Abstract
Objectives
To assess calcium status in healthy pregnant women during limited sun exposure time in winter, and to demonstrate the possible effect of serum albumin alterations on serum total calcium level and the role of albumin adjusted calcium concentration.
Methods
Subjects enrolled in the study included 160 apparently healthy women divided equally into four groups (I - IV), group I was considered as the control group, composed of non-pregnant women. Groups II-IV were composed of pregnant women in the first, second and third trimesters respectively. Semiquantitative urine protein determination and measurement of serum total calcium, ionized calcium, albumin, phosphorous and creatinine with calculation of corrected calcium were performed in all groups. The results were statistically evaluated by standard statistical methods.
Results
There was no significant difference in serum ionized calcium, corrected calcium and phosphorous during pregnancy. However, there was a significant reduction of serum total calcium, albumin and creatinine in pregnant women at second and third trimesters. In each group, a significant positive correlation was observed between total calcium with corrected and ionized calcium.
Conclusion
In healthy pregnant women even during limited sun exposure time in winter, there was no need for calcium supplementation in spite of the continuous and progressive reduction of serum measured total calcium during the second and third trimesters due to dilutional hypoalbuminemia. During pregnancy, measured calcium is parallel to both corrected and ionized calcium and since there was no significant difference between measured and corrected calcium, therefore, measured calcium is a useful test in assessing calcium status and suggests the need to establish a reference range for pregnant women.
Introduction
Pregnancy is a normal physiological phenomenon with many biochemical changes including calcium (Ca) metabolism. The results of biochemical tests during pregnancy may therefore differ from the normal reference ranges so they may be mistakenly interpreted as abnormal leading to unnecessary and potentially dangerous therapeutic actions.1
Ca, the most abundant mineral in the human body, has several important functions.2 More than 99% of total body Ca is stored in the bones and teeth supporting their structure.3 The remaining 1% is found throughout the body in blood, muscle, and the fluid between cells needed for muscle contraction, blood vessel contraction and expansion, secretion of hormones and enzymes, and sending messages through the nervous system so that these vital body processes function efficiently.4 A constant level of Ca is maintained in body fluid and tissues within a narrow limit for normal physiological functioning so that when blood Ca decreases it stimulates the secretion of parathyroid hormone (PTH) which stimulates the conversion of vitamin D to its active form (calcitriol) in the kidneys.5,6 Calcitriol increases intestinal Ca absorption, which in turn stimulates bone Ca release by activating osteoclasts and decreasing urinary Ca excretion.2,6,7
On the other hand, when blood Ca rises to normal level, the parathyroid glands stop secreting PTH and the kidneys begin to excrete any excess Ca in the urine.2,6,7 Although this complex system allows for rapid and tight control of blood Ca levels, it does so at the expense of the skeleton.5 Total blood Ca represents the three forms in equilibrium with one another; ionized Ca (Ca+2) represents about 50-65% of total Ca, Ca bound to plasma protein mainly albumin (Alb) represents about 30-45% of total Ca and Ca complex with an ion as citrate represents about 5-10% of total Ca.2
Therefore, abnormal total blood Ca concentration may arise from alteration of plasma Alb concentration or Ca+2concentration. It is important to identify the latter group since the pathological levels of Ca+2 may be life threatening and the conditions themselves are amenable to treatment.2 Therefore, the aims of this study are to assess Ca status in healthy pregnant women during limited sun exposure time in winter, demonstrate the possible effect of serum Alb alterations occurring during pregnancy on serum total Ca level and the role of Alb adjusted Ca concentration.
Methods
The study was conducted from the period of December 2008 to February 2009. Subjects enrolled in the study included 160 women who attended a private laboratory in Mosul. The subjects were divided into four groups (group I - IV).
Group I was considered as the control group, composed of 40 apparently healthy non-pregnant women, their ages ranged from 18-38 years.
Group II was composed of 40 apparently healthy women in the first trimester pregnancy, their age ranged from 18-38 years.
Group III was composed of 40 apparently healthy women in the second trimester of pregnancy, their ages ranged from 18-38 years.
Group IV was composed of 40 apparently healthy women in the third trimester pregnancy, their ages alsoranged from 18-38 years.
A complete record of medical history was obtained for each patient, including name, age, duration of pregnancy, dietary habit, pastmedical, and pastsurgical and drug history. Members of the four groups had the same dietary habit, no pastmedical history of renal, pancreatic, hepatic and parathyroid disorders or any pastsurgical history of thyroid or drug history affecting the parameters included in the study.
