Abstract
Purposive action requires the selection of a single movement goal from multiple possibilities. Neural structures involved in movement planning and execution often exhibit activity related to target selection. A key question is whether this activity is specific to the type of movement produced by the structure, perhaps consisting of a competition among effector-specific movement plans, or whether it constitutes a more abstract, effector-independent selection signal. Here, we show that temporary focal inactivation of the primate superior colliculus (SC), an area involved in eye-movement target selection and execution, causes striking target selection deficits for reaching movements, which cannot be readily explained as a simple impairment in visual perception or motor execution. This indicates that target selection activity in the SC does not simply represent a competition among eye-movement goals and, instead, suggests that the SC contributes to a more general purpose priority map that influences target selection for other actions, such as reaches.
Goal-directed behavior requires the serial selection of individual targets embedded in visual scenes that are often crowded with many different objects. Target selection is usually conceived of as a competition among potential movement goals (1–5), occurring in a priority map that encodes both the physical salience and behavioral relevance of each goal (6–10).
Much of the research on the neural mechanisms of target selection has focused on selection-related signals in brain areas involved in planning and executing the resultant motor response. For example, activity related to eye-movement target selection has been identified in the superior colliculus (SC) (7, 11–17), frontal eye field (18–20), lateral intraparietal area (10, 21–23), and supplementary eye fields (24), all areas in which signals related to eye movement execution are seen and in which electrical microstimulation and/or temporary inactivation affects the execution of eye movements (25–31). For reaching movements, target selection activity has been observed in the dorsal premotor area (32–34), a region from which reaches can be electrically elicited (35), as well as in the parietal reach region (36, 37), which exhibits reach-related planning and execution signals (38, 39).
Clear evidence for a more abstract, effector-independent priority map used for target selection has generally been limited to higher level cortical association areas, such as the dorsolateral prefrontal cortex, which shows activity related to both saccade and reach target selection (40–42) but from which neither saccades nor reaches can be evoked. This suggests a hierarchical model for target selection, in which effector-independent selection signals in higher level areas are selectively transmitted to appropriate lower level, effector-specific structures, which, in turn, finalize the selection process, plan, and execute the desired movement. Under this view, activity in these effector-specific structures, such as the SC, can be regarded as reflecting a competition among competing plans or goals for effector-specific movements.
Here, we challenge this hierarchical view of target selection by showing that temporary focal inactivation of the SC systematically influences the choice of goals for direct reaching movements without affecting lower level motor execution of reaches. In one task, a target and distractor were sequentially presented and monkeys were rewarded for reaching to touch the target while maintaining fixation. In the second task, monkeys also maintained fixation throughout each trial and a foveal cue indicated the reach target. In both tasks, when the reach goal was located in the affected part of the visual field, SC inactivation caused monkeys to make significantly more reaching errors to the distractor. This deficit was not attributable to an impairment in executing the reaches, because when the same target was presented alone, obviating the need to select the target from a distractor, reaches were always correctly directed to the target. Moreover, we recorded and analyzed the trajectories of the reaches and found that their velocity and end point accuracy were unaffected during SC inactivation, in contrast to what is seen for eye movements. The deficit is unlikely to be solely attributable to a simple impairment in visual processing or to a change in the subjective appearance of peripheral stimuli in the inactivated visual field, because in the second task, we explicitly trained the monkeys to ignore variations in the relative physical salience of the target and distractor stimuli and to respond only according to the direction of a foveal cue, and we verified that they did so.
These results establish that intermediate-layer SC activity has a robust influence on reach target selection even though it does not play a significant role in the execution of reaches, as shown by the lack of a motor impairment during SC inactivation. This indicates that target selection signals in the SC do not simply represent a competition among eye-movement goals and, instead, suggests that the SC is part of a more abstract, effector-independent priority map that influences target selection for reaches as well as eye movements.
Results
Effects of SC Inactivation on Reach Target Selection in a Distractor Task.
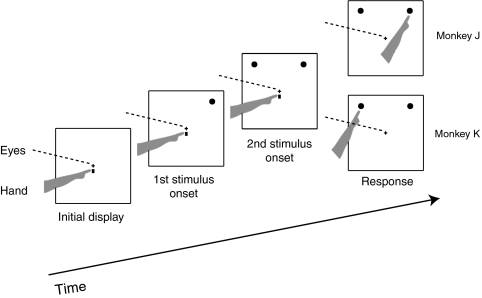
To investigate the effects of SC inactivation on reach target selection, we first used a distractor task (Fig. 1), in which two identical stimuli were sequentially presented with a variable stimulus onset asynchrony (SOA). One stimulus served as the target and the other as a distractor, as determined by the order of their presentation (Materials and Methods). In each trial, either the target or distractor was presented in the part of the visual field affected by the inactivation, whereas the other stimulus was presented in the opposite hemifield. For monkey J, the target was defined as the stimulus presented first, whereas for monkey K, the target was defined as the stimulus presented last. SOA was defined as an unsigned interval between the two sequentially presented stimuli; thus, for both monkeys, target selection difficulty increased as SOA decreased.
Fig. 1.
Distractor task. One stimulus is presented first, followed after a variable SOA by the second one. For monkey J, the target was the stimulus presented first, whereas for monkey K, the target was the stimulus presented second. Both monkeys were required to reach to the target while maintaining eye fixation at the center.
