Abstract
Few studies have examined the effects of chronic perchlorate exposure during growth and development, and fewer still have analyzed the effects of perchlorate over multiple generations. We describe morphological and developmental characteristics for threespine stickleback (Gasterosteus aculeatus) that were spawned and raised to sexual maturity in perchlorate-treated water (G1,2003) and for their offspring (G2,2004) that were not directly treated with perchlorate. The G1,2003 displayed a variety of abnormalities, including impaired formation of calcified traits, slower growth rates, aberrant sexual development, poor survivorship, and reduced pigmentation that allowed internal organs to be visible. Yet these conditions were absent when the offspring of contaminated fish (G2,2004) were raised in untreated water, suggesting a lack of transgenerational effects and that surviving populations may be able to recover following remediation of perchlorate-contaminated sites
Keywords: Perchlorate, Threespine stickleback, Gasterosteus aculeatus, Endocrine disruption, Morphology
INTRODUCTION
Perchlorate is used by the National Aeronautics and Space Administration and the U.S. Department of Defense as an oxidizer in the solid propellant of rockets, missiles, the space shuttle, and in artillery and other explosives [1]. It is also found in many common household and industrial products [1,2]. Natural sources of perchlorate were previously known to occur in sodium nitrate deposits of Chilean caliche (which is exported in Chilean fertilizers), in nitrates from Death Valley, California, USA, and in potash deposits from Carlsbad, New Mexico, USA [2–5]. However, recent methodological advances are revealing the geographically extensive occurrence of perchlorate at low levels (<4 µg/kg [ppb]), and elevated levels occur in some arid and semiarid regions of the American Southwest [6,7]. The highest concentrations of environmental pollution originate from industrial contamination of the Colorado River [8] and from military storage and disposal practices [9]. In 2005, perchlorate was reported in the Canadian Great Lakes, marking the first time that perchlorate contamination was identified outside the United States [10]. As of March 2005, perchlorate contamination had been reported in 36 states of the U.S. (http://www.epa.gov/fedfac/documents/known_perchlorate_releases_in_the_us_09_23_2004.xls).
Perchlorate is highly soluble in water [11], typically remains unaltered for several decades in solution [12,13], and can persist for millennia in groundwater [14]; it causes deleterious effects at low levels [15–17] and remains unregulated because the U.S. Environmental Protection Agency (U.S. EPA) has not established regulatory maximum exposure levels. The U.S. EPA’s most recent (2005) perchlorate reference dose is 24.5 µg/L (http://www.epa.gov/iris/subst/1007.htm#reforal).
Many acute toxicity studies have been conducted on a variety of vertebrates, showing that environmentally relevant concentrations of perchlorate (i.e., levels found in contaminated ground and surface water) affect the thyroid gland and alter concentrations of circulating thyroid hormones ([THs]; triiodothyronine [T3] and thyroxine [T4]). Perchlorate delivered via water reduces plasma TH concentrations among fish [18,19], amphibians [16,20], birds [21], and mammals [17,22,23], including humans [23]. It disrupts the thyroid cascade by competitively inhibiting iodide uptake [22] and has been reported to stimulate the discharge of inorganic iodine from the thyroid gland by an as-yet unidentified mechanism [2,9,24–26]. These conditions can lead to the production of insufficient levels of THs. This in turn typically leads to the upregulation of thyroid stimulating hormone (TSH) by the anterior pituitary and can result in thyroid hypertrophy, hyperplasia, angiogenesis, colloid depletion, and goiter [18,19,22,27,28]. Chronic thyroid hypertrophy can lead to hyperplasia, which increases the likelihood that organisms will develop tumors [29].
Maintaining proper thyroid hormone homeostasis is essential for normal development since THs mediate a wide range of developmental processes. In fish, many biological processes are believed to be at least partially under the influence of thyroid hormones, including metamorphosis; formation of the gastric organ (stomach); morphogenesis; temperature tolerance; skeletal and somatic growth; muscular development; calcification; locomotor activity; behavioral activity, including migration and reproduction; osmoregulation; lipid, carbohydrate, protein, and vitamin metabolism; smoltification; ovarian maturation and oogenesis; gonadal recrudescence; reproduction; and integumentary silvering and melanophore function [19,30–43].
Goleman et al. [16] demonstrated that perchlorate exposure altered sex ratios in postmetamorphosed Xenopus laevis with a bias toward females. Mukhi et al. [44] demonstrated that administration of perchlorate to zebrafish (Danio rerio) during larval-juvenile development produced a female-biased sex ratio at 43 d postfertilization (dpf), and perchlorate exposure with supplemental TH administration produced a male-biased sex ratio. This finding suggests that altered TH levels play a key role in the sexual development of at least some teleosts, although the outcome (feminization or masculinization) may vary. Bernhardt et al. [45] demonstrated that chronic perchlorate exposure masculinized genotypic female threespine stickleback (Gasterosteus aculeatus) to the point that they produced both functional sperm and eggs.
