Abstract
Pronounced feeding can be elicited by injections of the GABAA agonist muscimol into the medial shell region of the nucleus accumbens (AcbSh). This region of AcbSh has been shown to project to both the lateral hypothalamus (LH) and the medial ventral pallidum (VPm). The current study examined the effects of unilateral LH or VPm lesions on the ingestive responses induced by injections of muscimol into the AcbSh on either the same or the opposite side of the brain. We found that lesions of either of these structures drastically attenuated feeding induced from the ipsilateral, as compared to the contralateral, AcbSh. The “ipsilateral/contralateral disruption design” employed here virtually rules out the possibility of nonspecific effects of the lesions and suggests that the VPm and LH play essential roles in mediating the ingestive effects of inactivation of the AcbSh.
Keywords: Food Intake, Eating, Ingestive Behavior, Ventral striatum, NeuN
1. INTRODUCTION
Although the nucleus accumbens is often assumed to exert a generalized influence on motivational or reward mechanisms, studies have demonstrated that pharmacological inactivation of the shell subregion of the accumbens (AcbSh) with GABA agonists or glutamate receptor antagonists induces a large, and remarkably specific, increase in feeding behavior in satiated rats [1–4]. The active site in the ventral striatum at which these drugs elicit feeding has been localized to the rostral medial AcbSh [1,5,6,7] and the fact that large, behaviorally specific, increases in food intake can be elicited by blocking the actions of endogenous glutamate, or by increasing levels of endogenous GABA [1], indicate that the AcbSh is likely to play a role in the normal physiological control of feeding behavior. The importance of the AcbSh in the physiological control of feeding behavior is underscored by the fact that changes in food intake have also been observed after local injections of a variety of feeding related compounds including nociceptin, cocaine- and amphetamine-related transcript protein, melanin-concentrating hormone, orexin, opioids, amylin, and endocannabinoids (see [8] for review).
An obviously important line of investigation involves the determination of the functional circuitry through which the AcbSh mediates food intake. The AcbSh sends substantial projections to at least two brain regions known to play a role in the control of feeding; the lateral hypothalamus (LH) and medial ventral pallidum (VPm) [9–13]. While the role played by the VP in the expression of feeding induced by manipulations of amino acid-coded circuits in the AcbSh has never been investigated, the available data suggest the occurrence of a functional relationship between the AcbSh and the LH. Thus, feeding induced by inactivation of the AcbSh can be significantly attenuated by acute suppression of LH activity through local drug injections [2,14]. While these findings suggest that the feeding behavior elicited by inhibition of neurons in the AcbSh is dependent on activation of LH neurons, they are, unfortunately, open to alternative explanations. It is quite possible, for example, that the LH does not play a direct role in mediating effects obtained from the AcbSh, but that the LH manipulations performed in these studies may have suppressed feeding as a result of some “nonspecific” effect, such as sedation, malaise, alterations in hormonal or autonomic reactivity, or changes in gustatory or olfactory processing.
One way to circumvent these interpretive difficulties is through the use of what could be called an “ipsilateral/contralateral disruption design” (ICD) which allows for an evaluation of whether lesion effects are due to a specific disruption of lateralized circuitry activated by the AcbSh injections. This approach requires that the AcbSh on one side of the brain exerts a larger influence on the ipsilateral LH than on the contralateral LH. The available anatomical data supports this possibility with respect to direct projections, since very few fibers originating in the AcbSh appear to cross the midline to terminate in the contralateral LH [13,15,16]. The uncrossed functional nature of these pathways is further supported by the observation that unilateral injections of muscimol into the AcbSh produce a much larger increase in the number of cells expressing the nuclear protein Fos, a marker of neural activity [17,18], in the ipsilateral LH than the contralateral LH [19]. If muscimol-induced feeding is mediated through a predominantly unilateral activation of the LH, one would expect that lesions of this structure on the same side as the muscimol injection would produce a much greater disruption of the feeding response than would lesions on the contralateral side. Conversely, if AcbSh-mediated food intake were suppressed because a particular lesion elicited a “nonspecific” response, such as sedation or malaise, one would expect that the “nonspecific” response would be elicited in a similar manner by lesions on either side of the brain and therefore, that equivalent reductions in food intake would be observed after either ipsilateral or contralateral lesions.
The AcbSh also projects heavily to the VPm and both lesioning and drug microinjection studies strongly implicate this region in the control of feeding [12,20,21]. Considerably fewer Fos-immunoreactive cells are seen in the VPm than the LH after intra-AcbSh muscimol injections, but those cells that are present are located primarily ipsilateral to the injection site [19]. Anatomical data also suggest that the direct projection from the AcbSh to the VPm is strongly unilateral [16]. These findings suggest that the ICD experimental design may again be useful for evaluating the role of the VPm in mediating the feeding induced by GABAergic inactivation of the AcbSh.
In light of these considerations, we conducted three experiments to evaluate the connections through which the AcbSh is able to influence food intake. First, we examined feeding following unilateral injections of muscimol into the AcbSh, to verify that unilateral inactivation of the AcbSh is able to induce feeding in our current testing situation. We then examined the effects of unilateral lesions of the LH and of the VPm using the ICD approach outlined above.
