Abstract
The herbicide paraquat (PQ) has increasingly been reported in epidemiological studies to enhance the risk of developing Parkinson's disease (PD). Furthermore, case-control studies report that individuals with genetic variants in the dopamine transporter (DAT, SLC6A) have a higher PD risk when exposed to PQ. However, it remains a topic of debate whether PQ can enter dopamine (DA) neurons through DAT. We report here a mechanism by which PQ is transported by DAT: In its native divalent cation state, PQ2+ is not a substrate for DAT; however, when converted to the monovalent cation PQ+ by either a reducing agent or NADPH oxidase on microglia, it becomes a substrate for DAT and is accumulated in DA neurons, where it induces oxidative stress and cytotoxicity. Impaired DAT function in cultured cells and mutant mice significantly attenuated neurotoxicity induced by PQ+. In addition to DAT, PQ+ is also a substrate for the organic cation transporter 3 (Oct3, Slc22a3), which is abundantly expressed in non-DA cells in the nigrostriatal regions. In mice with Oct3 deficiency, enhanced striatal damage was detected after PQ treatment. This increased sensitivity likely results from reduced buffering capacity by non-DA cells, leading to more PQ+ being available for uptake by DA neurons. This study provides a mechanism by which DAT and Oct3 modulate nigrostriatal damage induced by PQ2+/PQ+ redox cycling.
Keywords: neurodegeneration, extraneuronal monoamine transporter, astrocytes, in vivo microdialysis
Parkinson's disease (PD) is characterized primarily by the loss of dopamine (DA) neurons in the substantia nigra pars compacta (1). Although in past decades discoveries of genetic mutations linked to PD have significantly impacted our current understanding of the pathogenesis of this devastating disorder, it is likely that the environment plays a critical role in the etiology of sporadic PD. Human epidemiological studies indicate that exposure to herbicides, pesticides, and heavy metals increase the risk of PD. One such environmental toxicant is paraquat (PQ2+, N,N′-dimethyl-4–4′-bipiridinium) (2, 3). This molecule exists natively as a divalent cation, but can undergo redox cycling with cellular diaphorases such as NADPH oxidase and nitric oxide synthase (4) (NOS) to yield the monovalent cation PQ+. From this redox cycle, superoxide is generated, leading to oxidative stress-related cytotoxicity. (For clarity and brevity, the abbreviations PQ2+ and PQ+ will be used to signify the respective cations, whereas PQ represents a general term when the valency is ambiguous.) On the basis of its structural similarity to 1-methyl-4-phenylpyridinium (MPP+), an active metabolite of the parkinsonian agent 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (5), PQ2+ has been predicted to be a potential environmental parkinsonian toxicant (6), and with subsequent recent epidemiological studies (2, 3), there has been increasing interest in this herbicide as a potential pathogenic agent in PD.
When PQ2+ is injected into mice, it induces a loss of nigral DA neurons, but the striatum is spared (7, 8), most likely due to compensatory striatal sprouting in the remaining neurons (9). In addition, PQ2+ induces α-synuclein up-regulation and aggregation (10), a neuropathological feature detected in PD patients. In a recent case-control study (2), PQ2+ was reported to increase the risk of PD in subjects with certain genetic variants in the dopamine transporter (DAT). Together, these studies support the neurotoxic role of PQ2+ in the nigrostriatal system and highlight the need to understand the mechanism by which PQ2+ induces toxicity. However, to date, the very fundamental questions of whether and, if so, how PQ2+ enters DA neurons remain unanswered (11).
In the present study, we describe a mechanism by which PQ2+ enters DA neurons. We propose that, in the brain, PQ2+ is reduced to PQ+ extracellularly by enzymes such as NADPH-oxidase on microglia. In contrast to its parent compound, PQ+ is a DAT substrate and is therefore accumulated in DA neurons where it establishes a new redox cycle intracellularly, leading to the generation of superoxide and DA reactive species and, ultimately, neurotoxicity. Blocking DAT function abolished PQ+ neurotoxicity in both cells and living mice. In addition to DAT, PQ+ is also a substrate for the organic cation transporter-3 (Oct3), a bidirectional transporter that is highly expressed in astrocytes and GABAergic neurons in the nigrostriatal regions (12, 13). Together, these two transporters function in a concerted manner to mediate nigrostriatal damage. Collectively, our data point to an interplay between DA and non-DA cells mediated, respectively, by DAT and Oct3, which modulate the function and viability of the nigrostriatal pathway.
Results
PQ2+ Induces Striatal Neurotoxicity and DA Overflow in Mice with Oct3 Deficiency.
