Abstract
Cellular functions and survival are dependent on a tightly controlled redox potential. Currently, an increasing amount of data supports the concept of local changes in the redox environment and specific redox signaling events controlling cell function. Specific protein thiol groups are the major targets of redox signaling and regulation. Thioredoxins and glutaredoxins catalyze reversible thiol-disulfide exchange reactions and are primary regulators of the protein thiol redox state. Here, we demonstrate that embryonic brain development depends on the enzymatic activity of glutaredoxin 2. Zebrafish with silenced expression of glutaredoxin 2 lost virtually all types of neurons by apoptotic cell death and the ability to develop an axonal scaffold. As demonstrated in zebrafish and in a human cellular model for neuronal differentiation, glutaredoxin 2 controls axonal outgrowth via thiol redox regulation of collapsin response mediator protein 2, a central component of the semaphorin pathway. This study provides an example of a specific thiol redox regulation essential for vertebrate embryonic development.
Keywords: embryonic development, axonogenesis
The development of any multicellular organism depends on the complex interaction of fundamental cellular processes like proliferation, differentiation, migration, and apoptosis. Intensive research during the last decade has established an essential role of the cellular redox state in all these processes and thereby in embryonic development (1, 2). It has been shown that the redox state regulates differentiation of neural progenitors, naturally occurring death of postmitotic neurons during development, and neuronal function via redox regulated transcription factors (3, 4). Currently, we experience a paradigm shift from the importance of a global cellular oxidant/antioxidant balance toward specific signaling events mediated by reversible redox modifications of protein thiol groups (5, 6). The oxidoreductases that control the redox state of these thiol groups are thioredoxins (Trxs) and glutaredoxins (Grxs). They are members of the thioredoxin family of proteins which are characterized by a common fold of three beta sheets and four alpha helices including a cis-proline and a C-X-X-C (where C is cysteine and X any other amino acid) active site motif (7). Grxs reduce protein disulfides using both cysteinyl residues of their active site, whereas the reduction of disulfides between protein thiols and glutathione depends only on the N-terminal cysteinyl residue (6). The genome of most vertebrates encodes four Grxs, the dithiol Grxs 1 and 2 and the monothiol Grxs 3 and 5, with the latter being a subfamily characterized by a C-X-X-S active site motif (6).
Human Grx2 contains an atypical active site motif (C-S-Y-C, consensus sequence: C-P-Y-C) and two extra cysteines forming a structural disulfide bond (8–10). Alternative splicing and transcription initiation of the human gene GLRX2 gives rise to three different isoforms, the mitochondrial Grx2a and the cytosolic Grx2b and Grx2c (11). It was proposed that under conditions of oxidative stress Grx2a is activated by the loss of its [2Fe2S]2+ cluster, which might therefore serve as a redox sensor (12, 13). Knockdown of the mitochondrial Grx2 isoform sensitized HeLa cells toward oxidative stress-induced apoptosis, whereas its overexpression increased resistance against various apoptotic stimuli (14, 15). Protection against mitochondrial oxidative stress is connected to Grx2-mediated redox modifications of specific cysteine residues of mitochondrial complex I (16).
Although the importance of cellular redox control during embryonic development is well-established, no specific thiol redox regulation events have been identified so far. Here, we have discovered an essential role of the vertebrate-specific oxidoreductase Grx2 for brain development. Using the powerful model organism zebrafish (zf), Danio rerio, as well as a human cellular model, we discovered that regulation of axonal outgrowth, survival of neuronal cells, and subsequent formation of a functioning neuronal network depends on the enzymatic activity of cytosolic Grx2.
Results
The genome of zebrafish is fully sequenced and its latest annotation (zv9) contains open reading frames for two dithiol Grxs. Sequence analysis revealed that the protein encoded by the gene 436677 (zgc: 92698) is the zebrafish homologue to hGrx2 (17). It lacks a mitochondrial target sequence and is most closely related to hGrx2c (Fig. S1A). In contrast to hGrx2, zfGrx2 possesses the common Grx active site motif C-P-Y-C. The two conserved extra structural cysteines are present, which connected zfGrx2 to a vertebrate-specific group (Fig. S1B). We determined with the Grx-specific hydroxyethyl disulfide (HED) assay an enzymatic activity of kcat = 4.23 ± 1.08 s-1, which is comparable to mammalian Grx2 (18).
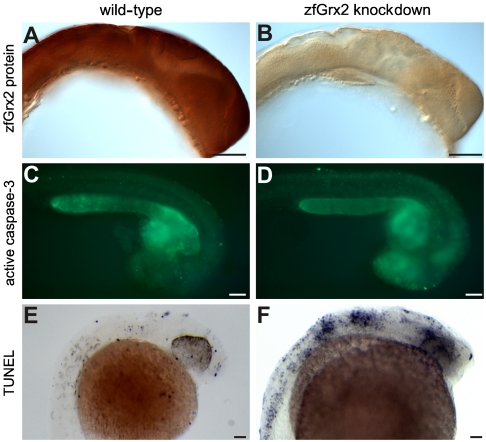
ZfGrx2 was expressed in the four cell stage and ubiquitously distributed in zebrafish embryos throughout development (Fig. 1A, Fig. S2). Silencing of the zfGrx2 expression by injection of a specific antisense morpholino (Fig. 1 A and B) induced apoptosis in the CNS from midsomitogenesis until approximately 30 hours post fertilization (hpf) (Fig. 1 C–F). Apoptotic cells were confined to the eye, the anterior brain, the hindbrain, and the spinal cord in all embryos lacking zfGrx2 (n = 125), whereas uninjected control embryos showed only few scattered apoptotic cells (n = 76).
