Abstract
Background
Atherosclerosis is a chronic inflammatory disease of artery wall characterized by infiltration of monocytes into subendothelial space and their differentiation into macrophages. Since rupture-prone plaques commonly contain high amounts of activated macrophages, imaging of the macrophage content may provide a useful tool for the evaluation of plaque vulnerability. The purpose of this study was to explore the uptake of 68gallium (68Ga) in atherosclerotic plaques in mice.
Methods
Uptake of ionic 68Ga was investigated in atherosclerotic LDLR-/-ApoB100/100 and C57BL/6N control mice at 3 h after injection. The ex vivo biodistribution of the 68Ga was assessed and autoradiography of aortic cryosections was defined. In vivo imaging of 68Ga was performed using a small animal positron emission tomography PET/CT scanner.
Results
Our results revealed that the uptake of 68Ga-radioactivity was higher in atherosclerotic plaques than in healthy vessel wall (ratio 1.8 ± 0.2, p = 0.0002) and adventitia (ratio 1.3 ± 0.2, p = 0.0011). The autoradiography signal co-localized with macrophages prominently as demonstrated by Mac-3 staining. In both mice strains, the highest level of radioactivity was found in the blood.
Conclusions
We observed a moderate but significantly elevated 68Ga-radioactivity uptake in the aortic plaques of atherosclerotic mice, especially at the sites rich in macrophages. While the uptake of 68Ga was promising in this animal model, the slow blood clearance may limit the usability of 68Ga as a PET tracer for clinical imaging of atherosclerotic plaques.
Keywords: atherosclerosis, plaque, autoradiography, gallium-68, PET
Background
Atherosclerosis is a chronic inflammatory disease of artery wall with infiltration of monocytes into subendothelial space and their differentiation into macrophages. Macrophages express pro-inflammatory and chemotactic molecules thus playing an essential role in the formation, progression, and pathogenicity of atherosclerotic plaques. The population of macrophages in atherosclerotic lesions is heterogeneous. The majority of macrophages transform into foam cells by accumulating lipids in their cytoplasm, while other macrophages may have vascular protective and repair properties. Furthermore, some macrophages may secrete enzymes that degrade the extracellular matrix and fibrous cap, possibly leading to a rupture of the atherosclerotic plaque. Significantly higher quantities of macrophage-rich plaque areas have been reported in atherosclerotic patients with acute coronary syndromes than in patients with stable angina. Since the rupture-prone plaques commonly contain high amounts of activated macrophages, imaging of the macrophages may provide a useful tool for determining plaque vulnerability [1-5].
68Gallium (68Ga, T1/2 68 min) is a generator-produced, positron-emitting radionuclide with a high positron yield (89%) possessing suitable physical properties for in vivo PET imaging. 67Gallium (67Ga) has traditionally been used to image inflammation with gamma cameras. Since 68Ga owns the same chemical characteristics as 67Ga, it is assumed that ionic 68Ga could accumulate at the sites of macrophage-rich areas in inflammatory lesions. Furthermore, 68Ga-based PET may have some added advantages compared to 67Ga-based SPECT, such as a better resolution for the detection of inflammatory plaques [6]. The aim of this study was to determine the uptake of ionic 68Ga in the atherosclerotic plaques in mice.
Methods
Production of ionic 68Ga
68Ga radionuclide is readily available by elution from a 68Ge/68Ga generator (Cyclotron Co., Obninsk, Russia) with 0.1 M HCl. The radioactive elution peak, monitored online with a positron-sensitive photodiode detector (Hamamatsu S5591, Hamamatsu Photonics K.K. Solid State Division, Hamatsu City, Japan), was collected and the 68Ga-chloride was neutralized with 1 M NaOH to give pH value of 7, as described earlier [7]. The quantity of colloidal 68Ga in the final product was measured by ultrafiltration, as indicated by the activity retained on the 0.2- μm ultrafilter. The final 68Ga product contained 13% of colloidal forms of 68Ga.
Ex vivo biodistribution of 68Ga
Nine 10-11-months-old male atherosclerotic LDLR-/-ApoB100/100 mice (weight 35 ± 5 g, mean ± SD) [8,9] and six 2.5-month-old male non-atherosclerotic C57BL/6N control mice (weight 28 ± 1 g) were ex vivo investigated. LDLR-/-ApoB100/100 mice were kept on a high-fat, Western-type diet (TD 88173, Harlan Teklad) for 3-4 months, starting at 7 months of age. C57BL/6N mice were fed with regular diet. The experiments were approved by the Laboratory Animal Care and Use Committee of the University of Turku, Finland.
