Layer-by-layer (LbL) assembly has attracted much attention because of its ability to create multifunctional films on surfaces while maintaining bulk properties.[1] The method relies on sequential adsorption of polymers onto bulk surfaces from solution, giving rise to complex multilayered films. LbL assembly is simple to implement and offers extensive control over film properties and composition during stepwise adsorption of components. Although the vast majority of LbL films are built from polyelectrolytes via electrostatic interaction between layers, more recently LbL films have been made with hydrogen-bonding of polymers,[2] and other building blocks such as inorganic nanoparticles have been used, giving access to even greater control of chemical and physical properties of LbL films.
In principle, LbL assembly can be performed on a wide variety of substrates, including noble metals (e.g., Au, Pt), oxides (e.g., quartz, Si, TiO2, mica), and synthetic polymers (e.g., poly(ethylene terephthalate) (PET), poly(methyl methacrylate) (PMMA), polyetherimide).[3,4] In practice, however, formation of well-ordered LbL layers on many polymeric surfaces has proven challenging,[5–7] and LbL assembly on hydrophobic polymers such as poly(tetrafluoroethylene) (PTFE), and polyethylene (PE) often requires aggressive “priming” methods such as plasma treatments,[5,7] oxidative chemical reactions (piranha/persulfonation),[8,9] or polymeric adsorption.[6,10,11] Our goal is to develop a simple, nondestructive and versatile method that enables LbL assembly to be performed on virtually any substrate (noble metals, semi-conductors, metal oxides, synthetic polymers, ceramics, and composites) as a useful addition to the LbL toolbox.
The adhesive proteins of mussels contain unusually high concentrations of catechol and amine functional groups[12] and are capable of mediating adhesion to most organic and inorganic surfaces.[13] Low-molecular-weight catecholamine mimics of these proteins such as dopamine self-polymerize at alkaline pH to form adherent polymer coatings on a large variety of substrates,[14] implying that synthetic polymers with catechol and amine functionalities may be useful as “universal” LbL primers. Here we show that a synthetic catecholamine polymer inspired by mussel adhesive proteins adsorbs to virtually all surfaces and can serve as a platform for LbL assembly in a surface-independent fashion.
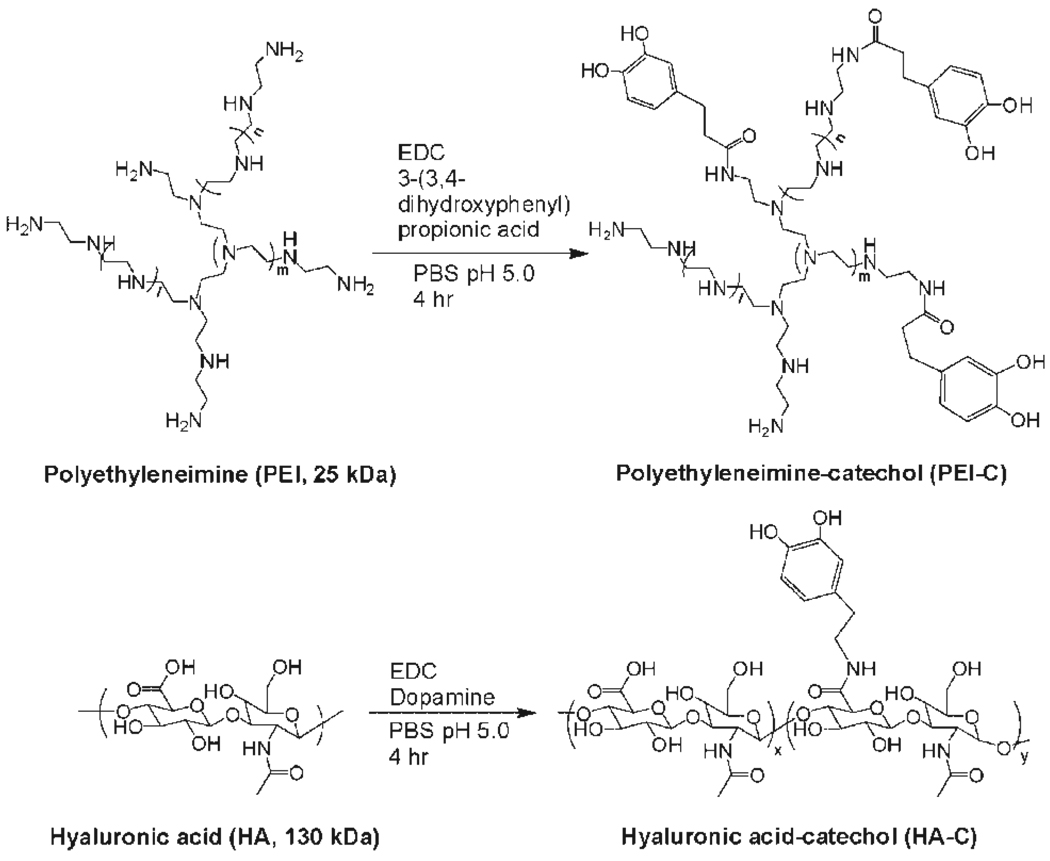
