Abstract
Granulomatosis with polyangiitis (Wegener's) is a rare autoimmune neutrophil-mediated vasculitis that can cause renal disease and mucosal manifestations. Antineutrophil cytoplasmic antibodies (ANCA) are present in many patients, vary in level over time, and induce neutrophil activation through engagement with Fc receptors (FcRs). Given roles for FcRs in ANCA-mediated neutrophil activation and IgA antibodies in mucosal immunity, we hypothesized that FcR genetics and previously unappreciated IgA ANCA affect clinical presentation. We assembled a total of 673 patients and 413 controls from two multicenter cohorts, performed ELISA and immunofluorescence assays to determine IgA and IgG ANCA positivity, and used Illumina, TaqMan, or Pyrosequencing to genotype eight haplotype-tagging SNPs in the IgA FcR (FCAR) and to determine NA1/NA2 genotype of FCGR3B, the most prevalent neutrophil IgG FcR. We evaluated neutrophil activation by measuring degranulation marker CD11b with flow cytometry or neutrophil extracellcular trap formation with confocal microscopy. Functional polymorphisms in FCGR3B and FCAR differed between patient groups stratified by renal involvement. IgA ANCA were found in ∼30% of patients and were less common in patients with severe renal disease. Neutrophil stimulation by IgA or IgG ANCA led to degranulation and neutrophil extracellcular trap formation in a FcR allele-specific manner (IgA:FCAR P = 0.008; IgG:FCGR3B P = 0.003). When stimulated with IgA and IgG ANCA together, IgG ANCA induced neutrophil activation was reduced (P = 0.0001). FcR genotypes, IgA ANCA, and IgG ANCA are potential prognostic and therapeutic targets for understanding the pathogenesis and presentation of granulomatosis with polyangiitis (Wegener's).
Keywords: genetic association, functional genomics, Fc receptor alleles
Granulomatosis with polyangiitis (Wegener's) (GPA), formerly known as Wegener's granulomatosis (1), is a rare autoimmune vasculitis marked by neutrophil-related tissue damage to small- and medium-sized vessels. Patients with GPA can experience a broad range of clinical manifestations, predominately affecting the mucosal upper airways and kidneys (2). Over half of these patients develop some form of renal involvement, ranging in severity from mild renal insufficiency to rapidly progressing glomerulonephritis, culminating in end-stage renal disease (3, 4). The basis for the varied clinical manifestations and disease severity is not well understood but may result from both genetic and environmental factors (5).
Antineutrophil cytoplasmic antibodies (ANCA) are frequently observed in patients with GPA. ANCA primarily target proteinase 3 (PR3), a serine protease expressed in azurophilic granules of neutrophils. Serum anti-PR3 antibodies produce a cytoplasmic staining pattern on immunofluorescence assays, explaining the designation cANCA. These antibodies occur in over 90% of patients with active systemic disease and 40% of patients in remission (6), and may influence disease pathogenesis. When activated, neutrophils display the majority of granular PR3 on their cell-surface membranes (7); anti-PR3 antibodies then bind to the surface of primed neutrophils, resulting in initiation of subsequent neutrophil-effector programs, such as an oxidative burst (8).
Such ANCA-induced effector mechanisms involve engagement of activating IgG Fc receptors (FcRs) (9), namely FCGR2A (CD32A) and FCGR3B (CD16B) by anti-PR3 antibodies. Anti-PR3 antibodies preferentially engage FCGR3B because of its numeric predominance on the neutrophil cell surface (10). FCGR3B copy number variation (CNV) has been associated with development of systemic autoimmune conditions, including GPA (11). Within FCGR3B, two common genetic variants (named NA1 and NA2) influence the ANCA-effector response, with the NA1 allele producing stronger phagocytosis, respiratory burst, and neutrophil degranulation compared with the NA2 allele (12), suggesting these alleles influence the strength of ANCA-induced neutrophil activation and, subsequently, disease severity.
Ig isotypes of ANCA other than IgG have yet to be observed in patients with GPA. Given the mucosal manifestations of GPA, IgA is an isotype of interest; however, IgA ANCA have not been previously reported in GPA (13, 14). IgA can induce a wide range of immune mechanisms, including phagocytosis, respiratory burst, cytokine release, and antibody-dependent cell-mediated cytotoxicity (15), as well as mediate an anti-inflammatory response (16, 17); therefore, IgA ANCA are plausible mediators of disease activity and severity in GPA. Serum IgA ANCA have been associated with erythema elevatum diutinum (a neutrophil-associated vascu-litis of the skin) (18), cutaneous vasculitis (19), ulcerative colitis (20), and Henoch-Schönlein purpura (21). Some of these IgA ANCA-associated diseases have clinical presentations similar to GPA, with renal and mucosal involvement because of vascular damage from excessive neutrophil activation (4, 18). The proinflammatory and anti-inflammatory effects of IgA are determined through engagement with the FcR for IgA: FCAR (also known as FcαRI and CD89) (16). Alleles of a SNP in FCAR, rs16986050, alter the protein sequence of its cytoplasmic tail. The “A” (serine) allele results in less immune activation as measured by proinflammatory cytokine release and the “G” (glycine) allele causes cellular activation and increased phagocytosis (22). Similar to the biology of FCGR3B, IgA ANCA and FCAR genotypes may influence disease susceptibility and severity.
