Abstract
Neuroglobin (Ngb) is a globin protein that is highly and specifically expressed in brain neurons. A large volume of evidence has proven that Ngb is a neuroprotective molecule against hypoxic/ischemic brain injury and other related neurological disorder; however, the underlying mechanisms remain poorly understood. Aiming to provide more clues in understanding the molecular mechanisms of Ngb’s neuroprotection, we performed yeast two-hybrid screening to search for proteins that interact with Ngb. From a mouse brain cDNA library, we found totally 36 proteins that potentially interact with Ngb, and 10 of them were each identified in multiple positive clones. The shared sequences within these multiple clones are more likely to be Ngb-interacting domains. In primary cultured mouse cortical neurons, immuno-precipitation was performed to confirm the interactions of selected proteins with Ngb. The discovered Ngb-interacting proteins in this study include those involved in energy metabolism, mitochondria function and signaling pathways for cell survival and proliferation. Our findings provide molecular targets for investigating protein interaction-based biological functions and neuroprotective mechanisms of Ngb.
Keywords: Neuroglobin, yeast two-hybrid screening, molecular interaction
Introduction
Neuroglobin (Ngb) is a globin family member identified in 2000 (Burmester et al., 2000) that is predominantly expressed in neurons of the neural systems including retina, and some endocrine tissues (Reuss et al., 2002, Wystub et al., 2003, Fordel et al., 2004, Brunori and Vallone, 2006). As a globin protein, Ngb binds with high affinity to various gaseous ligands such as O2, CO and NO (Dewilde et al., 2001). During the past decade, accumulating evidences have demonstrated that Ngb is protective for neurons against hypoxic/ischemic insults (Khan et al., 2006, Greenberg et al., 2008, Burmester and Hankeln, 2009, Yu et al., 2009a). Enhanced Ngb gene expression inversely correlates with the severity of histological and functional deficits after ischemic stroke (Sun et al., 2001, Sun et al., 2003, Hundahl et al., 2006, Peroni et al., 2007, Wang et al., 2008). Moreover, Ngb has been speculated to have translational importance with broad impact on neurological disorders. For example, Ngb over-expression has been found to be protective against beta-amyloid-induced neurotoxicity in mouse (Khan et al., 2007).
As an oxygen-binding protein, Ngb was originally thought to function in O2 storage and transportation. However, due to its high O2 binding rate and low O2 dissociation rate, plus its low protein level (~1uM) in the brain (Brunori and Vallone, 2006), Ngb is more likely to function in O2 sensing rather than O2 storage and transportation (Kriegl et al., 2002, Fago et al., 2004). Further study suggested Ngb may serve as a hypoxia sensor and initiate signal transduction in neuronal cells (Wakasugi and Morishima, 2005). It was reported that ferric human Ngb exerts guanine-nucleotide dissociation inhibitor (GDI) activity by preventing the Gα subunit from binding to the Gβγ complex and thus activates downstream signal transduction pathway, which is protective against oxidative stress (Schwindinger and Robishaw, 2001, Wakasugi et al., 2003).
Ngb was also proposed to modulate nitric oxide (NO) homeostasis since the oxygenated derivative of Ngb, Ngb-O2, reacts with NO rapidly to produce NO3− and met-Ngb (Brunori et al., 2005). This pathway disposes of NO, which may in turn protect cellular respiration jeopardized by the inhibitory effect of NO on cytochrome c oxidase activity (Moncada and Erusalimsky, 2002, Brunori et al., 2004).
Another important physiological implication of Ngb is its effect in maintaining mitochondrial function in brain under hypoxic/ischemic condition, and this may be related to Ngb’s role in reactive oxygen species (ROS) scavenging (Herold et al., 2004, Rayner et al., 2006, Fordel et al., 2007). At the subcellular level, Ngb is associated with mitochondria and linked to the oxidative metabolism (Burmester et al., 2007). Our lab has demonstrated that Ngb over-expression improved mitochondrial function and reduced oxidative stress in primary cultured neurons after hypoxia (Liu et al., 2009). Ngb over-expression also protected PC12 cells against beta-amyloid toxicity and attenuates beta-amyloid-induced mitochondrial dysfunction(Li et al., 2008).
Importantly, although the above hypotheses are inspiring in explaining Ngb’s neuroprotection mechanisms, most of them are based on indirect or correlative experimental data. Thus better understanding of the molecular mechanism of Ngb’s biological function and neuroprotective roles would have fundamental and translational significance, which may eventually improve the development of Ngb-targeted therapeutics against stroke and other neurological disorders. We strongly believe that the molecular interaction between Ngb and other proteins is an important basis on above regards. As the first step, in this study we aimed to identify the Ngb-interacting proteins using yeast two-hybrid screening system and further validate their bindings in primary cultured mouse cortical neurons.
