Abstract
In recent years, a number of investigators have studied the relationship between IGF-I and risk of developing cancer, diabetes or cardiovascular disease. Upper tertile, quartile, and quintile IGF-Is were associated with higher risk of developing cancer, and lowest quartile with cardiac disease and diabetes. As part of a study to correlate serum IGF-Is and growth bormone dynamics in aging, we measured fasting serum IGF-I at baseline and two weeks later in a group of 84 normal volunteers between the ages of 50 and 90 years. Although the correlation between the two IGF-Is was high (r=0.922; p<0.0001) there were substantial differences between the two IGF-I values ranging from -36.25 to +38.24% between individual IGF-I values at the two blood draws and a significant difference between the mean IGF-Is at visits 1 and 2 (mean l20.28±53.5 vs. l14.95±50.03; p=0.03). When considered in quartiles, IGF-I changed from one quartile to another in 34/84 (40.5%) of the volunteers. When the group was divided in halves, tertiles, quartiles, or quintiles there was an increasing number of subjects who changed from one subdivision to another as the number of gradations increased. These results suggest that the predictive outcomes of earlier studies that used single IGF-I samples for analysis of risk ratios according to tertiles, quartiles, or quintiles could have been different if a second IGF-I was used to establish the risk ratio. The results also suggest that variability in IGF-I should be taken into account when designing such studies.
INTRODUCTION
In recent years, high or low serum insulin–like growth factor-I (IGF-I) levels have been reported to be predictive of an increased risk of developing cancer (1-5), or diabetes and cardiovascular disease (6-8), respectively. Upper tertile, quartile, or quintile IGF-Is were associated with a higher risk of developing prostate, breast, colon, and lung cancer; and lower quartile IGF-Is were associated with cardiac disease and diabetes. During a study designed to relate serum growth hormone (GH) responses to GH secretagogues to serum IGF-I values in an aging population, we measured serum IGF-I twice. We report here the observed differences and similarities between the two IGF-I values.
METHODS
We measured fasting serum IGF-I at baseline and two weeks later in a group of 84 normal volunteers between the ages of 50 and 90 years. The mean age of the men was 65.7 years (range: 50-86). The mean for the women was 66.1 (range: 50 - 88). Mean BMI was 27.3 for males and 27.8 for females. Fasting samples were drawn at visit 1 and 2 between the hours of 8:00AM and 10:00AM. The samples were allowed to clot for 30 minutes and were then spun at 3000 rpm for 15 minutes. Serum was then frozen at -70 degrees C. Quest Diagnostics then picked up the frozen samples. Samples were measured individually in the order drawn.
The Nichols Advantage® IGF-I Assay is a two -site chemiluminescent immunoassay. The antibody to the C-terminal 62-70 amino acid sequence is biotinylated for capture and the antibody to the amino acid sequences of 1-23 and 42-61 is labeled with acridinium ester for detection. Patient samples were acidified to separate IGF-I from IGFBPs. The acidified samples were incubated simultaneously with the biotinylated capture antibody, excess IGF-II, and acridinium ester labeled antibody. After the initial incubation period, streptavidin-coated magnetic particles were added to the reaction mixture and further incubated. Free, labeled antibody was then separated from the labeled antibody bound to the magnetic particles by aspiration and washing. The amount of bound, labeled antibody is directly proportional to the concentration of IGF-I in the sample.
According to Nichols’ data (9), inter-assay variation was 6.7-8.4% (range 44.3-771.2 ng/mL) and intra-assay variation 6.0-9.4% (range 43.3-793.5 ng/mL.) The limit of quantification of 3.4 ng/mL at 20% coefficient of variation was established by running five low pools four times in five assays.
Ranges for halves, tertiles, quartiles, and quintiles were established based on the IGF-I value obtained at visit 1, with the group divided equally into subdivisions. Unequal distribution of subjects into subdivisions was due to identical IGF-I results from multiple subjects at the dividing IGF-I value. The Pearson two-tailed correlation coefficient and the Student's t-test were used for statistical analysis.