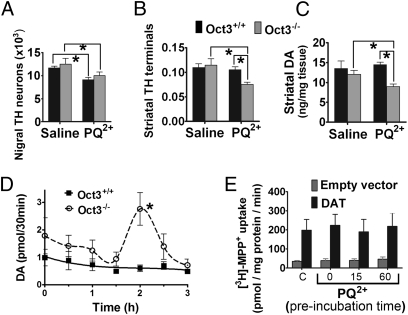
We recently reported that Oct3 can modulate toxicity in the dopaminergic system through its bidirectional transport capability of various toxic cations (12). In the present study, we asked whether this mechanism was also relevant to PQ2+. To this end, we injected Oct3-null (Oct3−/−) mice and their Oct3+/+ wild-type littermates intraperitoneally (i.p) with PQ2+. Consistent with the neurotoxic features of PQ2+ on the nigrostriatal system (7, 14), we detected a loss of nigral DA neurons (∼22%) (Fig. 1A). Quantification of total Nissl-positive neurons confirmed that this reduction was due to cell loss, and not to down-regulation of the phenotypic marker tyrosine hydroxylase (TH) (saline control group: 15,438 ± 532; PQ2+ treated group: 12,364 ± 522, n = 5 mice/group; data represent mean ± SEM). Although comparable loss of DA neurons was also observed in Oct3−/− mice treated with PQ2+, we also observed a significant reduction (∼40%) in immunoreactivity of striatal TH (the rate limiting enzyme for DA production) in Oct3−/− but not in Oct3+/+ mice (Fig. 1B). HPLC analysis confirmed a corresponding reduction in total striatal DA content (Fig. 1C). This lack of DA reduction in wild-type mice despite a loss of DA neurons is proposed to be related to the compensatory up-regulation of TH activity in the striatum after PQ2+ injection (9). On the basis of our previously proposed function of Oct3 in mediating the neurotoxicity of methamphetamine (12), it is possible that the striatal neurotoxicity observed in the Oct3−/− mice was due to a reduced PQ buffering capacity in non-DA cells, and, hence, more PQ was available for DAT-mediated transport into DA terminals. To lend further support for this argument, we performed in vivo microdialysis to compare the functional effects of PQ2+ treatment in freely moving Oct3−/− and Oct3+/+ mice. Using DA overflow as a functional response of DA neurons to PQ2+ (15, 16), we detected an increase in extracellular DA ∼120 min after a single PQ2+ injection (i.p.) in Oct3−/− mice, but not in their Oct3+/+ littermates (Fig. 1D). These results suggest that PQ2+ exerted a more dramatic effect on striatal DA terminals in Oct3−/− mice. To test whether this enhanced DA overflow was the result of an interaction between PQ2+ and DAT, we used stable cells expressing DAT to assess the ability of PQ2+ to compete with transport of the DAT substrate MPP+. Preincubation of cells with PQ2+ for up to 1 h did not affect the uptake of MPP+ mediated by DAT (Fig. 1E). Additionally, we found no difference in the kinetics of PQ2+ reaching the striatum in Oct3−/− and Oct3+/+ mice using in vivo microdialysis and HPLC to quantify PQ levels in mice injected with PQ2+ (Fig. S1). Thus, although the microdialysis data suggest that, in the absence of Oct3 function, PQ somehow leads to striatal DA release and subsequent neurotoxicity, the mechanism for these effects is unclear, given that PQ2+ does not appear to interact functionally with DAT on the basis of its inability to inhibit MPP+ transport by DAT (Fig. 1E).
Fig. 1.
PQ2+ injection increases striatal neurotoxicity and DA overflow in mice with Oct3 deficiency. (A–C: neurotoxicity study) Oct3−/− mice and Oct3+/+ littermates (10–12 wk old) were injected with PQ2+ (10 mg/kg, i.p., every second day for a total of 10 injections) or saline. Seven days after the last injection, mice were processed for stereological cell counting (A), striatal tyrosine hydroxylase immunoreactivity (B, optical density), or HPLC measurement of DA content (C). n = 5 animals per group for A and B and n = 5–9 per group for C. *P < 0.05, analyzed by two-way ANOVA followed by the Newman–Keuls post hoc test. (D: in vivo microdialysis study) Oct3−/− and Oct3+/+ littermates (10–12 wk old) were stereotactically implanted with microdialysis probes into the right striatum. After 2 h of equilibration, dialysates were collected every 30 min for 1 h before PQ2+ injection (15 mg/kg, i.p.) for baseline measurements (pooled for 0 time point) and for an additional 3 h after the injection, followed by HPLC analyses for DA. n = 7 animals per group. Area under the curve was generated using GraphPad Prism followed by a two-tailed t test. *P < 0.05 compared with the Oct3+/+ group. (E: transport study) Uptake of tritiated MPP+ was assessed in EM4 cells (modified human embryonic kidney cells; SI Materials and Methods) with stable expression of DAT or empty vector control. Uptake reaction mediated by DAT was assessed in cells preincubated with 500 μM PQ2+ up to 60 min and compared with the control group (“C”) without PQ2+ preincubation. n = 3–5 independent experiments in quadruplicate.