To better understand the pathogenesis, varied clinical presentations, and degrees of disease severity observed among patients with GPA, we sought to characterize potential FcR genotypes and ANCA isotypes influencing this disease. We studied biological materials and linked clinical data from two large cohorts: (i) the Wegener's Granulomatosis Genetics Repository (WGGER) that included 477 patients with GPA and 413 matched controls; and (ii) samples from patients with GPA enrolled in the longitudinal study of the Vasculitis Clinical Research Consortium (VCRC) (n = 263). With these samples and medical record data, we associated proinflammatory genetic variants of FCGR3B and FCAR with renal presentations and observed that serum IgA ANCA were present in patients with GPA. We showed that stimulation with IgA or IgG ANCA affected neutrophil activation measures, such as degranulation and neutrophil extracellular trap (NET) formation, in FcR-genotype–dependent fashion, suggesting that the pathogenesis of GPA is influenced by multiple ANCA isotypes and FcR genotypes.
Results
Patient Samples and Clinical Data.
We assembled samples and data from a total of 673 patients with GPA and 413 healthy controls from WGGER and VCRC. Chart review confirmed disease presence using the modified American College of Rheumatology diagnostic criteria and the Chapel Hill Consensus definitions of disease for vasculitis (2, 23).
WGGER is a cross-sectional collection of 477 patients and 413 healthy controls enrolled at the Beth Israel Medical Center (New York, NY), Boston University (Boston, MA), the Cleveland Clinic Foundation (Cleveland, OH), Duke University Medical Center (Durham, NC), John Hopkins University (Baltimore, MD), the Lahey Clinic (Burlington, MA), the Mayo Clinic (Rochester, MN), and the University of Alabama at Birmingham (Birmingham, AL). Of the patients, 463 (93%) are Caucasian, and 48% are male. Only self-identified Caucasians were analyzed in genetic studies.
The VCRC collects longitudinal clinical data and biological samples from patients with various vasculitides, including GPA, at Boston University (Boston, MA), the Cleveland Clinic (Cleveland, OH), The Johns Hopkins University (Baltimore, MD), the Mayo Clinic (Rochester, MN), McMaster University (Hamilton, ON, Canada), and the University of Toronto (Toronto, ON, Canada). There were 1,470 longitudinal samples from 263 GPA patients. Subjects with GPA averaged 5.6 visits (range 1–19; median, 4) with visit intervals most frequently at 3 mo but ranging from 1 mo to 1 y. Sixty-seven patients with GPA were enrolled in both WGGER and VCRC and, where appropriate, were included for analyses only within WGGER.
The patient characteristics in WGGER and VCRC are comparable in terms of the prevalence of upper airway and renal involvement, and are presented in Tables S1 and S2. In both sets of patients, slightly over half of patients with GPA exhibited renal involvement (WGGER: 58.5%; VCRC: 53.8%). Renal involvement was defined as “renal disease” in the medical record when not explained by other non-GPA factors. Among patients in WGGER, the mean peak serum creatinine recorded was 3.07 mg/dL (range: 0.7–22.0 mg/dL; median: 1.7 mg/dL). Of patients with GPA in WGGER, 83.1% manifested upper airway mucosal inflammation; 84.7% in VCRC manifested ear-nose-throat symptoms.
Proinflammatory FCGR3B NA1 Allele Associates with Severe Renal Disease.
Genotyping the NA1 allele of FCGR3B, which produces a stronger phagocytic, respiratory burst, and degranulation response compared with the NA2 allele (12), revealed similar allele frequencies for the WGGER (0.38), VCRC (0.36), and control (0.41) populations, indicating that this locus does not associate with overall disease susceptibility, similar to a previous study of 101 European patients with multiple forms of ANCA-associated vasculitis (24). However, among our patients with GPA, the presence of this proinflammatory allele was related to the presence of severe renal disease in GPA: 26% of NA1 homozygote-positive patients developed severe renal disease, compared with 11.5% among those not homozygous for NA1 (P = 0.064). Among patients in WGGER, the average peak serum creatinine level was also higher among the NA1 homozygotes (4.51 mg/dL) compared with all (renal and nonrenal) patients (3.07 mg/dL), or even with non-NA1 patients with renal disease (3.67 mg/dL) (data not available for VCRC). Homozygosity for the less activating allele, NA2, was present in 38% of patients with mucosal upper-airway manifestations compared with 30% of patients without this type of mucosal involvement (P = 0.091).