2. METHODS
2.1 Subjects
Male Sprague-Dawley rats (Charles Rivers, Wilmington, MA) weighing between 280 and 337 g at the time of surgery served as subjects. The rats were housed individually in plastic cages on a 12 h light:12 h dark cycle at a constant room temperature (~ 21° C) with food (Harlan Teklad) and tap water available ad libitum, except as noted below. All experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
2.2 Surgery
Surgery was performed using standard, aseptic, flat-skull stereotaxic techniques. Subjects in Experiment 1 received only bilateral AcbSh cannula implants, whereas subjects in Experiments 2 and 3 received unilateral excitotoxic lesions followed immediately by cannula implants. Rats were anesthetized with sodium pentobarbital (60 mg/kg). In order to produce excitotoxic lesions, a 28 ga stainless steel injector was lowered into either the VPm (AP: −0.1, LM: 1.8, DV: −8.8; mm from bregma) or LH (AP: −2.5, LM: 1.8, DV:−9.1) and ibotenic acid (Sigma, Saint Louis, MO; 10 μg/μl) was infused at a rate of 0.25 μl/min. The total amount of ibotenic acid infused was 5.0 μg into the VPm and 15 μg into the LH. Approximately half the animals were lesioned on the left and the remainder on the right. The injector was allowed to remain in place for 5 minutes after the infusion to minimize diffusion up the injector path. Bilateral 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA), aimed so as to terminate 2.0 mm dorsal to the AcbSh, were implanted at coordinates of AP: 1.6, LM: ±0.9, DV: −6.1. The guide cannulae were held in place using stainless steel screws and denture lining material and a stainless steel obturator was inserted into the lumen of each cannula to help maintain patency. After surgery, the rats received an injection of carprofen (5 mg/kg, sc) to help alleviate postoperative pain. Each rat was allowed to recover for at least seven days before the start of behavioral testing.
2.3. Intracerebral injections
During the intracerebral injections, the rats were gently restrained, the obturators removed, and 28-gauge injection cannulae, extending 2.0 mm beyond the ventral tip of the guide, were inserted into the guide cannulae. All injections were made in a volume of 0.5 μl at a rate of 0.33 μl/min using motor driven microsyringes attached to the injection cannulae through lengths of polyethylene tubing. Injections on the two sides were made simultaneously. After the infusion, the injection cannulae were left in place for an additional 30 seconds in order to minimize leakage up the cannula track, at which time the obturators were replaced and the animals placed in the testing cages.
2.4 Feeding measurments
Test chambers (pelletometers) consisted of plastic shoebox cages (L 43 X W 22 X H 21cm) equipped with metal trough into which individual 45 mg food pellets (Precision Dustless pellets, Bio-Serve, Frenchtown, NJ) could be deposited by automated dispensers (Med-Associates, St. Albans, VT) under computer control. Removal of the pellet unblocked an infrared photobeam; the time of these events was recorded to the nearest 0.1 sec, after which another pellet was delivered. Test sessions lasted for 120 min after introduction of the animal to the pelletometer. In order to acclimate the rats to the apparatus and procedure, they were placed in the pelletometers and allowed to eat for 120 min on at least five days before the start of testing. The rats were mildly food-deprived for the first one or two acclimation trials and non-deprived for the remainder. In order to accustom them to the injection procedure, each rat, on the final acclimation day, received bilateral 0.25 μl intracerebral injections of sterile 0.15 M saline.
2.5. Experimental proceedure
Experiment 1. Injections in unlesioned animals
Seven animals received two feeding tests that were presented in a counterbalanced order and separated from each other by at least 48 hr. On one test day, animals received simultaneous bilateral injections of sterile saline and on the other an injection of saline into the AcbSh on one side of the brain and an injection of 100 ng of muscimol into the AcbSh on the other side.
Experiment 2. Unilateral muscimol injections in rats with unilateral lesions of the LH
Eleven animals with unilateral LH lesions were studied. Each rat received the following three combinations of intracerebral injections in counterbalanced order: (1) bilateral saline injections, (2) injections of 100 ng of muscimol on the intact side and saline on the lesioned side, (3) injections of 100 ng of muscimol on the lesioned side and saline on the intact side. Tests were separated from each other by at least 48 hours.
Experiment 3. Unilateral muscimol injections in rats with unilateral lesions of the VPm
Fifteen rats with unilateral VPm lesions were studied who received bilateral saline and unilateral muscimol injections as described above under Experiment 2.
2.6. Histology
When behavioral testing was completed, each of the rats was deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused transcardially with 50 ml of a 0.15 M saline solution followed immediately by 200 ml of a 10% buffered formalin solution at pH 6.5 and then 300 ml of a 10% buffered formalin solution at pH 9.0 [22]. The brains were removed, postfixed for 1 hr and then stored in phosphate buffered saline (PBS) with 20% sucrose at 4°C for at least two days, after which they were frozen and 35 μm-thick coronal sections taken throughout the extent of the AcbSh and the lesion sites. Sections through the injection sites were stained with cresyl violet, and those through the lesions were processed for the immunohistochemical detection of neuronal nuclear protein (NeuN), a sensitive marker of undamaged neurons [23] which we have found to be particularly useful in evaluating excitotoxic lesions. Briefly, the sections were rinsed in PBS and incubated on a rotary shaker table for 24 hours at 4 °C in a monoclonal mouse anti-NeuN primary antibody (diluted 1:20,000 with 0.01 M PBS containing 0.2% Triton X-100 and 4% normal horse serum; Oncogene Research Products, La Jolla, CA). The sections were rinsed in PBS and incubated in the biotinylated horse anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA, diluted 1:200 with PBS containing 4% normal horse serum) for 60 min at room temperature. They were then rinsed in PBS (3×10 min), and incubated in the avidin-biotin complex solution (Vector) for 60 min. Following another series of rinses in PBS, the peroxidase was visualized by incubating the tissue for 5 min in a nickel-enhanced chromogen solution obtained from a Vector 3,3'-diaminobenzidine tetrahydrochloride peroxidase substrate kit. The sections were mounted on chrome-alum coated slides, air-dried, cleared in xylene, and coverslipped with Eukitt mounting medium. The lesion boundaries were mapped and the AcbSh injection sites were examined for placement with reference to the atlas of Paxinos ad Watson [24]. Two animals from Experiment 2 and four from Experiment 3 were removed from the studies due to misplacement of the lesions or to extensive spread of damage along the cannula tracks.