Blood and urine samples were taken from all groups. Blood collection was carried out by venepuncture technique in the erect posture without tourniquet application for the measurement of total Ca, Ca+2, creatinine(cr), Alb and phosphorous(P). Colorimetric measurement of serum total Ca, cr, Alb and P were done by O-cresolphthalein complexone dye binding, Jaffe’s reaction kinetic, bromocresol green dye binding and ammonium molybdate methods respectively using a kits supplied by Biolabo Company (France) and are performed using Cecil spectrophotometer-CE 1011 in the biochemistry laboratory at Ninevah College of Medicine in Mosul.8-10 Serum Ca+2 measurement was done by ion-selective electrode method using Combysis II instrument in the biochemistry laboratory at Al-Batool obstetric and gynecological hospital in Mosul.8 Random urine samples were collected from all groups were estimated semiquantitatively for protein using urine strips supplied by Cybow Company (South Korea).11
The results were statistically evaluated by standard statistical methods using the mean (X), standard deviation (SD), range (minimum-maximum), reference range, linear regression analysis (Pearson correlation coefficient r), student’s t-test and normal distribution curve with computer software programs including Microsoft excel 2003 and SPSS 11.5 to evaluate the relation between the different parameters. Differences between the observations were considered not significant at p>0.05.12-15
Results
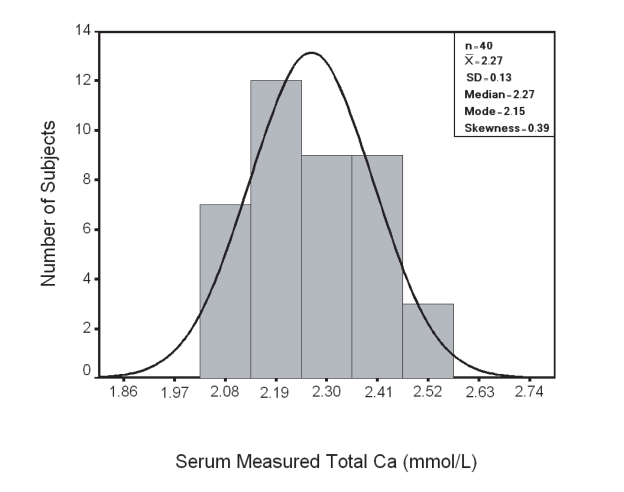
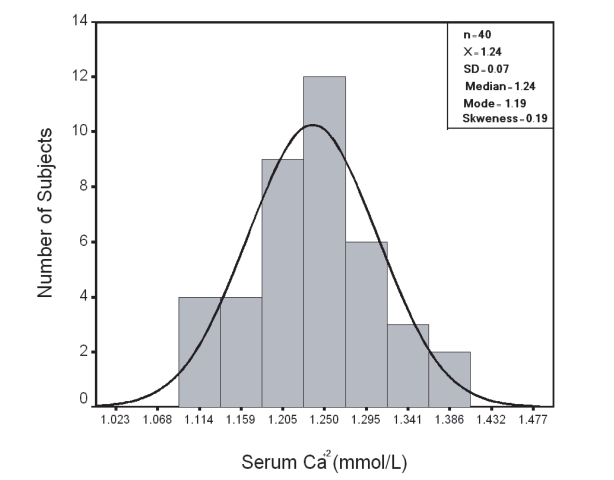
In group I; results of serum measured total Ca and Ca+2 showed a Gaussian distribution pattern (Fig. 1,2 respectively) with a reference range (95th centile confidence limit) calculated as X ± 2SD was 2.00-2.54 mmol/L and 1.10–1.38 mmol/L respectively.
Figure 1.
Distribution of Serum Total Ca in Group I
Figure 2.