68Ga (17 ± 2 MBq) was intravenously administered via a tail vein in non-anesthetized mice. Three hours after injection the mice were sacrificed under deep isoflurane anesthesia by cervical dislocation. Samples of blood and various tissues were excised, patted dry, weighed, and measured for radioactivity using an automatic gamma counter (1480 Wizard 3" Gamma Counter; EG & G Wallac, Turku, Finland) cross-calibrated with a dose calibrator (VDC-202, Veenstra Instruments, Joure, The Netherlands). The radioactivity remaining in the tail was compensated, background counts were subtracted, and the radioactivity decay was corrected to the time of injection. The concentration of the radioactivity accumulated in the various tissues over the 3-h period following 68Ga injection was expressed as a percentage of the injected activity per gram of tissue (% IA/g).
Autoradiography analysis of aortic cryosections
The distribution of 68Ga-radioactivity in the aortic tissue was studied by combining digital autoradiography and hematoxylin and eosin (HE) staining. The aorta and internal control muscle sample from the same animal were frozen and sequential longitudinal 8- and 20- μm sections were cut with a cryomicrotome at -15°C, thaw-mounted onto microscope slides. The sections were air dried for 5 min and apposed to an imaging plate (Fuji Imaging Plate BAS-TR2025, Fuji Photo Film Co., Ltd., Japan). After an exposure time of 2.5 h, the imaging plates were scanned with Fuji Analyser BAS-5000 (Fuji Tokyo, Japan; internal resolution 25 μm). The 20- μm cryosections were stained with HE and examined for morphology under a light microscope. After carefully co-registration of the autoradiographs and HE images, 68Ga-radioactivities were measured in the following regions of interest (ROIs): (1) plaque (excluding media), (2) healthy vessel wall (no lesion formation), (3) adventitia (including adjacent fat), and (4) muscle (internal control tissue). In control mice, no plaque areas were seen and ROIs were defined for healthy vessel wall, adventitia, and muscle. In each mouse, the uptakes of 68Ga-radioactivities in the regions of interest were calculated and normalized against internal control tissue (muscle). The results of digital autoradiography analysis were expressed as photostimulated luminescence per square millimeter (PSL/mm2).
Assessment of degree of inflammation in atherosclerotic plaques
Immunohistochemical staining with the mouse macrophage specific antibody (Mac-3, clone m3/84, BD Pharmingen, BD550292, USA - BD Biosciences Pharmingen, Franklin Lakes, NJ, USA) was performed using aortic longitudinal 8- μm cryosections in order to detect activated macrophages, as described earlier [10]. Based on the Mac-3 staining, plaque areas were divided into four categories for the semiquantitative estimation of the degree of plaque inflammation; (1) no inflammation = no macrophages, (2) mild inflammation = occasional macrophages, (3) moderate inflammation = occasional and some groups of macrophages, (4) severe inflammation = abundant infiltration of macrophages. The uptake of 68Ga-radioactivity in the different categories was studied with digital autoradiography from 8- μm cryosections combined with images of Mac-3 immunostained sections. The results of digital autoradiography analysis were normalized against internal control tissue (muscle) and expressed as PSL/mm2.
In vivo imaging
Three additional female LDLR-/-ApoB100/100 mice, aged 14 to 15 months and kept on a high-fat, Western-type diet for 5 to 6 months (weight 36 ± 10 g, mean ± SD) were in vivo imaged with the Inveon small animal PET/CT scanner (Siemens Medical Solutions, Knoxville, TN, USA) at 3 h after injection of 68Ga (16 ± 3 MBq). PET images were acquired for 15 min, followed by CT angiography (10 min) without moving the animal. To obtain vascular contrast, 0.2 ml of iodinated intravascular contrast agent eXIATM160XL (Binitio Biomedical Inc, Ottawa, ON, Canada) was injected. CT acquisition consisted of 270 projections acquired with exposure time of 400 ms, x-ray voltage of 80 kVp, and anode current of 400 μA for a full 360° rotation. Mice were kept fully sedated with 1.5% isoflurane during imaging. PET image was reconstructed using OSEM3D algorithm. The resulting matrix was 128 × 128 pixels with 159 transverse slices in a transaxial field of view of 12.7 cm (pixel size 0.87 × 0.87 × 0.80 mm). CT image was reconstructed using a modified Feldkamp algorithm. The resulting matrix was 256 × 256 pixels with 384 transverse slices (pixel size 0.17 × 0.17 × 0.17 mm). Co-registration of PET and CT images was done using an automatic weighted mutual information algorithm and confirmed visually on the basis of anatomical landmarks. Quantitative PET analysis was performed by drawing ROIs of the same size in the left ventricle (blood pool), aortic arch, and brachiocephalic artery as identified on the basis of the CT angiography by using the Inveon Research Workplace software (Siemens Medical Solutions, Knoxville, TN, USA). Radioactivity concentrations were corrected for injected radioactivity, and the results were expressed as percentage of injected radioactivity per gram of tissue (% IA/g) assuming 1 mL equals 1 g.
