Abstract
During endochondral ossification, the cartilage is surrounded by a layer of cells that constitute the perichondrium. Communication between osteoblasts in the perichondrium via N-cadherin adherens junctions is essential for endochondral bone growth. We observed that adherens junction molecule N-cadherin and its interacting partners p120, β-catenin and PTEN are expressed by cells present in the perichondrium. To study if N-cadherin mediated adherens junctions play a role in mediating signal transduction events during bone development, we utilized MC3T3E1 preosteoblasts plated at sub confluent (low) and confluent (high) densities to mimic adherens junction formation. When MC3T3E1 cells were plated at high density we observed an increase in phosphorylation of AKTSer473 and its downstream target GSK3Ser9, which coincided with an increase in Osterix, Osteomodulin and Osteoglycin gene expression. Using immunofluorescence, we identified N-cadherin, p120 and β-catenin localized at the membrane of MC3T3E1 cells. Treatment of confluent MC3T3E1cells with an N-cadherin junction inhibitor-EGTA and a PI3K inhibitor LY294002 resulted in reduction of phosphorylation levels of AKT and GSK3 and expression of Osterix, Osteomodulin and Osteoglycin. Furthermore, utilizing an N-cadherin blocking antibody resulted in reduced AKT signaling and Osterix gene expression, suggesting that osteoblast junction formation is linked to activation of PI3K signaling, which leads to osteoblast differentiation. To further explore the strength of this linkage, we utilized a conditional knockout approach using Dermo1cre to delete β-catenin and PTEN, two important proteins known to be essential for adherens junctions and PI3K signaling, respectively. In the absence of β-catenin, we observed a decrease in adherens junctions and AKT signaling in the perichondrium. PTEN deletion, on the other hand, increased the number of cells expressing N-cadherin in the perichondrium. These observations show that N-cadherin mediated junctions between osteoblasts are needed for osteoblast gene transcription.
Keywords: N-cadherin, Osteoblasts, Osterix, Adherens junctions
Introduction
Endochondral ossification is a process through which most of the long bones in vertebrates develop. During this process mesenchymal cells condense and form cartilage in the shape of the future long bones [1]. The mesenchymal cells in the center of these condensations start expressing N-cadherin which initiates chondrogenesis leading to the establishment of the growth plate. The cells that surround these nascent bones are fibroblastic in nature and form a layer called the perichondrium. As the development of the long bone continues, osteoprogenitors from the perichondrial region invade the hypertrophic chondrocytes along with blood vessels leading to the formation of the primary ossification center [2]. At this time of forming the primary ossification center, osteoblasts that are present in the perichondrium adjacent to the growth plate start expressing N-cadherin adherens junctions. N-cadherin (Cadherin2) is a calcium (Ca+2) dependent classical adherens junction protein, which plays an important role during endochondral ossification [3]. Two other forms of cadherin, Cadherin4 and Cadherin11 [4] are also expressed by osteoblasts along with - N-cadherin (Cadherin2). Recent studies have shown that both in vitro and in vivo overexpression of a dominant negative form of N-cadherin leads to delayed osteogenesis [5,6,7,8]. Conversely, overexpression of N-cadherin leads to inhibition of β-catenin dependent gene transcription and osteoblast differentiation, because of N-cadherin binding to LRP5 a Wnt signaling coreceptor [9]. These authors further showed that N-cadherin overexpression leads to inhibition of osteoblast proliferation because of decreased MAPK and PI3K/AKT signaling [10].
N-cadherin is a single transmembrane protein. The extracellular domain forms homophilic interactions with opposing cells. The intracellular domain of N-cadherin interacts with a number of key signaling molecules like β-catenin, α-catenin, EPLIN and p120 through which it can interact with the actin cytoskeleton and microtubules to anchor cells [11,12]. Interaction of N-cadherin with key signaling molecules raises the possibility that formation of adherens junctions modulates cellular signaling processes. To investigate, if formation of N-cadherin adherens junctions play a role during osteogenesis, we utilized preosteoblastic MC3T3E1 cells by plating them at low and high density. We observed N-cadherin junctions formation, coincided with an increase in pAKTSer473 and an increase in expression of osteoblast transcriptional factors Osterix, Osteomodulin and Osteoglycin. Blocking N-cadherin junctions directly using EGTA and N-cadherin blocking antibody [13] in preosteoblast cells resulted in decreased pAKTSer473 and pGSK3S9 and Osterix gene expression. Furthermore, conditional deletion of both β-catenin a key adherens junction protein and PTEN a negative regulator of PI3K signaling which interact at the adherens junction signalosome, showed defects in signaling mediated by N-cadherin. Deletion of β-catenin using Dermo1cre resulted in a decrease in N-cadherin expressing cells in the perichondrium and conditional knockout of Pten using Dermo1cre leads to an increase in N-cadherin expressing cells in the perichondrium with an increase in Osterix gene expression.
Material and Methods
Cell culture
MC3T3E1C14/C4 cells obtained from ATCC were cultured in 10% FBS α-MEM (modified Eagle’s medium) with 1% penicillin and streptomycin at 37°C in a 5% CO2 incubator. When performing cell density experiments, MC3T3E1C14 cells were plated at a density of 2×106 in a 10 cm diameter culture plate for confluent samples. For sub confluent samples cells were plated at 0.6×106 in 10 ml of growth media. Indirect immunofluorescence was done by culturing cells on cover slips coated with gelatin in 12 well plates. The coverslips with the cells adhering to them were treated with EGTA (1.5mM, 3hrs) followed by fixation and processing for immunofluorescence. Cells for western blotting were plated in 10 cm diameter culture plates at 2×106 in 0.5% FBS α-MEM. EGTA (1.8mM, overnight) and LY294002 (20µM) treatments were done to look at effects of junction formation on PI3K signaling. Calvarial osteoblasts were isolated using standard protocols reported in Yeh et al [14]. The LY294002 treatment for calvarial osteoblasts was 50 µM.
Total RNA isolation and Real time PCR
Total RNA was extracted using an RNAeasy kit from Qiagen following the protocol. 1µg of RNA was used to reverse transcribe the cDNA (Reverse transcription kit from Applied biosystems) in a total volume of 25ul. 3.5µl of the cDNA was added to a mastermix containing SYBR green and primers both forward and reverse mixed at a concentration of 2.5µM. The cycling conditions used were 50°C for 2 min, 95°C for 10 min, 40 cycles of 15sec at 95°C and 1 min at 60°C. The machine utilized was ABI 7000 sequence detection system, data obtained was analyzed using excel. The primers utilized for the Real time PCR were generated by using the software primer express supplied by Applied biosystems.