Material and methods
Yeast two-hybrid screening
Yeast two-hybrid screening was carried out using a GAL4-based yeast two-hybrid system (MATCHMAKER Two-Hybrid System 3; Clontech, Palo Alto, CA); screening and assays were performed following the manufacturer’s instruction (Clontech). Mouse Ngb cDNA was amplified by PCR; the PCR fragment was then digested with NdeI and BamHI, and inserted into the pGBKT7 vector (Clontech) to generate a construct of mouse Ngb cDNA fused in-frame to the GAL4 DNA-binding domain (BD) (amino acids [a.a.] 1–147 of GAL4) as the bait. The vector was then transformed into yeast strain Y187 and the transformants were plated on dropout medium lacking tryptophan (SD/Trp) because the pGBKT7 vector had a selectable TRP1 marker. The pre-transformed Mouse Brain Matchmaker cDNA Library in pGADT7 vector was purchased from Clontech.
For yeast two-hybrid screening, the mating reaction between Y187 transformed with pGBKT7-Ngb construct and AH109 pre-transformed with mouse brain library (in pGADT7) was performed and selected on Quadruple Drop Out (stringent selection) medium. Positive clones were further tested on medium containing X-alpha-Gal, which tests alpha-glactosidase activated by positive Ngb-target protein interaction. The pGADT7 plasmids encoding the library clones were isolated and sequenced using an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster city, CA), and homology searches against database sequences were performed using the BLAST algorithm on NCBI (National Center for Biotechnology Information).
Primary cortical neuronal culture
All animal experiments were performed following protocols approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Primary neuronal culture was prepared from the cortex of embryonic day 15 mouse. In brief, the cortical neurons were suspended in neuron-defined culture medium and plated onto poly-D-lysine-coated 35-mm dishes (3×105 cells per dish). Neural basal medium supplemented with 2% B27, 0.3 mM L-Glutamine and 1% Penicillin-Streptomycin was used. Half of the medium was replaced every 3 days. Protein extraction was carried out at day 8 of neuronal culture.
Western Blot
Western blot was performed as previously described (Yu et al., 2009b). Ngb protein levels were examined by Western blot using mouse anti-Ngb antibody (santa cruz). Selected Ngb-interacting proteins were detected with their respective antibodies after immuno-precipitation with anti-Ngb antibody.
Co-immunoprecipitation (Co-IP)
Proteins were extracted from primary cultured mouse cortical neurons, and immuno-precipitation were performed using 2 μg polyclonal antibodies against mouse Ngb (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After 3 hr incubation, protein G sepharose was added and incubated overnight at 4°C, and then centrifuged for 1 min at 12 000 g. The precipitates were rinsed with immuno-precipitation buffer (0.5% NP-40, Tris–Cl pH 8.0, 0.15 M NaCl) four times to remove non-specific binding molecules. IgG was used as negative control for precipitation. The co-immunoprecipitates were analyzed by Western blot. Antibodies against Atp1b1, Cyc1, Ubc, Dvl1, Etfa, Gabarap1 and VDAC antibody were used to detect these proteins.
Results
Expression of Ngb protein in yeast strain Y187 transformed with pGBKT7-Ngb vector
To conduct yeast two-hybrid screening, we first cloned mouse Ngb cDNA into pGBKT7 vector. The resulted pGBKT7-Ngb vector was transformed into yeast strain Y187 and Ngb expression was confirmed by Western blot (Figure.1).
Figure 1.
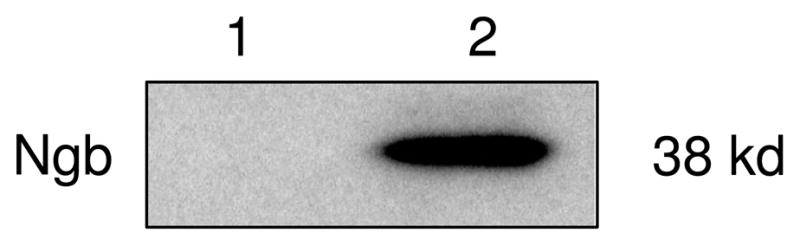
Expression of Ngb-Gal4 BD fusion protein in yeast. Yeast strain Y187 was transformed with pGBKT7-Ngb and transformants were selected on appropriate SD medium. Soluble protein extracts were prepared and protein samples were subjected to SDS-PAGE. Ngb expression was detected using anti-Ngb antibody. Lane 1: control Y187 transformed with pGBKT7 vector. Lane 2: Y187 transformed with pGBKT7-Ngb.