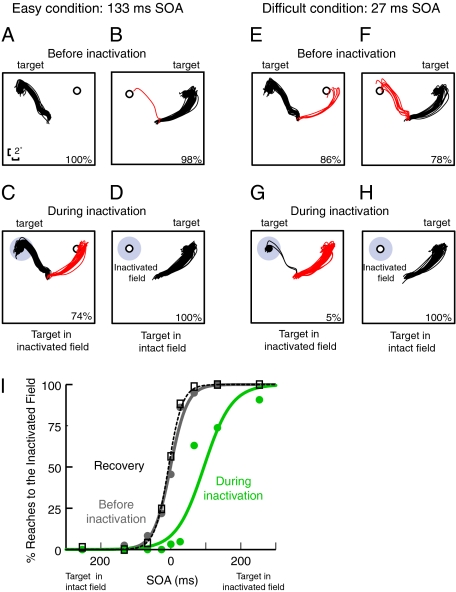
Fig. 2 shows results for one SC inactivation site. Each panel shows reach trajectories when the target was in one of the two potential locations, either at the center of the region affected by the SC inactivation or in the opposite hemifield. When SOA between the target and distractor was relatively long (e.g., 133 ms), target selection was easy, and before SC inactivation, reaches were nearly always directed to the target (Fig. 2 A and B). However, during SC inactivation, when the target was in the inactivated field, there was a significant increase in the number of erroneous reaches to the distractor (Fig. 2C; z-test: P < 0.002). On the other hand, when the target was located outside the inactivated field and the distractor was in the affected field, there was no significant change in performance (z-test: P < 1.0) and reaches were still consistently directed to the target (Fig. 2D). This pattern of results suggests that SC inactivation causes a bias in reach target selection against targets in the inactivated field.
Fig. 2.
Reach target selection in the distractor task for a representative SC inactivation site from monkey J. (A–H) Each trace represents a single reach trajectory from the center to a peripheral stimulus. Black traces indicate correct reaches to the target, and red traces indicate incorrect reaches to the distractor. Percentages in each panel indicate the percentage of correct responses in the corresponding condition. (A–D) Reach target selection in the distractor task when target selection was fairly easy (SOA = 133 ms). (A) Reach target selection performance before SC inactivation when the target was in the location corresponding to the injection site. (B) Performance in the same task when the target was in the opposite hemifield. (C) Performance in the same task during SC inactivation when the target was in the inactivated field (marked in gray). During SC inactivation, monkeys are biased against choosing the stimulus in the inactivated field. (D) Performance during SC inactivation when the target was in the opposite hemifield. (E–H) Conventions are as in A–D, except that performance is shown when target selection was difficult (SOA = 27 ms). (I) Summary data for this site. Percentage of reaches into the inactivated field is plotted as a function of SOA. Values of SOA to the left of zero on the abscissa denote trials in which the target was presented in the intact field and the distractor was in the inactivated field. Values of SOA to the right of zero denote the opposite case. Gray, green, and black dotted curves represent preinactivation, during inactivation, and recovery conditions, respectively.
This bias is even more pronounced when target selection is made more difficult by shortening the SOA between the target and distractor. Fig. 2 E–H shows data from the same injection site when the SOA was short (27 ms). Here, before inactivation, the majority of reaches were made to the correct target for both target locations (Fig. 2 E and F). However, during SC inactivation, there was a dramatic shift in the monkeys’ target selection behavior: Only a few reaches were directed to the target when it was in the inactivated field, resulting in a significant target selection deficit compared with preinactivation performance (Fig. 2G; z-test: P < 0.000001). These erroneous reaches are clearly not inaccurate attempts to reach the target; rather, they are accurate movements that are directed to the wrong stimulus, consistent with a deficit in target selection. When the target was located in the intact hemifield and the distractor was in the inactivated field, significantly fewer erroneous reaches were made to the distractor during inactivation than before (Fig. 2H; z-test: P < 0.01). This shows that SC inactivation does not simply cause monkeys to produce errors randomly, and it does not produce a generalized confusion in temporal order perception. Rather, it results in a systematic bias against selecting reach targets in the inactivated field. To illustrate this, Fig. 2I shows the percentage of reaches to the inactivated field as a function of SOA for this site: preinactivation, during inactivation, and after recovery from inactivation. To produce this psychometric function across a broad range of SOAs, we supplemented the data collected using staircase procedures (Materials and Methods) with additional data collected in the same session using the method of constant stimuli. On the abscissa, SOA values to the left of zero denote trials in which the target was presented in the intact field and the distractor was in the inactivated field, whereas SOA values to the right of zero denote the opposite case. Thus, the ordinate (percentage of reaches to the inactivated field) corresponds to the error rate for the former and percent correct for the latter. The plot shows a clear shift in tendency to select the stimulus in the inactivated field, confirming that SC inactivation causes a bias in reach target selection.
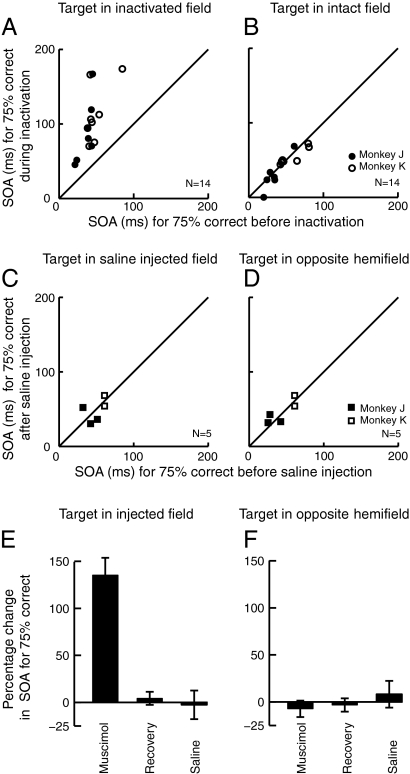
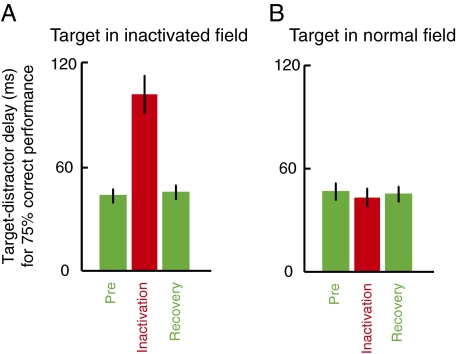
To quantify this bias across sites, we measured the SOA associated with 75% correct performance at each target location (Materials and Methods). Fig. 3 A and B compare this criterion SOA before and during SC inactivation for each injection site (n = 14). The data from monkey J (Fig. 3 A and B, ●) were collected in a variant of the task in which the target was defined as the stimulus appearing first, whereas the data from monkey K (Fig. 3 A and B, ○) were collected in a variant in which the target was defined as the stimulus appearing second. This task manipulation was performed to test whether apparent changes in reach target selection might have been attributable to systematic changes in the perceived temporal order of the stimuli resulting, for example, if SC inactivation had delayed visual information coming from the inactivated field. Differences in sign of the SOA for the two monkeys were removed for the purposes of Fig. 3 and subsequent analyses; for both monkeys, increases in SOA denote larger asynchronies between the stimuli, providing a stronger signal to guide target selection.