3. Results
3.1. Cannula placements
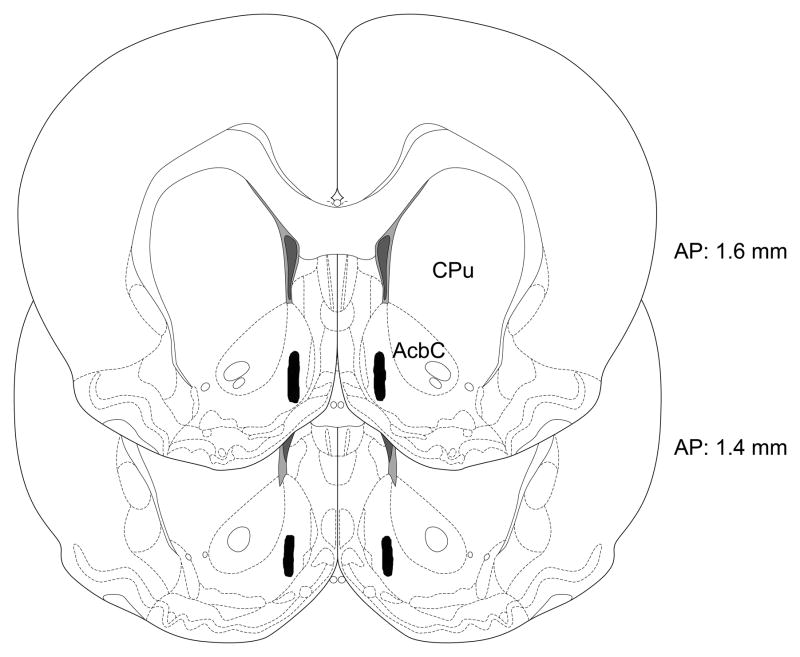
Histological analysis revealed that all cannula placements were located in the medial AcbSh within the region depicted in Fig. 1. The distribution of cannula placements was very similar in the animals examined in each of the three experiments.
Fig. 1.
Schematic illustration of the location of bilateral accumbens shell injection sites in the current study. All cannulae in the three experiments terminated within the shaded region. Sections modified from [24] and numbers indicate distance from bregma in mm.
3.2. Response to unilateral muscimol injections in unlesioned rats
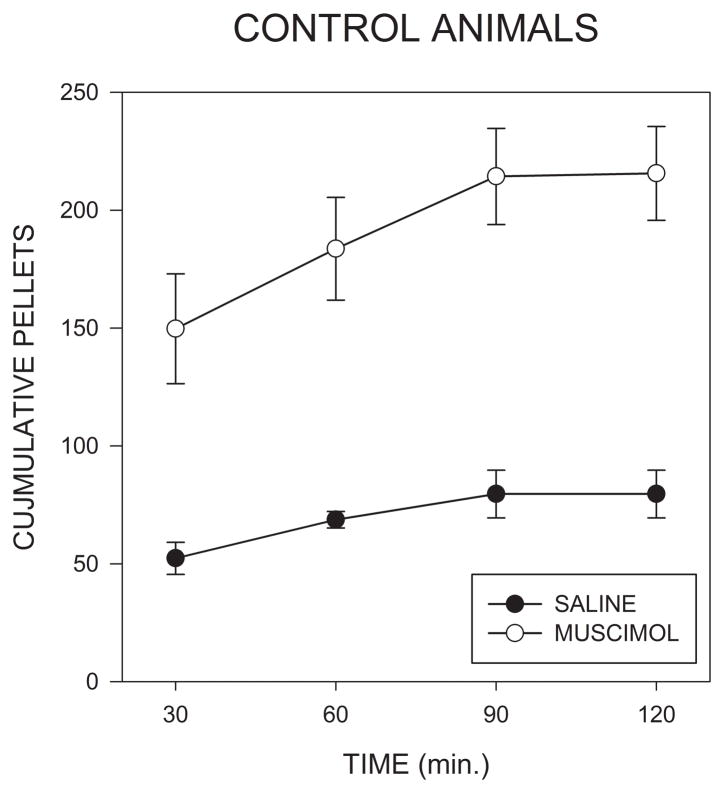
As can be seen in Fig. 2, unilateral injections of muscimol into the AcbSh induced a pronounced increase in food intake, the majority of which occurred in the first hour after injections. Analysis of this data by means of a 2 X 4 (muscimol X 30 min time bin) repeated measures analysis of variance (ANOVA) indicated a significant effect of muscimol (F(1,6)=34.4; p<0.001), of time (F(1,6)=20.3; p<.001) and of the muscimol X time interaction (F(3,18)=3.57; p<.035.) Post hoc comparisons indicated that the effect of muscimol was significant within the first 30 min time bin (F(1,6)=11.4; p<.015). Mean latency to begin eating following placement in the pelletometers tended to be lower after muscimol than after saline injections (42 ± 12 vs. 149 ± 96 sec), and an ANOVA conducted on log transformed latency scores indicated that this difference was significant (F(1,6)=7.21, p<.05).
Fig. 2.
Cumulative numbers of 45 mg food pellets consumed in the 2 hrs following injections of saline or muscimol (100ng/side) into the accumbens shell in Experiment 1.