Poly(ethylenimine) (PEI), a cationic polymer with a history of use in LbL assembly,[10,15] was conjugated with 3-(3,4-dihydroxyphenyl)propionic acid to make catechol-functionalized PEI (PEI-C) (Scheme 1). The degree of catechol modification in PEI-C was 63%, as determined by the ninhydrin test, thereby preserving the cationic character of the polymer for use in LbL while at the same time mimicking the high catechol content of mussel adhesive proteins.[12] For an anionic polymer we chose hyaluronic acid (HA), a linear polysaccharide found in extracellular matrix (ECM) of connective tissues which has also been used in LbL assembly.[16,17] A catechol-modified HA was synthesized by reacting dopamine with HA in the presence of EDC, yielding HA-catechol (HA-C) with 35.6% of carboxyl groups modified by dopamine (Scheme 1, bottom).
Scheme 1.
Synthesis of catechol-containing polymers for surface-independent layer-by-layer assembly (siLbL).
We first demonstrated LbL assembly on PTFE, chosen as an example of a particularly challenging substrate for LbL owing to its anti-adhesive property.[18] The progress of LbL assembly was monitored by X-ray photoelectron spectroscopy (XPS), as shown in Figure 1. The intensity of fluorine 1s (F1s) (690 eV) and carbon 1s (C1s) (292 eV, C–F) peaks from bare PTFE (Fig. 1A, top) decreased after the first cycle of PEI-C/HA-C assembly (Fig. 1A, middle) and completely disappeared after only three cycles (Fig. 1A, bottom). The fluorine composition at the PTFE surface decreased from 69 percent initially to only 1.6 percent after two-cycles of PEI-C/HA-C assembly (B), demonstrating well-controlled LbL deposition on untreated PTFE. Contact-angle measurements clearly showed the stark contrast in wetting characteristics of the PTFE surface before and after LbL assembly (panel C–D); the advancing contact angle (θadv) decreased from 115° for unmodified PTFE to 27.8° after three-cycle assembly (PEI-C/HA-C)3. The importance of the catechol functionality in effective LbL on PTFE was illustrated by poor wetting (θadv = 69.5°) when unmodified PEI and HA were used under the condition of significantly extended adsorption times (18–24 h per each assembly) (E).
Figure 1.
Layer-by-layer assembly on PTFE. A) XPS spectra of bare PTFE (top), after the first cycle assembly of PEI-C/HA-C (middle), and after three cycles (bottom). B) Surface composition of fluorine (F1s) as a function of the number of LbL deposition cycles of PEI-C/HA-C. C)–E) Wetting of water on bare PTFE (C, θstat = 106°), PTFE after three cycles of LbL assembly using PEI-C and HA-C (D, θstat = 19.7°), and PTFE after three cycles assembly using PEI and HA (E, θstat = 55.4°).