RESULTS
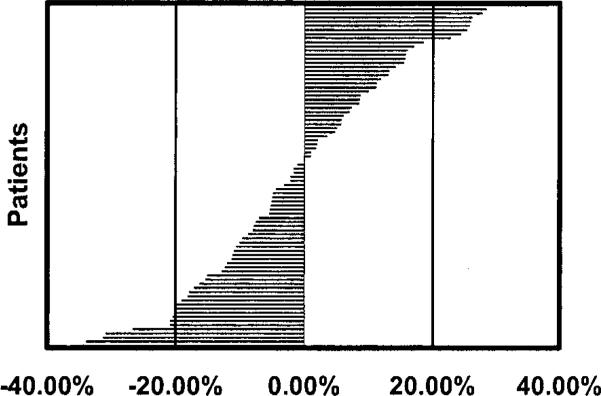
Significant differences between the mean IGF-Is at visits 1 and 2 were found (mean 120.28±53.5 vs. 114.95±50.03; p=0.03). Also noted was a surprising variability between IGF-I measurements drawn at baseline and at 2 weeks (Figure 1). The percent change varied form –36.25 to +38.24. Forty-six of 84 volunteers had serum IGF-Is that varied by more than 10% between visit 1 and visit 2 (five had changes of >30%, twelve >20%, and twenty-nine > 10%). Despite this variability the correlation coefficient (r) was 0.922 (p<0.0001).
Figure 1.
Percent Change of IGF-I from Visit 1 to Visit 2
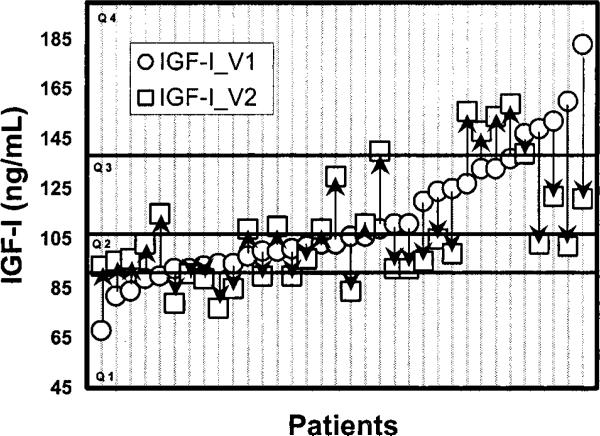
Notably, 40.5% of the volunteers changed from one quartile at the first draw to another at the second draw (Table 1). Figure 2 shows the changes and direction of change in those individuals changing from one quartile to another. When the entire group was examined using halves, quartiles and quintiles, there was an Increasing number of subjects who changed from one subdivision to another (Table 2).
Table 1.
Distribution of subjects in quartiles at Draw 2 compared to Draw 1.
| Visit 1 | Number of Subjects Changing Quartiles [n/total per quartile(%)] | Quartile Distribution for IGF-I at Visit 2 (n) | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Quartile 1 n=21 | 5/21 (24) | 16 | 4 | 1 | 0 |
| Quartile 2 n=22 | 13/22 (59) | 8 | 9 | 5 | 0 |
| Quartile 3 n=20 | 11/20 (55) | 1 | 5 | 9 | 5 |
| Quartile 4 n=21 | 5/21 (24) | 0 | 2 | 3 | 16 |
| TOTALS | 34/84 (40.5) | n=25 | n=20 | n=18 | n=21 |
IGF-I values of 14 patients (33%) moved from low risk (Quartiles 2 and 3) to high risk quartiles (Quartiles 1 or 4), and ten patients (24%) moved from high risk to low risk quartiles.
Figure 2.
Subjects who changed quartile from visit 1 (IGF-I_V1) to visit 2 (IGF-I_ V2). Arrows indicate direction of change for each subject. Quartile 1 (Q1) range: 45-92 ng/ml. Quartile 2 (Q2) range: 93-107 ng/ml. Quartile 3 (Q3) range: 108-139 ng/ml. Quartile 4 (Q4) range: 140-351 ng/ml.
Table 2.
Distribution of IGF-I values according to different subdivisions.
| Statistical Categories | N Switching Categories/Total | % Changed |
|---|---|---|
| Halves | 14/84 | 16.7% |
| Tertiles | 25/84 | 29.8% |
| Quartiles | 34/84 | 40.5% |
| Quintiles | 50/84 | 59.5% |
DISCUSSION
IGF-I varies depending upon the physiological state. IGF-I is relatively low during childhood, but reaches a peak during late puberty. (10,11) Following this, peak serum GH falls steadily as one ages. Other physiological and pathophysiological states that affect IGF-I include nutritional intake, body composition, pregnancy, hypothyroidism, renal failure, diabetes mellitus, severe liver disease, acromegaly, and GH dependent growth failure, among others. (12) Serum lGF-I concentrations are also affected by IGF binding proteins even in assays that attempt to correct for these proteins by extraction methods. (13)
Reliability of assays themselves ranges from 3-7% (inter-assay) and 7-13% (intra-assay). (12) Previous studies regarding the reliability of repeated IGF-I measurements have shown a high degree of correlation. (1,14) While the present study showed a high degree of correlation between the two values in the group as a whole, it revealed a high degree of individual variability between the two IGF-I results for the same subjects. The variability was substantial enough to lead to changes into and out of tertiles, quartiles, and quintiles. Variability in IGF-I may have, in part, been due to handling, storage, or shipping.