Genes found from yeast two-hybrid screening with multiple positive colonies
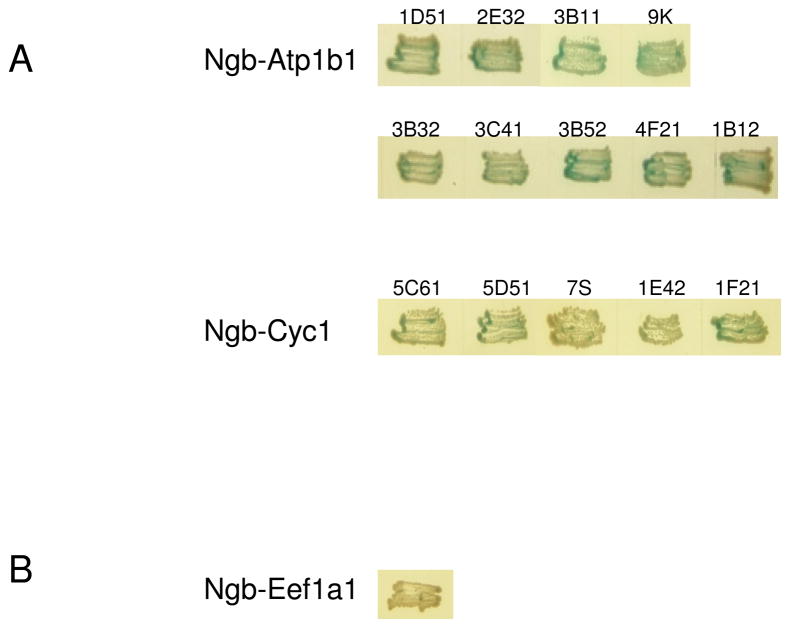
After mating reaction between pGBKT7-Ngb-transformed Y187 and AH109 containing mouse cDNA library, examples of clone selection were shown in Figure. 2. It shows 9 clones containing Atp1b1 sequence and 5 clones containing Cyc1 sequence are positive for both Drop Out medium and X-alpha-Gal selection (showing blue color), and they are considered potential Ngb-interacting proteins. However, for Eef1a1, it can grow in Quadruple Drop Out medium, but negative in X-alpha-Gal assay, so is not considered an Ngb-interacting protein.
Figure 2.
Selection of Ngb-interacting gene products in yeast two-hybrid screening. Mating reaction was set up between Y187 transformed with pGBKT7-Ngb vector and AH109 pretransformed with mouse cDNA library. Representative selected clones were shown. (A) Positive clones in both Drop Out medium and alpha-glactosidase activity test. (B) Clones positive in Drop Out medium selection but negative in alpha-glactosidase activity.
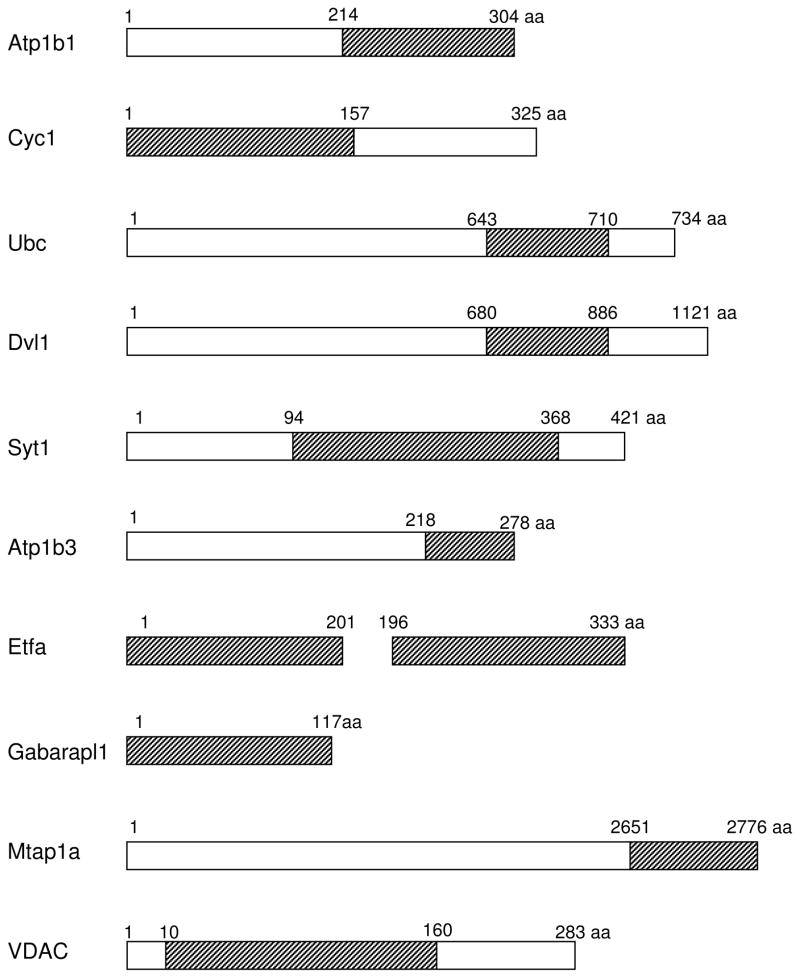
Totally 36 proteins were found to interact with Ngb. Among them, 10 proteins were each found in multiple positive clones (Table 1), and 26 were each found in one positive clone (Table 2). For proteins found in multiple clones, the gene inserts correspond to different parts of the same gene, most of them have common sequences and may designate the potential Ngb-interacting domains. These proteins with positive clone numbers from high to low were Na+/K+ ATPase beta 1 Subunit (Atp1b1), cytochrome c-1 (Cyc1), ubiquitin C (Ubc), disheveled-dsh homolog 1 (Dvl1), synaptotagmin I (Syt1), Na+/K+ ATPase beta 3 polypeptide (Atp1b3), electron transferring flavoprotein alpha subunit (Etfa), GABA(A) receptor-associated protein like 1 (Gabarapl1), microtubule-associated protein 1A (Mtap1a), and voltage-dependent anion channel 1 (VDAC1). The shared amino acid (a.a.) sequences from the multiple clones for each protein were depicted in Figure.3. All genes inserts share common sequences except Etfa. Among them, Atp1b1 was found in 9 positive clones, and the overlapping sequences correspond to the 91 a.a. at the C terminus. Cyc1 was found in 6 positive clones, and the overlapping sequence is 1–157 a.a. out of total 325 a.a.. Ubc appeared in 4 clones, with common sequences being the 643–710 a.a. out of total 734 a.a.. Both of Dvl1 and Syt1 were found in 3 positive clones. The shared sequence of Dvl1 clones corresponds to 680–886 a.a. out of total 1121 a.a., and Syt1 shared sequence corresponds to 94–368 a.a. out of 421 a.a.. Five genes were found in 2 positive clones, including Atp1b3, Etfa, Gabarapl1, Mtap1a and VDAC. Atp1b3 common sequence corresponds to 218–278 a.a. out of total 278 a.a.. Gabarapl1 shared sequence was the whole protein of 117 a.a.. Mtap1a shared sequence corresponds to 2651–2776 a.a. of total 2776 a.a.. VDAC common sequence is the 10–160 a.a. of total 283 a.a.. For Etfa, there was only 6a.a. of shared sequence between the two positive clones. One clone is the 1–201 a.a., and the other is 196–333 a.a. out of total 333 a.a.. These data suggest that Ngb might interact with multiple proteins and function in modulating energy metabolism, mitochondria function and other physiological processes.