Fig. 1.
Knockdown of zfGrx2 induced apoptosis in central nervous system. Injection of a morpholino blocking zfGrx2 translation reduced the levels of the corresponding protein as visualized by whole mount immunohistochemistry (A and B). Loss of zfGrx2 induced apoptosis in the central nervous system as demonstrated by in vivo caspase 3 measurements (C and D) and TUNEL staining (E and F). Scale bar, 50 μm.
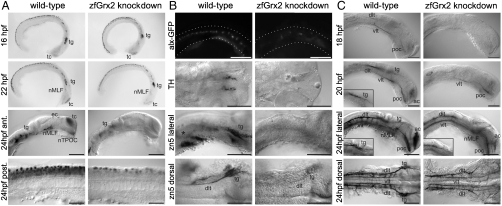
Knockdown of zfGrx2 was accompanied by extensive loss of early differentiated neurons at 24 hpf in 97% (n = 34) of analyzed embryos (Fig. 2A). The number and distribution of newborn neurons did not substantially differ between morpholino injected and uninjected embryos at 16–22 hpf (Fig. 2A), indicating that the majority of neurons were lost between 22 and 24 hpf. In fact, neurons were identified as the predominant cell type undergoing apoptosis at 24 hpf following knockdown of Grx2 (Fig. S3).
Fig. 2.
Knockdown of zfGrx2 suppressed formation of a neuronal network. Morpholino-induced knockdown of zfGrx2 led to loss of early HuC/D positive neurons between 22 and 24 hpf (A). As demonstrated by different techniques, all tested neuronal subgroups were affected at 24 hpf: glutamatergic excitatory interneurons, dopaminergic neurons, and secondary motor neurons (B). Embryos lacking zfGrx2 were not able to develop an axonal scaffold (C). Ac, anterior commissure; dlt, dorsal longitudinal tract; nMLF, nucleus of the medial longitudinal fascicle; poc, posterior commissure; tg, trigeminal ganglion; vlt, ventral longitudinal tract. Scale bar, 50 μm.
To investigate if specific neuronal subpopulations were affected by reduced levels of zfGrx2, we examined secondary motor neurons, dopaminergic neurons, and glutamatergic excitatory interneurons. Independent from neuronal cell type, the number of those specific neurons was dramatically diminished (Fig. 2B), which resulted in severe behavioral changes of embryos. In contrast to the stereotypical fast escape response which wild-type embryos display upon mechanical stimuli, embryos lacking zfGrx2 reacted to the stimulus but swam away slowly in an uncoordinated, circlewise fashion (Movie S1).
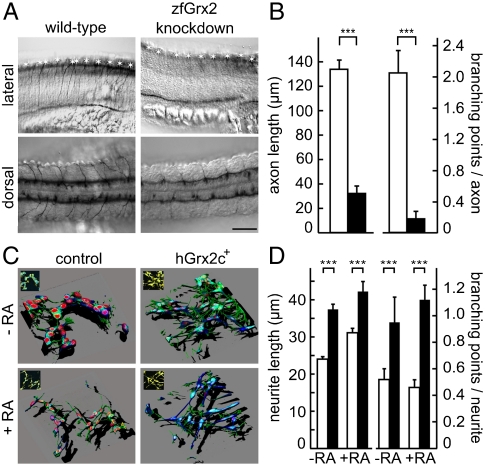
The onset of neuronal degradation coincides with the time window of initial axon tract formation. Therefore we visualized the axon scaffold and revealed a severe reduction of tracts in nearly all zfGrx2 knockdown embryos at 24 hpf (94%, n = 49) compared to uninjected controls (n = 21). Both anterior brain commissures as well as the major longitudinal axon tracts were strongly reduced in knockdown embryos and the trigeminal ganglion was almost undetectable (Fig. 2C). Already the maturation of axon tracts was disturbed as essentially no axons could be detected at 18, 20, and 22 hpf, the time frame when the scaffold developed in wild-type embryos (Fig. 2C). Length and number of branching points of single axons were significantly reduced in embryos lacking zfGrx2 (32.86 ± 5.50 μm, 0.18 ± 0.09) compared to wild-type embryos (133.76 ± 7.73 μm, 2.04 ± 0.29) (mean ± SEM, n = 25, Fig. 3 A and B).
Fig. 3.
Outgrowth of neurites depends on the presence of Grx2. (A) Length and number of branching points of axons in embryos with silenced zfGrx2 expression were dramatically reduced 24 hpf, as demonstrated by immunohistochemistry using antiacetylated tubulin antibodies (asterisks mark cell bodies). (B) Quantitative data obtained from A. White bars, wild-type; black bars, zfGrx2 knockdown (mean ± SEM, n = 25, two-tailed Student’s t test, *** p < 3 × 10-7). (C) Three-dimensional reconstruction of the cytoskeleton (red, DNA; blue, tubulin; green, actin; single confocal layers as Insets; blue, DNA; yellow, actin) of SH-SY5Y cells grown for 8 d +/- RA. (D) Quantification of axon lengths (−RA, n = 247; +RA, n = 156) and branching points (−RA, n = 106; +RA, n = 213) of three independent experiments corresponding to C; white bars, control cells; black bars, hGrx2c+ cells (mean ± SEM, two-tailed Student’s t test, ***p < 0.008).