Statistical methods
All the results are expressed as mean ± SD. Student's t-test and one-way ANOVA were applied when comparing the biodistribution results of the atherosclerotic and control mice. T test with a mixed Dunnett's model was used to correct the p values of biodistribution of aortic uptake versus different tissue uptakes in the atherosclerotic and control mice groups. In the autoradiographic analysis, a mixed model with Tukey-Kramer corrected p values was applied to individual mean values of ROIs. A p value less than 0.05 was considered as statistically significant.
Results
Characterization of atherosclerotic plaques
After 3 to 4 months on a Western-type diet, the LDLR-/-ApoB100/100 mice had developed areas covered with atherosclerotic plaques in aortic arch, aortic arteries, and also in the distal part of aorta. Histologically, the plaques were of fibroatheroma type and some of them contained calcifications. In the aortas of the control mice, no atherosclerotic plaques were detected.
Ex vivo biodistribution of 68Ga-radioactivity
The biodistribution of 68Ga-radioactivity at 3 h after i.v. injection is shown in Table 1. The highest level of 68Ga-radioactivity was found in the blood in both mouse strains. The uptake in the whole aorta was higher in LDLR-/-ApoB100/100 mice than in control mice, but the difference did not reach statistical significance. The aorta/heart and aorta/blood uptake ratios were 1.6 ± 0.7 and 0.2 ± 0.1 in atherosclerotic mice and 1.2 ± 0.2 and 0.2 ± 0.1 in control mice, respectively. The measured radioactivities in the bone and liver of atherosclerotic mice were significantly lower than in the control mice.
Table 1.
Ex vivo biodistribution of radioactivity in atherosclerotic LDLR-/-ApoB100/100 and C57BL/6N control mice
|
LDLR
-/-
/ApoB
100/100
(n = 9, urine n = 8) |
C57BL/6N (n = 6, urine n = 5) |
p value | |
|---|---|---|---|
| Aorta | 1.99 ± 0.73 | 1.52 ± 0.40 | NSa |
| Bladder | 2.50 ± 0.62 | 1.92 ± 0.29 | NS |
| Blood | 8.60 ± 1.29 | 8.07 ± 0.55 | NS |
| Bone | 2.25 ± 0.50 | 4.08 ± 0.49 | < 0.05 |
| Fat | 0.46 ± 0.21 | 0.51 ± 0.07 | NS |
| Heart | 1.26 ± 0.26 | 1.29 ± 0.20 | NS |
| Intestine | 2.71 ± 0.70 | 3.16 ± 0.36 | NS |
| Kidney | 4.07 ± 0.78 | 4.25 ± 0.33 | NS |
| Liver | 2.69 ± 1.14 | 8.34 ± 4.33 | < 0.05 |
| Lungs | 3.28 ± 1.10 | 3.58 ± 0.33 | NS |
| Lymph nodes | 2.03 ± 0.51 | 2.35 ± 0.34 | NS |
| Muscle | 0.71 ± 0.24 | 0.71 ± 0.08 | NS |
| Pancreas | 1.21 ± 0.25 | 1.48 ± 0.21 | NS |
| Spleen | 3.20 ± 1.28 | 4.51 ± 1.63 | NS |
| Thymus | 1.89 ± 0.96 | 1.18 ± 0.15 | NS |
At 3 h after intravenous injection of ionic 68Ga. Results are expressed as % IA/g (mean ± SD). NS, not significant. aDifference between atherosclerotic and control mice.
