxyribose ring and the phosphate group of G1 cover the base of G2 in a buried environment, whereas the backbones of G3 and T4 sit on a hydrophobic platform formed by the side chains of F241 and Y249 from L12 of Teb1A (Fig. 1D). Notably, the phosphate group of T4 also accepts a hydrogen bond from the side chain of Y249 (Fig. 1D). Consistent with the crystal structure, removal of the ssDNA G1 or alanine substitution of Teb1 F241 or Y249 greatly weakened protein–ssDNA interaction (Fig. 1E and Fig. S1).
In addition to these interactions, Teb1A also makes extensive sequence-specific contacts with the ssDNA through a total of 10 hydrogen bonds (Fig. 1D). These hydrogen-bonding interactions are entirely mediated by the Watson–Crick donor/acceptor groups of the bases of G2-T4 and Teb1A residues located on the surface of the ssDNA-binding groove. Amino acid substitutions of any of the ssDNA-interacting residues of Teb1A either greatly weakened or completely abolished the interaction of Teb1A with ssDNA (Fig. 1E). The edge of the base of G3 is surrounded with four hydrogen-bonding interactions that provide highly sequence-specific recognition of G3 (Fig. 1D). Notably, one of these hydrogen bonds involves a non-Watson–Crick base pair between the 2-amino group of G3 and the 4-carbonyl group of T4. This unusual base-pairing interaction links G3 and T4 together and allows them as a single unit to interact with Teb1A with high sequence specificity. The same sort of DNA “self-recognition” interaction also occurs with the bases of G1-T4 in the structure of the Schizosaccharomyces pombe Pot1pN–ssDNA complex (13).
Crystal Structure of Teb1B and Determination of Its Requirements for ssDNA Binding.
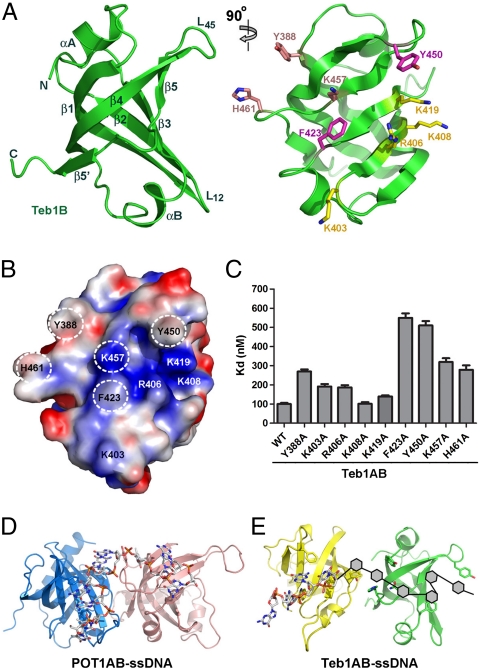
Similar to Teb1A, the structure of Teb1B showed it to be a single OB fold (Fig. 2A). The OB fold of Teb1B is slightly deformed by a very short loop L12, so that the canonical ssDNA-binding groove is much shallower than that in Teb1A (compare Fig. 1B with Fig. 2A). Nonetheless, our previous studies indicated that Teb1B is a ssDNA-binding module of Teb1 (11) and the surface of the putative ssDNA-binding groove is highly positively charged (Fig. 2B). Close inspection of this surface (Fig. 2 A and B) reveals two conserved aromatic residues F423 and Y450 that, in other ssDNA-binding OB-fold domains including those of RPA70 (14), mediate stacking interactions with ssDNA bases. Alanine substitution of either of these residues in Teb1B led to an approximately fivefold decrease Teb1AB ssDNA-binding affinity as determined by filter binding (Fig. 2C). Two additional aromatic residues Y388 and H461 on the edge of the predicted ssDNA-binding surface also play important roles in Teb1AB–ssDNA interaction (Fig. 2 A and C). In addition to these aromatic residues, a cluster of positively charged residues on the ssDNA-binding surface could contribute to ssDNA contact (Fig. 2 A and B). Individual alanine substitutions were introduced for five positively charged residues (K403A, R406A, K408A, K419A, and K457A), which, with the exception of K408A, reduced ssDNA binding (Fig. 2C). Together these results confirm that Teb1B utilizes a similar OB-fold surface for ssDNA binding as Teb1A.
Fig. 2.