To confirm the effect of cytosolic Grx2 on the outgrowth of neurites in a cellular system, we chose the human cell line SH-SY5Y as a model for neuronal differentiation. Cells were transfected with constructs allowing transient expression of the cytosolic isoform of human Grx2, hGrx2c. Morphology of control cells and hGrx2c overexpressing cells was compared with and without induction of differentiation by RA (Fig. 3C). hGrx2c+ SH-SY5Y cells displayed significantly longer neurites with 42.2 ± 2.7 μm (37.4 ± 1.4 μm without RA) compared to those of wild-type cells with 31.1 ± 1.2 μm (24.0 ± 0.7 μm without RA). The number of branching points per neurite increased significantly from 0.46 ± 0.059 (0.52 ± 0.08 without RA) in control cells to 1.12 ± 0.11 (0.95 ± 0.19 without RA) in hGrx2c+ cells (Fig. 3D).
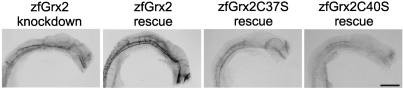
We verified that the above described phenotypes in zebrafish embryos were caused specifically by the loss of zfGrx2 by injection of zfGrx2 capped mRNA together with the morpholino (Fig. 4, Fig. S3B). Whereas injection of the morpholino alone resulted in the knockdown phenotype in all embryos (n = 15), simultaneous injection with zfGrx2 mRNA inhibited significantly induction of apoptosis (in 86% of embryos, n = 15) as well as reduction of axon tracts (in 68% of embryos, n = 22). Moreover, oxidoreductase activity of zfGrx2 was required to rescue the knockdown phenotype. Simultaneous injection of mRNAs coding for mutants lacking the N- (C37S) or the C-terminal active site cysteine residue (C40S) rescued formation of the axon scaffold only in 8% (n = 45) and 17% (n = 26) of the embryos, respectively (Fig. 4).
Fig. 4.
Formation of the axonal network depends on oxidoreductase activity of zfGrx2. Impaired formation of an axonal network (antiacetylated tubulin antibodies) could be rescued by injection of 20 pg/embryo capped zfGrx2 mRNA simultaneously to morpholino injection. Injection of mRNA coding for active site mutants of zfGrx2 (zfGrx2C37S, zfGrx2C40S) did not rescue. Scale bar, 50 μm.
Because the oxidoreductase activity of zfGrx2 was essential, we wondered if a general increase in oxidative stress was a cause of the observed phenotype. However, determination of carbonylated proteins (Fig. S4A) and rescue experiments with zfGpx1 mRNA (Fig. S4B)—glutathione peroxidase (Gpx1) is a general antioxidant protein reducing hydrogen peroxide and is used to rescue oxidative stress-induced phenotypes (19)—demonstrated that loss of zfGrx2 did not induce oxidative stress. Moreover, comparison of protein thiols in the whole zebrafish proteome by redox differential gel electrophoresis (redox-DIGE) (Fig. S4C) excluded an effect of zfGrx2 knockdown on the overall redox state of the proteome.
The lack of a general effect on the redox state as well as requirement of both active site cysteine residues to rescue the zfGrx2 knockdown phenotype suggested that redox regulation of a specific protein thiol group caused the disturbed formation of the neuronal network.
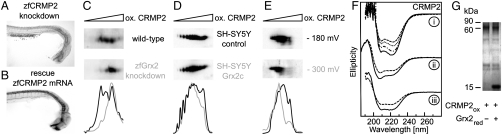
Coimmunoprecipitation experiments identified collapsin response mediator protein 2 (CRMP2) as potential interaction partner of hGrx2 in HeLa cells [Schütte and Lillig (20)]. We detected colocalization of zfGrx2 and zfCRMP2 in the cytosol of developing neurons (Fig. S5). Knockdown of zfCRMP2 by injection of a specific antisense morpholino as well as inhibition of zfCRMP2 by lacosamide mimicked the zfGrx2 knockdown phenotype. Axonal tracts and anterior brain commissures were reduced in 64% of 55 analyzed embryos by zfCRMP2 knockdown (Fig. 5A) and in 97% of 46 analyzed embryos by treatment with 2 μM lacosamide (Fig. S6A). Simultaneous injection of zfCRMP2 mRNA together with the morpholino blocking zfGrx2 expression decreased the amount of embryos with reduced axon scaffold from 81% in controls (n = 16) to 37% (n = 19) (Fig. 5B). This rescue indicated a functional interaction of zfGrx2 and zfCRMP2 during axonal outgrowth. To investigate if Grx2 changed the thiol redox state of CRMP2, we developed a two-dimensional (2D) redox blotting technique which is based on different pI values dependent on the thiol redox state via addition of two carboxyl groups to oxidized protein thiols and found indeed that zfCRMP2 thiols in zfGrx2 knockdown embryos were more oxidized compared to those in control embryos (Fig. 5C). Accordingly, rescue of the zfGrx2 knockdown phenotype by coinjection of zfCRMP2 mRNA was explained by increased amounts of reduced forms of zfCRMP2 (Fig. S6B). The redox state of CRMP2 was shifted to reduced forms in SH-SY5Y cells overexpressing hGrx2c (Fig. 5D). To investigate, if CRMP2 is a direct substrate of Grx2, we incubated recombinantly expressed and purified proteins in different redox buffers based on the glutathione/glutathione disulfide (GSH/GSSG) couple (−180 and -300 mV) and revealed a direct reduction of CRMP2 by the GSH/zfGrx2 redox system (Fig. 5E). The redox state affected the secondary structure of zfCRMP2. Using CD spectroscopy, we detected a time-dependent increase in ellipticity between 210 and 230 nm when reduced zfCRMP2 was incubated with H2O2 for 1, 15, or 30 min (Fig. 5F). These spectroscopic changes induced by oxidation indicated a conformational change of the protein that was reversed by the GSH/zfGrx2 redox system, or zfGrx2 alone (Fig. 5F). Observed changes in both, thiol redox state and conformation were not dependent on the formation of a mixed disulfide between oxidized zfCRMP2 and zfGrx2 (expected molecular mass 75 kDa) (Fig. 5G).