Digital autoradiography of aortic cryosections
A total of 8 to 12 autoradiographs of aortic cryosections from eight LDLR-/-ApoB100/100 mice (one sample was discarded from the analysis because of remaining blood clot inside the aorta) and 8 autoradiographs of aortic sections from six control mice were generated and superimposed on corresponding digitalized HE staining images. In total, 1,208 ROIs (plaque n = 384, healthy vessel wall n = 396, adventitia n = 406, muscle n = 56) were analyzed from 14 mice. The autoradiography analysis revealed a significantly higher uptake of 68Ga-radioactivity in plaques compared with healthy vessel wall (3.4 ± 0.6 vs. 1.9 ± 0.3 PSL/mm2, p = 0.0002) and adventitia (3.4 ± 0.6 vs. 2.6 ± 0.3 PSL/mm2, p = 0.0011). The plaque/wall ratio was 1.8 ± 0.2 and plaque/adventitia ratio 1.3 ± 0.2 in LDLR-/-ApoB100/100 mice (Figure 1). The difference between the adventitia and the healthy vessel wall was not statistically significant in either of the mouse strains. A relatively high uptake of 68Ga-radioactivity was detected in the calcified areas (n = 55 from the total 384 plaque ROIs) of atherosclerotic plaques vs. healthy vessel wall (ratio 2.1 ± 0.6, p = 0.0010). Calcified areas did not contain any macrophages. No statistically significant difference was detected in calcified areas of plaques vs. non-calcified plaques. The variability of the used digital autoradiography analysis has been tested and described earlier [10].
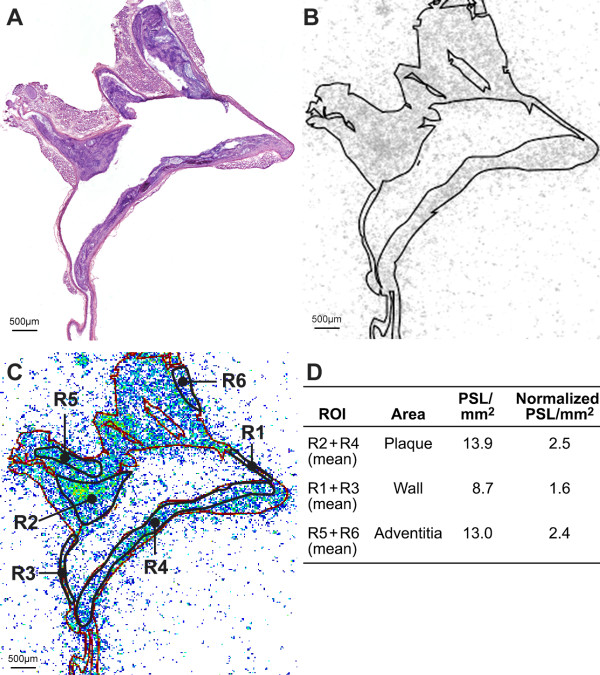
Figure 1.
An example of ex vivo aortic autoradiographic (ARG) analysis. (A) HE staining of a 20-μm cryosection, showing the aortic arch and branches of arteries (B) autoradiograph (grayscale) from section (A) superimposed with the patch image made from the HE-stained section (A). (C) Autoradiograph superimposed with contour image and an example of ROI analysis: R2 and R4, plaque; R1 and R3, healthy vessel wall; R5 and R6, adventitia. (D) Panel shows the ARG results of section (C). Results are expressed as PSL/mm2 (mean ± SD), normalized against the internal control tissue (muscle). In this representative section, the plaque/wall ratio is 1.6 and plaque/adventitia ratio 1.1, whereas in the whole study population, the corresponding ratios were 1.8 ± 0.2 and 1.3 ± 0.2.
Assessment of 68Ga-radioactivity uptake in macrophage-rich areas of atherosclerotic plaques
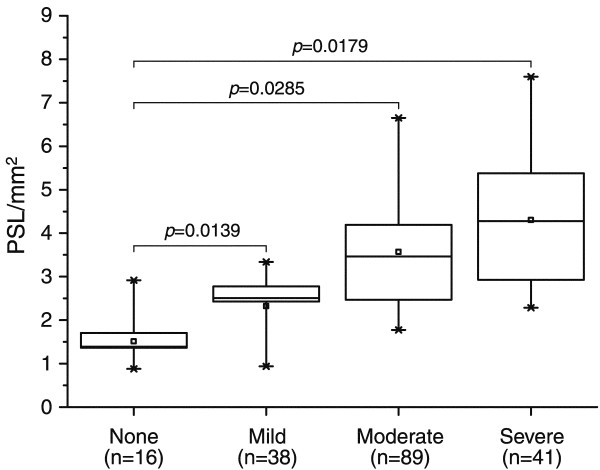
A total of 184 plaques obtained from four aortic cryosections of each LDLR-/-ApoB100/100 mouse were analyzed and normalized against internal control tissue (muscle). According to the autoradiographic analysis, the uptake of 68Ga in plaques with different degrees of inflammation was 1.5 ± 0.7 PSL/mm2 in non-inflamed plaques (n = 16), 2.3 ± 0.8 PSL/mm2 in mildly inflamed plaques (n = 38), 3.6 ± 1.5 PSL/mm2 in moderately inflamed plaques (n = 89), and 4.3 ± 1.9 PSL/mm2 in severely inflamed plaques (n = 41). Figure 2 shows the co-localisation of the 68Ga-radioactivity uptake and degree of macrophage-rich areas in atherosclerotic plaques. Representative Mac-3 stainings of non-inflamed and severely inflamed plaques are shown in Figure 3.