Osterix Fw ;5’CCA CTG GCT CCT CGG TTC T3’ Rv 5’GTC CCG CAG AGG GCT AGA G3’
Osteomodulin ;Fw 5’ CCA GCC CGA GGA CAA GAAA 3’ Rv 5’ TCG GTA AGC TTC ATA TTG GCA GTA 3’
18s ; Fw 5’ CAT GTG GTG TTG AGG AAA GCA 3’ Rv 5’ GTC GTGGGT TCT GCA TGA TG3’, Osteoglycin;Fw5’TTACCAACCAAGAAAGAAAATGATGA3’Rv5’GTGGTACAGCATCAATG TCAACTTC3’ Col1a1, ATF4, Runx2 and Osteocalcin primers have been described in Kawai et al [15]. The primers showed a single dissociation curve, the expression shown is relative to 18s used as an internal control and between control and experimental the lower value was set to one. The data was analysed using the method described by Livak and Schmittgen [16].
Immunofluorescence and Immunohistochemistry
Cells plated on cover slips were fixed with 4% PFA and then permeablized with 1% SDS. Then the coverslips were blocked in a blocking solution containing goat serum, BSA and PBS. After incubation with the primary antibody overnight at 4°C the cells were washed three times 10min each with 1xPBS containing 0.1% Triton-X-100 (wash buffer). This was followed by incubation with the cy3-conjugated secondary for 1hour at room temperature. The cells were then washed with the wash buffer three times and then mounted in prolong antifade mounting solution. Images were captured using a Zeiss Axioscope epifluorescence microscope, the brightness and contrast of the images was adjusted in Adobe photoshop 7.0. Confocal images were captured and processed as mentioned in [14]. Immunofluorescence on 5 micron thick paraffin embedded tissue sections was done utilizing Tyramide signal amplification fluorescence. The slides were heated to around 55–60°C for 12min, deparafinized by two consecutive washes in xylene for 5min each. The slides were then rehydrated with a series of decreasing alcohol concentration, followed by antigen unmasking by citrate retrieval in 10mM citrate buffer pH 6.0. The endogenous peroxidase activity was quenched with 1% H2O2 for 1hour. This was followed by washing with wash buffer (0.1M Tris-HCL pH 7.5, 0.15M Nacl with 0.05% Tween-20). The tissue sections were then blocked with TNB blocking buffer (0.1M Tris-HCl pH7.5, 0.15M NaCl, 0.5% blocking reagent) supplied by Perkin Elmer along with the tyramide kit followed by the primary antibody diluted in the TNB blocking buffer overnight at 4°C. The secondary biotinylated antibody incubation was done for 1hour at room temperature in a humidified chamber. This was followed by washing three times with the wash buffer for 5min each. The streptavidin horse radish peroxidase supplied with the kit was added at a dilution of 1/100 and incubated at room temperature for 30min. After three washes, the tyramide amplification reagent was added for 6 min at RT and mounted with prolong antifade reagent and cured over night before observing under the microscope [19].
Western Blotting
Protein lysates were prepared by lysing the cells in 2X Laemmli (125mM Tris HCl pH 6.8, 4% SDS and 20% Glycerol) buffer. The protein lysates were then reduced by adding 30mM DTT and heating the samples on heat block at 92°C for 10 minutes, EGTA treatments were done on confluent cells overnight at 1.8mM concentrations [17]. The western blot data shown is representative of data from at least two different experiments.
Antibodies
Anti-p120 (rabbit polyclonal, Santa Cruz), anti-β-catenin (mouse monoclonal, Santa Cruz), pan-cadherin, anti-N-cadherin mouse monoclonal (BD biosciences), anti-N-cadherin blocking antibody (Sigma). AntipAktSer473 (cell signaling, rabbit polyclonal 9271), anti-Akt (cell signaling, 9272 rabbit polyclonal), anti-Actin (mouse monoclonal, Chemicon international) anti-Erk (Zymed laboratories) Mouse anti MAP Kinase (ERK1+ERK2).
Mouse strains utilized; β-catenin flox/flox, Pten flox/flox , and Dermo1cre mice were described in [16,17,18] The animals were maintained in accordance with protocols approved by the animal care committee at the University of Texas Health Science Center at San Antonio.