Fig. 5.
Grx2 acts via CRMP2. Knockdown of zfCRMP2 by morpholino injection impaired formation of axon scaffold (A). Impaired formation of axon scaffold by knockdown of zfGrx2 was rescued by simultaneous injection of 20 pg/embryo capped zfCRMP2 mRNA (B). Densitometric analyses of the resulting pattern of CRMP2-specific spots after separation by 2D redox blots demonstrated Grx2-dependent changes in the thiol redox state of CRMP2 (C–E). At 24 hpf zfCRMP2 was more oxidized in embryos lacking zfGrx2 compared to wild-type (C), whereas in SH-SY5Y cells overexpressing hGrx2c the redox state of CRMP2 was more reduced compared to control cells (D). The redox state of recombinant zfCRMP2 was more reduced after incubation with recombinant zfGrx2 for 1 h under anaerobic conditions in a GSH/GSSG buffer adjusted to -300 mV compared to a buffer with a potential of -180 mV (E). Thiol redox modifications changed secondary structure of zfCRMP2 (F). (F, I) Ellipticity of reduced recombinant zfCRMP2 (solid line) and after incubation with H2O2 for 1 min (dashed/dotted line), 15 min (dotted line), and 30 min (dashed line). (F, ii) Spectra of oxidized zfCRMP2 before (dashed line) and after incubation with GSH/zfGrx2 (solid line). (F, iii) Spectra of oxidized zfCRMP2 before (dashed line) and after incubation with reduced zfGrx2 at a ratio of 1∶1.2 (solid line). (G) A Coomassie blue-stained PAGE comparing quarternary structure of oxidized zfCRMP (60 kDa) before and after incubation with reduced zfGrx2 (16 kDa) at a ratio of 1∶1.2.
Discussion
This study provides clear evidence that proper development of the embryonic nervous system is dependent on the oxidoreductase activity of cytosolic Grx2. We found that Grx2 regulates axonal outgrowth, survival of neurons, and development of a functional brain via the thiol redox state of CRMP2 representing a thiol redox regulated pathway essential for vertebrate embryonic development.
So far, only monothiol Grxs were described to be essential for vertebrate development (21, 22). In mice, transcription of Grx1 as well as mitochondrial and cytosolic Grx2 have been demonstrated in early embryogenesis (23). Our analysis showed that also zfGrx2 is already present in the fertilized zebrafish egg and expressed ubiquitously throughout the first 24 h of development, the critical period when all major organs, including the brain, are formed (24). Zebrafish embryos lacking zfGrx2 lost virtually all types of neurons by apoptosis and the ability to establish an axonal scaffold, which severely impaired larval movements. We were not able to detect an increase of general oxidative stress in embryos lacking zfGrx2, but we could confirm that development of axon tracts depends on the oxidoreductase activity of Grx2 indicating that knockdown of zfGrx2 disrupts specific signaling pathways.
Axon growth and guidance depends strongly on dynamic modifications of the cytoskeleton (25). It was described before that activation of actin and tubulin, the essential components of the cytoskeleton, are regulated by the GSH/Grx system (26, 27). Furthermore, a Grx-like domain of TXNRD1_v3, an isoform of thioredoxin reductase 1, induces formation of cytoplasmic filaments and filopodia in HEK293 cells (28). Interestingly, this domain shares high homology with hGrx2 (29) and zfGrx2.
One of the most important signaling pathways for the establishment of an axonal scaffold is the semaphorin pathway (30). When axon steering is required, the guidance cue semaphorin 3A (Sema3A) binds to the receptor pair neuropilin-1/plexin3A. Subsequently, plexin3A conveys the signal via molecule interacting with CasL (MICAL) to CRMP2, which induces growth cone collapse (31). Thereby CRMP2 controls axonal branching, guidance, and number of neurites (32–34). CRMP2 is essential during brain development (35) and highly conserved across vertebrate species (36). It is expressed in the major neural clusters of developing zebrafish embryos, especially between 16 and 24 hpf when the formation of the neuronal network takes place (37). The activity of CRMP2 is subject to complex regulation, e.g., phosphorylation and alternative splicing (38). Here, we demonstrated that activity of CRMP2 also depends on redox regulation by Grx2 both in zebrafish and human cells. Our results show that one or more disulfides formed by the eight (mammalian CRMP2), respectively nine (zfCRMP2) cysteine residues are reduced by Grx2. Oxidation of CRMP2 through MICAL was suggested before (39). During preparation of this manuscript it has been shown that CRMP2, which was oxidized via hydrogen peroxide formation by MICAL upon Sema3A stimulation, is a substrate for Trx (40). It was proposed that oxidized CRMP2 forms a complex with Trx which facilitates phosphorylation and growth cone collapse. Because of the different pKa values of the active site cysteine residues and the resulting reaction mechanism and kinetics of Trx and other oxidoreductases, a stable mixed disulfide complex between Trx and CRMP2 is highly unlikely (41). Between Grx2 and CRMP2 there is no formation of a stable complex. Therefore, we favor an alternative model in which redox regulation of CRMP2 induces conformational changes supported by the in vitro data using CD spectrocopy. These changes could, for instance, be essential for the exposure of phosphorylation sites.