To demonstrate the substrate versatility of LbL using catechol functionalized polymers, LbL assembly was also performed on several other polymeric surfaces (PE, PET, and polycarbonate (PC)) generally considered to be difficult to functionalize without prior surface modification. Comparative XPS studies of PEI vs. PEI-C adsorption on these substrates confirmed the importance of catechol residues in first layer adsorption. For example, the nitrogen signal (N1s), a useful indicator because of its presence in PEI-C chains but not in the substrate, showed that PE was anti-adsorptive to PEI but was readily modified by PEI-C (Fig. 2A). On PET and PC, trace amounts of nitrogen were detected by following PEI adsorption, but PEI-C showed enhanced absorption, providing a robust platform for LbL assembly. Quantitative XPS analysis of surfaces (Supporting Information) modified by PEI-C all contained similar nitrogen levels (5–7 percent) regardless of substrate, whereas PEI modification of the same surfaces yielded uniformly low nitrogen content (0–2 percent; Fig. 2A).
Figure 2.
Substrate-independent LbL assembly using PEI-C and HA-C. A) XPS surface nitrogen composition on various organic polymer surfaces after adsorption of PEI (black) or PEI-C (gray). B) Ellipsometric polymer film thickness versus number of cycles of PEI-C/HA-C adsorption on SiOx (circles), Au (triangles), and PMMA (squares).
In this manner, LbL assembly on a variety of organic and inorganic surfaces was facilitated using alternating cycles of PEI-C/HA-C adsorption. We used Au, SiOx, and PMMA as representatives of noble metal, oxide, and polymer substrates, respectively. Ellipsometric measurement of film thickness resuling from PEI-C/HA-C adsorption revealed a film deposition rate of 2.1 nm/cycle (n) regardless of substrate (Fig. 2B). The results suggest that catecholamine polymers such as PEI-C facilitate LbL assembly on a wide variety of substrates, a strategy we refer to as substrate-independent layer-by-layer (siLbL) assembly.
PEI-C also functions as a universal primer to facilitate subsequent LbL with other polymers. We demonstrated this concept on a silicon wafer with poly(acrylic acid) (PAA) and poly-L-lysine (PLL), two polymers that have a history of use in LbL assembly.[19] First, PEI-C was adsorbed as a primer layer on SiOx, after which XPS analysis revealed peaks representative of both substrate (99.5 eV for Si2p, and 143 eV for Si2s) and polymer (285 eV for C1s and 400 eV for N1s; Fig. 3A). The strong oxygen 1s (O1s) peak at 535 eV contains contributions from the silicon oxide and hydroxyl groups of the catechol. Adsorption of PAA followed by ten subsequent cycles of PLL/PAA adsorption [(PEI-C/PAA)1-(PLL/PAA)10] and XPS analysis resulted in complete suppression of substrate signals (Si2p,2s), leaving only C1s, N1s and O1s peaks corresponding to PAA and PLL (Fig. 3B). The thickness of the multilayer film was monitored by spectroscopic ellipsometry during LbL assembly, revealing a roughly linear increase in thickness with PLL/PAA deposition (Fig. 3C). Atomic force microscopy (AFM) imaging revealed a morphological transition from rough at an early stage to uniform film formation after many layers (Fig. 3D–F). The change of surface morphology could influence contact angle measurements.
Figure 3.
Layer-by-layer assembly of PAA and PLL on PEI-C primed SiOx. A) XPS spectrum after single-step PEI-C adsorption on SiOx B) XPS spectrum of (PEI-C/PAA)1-(PLL/PAA)10 adsorption on SiOx. C. Ellipsometry thickness of (PEI-catechol/PAA)1-(PLL/PAA)n. AFM image of a bare SiOx substrate (D), after (PEI-C/PAA)1 deposition (E), and after (PEI-C/PAA)1-(PLL/PAA)10 deposition (F). AFM images showed relatively smooth topography of the polymeric deposition.