Table 1.
Genes found from yeast two-hybrid assay with two or more positive clones.
| symbol | Accession # | Gene ID | Gene description | Redundancy |
|---|---|---|---|---|
| Atp1b1 | NM_009721 | 11931 | Na+/K+ ATPase, beta 1 polypeptide | 9 |
| Cyc1 | NM_025567 | 66445 | cytochrome c-1 | 5 |
| Ubc | NM_019639 | 22190 | ubiquitin C | 4 |
| Dvl1 | NM_010091 | 13542 | dishevelled, dsh homolog 1 (Drosophila) | 3 |
| Syt1 | NM_009306 | 20979 | synaptotagmin I | 3 |
| Atp1b3 | NM_007502 | 11933 | Na+/K+ ATPase, beta 3 polypeptide | 2 |
| Etfa (MADD) | NM_145615 | 110842 | Electron transferring flavoprotein, alpha subunit | 2 |
| Gabarapl1 | NM_020590 | 57436 | (GABA(A)) receptor-associated protein-like 1 | 2 |
| Mtap1a | NM_001173506 | 17754 | microtubule-associated protein 1 A | 2 |
| Vdac1 | NM_011694 | 22333 | voltage-dependent anion channel 1 | 2 |
Table 2.
Genes found from yeast two-hybrid assay with one positive clone.
| symbol | Accession# | Gene ID | Gene description |
|---|---|---|---|
| Actr2 | NM_146243 | 66713 | ARP2 actin-related protein 2 homolog (yeast) |
| Ahsa2 | NM_172391 | 268390 | AHA1, activator of heat shock protein ATPase homolog 2 (yeast) |
| Cmpk | NM_025647 | 66588 | Cytidylate kinase |
| Cnrip1 | NM_029861 | 380686 | cannabinoid receptor interacting protein 1 |
| Cplx1 | NM_007756 | 12889 | complexin 1 |
| Crlf3 | NM_018776 | 54394 | cytokine receptor-like factor 3 |
| Dmap1 | NM_023178 | 66233 | DNA methyltransferase 1-associated protein 1 |
| Dnaja1 | NM_001164671 | 15502 | DnaJ (Hsp40) homolog, subfamily A, member 1 |
| Dync1h1 | NM_030238 | 13424 | dynein cytoplasmic 1 heavy chain 1 |
| Eno1 | NM_023119 | 13806 | enolase 1, |
| Fgd1 | NM_008001 | 14163 | FYVE, RhoGEF and PH domain containing 1 |
| Fkbp1a | NM_008019 | 14225 | FK506 binding protein 1a |
| Fkbp8 | NM_001111066 | 14232 | FK506 binding protein 8 |
| Kifc3 | NM_001145831 | 16582 | kinesin family member C3 |
| March7 | NM_020575 | 57438 | membrane-associated ring finger |
| Ncam2 | NM_001113208 | 17968 | neural cell adhesion molecule 2 |
| Nr2c2ap | NM_001025586 | 75692 | nuclear receptor 2C2-associated protein |
| Nudc | NM_010948 | 18221 | nuclear distribution gene C homolog (Aspergillus) |
| Pcca | NM_144844 | 110821 | propionyl-Coenzyme A carboxylase, alpha polypeptide |
| Pja1 | NM_001083110 | 18744 | praja1, RING-H2 motif containing |
| Rpa1 | NM_001164223 | 68275 | replication protein A1 |
| Sec61b | NM_024171 | 66212 | Sec61 beta subunit |
| Trim3 | NM_018880 | 55992 | tripartite motif protein 3 |
| Ube2e1 | NM_009455 | 22194 | ubiquitin-conjugating enzyme E2E 1 |
| Ube2l3 | NM_009456 | 22195 | ubiquitin-conjugating enzyme E2L 3 |
| Zfp668 | NM_146259 | 244219 | zinc finger protein 668 |
Figure 3.