The responses of a variety of fish species, including fathead minnows (Pimephales promelas) [19], zebrafish [18,46], and Eastern mosquitofish (Gambusia holbrooki) [28,47] have been analyzed following perchlorate exposure. These studies illustrate that fish respond in a typical manner to perchlorate exposure; that is, all species display thyroid follicular hypertrophy, hyperplasia, and altered TH levels. Therefore, we assume that similar effects occur in threespine stickleback exposed to perchlorate and examine whether endpoints typically mediated by THs are affected by chronic perchlorate exposure. We employed the threespine stickleback as our model organism to test the hypothesis that chronic perchlorate exposure creates developmental abnormalities that become more prevalent and more severe as the concentration of perchlorate exposure increases. We also tested for transgenerational effects by analyzing whether stickleback raised in water lacking detectable concentrations of perchlorate (<1.1 µg/L) are affected by parental exposure.
MATERIALS AND METHODS
Overview
To determine how exposure to sublethal concentrations of perchlorate (sodium perchlorate; Sigma Aldrich Batch: 12802 TA) affects growth and development, we conducted a series of experiments on the threespine stickleback between 2002 and 2004. The 2002 sample sizes were small and were therefore treated as pilot data that helped to guide the design of the 2003 to 2004 study presented here [48]. All perchlorate concentrations are reported as mass of the perchlorate ion per unit, rather than mass of the sodium- or ammonium-perchlorate molecule.
Sexually mature anadromous threespine stickleback (G0,2003) were captured from Rabbit Slough, Alaska (61°32′12″N, 149°15′17″W) in June to July, 2003 as they returned to spawn. These fish were acclimated to captivity in negative control water (less than the method detection limit [MDL] of 1.1 µg perchlorate/L) for approximately three weeks before spawning trials began. Then they were exposed to perchlorate for approximately three weeks while they spawned and tended to their young. Their offspring (G1,2003) were raised in negative control water or perchlorate-treated water for approximately four months prior to euthanasia and morphometric analysis.
A subset of the G1,2003 was raised to sexual maturity at one year of age in negative control or perchlorate-treated water. Upon reaching sexual maturity, these fish were allowed to reproduce in 38-L aquaria containing negative control water, one male per aquarium. Therefore, gametogenesis for the G1,2003 occurred in perchlorate-treated water, but oviposition and fertilization occurred in untreated water. The resulting G2,2004 fish were maintained in negative control water until 25 weeks of age (from June 8 to December 28, 2004), at which time they were euthanized and stored at −80°C until their morphology could be analyzed for evidence of transgenerational effects.
Animals and husbandry
Experimental fish were housed in 400-L pools, 1,600-L pools, and/or 38-L aquaria containing fortified tap water (3–4 g/L Instant Ocean sea salt) that was continuously filtered and aerated through Azoo biofilters (Aquatic Ecosystems). All 38-L aquaria contained one biofilter (65 mm diameter), nesting material, and a sandy substrate; 400-L and 1,600-L pools each contained two biofilters (150 mm diameter each).
Daily observations were made throughout the experiment for viability and behavioral abnormalities. Dead and moribund fish were recorded and removed upon detection. Analysis of the negative control water was conducted by ion chromatography in tandem with electrospray ionization mass spectrometry (n = 21 samples). Temperature and perchlorate concentrations were monitored daily using an Acorn 6 potentiometer with a perchlorate ion-sensitive electrode (Cole-Parmer). Dissolved oxygen, pH, and salinity were checked every two to three months. The only exchange of perchlorate-treated water occurred on October 5, 2003 (experimental day 120) when a subset of the G1,2003 fish were moved indoors and placed in 400-L tanks at the same perchlorate concentrations as they had been raised outdoors. On May 21, 2004 (experimental day 349), mature, G1,2003 males were removed from their treated water and isolated in individual 38-L aquaria containing fortified tap water (2 g/L Instant Ocean without perchlorate added) in order to produce the G2,2004 fish.
Adults and juveniles (>2 months old) were fed frozen brine shrimp daily (Brine Shrimp Direct), while fry (<2 months old) were fed a mixture of Golden Pearls 100, Artemia food (both from Aquatic Ecosystems), and ground brine shrimp. Tanks were cleaned at approximately three-week intervals. Perchlorate-treated water lost during routine aquarium maintenance was replaced with fresh perchlorate-treated water to maintain the desired perchlorate concentrations. With those exceptions, water was only added to offset evaporative water loss.
2003 Experimental groups
Wild-caught adult males (G0,2003) in six experimental groups were distributed among 15 1,600-L outdoor pools, and each pool was partitioned into four quadrants with one adult male per quadrant. The six experimental groups consisted of negative controls (three pools/12 quadrants), and nominal perchlorate concentrations of 3.6 mg/L (three pools/12 quadrants), variable(0–4.5) mg/L (one pool/four quadrants), 30 mg/L (three pools/12 quadrants), variable(0–60) mg/L (three pools/12 quadrants), and 100 mg/L (two pools/eight quadrants). The G0,2003 exposures took place under ambient photoperiod (18:6 h light:dark increasing to 20:4 h and then decreasing to 18.5:5.5) and temperature (14–20°C) through mid-July.