Certain functional properties of LbL films may be enhanced by incorporation of catechol residues into LbL films. For example, the strong interaction of catechols with surfaces[20,21] suggests that LbL films deposited onto a primer layer of PEI-C should enhance adhesion and help prevent delamination of LbL films from substrate surfaces.
Likewise, catechols could enhance mechanical properties within LbL composite films- a preliminary report of enhanced mechanical properties of catechol-containing LbL nanocomposite multilayer films has recently appeared.[22]
Here, we demonstrate a useful functional property of LbL multilayer films constructed from catechol containing polymers. The catechol groups in the LbL film are redox active and therefore can function as a reducing agent to oxidize metal ions, as we previously demonstrated for spontaneous electroless Ag and Cu metallization of catecholamine polymer coated surfaces from aqueous metal salt solutions.[14] In this case, we employed the latent reactivity of catechol functional groups in PEI-C/HA-C LbL films for in-situ reduction of Ag(I) to Ag(0) within the LbL multilayer (Fig. 4A). First, LbL films of PEI-C/HA-C (n = 20) were assembled on SiOx. Subsequently, the LbL film and substrate were transferred to a silver nitrate solution (1 mM), upon which AFM imaging of the surface revealed topological changes corresponding to Ag nanoparticle formation. XPS analysis indicated a strong signal at 368.4 eV (Fig. 4E), corresponding to the reported binding energy of metallic silver (3d5/2).[23] Given the antimicrobial activity of metallic silver,[24] the bactericidal effect of the incorporated silver particles in the LbL film was tested in an in vitro adhesion experiment with Escherichia coli. Surfaces were inoculated with 105 CFU of E. coli for four hours and then the number of dead bacteria attached to the surface counted. Ag nanoparticle-embedded LbL films showed enhanced antibacterial effects compared to the LbL film without Ag and the bare SiOx surface (Fig. 4F).
Figure 4.
Catechol-mediated silver nanoparticle formation in LbL films of PEI-C/HA-C and antibacterial activity of the nanocomposite films. A) Schematic illustration of Ag nanoparticle formation in LbL film via catechol oxidation in the presence of Ag(I). B)–D) Topographic AFM images of the LbL film after PEI-C/HA-C (n = 20) deposition (B), and the same film incubated in 1 mM AgNO3 solution for 30 min (C) and 18 h (D). E. XPS spectra of the silver incorporated LbL film shown in D (18 h). Metallic silver photoelectron (3d5/2) was detected at the binding energy of 368.4 eV. F) Live-dead assay of adhered E. coli on bare Si, LbL (n = 20), and LbL+Ag (n = 20, 18 h) surfaces.
In conclusion, we described a simple approach to substrate-independent LbL assembly by exploiting the strong interfacial binding property of catechol containing polymers. In particular, use of the catecholamine polymer PEI-C as a universal surface primer facilitated LbL assembly on metal, oxide and polymer substrates. The strategy avoids the need for aggressive chemical or physical pre-treatment regimens normally required for LbL on challenging substrates such as neutral and hydrophobic polymers. Finally, the latent redox activity of catechol groups incorporated throughout the LbL film was exploited for in-situ deposition of Ag nanoparticles, imparting an antibacterial property to the multilayer film. With further improvements and through full exploitation of the substrate versatility afforded by siLbL, silver incorporated LbL films may be employed in the future to minimize bacterial fouling on a variety of materials.
Experimental
PEI-C Synthesis
3 g of PEI (Mw = 25 kDa, Sigma–Aldrich) was dissolved in 300 mL of PBS solution adjusted to pH 5.5 using 1 N HCl solution. 1.52 g (17.4 mmol) of 3-(3,4-dihydroxyphenyl)propionic acid, and 2.71 g (34.9 mmol) of EDC were added, and the pH of the reaction solution was maintained at 5.5 for 2 h with 1.0 N NaOH. Unreacted chemicals and urea byproducts were removed by extensive dialysis. The degree of substitution was determined by ninhydrin test.