3.3 Response to unilateral muscimol injections in rats with unilateral lesions of the LH
Histological examination
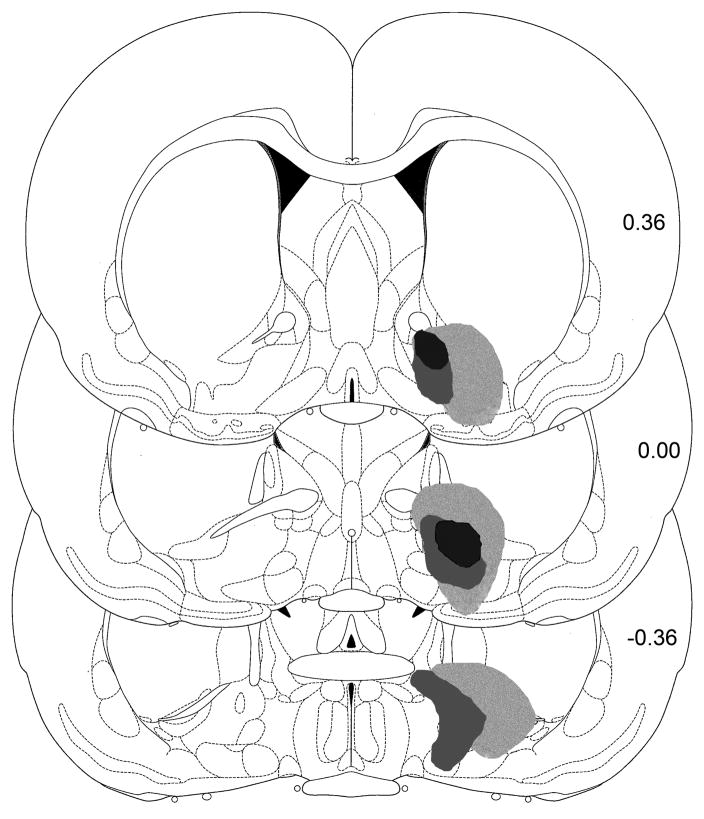
Ibotenic acid lesions (Figs. 3,4) were centered in the peduncular region of the LH at the of the perifornical nucleus, about 2.8 mm caudal to bregma [24]. The lesions extending approximately 2.0–2.5 mm in the anteroposterior plane. The smallest lesions were confined largely to the tuberal and peduncular regions of the LH. The larger lesions additionally damaged the medial perifornical region, the dorsal aspect of the LH, the most ventral part of the medial zona incerta, and produced minor damage to the overlying thalamus along the injector track. No obvious relations were apparent between damage to structures outside of the intended target area and the observed behavioral effects.
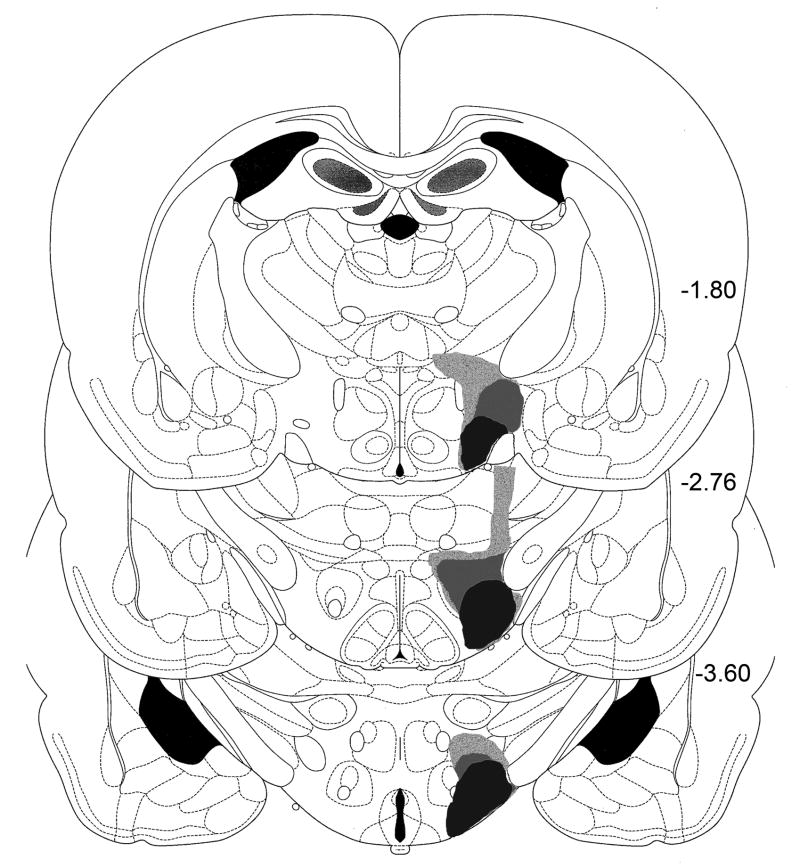
Fig. 3.
Illustrations of the largest, smallest, and intermediate excitotoxic lesions of the lateral hypothalamus from the animals studied in Experiment 2. Numbers indicate distance from bregma in mm.
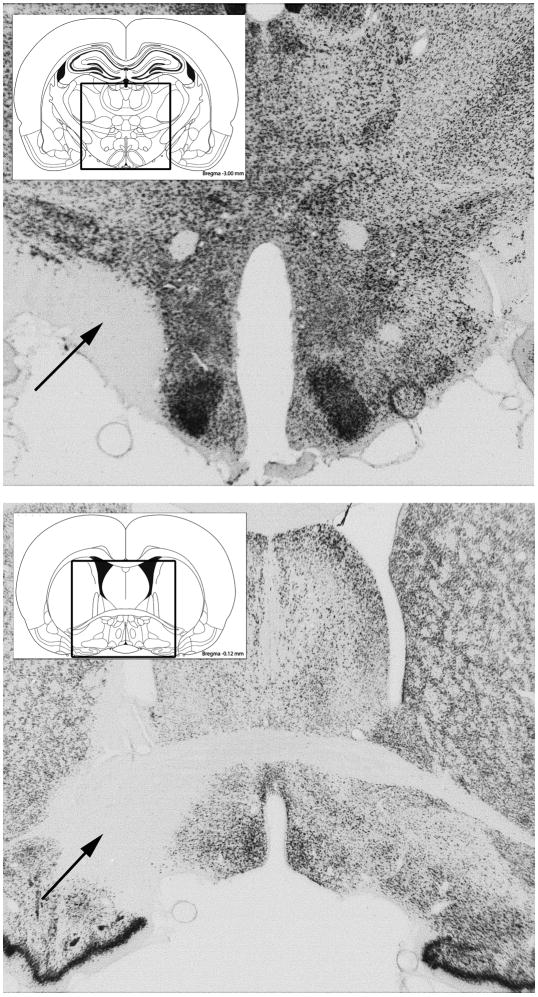
Fig. 4.