In 2003, sodium perchlorate was gradually added to the variable(0–60) mg/L experimental group to mimic the increasing concentrations recorded in our pilot 2002 study as perchlorate leached from 3.70 g cores of hydroxyl-terminated polybutadiene (HTPB) solid rocket propellant [48]. The concentration in the variable(0–60) mg/L treatment increased from <MDL on June 1, 2003 to 60 mg/L by October 14, 2003 (experimental day 129). The fifteenth pool (variable(0–4.5) mg/L) contained the partially spent HTPB cores from the 2002 trials, causing its perchlorate concentration to increase from undetectable levels on June 1, 2003 to 4.5 mg/L by August 22, 2003 (experimental day 76) as ammonium perchlorate continued to leach from the cores.
G1,2003 husbandry
After conducting 5 d of fry guarding (generally 11–12 dpf), adult G0,2003 males were removed from their pools and euthanized. Their offspring (G1,2003) remained outdoors in their parents’ treatment groups under ambient photoperiod (20:4 h light:dark declining to 11:13 h) and temperature (20°C declining to 8°C) through October 5, 2003. When the G1,2003 reached 15 weeks of age, only 200 fish from four experimental groups could be brought indoors due to space limitations. Fifty fish each from the negative control group, the 30 mg/L, variable(0–60), and the 100 mg/L groups were selected. These fish provided the opportunity to assess survival rates while being raised to sexual maturity. They continued to be maintained under simulated natural photoperiod throughout the winter but were kept between 17 and 19°C until they reached sexual maturity in the spring. Their offspring (G2,2004) were used to assess potential transgenerational effects from parental perchlorate exposure.
Morphological analysis
In all years, Fowler Sylvac digital calipers (Model S 235 PAT) were used to measure character lengths on whole fish. Growth rates were determined by taking repeated measures on live fish, but all other measurements were performed on intact, preserved fish. Bilateral traits were summed to produce a total length for each character and treated as a single trait. Fluctuating asymmetry did not differ between treatments in the pilot study [48], so it was not analyzed in 2003 or 2004. To test for transgenerational effects in 2004, we analyzed the 16 traits that varied the most among the pilot study fish and G1,2003 for transgenerational effects. We included two additional traits (dorsal fin length and total pelvic score) in 2004 that did not meet these criteria, but are frequently used in studies of comparative stickleback morphology.
In addition to analyzing the characters shown in Figure 1, a fish was considered to have a completely armored ring if the pelvic girdle, anterior lateral plates, and second dorsal spine formed an overlapping calcified ring entirely around the fish. Total pelvic score was determined by adding one point for each bilateral component of the pelvic girdle, including the two ascending branches, two anterior processes, two posterior processes, and the two pelvic spines for a maximum of eight points [49]. A fish was considered to be transparent when vertebrae, ribs, internal tissues or organs were externally visible.
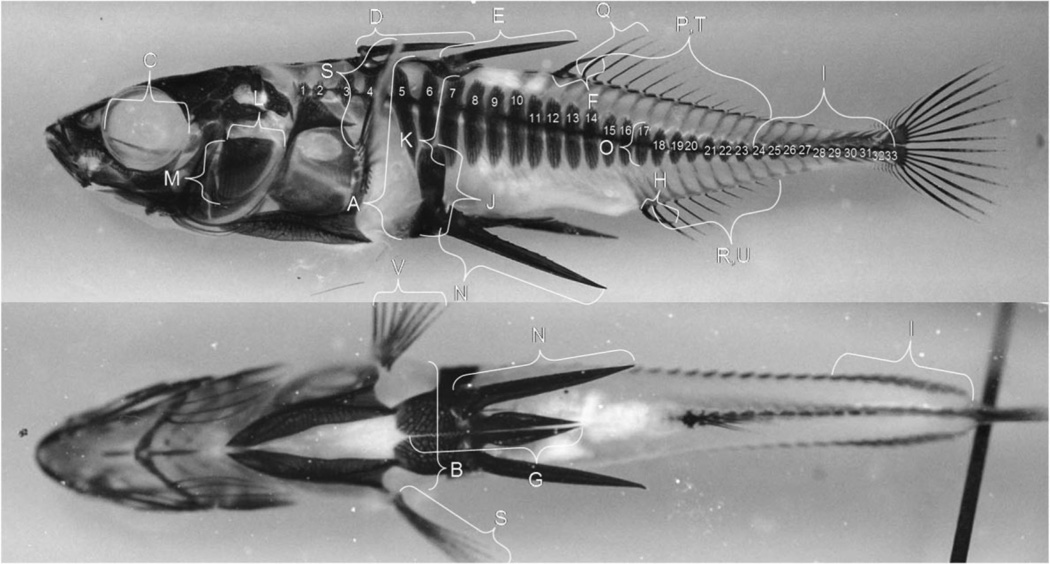
Fig. 1.