Fig. 3.
Reach target selection performance in the distractor task for all sites. (A) Criterion SOA needed for 75% correct performance in the distractor task when the target was in the inactivated field. Performance before SC inactivation is plotted along the abscissa, whereas performance during inactivation is plotted along the ordinate. Each circle represents one experiment (injection site). Filled circles (●) show data from monkey J in the task variant in which the target was the stimulus presented first, and open circles (○) represent data from monkey K in the other variant in which the target was the stimulus presented second. For both monkeys, larger SOAs denote longer time intervals between the presentation of the target and distractor, leading to easier target selection. (B) Criterion SOA for 75% correct performance when the target was in the intact hemifield. Conventions are as in A. (C and D) Plots comparing criterion SOA before and after saline injection. Conventions are the same as in A and B. Filled squares (■) show data from monkey J, and open squares (□) show data from monkey K. (E and F) Mean percentage change in criterion SOA across sites during SC inactivation and after recovery, compared with preinjection. For E, the target was in the inactivated field, whereas for F, the target was in the intact field. The right-most bars of the plots show the mean percentage change for the saline sites. Error bars indicate SEM.
If the decrement in performance during SC inactivation resulted from a deficit in temporal order perception attributable, for example, to slowed processing of visual signals from the inactivated field, we would expect opposite effects in the two monkeys (a bias against the stimulus in the inactivated field for the monkey trained to reach to the stimulus appearing first and a bias in favor of the stimulus in the inactivated field for the monkey trained to reach to the stimulus appearing last). Because the data from the two monkeys were indistinguishable (Wilcoxon rank sum test: P > 0.8), we ruled out this explanation and combined the data for further analysis.
To test the statistical significance of the target selection deficit for individual sites, we applied a permutation test with 2,000 iterations (43), with a Bonferroni correction, to the 75% correct SOA measurements. When the target was presented in the inactivated field (Fig. 3A), the criterion SOA was significantly higher during SC inactivation than before inactivation (permutation tests: P < 0.00001 for 14 of 14 sites). To gain an estimate of the inherent variability in the data, we also compared preinjection performance with performance on the following day, after recovery from the muscimol injection. We found that there was no difference between preinjection and recovery performance for any of the sites (permutation tests: P > 0.24 for all sites).
When the target appeared in the intact hemifield (Fig. 3B), there was no significant difference in performance before vs. during inactivation for 9 of 14 sites (P > 0.26), whereas for 5 sites (P < 0.04), SC inactivation slightly improved performance. This is likely attributable to effective inhibition of the distractor in the inactivated field, consistent with an effect of SC inactivation on the priority map representation of the stimulus in the affected field. As expected, the difference between preinjection and recovery performance when the target was in the intact hemifield was not significant for any of the sites (P > 0.20 for all sites).
In control experiments, we tested whether the injection procedure itself caused deficits in reach target selection. Saline injections of volumes equal to those used in the muscimol experiments were made at five sites (3 in monkey J and 2 in monkey K). We found that after injections of saline, performance in the task was not altered compared with preinjection performance (Fig. 3 C and D; Wilcoxon signed rank test: P = 0.85). These results verify that the observed deficits are attributable to SC inactivation rather than to the injection procedure per se.
Summary results across all sites are shown in Fig. 3 E and F, which plot the percentage change in criterion SOA during inactivation and after recovery compared with preinjection values. Overall, when the target was presented in the inactivated field (Fig. 3E), monkeys required an SOA between the target and distractor 135% larger than preinactivation to achieve 75% correct reach target selection performance (Wilcoxon signed rank test: P = 0.0001). After recovery from inactivation, performance was equivalent to preinjection levels (Wilcoxon signed rank test: P = 0.25). A contrast analysis, testing the hypothesis that performance during inactivation was significantly worse than preinactivation and that preinjection and recovery performance was equal, was significant across sites (P < 0.00002). On the other hand, when the target was in the intact hemifield (Fig. 3F), the contrast analysis was not significant (P = 0.42). Thus, the summary data support the idea that SC inactivation leads to a clear and robust reach target selection bias against targets in the inactivated field. Finally, the right-most bars in Fig. 3 E and F confirm that there were no appreciable changes across sites in criterion SOA after saline injection compared with preinjection at either target location (Wilcoxon signed rank tests: P > 0.74).
Performance in a Centrally Cued Reaching Task.
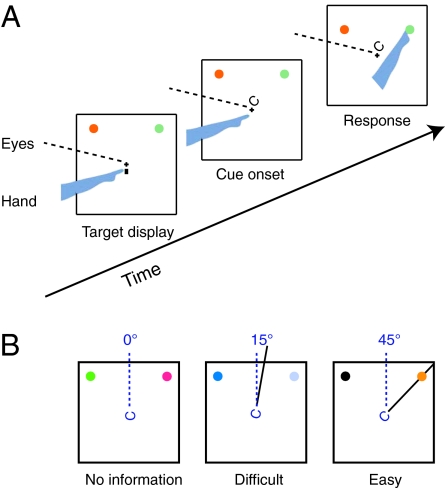
In addition to testing the effects of SC inactivation on reach target selection guided by peripheral cues, we measured its effects when the cue was presented at the fovea (Fig. 4A). This allowed us to distinguish a possible effect of SC inactivation on visual processing of the cue from a bias in target selection. Locating the cue at the fovea meant that our peripheral SC inactivations were unlikely to affect perception of the cue. More importantly, if impairments in this task were attributable to a deficit in perceiving the foveal cue, performance would be degraded for both target locations rather than showing a systematic bias against targets in the inactivated field.