Crystal structure of Teb1B and its ssDNA-binding requirements. (A) Perpendicular views of the Teb1B structure. Secondary structure elements of the protein are labeled in the left panel. In the right panel, Teb1B side chains are shown in stick model and highlighted in three colors according to their importance for ssDNA binding (magenta, very important; salmon, important; yellow, less important). (B) Electrostatic potential surface representation of Teb1B. Orientation is the same as in the right panel of A. Putative ssDNA-binding residues are labeled, with the most important residues highlighted by dashed white circles. (C) Teb1B ssDNA interaction requirements. Teb1AB proteins with sequence substitutions in Teb1B were used in twofold concentration steps over the range of 5–1,000 nM with 100 pM 5′-GGGTTGGGGT-3′. (D) Ribbon diagram of the POT1AB–ssDNA complex with POT1A in light blue and POT1B in salmon. The ssDNA is in stick representation and colored as in Fig. 1B. (E) Structural model of the Teb1AB–ssDNA complex. The relative positioning of Teb1A (yellow) and Teb1B (green) was based on POT1AB and the path of ssDNA was modeled from Teb1 domain mutational analysis of ssDNA binding.
Overall our findings above suggest that the mechanism of telomeric-repeat ssDNA recognition by Teb1AB has parallels with ssDNA recognition by the two N-terminal OB folds of human POT1 (hereafter designated POT1AB) and less similarity with ssDNA recognition by RPA70AB (Fig. S3). First, Teb1A (Fig. 1) and POT1AB (12) employ an extensive network of electrostatic interactions to sequence-specifically recognize their cognate telomeric-repeat ssDNAs. In contrast, most of the electrostatic contacts observed in the RPA70AB–ssDNA complex are sequence-nonspecific interactions with the ssDNA backbone (14). Second, in addition to the aromatic residues that are common to ssDNA-binding OB folds (5), represented by W267 and F293 in Teb1A and F423 and Y450 in Teb1B, Teb1AB has three key ssDNA-binding residues analogous to ssDNA-binding residues of POT1AB (Y236, Y388, and H461 in Teb1AB; F31, Y161, and Y271 in POT1AB) that are not similarly represented in RPA70AB (Fig. S3). In POT1AB, all three of these residues mediate stacking interactions with ssDNA bases (12). Substitution of Teb1AB Y388 or H461 weakened ssDNA binding (Fig. 2C), supporting a role for these two residues in Teb1–ssDNA interaction. Based on these observations, we propose that similar to ssDNA recognition by POT1AB (12), each of the Teb1AB OB-fold domains contacts 4–5 nt to establish the overall sequence-specificity of ssDNA binding (Fig. 2 D and E).
Crystal Structure of Teb1C.
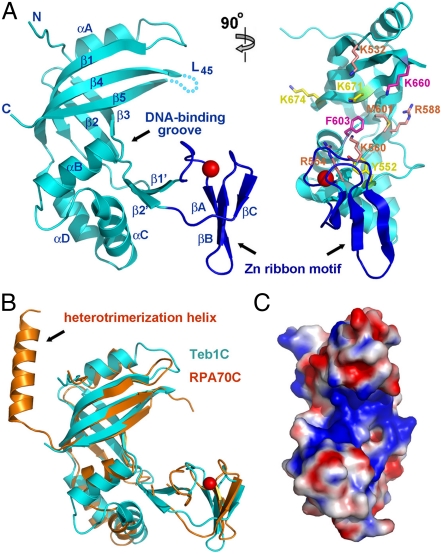
In contrast to Teb1A and Teb1B, Teb1C (Teb1 residues 505–701) by itself exhibited no detectable ssDNA-binding activity (11). However, the C-terminal half of Teb1 including both Teb1B and Teb1C (Teb1BC, residues 324–701) showed improved DNA-binding affinity compared to Teb1B alone, suggesting that Teb1C could contribute weak ssDNA binding. Teb1C also functions as a processivity factor, dramatically increasing telomerase holoenzyme RAP (11). To investigate the structural basis of Teb1C ssDNA-binding and processivity-factor activities, we solved its crystal structure using SAD and a platinum derivative (K2PtCl6) at a resolution of 2.5 Å. The final model contains Teb1 residues 511–678 with good geometry (Table S1).