Figure 2.
Uptake of 68Ga-radioactivity and the degree of inflammation in atherosclerotic plaques. Uptake of 68Ga-radioactivity in atherosclerotic plaques of LDLR-/-ApoB100/100 mice in comparison to the degree of inflammation assessed on the basis of Mac-3 immunohistochemistry. None, no macrophages; Mild, occasional macrophages; Moderate, occasional and some groups of macrophages; Severe, abundant infiltration of macrophages. The 68Ga-radioactivity uptake results are expressed as PSL/mm2(mean ± SD), normalized against internal control tissue (muscle).
Figure 3.
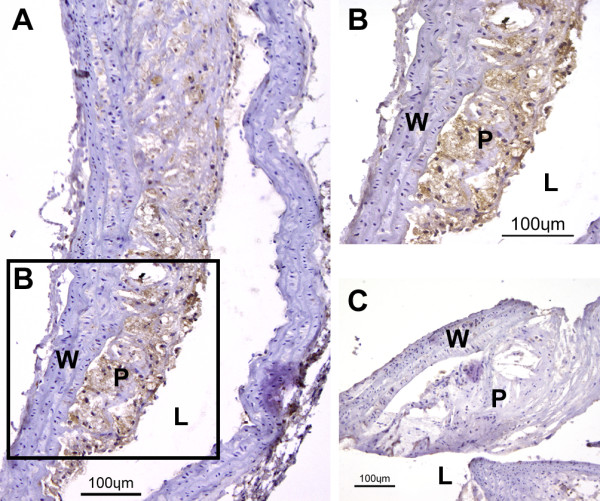
Mac-3 immunostained atherosclerotic plaques. (A) Representative Mac-3 immunostainings of severely inflamed plaque. Macrophages are shown in brown color. Adventitia and healthy vessel wall areas were Mac-3 negative. (B) An enlarged area rich in macrophages marked in (A). (C) Non-inflamed plaque with only scanty immunopositivity for Mac-3. L, lumen; P, plaque; W wall.
In vivo imaging
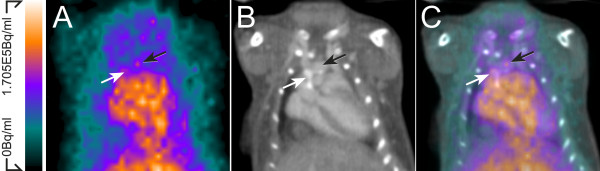
In vivo PET/CT imaging demonstrated high radioactivity in the blood pool (8.1 ± 2.2% IA/g, n = 3) at 3 h after 68Ga injection as seen in the aorta and heart of LDLR-/-ApoB100/100 mouse (Figure 4). The in vivo uptake of 68Ga-radioactivity in the aortic arch and brachiocephalic artery was 6.4 ± 3.0 and 5.8 ± 2.5% IA/g, respectively.
Figure 4.
In vivo imaging. (A) In vivo PET, (B) CT angiography, and (C) fused PET/CT images demonstrate the high 68Ga signal in the blood pool as seen in the aorta and heart. Calcified (white arrows) and non-calcified (black arrows), aortic arch and aortic root can be seen in contrast-enhanced CT angiography.
Discussion
Macrophages play a crucial role in the progression and development of atherosclerosis from early fatty-streak lesions to advanced atherosclerotic plaques [11]. Ruptured plaques, in particular, commonly contain high quantities of macrophages [3]. Therefore, the contents of macrophages may be an excellent imaging biomarker for the vulnerability of atherosclerotic plaques. The present results revealed that the 68Ga-radioactivity uptake in atherosclerotic plaques, especially in their macrophage-rich areas, was higher when compared to healthy vessel wall. This observation strengthens our conception that 68Ga-radioactivity accumulates at the site of inflammation.