Because a null allele at FCGR3B has been associated with susceptibility to GPA in some studies but not in others, such structural variation could potentially confound our ability to calculate accurately the frequency of NA1/NA2 alleles. Using gene-specific primers in Pyrosequencing, we observed no difference in CNV between patients and controls, thereby negating any confounding effects of potential CNV (Fig. S1).
IgA ANCA Are Less Prevalent Among Patients with Severe Renal Disease.
IgA anti-PR3 antibodies were present in 27% of patients tested by ELISA in two GPA collections (WGGER: n = 335; VCRC: n = 196) (Table 1). Titers varied over time in samples from the same patient within the longitudinal VCRC. Because we defined positive as “ever positive,” the higher prevalence of IgA anti-PR3 antibodies in VCRC likely resulted from testing multiple samples. Over half of patients tested in the combined groups were positive for IgG anti-PR3 antibodies, consistent with previous studies (25). We confirmed a cytoplasmic staining pattern in selected IgA anti-PR3–positive samples using immunofluorescence assays. IgA and IgG ANCA both targeted PR3 instead of myeloperoxidase (MPO), defined by antigen-specific ELISA. Capture ELISAs using recombinant PR3 as antigen, with previously described methods (26), detected IgA anti-PR3 antibodies in 11 of 35 GPA patients (31.4%) from the Mayo Clinic (Rochester, MN), replicating a similar prevalence with independent samples and techniques.
Table 1.
IgA and IgG isotypes of anti-PR3 antibodies are present in patients with GPA
| Controls | WGGER | VCRC | |
| IgA positive only | 1 (1%) | 12 (3.6%) | 45 (17.1%) |
| IgG positive only | 1 (1%) | 151 (45.1%) | 80 (30.4%) |
| IgA and IgG positive | 0 (0%) | 49 (14.6%) | 56 (21.3%) |
| Negative | 97 (98%) | 123 (36.7%) | 82 (31.2%) |
| Total | 99 | 335 | 263 |
Data were obtained by ELISA. Of the 463 Caucasian patients with GPA in WGGER, 335 had single samples available. Patients from the VCRC cohort typically contributed multiple samples (263 participants, 1,470 samples), and a patient was counted as positive if any sample tested positive. Healthy controls were assayed to determine a threshold for positivity, as described in Methods.
We next determined the relationship of IgA anti-PR3 antibodies to renal and upper-airway manifestations. Within WGGER, 11.5% of IgA anti-PR3–negative patients developed severe chronic renal disease requiring dialysis, compared with 4.5% of IgA anti-PR3–positive patients (P = 0.059). Within VCRC, 20.8% of IgA anti-PR3–negative patients developed severe chronic renal disease requiring dialysis, compared with 4.9% of IgA anti-PR3–positive individuals (P = 0.006). Among WGGER patients, severe renal damage was defined as an irreversible dialysis requirement (end-stage renal disease), but among VCRC patients, severe renal disease may have improved reversing the requirement for dialysis.
Mucosal upper airway inflammation occurred in 98.0% of IgA anti-PR3–positive patients compared with 81.9% of IgA anti-PR3–negative patients in WGGER (P = 0.004). Within VCRC samples, development of ear-nose-throat involvement was not significantly stratified by presence of IgA anti-PR3 antibodies (84% vs. 86%). Therefore, the presence of IgA anti-PR3 antibodies is less frequently observed in individuals with severe renal disease and more common with mucosal upper airway manifestations.
Inflammatory Allele of FCAR Associates with Renal Disease in GPA.
We genotyped eight haplotype-tagging SNPs (htSNPs) covering FCAR (CD89) in patients with GPA and healthy controls. A coding SNP that alters the cytoplasmic tail of CD89, rs16986050, was associated with overall susceptibility to GPA (P = 0.00005, odds ratio: 1.61, 95% confidence interval: 1.27–2.05). Furthermore, its G allele, which generates an increased inflammatory response to IgA, was found in 18.9% patients with renal disease compared with 10.9% of patients with GPA without any renal manifestation (P = 0.01) (Table 2). Like FCGR3B, there were also significantly different frequencies for the proinflammatory allele between the mucosal upper airway involvement (15.4%) and nonmucosal (21.6%) patient groups (P = 0.02). We did not detect a significant difference in allele frequency among other htSNPs in FCAR.