The crystal structure of the Teb1C OB-fold domain (Fig. 3A) closely resembles that of RPA70C (Fig. 3B), with an rmsd value of 2.1 Å between them. The structural conservation includes not only the central β-barrel of the OB fold but also peripheral structural elements. First, both Teb1C and RPA70C contain a zinc ribbon motif embedded in the OB fold between strands β1 and β2 (Fig. 3 A and B). Second, in both structures, a large three-helix insertion between strands β3 and β4 (αB-αC-αD in Teb1C) caps the bottom of the OB fold while an α-helix (αA in Teb1C) covers the top (Fig. 3B). Deviating from this conservation, Teb1C and RPA70C structures differ at their extreme C termini. The C-terminal helix in RPA70C protrudes away from the β-barrel core to interact with the other two components of the RPA complex, RPA32 and RPA14, through an intermolecular three-helix bundle (15). Teb1C structures were the same with or without the region corresponding to the RPA70 C-terminal helix included, suggesting that either Teb1C lacks a C-terminal helix or it is not ordered in the crystal (Fig. 3B). This difference from RPA70C can account for how Teb1 avoids assembly with subunits of Tetrahymena RPA and why truncation of the extreme C terminus of Teb1 did not prevent its function as a processivity factor (10, 11).
Fig. 3.
Crystal structure of Teb1C. (A) Two perpendicular views of the Teb1C structure. Teb1C is in cyan except for the zinc ribbon motif in blue; the zinc ion is represented as a red sphere. Secondary structure elements are labeled in the left panel; the cyan dashed line indicates the unstructured loop L45. In the right panel, putative ssDNA-binding residues are highlighted as in Fig. 2A according to their importance in ssDNA binding. (B) Superposition of Teb1C (cyan) and RPA70C (orange) structures. The superposition is based on the OB-fold β-barrels of the proteins. (C) Electrostatic potential surface representation of Teb1C. The orientation of Teb1C is the same as in the right panel of A.
Structural Requirements for Teb1C Activities.
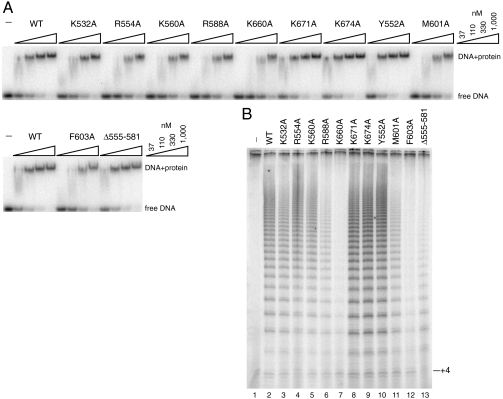
Surface electrostatic potential analysis of Teb1C revealed a highly positively charged patch suggestive of a potential site for ssDNA binding (Fig. 3C). To examine whether this surface contributes to the ssDNA-binding activity of Teb1BC, we introduced individual substitutions of positively charged or hydrophobic residues and assessed their effects on ssDNA binding by EMSA (Fig. 4A). The presence of Teb1C increases Teb1BC affinity for ssDNA by roughly 10-fold compared to Teb1B alone (11). Teb1C domain substitutions F603A and K660A imposed the most significant loss of binding affinity (Kd change of more than sixfold, calculated using the free DNA signal), and alanine substitutions of K532, R554, R588, K560, and M601 also reduced Teb1BC–ssDNA interaction (Fig. 4A). These results confirm that the positively charged surface of the Teb1C OB-fold domain does indeed contribute to ssDNA interaction. Given that an optimal ssDNA-binding site for full-length Teb1 contains at least 18 nt (10, 11) and that Teb1AB recognizes two telomeric repeats (Fig. S1), it is likely that, in the context of full-length Teb1, Teb1C is responsible for interaction with the third telomeric repeat. However, an indirect effect of Teb1C on Teb1B–ssDNA interaction has not been excluded.
Fig. 4.
Sequence requirements for ssDNA-binding and processivity-factor activities of Teb1C. (A) DNA-binding defects imposed by Teb1C amino acid substitutions assayed by EMSA. Teb1BC proteins were used in threefold concentration steps over the range of 37–1,000 nM with 10 pM 5′-GGGGTTGGGGTT-3′. (B) Processivity-factor defects imposed by Teb1C amino acid substitutions. Reconstituted telomerase holoenzyme was assayed with 200 nM (GT2G3)3 primer and 200 nM of the indicated Teb1BC polypeptide.