Morphological landmarks shown on a cleared and stained (Alizarin red S) stickleback. The 45 characters that were measured on each of the G1,2003 fish are shown. A = body depth, B = body width, C = orbit length, D = 1st dorsal spine length, E = 2nd dorsal spine length, F = 3rd dorsal spine length, G = pelvic girdle length, H = anal spine length, I = keel length, J = ascending branch length, K = anterior lateral plate length, L = opercular length, M = opercular depth, N = pelvic spine length, O = anal plate length, no. 1–33 = number of lateral plates, no. 24–33 = number of keel plates, P = dorsal fin length, Q = dorsal fin height, R = anal fin length, S = pectoral fin length, T = number dorsal fin rays, U = number anal fin rays, V = number pectoral fin rays. The total pelvic score was determined by adding the presence or absence of J, N, and the two components represented by G (anterior and posterior processes of the pelvic girdle) for each side of the fish. An armored ring was considered to be completely developed when the calcified structures represented by E, 6, G, and J overlapped one another without gaps. See text for the criteria used to determine transparency. Photo courtesy of W. Cresko.
Unlike meristic traits, morphometric traits are impacted by the size of the fish, and therefore had to be adjusted for standard length (distance from the anterior tip of upper jaw to posterior end of hypural plate; standard length [SL]). This was done by converting traits to their expected value at the mean SL for all fish (global mean; see von Hippel and Weigner [50]). First, each of the morphometric traits was entered into a regression, with SL as the independent variable, and residuals were recorded. Second, for each trait, the y-value at the global mean SL was recorded. Finally, residuals were added to the y-value at the global mean, essentially converting all specimens into a common SL while preserving the individual morphological differences between them.
2003 Condition indices
Condition indices for every surviving G1,2003 fish were periodically assessed. These included survivorship, SL, transparency, gravidity, swimming behavior, and the presence of fungal infections. Censuses of the G1,2003 fish were conducted monthly. The SL of each G1,2003 fish was measured five times between August 30, 2003 (experimental day 84) and June 10, 2004 (experimental day 369). On each occasion, all surviving G1,2003 fish were removed from their tanks and measured with digital calipers.
Statistical analyses
Prior to conducting statistical analyses, homogeneity of variances was evaluated using Levene’s tests, and data normality was evaluated using Kolmogorov–Smirnov tests. Kruskal–Wallis tests were used when violations to normality, homogeneity of variances, or low sample sizes (n < 20) occurred with continuous data. Frequency data, such as the number of transparent fish and the number with completely armored ring development were compared using chi-square tests. When data were normally distributed with homogeneous variances and sample sizes ≥20, data were analyzed using analysis of variance (ANOVA) tests. Bonferroni multiple comparison tests revealed which groups differed when ANOVAs indicated overall significance, and Dunnett’s tests followed significant Kruskal–Wallis tests. An analysis of covariance (ANCOVA) was used to determine the significance of perchlorate concentration (factor) and fish density (covariate) on growth (dependent variable) of the G1,2003. SPSS v. 15.0 was used for all statistical procedures, and all tests were two-tailed.
To reduce the likelihood of committing type I errors, α values were adjusted downward to equal the probability of having one false positive in the number of annual tests plus one. The greatest number of statistical tests (n = 29) were run in 2003, and a cutoff value (α) of 0.038 (i.e., 1/30 = 0.038) was deemed to be the most appropriate measure of significance. A similar correction in 2004 would have caused the α value to rise above 0.05; therefore, the α value was not adjusted in 2004.
RESULTS
Perchlorate measurements
Spectrophotometric analysis revealed that the level of background perchlorate in untreated tap water used in all experimental groups was less than the method detection limit (<1.1 µg perchlorate/L). The mean perchlorate concentration of the negative control water based on 18 Acorn 6 readings was 0.15 mg/L (standard error [SE] 0.01), yet this value is less than the lowest calibration standard used (1 mg/L) and is less reliable than the spectrophotometric analyses. The results of the daily perchlorate readings from the Acorn 6 perchlorate potentiometer show that the nominal 1.6, variable0–4.5, 30, and 100 mg/L treatments had mean perchlorate concentrations of 1.6 mg/L (n = 85, SE = 0.05), 2.2 mg/L (n = 85, SE = 0.08), 32.0 mg/L (n = 334, SE = 0.20), and 102.9 mg/L (n = 334, SE = 0.57), respectively. The variable(0–60) mg/L treatment reached a maximum concentration of 71.8 mg/L on experimental day 179 (December 3, 2003) with a mean perchlorate concentration of 51.1 mg/L (n = 324 readings, SE = ± 1.00 mg/L). After experimental day 179, the perchlorate readings remained relatively stable (mean2003 = 61.8 mg/L, n = 185, SE = 0.19). The rates of increase between the 2002 HTPB and 2003 variable(0–60) mg/L treatments were similar (y2002 = 0.32x – 12.04, r2 = 0.92 vs y2003 = 0.31x – 9.44, r2 = 0.88; y = perchlorate concentration, x = number of experimental dpf).