Common sequences of gene inserts found in multiple positive clones in yeast two-hybrid assay. The total length of amino acid sequence and the position of common sequence (shadowed region) of positive clones are indicated.
Co-immunoprecipitation to confirm the interaction between Ngb and selected proteins found in yeast two-hybrid screening with multiple clones
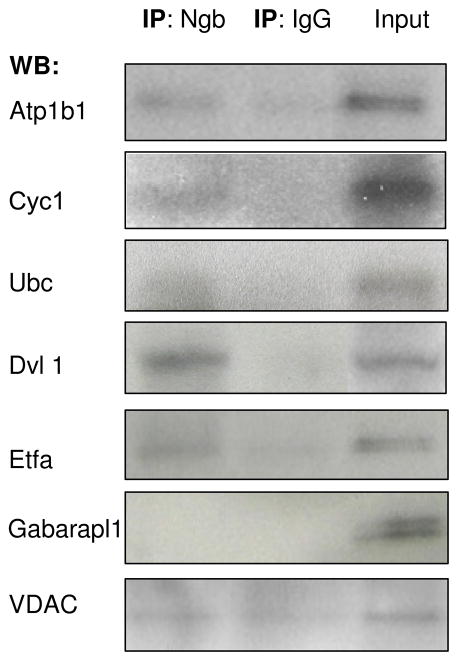
To validate our results of yeast two-hybrid screening, we conducted Co-immunoprecipitation (co-IP) for Ngb and those selected proteins with multiple positive clones to confirm their interaction, based on the commercial availability of specific antibodies against the discovered proteins. Our results showed that Atp1b1, Cyc1, Ubc, Dvl1, Etfa and VDAC can be precipitated by anti-Ngb antibody from primary cultured mouse cortical neurons (Figure 4). However, Gabarapl1, which has 2 positive clones determined by yeast two-hybrid, could not be precipitated by anti-Ngb antibody, indicating a false positive result of yeast two-hybrid assay.
Figure 4.
Binding of Ngb with gene products identified in yeast two-hybrid assay detected by Co-immunoprecipitation. Cell lysates of primary cortical neurons were incubated with anti-Ngb antibody. Protein precipitates were subjected to SDS-PAGE, and potential Ngb-interacting proteins were detected using their respective antibodies. The Co-IP experiments were repeated three times and representative images were shown.
Discussion
In this study, we identified a group of proteins that can potentially interact with Ngb using yeast two-hybrid screening. All the positive clones were selected under stringent selection condition using Quadruple Drop Out selection medium, combined with alpha-glactosidase activity test. Some of the proteins were found in multiple positive clones. We further used Co-IP to confirm several pairs of interactions between Ngb and these discovered proteins. Our results provide initial information of potential Ngb-interacting proteins, which might be new clues in investigating the role and molecular mechanisms of Ngb’s biological functions and neuroprotection effects.
The potential Ngb-interacting proteins determined from the present study have been reported to be functional in a wide range, suggesting Ngb may have broad physiological functions through interacting with multiple cellular proteins. For example, it has been well documented that Atp1b1 and Atp1b3 are two polypeptides of the beta subunit of Na+/K+ ATPase, which is a transmembrane heterodimer protein composed of alpha and beta subunits and mainly functions in maintaining ionic homeostasis (Dobretsov and Stimers, 2005, Zhan et al., 2011). The alpha-subunit is a multispanning membrane protein responsible for the catalytic and transport properties of the enzyme; while the beta-subunit is responsible for correct folding of the enzyme and regulated the number of sodium pumps transported to plasma membrane (Blanco and Mercer, 1998, Rajasekaran et al., 2001). Although the physiological role of Ngb interacting with Na+/K+ ATPase is yet to be investigated, it is interesting to see some functional connections between these two proteins. For instance, Duong et al showed that Ngb over-expression preserves ATP level in neuron cells after hypoxia. Furthermore, their study revealed that Ngb over-expression inhibited hypoxia-induced increase of intracellular iron (Fe), Copper (Cu) and zinc (Zn), but sulphur (S), chlorine (Cl) and potassium (K) were significantly decreased (Duong et al., 2009). Since Ngb is an iron-binding protein, it may play a role in ionic homeostasis maintenance through interaction with Na+/K+ ATPase, however the detailed mechanism warrants further study.