Distribution of Serum Ca+2 in Group I
Adjustment of serum measured total Ca to Alb concentration was done in all groups to obtain serum corrected total Ca using the formula. Corrected Ca = Measured Ca + 0.02(40 – Al).2
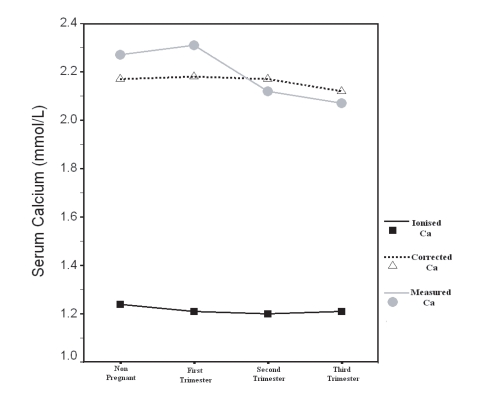
Table 1 illustrates X ± SD of the age, serum measured total Ca, corrected total Ca, Ca+2, Alb, P and cr in all groups with statistical comparison. There were no significant difference (p>0.05, Table 1, Fig. 7, 8) in the age, serum Ca+2, serum corrected total Ca and serum P between the groups.
Figure 7.
Measured, Corrected and Ionized Ca in Non-pregnant and Pregnant Women included in the Study
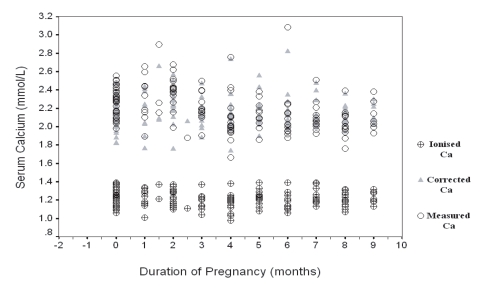
Figure 8.
Mean of Measured, Corrected and Ionized Ca in Non-pregnant and Pregnant Women in the First, Second and Third Trimesters
Table 1. Comparison of Parameters in Different Groups.
| Parameter | Group I Non Pregnant n = 40 |
Group II First Trimester n = 40 |
Group III Second Trimester n = 40 |
Group IV Third Trimester n = 40 |
P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Group III, IV | Group II, III | Group I, IV |
Group I, III |
Group I, II |
|||||
| Age (Years) | 24.18±5.11 | 24.25±4.83 | 24.95±6.03 | 25.80±5.68 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| S. Measured Total Ca (mmol/L) | 2.27±0.13 | 2.31±0.22 | 2.12±0.25 | 2.07±0.14 | > 0.05 | <0.001 | <0.001 | <0.01 | > 0.05 |
| S. Corrected Total Ca (mmol/L) | 2.17±0.15 | 2.18±0.20 | 2.17±0.21 | 2.12±0.12 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
| S. Ca+2 (mmol/L) | 1.24±0.07 | 1.21±0.09 | 1.20±0.09 | 1.21±0.08 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | >0.05 |
| S. Alb (g/L) | 44.8±4.8 | 46.5±4.4 | 37.7±4.4 | 37.4±3.2 | > 0.05 | <0.001 | <0.001 | <0.001 | > 0.05 |
| S. P (mmol/L) | 1.35±0.26 | 1.28±0.22 | 1.32±0.21 | 1.25±0.24 | > 0.05 | > 0.05 | > 0.05 | >0.05 | > 0.05 |
| S.cr (µmol/L) | 83.71± 18.93 | 82.69±18.00 | 74.87±10.01 | 72.15±10.33 | > 0.05 | <0.05 | <0.01 | <0.05 | > 0.05 |
Values are presented as Mean ± SD
SD: Standard Deviation
Serum measured total Ca (Table 1, Fig. 7,8) was significantly lower in group III and IV than in group I (p<0.01, p<0.001 respectively) and in group III than group II (p<0.001), however, there was no significant difference in group II compared with group I, and in group IV compared with group III (p>0.05).
Serum Alb (Table I) was significantly lower in group III and IV than in group I (p<0.001) and in group III than group II (p<0.001), however, there was no significant difference in group II compared with group I and in group IV compared with group III (p>0.05).
Serum cr (Table I) was significantly lower in group III and IV than in group I (p<0.05, p<0.01 respectively)and in group III than group II (p<0.05), but there was no significant difference in group II compared with group I and in group IV compared with group III (p>0.05).