PQ2+ Is a Poor Substrate for Both Oct3 and DAT, Unless Converted to PQ+.
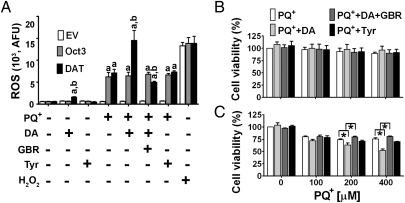
To further investigate directly whether PQ2+ is a substrate for Oct3 and DAT, we assessed uptake of PQ2+ in stable EM4 cells (modified HEK293) expressing Oct3, DAT, or an empty vector control (12). Despite its similar structure to MPP+, which is an excellent substrate for these transporters, we found that PQ2+ was not taken up into cells through these transporters, as shown in the dose–response studies (Fig. 2 A–C). This is consistent with the inability of PQ2+ to interfere with MPP+ uptake (Fig. 1E) and a previous study reporting that PQ2+ was not transported by DAT (11). We hypothesized that the two positive charges on PQ2+ may interfere with its ability to be transported by Oct3 and DAT, as both transporters favor monovalent cations as substrates. To test this hypothesis, we performed transport studies in the presence of sodium dithionite (SDT). This reducing agent was used to donate an electron and hence convert PQ2+ to PQ+ as previously described (17). After PQ2+ was converted to PQ+, the intracellular content of PQ was dramatically higher in stable cells expressing Oct3 (Fig. 2B) or DAT (Fig. 2C) as determined by HPLC. That Oct3 accumulated more PQ+ than did DAT could relate to transporter expression level, the affinity and maximal transport rates of the transporters, and the different driving forces. To rule out the possibility that the observed increased uptake in the presence of SDT was due to redox action of this agent on the transporters, we performed a transport study using stable DAT cells and MPP+ as a substrate because this molecule is not affected by SDT (Fig. S2). Intracellular levels of MPP+, as measured by HPLC, were comparable between the groups of cells treated with or without SDT, indicating that this reducing agent did not affect the function of DAT itself. Together, our results strongly support that PQ2+, once converted to PQ+, is capable of entering cells through DAT and Oct3.
Fig. 2.
PQ+ is transported by both Oct3 and DAT to induce cytotoxicity. Stable EM4 cells expressing empty vector control (A), Oct3 (B), or DAT (C) were cultured in 24-well plates for 24 h. Cells were then washed and incubated with varying concentrations of PQ2+ with or without 0.5 mM sodium dithionite (SDT) in degassed Krebs Ringer Hepes (KRH) buffer to convert PQ2+ to PQ+. After 20 min, cells were washed and then collected for HPLC analysis. To determine the effects of PQ+ on the formation of reactive oxygen species (ROS) (D–F), these cell types were treated for 24 h with the indicated concentrations of PQ2+ with or without 0.5 mM SDT. After 20 min, cells were washed and grown in regular medium. After 24 h, cells were incubated with 5 μM dihydroethidium and analyzed for ROS levels using flow cytometry (AFU: arbitrary fluorescence unit). H2O2 (30 μM) was incubated with cells for 20 min as a positive control for ROS production. To assess whether the observed ROS production would lead to cytotoxicity, cell viability was performed (G–I). Cells were treated with PQ2+ and SDT as shown in D–F, washed, and grown in cell culture medium for another 48 h before a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed. n = 4 independent experiments in quadruplicate, analyzed by two-way ANOVA followed by the Newman–Keuls post hoc test. *P < 0.05 compared with the respective control groups without SDT.
To determine whether higher uptake of PQ+ in cells would lead to more oxidative stress and cytotoxicity, we assessed the levels of reactive oxygen species (ROS) and cell viability. Cells were treated with PQ2+ for 20 min with or without SDT as performed for Fig. 2 A–C. This short treatment of SDT with PQ2+ dramatically increased intracellular levels of ROS in stable cells with Oct3 or DAT expression, but not in those with empty vector (Fig. 2 D–F). As expected, the control treatment of H2O2, which does not need a transporter to enter cells, increased ROS production in all cell types. The increase in ROS levels induced by PQ+ subsequently led to higher cytotoxicity (Fig. 2 G–I). These results indicate that PQ+, but not PQ2+, enters cells through both Oct3 and DAT to induce oxidative stress and cell death.
DA Enhances Cytotoxicity Induced by PQ+.