More interestingly, some of the potential Ngb-interacting proteins found in this study are mitochondrial proteins, such as Cyc1, Etfa and VDAC. Although Ngb has been traditionally considered a cytoplasmic protein, previous studies have shown that Ngb is closely associated with the presence mitochondria (Burmester et al., 2007). Studies from our lab and others have shown that Ngb over-expression preserved mitochondria function after hypoxic or beta-amyloid-induced neuron injury, which are supportive of the close link between Ngb and mitochondria (Li et al., 2008, Liu et al., 2009). Furthermore, a very recent report suggested Ngb protein is localized inside mitochondria (Hundahl et al., 2010). Our lab recently obtained more direct and convincing evidence showing that Ngb protein is physically localized inside mitochondria using multiple methods including immuno-staining, immuno-electron microscopic imaging and Western blot (unpublished data). The mitochondrial proteins found in our yeast two-hybrid screening further support the physical and functional association between Ngb and mitochondria, but the detailed interaction modes are yet to be further investigated.
Previous investigations have shown that Cyc1 is a subunit of mitochondrial complex III, a component of mitochondrial electron transfer and respiration chain, which is required for hypoxia-induced ROS production (Guzy et al., 2005). Our lab showed that Ngb over-expression ameliorated hypoxia-induced ROS in primary neurons (Liu et al., 2009). More interestingly, our preliminary data showed that Ngb-Cyc1 interaction was increased after OGD treatment and Ngb over-expression ameliorated OGD-induced increase of complex III activity and ROS level (unpublished data), thus the interaction between Ngb and Cyc1 may be responsible for Ngb’s role in decreasing hypoxia/OGD-induced ROS production.
Etfa is a subunit of electron transferring flavoprotein (ETF), which functions as a specific electron acceptors for primary dehydrogenases, transferring electrons to terminal respiratory systems such as electron-transferring-flavoprotein dehydrogenase (ETFDH) (Tsai and Saier, 1995). ETFDH further links the oxidation of fatty acids and some amino acids to oxidative phosphorylation in the mitochondria. The interaction between Ngb and Etfa suggests that Ngb may play roles in mitochondrial respiration and energy metabolism. Furthermore, VDAC is a key component of mitochondria permeability transition pore (mPTP) (Shimizu et al., 1999). Hypoxia/ischemia insults for neurons can cause mPTP opening, which leads to mitochondria dysfunction and subsequent apoptotic and necrotic cell death (Crompton, 1999). Inhibition of mPTP opening by cyclosporin A that binds to CyP-D, another mPTP component, and by Bcl-2 that binds to VDAC are neuroprotective that invoke complex signaling pathways such as blockage of Cytochrome c release to reduce neuron death (Tsujimoto and Shimizu, 2000, Precht et al., 2005). It is possible that the interaction of Ngb with VDAC may play a potential important role in mPTP opening following hypoxia/ischemia insults for neurons.
Other positive proteins are also thought important molecules in cellular functions. For instance, Dvl1 is the human homolog of the Drosophila dishevelled gene (dsh) and acts as a transducer molecule for cell proliferation and developmental processes, including segmentation and neuroblast specification (Malbon and Wang, 2006, Etheridge et al., 2008). Dvl1 functions as essential scaffold proteins that interact with diverse proteins in a few signaling pathways, including Wnt pathway (Wallingford and Habas, 2005). Further studies would be important in defining Ngb’s roles in these Dvl1-involved physiological processes and signaling pathways. Another positive molecule Ubc is an important source for ubiquitin (Ub) generation, the latter having critical functions in eukaryotic cells (Hochstrasser, 1996, Hershko and Ciechanover, 1998). Maintenance of cellular Ub levels to sustain its multiple cellular functions under all metabolic conditions is important for cellular survival. The expression of Ubc is induced by heat shock and certain other stresses (Fornace et al., 1989). The interaction between Ubc and Ngb may imply a possible function of Ngb in Ub-mediated cellular signaling in response to stress, not limited to hypoxic/ischemic insult.
Indeed, emerging evidence have suggested Ngb may function in multiple pathways leading to its neuroprotection role (Greenberg et al., 2008, Yu et al., 2009a, Brittain et al., 2010). For example, our laboratory performed a microarray screening to examine the effect of Ngb over-expression on the expression of hypoxic-response genes in mouse cortical neurons. We found that 20 genes were downregulated at early phase of OGD/Reoxygenaton in wild type neurons, while 12 of them were no longer significantly changed in Ngb-overexpressing neurons. These genes are broadly involved in neuronal function and survival, indicating the possible involvements of Ngb in multiple cell survival signaling pathways (Yu et al., 2009b). However, none of these genes were discovered as Ngb-interacting proteins in the present study. One explanation could be that the role of Ngb in other genes’ expression is mostly indirect, normally mediated through transcription factors, nuclear receptors and other DNA transcription regulators. Indeed, among the discovered Ngb-interacting proteins found in our current study, Dmap1 (DNA methyltransferase 1-associated protein 1) can directly influence target gene’s expression (Mohan et al., 2011). Another gene, Nr2c2ap (nuclear receptor 2C2-associated protein), may also affect other genes’ expression, although its detailed function is unclear (Wang et al., 2006). The physiological implication of Ngb interaction with these proteins needs to be further investigated.
