Abstract
Fetomaternal hemorrhage refers to the entry of fetal blood into the maternal circulation before or during delivery. Very small amount of fetal red cells are normally detectable in all pregnancies. Massive fetomaternal bleed is very rare and even rarer is the resultant severe anemia causing early neonatal death, despite an uneventful normal pregnancy until the end. Antenatal fetomaternal hemorrhage is a pathological condition with a wide spectrum of clinical variation. Secondary to the resultant anemia, fetomaternal hemorrhage may have devastating consequences for the fetus such as neurologic injury, stillbirth, or neonatal death. The Presentation is frequently without an evident precipitating factor. Recognition may become apparent only after injury has occurred, if at all. The most common antenatal presentation is decreased fetal activity and a heightened index of suspicion is warranted in cases of persistent maternal perception of decreased fetal movements.
Keywords: Fetomaternal hemorrhage (FMH), Fetal anemia, Hydrops fetalis, Kleihaure-Betke test (KBT), Cardiotocograph (CTG)
Introduction
Small amount (<0.1 ml) of fetal blood is commonly found in maternal circulation.1 Massive fetomaternal hemorrhage (FMH) involves fetal blood loss into the maternal circulation of more than 150 ml or otherwise more than half the fetal blood volume.2 Large bleeds can be a cause of intrauterine death in up to 0.04% of all births.3 Most cases of acute and chronic fetomaternal hemorrhage are idiopathic in origin, most often spontaneous and involve uncomplicated near term pregnancies.4,5 We report a case of severe and fatal fetomaternal transfusion, which was proved by Kleihaure-Betke test. The purpose of this report is to make others aware of this rare but potentially fatal condition.
Case Report
A 37-year-old female in her third pregnancy at 35 weeks gestation by menstrual dates presented to her obstetrician with markedly decreased fetal movements. There was no history of pain, trauma or vaginal bleeding. The patient was known to be Rhesus positive. Past obstetric history revealed a normal delivery at term in her first two pregnancies. Current pregnancy was uneventful until 2 days before delivery when she started to feel reduced fetal movements.
On arrival at the delivery unit, cardiotocograph (CTG) showed reduced beat to beat variability with variable deceleration for which the mother underwent emergency lower segment cesarean section (LSCS) at 35 weeks of gestation. Outcome was a baby girl. Apgar scores were 2 and 3 at 1 and 5 minute respectively.
On examination, the baby was markedly pale with respiratory depression not responding to bag and mask ventilation for which the baby was immediately intuabated and connected to mechanical ventilation. The liver was palpable 4 cms below the right costal margin (suggestive of congestive cardiac failure). The spleen was not palpable, and no petechiae was noted. Birth weight was 2.8 kg. Subcutaneous edema was also noted. Cord Blood gas Ph: 7.063; HCO3: 14.2; base excess was -12.2; blood sugar: 2 mol; and lactate: 15 mmol.
Our initial impression was severe fetal anemia leading to birth asphyxia. The cause of fetal anemia was most likely to be due to fetomaternal hemorrhage. Hematological results were as follow: Anemia with higher reticulocyte counts was suggestive of chronicity of inutero bleed. (Table 1)
Table 1. Hematological results.
| Parameter | HB (g/dl) |
Hematocrit (%) | Retics (%) | Platelets (10*9/L) |
|---|---|---|---|---|
| Cord Blood | 3.2 | 10 | Not done | 110 |
| At 6 hrs. | 7.3 | 22 | 8.6 | 113 |
| At 24 hrs. | 10.1 | 31 | 6.0 | 82 |
There was evidence of multiorgan involvement in the form of deranged coagulation (Table 2), markedly deranged liver function test (Table 3), and marked hematuria (++++ on urine dipstick examination), which were consistent with severe degree of birth asphyxia affecting more than one organ systems.