Upper Panel: Photomicrograph of section stained for NeuN taken through the center of a typical excitotoxic lesion of the lateral hypothalamus in Experiment 2. Lower Panel: Photomicrograph of a section stained for NeuN through a typical excitotoxic lesion of the medial ventral pallidum in Experiment 3.
Ingestive responses
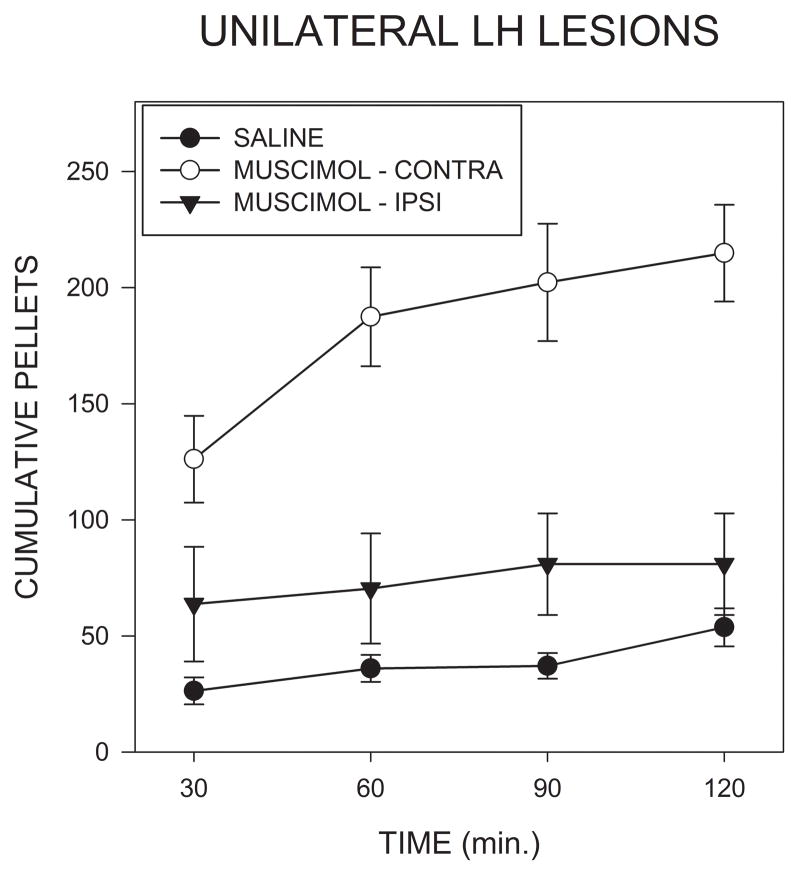
Fig. 5 shows that injections of muscimol into the AcbSh on the side contralateral to the LH lesions induced a much larger increase in food intake than did injections on the lesioned side. Analysis of total intakes indicated a significant effect of treatment condition (F(2,16)=18.59; p<0.001). Post hoc comparisons, using the Bonferroni correction, indicated that muscimol injections on the side contralateral to the lesion resulted in significantly higher intakes then seen after either saline injections (F(1,8)=33.84; p<.001) or after ipsilateral muscimol injections (F(1,8)=23.7; p<0.005). In contrast, muscimol injections on the side of the lesion did not significantly increase intake relative to saline injections (F<1). Latencies to begin feeding did not differ significantly between groups.
Fig. 5.
Cumulative numbers of 45 mg food pellets consumed in the 2 hrs following bilateral injections of saline, or unilateral injections of muscimol into the accumbens shell ipsilateral or contralateral to lesions of the lateral hypothalamus in Experiment 2.
3.4. Response to unilateral muscimol injections in rats with unilateral lesions of the VPm
Histological examination
Lesions (Figs. 4,6) were centered in the VPm, at approximately the level of bregma [24], leaving an aneuronal region extending approximately 0.5 to 1.0 mm in all planes. In the smallest lesions, damage was limited to the VPm and the ventral bed nucleus of the stria terminalis. In the largest lesions, additional damage also occurred in the substantia innominata, the ventral pole of the striatum, the lateral preoptic area, and the caudal pole of the horizontal limb of the diagonal band. No obvious relationship was apparent between damage to these structures outside of the VPm and the resultant behavioral effects. No overlap occurred between these lesions and those examined in Experiment 2.
Fig. 6.
Illustrations of the largest, smallest, and intermediate excitotoxic lesions of the medial ventral pallidum from the animals studied in Experiment 2. Numbers indicate distance from bregma.