Fig. 4.
Centrally cued task. (A) Task procedure: The two potential reach targets are presented at the beginning of each trial. After a fixation period, a Landolt-C cue is presented at the fovea. The gap of the C points toward the reach target. Monkeys are required to reach to the target indicated by the central cue while maintaining eye fixation. (B) Manipulation of target selection difficulty in the central cueing task. When the cue is more strongly tilted toward the target, target selection is easier.
The foveal cue consisted of a Landolt-C figure, and the difficulty of target selection was manipulated by changing the amount by which the gap in the Landolt-C figure was tilted toward the target (Fig. 4B). The strongest target selection information was provided when the gap was pointed directly at the target, and target selection difficulty increased as the gap tilted toward intermediate locations. This task was performed for 12 SC injection sites: for 10 injection sites, we varied cue tilt using staircase procedures and measured target selection performance at each site by calculating the cue tilt needed for 75% correct performance for reaches into the inactivated field and for reaches into the intact field. For 2 other sites, the central cue always pointed directly at the reach target; thus, target selection difficulty was held constant. For these two sites, our performance measure was the percentage of trials in which monkeys correctly reached to the target during SC inactivation compared with preinjection.
To minimize the effect on performance of possible changes in the perceptual appearance of stimuli in the inactivated field, we designed the task so that monkeys were required to ignore the perceptual salience and subjective appearance of the target and distractor in making their decisions. Specifically, in each trial, the contrast and color of the target and distractor stimuli were randomly and independently varied. As a result, on a trial-to-trial basis, the target could appear either brighter or dimmer than the distractor and the target and distractor stimuli randomly differed in color from each other. Thus, to learn the task successfully, the monkeys had to ignore variations in the subjective appearance of the peripheral stimuli, as well as differences in the perceptual salience of the target vs. the distractor.
When the target was presented in the inactivated field (Fig. 5A), the results show a clear target selection bias during SC inactivation compared with preinjection. Indeed, this bias against reaching to the target in the inactivated field was statistically significant for 11 of 12 individual sites (permutation tests: P < 0.000001). The magnitude of the bias, expressed as the percentage change in criterion cue tilt, was positively correlated with the amplitude of the injection site on the SC map (Pearson correlation: P < 0.01), supporting the idea that the deficit was not attributable to encroachment of the inactivation effect on foveal sites in the SC. Further support for this point comes from the fact that a deficit in foveal vision would be expected to lead to worse performance for both target locations. However, when the target appeared in the intact hemifield (Fig. 5B), 8 of 12 sites (permutation tests: P > 0.1) did not show any significant change in performance during inactivation, whereas for 4 sites (permutation tests: P < 0.015), SC inactivation slightly improved performance. This trend toward improvement in performance for the opposite-hemifield target is consistent with a bias against selecting the stimulus in the inactivated field. Finally, as expected, when we tested performance on the following day, after recovery from the muscimol injection, this bias disappeared and we found no difference in performance preinjection vs. recovery (permutation tests: P > 0.20 for all sites).
Fig. 5.
Reach target selection performance in the centrally cued task. (A and B) Comparison of criterion cue tilt required for 75% correct performance before and during inactivation in the centrally cued task for each injection site. Conventions are the same as in Fig. 3A. (C and D) Comparison of criterion cue tilt before and after saline injection. Conventions are the same as in Fig. 3 A and B. Filled squares (■) show data from monkey J, and open squares (□) show data from monkey K. (E and F) Mean percentage change in criterion cue tilt across sites during SC inactivation, after recovery, and after saline injection, compared with the corresponding preinjection conditions. Conventions are the same as in Fig. 3 E and F.
As before, to test whether these effects were attributable to muscimol inactivation or to the injection procedure itself, we performed control injections of saline at five sites. Using the same testing protocol as for the muscimol injections, we found that performance after saline injection was not significantly different from preinjection performance at any of the sites (permutation tests: P > 0.23 for all sites) even when the target was presented in the part of the visual field represented at the injection site (Fig. 5 C and D).
Fig. 5 E and F show the mean percentage change in criterion cue tilt during inactivation and after recovery compared with the preinjection values. When the target was in the inactivated field (Fig. 5E), a contrast analysis confirmed that target selection performance during inactivation was significantly worse than preinactivation, and that preinactivation and recovery performance were equal across sites (P < 0.0001). This target selection deficit was spatially localized to the inactivated field: When the target appeared in the intact hemifield (Fig. 5F), the contrast analysis across sites was not significant (P = 0.1 for all data). Finally, the right-most bars in Fig. 5 E and F show that after saline injection, there were no appreciable changes in criterion cue tilt across sites at either target location (Wilcoxon signed rank tests: P > 0.31). These results are consistent with what we observed in the distractor task.
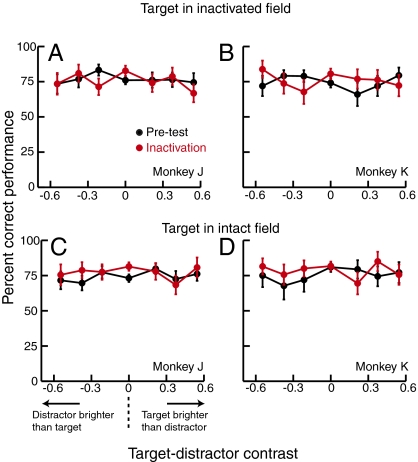
As described above, monkeys were trained to ignore the properties of the peripheral target and distractor stimuli and to base their responses solely on the orientation of the central cue. To verify that they successfully ignored the perceptual salience of the target and distractor in making their target choices, we analyzed the effect of the relative luminance contrast of the target vs. distractor (as well as the effect of cue tilt) on performance. If the monkeys had tended to reach for the brighter stimulus, they should have performed better with larger values of target-distractor contrast (corresponding to trials in which the target was brighter than the distractor) and worse with smaller values (corresponding to trials in which the distractor was brighter than the target). This trend is not evident in plots of percent correct performance vs. target-distractor contrast (Fig. 6).
Fig. 6.