HA-C Synthesis
1 g of HA (Mw = 130 kDa, Lifecore) was dissolved in 100 mL of PBS solution adjusted to pH 5.5 using 1 N HCl solution. 388.1 mg (2.5 mmol) of EDC and 474.1 mg (2.5 mmol) of dopamine hydrochloride were added, and the pH of the reaction solution was maintained at 5.5 for 2 h with 1.0 N NaOH. This reaction resulted in modification of 35.6% of primary amine groups.
Layer-by-Layer Assembly
PTFE, PE, PC, PET, PMMA, Si, and Au surfaces were ultrasonically cleaned in deionized water for 5 min and transferred to the PEI-C and HA-C solutions (5 mg mL−1 in water, pH 6.5) for LbL assembly. The following cycle was generally used: (1) PEI-C for 3 min, (2) wash in water for 1 min, (3) HA-C for 3 min, and (4) wash in water for 1 min. For PTFE, the first PEI-C/HA-C adsorption was carried out for 2 h, and subsequent steps were same as described. A control experiment involving LbL on PTFE using as-supplied PEI (no catechol) in each assembly step was performed with overnight adsorptions (18–24 h). The same method was used for heterogeneous assembly of PEI-C/PAA (Mw = 90 kDa, Polysciences) followed by alternating PLL/PAA adsorption. Concentrations of PAA and PLL (Ave Mw = 28,000 Da, Sigma–Aldrich) were 3 mg mL−1 in 10 mM Tris, pH 7.0.
Characterization
Spectroscopic ellipsometry (Woollam Co., Inc. Lincoln, NE) was used to determine the film thickness. AFM surface topography was measured in air using an MFP-3D atomic force microscopy (Asylum Research, San Diego, CA) operated in AC and contact modes. X-ray photoelectron spectroscopy (Omicron ESCA-LAB) (Omicron, Taunusstein, Germany) was performed to measure surface atomic composition. XPS is configured with a monochromated Al Kα (1486.8 eV) 300 W X-ray source with an ultrahigh vacuum (<10−8 Torr (1 Torr = 1.333 × 102 Pa)). The takeoff angle was fixed at 45°, and all spectra were calibrated using the hydrocarbon C(1s) peak (284.5 eV).
Bactericidal Testing
E. coli (ATCC 35218) was grown in MHB (Mueller-Hinton Broth, cation adjusted) at 37 °C for 24 h from previously frozen inoculums. Substrates were sterilized by UV treatment and incubated at 37 °C with 1 mL of phosphate buffered saline (PBS) containing ~105 CFU mL−1 E. coli for 4 h with mild agitation. Substrates were rinsed with PBS and stained with Syto 9 and propidium iodide in PBS (2 uL mL−1) for 10 min and then mountained on glass slides. Attached bacteria were imaged using a Leica epifluorescence microscope (40× magnification).
Footnotes
This work was supported by grant DE014193 from the NIH (USA) and the BK21 program (Korea). Portions of this work were performed in the Keck-II facility of NUANCE at Northwestern University, which is supported by NSF-NSEC, NSF-MRSEC, the Keck Foundation, the State of Illinois, and Northwestern University. Supporting Information is available online from Wiley InterScience or from the authors.
Contributor Information
Haeshin Lee, Biomedical Engineering Department, Northwestern University, 2145 Sheridan Rd., Evanston, IL 60208 (USA).
Yuhan Lee, Department of Biological Sciences, Korea Advanced Institute of Science and Technology, 373-1 Guseong-dong, Yuseong-gu, Deajeon 305-701 (Korea).
Andrea R. Statz, Biomedical Engineering Department, Northwestern University, 2145 Sheridan Rd., Evanston, IL 60208 (USA)
Junsung Rho, Biomedical Engineering Department, Northwestern University, 2145 Sheridan Rd., Evanston, IL 60208 (USA).
Tae Gwan Park, Department of Biological Sciences, Korea Advanced Institute of Science and Technology, 373-1 Guseong-dong, Yuseong-gu, Deajeon 305-701 (Korea).