Table 2.
Proinflammatory allele of FCAR associates with renal involvement in GPA
| Genotype |
Allele |
||||
| Cases | AA | AG | GG | A | G |
| WEGGER | |||||
| No renal involvement | 149 (78.4%) | 38 (20%) | 3 (1.6%) | 336 (88.4%) | 44 (11.6%) |
| Renal involvement | 171 (67.1%) | 77 (30.2%) | 7 (2.7%) | 419 (82.1%) | 91 (17.8%) |
| All cases | 320 (71.9%) | 115 (25.8%) | 10 (2.2%) | 755 (84.8%) | 135 (15.2%) |
| Controls | 249 (60.3%) | 149 (36.1%) | 15 (3.6%) | 647 (78.3%) | 179 (21.7%) |
| VCRC | |||||
| No renal involvement | 83 (83.8%) | 13 (13.1%) | 3 (3.0%) | 179 (90.4%) | 19 (9.6%) |
| Renal involvement | 55 (59.8%) | 34 (37.0%) | 3 (3.3%) | 144 (78.3%) | 40 (21.7%) |
| All cases | 135 (71.1%) | 49 (25.8%) | 6 (3.2%) | 319 (83.9%) | 61 (16.1%) |
| WGGER and VCRC | |||||
| No renal involvement | 232 (80.3%) | 51 (17.6%) | 6 (2.7%) | 515 (89.1%) | 63 (10.9%) |
| Renal involvement | 226 (65.1%) | 111 (32.0%) | 10 (2.9%) | 563 (81.1%) | 131 (18.9%) |
| All cases | 455 (71.7%) | 164 (25.8%) | 16 (2.5%) | 1074 (84.6%) | 196 (14.4%) |
| HapMap Caucasians | 75 (66.4%) | 32 (28.3%) | 6 (5.3%) | 182 (80.5%) | 44 (19.5%) |
The FCAR G allele (rs16986050), functionally related to proinflammatory neutrophil responses, associates with predisposition for renal involvement. All samples were genotyped using an Illumina GoldenGate BeadXpress system for WGGER and Pyrosequencing for VCRC. Sixty-seven patients with GPA were enrolled in both studies and have been excluded from the VCRC dataset here.
Neutrophil Activation Studies.
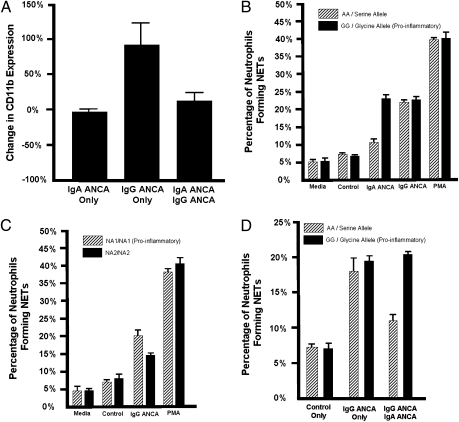
To characterize IgA and IgG ANCA as neutrophil activation stimuli, we incubated neutrophils in blood from healthy donors (n = 4) with column-purified IgA and IgG antibody fractions from patients with known high anti-PR3 titers and from ANCA-negative controls. We then measured the percent increase of degranulation marker expression (CD11b) by flow cytometry. Neutrophil stimulation by the IgG anti–PR3-containing antibody fraction resulted in a 100% CD11b increase compared with isotype control IgG; however, stimulation with the IgA anti-PR3–containing antibody fraction led to virtually no change in CD11b compared with control IgA (Fig. 1A), indicating that neutrophil activation is enhanced by stimulation from IgG ANCA but not IgA ANCA. Because activation was measured in neutrophils from donors with the more common yet less inflammatory FCAR A allele of rs16986050, we repeated using donors with the proinflammatory variant and found that IgA anti-PR3 antibody containing fractions can increase neutrophil degranulation, albeit in an allele-specific manner.
Fig. 1.