Performance as a function of target-distractor contrast. Each plot shows performance for an individual monkey, averaged across injection sites, preinjection (black points), and during SC inactivation (red points), when the target was in the inactivated field (A and B) or in the opposite hemifield (C and D). Target-distractor contrast was defined as: (target contrast − distractor contrast)/(target contrast + distractor contrast). Negative values of target-distractor contrast indicate trials in which the distractor was higher contrast than the target, and vice versa. Overall performance was maintained near 75% correct by the staircase procedures, which varied cue tilt. Target-distractor contrast was independent of the staircases and was randomly varied on a trial-by-trial basis.
To verify this impression statistically, we conducted logistic regressions examining the influence of target-distractor contrast and cue tilt on trial outcome (correct vs. incorrect). Separate regressions were performed for each monkey and for each of the two target locations (i.e., in vs. out of the affected field), on data pooled across the 10 sites in which cue tilt was varied. We found that although cue tilt significantly affected performance (preinjection: P < 0.01; during inactivation: P < 0.03), there was no significant effect of target-distractor contrast, for both the preinjection and inactivation conditions, in either monkey (preinjection: P > 0.29; during inactivation: P > 0.21). We used power analyses to estimate the sensitivity of these logistic regressions. Because our goal was to verify that performance was driven primarily by cue tilt with little contribution of target-distractor contrast, we computed the power (1-β) of the regressions to detect an effect size for target-distractor contrast that was 25% of the observed effect size for cue tilt, using a large sample approximation method (44). Using our significance criterion (α) of 0.05, the analyses revealed that the statistical power to detect an effect of target-distractor contrast of this magnitude was high, ranging from 0.96 to 0.99 (for reference, a power of 0.80 is conventionally considered sufficient). These analyses confirm that, consistent with their training, both monkeys largely ignored the relative perceptual salience of the target and distractor in making their target choices. Thus, even if SC inactivation had affected the perceived contrast of the stimulus in the inactivated field, such a perceptual deficit would have had a negligible effect on performance and could not fully explain the target selection deficits observed.
Effects of SC Inactivation on Execution of Reaching Movements.
To determine whether the muscimol-related changes in performance could be attributed to impairments in the motor execution of reaches during SC inactivation, we optically tracked the reach trajectories and examined reaching performance when a single target was presented without distractors (Fig. 7 A and B). Specifically, during both of our target selection tasks, we randomly interleaved trials in which the target appeared without a distractor and monkeys were required to maintain fixation and reach to the target. In contrast to the movement execution deficits that are observed for saccades, SC inactivation did not impair reaching movements to the target in the inactivated field (Fig. 7B). Indeed, we found no significant differences in reach reaction time (Wilcoxon signed rank test: P = 0.45), movement duration (P = 0.13), end point variance in both horizontal (P = 0.76) and vertical (P = 0.69) directions, end point error (P = 0.84) (Fig. 7C), or peak movement velocity (P = 0.23) (Fig. 7D) for reaches to single targets during SC inactivation vs. before inactivation. This indicates that SC inactivation does not cause a motor deficit for reaching movements; instead, the impairment is restricted to the target selection stage of processing.
Fig. 7.
Effects of SC inactivation on reach execution. Trajectories of reaches made before SC inactivation (A) or during SC inactivation (B) to single targets presented in the part of the visual field represented at the injection site (approximate center shown in gray) for a representative site. (C) Comparison across sites of the end point error of reaches made to single targets presented in the part of the visual field represented at the injection site before and during SC inactivation. Each data point represents the mean for one injection site. (D) Comparison of the mean peak velocity of reaches to a single target in the inactivated field before and during inactivation. Each data point represents the mean for one injection site.
Discussion
Previous recording studies have identified neural correlates of target selection for saccadic and pursuit eye movements in the SC (7, 11–17). Furthermore, inactivation and microstimulation studies have demonstrated that manipulations of SC activity can actually change the eye movement choices made by animals, establishing that the SC plays a causal role in target selection for eye movements (45–47). These manipulations of SC activity also affect the execution of saccadic and pursuit eye movements (25–27). Because the primate SC has been traditionally viewed as an oculomotor structure, these findings fit well with a hierarchical model for target selection, in which selection signals from higher level, effector-independent brain areas are transmitted to the motor structures, such as the SC, involved in producing the required response, and these effector-specific structures finalize the target selection process and produce the resultant movement.
In the current study, we challenge this view of the SC by demonstrating that SC activity has a strong effect on target selection for arm-reaching movements, despite the fact that the SC does not contribute significantly to the execution of these movements. This conclusion is supported by the fact that SC inactivation causes a clear bias in the selection of reach targets without causing any notable impairment or change in the execution of the reaching movements. This is in stark contrast to the deficits in saccade and pursuit execution seen during SC inactivation. These findings suggest that beyond its role in eye movements, the SC forms part of a more general purpose priority map that governs target selection for other effectors.
The concept of a priority map used for target selection has been proposed by a number of groups (6–10). Activity in such a map does not encode the specific object features (e.g., color, shape) of potential movement goals; rather, it represents the physical salience and behavioral relevance of the goals. Target selection has been hypothesized to involve a competition among the goals in the priority map, leading to the selection of a single movement goal (1–5). Current evidence suggests that priority maps for target selection are distributed across a number of brain regions (6–10), and oculomotor areas, such as the SC, have been shown to possess the characteristics of a priority map for eye-movement tasks (7, 11–17). If SC activity also participates in a priority map used for reaching movements, inactivation of a portion of the SC would be expected to reduce activity in the map for reach goals located in the inactivated field and, as a result, would bias reach target selection against such goals. This is exactly the pattern of behavior observed in our reach target selection tasks during SC inactivation. Thus, we speculate that feedback projections from the disrupted SC priority map to the cortex (via the thalamus) allowed our perturbation of SC activity to influence and corrupt activity in cortical areas involved either in effector-independent target selection or in reach target selection specifically. Although the exact pathways underlying this influence are currently unknown, it is clear from the reaching behavior observed here that SC activity has a significant impact on the choice of reach targets.