68Ga radionuclide is readily available by elution from a 68Ge/68Ga generator with 0.1 M HCl in the form of 68Ga-chloride. However, after neutralization, 68Ga-chloride is hydrolyzed in situ or after administration in vivo. In general, ionic 68Ga3+ is in the form of soluble anion called gallate, 68Ga(OH)-4, and/or insoluble colloidal particles 68Ga(OH)3. In the present study, the final 68Ga product contained 13% of colloidal forms of 68Ga. Uncharged gallium hydroxide or gallium nanoparticles are capable of penetrating the cell. Under the lower pH conditions, inside the cell, the material ionizes and becomes bound. Accordingly, accumulation into the liver, spleen, and bone marrow would be expected [12,13].
In blood, 68Ga-radioactivity can circulate also as a free 68Ga3+ and mimic ferric ions. In spite of their physical similarities, gallium and iron behave differently in vivo. The major difference is the inability of gallium to be reduced in vivo wherefore gallium remains bound to iron-transport proteins and carrier molecules with the inability to interact with protoporphyrin IX to form heme. A high blood uptake of gallium is explained by its binding to iron-binding molecules; transferrin, lactoferrin, and ferritin. One way to facilitate the solubilization of 68Ga may be its chelation with citrate. For SPECT imaging, 67Ga is indeed administrated as citrate to avoid protein binding. Since citrate is only a weak chelator in vivo, 67Ga is rapidly released, hydrolyzed, and bound to transferrin and other iron-binding proteins [12,14-16], suggesting that chelation does not improve the uptake of the tracer. We have compared uptakes of 68Ga and 68Ga-citrate in rat osteomyelitis model (unpublished data), and ascertained that there were no differences between 68Ga and 68Ga-citrate uptakes in inflammation lesions.
In comparison to 67Ga-based SPECT, 68Ga-based PET may have some advantages, such as a better resolution for the detection of inflammation and a smaller radiation exposure for the subject. However, the blood clearance of 68Ga-radioactivity was slow as demonstrated by ex vivo measurement and by in vivo PET/CT imaging. Unfortunately, the relatively short half-life of 68Ga does not allow delayed imaging times for better blood clearance. In an inflamed lesion, 67Ga is accumulated in the areas rich in infiltrated macrophages. Interestingly, 67Ga is not accumulated at the sites of crowded neutrophils, suggesting that the polymorphonuclear leukocytes do not play a major role in the accumulation of 67Ga in inflammatory lesions [17].
In the present study, the plaque/wall ratio of 68Ga was 1.8 ± 0.2. As compared to 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG), we have measured the plaque/wall ratio of 2.7 ± 1.1 approximately in the same aged LDLR-/-ApoB100/100 mice as used in the present study [18]. It has been shown that the clinical image qualities of 68Ga-tracers (β + decay 97%, Eβ + max 0.64 MeV) and 18F-tracers (β + decay 97%, Eβ + max 0.64 MeV) are not necessarily very much different from each other [19]. However, 68Ga has a higher kinetic energy compared to 18F, which could decrease the imaging spatial resolution, particularly when a small plaque lesion is imaged with a high resolution small PET/CT. Based on these earlier observations, 18F-FDG seems to be more promising PET tracer for imaging of atherosclerotic plaques than 68Ga.
Clinical observations indicate that in normal circumstances, the major proportion of the gallium retained in the body is localized in the bone and liver, and that inflammatory lesions alter this biodistribution, frequently causing decreased hepatic uptake [15]. In the present study, a difference in the uptake of 68Ga between the atherosclerotic mice and controls was found in bone and liver. The uptake of 68Ga-radioactivity in the whole aorta of atherosclerotic mice was higher than that in the control mice, but the difference was not significant. The uptake in the inflammatory plaques could partly explain the lower uptake of 68Ga in the liver of atherosclerotic mice. More likely this may be due to liver failure caused by high fat content, which can occur in older LDLR-/-ApoB100/100 mice fed with a Western-type diet. Concerning the uptake of 68Ga in the bone, we assume that in the young control mice (aged 2.5 months), active bone remodeling may have contributed to the higher bone uptake as compared to the older LDLR-/-ApoB100/100 mice (aged 10 to 11 months).