PQ+ induced the same extent of toxicity in EM4 cells regardless of whether it entered cells through Oct3 or DAT (Fig. 2). These results raise the question of why in animal models DA neurons are more vulnerable to PQ toxicity (7). DA itself has been suggested to contribute to the enhanced cytotoxicity of PQ in DA neurons as DA is known to generate reactive species through processes such as auto-oxidation, enzymatic metabolism, or interactions with the products (such as superoxide) generated from the redox cycling of PQ2+/PQ+ (18). To test this hypothesis, ROS and cell viability was measured with or without the addition of 50 μM DA. In the presence of this nontoxic concentration of DA, PQ+ induced a dramatic increase in ROS (Fig. 3A) and cell death (Fig. 3C) in DAT cells, but not in EV cells (Fig. 3B). This enhanced ROS production and cell death was blocked by the DAT inhibitor GBR12909 and was absent when DA was replaced by the DAT-substrate tyramine, which has a similar structure to DA (but only one hydroxyl substituent on the phenyl ring). The metabolism of tyramine requires monoamine oxidase; however, EM4 cells contain only catechol-O-methyltransferase, and thus, only DA can be metabolized to reactive species in these cells. Consistent with the low affinity of Oct3 for DA (Km = 1.5 mM) (19), we observed that addition of 50 μM DA did not further increase ROS (Fig. 3A) in Oct3-expressing cells. Together, these results support the significant role that DA contributes to selective cell death in PQ toxicity.
Fig. 3.
DA enhances ROS production and cytotoxicity induced by PQ+. EM4 cells with empty vector, Oct3, or DAT expression were treated with 200 μM PQ2+ plus 0.5 mM SDT for 20 min. Then the cells were washed and incubated in culture medium with or without 50 μM DA, 50 μM tyramine, or 1 μM GBR12909 (a DAT inhibitor). H2O2 (30 μM for 1 h) was used as a positive control for ROS production. After 48 h, cells were assessed for ROS levels using flow cytometry (A). In a parallel set of experiments, cells with empty vector (B) and DAT (C) treated with varying concentrations of PQ2+ as described in A were assessed for cell viability using a MTT assay. n = 4 independent experiments in quadruplicate, analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test. aP < 0.05 compared with respective EV; bP < 0.05 compared with the respective Oct3 groups; *P < 0.05.
Human Microglia Are Capable of Converting PQ2+ to PQ+ Through Their Intrinsic NADPH Oxidase Activity.
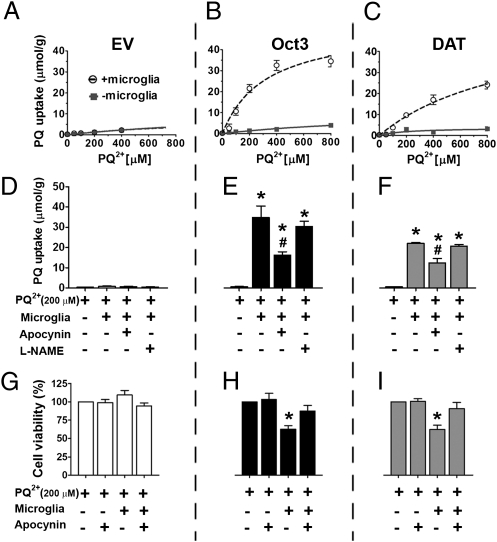
To demonstrate that the conversion of PQ2+ to PQ+ can take place in the brain and is relevant to humans, we cocultured human microglia with stable EM4 cells expressing Oct3 or DAT. After 24 h of treatment with PQ2+, EM4 cells were collected for the measurement of PQ uptake using HPLC. As demonstrated in Fig. 4 A–C, higher uptake was detected in Oct3- and DAT-expressing cells when cocultured with microglia, suggesting that microglia are capable of converting PQ2+ to PQ+, which is then transported into cells through these transporters. The patterns of uptake in these cells are similar to those in Fig. 2, which shows the conversion of PQ2+ to PQ+ by SDT. This conversion was mediated at least partly by NADPH oxidase on microglia because inhibiting the activity of this enzyme by apocynin significantly attenuated the uptake of PQ+ through Oct3 and DAT (Fig. 4 D–F). The reduction of intracellular PQ levels resulted in significant attenuation of cell death (Fig. 4 G–I). Because NOS is also capable of reducing PQ2+ and blocking this enzyme in mouse microglia decreases ROS production (18), we assessed the role of NOS in converting PQ2+ to PQ+ in our cell culture paradigm. Our results indicate that, after treating human microglia with the same concentration of N-nitro-l-arginine methyl ester (l-NAME) (a NOS inhibitor) as previously described (18), we did not see a decrease in PQ+ uptake into cocultured Oct3 or DAT stable cells. These results suggest that human microglia are not capable of taking up PQ to interact with intracellular NOS. Alternatively, our results are consistent with previous studies reporting that human microglia either have no or very low NOS activity (20–22), in contrast to rodent microglia (23, 24). Regardless of whether human microglia are capable of performing this conversion via NOS, our results support that, under in vivo conditions, it is likely that PQ2+ can be converted to PQ+ at least by NADPH oxidase before being transported into DA neurons to induce neurotoxicity.