A class of deep-layer SC neuron that is selectively active during reaching movements has previously been identified (48, 49). At first glance, one might suspect that the effects reported here are attributable to inactivation of these cells, but this seems unlikely for several reasons. First, these neurons are found predominantly in the deep SC and underlying mesencephalic reticular formation, whereas our injections were centered in the intermediate layers, where saccades are evoked with low-current electrical microstimulation (Materials and Methods). Second, even in the deeper layers of the SC, these cells are sparsely scattered and do not follow the orderly retinotopic organization shared by the other layers of the SC. In contrast, the reach target selection deficits that we observed always occurred when the target was located in the part of the visual field corresponding to the injection location on the traditional retinotopic SC map. Thus, we conjecture that these deep-layer SC reach-related neurons might play a role in coordinating eye and hand movements toward a common goal but are unlikely to underlie the reach target selection deficits observed here.
Evidence that the functions of the primate SC extend beyond eye movements has recently come from studies showing that manipulations of SC activity can influence covert attention, leading to changes in performance for difficult perceptual tasks (50–52). Furthermore, it has been suggested that the same covert attention mechanisms involved in perception might also be involved in selecting movement goals (53–55), although some physiological evidence argues against this proposition (56). The finding that the SC plays a causal role both in perceptual selection and in selection for action suggests that these two functions may be governed by a common mechanism.
Nummela and Krauzlis (47) examined the effects of SC inactivation on target selection for saccadic and pursuit eye movements, as well as on a task in which monkeys reported the position (left vs. right of fixation) of a target presented with a distractor by pressing a corresponding button outside the monkeys’ field of view. They found that SC inactivation strongly affected saccadic and pursuit eye movements, as expected (45, 46); however, for the task involving button presses, they found only mild effects, which were significant in a minority of sites. We speculate that this was attributable to the fact that in their task, neither of the action goals (response buttons) was located in the inactivated field. Importantly, their experimental task could not distinguish effects of SC inactivation on the subjective appearance or perceptual processing of peripheral stimuli in the inactivated field from effects on target selection per se.
A key difference between a perceptual deficit, such as failing to detect a signal or perceiving it as dimmer, and a target selection deficit is that a perceptual deficit will, by definition, be influenced by perceptual factors, such as the contrasts of the target and distractor. On the other hand, target selection is strongly influenced by behavioral relevance: When the animal's task demands that it ignore target and distractor contrast, this irrelevant factor will have minimal influence on target selection in a well-trained animal, even though the animal perceives the variations in contrast. Although we cannot rule out the possibility that our SC inactivations caused perceptual deficits, we chose a set of tasks intended to minimize the influence of potential visual deficits on performance, and the pattern of results that we obtained would be difficult to explain solely on the basis of simple visual deficits. Specifically, in the distractor task, if SC inactivation had affected reach target selection by slowing down perception of the stimulus in the inactivated field, the two monkeys would have shown opposite changes in performance, because one was trained to reach to the first stimulus and the other to the second stimulus. If SC inactivation, instead, had produced a more generalized confusion in temporal order perception, this would have worsened performance both when the target was in the inactivated field and when it was in the intact field. We also think it is unlikely that a weaker percept of stimuli in the inactivated field can account for our findings, because in the second experiment, animals were explicitly trained to ignore the strength or weakness of the target and distractor signals, and, as shown in Fig. 6, they successfully did so. This finding also makes it unlikely that the results are attributable to increased vulnerability of targets in the inactivated field to perceptual competition from distractors, because a purely perceptual competition should be influenced by the relative contrasts of the target vs. the distractor. Finally, by measuring the kinematics and metrics of the reaches, we can firmly rule out the presence of an impairment in reach execution during SC inactivation. Thus, our results argue that regardless of perceptual effects that may (or may not) have occurred, SC inactivation caused a target selection deficit for reaching movements.
Materials and Methods
Physiological Methods.
The experiments were carried out at the Smith–Kettlewell Eye Research Institute. All experimental protocols were approved by the Smith–Kettlewell Institutional Animal Care and Use Committee and complied with the guidelines of the US Public Health Service policy on Humane Care and Use of Laboratory Animals. We used standard methods for recording single neurons, microstimulating, and microinjecting (45) in two monkeys (Macaca mulatta) implanted with a head restraint and a recording chamber to access the SC. Microinjections were made through a 33-gauge metal cannula with an attached microelectrode. In each session, an injection of 0.5 μL of muscimol (0.5-μg/μL concentration) was delivered at a rate of 0.5 μL/min at a site at which saccade-related activity was recorded and saccades were reliably elicited with low-current electrical stimulation (less than 30 μA at 400-Hz bipolar stimulation). During control experiments, injections of 0.5 μL of saline were made instead of muscimol. The injection sites were located 1.5–2.5 mm below the SC surface. The location of the injection site within the SC motor map was estimated by measuring the end points of saccades elicited by microstimulation using currents of twice the threshold current. Stimulation train duration was varied to obtain the site-specific maximal amplitude at each site (57, 58). The region of the visual field corresponding to the injection site was then estimated as the median end point of the saccades evoked using these current and duration parameters. A list of injection sites is provided in Table S1. A significant decrease in peak velocity for saccades into the inactivated field was used as our criterion for determining that the SC had been successfully inactivated (26). Testing before and during inactivation was conducted in the same session. Recovery data were collected on the following day.
Behavioral Testing.
During experiments, monkeys were seated in a primate chair, with the head restrained and the left arm loosely restrained. Eye position was sampled at 1 kHz using an EyeLink 1000 high-speed video tracker (SR Research). A small, reflective hemisphere was affixed during each testing session to the right hand, near the tip of the index finger (D2), to measure reach movement trajectories. The hemisphere was illuminated by infrared light and optically tracked in three dimensions at a rate of 60 Hz using a Polaris tracker (Northern Digital, Inc.). Visual stimuli were presented on a 17-in color cathode ray tube touch-sensitive monitor (ELO Touch Systems), positioned 25.5 cm in front of the monkeys.
Distractor task.