IgA ANCA, IgG ANCA, and their Fc receptors influence neutrophil activity. (A) Human IgG and IgA anti-PR3 containing antibody fractions impact degranulation in neutrophils from healthy donors (n = 4) measured as change in CD11b surface expression by flow cytometry. These differences in degranulation were compared with stimulation with IgG and IgA antibody fractions not containing anti-PR3. (B) When stratifying by FCAR genotype (rs16986050), the homozygous G allele, which results in a proinflammatory response, was associated with a higher percentage of cells with NET formation when stimulated with IgA anti-PR3 antibody fractions (P = 0.008). Stimulating with media or antibody fractions from healthy controls was used as a negative control. Phorbol myrisitate acetate (PMA) was used as a positive control. (C) When stratifying by FCGR3B NA1/NA2 genotype, the more proinflammatory NA1 genotype results in a higher percentage of cells with NET formation when stimulated by IgG anti-PR3 antibodies (P = 0.003). (D) Stimulating with IgA anti-PR3 antibody fractions can reduce the NET formation potential of IgG ANCA stimulation in a genetically dependent manner. Stimuli included antibody fractions from healthy controls, antibody fractions from IgG anti-PR3–positive patients, and a combination of antibody fractions from an IgG anti-PR3–positive patient and an IgA anti-PR3–positive patient. The presence of the A allele of rs16986050 in FCAR significantly reduced the potential of IgG anti-PR3 antibodies to induce NET formation when costimulated with IgA anti-PR3 antibodies (P = 0.0001).
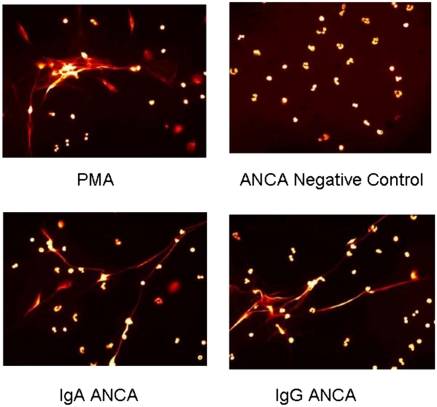
In addition to degranulation, when IgG ANCA stimulate neutrophils they release chromatin fibers known as NETs, which promote renal inflammation in small vessel vasculitis (27). We stimulated isolated neutrophils with IgA anti-PR3–containing fractions and observed enhanced NET formation in individuals with the proinflammatory FCAR allele (P = 0.008) (Fig. 1B and Fig. 2). Stimulation with IgG anti-PR3–containing fractions increases NET formation in FCGR3B NA1-homozygotes more than NA2-homozygous individuals (P = 0.003) (Fig. 1C).
Fig. 2.
Fluorescent microscopy displaying NET formation based on 180-min stimulation with control serum, PMA-positive control, IgA anti-PR3 antibody fractions, and IgG anti-PR3 antibody fractions.
When we stimulated neutrophils with IgA and IgG anti-PR3–containing fractions simultaneously, we measured a reduction in NET formation that was dependent upon FCAR genotype (P = 0.0001) (Fig. 1D) and a change in CD11b expression that was reduced compared with stimulation with the IgG anti-PR3 fraction alone (P = 0.031) (Fig. 1A).
Conclusions
The pathogenesis of GPA is complex, involving multiple genetic and environmental factors (5). Our finding of IgA ANCA in patients with GPA, their association with clinical presentation, and the genetic predisposition conferred by their cognate receptor, uncover unique pathogenic mechanisms and offer a previously undescribed framework for understanding the biology of ANCA and GPA. Additional study of IgA ANCA, IgG ANCA, and FcR genotypes may leverage this framework to develop future prognostic tools and point to novel therapeutic interventions.
The role of ANCA in GPA appears dependent upon interactions with FcRs. When neutrophils are simultaneously stimulated with IgG ANCA and FcR blocking agents, such as the IgG:FcR engagement-inhibiting peptide TG19320 or soluble IgG FcRs (FCGR2A/FCGR2B), there is a dose-dependent reduction in degranulation measured as change in CD11b expression, demonstrating IgG ANCA activation signals via a FcR (28). We found that genotypes of one such FcR, specifically the NA1 allele of FCGR3B, influences development of severe renal disease, suggesting therapeutic opportunities for FcR blockers such as TG19320 in GPA-related renal disease, particularly in NA1-positive patients. The mechanisms underlying the effects of IgA ANCA on inflammation also likely involve FcR engagement, given the different levels of NET formation among individuals according to the presence of the functional FCAR allele (rs16986050). In addition, the dual inhibiting/activating potential of FCAR to regulate immunoreceptor tyrosine-based activation motif (ITAM) function (16), or cross-regulation between ITAM-containing receptors, such as FcRs (29), may provide a mechanism for IgA ANCA and FcRs to modulate disease severity.