Table 2. Coagulation profile.
| Test | Value | Normal range |
|---|---|---|
| Prothrombin Time | 36.8 sec | 9.9 – 12.4 |
| Activated Partial Thromboplastin Time | 61 sec | 31 - 55 |
| INR | 3.31 | 0.91 – 1.08 |
| Fibrinogen | 0.9 g/L | 1.7 – 3.6 |
Table 3. Liver function tests.
| Test | Value | Normal range |
|---|---|---|
| Alanine aminotransferase | 333 IU/L | 0 - 31 |
| Aspertate aminotransferase | 1970 U/L | 0 - 32 |
| Albumin | 16 g/L | 28 - 44 |
| Total protein | 25 g/L | 46 - 70 |
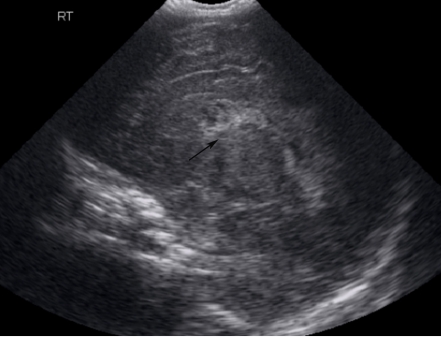
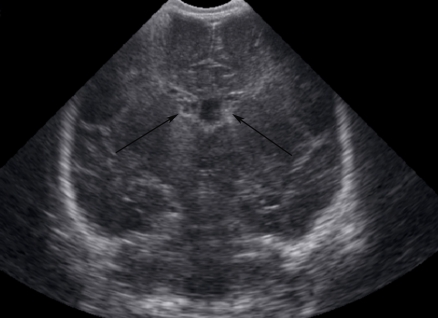
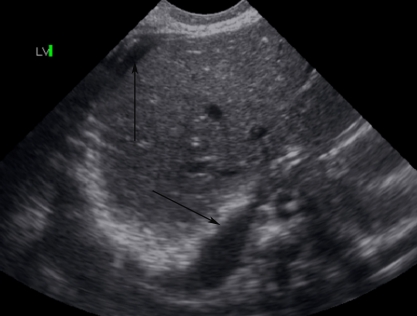
Cranial ultrasonography showed grade 1 Intraventricular hemorrhage (Figs. 1 & 2), which was most likely relate to severe birth asphyxia. Right sided pleural effusion and ascites were also confirmed by ultrasonography. (Fig. 3)
Figure 1.
Cranial US, sagittal view showing Intraventricular hemorrhage (arrow).
Figure 2.
Cranial US, coronal view showing bilateral Intraventricular hemorrhage (arrows).
Figure 3.
Ultrasound showing mild peritoneal fluid (arrow above) and pleural fluid (arrow below).
The presence of subcutaneous edema and fluid in two body cavities (peritoneal and pleural) were suggestive of hydrops fetalis. The baby required high ventilatory settings and 100% oxygen with oxygen saturartions around 80%. Blood pressure was irrecordable with refractory hypotension due to blood loss. Two boluses of saline, each 10 ml per kg were given as well as packed red blood transfusion and inotropic support with dopamine; dobutamine and adrenaline were administered without any improvement in clinical condition.
The Kleihaure-Betke test (KB test) was done on the mother’s blood, which revealed that maternal circulation had 6% of fetal blood, which is nearly 140 ml of fetal blood. This confirms a very large amount of fetomaternal hemorrhage, reaching up to 50 ml/kg of fetal blood. When the transfused volume exceeds or equals 20 ml/kg, massive fetomaternal hemorrhage may lead to severe prenatal or neonatal complications including early neonatal death.
Inspite of extensive supportive measures being taken, the baby’s condition continued to deteriorate and the baby expired at 28 hrs of life. The final impression in our case was chronic fetomaternal hemorrhage leading to severe anemia causing birth asphyxia and non-immune hydrops.