Ingestive responses
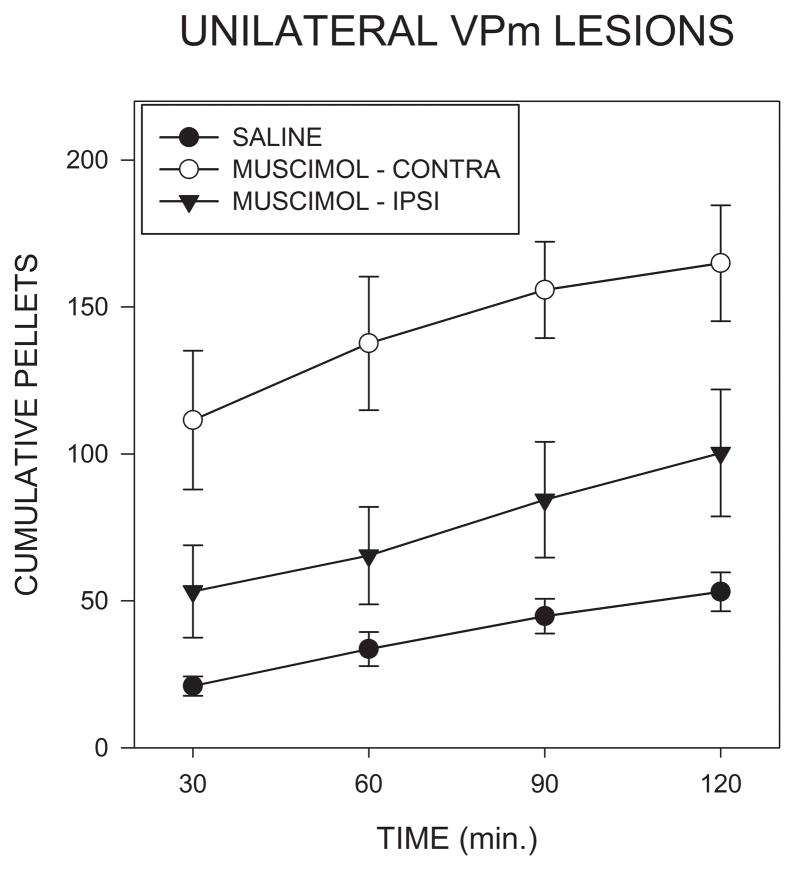
Food intake following intra-AcbSh injections of muscimol in rats with unilateral VPm lesions is shown in Fig. 7 where it can be seen that a contralateral injections produced a much larger response than did ipsilateral injections (F(2,16)=11.51; p<0.001). Post hoc contrasts indicated that food intake after contralateral muscimol injections was significantly larger than that seen after either saline injections (F(1,8)=74,6; p<0.001) or after ipsilateral muscimol injections (F(1,8)=11.51; P<0.03.) Injection of muscimol on the side of the lesion did not significantly increase intake (F(1,8)=1.10; p>0.5), although a trend for a small increase was apparent in the data, as can be seen in Fig. 7. Latencies to begin feeding did not differ significantly between groups (p<.1), although there was a trend for them to be shorter after contralateral muscimol than in the other conditions.
Fig. 7.
Cumulative numbers of 45 mg food pellets consumed in the 2 hrs following bilateral injections of saline, or unilateral injections of muscimol into the accumbens shell ipsilateral or contralateral to lesions of the medial ventral pallidum in Experiment 3.
4. Discussion
The current results confirm previous reports that injections of muscimol into the nucleus accumbens shell (AcbSh) produce marked, short latency, increases in food intake [1,5,14,25] which can be observed even after unilateral injections [19]. The most important results of the current study, however, consist of the demonstrations that unilateral lesions of either the LH or the VPm have drastically different effects on muscimol induced feeding depending on whether the GABA agonist was injected on the lesioned or unlesioned side. The simplest interpretation of these results is that both the VPm and the LH play a direct role in mediating the ingestive effects of intra-AcbSh muscimol.
It would have been possible to have conducted a study conceptually similar to the present one in which the VPm or the LH would have been rendered nonfunctional not by excitotoxic lesions but by acute injections of a drug such as muscimol or 2-APV. The current technique has the advantage that the exact extent of the lesions can be determined histologically, whereas it is always difficult to ascertain the effective spread of an injected compound. In contrast, permanent lesions have the drawback that they may allow for compensatory plastic changes in residual tissue elements which might tend to obscure the functional role of the lesioned structure. Therefore, although acute injections are likely to be a more sensitive technique for identifying essential output pathways, lesions, if successful, allow for a more accurate delineation of the critical region.