Statistical analysis
For statistical significance p value was calculated using student’s t-test, the null hypothesis was rejected for p values < 0.05 and is shown as * on the chart using both graph pad and excel.
Results
Adherens junction proteins are expressed in the perichondrium
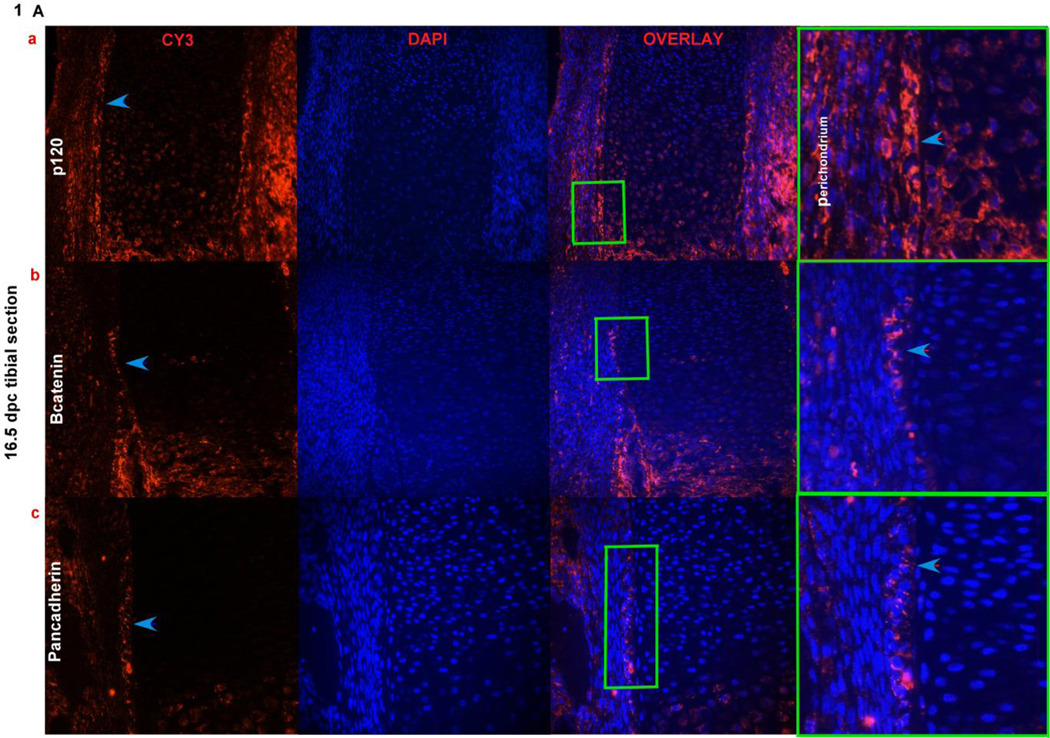
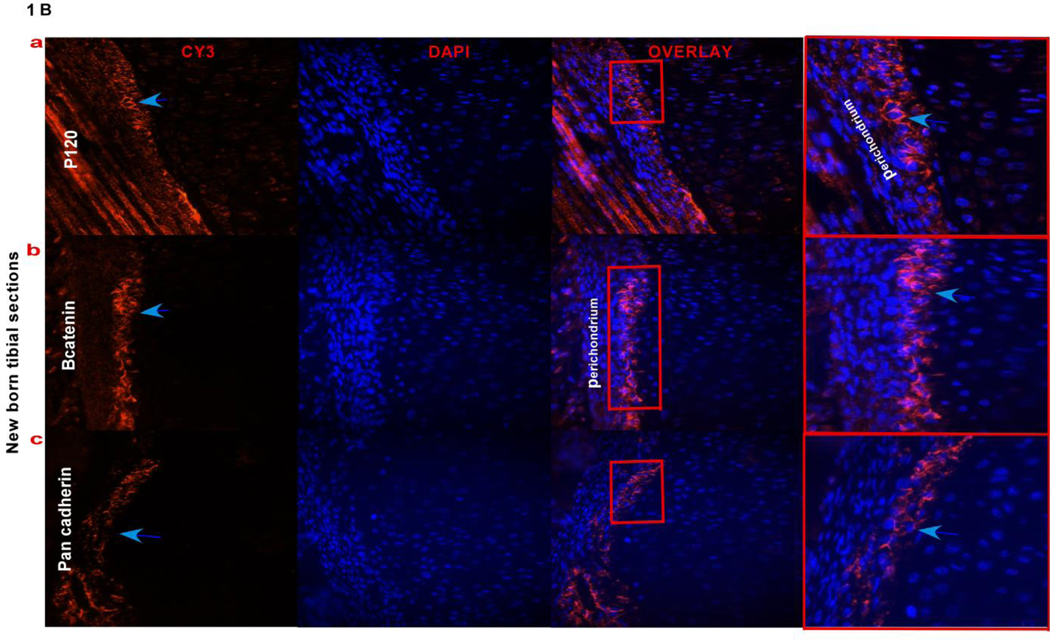
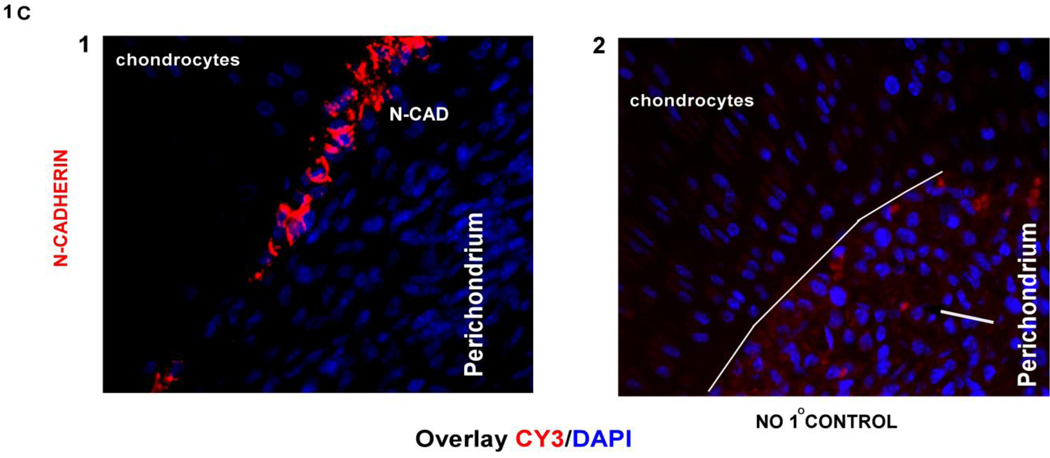
The developing long bone is surrounded by a sheath of cells called the perichondrium that contain osteoprogenitors. To investigate if adherens junction proteins are expressed in the perichondrium, 16.5 dpc and new born tibial sections were stained with p120 and β-catenin, as shown in (Fig. 1A a,b and Fig. 1B a,b) there is expression of these proteins in perichondrial cells. We further identified cells in the perichondrium of the long bone expressing N-cadherin in a very small subset of cells adjacent to the chondrocytes of the growth plate. Cadherin expression can be seen in 16.5 dpc perichondrial cells when stained with a pan-cadherin antobody (Fig. 1A c) and N-cadherin staining can be seen in new born tibiae (Fig. 1B c and Fig. 1C). The immunofluorescence data showed that N-cadherin adherens junctions form in the perichondrium. These observations suggest that as osteogenesis progresses during endochondral ossification, N-cadherin adherens junction’s form between cells in the perichondrium.
FIG. 1). Adherens junction proteins in the perichondrium.
Fig. 1Aa,b,c) Expression of p120, β-catenin and pan-cadherin in cells of the perichondrium (overlay last panel) where cells are counterstained with DAPI to indicate nuclei, using immunofluorescence on 16.5 dpc paraffin embedded tibial sections. Blue arrows point out the fluorescence signal for the antibodies used in the first panel; the boxed regions (green) in the overlay panel are magnified in the last panel showing the antibody signal pointed out with blue arrow heads. Fig. 1B a,b,c) Expression of p120, β-catenin and pan-cadherin in cells that are expressed in the perichondrial region of new born tibial sections. Blue arrows point out the signal for each of the antibodies used in the perichondrium. The boxed regions (red) in the overlay panel are magnified in the last panel showing the antibody signal pointed out with blue arrow heads. Fig. 1C) N-cadherin expression using immunofluorescence in new born tibial sections, showing signal in the perichondrium, pointed out by a arrow, the second panel shows a no primary antibody control with the perichondrium outlined by a white line. (DIC, differential interference contrast)
Activation of Osteogenesis in a P13K dependent manner
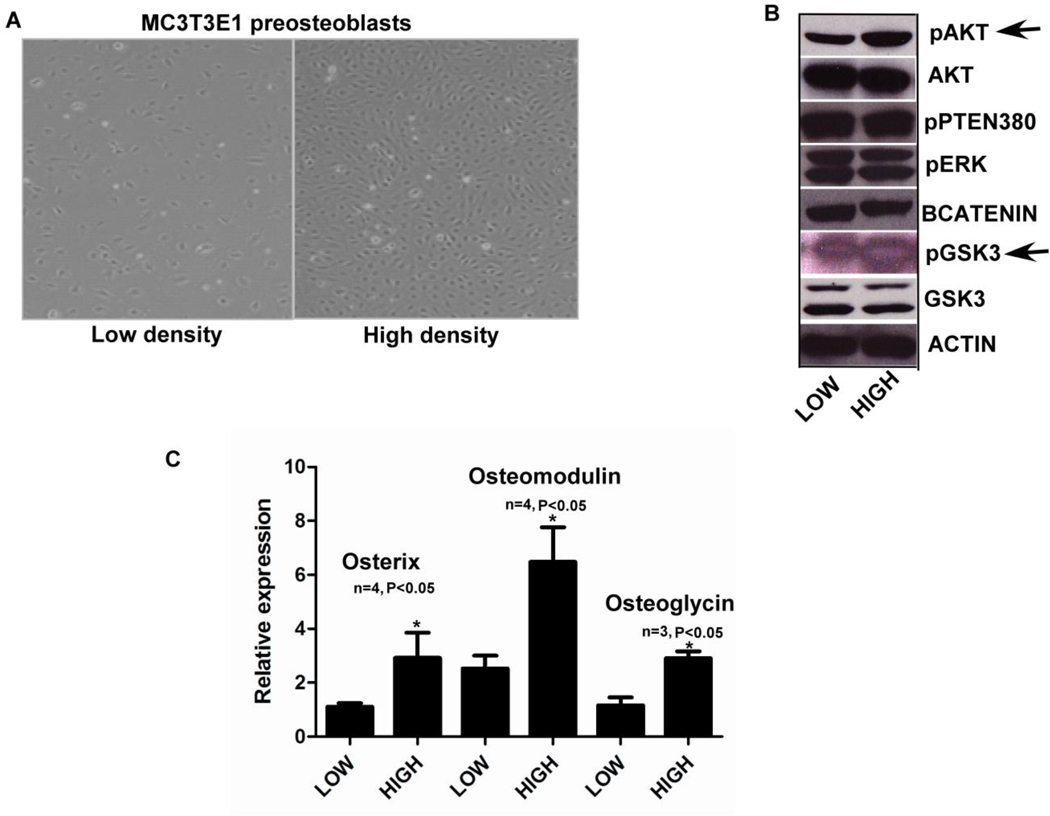
To study the relationship between adherens junction formation and osteoblast differentiation MC3T3E1 cells were plated at sub confluent (low density) and confluent (high density) densities to mimic formation of adherens junctions (Fig. 2A). To test if signaling molecules at the protein level are sensitive to cell density, we checked the activation status of a number of key signaling markers and observed an increase in pAKTSer473 and pGSK3Ser9 during confluent conditions (high density). Significantly, we did not observe any changes in other major signaling molecules like pERK (Fig. 2B). Using the low and high density conditions we studied the expression of osteoblast differentiation markers using QPCR and observed, Osterix an important transcriptional factor, Osteomodulin and Osteoglycin important osteoblast secreted matrix proteins were upregulated along with osteoblast specific markers like Runx2, and Osteocalcin (Supplemental Fig. 2A), indicating that when cells are plated at high density, they have an increase in pAKT along with increased osteoblast differentiation.