Untransformed morphometric results
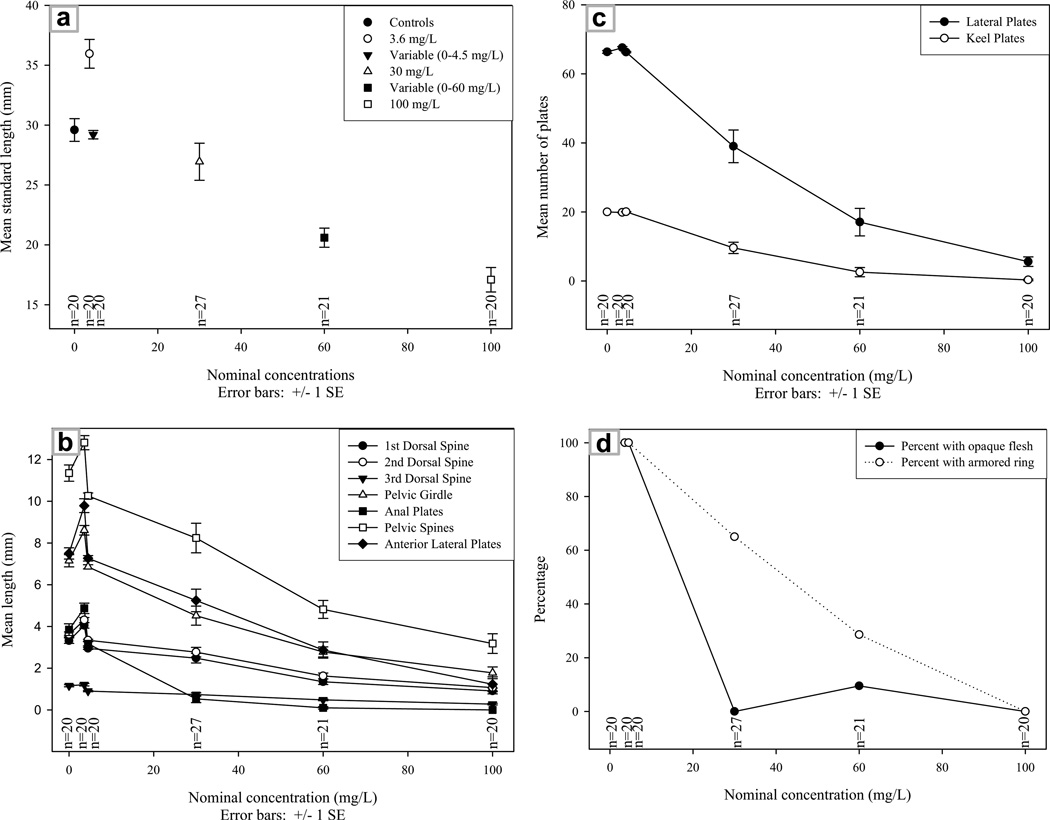
In 2002, fish raised from syngamy through 5.5 months of age in negative control water, 1.5 mg/L, or 12.0 mg/L treatments showed significant developmental differences in 9 of 39 morphological characters, with fish from higher treatments generally showing greater impairments of growth and calcified structure development. The experiments of 2003 repeated and expanded upon the 2002 experiments with larger sample sizes. In 2003, fish raised from syngamy to 15 weeks of age showed highly significant statistical differences (p < 0.0001) in developmental patterns for 27 of 29 morphological characters (Table 1, Fig. 2). The dose-dependent relationship noted in 2002 was replicated in 2003, as a greater proportion of fish from higher treatments displayed transparency, incomplete keel plate development, fewer and smaller lateral plates, incomplete armored ring formation, and smaller fins and spines (Table 1, Fig. 2).
Table 1.