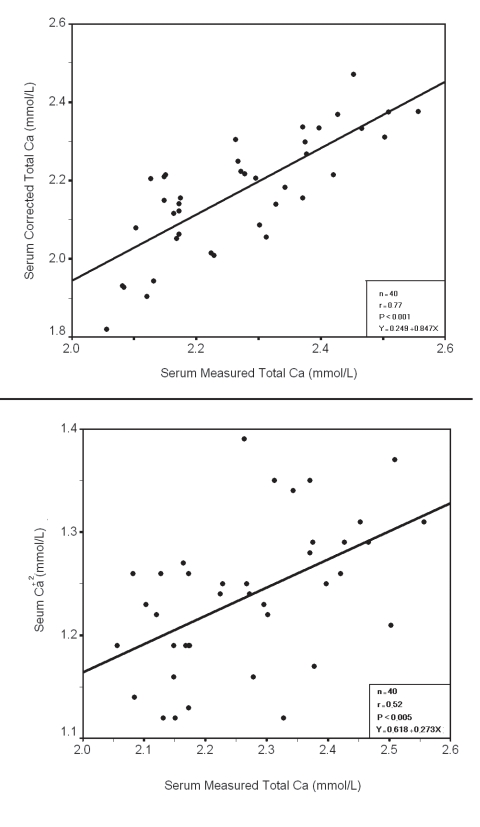
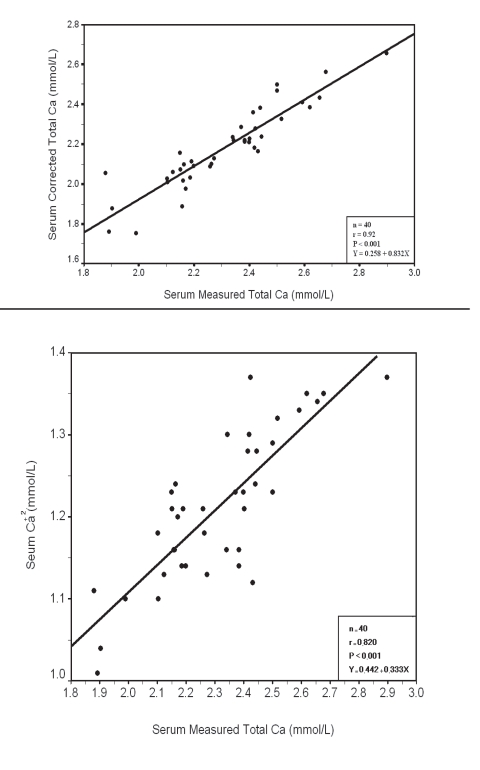
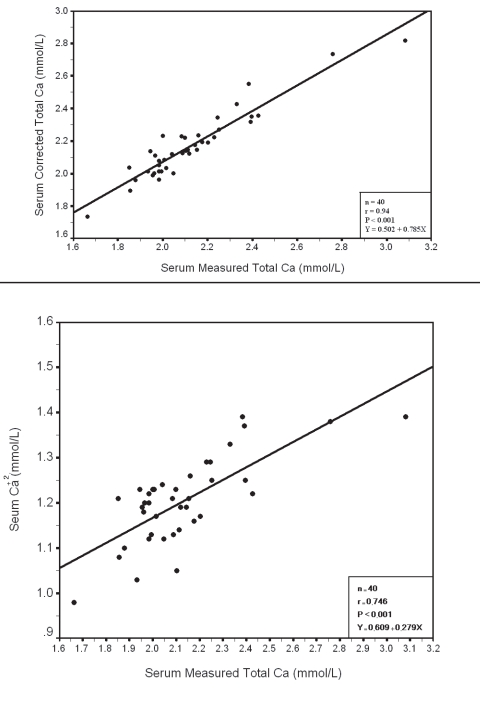
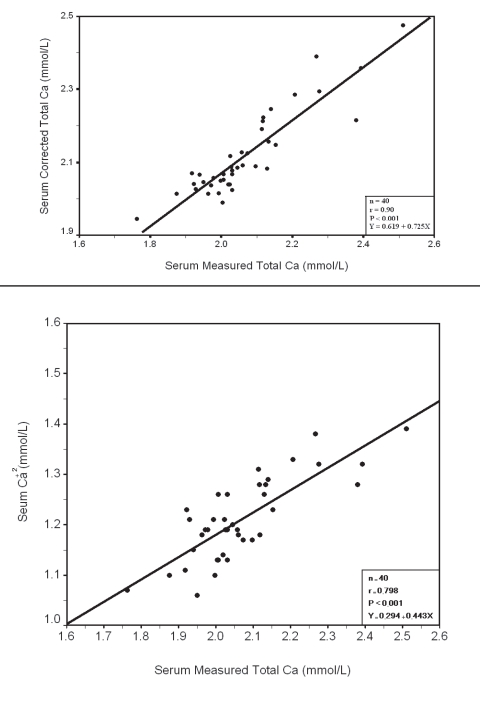
Within each group a significant positive correlation was observed between serum measured total Ca with corrected total Ca and Ca+2 (Fig. 3, 4, 5, 6), however there was no significant difference between measured and corrected Ca (p>0.05, Fig. 7, 8). Random urine samples of all groups showed no significant proteinuria.