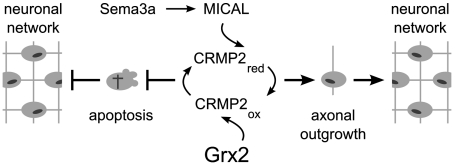
A delicate balance of Sema3A signaling is also essential for survival of immature neurons (42, 43). Disruption of the semaphorin signaling pathway decreases the rate of cell death in dorsal root ganglions (DRG), whereas overexpression of Sema3A induces apoptosis in embryonic DRG (44, 45). This overexpression might lead, as described above, to overactivation of MICAL and subsequently to an increased pool of oxidized CRMP2, which we also have observed in zfGrx2 knockdown embryos. Therefore, a tightly controlled balance of oxidized and reduced CRMP2 might be essential to regulate both neuronal survival and axonal outgrowth (Fig. 6).
Fig. 6.
Model of the role of Grx2 during brain development. A redox circuit consisting of Grx2 and MICAL regulates the thiol redox state of CRMP2. In response to semaphorin signaling MICAL oxidizes CRMP2, whereas Grx2 reduces CRMP2. The balance between reduced and oxidized CRMP2 regulates axonal outgrowth. Disturbance of thiol redox regulation of CRMP2 can lead to loss of the axonal scaffold and to neuronal apoptosis. Therefore, oxidoreductase activity of Grx2 is essential for the formation of a neuronal network.
CRMP2 is involved in several neurological disorders and it has been proposed that manipulation of CRMP2 activity, e.g., by the epilepsy drug lacosamide (46), may offer unique opportunities for therapy against some of these disorders (35, 47).
In the brains of adult mice, rats, and humans Grx2 is mainly expressed in neurons (48–50) and it is suggested that mitochondrial Grx2 may protect neurons against cell death in Parkinson’s disease by maintaining function of mitochondrial complex I (48, 51). The interaction demonstrated here between Grx2 and CRMP2 indicates that also cytosolic Grx2 might be important for protection against neurological disorders. Alzheimer’s disease patients displayed a larger pool of oxidized CRMP2 (52) suggesting a relation between a functional Grx2-dependent regulation of CRMP2 and the ability to regenerate damaged adult neuronal networks.
In synopsis, our report highlights the significance of glutaredoxin-based thiol redox regulation during vertebrate embryonic development and its potential importance in physiology and pathophysiology of the brain.
Materials and Methods
Zebrafish Maintenance and Cell Culturing.
Zebrafish maintenance and staging followed standard protocols (24). SH-SY5Y cells were propagated in MEM medium including 10% fetal calf serum, 2 mM penicillin/streptavidin, and 2 mM glutamine. For transient expression of tag-free human Grx2c, 5.5 × 106 SH-SY5Y cells were resuspended in 550 μL transfection buffer (21 mM Hepes, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM d-glucose, pH 7.15), mixed with 20 μg of plasmid pEGFP-N1-Grx2c (11) and electroporated by a single pulse of 250 V and 1,500 μF. Differentiation of SH-SY5Y cells was induced by 10 μM RA.
Cloning and Recombinant Protein Expression.
The ESTs IMAPp9981714884Q1 (zfGrx2) and IRAKp961K11293Q (zfCRMP2) were purchased through ImaGenes and the respective ORFs amplified by PCR (for all primer sequences see Table S1). Mutations were generated by rolling circle PCR. zfGpx1 was amplified from whole adult zebrafish cDNA. PCR products were subcloned into pet15b (Novagene) to express the respective His-tagged proteins in Escherichia coli BL21 codon plus (DE3)-RIL cells (Stratagene) as previously described (13). Protein concentrations were determined by absorption coefficients (zfGrx2, 3,480 M/cm at 280 nm; zfCRMP2, 64,200 M/cm at 280 nm ). The enzymatic activity of glutaredoxins was measured by the HED assay (53).
Treatment of Zebrafish Embryos.
The morpholino oligomer knocking-down zfGrx2 (5′-GTTGAAGATACTAGGAAAGCAAACG-3′) targets the direct upstream sequence of the ATG codon, the morpholino knocking-down zfCRMP2 (5′-TCACTCTGGAAACACAGATAAACAC-3′) targets the splice acceptor of the second exon and thereby inhibits correct splicing of both isoforms zfCRMP2a and b. Both morpholinos (GeneTools) were dissolved to a concentration of 3 mM in dH2O and diluted 1/40 (Grx2) or 1/10 (CRMP2) in injection buffer (9 μM spermine, 0.21 mM spermidine, and 0.3% phenol red in PBS). Single cell eggs were injected with 1.5 nL using a Femtojet microinjector (Eppendorf). Capped mRNA was generated with the mMessage/Machine Kit (Ambion). The ORFs of zfGrx2, zfGrx2C37S, zfGrx2C40S, zfCRMP2, and zfGpx1 were cloned into pCS2 and used as template. Lacosamide (VIMPAT) was purchased through a local pharmacy.
In Situ Hybridization and Immunohistochemistry.