To address the mechanism of Teb1-dependent RAP, we investigated whether the ssDNA-binding activity of Teb1C is required using a previously established telomerase holoenzyme reconstitution system (11). We combined the recombinant Tetrahymena telomerase ribonucleoprotein (RNP) catalytic core produced in rabbit reticulocyte lysate with TASC isolated from Tetrahymena and Teb1BC purified from Escherichia coli. Individual amino acid substitutions within the Teb1C domain inhibited high-RAP activity reconstitution in a manner that generally paralleled the severity of the introduced ssDNA-binding defect: The F603A and K660A substitutions with the greatest impact on ssDNA binding also imposed the largest defect in high-RAP activity reconstitution (Fig. 4B).
Teb1C and RPA70C harbor a zinc ribbon motif comprised of a three-stranded antiparallel β-sheet with a coordinated zinc ion (Fig. 3 A and B). We deleted this zinc ribbon motif (Teb1 residues 555–581) in the soluble Teb1BCΔ555–581 purified from E. coli. Gel filtration analysis showed that Teb1BCΔ555–581 was a well-behaved protein in solution, suggesting that the zinc ribbon motif is not required for stability of the Teb1C OB fold. Despite a complete lack of impact on Teb1BC ssDNA binding (Fig. 4A), removing the Teb1C zinc ribbon motif greatly reduced Teb1BC function as a processivity factor (Fig. 4B). As a working model, we suggest that the zinc ribbon motif directly or indirectly contributes to the physical association of Teb1C with other telomerase holoenzyme subunits, including the TASC subunits required for bridging Teb1 to the RNP catalytic core.
Discussion
Telomerase function involves only transient association with telomeric DNA, whereas end-capping factors such as POT1 or the ciliate telomere end-binding protein heterodimer protect chromosome ends from inappropriate degradation or repair reactions throughout the cell cycle. Nonetheless, the initial recruitment of telomerase or an end-capping factor to the telomere would share a requirement for discrimination of telomeric-repeat DNA from the vast excess of other DNA present in a cell. Here we show that the two OB-fold domains of Teb1AB together recognize telomeric-sequence ssDNA with a dependence on repeat permutation and length that is comparable to ssDNA recognition by human POT1. We suggest that the double OB fold is a convergent evolutionary solution for biologically selective discrimination of Tetrahymena T2G4 or human T2AG3 repeats.
In contrast to the ssDNA-binding domains of Teb1AB or POT1, Teb1C has sizeable structural elaborations added to the OB fold and provides only a weak stimulation of ssDNA interaction. We show that Teb1C ssDNA-binding activity and also its zinc ribbon motif play a critical role in promoting the high-RAP activity of Tetrahymena telomerase holoenzyme. We suggest that ssDNA contact by Teb1C is sufficient to support an initial threading of ssDNA through a cleft or channel in the high-RAP conformation of elongating holoenzyme that can then slide or translocate along the nascent product. This DNA threading would account for how high RAP is conferred without high-affinity ssDNA binding by the combination of Teb1C, TASC, and the RNP catalytic core. We propose that the initial recruitment of telomerase to G-rich telomeric-repeat tracts exposed as ssDNA by conventional DNA replication machinery involves Teb1AB recognition of telomeric repeats that are potentially somewhat internal to the extreme chromosome terminus. Subsequent capture of the chromosome 3′ end by the active site of the RNP catalytic core could then favor Teb1C–ssDNA contact and/or other changes in holoenzyme conformation associated with the gain of increased enzyme–DNA association stability. It is possible that interdomain coordination between Teb1AB and Teb1C is regulated in cellular context, potentially by the Teb1N domain and/or additional factors (8).
Materials and Methods
Teb1 polypeptides were expressed in N-terminal fusion to a His6 or His6-SUMO tag. Detailed protocols for protein expression, purification, and crystallization as well as for determination of protein and protein–DNA complex structures are provided in SI Text. Electrophoretic mobility shift assays and telomerase activity assays were done as described previously (11), with additional details in the figure legends. Filter-binding experiments were performed in a 96-well dot blot apparatus essentially as described (16) using 5′-end-labeled oligonucleotide at 100-pM final concentration in a 50-μL total volume of binding buffer [20 mM Tris·HCl (pH 7.5), 1 mM DTT, and 150 mM NaCl] for 1 h at room temperature. The mixtures were then filtered through a membrane sandwich containing a top layer of nitrocellulose membrane (GE), a middle of layer of positively charged nylon membrane (GE), and a bottom layer of filter paper (Whatman).