Figure 3.
Relation between Serum measured Total Ca
Figure 4.
Relation between Serum measured Total Ca with corrected total Ca and Ca+2 in group 1 Total and group 2
Figure 5.
Relation between Serum measured Total Ca
Figure 6.
Relation between Serum measured total with Corrected Total Ca and Ca+2 in Group III Ca with Corrected Total Ca and Ca+2 in Group IV
Discussion
In this study, serum measured total Ca and Ca+2 results in the control group showed a Gaussian distribution pattern with a reference range was 2.00-2.54 mmol/L and 1.10–1.38 mmol/L respectively. In comparison to other studies, the reference range for serum measured total Ca was 2.1-2.8 mmol/L, 2.15-2.57 mmol/L and Ca+2 was 1.03-1.23, 1.15-1.35 mmol/L.8,16,17 This variation may be attributed to analytical factors affecting the measurement of total Ca by O-cresolphthalein complexone dye binding method including PH, temperature and interfering substances such as myoglobin, hemoglobin and bilirubin in addition to the preanalytical factors affecting total Ca and Ca+2 measurement such as PH alteration, change in posture, exercise, hyperventilation, alteration in protein binding, contamination with Ca and diurnal variation.2,8,18
To overcome the effect of these factors, all the measurements were performed in the same laboratory, venepuncture was performed between 3-5 pm in the erect posture without stasis. Blood samples were collected in new plain plastic tube and the effect of PH alteration on Ca+2 was overcome by correction of the results to PH 7.4.
In this study, serum measured total Ca level was significantly and continuously reduced during the second and third trimesters compared with the first trimester and the non-pregnant group. To find the aetiological point behind that, other factors that may affect serum measured total Ca level were excluded, these included; factors affecting body Ca and vitamin D status, since all have the same dietary habit (including vitamin D containing diet), same sun exposure as the study done in the same season and locality, no drug history of vitamin D or Ca administration.2,19,20
Additionally, there was no significant difference in serum P among the groups (p>0.05), since it is reduced in vitamin D deficiency, parathyroid diseases affecting Ca levels were excluded since all showed no significant difference of serum P as it altered in parathyroid diseases with no history of either parathyroid disease or thyroid surgery injured parathyroid gland, pancreatic disease affecting Ca levels was excluded by history, renal failure as a leading cause of hypocalcemia was excluded by history and by measurement of serum cr which was considered as a surrogate marker of glomerular filtration rate (GFR) as it was not significantly elevated among pregnant groups compared with control group.2,19-22 However, it was reduced significantly during the second and third trimesters (p<0.05, p<0.01 respectively) because of the fact that cr synthesis increased during pregnancy as a result of hypercatabolic state. This elevation is offset by the combined effect of fluid retention and physiological elevation of GFR in addition to exclusion of factors affecting serum cr level, mainly drugs, age and sex, as all were women within the same age group in addition to the use of kinetic method for its measurement to eliminate the effect of interfering substances.2,9
In this study, the reduction of serum Alb concentration in the second and third trimesters compared with the first trimester and non-pregnant women (p<0.001) was attributed to physiological overhydration during pregnancy after exclusion of other factors affecting serum Alb mainly malnutrition, proteinuria and chronic liver diseases.23-25
After exclusion of all factors affecting total Ca in the second and third trimesters, the reduction was attributed to the expanded intravascular space occurring during pregnancy which was reflected by the reduction of serum albumin as Ca bound to plasma protein mainly Alb represents about 30-45% of total Ca.1,2 This explanation was further confirmed by the results of the estimation of serum corrected total Ca and Ca+2 independent on serum Alb showed no significant differences between pregnant women in the third trimester and non-pregnant women (p>0.05). Although the study was performed in winter time with limited sun exposure which is required for vitamin D synthesis, the findings were in agreement with other studies which showed a reduction in vitamin D synthesis and hypocalcemia in winter.26 This can be explained by the fact that all the participants in this study had vitamin D containing diets to compensate for low vitamin D synthesis in winter.27 Geographical variation is also a contributing factor, since other studies were done in western countries in addition to that, it has been shown that 15-30 minutes of sun exposure on the hands and face per day is adequate for vitamin D production.28,29