Phillip B. Messersmith, Email: philm@northwestern.edu, Biomedical Engineering Department, Northwestern University, 2145 Sheridan Rd., Evanston, IL 60208 (USA).
References
- 1.Decher G, Hong J-D. Makromol. Chem. Macromol. Symp. 1991;46:321. [Google Scholar]
- 2.Lutkenhaus JL, Hrabak KD, MccEnnis K, Hammond PT. J. Am. Chem. Soc. 2005;127:17228. doi: 10.1021/ja053472s. [DOI] [PubMed] [Google Scholar]
- 3.Hammond PT. Adv. Mater. 2004;16:1271. [Google Scholar]
- 4.Tang Z, Wang Y, Podsiadlo P, Kotov NA. Adv. Mater. 2006;18:3203. [Google Scholar]
- 5.Bergbreiter DE. Prog. Polym. Sci. 1994;19:529. [Google Scholar]
- 6.Raposo M, Pontes RS, Mattoso LHC, Oliveira ON. Macromolecules. 1997;30:6095. [Google Scholar]
- 7.Hsieh MC, Farris RJ, McCarthy TJ. Macromolecules. 1997;30:8453. [Google Scholar]
- 8.Price G, Keen F, Clifton AA. Macromolecules. 1996;29:5664. [Google Scholar]
- 9.Zhao H, Yang P, Deng J, Liu L, Zhu J, Sui Y, Lu J, Yang W. Langmuir. 2007;23:1810. doi: 10.1021/la061954r. [DOI] [PubMed] [Google Scholar]
- 10.Delcorte A, Bertrand P, Wischerhoff E, Laschewsky A. Langmuir. 1997;13:5125. [Google Scholar]
- 11.Khademhosseini A, Jon S, Suh KY, Tran T-NT, Eng G, Yeh J, Seong J, Langer R. Adv. Mater. 2003;15:1995. [Google Scholar]
- 12.Waite JH, Andersen NH, Jewhurst S, Sun C. J. Adhes. 2005;81:1. [Google Scholar]
- 13.Crisp DJ, Walker G, Young GA, Yule AB. J. Colloid Interface Sci. 1985;104:40. [Google Scholar]
- 14.Lee H, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318:426. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boura C, Menu P, Payan E, Picart C, Voegel JC, Muller S, Stoltz JF. Biomaterials. 2003;24:3521. doi: 10.1016/s0142-9612(03)00214-x. [DOI] [PubMed] [Google Scholar]
- 16.Picart C, Lavalle Ph, Hubert P, Cuisinier FJG, Decher G, Schaaf P, Voegel JC. Langmuir. 2001;17:7414. [Google Scholar]
- 17.Thierry B, Winnik FM, Merhi Y, Tabrizian M. J. Am. Chem. Soc. 2003;125:7494. doi: 10.1021/ja034321x. [DOI] [PubMed] [Google Scholar]
- 18.Vicente J, Ramirez-Camacho R, Trinidad A, Garcia-Berrocal JR, Lobo D, Pinilla M. Acta Oto-Laryngol. 2006;126:144. doi: 10.1080/00016480500312570. [DOI] [PubMed] [Google Scholar]
- 19.Pardo-Yissar V, Katz E, Lioubashevski O, Willner I. Langmuir. 2001;17:1110. [Google Scholar]
- 20.Lee H, Scherer NF, Messersmith PB. Proc. Natl. Acad. Sci. USA. 2006;103:12999. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Lee BP, Messersmith PB. Nature. 2007;448:338. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 22.Podsiadlo P, Liu Z, Paterson D, Messersmith PB, Kotov NA. Adv. Mater. 2007;19:949. [Google Scholar]
- 23.Murray BJ, Li Q, Newberg JT, Menke EJ, Hemminger JC, Penner RM. Nano Lett. 2005;5:2319. doi: 10.1021/nl051834o. [DOI] [PubMed] [Google Scholar]
- 24.Ho CH, Tobis J, Sprich C, Thomann R, Tiller JC. Adv. Mater. 2004;16:957. [Google Scholar]