The accumulation of 68Ga-radioactivity in inflammatory lesions is complex, and the majority of 68Ga is probably delivered into the inflammatory lesions through the increased permeability at the site of atherosclerotic arteries. In the present study, accumulated 68Ga-radioactivity was detected in the macrophages-rich plaques suggesting that 68Ga accumulates at the site of inflammation. In addition to that, calcified areas of atherosclerotic plaques were also detected by 68Ga, which may be explained by the competitive binding of gallium ions to the Ca2+ and Mg2+ binding sites [17]. Since the calcified areas can be localized with CT, this observation is not limiting the use of 68Ga to distinguish macrophages-rich plaques and calcified plaques.
To some extent, gallium may also be taken up by atherosclerotic plaques due to its binding to the circulating transferrin and avidly to the transferrin receptors at the site of atherosclerotic arteries. Transferrin receptor expression has been shown to correlate positively with macrophage infiltration in human carotid atherosclerotic lesions [20]. Moreover, symptomatic patients with carotid atherosclerosis have significantly higher expression of transferrin receptors than asymptomatic patients [21]. However, although the uptake of 68Ga in inflamed atherosclerotic plaques was promising as measured by autoradiography, the slow blood clearance limits the use of ionic 68Ga for in vivo imaging of atherosclerotic plaques.
Conclusions
Autoradiography analysis showed a moderate, but significantly higher 68Ga-radioactivity uptake in the aortic plaques of atherosclerotic mice, especially at the sites rich in macrophages, as compared to areas of healthy vessel wall. While the uptake of 68Ga-radioactivity was prominent in the atherosclerotic plaques of LDLR-/-ApoB100/100 mice, the slow blood clearance limits the feasibility of ionic 68Ga for clinical imaging of atherosclerotic plaques especially in coronary arteries.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JMUS contributed the conception and design, performed the experiments, analyzed and interpreted data and drafted the manuscript. IL performed the experiments, acquired, analyzed and interpreted data and participated in writing of the manuscript. HJS prepared the 68gallium and participated in writing of the manuscript. VJOL involved in designing the analysis and interpretation of data, and in drafting and revising the manuscript. PL involved in designing the experiments and revising the manuscript. SY-H, JK, and AR contributed the conception and design, critically contributed and revised the manuscript and enhanced its intellectual content. All authors have approved the final content of the manuscript.
Contributor Information
Johanna MU Silvola, Email: johanna.silvola@utu.fi.
Iina Laitinen, Email: Iina.Laitinen@lrz.tu-muenchen.de.
Henri J Sipilä, Email: hensip@utu.fi.
V Jukka O Laine, Email: vellai@utu.fi.
Pia Leppänen, Email: pia.leppanen@uku.fi.
Seppo Ylä-Herttuala, Email: Seppo.YlaHerttuala@uku.fi.
Juhani Knuuti, Email: juhani.knuuti@utu.fi.
Anne Roivainen, Email: anne.roivainen@utu.fi.
Acknowledgements
The authors would like to thank Erja Mäntysalo and Erica Nyman for technical assistance, and Ville Aalto for statistical analyses. This work was funded by the Instrumentarium Foundation, the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, Aarne Koskelo Foundation, and Ida Montin Foundation and it was conducted in the Finnish Centre of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research, supported by the Academy of Finland, the University of Turku, the Turku University Hospital, and the Åbo Akademi University.