Fig. 4.
Microglia promote the conversion of PQ2+ to PQ+ through NADPH oxidase. Stable EM4 cells expressing Oct3, DAT, or empty vector control were grown on glass coverslips for 24 h. These coverslips were then transferred to six-well plates on which a monolayer of human microglial immortalized cells (CHME5) had been plated for 24 h. Different concentrations of PQ2+ were added to these cocultures. After 24 h of PQ2+ addition, the uptake of PQ+ into EM4 cells was evaluated by removing the coverslips from the cocultures and collecting cells for HPLC measurement (A–C). To determine whether increased uptake of PQ+ was mediated by NADPH oxidase or nitric oxide synthase (NOS), apocynin and l-NAME, respectively, were added to the cocultures (D–F). Inhibition of NADPH oxidase by apocynin, but not NOS by l-NAME, significantly reduced PQ+ transport into Oct3 or DAT cells. n = 4 independent experiments, analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test. *P < 0.05 compared with the PQ2+ alone group; #P < 0.05 compared with PQ2+ plus microglia group as well as PQ2+ plus microglia plus the l-NAME group. The reduced uptake of PQ+ in the presence of apocynin resulted in less cytotoxicity (G–I) as assessed by using an MTT assay 48 h later. n = 4 independent experiments, analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test. *P < 0.05 compared with all other groups.
Mutant Mice with Hypomorphic DAT Are Resistant to PQ Neurotoxicity.
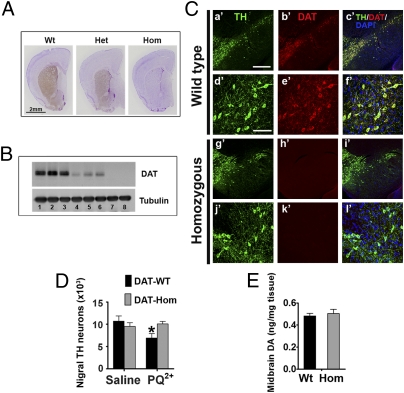
If PQ2+ could be converted to PQ+ in vivo and then taken up by DA neurons through DAT to induce toxicity, then reducing DAT function should reduce neurotoxicity. Therefore, we used a genetically engineered mouse model (Fig. S3) with an almost complete loss of DAT expression (Fig. 5 A–C). The small amount of residual DAT (< 5%) (Fig. 5 A and B) allows for normal survival rates and normal lactation in these mutant mice, which did not require cross-fostering at birth like DAT−/− mice (25). When injected with PQ2+, mice homozygous for hypomorphic DAT exhibited no loss in nigral DA neurons (Fig. 5D) in contrast to their wild-type littermates. Because DAT−/− mice have been reported to display modest reductions of TH in the midbrain (26) and because our in vitro data suggest that cytosolic DA can modulate PQ toxicity, we assessed whether such alterations were present in this hypomorphic DAT model. HPLC analyses of the midbrain demonstrate that DA levels (Fig. 5E) were comparable between genotypes, thereby eliminating the possibility that lower DA levels in the cell bodies may account for the resistance to PQ toxicity. We did observe significantly decreased striatal DA content in DAT hypomorphic mice (Fig. S4B), but, due to the lack of PQ toxicity observed in the striatum of wild-type and mutant mice (Fig. S4), the significance of the altered DA content in this region relating to cytotoxicity cannot be determined. In summary, the lack of PQ-induced neurodegeneration in DAT hypomorphic mice strengthens the role of this transporter in mediating PQ neurotoxicity.
Fig. 5.