At the beginning of each trial, two vertically adjacent fixation points were presented in the central position. The fixation points had a luminance of 1.5 cd/m2 against a black homogeneous background of 0.2 cd/m2; the upper fixation point subtended 0.25°, and the lower point subtended 1.5°. The monkeys were trained to fixate the upper fixation point with their eyes and touch the lower fixation point with their right index finger (e.g., Fig. 1). This hand/eye fixation position was held for a randomly varying period of 500–1,000 ms. At the end of this period, the lower fixation point was extinguished and a reach target was presented, followed after a variable delay by a distractor (or vice versa). The target and distractor stimuli were identical, with luminances of 1.2 cd/m2, and were distinguished only by their temporal order. Monkey J performed a version in which the target preceded the distractor, whereas monkey K performed a version in which the distractor was presented first, followed by the target. The monkeys were required to maintain fixation in the center and reach to the target, and target selection difficulty was manipulated by varying the SOA between the target and distractor. Monkeys were rewarded if the first point at which the hand touched the screen (after lifting off from the center point) was within 3–5° of the correct target location and if the positions of the eyes (at the center) and hand (at the target) after the reach ended were maintained for 300 ms. The target and distractor stimuli were scaled according to the cortical magnification factor to keep their salience constant across different eccentricities (59). At an eccentricity of 10°, target and distractor stimuli subtend 2.5°. In each trial, either the target or distractor was positioned at the part of the visual field estimated (using microstimulation, see above) to be represented at the center of the injection site and the other stimulus was positioned at an isoeccentric position in the opposite hemifield, separated by an angle of 90° (Fig. 1). To test for the presence of simple reaching motor deficits, we also randomly intermixed trials in which only a single target was presented. The procedure was the same as in the distractor task except that the distractor stimulus was not presented. The SOA required for 75% correct reaching to each target location was separately measured using two independent, randomly interleaved staircase procedures, one for each target location. The SOA was decreased after three consecutive correct responses and was increased after each incorrect response for that target location. For one injection site, additional data were collected using the method of constant stimuli to probe a broader range of SOAs.
Centrally cued task.
At the beginning of each trial, the eye and hand fixation points described above appeared along with two potential target stimuli. The color and luminance of the two potential targets were independently randomized in each trial so that the features of the stimuli and their perceptual salience relative to each other were irrelevant for the task. Color was chosen from six preselected values, roughly equally spaced in Commission Internationale de l'Éclairage (CIE) L*a*b* color space. Luminance of the stimuli ranged from 0.25 to 0.85 cd/m2, in steps of 0.3 cd/m2, yielding contrasts ranging from 11 to 62% against a background luminance of 0.2 cd/m2. As for the distractor task, one stimulus was positioned at the center of the region represented by the injection site and the other stimulus was positioned at an isoeccentric position in the opposite hemifield, separated by an angle of 90° (Fig. 4A). After the 500- to 1,000-ms initial fixation period, a Landolt-C cue was presented near the fovea and remained visible throughout the trial. The orientation of the gap of the Landolt-C figure indicated which one of the two stimuli was the reach target. As soon as the cue was presented, the lower fixation point disappeared, and monkeys were rewarded for reaching to the cued target location within 2 s, while maintaining eye fixation on the upper fixation point at the center of the screen. Target selection difficulty was manipulated by varying the angular tilt of the Landolt-C figure toward the target as shown in Fig. 4B. The tilt required for 75% correct reaching to each target location was separately measured using independent, randomly interleaved staircase procedures, as in the distractor task.
Data Analysis.
Trials in which monkeys reached to a location within 3–5° of the target were scored as correct, whereas trials in which monkeys reached to a location within 3–5° of the distractor were scored as incorrect. Trials in which monkeys broke fixation or reached elsewhere were aborted and did not affect the staircases. Aborted trials constituted less than 2% of trials overall, and the proportion of aborted trials was similar preinactivation vs. during inactivation (1.8% vs. 1.3%). Threshold SOA (for the distractor task) or tilt (for the centrally cued task) for 75% correct performance was estimated for each session from the staircase reversals (60). Performance in single-target trials did not affect the staircases. In the centrally cued task, the quantity referred to as “target-distractor contrast” was defined as: (target contrast − distractor contrast)/(target contrast + distractor contrast). Reach reaction time was defined as the interval between target and movement onset. Peak velocity was calculated as the maximum magnitude of the 3D hand velocity during the reach. End point accuracy was computed as the distance from the center of the target to the reach end point.
Statistical Analysis.
For individual injection sites, we compared target selection performance before and during SC inactivation as well as before inactivation and after recovery by applying a permutation test with 2,000 iterations (43) to the staircase reversals. Bonferroni correction was applied. In addition, contrast analysis was performed (61) to test at each target location the significance of the statistical hypothesis that preinactivation and recovery target selection performance was equal and that performance during inactivation was worse. Wilcoxon signed rank tests were conducted to compare target selection performance and reach movement parameters across sites. A statistical criterion of P < 0.05 was used to assess the significance of experimental effects.
Supplementary Material
Acknowledgments
We thank N. Takahashi for animal care. This work was supported by National Eye Institute Grant R01-EY014885 and by an R. C. Atkinson Fellowship Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 20293.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109656108/-/DCSupplemental.