It should be noted that our findings here just provide initial information of potential Ngb-interacting proteins, the actual binding between Ngb and these proteins have to be validated with multiple approaches such as Co-IP and double-immunostaining before further defining their physiological roles. We are aware that there could be false positives in yeast two-hybrid assay, such as Ngb and Gabarapl1 interaction. There could also be false negatives, for example, we did not find the previously reported Ngb-interacting protein, Cyt c (Bonding et al., 2008). Thus, the interaction network and functions of Ngb should not be limited to the proteins found in this study.
In summary, our present study for the first time identified potential Ngb-interacting proteins. The binding of Ngb to multiple proteins suggest Ngb may play multiple roles in energy metabolism, mitochondria function and signaling pathways for cellular physiological functions. Clearly, the roles and mechanisms of each individual Ngb-protein interactions need to be further validated and investigated both in vitro and in vivo. We believe our present study provided important fundamental basis for elucidating Ngb’s physiological function and neuroprotection mechanisms, it may also eventually help in development of therapeutic strategies for intervention and treatment of stroke and related neurological disorders.
Highlights.
A group of neuroglobin-interacting proteins were identified by yeast two-hybrid.
The interaction between Ngb and selected proteins were confirmed by Co-IP.
These proteins involve in energy metabolism, mitochondria function and cell signaling.
This study provides new clues for defining neuroglobin’s neuroprotection mechanism.
Acknowledgments
This work was supported in part by NIH grant R01-NS049476 (to X.W.), and Natural Science Foundation of Zhejiang Province, China (Y2090680). We appreciate Dr. Eng H. Lo for his very helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Bonding SH, Henty K, Dingley AJ, Brittain T. The binding of cytochrome c to neuroglobin: a docking and surface plasmon resonance study. Int J Biol Macromol. 2008;43:295–299. doi: 10.1016/j.ijbiomac.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Brunori M, Giuffre A, Forte E, Mastronicola D, Barone MC, Sarti P. Control of cytochrome c oxidase activity by nitric oxide. Biochim Biophys Acta. 2004;1655:365–371. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- Brunori M, Giuffre A, Nienhaus K, Nienhaus GU, Scandurra FM, Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc Natl Acad Sci U S A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M, Vallone B. A globin for the brain. Faseb J. 2006;20:2192–2197. doi: 10.1096/fj.06-6643rev. [DOI] [PubMed] [Google Scholar]
- Burmester T, Gerlach F, Hankeln T. Regulation and role of neuroglobin and cytoglobin under hypoxia. Adv Exp Med Biol. 2007;618:169–180. doi: 10.1007/978-0-387-75434-5_13. [DOI] [PubMed] [Google Scholar]
- Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341 ( Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden MC, Caubergs R, Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Stimers JR. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci. 2005;10:2373–2396. doi: 10.2741/1704. [DOI] [PubMed] [Google Scholar]
- Duong TT, Witting PK, Antao ST, Parry SN, Kennerson M, Lai B, Vogt S, Lay PA, Harris HH. Multiple protective activities of neuroglobin in cultured neuronal cells exposed to hypoxia re-oxygenation injury. J Neurochem. 2009;108:1143–1154. doi: 10.1111/j.1471-4159.2008.05846.x. [DOI] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fago A, Hundahl C, Dewilde S, Gilany K, Moens L, Weber RE. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance. J Biol Chem. 2004;279:44417–44426. doi: 10.1074/jbc.M407126200. [DOI] [PubMed] [Google Scholar]
- Fordel E, Geuens E, Dewilde S, De Coen W, Moens L. Hypoxia/ischemia and the regulation of neuroglobin and cytoglobin expression. IUBMB life. 2004;56:681–687. doi: 10.1080/15216540500037406. [DOI] [PubMed] [Google Scholar]
- Fordel E, Thijs L, Martinet W, Schrijvers D, Moens L, Dewilde S. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007;398:114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Fornace AJ, Jr, Alamo I, Jr, Hollander MC, Lamoreaux E. Ubiquitin mRNA is a major stress-induced transcript in mammalian cells. Nucleic Acids Res. 1989;17:1215–1230. doi: 10.1093/nar/17.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Jin K, Khan AA. Neuroglobin: an endogenous neuroprotectant. Current opinion in pharmacology. 2008;8:20–24. doi: 10.1016/j.coph.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. The Journal of biological chemistry. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- Hundahl C, Kelsen J, Kjaer K, Ronn LC, Weber RE, Geuens E, Hay-Schmidt A, Nyengaard JR. Does neuroglobin protect neurons from ischemic insult? A quantitative investigation of neuroglobin expression following transient MCAo in spontaneously hypertensive rats. Brain research. 2006;1085:19–27. doi: 10.1016/j.brainres.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Hundahl CA, Allen GC, Hannibal J, Kjaer K, Rehfeld JF, Dewilde S, Nyengaard JR, Kelsen J, Hay-Schmidt A. Anatomical characterization of cytoglobin and neuroglobin mRNA and protein expression in the mouse brain. Brain Res. 2010;1331:58–73. doi: 10.1016/j.brainres.2010.03.056. [DOI] [PubMed] [Google Scholar]