Mutant DAT hypomorphic mice are resistant to PQ neurotoxicity. (A) Coronal striatal sections from wild type (Wt), heterozygous (Het), and homozygous (Hom) mutant DAT mice were immunostained with a DAT antibody (brown, diaminobenzidine chromogen) and counterstained with Nissl to reveal the structure of other brain regions. (B) For a more quantitative comparison, Western blotting was performed. Lanes 1–3 (Wt), lanes 4–6 (Het), and lanes 7–8 (Hom) are from eight animals. (C) Immunofluorescence of TH and DAT in coronal midbrain sections containing the substantia nigra and ventral tegmental area. [Scale bars: 400 μm (a′–c′, g′–i′), 100 μm (d′–f′, j′–l′).] No apparent differences in morphology of midbrain DA neurons was noted between mutant and wild-type mice. Stereological cell counting confirmed comparable population of nigral DA neurons (D) and midbrain DA levels (E) in these mutant mice. (D) To assess the effects of PQ toxicity in these animals, DAT wild-type mice and their homozygous mutant littermates (∼10 wk old) were injected with PQ2+ (10 mg/kg, i.p., every second day for a total of 10 injections) or saline. Seven days after the last injection, mice were processed for stereological cell counting. n = 4–5 animals per group, analyzed by two-way ANOVA followed by the Newman–Keuls post hoc test. *P < 0.05 compared with the control saline-treated group.
Discussion
After the discovery of MPTP as the cause of acute parkinsonism in a group of drug abusers (5) and the subsequent characterization of MPP+ as the active metabolite of MPTP (27), a search for environmental contaminants with a similar structure to MPP+ was initiated. The widely used herbicide PQ2+ was identified as such an agent and was suggested to be a potential environmental parkinsonian toxicant (6). Supported by epidemiological studies, especially in recent years (2, 3), there has been an interest in further investigating the mechanism by which this molecule induces dopaminergic neurodegeneration.
In the present study, we demonstrated that, when PQ2+ was reduced to the monovalent cation PQ+, it was efficiently taken up by cells through DAT and Oct3. This conversion took place in the presence of either a reducing agent or NADPH oxidase on microglia. The increase in intracellular content of PQ resulted in higher ROS production and cytotoxicity. When PQ+ was combined with a nontoxic concentration of DA, significant increases in ROS levels and cell death were detected, suggesting that DA itself may contribute to the vulnerability of DA neurons to PQ toxicity. Either blocking the uptake of PQ+ through DAT or blocking the conversion of PQ2+ mediated by NADPH oxidase resulted in lower intracellular content of PQ and its subsequent ROS production and cell death. In aggregate, these results demonstrate that, although the initial divalent form of PQ2+ is not toxic to cells, its monovalent metabolite can enter DA neurons through DAT to induce oxidative damage. This scenario is analogous to the fact that, although MPTP itself is not toxic, its metabolite MPP+ is the culprit in DA cell death (28).
The toxic effect of PQ2+ mediated by microglia through redox cycling of NADPH oxidase has been reported (18, 29). Although the expression of this enzyme has been well documented in microglia, it is also present in astrocytes (30) and DA neurons (31). In mice deficient in gp91phox (a functional subunit required by NADPH oxidase), PQ2+ failed to induced neurotoxicity (29, 32). NADPH oxidase is composed of three cytosolic and two membrane-bound subunits. When activated, the cytosolic subunits translocate and bind to the membrane-bound subunits (p22 and gp91) and produce extracellular superoxide. Thus, this enzyme can induce redox cycling of PQ2+ extracellularly. Therefore, the mechanism of PQ2+ toxicity mediated by NADPH oxidase has been suggested to be mediated by extracellular superoxide (29, 32). Although it is likely that this oxidative stress pathway is involved in PQ2+ toxicity, it has been a topic of debate how PQ2+ can enter DA cells to induce oxidative stress intracellularly. On the basis of our data, we propose that PQ+ is generated from the extracellular redox cycling and then taken up into DA neurons where it establishes a new round of redox cycling and, together with DA, induces neurodegeneration. Consistent with our theory that PQ2+ is a protoxicant that must first be converted to an active metabolite, when we compared DA overflow induced by PQ2+ and MPP+ by separately infusing equimolar amounts of these molecules into the striatum using a microdialysis cannula (Fig. S5), a peak of DA was detected about 2 h after PQ2+ injection. This peak was much delayed and less pronounced than the one produced by MPP+.
Although our results outline one mechanism by which PQ2+ induces selective cell death in DA neurons, they do not address why the group of DA neurons in the ventral tegmental area (VTA) is insensitive to PQ2+ toxicity (14, 33). Di Monte and colleagues (33) previously reported that DA neurons expressing calbindin-D28K were resistant to neurotoxicity induced by PQ2+ and that the number of calbindin-D28K–containing DA neurons in VTA was five times higher than in their counterparts in the nigra. Calbindin is a Ca2+-buffering protein that has been shown to be correlated with cell viability in PD (34, 35). Consistent with these observations, recent studies demonstrated that the selective expression of L-type Ca2+ channels is responsible for higher cytosolic Ca2+ levels in nigral DA neurons (36). When combined with synuclein and DA, these factors account for the selective cell vulnerability of nigral DA neurons (37). Although the complex mechanisms of selective cell death in genetic or toxicant-induced models of PD will likely remain a topic of research for years to come, our demonstration of a mechanism through which PQ2+ enters DA cells is a critical step in elucidating the pathogenic mechanism of this toxicant and perhaps other toxic redox-cycling molecules as well.