In situ hybridization including generation of riboprobes as well as zebrafish whole mount immunohistochemistry was performed according to standard protocols. To stain sections, embryos were fixed in 4% paraformaldehyde and either embedded in paraffin, or in tissue tek (Sakura Finetek) and frozen in liquid nitrogen. Sections (5–10 μm) were cut with a microtom (Micron) or a CM1900 UV cryostat (Leica). Paraffin sections were deparaffinized and antigens were demasked by heating in 10 mM citrate buffer pH 6.0 for 10 min and nonspecific antibody binding sites were blocked with 5% normal goat serum (Invitrogen). For immunohistochemical analysis of SH-SY5Y cultures, cells were seeded on glass plates coated with 0.5 μg/μL fibronectin and propagated as described above. After washing with PBS and fixation with 4% paraformaldehyde, cells were permeabilized for 1 h in PBS containing 10 mM Hepes, 3% BSA, 0.3% Triton X-100. Primary antibodies were dissolved in this buffer and incubated overnight at 4 °C, secondary antibodies for 1 h at room temperature. Fixed cells and embryo sections were mounted using Mowiol 4–88 (Carl Roth). Rabbit anti-zfGrx2 was produced as described before (50), other primary antibodies obtained from different suppliers and sources: actin (sc-47778, Santa Cruz) , tubulin (T9026, Sigma), CRMP2 (C2993, Sigma; sc-25895, Santa Cruz), acetylated tubulin (T6793, Sigma), zn5 (ZIRC), HuC/D (A21271, Invitrogen). Nuclei were counterstained with Hoechst 33342 (Sigma), mitochondria with prohibitin (Calbiochem), cytosol/nuclei with DNaseI labeled with Alexa-488 (Invitrogen), and actin fibers with phalloidin labeled with Alexa Fluor-546 (Invitrogen). As secondary antibodies horseradish peroxidase conjugated IgGs (BioRad, Sigma), alkaline phosphatase conjugated antibodies (Sigma), Alexa Fluor-488 and -633 labeled antibodies, and Cy2, and Cy5 (Invitrogen), and Cy3 (Millipore) labeled antibodies were used.
Detection of Apoptosis.
Active caspase 3 was detected in vivo using the Caspase-3 DEVD-R110 Fluorometric HTS Assay Kit (Biotium). Embryos were dechorionized and transferred into 200 μL PBS containing 7 μL substrate solution, incubated 15 min and washed several times with PBS containing 0.1% Tween-20. Prior to analysis by fluorescence microscopy, embryos were anesthetized with tricaine and mounted in 3% methylcellulose. In addition, apoptotic cells were detected using the in situ cell death detection Kit (Roche) in conjunction with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate staining.
Microscopy.
Stained cells or zebrafish were analyzed under a Leica MZ16 microscope equipped with a Leica DFC300FX camera, a Zeiss Axioplan microscope equipped with a Zeiss Axiocam digital camera, a Leica Diaplan microscope equipped with a MicroPublisher camera (Qimaging), a Leica TCS SP2 instrument using a 40x oil plan apochromat lens (Leica), or an Olympus BX51 microscope equipped with a U-CMAD3 digital camera.
CD Spectroscopy.
Twenty-five micromolars recombinant zfCRMP2 were completely reduced by incubation with 1 mM DTT and 1 mM Tris(2 carboxyethyl)phosphine (TCEP). After removal of the excess reductant using an NAP-5 column (GE healthcare), spectra were recorded using a Jasco spectropolariometer. Subsequently, reduced protein was incubated with 0.5 mM H2O2. Next, in an enzymatic approach oxidized zfCRMP2 was incubated with 0.5 mM GSH and 0.1 μM recombinant zfGrx2 before ellipticity of rereduced zfCRMP2 was recorded. In a single turnover approach 10 μM oxidized zfCRMP2 was incubated with 12 μM reduced zfGrx2 (incubated for 30 min with 10 mM DTT and 10 mM TCEP followed by gel filtration using an NAP-5 column). Ellipticity of reduced zfGrx2 was used as reference.
Preparation of Whole Zebrafish Embryo Extracts and Cell Extracts.
Whole zebrafish embryo extracts were prepared following a previously published protocol (54) with some modifications. Embryos were dechorionated and incubated in preblocking buffer (100 mM N-ethyl maleimide in PBS) for 30 min. Yolk sack was removed by pipetting up and down in deyolking buffer (55 mM NaCl, 1.8 mM KCl, 1.25 mM NaHCO3) followed by centrifugation for 30 s at 300 g. SH-SY5Y cells were harvested by trypsination and washed with PBS. Pellets of zebrafish and SH-SY5Y cells were taken up in preblocking buffer and the fourfold volume of lysis buffer (150 mM NaCl, 5 mM EDTA, 100 mM N-ethylmaleimide, 8 M urea in 100 mM Na-phosphate buffer pH 7.2), and 2% (wt/vol) CHAPS was added followed by a 20 min anaerobic incubation at room temperature and snap freezing.
SDS-PAGE and Western Blotting.
SDS-PAGE and Western blotting were performed using Novex MiniCells (Invitrogen), precasted Precise gels (Thermo Scientific, Invitrogen), and PVDF membranes (Macherey and Nagel). Horse radish peroxidase labeled immunocomplexes were stained with SuperSignal West Pico/Femto (9∶1) luminescence kit (Thermo Scientific). Carbonylated proteins were visualized by formation and detection of derivatives with 2,4-dinitrophenylhydrazin after SDS-PAGE and Western blotting transfer of 6.4 μg zebrafish extract using antidinitrophenol antibodies (BP424, Acris Antibodies).
Two-Dimensional Redox Blot and Redox-DIGE.