References
- Bobryshev YV. Monocyte recruitment and foam cell formation in atherosclerosis. Micron. 2006;37:208–222. doi: 10.1016/j.micron.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Choudhury RP, Lee JM, Greaves DR. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2:309–315. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques - Role of extracellular lipid, macrophage, and smooth-muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes - Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- Wilson HM, Barker RN, Erwig LP. Macrophages: Promising targets for the treatment of atherosclerosis. Curr Vasc Pharmacol. 2009;7:234–243. doi: 10.2174/157016109787455635. [DOI] [PubMed] [Google Scholar]
- Al Nahhas A, Win Z, Szyszko T, Singh A, Khan S, Rubello D. What can gallium-68 PET add to receptor and molecular imaging? Eur J Nucl Med Mol Imaging. 2007;34:1897–1901. doi: 10.1007/s00259-007-0568-1. [DOI] [PubMed] [Google Scholar]
- Ujula T, Salomäki S, Autio A, Luoto P, Tolvanen T, Lehikoinen P, Viljanen T, Sipilä H, Härkönen P, Roivainen A. Ga-68-chloride PET reveals human pancreatic adenocarcinoma xenografts in rats-comparison with FDG. Mol Imaging Biol. 2010;12:259–268. doi: 10.1007/s11307-009-0267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan VM, Sziklas JJ, Spencer RP. Localization of Ga-67 in inflammations in absence of circulating polymorphonuclear leukocytes. J Nucl Med. 1978;19:292–294. [PubMed] [Google Scholar]
- Heinonen SE, Leppänen P, Kholova I, Lumivuori H, Häkkinen SK, Bosch F, Laakso M, Ylä-Herttuala S. Increased atherosclerotic lesion calcification in a novel mouse model combining insulin resistance, hyperglycemia, and hypercholesterolemia. Circ Res. 2007;101:1058–1067. doi: 10.1161/CIRCRESAHA.107.154401. [DOI] [PubMed] [Google Scholar]
- Haukkala J, Laitinen I, Luoto P, Iveson P, Wilson I, Karlsen H, Cuthbertson A, Laine J, Leppänen P, Ylä-Herttuala S, Knuuti J, Roivainen A. Ga-68-DOTA-RGD peptide: biodistribution and binding into atherosclerotic plaques in mice. Eur J Nucl Med Mol Imaging. 2009;36:2058–2067. doi: 10.1007/s00259-009-1220-z. [DOI] [PubMed] [Google Scholar]
- Saha P, Modarai B, Humphries J, Mattock K, Waltham M, Burnand KG, Smith A. The monocyte/macrophage as a therapeutic target in atherosclerosis. Curr Opin Pharmacol. 2009;9:109–118. doi: 10.1016/j.coph.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Hnatowich DJ. Review of radiopharmaceutical development with short-lived generator-produced radionuclides other than Tc-99 m. Int J Appl Radiat Isot. 1977;28:169–181. doi: 10.1016/0020-708X(77)90171-5. [DOI] [PubMed] [Google Scholar]
- Mäkinen TJ, Lankinen P, Pöyhönen T, Jalava J, Aro HT, Roivainen A. Comparison of F-18-FDG and Ga-68 PET imaging in the assessment of experimental osteomyelitis due to Staphylococcus aureus. Eur J Nucl Med Mol Imaging. 2005;32:1259–1268. doi: 10.1007/s00259-005-1841-9. [DOI] [PubMed] [Google Scholar]
- Green MA, Welch MJ. Gallium radiopharmaceutical chemistry. Nucl Med Biology. 1989;16:435–448. doi: 10.1016/0883-2897(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Hoffer P. Gallium - Mechanisms. J Nucl Med. 1980;21:282–285. [PubMed] [Google Scholar]
- Oyen WJG, Boerman OC, van der Laken CJ, Claessens RA, van der Meer JW, Corstens FH. The uptake mechanisms of inflammation- and infection-localizing agents. Eur J Nucl Med. 1996;23:459–465. doi: 10.1007/BF01247377. [DOI] [PubMed] [Google Scholar]
- Ando A, Nitta K, Ando I, Katsuda S, Tonami N, Hiraki T, Hisada K, Oqawa H. Ga-67 Accumulation in inflammatory lesion and its mechanism - Comparison with malignant-tumor. Eur J Nucl Med. 1987;12:560–566. doi: 10.1007/BF00296098. [DOI] [PubMed] [Google Scholar]
- Laitinen I, Marjamäki P, Haaparanta M, Savisto N, Laine VJ, Soini SL, Wilson I, Leppänen P, Ylä-Herttuala S, Roivainen A, Knuuti J. Non-specific binding of [18F]FDG to calcifications in atherosclerotic plaques: experimental study of mouse and human arteries. Eur J Nucl Med Mol Imaging. 2006;33:1461–1467. doi: 10.1007/s00259-006-0159-6. [DOI] [PubMed] [Google Scholar]
- Sánchez-Crespo A, Andreo P, Larsson SA. Positron flight in human tissues and its influence on PET image spatial resolution. Eur J Nucl Med Mol Imaging. 2004;3:1: 44–51. doi: 10.1007/s00259-003-1330-y. [DOI] [PubMed] [Google Scholar]
- Li W, Xu LH, Forssell C, Sullivan JL, Yuan XM. Overexpression of transferrin receptor and ferritin related to clinical symptoms and destabilization of human carotid plaques. Exp Biol Med. 2008;233:818–826. doi: 10.3181/0711-RM-320. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Iron and the sex difference in heart-disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]