These differences indicate that the predictive outcomes of earlier studies that used analysis of risk ratios according to quintiles, quartiles or tertiles could have been different had the lGF-Is from a visit 2 been substituted for those of visit 1. Since individuals changed into and out of high-risk quartiles in similar numbers, the predictive outcomes would be similar for the group but different for the individual who changed subdivision. One could have expected 24% of the subjects from quartile 1 and 24% from quartile 4 to change to other quartiles, thus decreasing their assigned risk of developing disease. At the same time, one could have expected 33% of the subjects from quartiles 2 and 3 to change to quartiles 1 or 4, thus increasing their assigned risk of developing disease. It should be pointed out that previous epidemiological studies were done with larger cohorts.
These results raise questions about the designs of studies that establish risk ratios to predict the chance of disease development based on single IGF-I measurements. They suggest that variability in IGF-I should be taken into account when designing such studies. Since our study reveals a high degree of variability, we believe that more than one IGF-I determination should be made if risk ratios are to be ascertained according to tertile, quartile, or quintile. We also suggest that studies designed to assess the number of IGF-I samples necessary to reliably predict a stable IGF-I for an individual to be consistently placed within the same subdivision should be done.
ACKNOWLEDGEMENTS
Supported by an Investigator Initiated Grant from Pfizer, Inc., NIH, NCRR-MOIRR00096 (SPlD# 1063) and the NIH G P30-AG 08051.
REFERENCE LIST
- 1.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 2.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 3.Janssen JA, Lamberts SWJ. Insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;352:490. doi: 10.1016/s0140-6736(05)79230-8. [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB. Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer [In Process Citation]. Journal of Clinical Endocrinology and Metabolism. 2000;85:4258–4265. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 5.Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Lukanova A, Shore RE, Zeleniuch-Jacquotte A. Serum insulin-like growth factor-I and breast cancer [In Process Citation]. International Journal of Cancer. 2000;88:828–832. doi: 10.1002/1097-0215(20001201)88:5<828::aid-ijc22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Spallarossa P, Brunelli C, Minuto F, Caruso D, Battistini M, Caponnello S, Cordera R. Insulin-like growth factor-I and angiographically documented coronary artery disease. Am J Cardiol. 1996;77:200–202. doi: 10.1016/s0002-9149(96)90600-1. [DOI] [PubMed] [Google Scholar]
- 7.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 8.Vaessen N, Heutink P, Janssen JA, Witteman JC, Testers L, Hofman A, Lamberts SW, Oostra BA, Pols HA, van Duijn CM. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- 9.Nichols Advantage Insulin-Like Growth Factor I, Chemiluminescence Immunoassay for the Quantitative Determination of Insulin-Like Growth Factor in Human Serum, Package insert, June 2001.
- 10.Cohick WS, Clemmons DR. The insulin-like growth factors. Annu Rev Physiol. 1993;55:131–153. doi: 10.1146/annurev.ph.55.030193.001023. [DOI] [PubMed] [Google Scholar]
- 11.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endoer Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 12.Clemmons DR. Commercial assays available for insulin-like growth factor I and their use in diagnosing growth hormone deficiency. Horm Res. 2001;55(Suppl 2):73–9. doi: 10.1159/000063480. 73-79. [DOI] [PubMed] [Google Scholar]
- 13.Ranke MB, Feldt-Rasmussen U, Bang P, Baxter RC, Camacho-Hubner C, Clemmons DR, Juul A, Orskov H, Strasburger CJ. How should insulin-like growth factor I be measured? A consensus statement. Horm Res. 2001;55(Suppl 2):106–9. doi: 10.1159/000063485. 106-109. [DOI] [PubMed] [Google Scholar]
- 14.Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065. [DOI] [PubMed] [Google Scholar]