For 2D redox blots and redox-DIGE, pellets of N-ethyl maleimide treated proteins [2D redox blots, 200 μg of zebrafish extract; 10 μg of SH-SY5Y extract; or recombinant proteins, 4.8 μM zfCRMP2 was incubated for 1 h under anaerobic conditions with 0.048 μM zfGrx2 in GSH/GSSG buffers adjusted to −180 (6.06 mM GSH/3.94 mM GSSG) and -300 mV (9.999 mM GSH/0.001 mM GSSG); redox-DIGE, 10 μg zebrafish extract] were resuspended in 100 mM Na-phosphate buffer pH 7.2 containing 150 mM NaCl, 5 mM EDTA, and 8 M urea. Previously oxidized thiols were reduced with 10 or 0.2 mM TCEP. For 2D blots, the newly generated thiols were labeled by incubation with 20 mM 5-maleimido isophtalic acid. After precipitation with 12% trichloroacetic acid, proteins were washed with aceton, rehydrated in 8 M urea, 1% Nonidet P-40 (NP-40), 20 mM DTT, 0.5% ampholytes, separated by two dimensions (first pH 3–10 for fish extracts, pH 4–7 for SH-SY5Y extracts, second molecular weight) according to supplier’s manual (Invitrogen), and transferred to PVDF membrane as described above. For redox-DIGE, the newly generated thiols in the different samples were labeled by incubation with 0.4 mM Cy3, respectively, Cy5 minimal CyDye (GE healthcare). After inactivation of free dye by 65 mM DTT, unlabeled extract (40 μg) was added, the two samples were pooled in 8 M urea, 20 mM DTT, 1% NP-40, 0.5% ampholytes, and separated as described before at the same gel. The 2D gel was scanned using a Typhoon Trio scanner (GE healthcare). Yellow color is the result of an overlay of Cy3 and Cy5.
Computational Analysis and Statistics.
Primary sequence data was analyzed using BLAST and Align2seq. Deconvolution, three-dimensional reconstruction, and maximum intensity projection of confocal microscopy pictures were computed using the software package Huygens (Scientific Volume Imaging). Neurite lengths were measured using Adobe Photoshop CS5, densitometric measurements were performed using ImageJ. For statistical analyses the two-tailed Student’s t test was used.
Supplementary Material
Acknowledgments.
We thank Sabrina Oesteritz, Gisela Lesch (Philipps-University) and Iris Söll, Lena Ringdén (Karolinska Institutet) for technical and administrative assistance, Vadim N. Gladyshev, Leonard I. Zon (Harvard Medical School), and Orhan Aktas (Heinrich-Heine University) for valuable discussions as well as Shin-ichi Higashijima (Okazaki Institute for Integrative Bioscience) for providing the zebrafish alx-GFP transgenic line. This work was supported by the Deutsche Forschungsgemeinschaft BE 3259/2 (to C.B.), SFB593-N01 (to C.H.L.), the Karolinska Institutet (G.H., C.B.), Karolinska Institutet fellowship for new PhD students Dnr. 2379/07-225 (to L.B.), the Swedish Cancer Society Grant 961 (to A.H.), the Swedish Research Council (A.H.), and the P. E. Kempkes foundation (C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110085108/-/DCSupplemental.
References
- 1.Covarrubias L, Hernández-García D, Schnabel D, Salas-Vidal E, Castro-Obregón S. Function of reactive oxygen species during animal development: Passive or active? Dev Biol. 2008;320:1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 2.Dennery PA. Oxidative stress in development: Nature or nurture? Free Radical Biol Med. 2010;49:1147–1151. doi: 10.1016/j.freeradbiomed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Prozorovski T, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 4.Castagne V, Gautschi M, Lefevre K, Posada A, Clarke PG. Relationships between neuronal death and the cellular redox status. Focus on the developing nervous system. Prog Neurobiol. 1999;59:397–423. doi: 10.1016/s0301-0082(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 5.Jones DP. Redefining oxidative stress. Antioxid Redox Signaling. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 6.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta Gen Subj. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren A. Thioredoxin structure and mechanism: Conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure. 1995;3:239–243. doi: 10.1016/s0969-2126(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg M, et al. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J Biol Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 9.Gladyshev VN, et al. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx. J Biol Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- 10.Hashemy SI, Johansson C, Berndt C, Lillig CH, Holmgren A. Oxidation and S-nitrosylation of cysteines in human cytosolic and mitochondrial glutaredoxins: Effects on structure and activity. J Biol Chem. 2007;282:14428–14436. doi: 10.1074/jbc.M700927200. [DOI] [PubMed] [Google Scholar]
- 11.Lönn ME, et al. Expression pattern of human glutaredoxin 2 isoforms: Identification and characterization of two testis/cancer cell-specific isoforms. Antioxid Redox Signaling. 2008;10:547–557. doi: 10.1089/ars.2007.1821. [DOI] [PubMed] [Google Scholar]
- 12.Lillig CH, et al. Characterization of human glutaredoxin 2 as iron-sulfur protein: A possible role as redox sensor. Proc Natl Acad Sci USA. 2005;102:8168–8173. doi: 10.1073/pnas.0500735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berndt C, et al. How does iron-sulfur cluster coordination regulate the activity of human glutaredoxin 2? Antioxid Redox Signaling. 2007;9:151–157. doi: 10.1089/ars.2007.9.151. [DOI] [PubMed] [Google Scholar]
- 14.Lillig CH, Lönn ME, Enoksson M, Fernandes AP, Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc Natl Acad Sci USA. 2004;101:13227–13232. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enoksson M, et al. Overexpression of glutaredoxin 2 attenuates apoptosis by preventing cytochrome c release. Biochem Biophys Res Commun. 2005;327:774–779. doi: 10.1016/j.bbrc.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 16.Beer SM, et al. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: Implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 17.Sagemark J, et al. Redox properties and evolution of human glutaredoxins. Proteins. 2007;68:879–892. doi: 10.1002/prot.21416. [DOI] [PubMed] [Google Scholar]