Our results also demonstrate that PQ+ is a substrate for Oct3. The significance of Oct3 in PQ2+ toxicity is illustrated by the observation that, in Oct3−/− mice, PQ2+ injection induced striatal damage in DA terminals. As discussed, this is an atypical toxic profile in the PQ2+ mouse model of PD. That PQ+ is a substrate for both Oct3 and DAT suggests opportunities for crosstalk between glia, non-DA neurons, and DA neurons. As proposed in Fig. S6, when PQ2+ reaches the striatum after systemic injection, it can be converted to PQ+ by microglia and subsequently taken up into DA neurons by DAT and into astrocytes as well as medium spiny neurons by Oct3. Therefore, the initial direct target of PQ is not only DA neurons. Because Oct3 is bidirectional, the neighboring non-DA cells may act as a “sink” to store the excess of PQ right after injection and then act as a “source” to slowly release this cation (in the monovalent form) to neighboring DA neurons. Therefore, this slow release could induce a constant chronic state of oxidative stress to DA neurons. Consistent with this theory, for cell death to occur, animals have to be treated with PQ2+ for at least 3–4 wk.
Although our results further support the role of Oct3 in mediating neurotoxicity, it highlights the intriguing question of why striatal DA terminals are usually so resistant to PQ toxicity. It has been noted that different toxicants seem to affect the nigrostriatal structure differentially (9). In the MPTP model, for example, both striatal terminals and nigral cell bodies are affected. In methamphetamine toxicity, only the terminals are damaged (unless a very high dose is used). Other neurotoxic models such as 6-hydroxydopamine and rotenone also induce more toxicity in the striatum. In PQ toxicity, however, only the cell body is affected, not the striatal terminals. Addressing the issue of regional vulnerability is beyond the scope of this study; however, Oct3−/− mice may provide a useful model to study PQ toxicity when damage to both DA terminals and cell bodies is desired. It is worthwhile to note that Oct3 immunoreactivity has been reported to be higher in the striatum than in the niga (13). A lower level of Oct3 (and hence, less buffering capacity) in combination with a high microglia population in the nigra might contribute to a higher sensitivity of the DA cell body compared with DA terminals when treated with PQ. This expression pattern is consistent with our finding that there is no damage to striatal terminals in wild-type mice, but significant damage in Oct3−/− mice when treated with PQ.
In a case-control study, Ritz et al. (2) report that individuals with certain allelic variants belonging to clade A of the DAT gene are more likely to develop PD when they also have “high” exposure to PQ and maneb, but not in the absence of this gene/pesticide combination, suggesting a gene–environment interaction. This study also reports that a clade A diplotype is more strongly associated with the development of PD than clade B. A gene-dose effect is also observed. Combined with the in vitro observation that clade A haplotypes have higher transcriptional activity than clade B (38), these two studies are consistent with our observation that PQ toxicity requires DAT function. However, Ritz et al. (2) speculated that clade A variants with increased risk of PD in their study would result in a lower DAT expression (and, hence, in overall reduced function) on the basis in part of the following two studies. First, clade B variants increase the level of DAT expression as measured by PET imaging and binding studies in humans (39). However, the impact of these genetic variations on levels of functional surface-expressed DAT in neurons is unknown. Second, on the basis of a cell culture study, PQ2+ is not a substrate for DAT (11). Although our findings are fully in agreement with this latter study, we have shown that PQ+ is a substrate for DAT, that PQ+ elevates reactive oxygen species, and that lowering DAT expression eliminates the neurotoxicity of PQ. The mechanism that we have discovered makes it more likely that disease-associated DAT variants have an overall DAT-enhanced function through which more PQ+ is accumulated. Additional studies are required to directly test this hypothesis.
In summary, the present study describes a mechanism by which PQ enters DA cells to induce neurotoxicity. This information is critical to the paradoxical observations of a higher risk of developing PD in individuals with genetic variants in DAT and in individuals exposed to PQ2+ (2), despite the lack of PQ2+ uptake by DAT (11). By extension, other toxic molecules with the redox-cycling property might also use this mechanism to induce neurotoxicity. Combined with our previous study (12) demonstrating that both Oct3 and DAT functionally coordinate to modulate neurotoxicity of MPTP and methamphetamine (Fig. S6), our present study has a broad mechanistic implication for neurotoxicity in the nigrostriatal pathway.
Materials and Methods
See SI Materials and Methods for description of animal models, paraquat treatment, stereological cell counts, in vivo microdialysis, HPLC measurements of striatal DA and its metabolites, cell cultures, transport assays, and oxidative stress assessment.