The role of ANCA in GPA also involves more than the IgG isotype. Although IgA ANCA are underrepresented in patients with severe renal disease, their contribution to the pathogenesis of GPA involves interaction with both IgG ANCA and FcRs. IgA ANCA may compete with IgG ANCA for PR3 binding, and therefore limit IgG ANCA engagement, subsequent neutrophil activation, and resulting renal damage, given the robust neutrophil response to IgG ANCA stimulation and the reduced neutrophil activation when stimulated by both antibody classes. Such evidence that IgA ANCA can reduce IgG ANCA-related proinflammatory neutrophil responses suggests potential for IgA:FcR-based therapies. The cytoplasmic tail of FCAR can signal via Bruton a-γ-globulinemia tyrosine kinase (BTK) activation in an allele-dependent manner. Because the FCAR SNP we associated with renal severity influences cytoplasmic tail function, a BTK inhibitor currently in preclinical testing for autoimmune diseases may provide another treatment option for GPA (30). Furthermore, given the fluctuation in IgA anti-PR3 antibody levels (and presence) within individual patients measured at sequential office visits, similar to that observed with IgG ANCA (6, 31), the prevalence of IgA ANCA in GPA may be higher than reported and impact disease pathogenesis in a greater percentage of patients.
The pathogenesis of GPA is complex and multifaceted, involving more than IgA and IgG ANCA-induced neutrophil activation. Factors influencing NET degradation, such as inhibitors (e.g., antibodies) preventing DNase1 break-down of NETs, which are implicated in lupus nephritis (32), may be important in autoimmune renal diseases such as GPA. Understanding such additional aspects of the etiology of GPA, along with appreciating a role for IgA ANCA, IgG ANCA, and their respective FcRs, may provide new insights into the pathogenesis and suggest new options for the diagnosis and treatment of granulomatosis with polyangiitis.
Methods
All patients were recruited with informed consent with the approval of each respective Institutional Review Board. Genomic DNA was isolated from EDTA-treated whole blood using the PureGene DNA Isolation Kit (Gentra Systems) and plasma removed by standard methods. After extraction, genomic DNA, buffy coats, and plasma were stored at −80 °C.
ELISAs were performed in duplicate following the manufacturer's protocol on QUANTA Lite PR-3 and MPO ELISA kits (INOVA Diagnostics). Because IgA anti-PR3/anti-MPO positivity is a unique finding in GPA, the manufacturer's protocol does not evaluate IgA. We substituted with a manufacturer-provided HRP IgA conjugate validated for the Gliadin IgA ELISA kit (INOVA). For IgG, the kits establish positivity by comparing patient specific spectrophotometric values to those measured from provided standards (low and high positive). To determine a positive cutoff value for IgA, we first performed ELISAs (both IgA and IgG) on 90 WGGER control samples; we defined a positive threshold as a value above the average spectrophotometric values (OD450) from the 90 controls plus two SDs. To test this definition, we applied it to all IgG-based ELISAs and found 98% agreement between this method and the manufacturer's method to define positivity. For replication, c-myc capture ELISAs using recombinant PR3 as antigen were performed on 35 patients with GPA taken from the Wegener's Granulomatosis Etanercept Trial (33) in a separate laboratory (the Mayo Clinic) (34).
Immunofluorescence assays were performed using neutrophil substrate from NOVA Lite ANCA (INOVA) and read on a Nikon fluorescence microscope. We used goat anti-human anti-IgG conjugated with FITC (INOVA) and goat anti-human anti-IgA conjugated with Texas Red (Southern Biotech) as secondary antibodies.
Genotyping of htSNPs spanning FCAR was performed using an Illumina GoldenGate BeadXpress system following all manufacturer's protocols on 463 Caucasian patients and 413 Caucasian controls from WGGER and using Pyrosequencing (Qiagen) for 261 Caucasian patients from VCRC. htSNPs were selected using pair-wise comparisons with the Tagger algorithm using a r2 threshold of 0.8 and minor allele frequencies ≥ 0.05 in the HapMap Caucasian population. SNPs genotyped included: rs11666735, rs12975083, rs16986050, rs1865096, rs2304225, rs3816051, rs4806608, and rs7259347. One SNP (rs11666735) was not compatible with Illumina and was genotyped with a TaqMan assay (Applied Biosystems) using an ABI7900HT. All SNPs were in Hardy–Weinberg equilibrium. Assays to determine FCGR3B genotype were performed as previously described (28). In brief, we used allele-specific PCR reactions and direct sequencing of gene-specific amplicons to determine the FCGR3B NA1 and NA2 alleles. The PCR products were gel purified with the QIAquick Gel Extraction Kit before sequencing. Sequencing was performed on an Applied Biosystems 377. Determination of CNV at FCGR3B was determined by Pyrosequencing (Qiagen).