Differences in morphological traits between G1,2003 experimental groups
| 2003 r2 | 2003 Untransformed K-W | 2003 Standardized K-W | |
|---|---|---|---|
| Characters | with SL | F / df / p values | F / df / p values |
| Standard length | 1** | 82.013 / 5 / <.0001 | |
| Body mass | 0.92** | 95.355 / 5 / <.0001 | 74.398 / 5 / <.0001 |
| Body depth | 0.982** | 78.258 / 5 / <.0001 | 28.762 / 5 / <.0001 |
| Body width | 0.948** | 71.495 / 5 / <.0001 | 45.421 / 5 / <.0001 |
| Sum orbit length | 0.945** | 79.965 / 5 / <.0001 | 72.798 / 5 / <.0001 |
| 1st dorsal spine | 0.95** | 89.930 / 5 / <.0001 | 94.643 / 5 / <.0001 |
| 2nd dorsal spine | 0.941** | 87.991 / 5 / <.0001 | 91.330 / 5 / <.0001 |
| 3rd dorsal spine | 0.892** | 85.844 / 5 / <.0001 | 89.296 / 5 / <.0001 |
| Pelvic girdle length | 0.925** | 91.927 / 5 / <.0001 | 77.253 / 5 / <.0001 |
| Anal spine length | 0.919** | 78.358 / 5 / <.0001 | 83.929 / 5 / <.0001 |
| Sum keel length | 0.86** | 87.127 / 5 / <.0001 | 101.491 / 5 / <0.001 |
| Sum ascending branch length | 0.98** | 76.800 / 5 / <.0001 | 68.160 / 5 / <.0001 |
| Sum anterior lateral plate length | 0.944** | 90.828 / 5 / <.0001 | 83.926 / 5 / <.0001 |
| Sum opercular length | 0.986** | 81.658 / 5 / <.0001 | 69.114 / 5 / <.0001 |
| Sum opercular depth | 0.984** | 83.004 / 5 / <.0001 | 75.883 / 5 / <.0001 |
| Sum pelvic spine length | 0.944** | 87.171 / 5 / <.0001 | 74.745 / 5 / <.0001 |
| Sum anal plate length | 0.79** | 103.441 / 5 / <.0001 | 78.943 / 4 / <.0001 |
| Total no. of lateral plates | 0.817** | 95.503 / 5 / <.0001 | 98.985 / 5 / <.0001 |
| Total no. of keel plates | 0.771** | 106.432 / 5 / <.0001 | 80.213 / 4 / <.0001 |
| Total Pelvic Score | 0.169 | 5.050 / 5 / .410 | |
| Dorsal fin length | 0.978** | 77.029 / 5 / <.0001 | 8.220 / 5 / .145 |
| Dorsal fin height | 0.952** | 71.192 / 5 / <.0001 | 61.350 / 5 / <.0001 |
| Anal fin length | 0.968** | 79.169 / 5 / <.0001 | 12.572 / 5 / .028 |
| Sum pectoral fin length | 0.888** | 97.389 / 5 / <.0001 | 96.658 / 5 / <.0001 |
| No. dorsal fin rays | 0.681** | 53.149 / 5 / <.0001 | 100.779 / 5 / <.0001 |
| No. anal fin rays | 0.611** | 34.096 / 5 / <.0001 | 72.172 / 5 / <.0001 |
| Total no. of pectoral fin rays | 0.004 | 9.589 / 5 / .088 | 50.449 / 3 / <.0001 |
| Transparent flesh | 0.59** | 112.817 / 5 / <.0001 | |
| Armored ring completely formed | 0.754** | 81.334 / 5 / <.0001 | |
| Significance summary | 27 of 29 | 24 of 25 |
Significant differences were identified for 27 of 29 untransformed characters and for 24 of 25 standardized characters in nominal treatments ranging from negative controls (<1.1 µg/L) to 100 mg/L perchlorate.