FIG. 2). Activation of Osterix, Osteomodulin and Osteoglycin in a P13K dependent manner.
Fig. 2A) MC3T3E1 cells plated at low and high density were then used for assaying gene expression using real time PCR and Western blotting for protein levels. Fig. 2B) When cells plated at low and high density were assayed for pAKT and total AKT levels using western blots we saw an increase in active AKT levels at high density along with increase in pGSK3S9 (pointed out with black arrows). There was no change in pERK or pPTEN levels (all the western blot images shown are representative of at least two different experiments). Fig. 2C) MC3T3E1 cells plated at sub confluent (low density) and confluent densities (high density), when assayed for osteoblast gene markers showed increases in Osterix, Osteomodulin and Osteoglycin gene expression using real time PCR. (n=3, p<0.05, all error bars indicate SD)
Effects of chemical inhibition on N-cadherin adherens junction formation
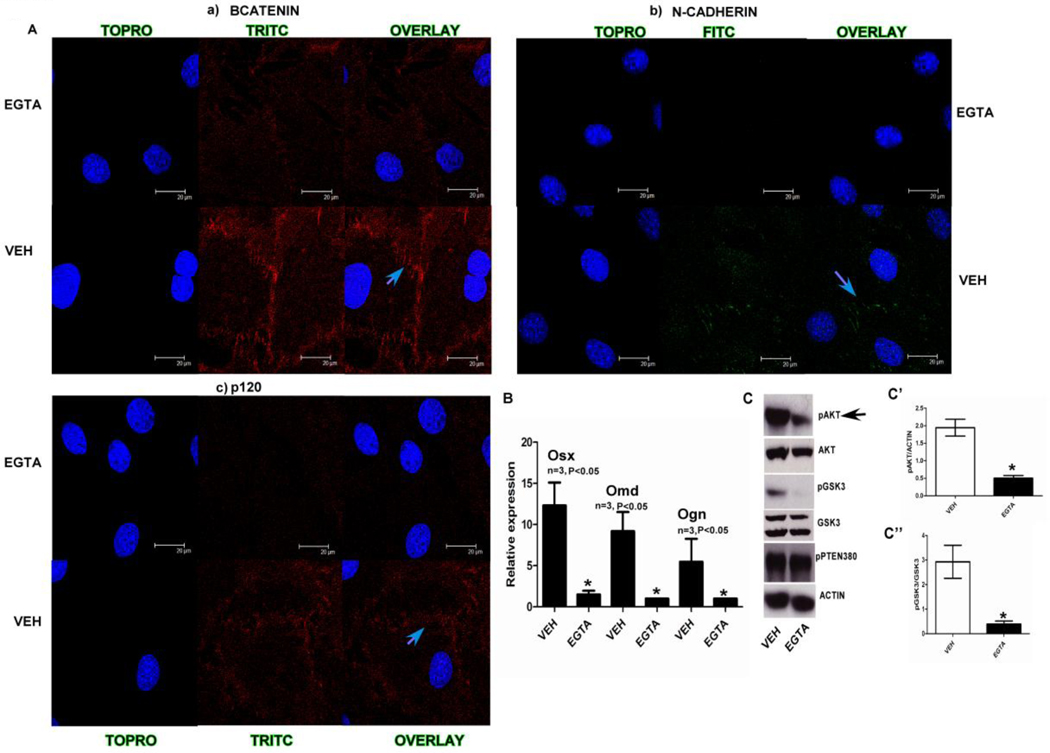
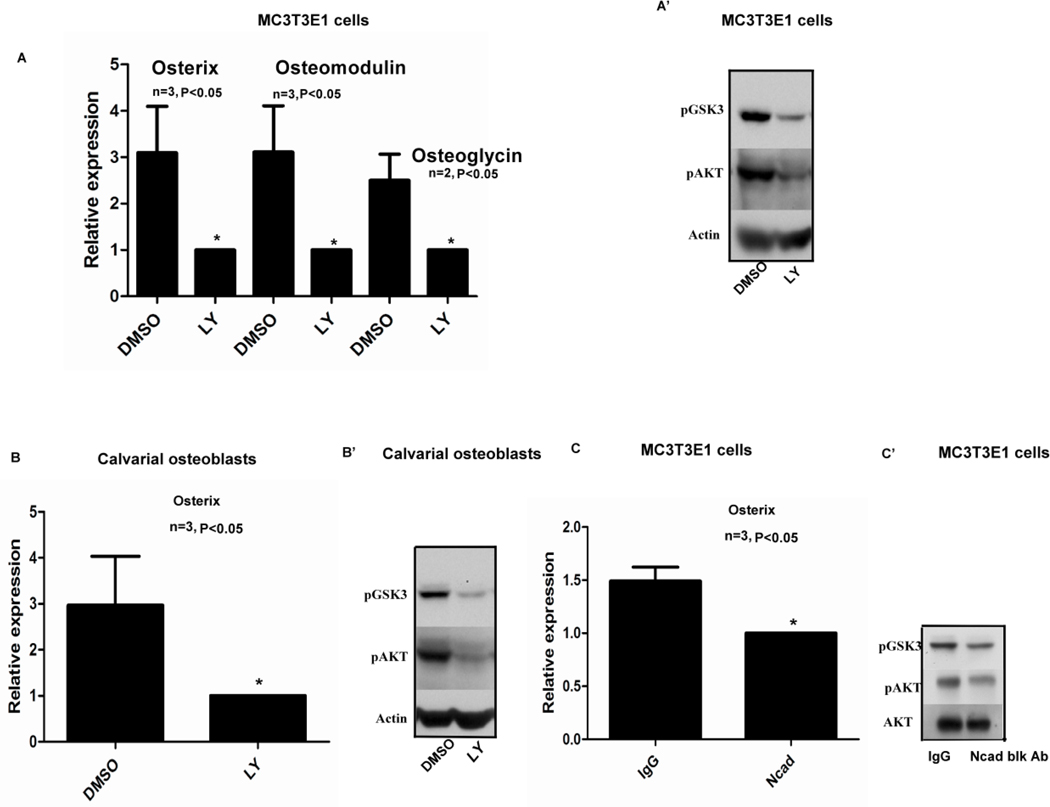
As there is an increase in AKT signaling and Osterix, Osteomodulin and Osteoglycin gene expression when adherens junction form, we tested the relationship between junction formation, activation of PI3K signaling and osteogenesis. To this end we used EGTA (1.5mM for 3hrs), a calcium chelator to inhibit adherens junction formation in MC3T3E1 cells. EGTA treatment abolished most of the signal for adherens junction molecules like β-catenin, N-cadherin and p120 from cell junctions. In Fig. 3A the blue arrows point out the cell-cell junctions that show expression of a) β-catenin, b) N-cadherin and c) p120 which is lost in the micrographs showing the EGTA treated samples. Furthermore, inhibition of N-cadherin junctions using EGTA when cells were plated at high density resulted in a decrease in pAKTSer473 and pGSK3Ser9 when compared to the control treatment (Fig. 3C). Inhibition of junctions with EGTA also caused decreased transcript levels of osteoblast differentiation markers Osterix, Osteoglycin, Osteomodulin, Osteocalcin and Col1a1 (Fig. 3B and Supplemental Fig.2B). Treating MC3T3E1 and calvarial osteoblasts plated at high density with a PI3K inhibitor LY294002 (Fig. 4 A’,B’) inhibited AKT signaling. Interestingly, Osterix and Osteomodulin mRNA levels could also be inhibited by treating both MC3T3E1 cells and primary calvarial osteoblasts with the LY294002 inhibitor as shown in (Fig. 4A,B). The above data showed us that when we inhibit adherens junction formation there is a loss of junctional proteins at the membrane which result, in a decrease in AKT signaling that leads to reduced expression of osteoblast differentiation markers like Osterix and Osteomodulin. To further elucidate the role of N-cadherin junctions, in osteogenesis directly, we utilized a monoclonal N-cadherin blocking antibody to inhibit junction formation in MC3T3E1 cells and observed that AKT signaling was inhibited at high cell densities along with a decrease in Osterix gene expression (Fig. 4C,C’).