The current results are consistent with previous data suggesting LH involvement in the effects of GABAergic inactivation of the AcbSh. Injections of muscimol into the AcbSh induce pronounced Fos expression in the LH [14,19,26,27], some of which occurs in cells expressing the peptide orexin [26,27]. Furthermore, unilateral injections of muscimol into the AcbSh induce much stronger Fos expression in the ipsilateral as compared to the contralateral LH [19], suggesting that the AcbSh exerts a primarily unilateral influence on the LH. Since a great deal of evidence has shown that electrical or chemical stimulation of cells within the LH can induce feeding [9,28], it is plausible that the orexigenic effects of AcbSh inactivation may result from LH activation. This conclusion has been supported by previous studies which have shown that bilateral inactivation of the LH is able to suppress the feeding induced by drug injections in AcbSh [2,14,29]. These findings, by themselves, however do not conclusively demonstrate a role for the LH for two related reasons. First, it is possible that intra-LH drug injections might suppress feeding as a result of generalized effects such as motor difficulties, sedation, or malaise. Effects of this type are not implausible; for example, intra-LH muscimol has been reported to induce pronounced autonomic changes [30]. Secondly, it is possible that the effects of LH inactivation are restricted to feeding, but that these treatments might suppress any form of feeding, regardless of its origin. In fact, suppression of LH activity has been found to inhibit feeding induced by a variety of treatments including deprivation and dark onset [28,30]. Generalized suppression of feeding might occur, for example, if inactivation of the LH were to induce a satiety-like effect or to alter gustatory processing or oral motor patterning. These types of effects are not inconsistent with LH mediation of the actions of intra-AcbSh muscimol, but their possibility renders uncertain the inference to this conclusion from the effects of bilateral LH inactivation. In contrast, the current design virtually rules out the possibility that the lesion effects were nonspecifically mediated. The simplest explanation of our results is that the effects of muscimol are mediated through activation of the LH, although it should be noted that there are other substantially more elaborate possibilities that are still consistent with the data. It is interesting that the LH may also mediate ingestive behavior induced by manipulations of other central structures: for example, we have found that bilateral LH lesions attenuate the hyperdipsia induced by injections of muscimol into the median raphe nucleus [31]
If the LH does serve as a critical output station for the AcbSh, activity could be transferred from the AcbSh to the hypothalamus either directly or through some intervening relay. The former possibility is supported by reports that the AcbSh sends a heavy projection to the LH [10,11,15], which presumably contains GABA colocalized with various peptides. Inactivation of the AcbSh would thus be expected to reduce GABA release within the LH, and intra-LH injections of GABA antagonists have been found to induce feeding [32–35], although this result has not always been obtained [14]. The reasons for discrepancies are unclear, and may relate to the exact doses and placements studied or the test diets employed. At any account, a failure to observe feeding following injections of GABA antagonists would certainly not rule out a role for direct projections from the AcbSh to the LH, as it is possible either that peptides contained in this projection might play a critical role, or that GABAergic inputs into the LH originating in a another site might affect feeding in a different manner.
A second possibility is that the influence of the AcbSh on the LH is at least partly relayed through an intermediate structure. The most obvious possibility is the VPm as this nucleus receives heavy projections from the AcbSh and, in turn, projects to the LH [36–38]. Inactivation of the AcbSh would be expected to reduce GABA release in the VPm, and it is striking in this regard that intra-VPm injections of the GABA antagonist bicuculline result in feeding which is at least as pronounced as that obtained from the AcbSh [12,21]. Our current finding that unilateral excitotoxic lesions of the VPm attenuate the ingestive response to ipsilateral, as compared to contralateral, injections of muscimol into the AcbSh strongly suggests that the VPm does play a critical, lateralized, role in the response to AcbSh inactivation which is unlikely to reflect nonspecific factors.
In contrast to the results observed here, Taha et al. [39] have reported that unilateral lesions of the VP do not differentially alter the ingestive response to intra-AcbSh DAMGO when the drug was injected on the lesioned, as compared to the intact side. There are a number of possible explanations for differences between their effects and those observed here. Although the lesions in the Taha et al. study appear to be at least as large as those we examined, these authors used quinolinic acid as the lesioning agent, in contrast to the ibotenic acid we employed, and previous studies have indicated that various excitotoxic agents may have differential effects on individual cell populations [40,41]. A more likely possibility comes from the fact that we studied the response to injections of the GABA-A agonist muscimol whereas Taha et al. [39] examined the response to injections of the mu-opioid agonist DAMGO, and it is quite possible that different pathways may mediate these two effects. Several lines of evidence support this possibility. The behavioral syndromes produced by injections of muscimol and DAMGO into the accumbens are by no means identical; the two drugs, for example, have very different effects on saccharine and ethanol intake [42–45]. Additionally, the ingestive response to muscimol appears sharply limited to the medial AcbSh, whereas opioid-induced feeding can be obtained from a much larger region including the accumbens core, lateral shell and even the dorsal striatum [45,46]. It is striking in this regard that while the ingestive response to intra-AcbSh muscimol is rapid in onset, as we observed here, the response to mu opioid agonists is frequently reported to be delayed by 30–60 min [25,39,46,47]. Finally, it is of note that mu-opioid and GABAA receptors are distributed in vezry different patterns within the ventral striatum [48]. These findings are all consistent with the possibility that the substrates of the muscimol and DAMGO responses may not be identical, in which case it would not be surprising if the VPm were to play an essential role in the response to the former, but not the latter drug.
The current results strongly suggest that both the VPm and the LH play an essential, lateralized, role in the feeding response to intra-AcbSh muscimol. Given that the VPm projects to the LH, the simplest explanation of these results, as mentioned above, is that the VPm provides an essential relay between these two structures. It is possible either that the effects of AcbSh inactivation on the LH may be entirely mediated through the VPm, or that this “indirect” pathway may function in parallel with direct projections from the AcbSh to the LH, with activity in both pathways necessary for a maximal response to be realized. In either case, this theory would predict that lesions of the LH would attenuate the ingestive response to stimulation of the VPm, a possibility we are currently investigating. Alternatively, the current results might also be anticipated if pathways passing through the LH and the VPm converge, in a lateralized fashion, at some point further downstream. For example, both the LH and the VPm project to the VTA; If this structure were to play an essential role in mediating the influence of the AcbSh, it is possible that simultaneous activation of VTA afferents arising in both the LH and the VPm might be required in order to induce feeding. Clearly further studies will be needed to clarify the pathways through which the AcbSh influences ingestion, but the current results do substantially advance this goal by demonstrating an essential role for both the LH and the VPm, structures that, on the basis of other evidence, are known to play an important role in ingestive behavior.
RESEARCH HIGHLIGHTS.