A significance value of α ≤ 0.038 was adopted for 2003.
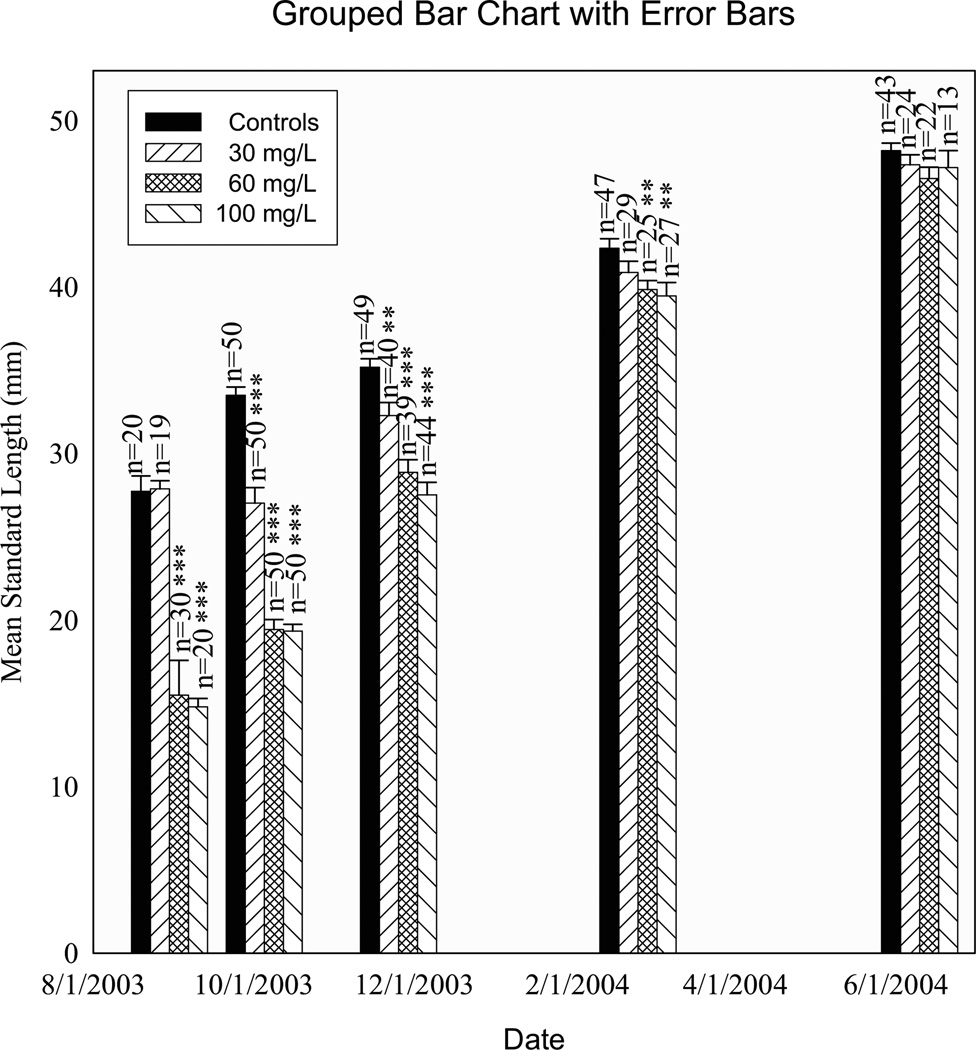
Fig. 2.
Typical developmental patterns for the G1,2003 fish showing dose-dependency: (a) mean standard length by experimental group; (b) mean length of select calcified structures by experimental group; (c) percent of fish with opaque flesh or armored ring by experimental group; and (d) mean number of lateral and keel plates by experimental group. Figures show nominal concentrations along the x axis (actual mean concentrations were 1.6 mg/L, 2.2 mg/L, 32.0 mg/L, 51.1 mg/L, and 102.9 mg/L). SE = standard error.
The G2,2004, which were maintained in water without detectable perchlorate concentrations to test for transgenerational effects, suffered early mortality. By the time they were euthanized in December 2004, only 32, 20, and 7 of the G2,2004 remained from parental groups in the negative control, 30, and variable(0–60) mg/L experimental groups, respectively. From these, 20, 20, and 7 G2,2004 progeny from the negative control, 30, and variable(0–60) mg/L experimental groups, respectively, were tested for morphological differences.
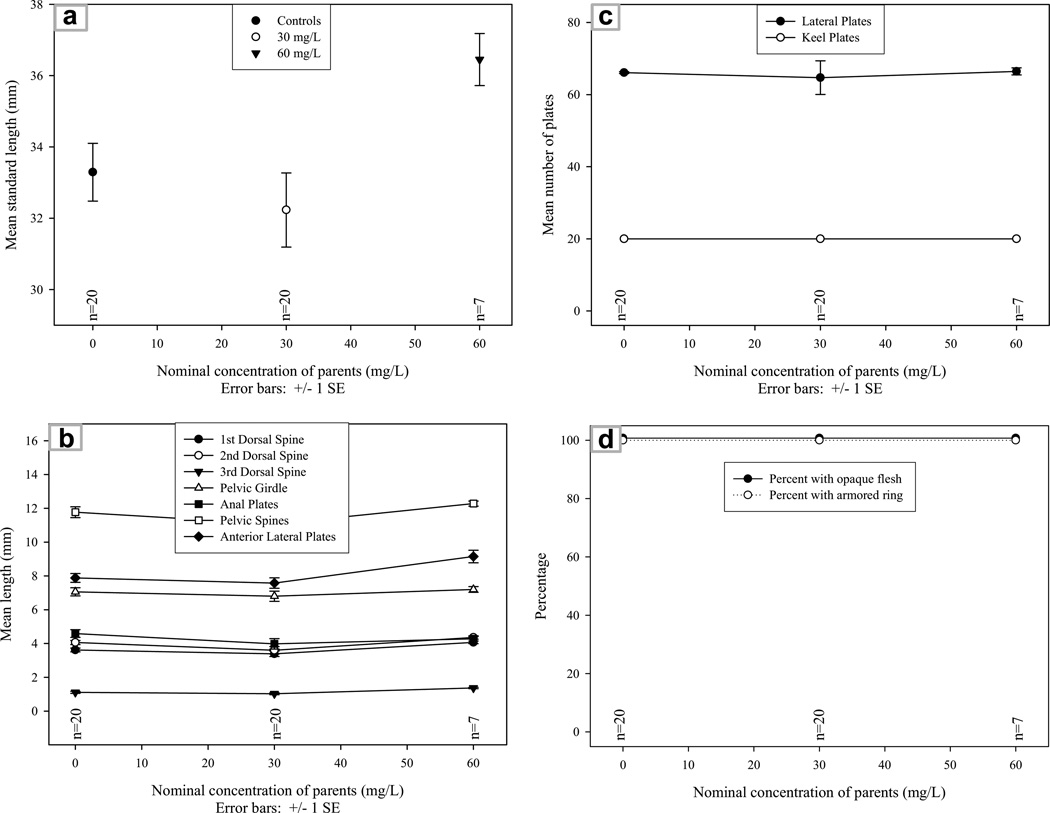
The G2,2004, all unexposed to perchlorate, showed growth patterns typical of fish being raised at different densities, but a typical of stickleback affected by perchlorate. Statistically, the negative control and progeny of fish exposed to 30 mg/L did not differ from each other (Table 2, Fig. 3), but both groups were generally smaller than the unexposed G2,2004 progeny of fish from the variable(0–60) mg/L treatment (Fig. 3). Although four of 18 characters were significantly different among groups of the G2,2004, this is due to the progeny of fish in the variable(0–60) mg/L group being larger than the progeny of fish exposed to negative control and 30 mg/L perchlorate. None of these four characters showed highly significant differences (p < 0.001) between experimental groups (Table 2), and none of the G2,2004 fish showed incomplete calcification, transparency, or impaired developmental patterns. One anomalous developmental event occurred, however, on one of the G2,2004 progeny of the variable(0–60) mg/L group. This fish developed a fourth dorsal spine (Fig. 4).
Table 2.
Differences in morphological traits between G2,2004 experimental groups
| 2002 r2 | 2004 Untransformed K-W | 2004 Standardized K-W | |
|---|---|---|---|
| Characters | with SL | F / df / p values | F / df / p values |
| Standard length | 1 | 6.717 / 2 / .035 | |