FIG. 3). Effect of EGTA on adherens junctions mediated signaling.
Fig. 3A) Using immunofluorescence we identified a number of different adherens junction interaction proteins like a) β-catenin, b) N-cadherin and c) p120 observed expression at the cell membrane. This was inhibited when cells were treated with 1.5 mM EGTA a calcium chelator, blue arrows in the images point out the cell-cell junctions with the presence (vehicle treated) and absence of signal (EGTA treated) for the respective antibodies. Fig. 3B) Osterix, Osteomodulin and Osteoglycin gene expression was suppressed when confluent (high density) samples were treated with 1.8mM EGTA (n=3, p<0.05, all error bars indicate SD). Fig. 3C) when cells plated at sub confluent (low) and confluent (high density) were treated with 1.8 mM EGTA there was a decrease in pAKT, pGSK3. Actin and total GSK3 were used as loading controls for western blots for pAKT and pGSK3 respectively the densitometric measurements are shown as bar graphs in C’ and C” (n=3 p<0.05).
FIG. 4.
A, A’) Treatment of MC3T3E1 cells with a PI3K inhibitor LY294002 (20µM) decreased Osterix Osteomodulin and Osteoglycin gene expression. Additionally, there was a decrease in pAKT and pGSK3 levels at high density. Fig. 4B, B’) Confluent primary calvarial osteoblasts when assayed for Osterix gene expression using real time PCR after treatment with LY294002 (50µM) showed a suppression of Osterix expression when compared to the DMSO treated sample. There was also a suppression of pAKT and pGSK3 levels when the cells were plated at high density and treated with LY294002. Fig. 4C,C’) Treatment of confluent (high density) MC3T3E1 cells with 130µg of N-cadherin blocking antibody showed a slight but significant decrease in Osterix gene expression levels using real time PCR. (n=3, p<0.05, all error bars indicate SD). There was also reduced phospho AKT and GSK3 levels in the N-cadherin blocking antibody (70µg) treated samples compared to the IgG treated controls. Western blot images are representative of two different experiments.
Effect of β-catenin and PTEN conditional deletion on N-cadherin junctions
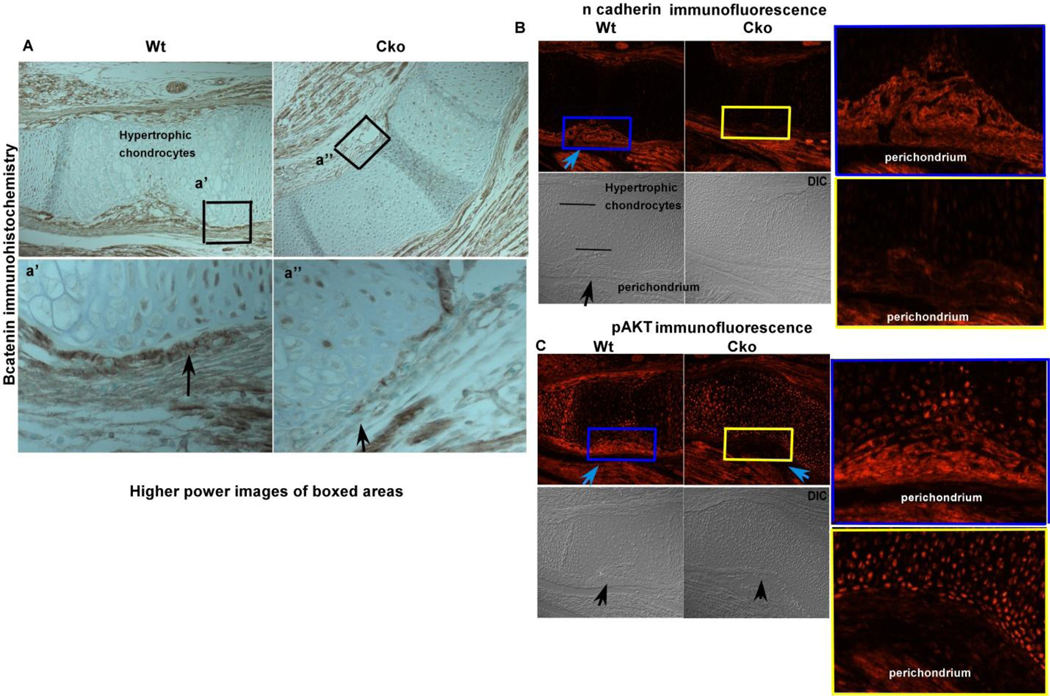
β-catenin and PTEN are two important proteins that are bound to the C-terminus of N-cadherin. To test if these proteins have any effect on the adherens junction complexes, we utilized Dermo1cre to conditionally delete β-catenin and Pten in mice. Conditional deletion of β-catenin using Dermo1cre at 15.5 dpc (Fig. 5A) resulted in decreased N-cadherin junctions in the β-catenin cko compared to control tibial perichondrium at 15.5 dpc (Fig. 5B). The perichondrial cells in the region where N-cadherin adherens junctions form in the wildtype show a decrease in activated AKT in the β-catenin cko (Fig. 5C). The conditional knockout data provide circumstantial evidence that when N-cadherin junctions form there is activation of osteoblast genes via AKT signaling.