The findings from this study on Ca are in agreement with other studies showing that serum measured total Ca decreased continuously during pregnancy, mainly in the third trimester, while corrected Ca, Ca+2 and P levels remained unchanged in spite of increased Ca demand which explained why calcitriol and the rate of intestinal Ca absorption at least doubled during pregnancy.3,24,30-36 In addition, the increased markers of bone resorption and formation during pregnancy compared to pre-pregnancy suggests that the maternal skeleton may contribute some mineral including about 80% of Ca to the fetus specially during the third trimester.24,37 For this reason, Ca recommendations established for pregnant women should not differ from that for non-pregnant women.4
However, other studies have reinforced the importance of vitamin D and Ca supplementation during pregnancy, suggesting that insufficient maternal intake and/or intestinal absorption in addition to increase fetal demand specially during the third trimester.31,36,38-40 A pregnant woman’s body requires 25-30 g of Ca to support the developing fetal skeleton which may contribute for this reduction.39,40 In contrast, other reports showed that maternal serum total Ca does not vary with increased in gestational age.41 While other reports have shown that there is increase in serum Ca in pregnant women compared to non–pregnant women.42 However, it should be noted that this observation was made in the Western (developed) countries where there is sufficient Ca and vitamin D intake during pregnancy since there is a direct linear relationship between dietary intake of Ca and its serum concentration.35,43
In this study, serum measured total Ca was significantly lowered during the second and third trimesters and by the calculation of corrected Ca and measurement of Ca+2 this reduction was eliminated. Serum measured total Ca is high significantly correlated with both corrected Ca and Ca+2 (Fig. 3, 4, 5, 6) with no significant difference between measured and corrected Ca (p>0.05) in each group (Fig. 7, 8). This finding is in agreement with other studies which showed that in spite of reduced serum measured total Ca results during pregnancy, it still remains within the reference range.1 However, there are contrasting views on the effects of gestational age and the interpretation of serum Ca levels as other studies have concluded that Ca homeostasis is considerably changed during pregnancy and non-pregnant reference limits are often invalid and the upper normal limit for total serum Ca during pregnancy is 2.37 mmol/L.30,40,41,44
Conclusion
In healthy pregnant women, even during limited sun exposure time in winter, there were no significant changes in both corrected and ionized Ca results. Therefore, there was no need for Ca supplementations in spite of serum measured total Ca continuously and progressively declining during the second and third trimesters due to dilutional hypoalbuminemia.
Since during pregnancy measured Ca is parallel with both corrected and ionized Ca with no significant difference between measured and corrected Ca, therefore, measured Ca is a useful test in assessing Ca status suggesting the need to establish a reference range for pregnant women.
Acknowledgements
The authors reported no conflict of interest and no funding has been received on this work.
References
- 1.Tran HA. Biochemical tests in pregnancy. Aust Prescr 2005;28:98-101 [Google Scholar]
- 2.Beckett G, Walker S, Rae P, Ashby P. Lecture notes clinical biochemistry. 4th ed. Blackwell Publishing 2005; 55, 72, 74, 82, 83, 87. [Google Scholar]
- 3.Shils ME, Oslen JA, Shike M, Ross AC. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins 1999. [Google Scholar]
- 4.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington DC: The National Academies Press 1997. [PubMed] [Google Scholar]
- 5.Weaver CM, Heaney RP. Calcium. In Modern Nutrition in Health and Disease (Shils M, Olson JA, Shike M, Ross AC, eds). 9th ed. Baltimore: Williams & Wilkins; 1999: 141-155. [Google Scholar]
- 6.Pearce SH, Thakker RV. The calcium-sensing receptor: insights into extracellular calcium homeostasis in health and disease. J Endocrinol 1997; 154: 371 c-378. [DOI] [PubMed]
- 7.Robert K, Murray RK, Daryl K, Granner DK, Rodwell VW. Harper’s illustrated biochemistry. 27th ed. McGraw-Hill Companies 2006; Chapter 41. [Google Scholar]
- 8.Endres DB, Rude RK. Mineral and bone metabolism. In: Tietz textbook of clinical chemistry (Burtis CA, Ashwood ER, eds). 2nd ed. Saunders com 1994: 1890, 1891, 1893, 1897-1904, 1908, 1909. [Google Scholar]
- 9.Whelton A, Watson JA, Robert RC. Nitrogen metabolism and renal function. In: Tietz fundamentals of clinical chemistry (Burtis CA, Ashwood ER, eds). 4th ed. Saunders com 1996: 578. [Google Scholar]
- 10.Silverman LM, Christenson RH. Amino acids and proteins. In: Tietz textbook of clinical chemistry (Burtis CA, Ashwood ER, eds). 2nd ed. Saunders com 1994: 702, 703. [Google Scholar]
- 11.Fischbach FT, Marshall BD. Manual of Laboratory & Diagnostic Tests. 7th ed. Lippincott Williams & Wilkins 2003; Chapter 3. [Google Scholar]
- 12.Chap T LE. Introductory biostatistics. Wiely interscience 2003: 283 – 292.