References
- 1.Clark JJ. Spatial attention and latencies of saccadic eye movements. Vision Res. 1999;39:585–602. doi: 10.1016/s0042-6989(98)00190-4. [DOI] [PubMed] [Google Scholar]
- 2.Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behav Brain Sci. 1999;22:661–674. doi: 10.1017/s0140525x99002150. discussion 674–721. [DOI] [PubMed] [Google Scholar]
- 3.Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Res. 2000;40:1489–1506. doi: 10.1016/s0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 4.Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci. 2010;30:2340–2355. doi: 10.1523/JNEUROSCI.1730-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch C, Ullman S. Shifts in selective visual attention: Towards the underlying neural circuitry. Hum Neurobiol. 1985;4:219–227. [PubMed] [Google Scholar]
- 6.Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Fecteau JH, Munoz DP. Salience, relevance, and firing: A priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb J. From thought to action: The parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Boehnke SE, Munoz DP. On the importance of the transient visual response in the superior colliculus. Curr Opin Neurobiol. 2008;18:544–551. doi: 10.1016/j.conb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–545. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- 12.Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- 14.Krauzlis R, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron. 2002;35:355–363. doi: 10.1016/s0896-6273(02)00756-0. [DOI] [PubMed] [Google Scholar]
- 15.McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- 16.Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J Neurophysiol. 2003;90:1392–1407. doi: 10.1152/jn.01049.2002. [DOI] [PubMed] [Google Scholar]
- 17.Kim B, Basso MA. Saccade target selection in the superior colliculus: A signal detection theory approach. J Neurosci. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- 19.Burman DD, Segraves MA. Primate frontal eye field activity during natural scanning eye movements. J Neurophysiol. 1994;71:1266–1271. doi: 10.1152/jn.1994.71.3.1266. [DOI] [PubMed] [Google Scholar]
- 20.Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg ME, Bisley JW, Powell KD, Gottlieb J. Saccades, salience and attention: The role of the lateral intraparietal area in visual behavior. Prog Brain Res. 2006;155:157–175. doi: 10.1016/S0079-6123(06)55010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78:1574–1589. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- 23.Thomas NW, Paré M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol. 2007;97:942–947. doi: 10.1152/jn.00413.2006. [DOI] [PubMed] [Google Scholar]
- 24.Olson CR, Gettner SN, Ventura V, Carta R, Kass RE. Neuronal activity in macaque supplementary eye field during planning of saccades in response to pattern and spatial cues. J Neurophysiol. 2000;84:1369–1384. doi: 10.1152/jn.2000.84.3.1369. [DOI] [PubMed] [Google Scholar]
- 25.Basso MA, Krauzlis RJ, Wurtz RH. Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol. 2000;84:892–908. doi: 10.1152/jn.2000.84.2.892. [DOI] [PubMed] [Google Scholar]
- 26.Hikosaka O, Wurtz RH. Effects on eye movements of a GABA agonist and antagonist injected into monkey superior colliculus. Brain Res. 1983;272:368–372. doi: 10.1016/0006-8993(83)90586-3. [DOI] [PubMed] [Google Scholar]
- 27.Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- 28.Robinson DA, Fuchs AF. Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol. 1969;32:637–648. doi: 10.1152/jn.1969.32.5.637. [DOI] [PubMed] [Google Scholar]
- 29.Dias EC, Kiesau M, Segraves MA. Acute activation and inactivation of macaque frontal eye field with GABA-related drugs. J Neurophysiol. 1995;74:2744–2748. doi: 10.1152/jn.1995.74.6.2744. [DOI] [PubMed] [Google Scholar]
- 30.Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- 31.Shibutani H, Sakata H, Hyvärinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp Brain Res. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- 32.Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408:466–470. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- 34.Song JH, McPeek RM. Roles of narrow- and broad-spiking dorsal premotor area neurons in reach target selection and movement production. J Neurophysiol. 2010;103:2124–2138. doi: 10.1152/jn.00238.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godschalk M, Mitz AR, van Duin B, van der Burg H. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 1995;23:269–279. doi: 10.1016/0168-0102(95)00950-7. [DOI] [PubMed] [Google Scholar]
- 36.Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherberger H, Andersen RA. Target selection signals for arm reaching in the posterior parietal cortex. J Neurosci. 2007;27:2001–2012. doi: 10.1523/JNEUROSCI.4274-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- 39.Hwang EJ, Andersen RA. Brain control of movement execution onset using local field potentials in posterior parietal cortex. J Neurosci. 2009;29:14363–14370. doi: 10.1523/JNEUROSCI.2081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iba M, Sawaguchi T. Involvement of the dorsolateral prefrontal cortex of monkeys in visuospatial target selection. J Neurophysiol. 2003;89:587–599. doi: 10.1152/jn.00148.2002. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa RP, Matsumoto M, Mikami A. Search target selection in monkey prefrontal cortex. J Neurophysiol. 2000;84:1692–1696. doi: 10.1152/jn.2000.84.3.1692. [DOI] [PubMed] [Google Scholar]
- 42.Hoshi E, Shima K, Tanji J. Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J Neurophysiol. 2000;83:2355–2373. doi: 10.1152/jn.2000.83.4.2355. [DOI] [PubMed] [Google Scholar]
- 43.Efron BTR. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 44.Demidenko E. Sample size determination for logistic regression revisited. Stat Med. 2007;26:3385–3397. doi: 10.1002/sim.2771. [DOI] [PubMed] [Google Scholar]
- 45.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- 46.Carello CD, Krauzlis RJ. Manipulating intent: Evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 47.Nummela SU, Krauzlis RJ. Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. J Neurophysiol. 2010;104:1538–1548. doi: 10.1152/jn.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: Comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]
- 49.Stuphorn V, Bauswein E, Hoffmann KP. Neurons in the primate superior colliculus coding for arm movements in gaze-related coordinates. J Neurophysiol. 2000;83:1283–1299. doi: 10.1152/jn.2000.83.3.1283. [DOI] [PubMed] [Google Scholar]
- 50.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13:261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- 54.Corbetta M, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 55.Schiegg A, Deubel H, Schneider WX. Attentional selection during preparation of prehension movements. Vis Cogn. 2003;10:409–431. [Google Scholar]
- 56.Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paré M, Crommelinck M, Guitton D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res. 1994;101:123–139. doi: 10.1007/BF00243222. [DOI] [PubMed] [Google Scholar]
- 58.Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: Evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol. 1996;76:3360–3381. doi: 10.1152/jn.1996.76.5.3360. [DOI] [PubMed] [Google Scholar]
- 59.Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Exp Brain Res. 1979;37:495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- 60.Falmagne J-C. Elements of Psychophysical Theory. New York: Clarendon; 1985. [Google Scholar]
- 61.Rosenthal R, Rosnow RL. Essentials of Behavioral Research: Methods and Data Analysis. New York: McGraw–Hill; 1984. [Google Scholar]