FIG. 5). Effect of loss of β-catenin on adherens junctions.
Fig. 5A) Loss of β-catenin protein shown using immunohistochemistry and counterstained with methyl green, the higher power images of the boxed (black) regions are shown in the bottom panel below. The black arrows point out the regions where there is less protein signal in the cko (right panel) compared to the wt control. Fig. 5B) Decreased N-cadherin expression in the β-catenin cko compared to the wild type control. The blue arrow shows the region of the perichondrium which shows N-cadherin signal, the bottom panels (DIC, differential interference contrast) show the same regions pointed out with black arrow, the blue and yellow colored boxed regions are magnified and shown on the right hand side for both the cko and wt samples. Fig. 5C) Decreased pAKT levels in the β-catenin cko (blue arrow) compared to the wildtype control, the bottom panels are the DIC images. The area between the blue and yellow boxes are enlarged and shown besides the fluorescence images for better visualization. (DIC, differential interference contrast)
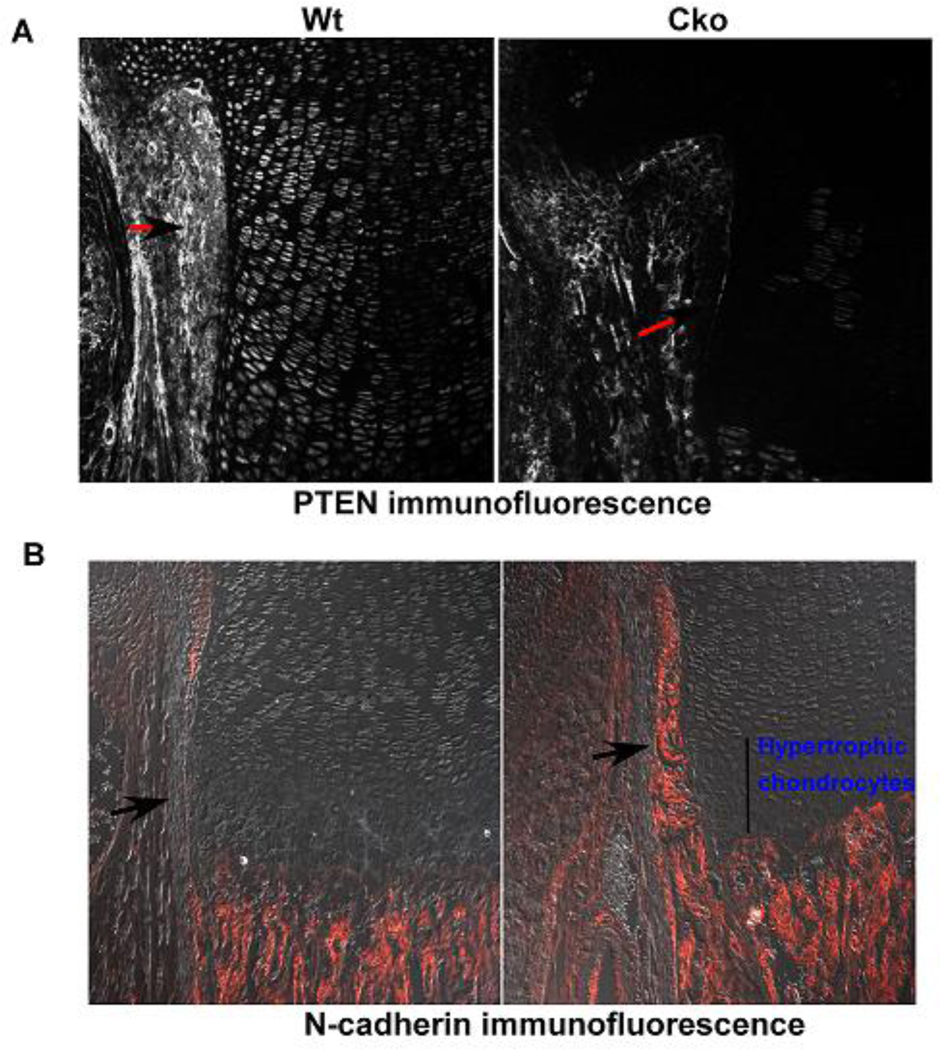
Conditional deletion of Pten using Dermo1cre resulted in a loss of PTEN protein in the perichondrium of the conditional knockout tibia (Fig. 6A) when compared to the wildtype control. We also observed that N-cadherin expressing cells in the perichondrium of the Pten cko increased compared to the wild type control (Fig. 6B). Furthermore, we have recently shown conditional knockout of Pten showed an increase in Osterix expression as seen by in situ hybridization suggesting that junction formation positively regulates osteogenesis [19]
FIG. 6). Effect of loss of PTEN on adherens junctions.
Fig. 6A) Wild type and Pten cko new born tibial sections, PTEN has been deleted with Dermo1cre, showing PTEN protein levels using immunfluorescence, arrows with black heads and red tails point out the cells in the perichondrium that have Pten protein expression in the wildtype and its loss in the cko Fig 6B) N-cadherin immunofluorescence in the Pten cko the perichondrium shows increased N-cadherin expression compared to the wildtype control, the panels show N-cadherin expression in the cells of the perichondrium (pointed out with black arrows) showing N-cadherin positive cells.
Discussion
In vertebrates, long bones develop through endochondral ossification where a chondrocyte template in the shape of the future long bone is replaced by mineralized bone. During endochondral ossification N-cadherin, cadherin11 and cadherin4 are the major cadherins that are expressed in osteoblasts. Recent studies using a conditional knockout approach to delete N-cadherin in bone suggested that N-cadherin is crucial for maintaining adhesion junctions when compared to other osteoblast specific junction cadherins like cadherin11 [20]. Therefore, N-cadherin was the focus of our studies. We observed expression of N-cadherin, β-catenin and p120 in 16.5 dpc tibia in vivo using immunofluorescence between cells present in the perichondrium (Fig. 1). When we looked at older tibial sections in the perichondrium (Fig. 1), we observed an increase in positive staining for adherens junction associated proteins between cells in the perichondrium, suggesting that N-cadherin adherens junctions may play a key role during endochondral ossification.