For neutrophil activation studies that measured degranulation (CD11b surface expression), we examined neutrophils in washed whole blood (28). In short, EDTA anticoagulated blood was chilled to 4 °C, washed twice in modified PBS (125 mM sodium chloride, 10 mM phosphate, 5 mM potassium chloride, 5 mM glucose, pH 7.35) kept at 4 °C, and then resuspended in the original volume. Washed cells were preincubated for 40 min at 37 °C to induce cell surface expression of PR3. Aliquots were then incubated with or without anti-PR3 mAb CLB 12.8 (10 μg/mL) or anti-PR3 containing antibody fractions for 45 min. We purchased anti-CD11b monoclonal antibodies from Caltag and anti-PR 3 mAb CLB 12.8 (mIgG1) from Research Diagnostics. Human anti-PR3 containing antibody fractions (IgA and IgG) were jacalin and protein G Sepharose column purified from two patients with known high measurements of anti-PR3 antibody; each sample was further reversely depleted to avoid contamination. We confirmed the isolation by SDS/PAGE and Western blot using Fcα- and Fcγ-specific antibodies (Jackson ImmunoResearch Laboratories) and confirmed reactivity with anti-PR3 ELISA. Endotoxin levels in all reagents were below detection limits by the limulus ameobocyte assay (Sigma). After stimulation with antibody fractions, samples were then treated with FACS Lysing Solution (Becton Dickinson Immunocytometry) for 10 min at room temperature, washed once, and analyzed by flow cytometry (FACSCaliber; Becton Dickinson). Neutrophils were identified by characteristic light-scatter properties and FCGR staining with mAbs IV.3 and 3G8 (Medarex). As previously reported, cell-surface expression of PR3 displayed a bimodal pattern that confirmed presence of ANCA target (28). Analysis of flow cytometry data were performed using CellQuest (Becton Dickinson). The results are expressed as mean fluorescence intensity of the histogram data.
To measure NETs, neutrophils isolated via a Ficoll-Hypaque density gradient (22) were primed with TNF and then IgA and IgG antibody fractions from healthy controls and from patients with known high anti-PR3 ELISA titers (either IgA or IgG), produced as described above. As previously reported, cell-surface expression of PR3 displayed a bimodal pattern (28). Analysis of flow cytometry data were performed using CellQuest (Becton Dickinson). The results are expressed as mean fluorescence intensity of the histogram data. These experiments were conducted using neutrophils isolated from three healthy volunteer donors with each genotype of interest.
Genotype and allele frequencies were determined by direct counting. Statistical analyses for genotype distribution were performed using χ2 for genotype based analyses with Prism GraphPad v4.03. For statistical analysis of neutrophil activation studies, we used a paired t test. To estimate statistical power to detect association at rs16986050, we calculated power as 0.9203 with α= 0.05 (35).
Supplementary Material
Acknowledgments
We thank Debbie McDuffie and Judy Cain [University of Alabama at Birmingham (UAB)] for technical support, Ed Phillips (UAB) for assistance with microscopy, and all of the patients and normal donors for their participation in these studies. The Vasculitis Clinical Research Consortium has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319), the National Center for Research Resources (U54 RR019497), and the Office of Rare Diseases Research. The Vasculitis Clinical Research Consortium is part of the Rare Diseases Clinical Research Network. This work was funded by National Institutes of Health Grants R01-AR33062 and R01-AR47799, as well as by support from the Ruthrauff family; the Methodology Core of the UAB Multidisciplinary Clinical Research Center was supported by Grant P60-AR48095; the Flow Cytometry Core of the UAB Rheumatic Diseases Core Center by Grant P30-AR48311; and the specimen repository was supported, in part, by Grants M01-RR00032 and 5UL1RR025777. J.M.K. was supported by Grant T32-AR07450, the University of Alabama Health Sciences Foundation, and the American College of Rheumatology Research and Education Fund; and P.A. Monach was supported by the Arthritis Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109227109/-/DCSupplemental.