- 18.Gallogly MM, Starke DW, Leonberg AK, Ospina SME, Mieyal JJ. Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: Implications for intracellular roles. Biochemistry. 2008;47:11144–11157. doi: 10.1021/bi800966v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schütte L, et al. Redox control of the cytoskeleton by glutaredoxins. Acta Microsc; Proceedings of the Tenth Inter-American Congress of Electron Microscopy and the First Congress of the Argentine Society of Microscopy; Rosario, Argentina. 2009. [Google Scholar]
- 21.Cheng N-H, et al. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progression during embryogenesis. FEBS J. 2011;278:2525–2539. doi: 10.1111/j.1742-4658.2011.08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingert RA, et al. Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- 23.Jurado J, Prieto-Alamo M-J, Madrid-Rísquez J, Pueyo C. Absolute gene expression patterns of thioredoxin and glutaredoxin redox systems in mouse. J Biol Chem. 2003;278:45546–45554. doi: 10.1074/jbc.M307866200. [DOI] [PubMed] [Google Scholar]
- 24.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 25.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 2009;122:3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, et al. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 27.Landino LM, Moynihan KL, Todd JV, Kennett KL. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun. 2004;314:555–560. doi: 10.1016/j.bbrc.2003.12.126. [DOI] [PubMed] [Google Scholar]
- 28.Damdimopoulou PE, Miranda-Vizuete A, Arnér ESJ, Gustafsson J-A, Damdimopoulos AE. The human thioredoxin reductase-1 splice variant TXNRD1_v3 is an atypical inducer of cytoplasmic filaments and cell membrane filopodia. Biochim Biophys Acta. 2009;1793:1588–1596. doi: 10.1016/j.bbamcr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Dammeyer P, et al. Induction of cell membrane protrusions by the N-terminal glutaredoxin domain of a rare splice variant of human thioredoxin reductase. J Biol Chem. 2008;283:2814–2821. doi: 10.1074/jbc.M708939200. [DOI] [PubMed] [Google Scholar]
- 30.Fiore R, Püschel AW. The function of semaphorins during nervous system development. Front Biosci. 2003;8:s484–499. doi: 10.2741/1080. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Gunput R-AF, Pasterkamp RJ. Semaphorin signaling: Progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki N, et al. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura T, et al. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 2003;5:819–826. doi: 10.1038/ncb1039. [DOI] [PubMed] [Google Scholar]
- 35.Charrier E, et al. Collapsin response mediator proteins (CRMPs): Involvement in nervous system development and adult neurodegenerative disorders. Mol Neurobiol. 2003;28:51–64. doi: 10.1385/MN:28:1:51. [DOI] [PubMed] [Google Scholar]
- 36.Schweitzer J, Becker CG, Schachner M, Becker T. Expression of collapsin response mediator proteins in the nervous system of embryonic zebrafish. Gene Expression Patterns. 2005;5:809–816. doi: 10.1016/j.modgep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Christie TL, Starovic-Subota O, Childs S. Zebrafish collapsin response mediator protein (CRMP)-2 is expressed in developing neurons. Gene Expression Patterns. 2006;6:193–200. doi: 10.1016/j.modgep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terman JR, Mao T, Pasterkamp RJ, Yu H-H, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/s0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 40.Morinaka A, et al. Thioredoxin mediates oxidation-dependent phosphorylation of CRMP2 and growth cone collapse. Sci Signaling. 2011;4:ra26. doi: 10.1126/scisignal.2001127. [DOI] [PubMed] [Google Scholar]
- 41.Kallis GB, Holmgren A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J Biol Chem. 1980;255:10261–10265. [PubMed] [Google Scholar]
- 42.Vanderhaeghen P, Cheng H-J. Guidance molecules in axon pruning and cell death. Cold Spring Harbor Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirvan A, et al. Semaphorins as mediators of neuronal apoptosis. J Neurochem. 1999;73:961–971. doi: 10.1046/j.1471-4159.1999.0730961.x. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Zvi A, et al. The semaphorin receptor PlexinA3 mediates neuronal apoptosis during dorsal root ganglia development. J Neurosci. 2008;28:12427–12432. doi: 10.1523/JNEUROSCI.3573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Zvi A, et al. Semaphorin 3A and neurotrophins: A balance between apoptosis and survival signaling in embryonic DRG neurons. J Neurochem. 2006;96:585–597. doi: 10.1111/j.1471-4159.2005.03580.x. [DOI] [PubMed] [Google Scholar]
- 46.Beydoun A, D’Souza J, Hebert D, Doty P. Lacosamide: Pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother. 2009;9:33–42. doi: 10.1586/14737175.9.1.33. [DOI] [PubMed] [Google Scholar]
- 47.Hensley K, Venkova K, Christov A, Gunning W, Park J. Collapsin response mediator protein-2: An emerging pathologic feature and therapeutic target for neurodisease indications. Mol Neurobiol. 2011;43:180–191. doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- 48.Karunakaran S, Saeed U, Ramakrishnan S, Koumar RC, Ravindranath V. Constitutive expression and functional characterization of mitochondrial glutaredoxin (Grx2) in mouse and human brain. Brain Res. 2007;1185:8–17. doi: 10.1016/j.brainres.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 49.Aon-Bertolino ML, et al. Thioredoxin and glutaredoxin system proteins-immunolocalization in the rat central nervous system. Biochim Biophys Acta. 2011;1810:93–110. doi: 10.1016/j.bbagen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Godoy JR, et al. Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2011;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Lee DW, Kaur D, Chinta SJ, Rajagopalan S, Andersen JK. A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: Implications for Parkinson’s disease. Antioxid Redox Signaling. 2009;11:2083–2094. doi: 10.1089/ars.2009.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sultana R, et al. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: An approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 53.Luthman M, Holmgren A. Glutaredoxin from calf thymus. Purification to homogeneity. J Biol Chem. 1982;257:6686–6690. [PubMed] [Google Scholar]
- 54.Link V, Shevchenko A, Heisenberg C-P. Proteomics of early zebrafish embryos. BMC Dev Biol. 2006;6:1. doi: 10.1186/1471-213X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.