We used MC3T3E1 preosteoblasts to study adherens junction formation and its effect on osteogenesis in vitro and observed that there is an activation of PI3K signaling, as measured by pAKTSer473 and pGSK3Ser9 (Fig. 2B) when cells were plated at a high density. There was no significant activation of other signaling markers that we surveyed such as pERK, which rules out the involvement of MAPK signaling. Additionally, as cells in the perichondrium form junctions, there is an increase in Osterix, an osteoblast transcription factor expressed in cells that are committed to the osteoblast lineage. We also found Osteomodulin and Osteoglycin, (Fig. 2C) which belong to the small leucine rich repeat family (SLRP) show increased expression in osteoblasts during differentiation [21,22]. Recent published studies show 1) that when 2T3 cells (preosteoblast cells) become confluent in culture, they increase expression of Osterix, DLX5, BMP2 and Osteomodulin [23] and 2) over expression of Osteomodulin in vitro leads to increased osteoblast differentiation [24]. We also observed that Runx2 the major osteoblast transcriptional factor and its target gene Osteocalcin are upregulated. Our observations show that there is an activation of P13K signaling when osteoblast cells are plated at a high density along with an increase in osteogenic markers. To see if there is a link between these two observations we first confirmed that different N-cadherin adherens junction proteins like p120 [25] which belongs to the catenin family and β-catenin are expressed in MC3T3E1 cells in vitro. To inhibit adherens junctions we used EGTA which acts as a calcium ion chelator leading to a loss in structural integrity of N-cadherin cell-cell adhesions [30]. EGTA treatment resulted in a loss of expression of N-cadherin, p120 and β-catenin from the cell membrane, (Fig. 3A and Supplemental Fig1B). Under similar EGTA treatment conditions we also observed a decrease in Osterix, Osteomodulin, Osteoglycin, and Osteocalcin gene expression. Interestingly, ATF4 did not show major suppression in expression on EGTA treatment potentially suggesting that it might not be responsive to junction formation (Fig. 3B and Supplemental Fig. 2B), These changes in osteoblast specific gene expression could be correlated with a decrease in PI3K dependent signaling (Fig. 3C). Furthermore, direct blockage of N-cadherin junctions, using a monoclonal N-cadherin blocking antibody [13] led to repression of AKT signaling and osteogenesis as measured by Osterix gene expression (Fig. 4C,C’). Treating MC3T3E1 cells and primary calvarial osteoblasts directly with a PI3K inhibitor LY294002 showed that PI3K signaling controls Osterix and Osteomodulin gene expression (Fig. 4A,A’,B,B’). Taken together these observations along with recent published data suggests that upregulation of Osterix needs an active PI3K component, though Mandal et al [26] suggest this regulation of Osterix gene expression is in concert with BMP2 signaling. Previous findings from other groups have show that engagement of E-cadherin junctions in epithelial cells [27] and N-cadherin in neural progenitor cells [28] leads to an increase in PI3K signaling. Our study reports that N-cadherin junction formation in osteoblasts leads to increased PI3K signaling.
Recent studies in bone showed that using over expression of N-cadherin as well as a dominant negative form of N-cadherin affect osteogenesis by inhibiting the Wnt/PI3K/MAPK signaling processes. N-cadherin binds LRP5, a Wnt signaling coreceptor thereby negatively regulating β-catenin signaling [6,7,8,9,10]. Because we observed expression of β-catenin a transcriptional co activator and adherens junctional protein in the perichondrium, we investigated the role of β-catenin on N-cadherin mediated adherens junctions in vivo using the Dermo1cre deleter strain. The utilization of this cre which begins to be expressed at around 9.5 dpc leads to deletion of β-catenin in mesenchymal cells [17, 29]. There was an overall decrease in the N-cadherin positive cells in the perichondrium of the β-catenin cko compared to the wild type control. There is also decreased pAKTSer473 expression in the cells of the perichondrium of the β-catenin cko compared to the wildtype control (Fig. 5B,C). Our studies cannot rule out the possibility of loss of β-catenin effecting osteoblast formation through its role as a coactivator in the nucleus as EGTA treatment of MC3T3E1 cells could affect localization of βcatenin. We next deleted PTEN a key component of the PI3K signaling process which is expressed in the perichondrium and studied its role in adherens junction mediated osteogenesis. We looked at the expression of N-cadherin in the cells that are present in the perichondrium of the Pten cko and observed increased number of N-cadherin expressing cells (Fig. 6B). This suggested to us that as junctions form, there is an activation of PI3K signaling leading to activation of osteogenesis driven by the increased Osterix (Fig. 6C) expression. There is also the possibility that knockout of Pten activates AKT independent of the adherens junctions, but the in vitro data supports our hypothesis that adherens junction formation is important for PI3K signaling and its effects on osteogenesis.
Highlights.
1) N-cadherin mediated adherens junctions are formed between cells in the perichondrium during osteogenesis 2) N-cadherin mediated adherens junctions activate PI3K signaling cascade in osteoblasts leading to increased osteogenesis 3) Knockout of β-catenin and Pten, adherens junction proteins conditionally in preosteoblasts using Dermo1cre affects N-cadherin adherens junction formation.
Supplementary Material
Supplementary FIG. 1) Expression of p120, βcatenin and pan-cadherin in the perichondrium. The immunoflourescence images are magnified images of the overlay of CY3/DAPI shown in Figure 1A, B for better visualization of the signal.
Supplementary FIG. 2) Effect of Low density and High density on osteogenesis
Fig A) Expression of Collagen1a1 (Col1a1), Osteocalcin (Ocn) and Runx2 using QPCR between low density and high density. Fig B) Expression of osteoblast markers between vehicle and EGTA treated samples in high density samples.