References
- 1.Falk RJ, et al. American College of Rheumatology American Society of Nephrology European League Against Rheumatism Granulomatosis with polyangiitis (Wegener's): An alternative name for Wegener's granulomatosis. Arthritis Rheum. 2011;63:863–864. doi: 10.1002/art.30286. [DOI] [PubMed] [Google Scholar]
- 2.Leavitt RY, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 3.Renaudineau Y, Le Meur Y. Renal involvement in Wegener's granulomatosis. Clin Rev Allergy Immunol. 2008;35:22–29. doi: 10.1007/s12016-007-8066-6. [DOI] [PubMed] [Google Scholar]
- 4.Reinhold-Keller E, et al. An interdisciplinary approach to the care of patients with Wegener's granulomatosis: Long-term outcome in 155 patients. Arthritis Rheum. 2000;43:1021–1032. doi: 10.1002/1529-0131(200005)43:5<1021::AID-ANR10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Mahr AD, Neogi T, Merkel PA. Epidemiology of Wegener's granulomatosis: Lessons from descriptive studies and analyses of genetic and environmental risk determinants. Clin Exp Rheumatol. 2006;24(2) Suppl 41:S82–S91. [PubMed] [Google Scholar]
- 6.Nölle B, et al. Anticytoplasmic autoantibodies: Their immunodiagnostic value in Wegener granulomatosis. Ann Intern Med. 1989;111:28–40. doi: 10.7326/0003-4819-111-1-28. [DOI] [PubMed] [Google Scholar]
- 7.Witko-Sarsat V, et al. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J Am Soc Nephrol. 1999;10:1224–1233. doi: 10.1681/ASN.V1061224. [DOI] [PubMed] [Google Scholar]
- 8.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porges AJ, et al. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994;153:1271–1280. [PubMed] [Google Scholar]
- 10.Kocher M, Edberg JC, Fleit HB, Kimberly RP. Antineutrophil cytoplasmic antibodies preferentially engage Fc gammaRIIIb on human neutrophils. J Immunol. 1998;161:6909–6914. [PubMed] [Google Scholar]
- 11.Fanciulli M, et al. FCGR3B copy number variation is associated with susceptibility to systemic, but not organ-specific, autoimmunity. Nat Genet. 2007;39:721–723. doi: 10.1038/ng2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmon JE, Edberg JC, Kimberly RP. Fc gamma receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J Clin Invest. 1990;85:1287–1295. doi: 10.1172/JCI114566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennings JG, Chang L, Savige JA. Anti-proteinase 3 antibodies, their characterization and disease associations. Clin Exp Immunol. 1994;95:251–256. doi: 10.1111/j.1365-2249.1994.tb06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baltaro RJ, et al. Immunoglobulin G antineutrophil cytoplasmic antibodies are produced in the respiratory tract of patients with Wegener's granulomatosis. Am Rev Respir Dis. 1991;143:275–278. doi: 10.1164/ajrccm/143.2.275. [DOI] [PubMed] [Google Scholar]
- 15.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasquier B, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: Dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 18.Ayoub N, et al. Antineutrophil cytoplasmic antibodies of IgA class in neutrophilic dermatoses with emphasis on erythema elevatum diutinum. Arch Dermatol. 2004;140:931–936. doi: 10.1001/archderm.140.8.931. [DOI] [PubMed] [Google Scholar]
- 19.Rovel-Guitera P, et al. IgA antineutrophil cytoplasmic antibodies in cutaneous vasculitis. Br J Dermatol. 2000;143:99–103. doi: 10.1046/j.1365-2133.2000.03597.x. [DOI] [PubMed] [Google Scholar]
- 20.Gigase P, et al. Anti-neutrophil cytoplasmic antibodies in inflammatory bowel disease with special attention for IgA-class antibodies. Dig Dis Sci. 1997;42:2171–2174. doi: 10.1023/a:1018803509150. [DOI] [PubMed] [Google Scholar]
- 21.Ozaltin F, et al. The significance of IgA class of antineutrophil cytoplasmic antibodies (ANCA) in childhood Henoch-Schönlein purpura. Clin Rheumatol. 2004;23:426–429. doi: 10.1007/s10067-004-0910-y. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, et al. FcalphaRI (CD89) alleles determine the proinflammatory potential of serum IgA. J Immunol. 2007;178:3973–3982. doi: 10.4049/jimmunol.178.6.3973. [DOI] [PubMed] [Google Scholar]
- 23.Jennette JC, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 24.Tse WY, et al. No association between neutrophil FcgammaRIIa allelic polymorphism and anti-neutrophil cytoplasmic antibody (ANCA)-positive systemic vasculitis. Clin Exp Immunol. 1999;117:198–205. doi: 10.1046/j.1365-2249.1999.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368:404–418. doi: 10.1016/S0140-6736(06)69114-9. [DOI] [PubMed] [Google Scholar]
- 26.Finkielman JD, et al. ANCA are detectable in nearly all patients with active severe Wegener's granulomatosis. Am J Med. 2007;120:643.e649–e614. doi: 10.1016/j.amjmed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka S, Edberg JC, Chatham W, Fassina G, Kimberly RP. Fc gamma RIIIb allele-sensitive release of alpha-defensins: anti-neutrophil cytoplasmic antibody-induced release of chemotaxins. J Immunol. 2003;171:6090–6096. doi: 10.4049/jimmunol.171.11.6090. [DOI] [PubMed] [Google Scholar]
- 29.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honigberg LA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tervaert JW, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–2465. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 32.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wegener's Granulomatosis Etanercept Trial (WGET) Research Group Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med. 2005;352:351–361. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 34.Finkielman JD, et al. WGET Research Group Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147:611–619. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- 35.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: Design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.