- Khan AA, Mao XO, Banwait S, DerMardirossian CM, Bokoch GM, Jin K, Greenberg DA. Regulation of hypoxic neuronal death signaling by neuroglobin. FASEB J. 2008;22:1737–1747. doi: 10.1096/fj.07-100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Mao XO, Banwait S, Jin K, Greenberg DA. Neuroglobin attenuates beta-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc Natl Acad Sci U S A. 2007;104:19114–19119. doi: 10.1073/pnas.0706167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegl JM, Bhattacharyya AJ, Nienhaus K, Deng P, Minkow O, Nienhaus GU. Ligand binding and protein dynamics in neuroglobin. Proc Natl Acad Sci U S A. 2002;99:7992–7997. doi: 10.1073/pnas.082244399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Pouranfar F, Lee SK, Morris MW, Wang Y, Gozal D. Neuroglobin protects PC12 cells against beta-amyloid-induced cell injury. Neurobiol Aging. 2008;29:1815–1822. doi: 10.1016/j.neurobiolaging.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu Z, Guo S, Lee SR, Xing C, Zhang C, Gao Y, Nicholls DG, Lo EH, Wang X. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J Neurosci Res. 2009;87:164–170. doi: 10.1002/jnr.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon CC, Wang HY. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 2006;72:153–166. doi: 10.1016/S0070-2153(05)72002-0. [DOI] [PubMed] [Google Scholar]
- Mohan KN, Ding F, Chaillet JR. Distinct roles of DMAP1 in mouse development. Mol Cell Biol. 2011;31:1861–1869. doi: 10.1128/MCB.01390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- Peroni D, Negro A, Bahr M, Dietz GP. Intracellular delivery of Neuroglobin using HIV-1 TAT protein transduction domain fails to protect against oxygen and glucose deprivation. Neuroscience letters. 2007;421:110–114. doi: 10.1016/j.neulet.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Precht TA, Phelps RA, Linseman DA, Butts BD, Le SS, Laessig TA, Bouchard RJ, Heidenreich KA. The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death Differ. 2005;12:255–265. doi: 10.1038/sj.cdd.4401552. [DOI] [PubMed] [Google Scholar]
- Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr, Bander NH, Peralta Soler A, Rajasekaran AK. Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell. 2001;12:279–295. doi: 10.1091/mbc.12.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner BS, Duong TT, Myers SJ, Witting PK. Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. Journal of neurochemistry. 2006;97:211–221. doi: 10.1111/j.1471-4159.2006.03726.x. [DOI] [PubMed] [Google Scholar]
- Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Robishaw JD. Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene. 2001;20:1653–1660. doi: 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MH, Saier MH., Jr Phylogenetic characterization of the ubiquitous electron transfer flavoprotein families ETF-alpha and ETF-beta. Res Microbiol. 1995;146:397–404. doi: 10.1016/0923-2508(96)80285-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Morishima I. Preparation and characterization of a chimeric zebrafish-human neuroglobin engineered by module substitution. Biochemical and biophysical research communications. 2005;330:591–597. doi: 10.1016/j.bbrc.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Nakano T, Morishima I. Oxidized human neuroglobin acts as a heterotrimeric Galpha protein guanine nucleotide dissociation inhibitor. J Biol Chem. 2003;278:36505–36512. doi: 10.1074/jbc.M305519200. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wang CP, Lee YF, Chang C, Lee HJ. Transactivation of the proximal promoter of human oxytocin gene by TR4 orphan receptor. Biochem Biophys Res Commun. 2006;351:204–208. doi: 10.1016/j.bbrc.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wystub S, Laufs T, Schmidt M, Burmester T, Maas U, Saaler-Reinhardt S, Hankeln T, Reuss S. Localization of neuroglobin protein in the mouse brain. Neuroscience letters. 2003;346:114–116. doi: 10.1016/s0304-3940(03)00563-9. [DOI] [PubMed] [Google Scholar]
- Yu Z, Fan X, Lo EH, Wang X. Neuroprotective roles and mechanisms of neuroglobin. Neurological research. 2009a;31:122–127. doi: 10.1179/174313209X389866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Liu J, Guo S, Xing C, Fan X, Ning M, Yuan JC, Lo EH, Wang X. Neuroglobin-overexpression alters hypoxic response gene expression in primary neuron culture following oxygen glucose deprivation. Neuroscience. 2009b;162:396–403. doi: 10.1016/j.neuroscience.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Peng W, Sun W, Xu E. Hypoxic preconditioning induces neuroprotection against transient global ischemia in adult rats via preserving the activity of Na(+)/K(+)-ATPase. Neurochem Int. 2011;59:65–72. doi: 10.1016/j.neuint.2011.04.016. [DOI] [PubMed] [Google Scholar]