- 13.Jones D. Pharmaceutical statistics. Pharmaceutical press 2002.
- 14.Khachatryan A, Nikos M. Biostatistics and epidemiology. McGraw-Hill 1998. [Google Scholar]
- 15.Landau S, Everitt BS. A Handbook of Statistical Analyses using SPSS. A CRC Press Company USA 2003. [Google Scholar]
- 16.Rao DA, Le T, Bhushan V. First Aid for the USMLE Step 1. McGraw-Hill Medical 2008. [Google Scholar]
- 17.Larsson L, Ohman S. Serum ionized calcium and corrected total calcium in borderline hyperparathyroidism. Clin Chem 1978. Nov;24(11):1962-1965 [PubMed] [Google Scholar]
- 18.Winkel P, Statland BE, Bokelund H. The effects of time of venipuncture on variation of serum constituents. Consideration of within-day and day-to-day changes in a group of healthy young men. Am J Clin Pathol 1975. Oct;64(4):433-447 [DOI] [PubMed] [Google Scholar]
- 19.Arneson W, Brickell J, Chandler K. Endocrine Disorders and Function. In Clinical Chemistry: A Laboratory Perspective (Arneson W, Brickell J eds) 1st ed. FA Davis Company Philadelphia 2007: 416. [Google Scholar]
- 20.Starchan MW, Walker BR. Endocrine disease. In: Davidson’s principle and practice of medicine (Boon NA, Colledge NR, Walker BR, Hunter J eds) 20th edition. Churchill Livinqstone 2006: 772-775. [Google Scholar]
- 21.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003. Jul;139(2):137-147 [DOI] [PubMed] [Google Scholar]
- 22.Orth SR, Ritz E. The renal risks of smoking: an update. Pharmacology and therapeutics. Curr Opin Nephrol Hypertens 2002;1:483-488 . 10.1097/00041552-200209000-00002 [DOI] [PubMed] [Google Scholar]
- 23.Harewood WJ, Gillin A, Hennessy A, Armitstead J, Horvath JS, Tiller DJ. The effects of the menstrual cycle, pregnancy and early lactation on haematology and plasma biochemistry in the baboon (Papio hamadryas). J Med Primatol 2000. Dec;29(6):415-420 10.1111/j.1600-0684.2000.290606.x [DOI] [PubMed] [Google Scholar]
- 24.Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab 2001. Jun;86(6):2344-2348 10.1210/jc.86.6.2344 [DOI] [PubMed] [Google Scholar]
- 25.Arneson W, Brickell J. Assessment of liver function. In Clinical Chemistry: A Laboratory Perspective (Arneson W, Brickell J eds) 1st ed. FA Davis Company Philadelphia 2007: 253. [Google Scholar]
- 26.Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorders of mineral metabolism. In: Williams’s textbook of endocrinology (Larsen PR, Kronenberg HM, Melmed S, Polonsky KS). 10th ed. Saunders com 2003: 1318. [Google Scholar]
- 27.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007. Jun;92(6):2130-2135 10.1210/jc.2006-2250 [DOI] [PubMed] [Google Scholar]
- 28.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int 1999;9(5):394-397 10.1007/s001980050162 [DOI] [PubMed] [Google Scholar]
- 29.Sayre RM, Dowdy JC, Shepherd J, Sadig I, Bager A, Kollias N. Vitamin D production by natural and artificial sources. Orlando, Florida, Photo Medical Society Meeting. 3-1-1998. Ref Type: Conference Proceeding. 1998. [Google Scholar]
- 30.Dahlman T, Sjöberg HE, Bucht E. Calcium homeostasis in normal pregnancy and puerperium. A longitudinal study. Acta Obstet Gynecol Scand 1994. May;73(5):393-398 10.3109/00016349409006250 [DOI] [PubMed] [Google Scholar]