Discussion
Fetomaternal hemorrhage can begin any time from the mid-first trimester onwards. The causative factor is usually a breach in the integrity of the placental circulation. The presence of fetal red cells in the maternal circulation is not abnormal. By term, 50% of mothers will have detectable fetal red cells. Though 96-98% of pregnancies have very small leaks of up to 2 ml.6
There is no universally accepted definition of the volume of fetal erythrocytes in the maternal circulation that constitutes a massive FMH; volumes of 10 to 150 mL have been proposed. FMH greater than 80 mL and greater than 150 mL is estimated to occur in 1 in 1000 deliveries and 1 in 5000 deliveries, respectively. Various studies have described the incidence of FMH >20-30 ml at delivery to be about 1 in 200-300 deliveries.4,7,8
Massive fetomaternal hemorrhage (>150 ml) [in this case 140 ml] occurs in 0.12 to 0.5% of pregnancies.9 A standard approach to assess the severity of FMH is to estimate the percentage of the fetal blood volume represented by the FMH. A FMH of 20 mL/kg, which represents 20% of the fetoplacental blood volume is considered massive because it is associated with significant fetal/neonatal morbidity or mortality.
Other associated factors include: direct trauma to the abdomen, motor vehicle accident, abruptio placentae, vasa previa with membranous insertion, choriocarcinoma, amniocentesis, chorionic villous sampling and external cephalic version.
In this case, placental analysis revealed umbilical cord with 3 blood vessels and no funisitis. The membranes have no chorioamnionitis. Placenta shows multiple chorionic villi with no villitis or intervillositis. However, in large proportion of cases (up to 82%), the cause of fetomaternal hemorrhage remains unknown.9
Pregnancies complicated by FMH often proceed normally, although signs of fetal compromise may appear if the degree of FMH is significantly high.10 In our case, this was evident by the absent fetal movements for two days before delivery. Further evidence of fetal compromise was the detection of abnormal fetal heart pattern and CTG showing reduced beat to beat variability and very small decelerations.
The exact time of FMH, in our case cannot be estimated but hematological parameters were suggestive of a relatively chronic onset, which caused fetal compromise just at the end of pregnancy, which was evident by predelivery obstetrical evidence in the form of abnormal CTG (cardiotocograph). Due to severe anemia, the baby was depressed at birth and showed significant evidence of birth asphyxia as evident by cord blood ph of 7.03, high lactate of 15 mmol/lit and multiorgan involvement (hematuria, deranged coagulation and deranged LFTs (liver function tests). The presence of subcutaneous edema with the fluid in two serous cavities suggested non-immune hydrops, which is a well known consequence of fetal anemia. Hydrops is a term used to describe generalized subcutaneous edema in the fetus or neonate, usually accompanied by ascites and pleural or pericardial effusions. Our case is a combination of subcutaneous edema, ascites and pleural effusion.
Many causes of non-immune hydrops have been described with varying associations. The baby presented within hrs of birth and there was no history of maternal exposure to viral infections including varicella, which makes neonatal varicella unlikely. Maternal Parvovirus IgG and IgM were negative. Our case is a typical example of how a single inutero event can lead to a series of devastating effects. Hydrops fetalis was first described by Ballantyne in 1892, which means universal edema of the newborn. Three major conditions are responsible for this state: anemia, hypoproteinemia and cardiac failure. More than one condition can coexist.11,12 In our case, the cause was fetal anemia which was secondary to fetomaternal hemorrhage. At 6 hrs of age, hemoglobin value was 7.3g/dl with a reticulocyte count of of 8.6%. These readings were consistent with bone marrow activation caused by chronic anemia (inutero onset).
After suspecting inutero onset of fetal anemia, we confirmed the FMH using the KB test. Various tests have been described in the literature to detect fetal blood cells in maternal circulation. The KB test is the standard method of detecting fetal-maternal hemorrhage (FMH). Comparison with other more expensive or technologically advanced methods such as flow cytometry has shown that the KB stain, like the more advanced methods, is almost equally sensitive in its detection of FMH.3
We used the KBT to diagnose FMH, which is an internationally recognized and standard test to diagnose this condition. KBT is equally helpful as other methods like flow cytometery, which is expansive and requires more technical support.