Acknowledgements
Part of this study was submitted as part of a PhD thesis to the Department of Biochemistry, UTHSCSA by ARG. This study was funded by NIH grant R01AR053100 to MCN, AR045433 and AR54604 to CJR. We acknowledge the use of core facilities funded by NIH grants P20 RR181789 to Dr. Don Wojchowski and P30 RRO30927 to Dr. Robert Friesel at MMCRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary: The authors have nothing to disclose
References
- 1.Kronenberg H. Developmental regulation of the growth plate. Nature. 2003;423(6937):332. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 2.Karsenty G. The genetic transformation of bone biology. Genes & development. 1999;13(23):3037–3051. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 3.Woodward WA, Tuan RS. N-Cadherin expression and signaling in limb mesenchymal chondrogenesis: Stimulation by Poly-l-Lysine. Developmental Genetics. 1999;24:178–187. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<178::AID-DVG16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Cheng S-L, Lecanda F, Davidson MK, Warlow PM, Zhang S-F, Zhang L, Suzuki S, St. John T, Civitelli R. Human Osteoblasts Express a Repertoire of Cadherins, Which Are Critical for BMP-2–Induced Osteogenic Differentiation. Journal of Bone and Mineral Research. 1998;13:633–644. doi: 10.1359/jbmr.1998.13.4.633. [DOI] [PubMed] [Google Scholar]
- 5.Lai CF, Cheng S-L, Mbalaviele G, Donsante C, Watkins M, Radice GL, Civitelli R. Accentuated Ovariectomy-Induced Bone Loss and Altered Osteogenesis in Heterozygous N-Cadherin Null Mice. Journal of Bone and Mineral Research. 2006;21:1897–1906. doi: 10.1359/jbmr.060906. [DOI] [PubMed] [Google Scholar]
- 6.Mbalaviele G, Shin CS, Civitelli R. Perspective: Cell-Cell Adhesion and Signaling Through Cadherins: Connecting Bone Cells in Their Microenvironment. Journal of Bone and Mineral Research. 2006;21:1821–1827. doi: 10.1359/jbmr.060811. [DOI] [PubMed] [Google Scholar]
- 7.Castro CHM, Shin CS, Stains JP, Cheng S-L, Sheikh S, Mbalaviele G, Szejnfeld VL, Civitelli R. Targeted expression of a dominant-negative N-cadherin in vivo delays peak bone mass and increases adipogenesis. J Cell Sci. 2004;117:2853–2864. doi: 10.1242/jcs.01133. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S-L, Shin CS, Towler DA, Civitelli R. A Dominant Negative Cadherin Inhibits Osteoblast Differentiation. Journal of Bone and Mineral Research. 2000;15:2362–2370. doi: 10.1359/jbmr.2000.15.12.2362. [DOI] [PubMed] [Google Scholar]
- 9.Hay E, Laplantine E, Geoffroy V, Frain M, Kohler T, Muller R, Marie PJ. N-Cadherin Interacts with Axin and LRP5 To Negatively Regulate Wnt/(Baron and Rawadi)-Catenin Signaling, Osteoblast Function, and Bone Formation. Mol Cell Biol. 2009;29:953–964. doi: 10.1128/MCB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haÿ E, Nouraud A, Marie PJ. N-Cadherin Negatively Regulates Osteoblast Proliferation and Survival by Antagonizing Wnt, ERK and PI3K/Akt Signalling. PLoS ONE. 2009;4:e8284. doi: 10.1371/journal.pone.0008284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin–catenin complex to F-actin and stabilizes the circumferential actin belt. Proceedings of the National Academy of Sciences. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aono S, Nakagawa S, Reynolds AB, Takeichi M. p120ctn Acts as an Inhibitory Regulator of Cadherin Function in Colon Carcinoma Cells. The Journal of Cell Biology. 1999;145:551–562. doi: 10.1083/jcb.145.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncharova EJ, Kam Z, Geiger B. The involvement of adherens junction components in myofibrillogenesis in cultured cardiac myocytes. Development. 1992;114:173–183. doi: 10.1242/dev.114.1.173. [DOI] [PubMed] [Google Scholar]
- 14.Yeh L, Adamo M, Kitten A, Olson M, Lee J. Osteogenic protein-1-mediated insulin-like growth factor gene expression in primary cultures of rat osteoblastic cells. Endocrinology. 1996;137:1921–1931. doi: 10.1210/endo.137.5.8612532. [DOI] [PubMed] [Google Scholar]
- 15.Kawai M, Green CB, Horowitz M, Ackert-Bicknell C, Lecka-Czernik B, Rosen CJ. Nocturnin: a circadian target of Pparg-induced adipogenesis. Annals of the New York Academy of Sciences. 2010;1192:131–138. doi: 10.1111/j.1749-6632.2009.05221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Reinhold MI, Naski CM. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. The Journal of biological chemistry. 2007;282:3653–3663. doi: 10.1074/jbc.M608995200. [DOI] [PubMed] [Google Scholar]
- 18.Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development (Cambridge, England) 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- 19.Guntur AR, Reinhold MI, Cuellar J, Naski MC. Conditional ablation of Pten in osteoprogenitors stimulates FGF signaling. Development. 2011;138:1433–1444. doi: 10.1242/dev.058016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Benedetto A, Watkins M, Grimston S, Salazar V, Donsante C, Mbalaviele G, Radice GL, Civitelli R. N-cadherin and cadherin 11 modulate postnatal bone growth and osteoblast differentiation by distinct mechanisms. J Cell Sci. 2010;123:2640–2648. doi: 10.1242/jcs.067777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 22.Wendel M, Sommarin Y, Heinegård D. Bone Matrix Proteins: Isolation and Characterization of a Novel Cell-binding Keratan Sulfate Proteoglycan (Osteoadherin) from Bovine Bone. The Journal of Cell Biology. 1998;141:839–847. doi: 10.1083/jcb.141.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W, Harris MA, Heinrich JG, Guo D, Bonewald LF, Harris SE. Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone. 2009;44:32–45. doi: 10.1016/j.bone.2008.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehn A, Cerny R, Sugars R, Kaukua N, Wendel M. Osteoadherin is Upregulated by Mature Osteoblasts and Enhances Their In Vitro Differentiation and Mineralization. Calcified Tissue International. 2008;82:454–464. doi: 10.1007/s00223-008-9138-1. [DOI] [PubMed] [Google Scholar]
- 25.Daniel JM, Reynolds AB. The Catenin p120ctn Interacts with Kaiso, a Novel BTB/POZ Domain Zinc Finger Transcription Factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandal C, Drissi H, Ghosh Choudhury G, Ghosh-Choudhury N. Integration of Phosphatidylinositol 3-Kinase, Akt Kinase, and Smad Signaling Pathway in BMP-2-Induced Osterix Expression. Calcified tissue international. 2010:1–8. doi: 10.1007/s00223-010-9419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. The Journal of biological chemistry. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Woodhead GJ, Swaminathan SK, Noles SR, McQuinn ER, Pisarek AJ, Stocker AM, Mutch CA, Funatsu N, Chenn A. Cortical Neural Precursors Inhibit Their Own Differentiation via N-Cadherin Maintenance of [beta]-Catenin Signaling. Developmental Cell. 2010;18:472–479. doi: 10.1016/j.devcel.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhold MI, Kapadia RM, Liao Z, Naski CM. The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. The Journal of biological chemistry. 2006;281(3):1381–1388. doi: 10.1074/jbc.M504875200. [DOI] [PubMed] [Google Scholar]
- 30.Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. The Journal of Cell Biology. 1986;102:1832–1842. doi: 10.1083/jcb.102.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary FIG. 1) Expression of p120, βcatenin and pan-cadherin in the perichondrium. The immunoflourescence images are magnified images of the overlay of CY3/DAPI shown in Figure 1A, B for better visualization of the signal.
Supplementary FIG. 2) Effect of Low density and High density on osteogenesis
Fig A) Expression of Collagen1a1 (Col1a1), Osteocalcin (Ocn) and Runx2 using QPCR between low density and high density. Fig B) Expression of osteoblast markers between vehicle and EGTA treated samples in high density samples.