- 31.Singh HJ, Mohammad NH, Nila A. Serum calcium and parathormone during normal pregnancy in Malay women. J Matern Fetal Med 1999. May-Jun;8(3):95-100 [DOI] [PubMed] [Google Scholar]
- 32.Gertner JM, Coustan DR, Kliger AS, Mallette LE, Ravin N, Broadus AE. Pregnancy as state of physiologic absorptive hypercalciuria. Am J Med 1986. Sep;81(3):451-456 10.1016/0002-9343(86)90298-6 [DOI] [PubMed] [Google Scholar]
- 33.Seki K, Makimura N, Mitsui C, Hirata J, Nagata I. Calcium-regulating hormones and osteocalcin levels during pregnancy: a longitudinal study. Am J Obstet Gynecol 1991. May;164(5 Pt 1):1248-1252 [DOI] [PubMed] [Google Scholar]
- 34.Seely EW, Brown EM, DeMaggio DM, Weldon DK, Graves SW. A prospective study of calciotropic hormones in pregnancy and post partum: reciprocal changes in serum intact parathyroid hormone and 1,25-dihydroxyvitamin D. Am J Obstet Gynecol 1997. Jan;176(1 Pt 1):214-217 10.1016/S0002-9378(97)80039-7 [DOI] [PubMed] [Google Scholar]
- 35.Ikechukwu IC, Chinyere U, Uzoma IC, Gilbert N, Hughs MA, Ekenedirichukwu OJ, et al. Does Pregnancy actually affect serum calcium and inorganic phosphate levels? Shiraz E-Medical Journal 2005;6. [Google Scholar]
- 36.Huang HM, Leung PL, Sun DZ, Zhu MG. Hair and serum calcium, iron, copper, and zinc levels during normal pregnancy at three trimesters. Biol Trace Elem Res 1999. Aug;69(2):111-120 10.1007/BF02783863 [DOI] [PubMed] [Google Scholar]
- 37.Reddy GS, Norman AW. Regulation of vitamin D Metabolism in Normal Human Pregnancy. Obstet Gynecol 1983;88:170-173 [DOI] [PubMed] [Google Scholar]
- 38.Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res 2007. Dec;22(Suppl 2):V39-V44 10.1359/jbmr.07s215 [DOI] [PubMed] [Google Scholar]
- 39.Trotter M, Hixon BB. Sequential changes in weight, density, and percentage ash weight of human skeletons from an early fetal period through old age. Anat Rec 1974. May;179(1):1-18 10.1002/ar.1091790102 [DOI] [PubMed] [Google Scholar]
- 40.Ardawi MS, Nasrat HA, BA’Aqueel HS. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 1997. Oct;137(4):402-409 10.1530/eje.0.1370402 [DOI] [PubMed] [Google Scholar]
- 41.Standley CA, Whitty JE, Mason BA, Cotton DB. Serum ionized magnesium levels in normal and preeclamptic gestation. Obstet Gynecol 1997. Jan;89(1):24-27 10.1016/S0029-7844(96)00380-8 [DOI] [PubMed] [Google Scholar]
- 42.Pedersen EB, Johannesen P, Kristensen S, Rasmussen AB, Emmertsen K, Møller J, et al. Calcium, parathyroid hormone and calcitonin in normal pregnancy and preeclampsia. Gynecol Obstet Invest 1984;18(3):156-164 10.1159/000299073 [DOI] [PubMed] [Google Scholar]
- 43.Easthan RD. Calcium. In: Biochemical values in clinic Medicine. 7th ed. (Brenda Slavin) John wright Sons 1985; 66 – 70, 281 – 284. [Google Scholar]
- 44.Olatunbosun DA, Adeniyi FA, Adadevoh BK. Serum calcium, phosphorus and magnesium levels in pregnant and non-pregnant Nigerians. Br J Obstet Gynaecol 1975. Jul;82(7):568-571 10.1111/j.1471-0528.1975.tb00688.x [DOI] [PubMed] [Google Scholar]