Supplementary Material
Acknowledgments.
M.L. acknowledges generous financial support from National Institutes of Health (NIH) R01 GM083015 and the American Cancer Society. M.L. is a Howard Hughes Medical Institute Early Career Scientist. The General Medicine and Cancer Institutes Collaborative Access Team has been funded in whole or in part with federal funds from the National Cancer Institute (Grant Y1-CO-1020) and the National Institute of General Medical Science (Grant Y1-GM-1104). Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02-06CH11357. K.C. laboratory support for Tetrahymena telomerase research is provided by NIH R01 GM54198.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Telomerase and Retrotransposons: Reverse Transcriptases that Shaped Genomes” held September 29–30, 2010, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/telomerase_and_retrotransposons.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.J.C. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3U4V, 3U4Z, 3U50, and 3U58).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113624108/-/DCSupplemental.
References
- 1.O’Sullivan RJ, Karlseder J. Telomeres: Protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croy JE, Wuttke DS. Themes in ssDNA recognition by telomere-end protection proteins. Trends Biochem Sci. 2006;31:516–525. doi: 10.1016/j.tibs.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Horvath MP. Single-stranded nucleic acid (SSNA)-binding proteins. In: Rice PA, Correll CC, editors. Protein-Nucleic Acid Interactions: Structural Biology. Cambridge, UK: The Royal Society of Chemistry; 2008. pp. 91–128. [Google Scholar]
- 4.Theobald DL, Mitton-Fry RM, Wuttke DS. Nucleic acid recognition by OB-fold proteins. Annu Rev Biophys Biomol Struct. 2003;32:115–133. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochkarev A, Bochkareva E. From RPA to BRCA2: Lessons from single-stranded DNA binding by the OB-fold. Curr Opin Struct Biol. 2004;14:36–42. doi: 10.1016/j.sbi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Flynn RL, Zou L. Oligonucleotide/oligosaccharide-binding fold proteins: A growing family of genome guardians. Crit Rev Biochem Mol Biol. 2010;45:266–275. doi: 10.3109/10409238.2010.488216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins K. Forms and functions of telomerase RNA. In: Walter NG, Woodson SA, Batey RT, editors. Non-Protein Coding RNAs. Vol. 13. Berlin: Springer; 2009. pp. 285–301. (Springer Series in Biophysics). [Google Scholar]
- 8.Collins K. Single-stranded DNA repeat synthesis by telomerase. Curr Opin Chem Biol. 2011;15:643–648. doi: 10.1016/j.cbpa.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob NK, Lescasse R, Linger BR, Price CM. Tetrahymena POT1a regulates telomere length and prevents activation of a cell cycle checkpoint. Mol Cell Biol. 2007;27:1592–1601. doi: 10.1128/MCB.01975-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min B, Collins K. An RPA-related sequence-specific DNA-binding subunit of telomerase holoenzyme is required for elongation processivity and telomere maintenance. Mol Cell. 2009;36:609–619. doi: 10.1016/j.molcel.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min B, Collins K. Multiple mechanisms for elongation processivity within the reconstituted Tetrahymena telomerase holoenzyme. J Biol Chem. 2010;285:16434–16443. doi: 10.1074/jbc.M110.119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei M, Podell ER, Cech TR. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11:1223–1229. doi: 10.1038/nsmb867. [DOI] [PubMed] [Google Scholar]
- 13.Lei M, Podell ER, Baumann P, Cech TR. DNA self-recognition in the structure of Pot1 bound to telomeric single-stranded DNA. Nature. 2003;426:198–203. doi: 10.1038/nature02092. [DOI] [PubMed] [Google Scholar]
- 14.Bochkarev A, Pfuetzner RA, Edwards AM, Frappier L. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature. 1997;385:176–181. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]
- 15.Bochkareva E, Korolev S, Lees-Miller SP, Bochkarev A. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. EMBO J. 2002;21:1855–1863. doi: 10.1093/emboj/21.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei M, Baumann P, Cech TR. Cooperative binding of single-stranded telomeric DNA by the Pot1 protein of Schizosaccharomyces pombe. Biochemistry. 2002;41:14560–14568. doi: 10.1021/bi026674z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.