Unilateral injections of muscimol into the AcbSh greatly increase food intake
Ipsilateral lesions of the LH or VPm block AcbSh elicited feeding
Contralateral lesions of the LH or VPm do not block AcbSh elicited feeding
The LH and VPm are essential components of the AcbSh feeding circuit
Acknowledgments
This publication is based upon the work supported by grants R01DK071738 from the National Institute of Diabetes and Digestive and Kidney Diseases and 0641943 from the National Science Foundation, and R03DA020802 from the National Institute for Drug Abuse. The authors thank Beth Cowgill and Ignacio R. Covelo for their help with these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratford TR, Swanson CJ, Kelley AE. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- 4.Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. NeuroReport. 2004;15:2673–2676. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- 5.Basso AM, Kelley AE. Feeding induced by GABAA receptor stimulation within the nucleus accumbens shell: Regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- 6.Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behav Bain Res. 1997;89:107–113. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds SM, Berridge KC. Positive and negative motivaton in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactionsplace preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratford TR. The nucleus accumbens shell as a model of integrative subcortical forebrain systems regulating food intake. In: Kirkham TC, Cooper SJ, editors. Appetite and Body Weight: Integrative Systems and the Development of Anti-Obesity Drugs. London: Elsevier; 2007. pp. 27–65. [Google Scholar]
- 9.Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: Ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 10.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neurosci. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 11.Kirouac GJ, Ganguly PK. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neurosci. 1995;67:625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 12.Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825:199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- 13.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 14.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domesick VB. Further observations on the anatomy of nucleus accumbens and caudato-putamen in the rat: Similarities and contrasts. In: Chronister RB, DeFrance JF, editors. The Neurobiology of the Nucleus Accumbens. Brunswick, Maine: Haer Institute for Electrophysiological Research; 1981. pp. 7–40. [Google Scholar]
- 17.Dragunow M, Faull RLM. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Meth. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 18.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 19.Stratford TR. Activation of feeding related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048:241–250. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus. Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 21.Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Europ J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- 22.Berod A, Hartman BK, Pujol JF. Importance of fixation in immunocytochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981;29:844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- 23.Jongen-Relo AL, Feldon J. Specific neuronal protein: A new tool for histological evaluation of excitotoxic lesions. Physiol Behav. 2002;76:449–456. doi: 10.1016/s0031-9384(02)00732-1. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. New York: Academic Press; 2007. [Google Scholar]
- 25.Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacol. 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic neurons and inhibits POMC neurons. Am J Physiol Regulatory Integrative Comp Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- 27.Baldo BA, Gual-Bonilla L, Sijapati K, Danies RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibiton of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- 28.Stanley BG, Willett VL, Donias HW, Dee MG, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol Reg Integr Comp Physiol. 1996;270:R443–R449. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- 29.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens u-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly J, Rothstein J, Grossman SP. GABA and hypothalamic feeding systems. I. Topographic analysis of the effects of microinjections of muscimol. Physiol Behav. 1979;23:1123–1134. doi: 10.1016/0031-9384(79)90306-8. [DOI] [PubMed] [Google Scholar]
- 31.Stratford TR, Wirtshafter D. Forebrain lesions differentially affect drinking elicited by dipsogenic challenges and injections of muscimol into the median raphe nucleus. Behav Neurosci. 2000;114:760–771. [PubMed] [Google Scholar]
- 32.Turenius CL, Htut MM, Prodon DA, Ebersole PL, Ngo PT, Lara RN, Wilczynski JL, Stanley BG. GABAA receptors in the lateral hypothalamus as mediators of satiety and body weight regulation. Brain Res. 2009;1262:16–24. doi: 10.1016/j.brainres.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Kelly J, Alheid GF, Newberg A, Grossman SP. GABA stimulation and blockade in the hypothalamus and midbrain: Effects on feeding and locomotor activity. Pharmacol Biochem Behav. 1977;7:537–541. doi: 10.1016/0091-3057(77)90250-7. [DOI] [PubMed] [Google Scholar]
- 34.Turenius CL, Charles JR, Tsai DH, Ebersole PL, Htut MH, Ngo PT, Lara RN, Stanley BG. The tuberal lateral hypothalamus is a major target for GABAA- but not GABAB-mediated control of food intake. Brain Res. 2009;1283:65–72. doi: 10.1016/j.brainres.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 35.Tsujii S, Bray GA. GABA-related feeding control in genetically obese rats. Brain Res. 1991;540:48–54. doi: 10.1016/0006-8993(91)90491-d. [DOI] [PubMed] [Google Scholar]
- 36.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- 37.Zahm DS, Heimer L. Two transpallidal pathways orginating in the rat nucleus accumbens. J Comp Neurol. 2011;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- 38.Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- 39.Taha SA, Katsuura Y, Noorvaash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 2009;161:718–733. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inglis WL, Semba K. Discriminable excitotoxic effects of ibotenic acid, AMPA, NMDA and quinolinic acid in the rat laterodorsal tegmental nucleus. Brain Res. 2011;755:17–27. doi: 10.1016/s0006-8993(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 41.Dunnett SB, Whishaw IQ, Jones GH, Bunch ST. Behavioural, biochemical and histochemical effects of different neurotoxic amino acids injected into nucleus basalis magnocellularis of rats. Neurosci. 1987;20:653–669. doi: 10.1016/0306-4522(87)90117-5. [DOI] [PubMed] [Google Scholar]
- 42.Stratford TR, Wirtshafter D. Activation of GABA-A receptors in the nucleus accumbens shell elicits opposite effects on consumption of sucrose and saccharin solutions. Neuroscience Meeting Planner. 2007 Program No. 630.5. [Google Scholar]
- 43.Stratford TR, Wirtshafter D. Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behav Brain Res. 2011;216:514–518. doi: 10.1016/j.bbr.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Kelley AE. Intake of saccharin, salt and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacol. 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 46.Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: Role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–1260. [PubMed] [Google Scholar]
- 47.Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacol. 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- 48.Herkenham M, Moon Edley S, Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetyhcholinesterase and subcorftical afferent terminations. Neuroscience. 1984;11:561–593. doi: 10.1016/0306-4522(84)90045-9. [DOI] [PubMed] [Google Scholar]