All statistical values are expressed as mean ± SEM. Differences between means were analyzed using either one-way or two-way ANOVA followed by Newman–Keuls post hoc testing for pairwise comparison using SigmaStat v 3.5. For in vivo microdialysis data, areas under the curve were generated using GraphPad Prism v 5.01 followed by a two-tailed t test. The null hypothesis was rejected when P value was < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Baek Kim (University of Rochester) for kindly sharing the human microglia CHME5 cells. We are also thankful to Dr. Christoph Kellendonk (Columbia University) for advice on characterizing the DAT hypomorphic mice and comments on the manuscript. This work was supported in part by National Institutes of Health Grants ES014899 and ES17470 (to K.T.); Grants DA022413 and DA12408 (to J.A.J.); Grant AG040903 (to P.M.R.); Grant ES020081 (to A.S.C.); and by training Grant TL1RR 024135 from the National Center for Research Resources (to P.M.R.). P.M.R. and A.S.C. are trainees in the Medical Scientist Training Program funded by National Institutes of Health Grant T32 GM07356. J.C.G. is the recipient of National Institutes of Health/National Institute on Environmental Health Sciences Undergraduate Award ES0017470-01S1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115141108/-/DCSupplemental.
References
- 1.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Ritz BR, et al. Dopamine transporter genetic variants and pesticides in Parkinson's disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanner CM, et al. Rotenone, paraquat and Parkinson's disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci USA. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 6.Snyder SH, D'Amato RJ. Predicting Parkinson's disease. Nature. 1985;317:198–199. doi: 10.1038/317198a0. [DOI] [PubMed] [Google Scholar]
- 7.McCormack AL, et al. Environmental risk factors and Parkinson's disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 8.Thiruchelvam M, et al. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 9.Tieu K. A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harbor Perspective in Medicine. 2011;1:a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning-Bog AB, et al. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: Paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 12.Cui M, et al. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci USA. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- 14.Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: Implications for Parkinson's disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu K, et al. Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res. 2003;976:243–252. doi: 10.1016/s0006-8993(03)02750-1. [DOI] [PubMed] [Google Scholar]
- 16.Faro LR, Alfonso M, Cervantes R, Durán R. Comparative effects of pesticides on in vivo dopamine release in freely moving rats. Basic Clin Pharmacol Toxicol. 2009;105:395–400. doi: 10.1111/j.1742-7843.2009.00468.x. [DOI] [PubMed] [Google Scholar]
- 17.Cochemé HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 18.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Amphoux A, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Colton C, et al. Species differences in the generation of reactive oxygen species by microglia. Mol Chem Neuropathol. 1996;28:15–20. doi: 10.1007/BF02815200. [DOI] [PubMed] [Google Scholar]
- 21.Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglial derived from postmortem human brains. J Neurosci Res. 1995;40:478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- 22.Lee SC, Dickson DW, Liu W, Brosnan CF. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993;46:19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- 23.Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson's disease. IUBMB Life. 2003;55:329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Dawson VL, Dawson TM. Role of nitric oxide in Parkinson's disease. Pharmacol Ther. 2006;109:33–41. doi: 10.1016/j.pharmthera.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 26.Jaber M, et al. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur J Neurosci. 1999;11:3499–3511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- 27.Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by MPP+, a metabolite of the neurotoxin MPTP. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 28.Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purisai MG, et al. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25:392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abramov AY, et al. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cristóvão AC, Choi DH, Baltazar G, Beal MF, Kim YS. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu XF, et al. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 33.McCormack AL, Atienza JG, Langston JW, Di Monte DA. Decreased susceptibility to oxidative stress underlies the resistance of specific dopaminergic cell populations to paraquat-induced degeneration. Neuroscience. 2006;141:929–937. doi: 10.1016/j.neuroscience.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 34.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 35.German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: Sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci. 1992;648(1):42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- 36.Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA. What causes the death of dopaminergic neurons in Parkinson's disease? Prog Brain Res. 2010;183:59–77. doi: 10.1016/S0079-6123(10)83004-3. [DOI] [PubMed] [Google Scholar]
- 37.Mosharov EV, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelada SN, et al. 5′ and 3′ region variability in the dopamine transporter gene (SLC6A3), pesticide exposure and Parkinson's disease risk: A hypothesis-generating study. Hum Mol Genet. 2006;15:3055–3062. doi: 10.1093/hmg/ddl247. [DOI] [PubMed] [Google Scholar]
- 39.Drgon T, et al. Common human 5′ dopamine transporter (SLC6A3) haplotypes yield varying expression levels in vivo. Cell Mol Neurobiol. 2006;26:875–889. doi: 10.1007/s10571-006-9014-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.