Conclusion
Alfa fetoprotein (AFP) is merely a supportive marker especially in very early pregnancy, not diagnostic of FMT. Its detection will not alter the management of the described condition. With increasing gestation, FMH was detected more readily by both tests; however, in evaluating FMH at less than 10 weeks gestation, AFP was found to be a more sensitive and reliable marker than the Kleihauer test.13
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
References
- 1.Kosasa TS, Ebesugawa I, Nakayama RT, Hale RW. Massive fetomaternal hemorrhage preceded by decreased fetal movement and a nonreactive fetal heart rate pattern. Obstet Gynecol 1993. Oct;82(4 Pt 2)(Suppl):711-714 10.1097/00006250-199310000-00059 [DOI] [PubMed] [Google Scholar]
- 2.Heise RH, Van Winter JT, Ogburn PL., Jr Identification of acute transplacental hemorrhage in a low-risk patient as a result of daily counting of fetal movements. Mayo Clin Proc 1993. Sep;68(9):892-894 [DOI] [PubMed] [Google Scholar]
- 3.Katiyar R, Kriplani A, Agarwal N, Bhatla N, Kabra M. Detection of fetomaternal hemorrhage following chorionic villus sampling by Kleihauer Betke test and rise in maternal serum alpha feto protein. Prenat Diagn 2007. Feb;27(2):139-142 10.1002/pd.1632 [DOI] [PubMed] [Google Scholar]
- 4.de Almeida V, Bowman JM. Massive fetomaternal hemorrhage: Manitoba experience. Obstet Gynecol 1994. Mar;83(3):323-328 [PubMed] [Google Scholar]
- 5.Fliegner JR, Fortune DW, Barrie JU. Occult fetomaternal haemorrhage as a cause of fetal mortality and morbidity. Aust N Z J Obstet Gynaecol 1987. May;27(2):158-161 10.1111/j.1479-828X.1987.tb00971.x [DOI] [PubMed] [Google Scholar]
- 6.Pauli RM. Fetomaternal hemorrhage and still birth. Newsletter of Wisconsin Still birth service program, 1993 Vol 1, N.3. [Google Scholar]
- 7.Rubod C, Deruelle P, Le Goueff F, Tunez V, Fournier M, Subtil D. Long-term prognosis for infants after massive fetomaternal hemorrhage. Obstet Gynecol 2007. Aug;110(2 Pt 1):256-260 10.1097/01.AOG.0000271212.66040.70 [DOI] [PubMed] [Google Scholar]
- 8.Sebring ES, Polesky HF. Fetomaternal hemorrhage: incidence, risk factors, time of occurrence, and clinical effects. Transfusion 1990. May;30(4):344-357 10.1046/j.1537-2995.1990.30490273444.x [DOI] [PubMed] [Google Scholar]
- 9.Giacoia GP. Severe fetomaternal hemorrhage: a review. Obstet Gynecol Surv 1997. Jun;52(6):372-380 10.1097/00006254-199706000-00022 [DOI] [PubMed] [Google Scholar]
- 10.Laube DW, Schauberger CW. Fetomaternal bleeding as a cause for "unexplained" fetal death. Obstet Gynecol 1982. Nov;60(5):649-651 [PubMed] [Google Scholar]
- 11.Lionnet C, Body G, Gold F, Paillet C, Vaillant MC, Alle C, et al. Fetal cerebral accident due to massive fetomaternal hemorrhage. A case report. J Gynecol Obstet Biol Reprod (Paris) 1995;24(5):553-556 [PubMed] [Google Scholar]
- 12.Lau MS, Tan JV, Tan TY, Gomez JM, Yeo GS. Idiopathic chronic fetomaternal haemorrhage resulting in hydrops–a case report. Ann Acad Med Singapore 2003. Sep;32(5):642-644 [PubMed] [Google Scholar]
- 13.Evaluating fetomaternal hemorrhage by alphafetoprotein and Kleihauer following therapeutic abortions . D.L. Hay, a, I. Horaceka and J. Paullb Department of Pathology, The Royal Women’s Hospital, Melbourne, Australia Department of Anaesthetics, The Royal Women’s Hospital, Melbourne, Australia. Received 18 May 1981; accepted 13 July 1981. Available online 10 April 2004.