| Body depth | 0.951 ** | 5.151 / 2 / .076 | 1.111 / 2 / .574 |
| 1st dorsal spine | 0.925 ** | 7.271 / 2 / .026 | 8.244 / 2 / .016 |
| 2nd dorsal spine | 0.895 ** | 5.906 / 2 / .052 | 7.120 / 2 / .028 |
| 3rd dorsal spine | 0.848 ** | 9.357 / 2 / .009 | 6.967 / 2 / .031 |
| Pelvic girdle length | 0.924 ** | 5.151 / 2 / .076 | 13.366 / 2 / .001 |
| Sum ascending branch length | 0.896 ** | 4.408 / 2 / .110 | 6.247 / 2 / .044 |
| Sum anterior lateral plate length | 0.899 ** | 6.857 / 2 / .032 | 0.409 / 2 / .815 |
| Sum pelvic spine length | 0.892 ** | 0.657 / 2 / .720 | .498 / 2 / .780 |
| Sum anal plate length | 0.844 ** | 3.174 / 2 / .205 | 7.554 / 2 / .023 |
| Total no. of lateral plates | 0.561 | 0.635 / 2 / .728 | 4.815 / 2 / .090 |
| Total no. of keel plates | 0.000 / 2 / 1.000 | ||
| Total pelvic score | 0.089 | 0.000 / 2 / 1.000 | |
| Dorsal fin length | 0.843 ** | 4.154 / 2 / .125 | 3.590 / 2 / .166 |
| Sum pectoral fin length | 0.87 ** | .080 / 2 / .961 | 12.115 / 2 / .002 |
| No. dorsal fin rays | 0.383 * | 2.222 / 2 / .329 | 0.424 / 2 / .809 |
| Transparent flesh | 0.000 / 2 / 1.000 | ||
| Armored ring completely formed | 0.000 / 2 / 1.000 | ||
| Significance summary | 4 of 18 | 7 of 13 |
All G2,2004 were raised in water without detectable levels of perchlorate, but their parents were raised from fertilization to sexual maturity in negative control or perchlorate-treated water. Significant differences were identified for four of 18 untransformed characters and for seven of 13 standardized characters. Significant differences were due to the offspring of the G1,2003 fish in the variable(0–60) mg/L treatment being larger, which was likely due to lower densities in their aquaria.
Differences were considered significant when α ≤ 0.05 in 2004.
Fig. 3.
Typical developmental patterns for G2,2004 fish: (a) mean standard length by experimental group; (b) mean length of select calcified structures by experimental group; (c) percent of fish with opaque flesh or armored ring by experimental group; and (d) mean number of lateral and keel plates by experimental group. The G2,2004 are categorized according to the nominal exposure of their parents. The progeny of fish exposed to 30 and variable(0–60) mg/L perchlorate show none of the aberrant patterns noted among their perchlorate-treated parents. When differences occurred, the G2,2004 progeny of fish from the variable(0–60) mg/L experimental group were larger than fish in the other treatments, probably reflecting the effects of unequal fish densities. SE = standard error.
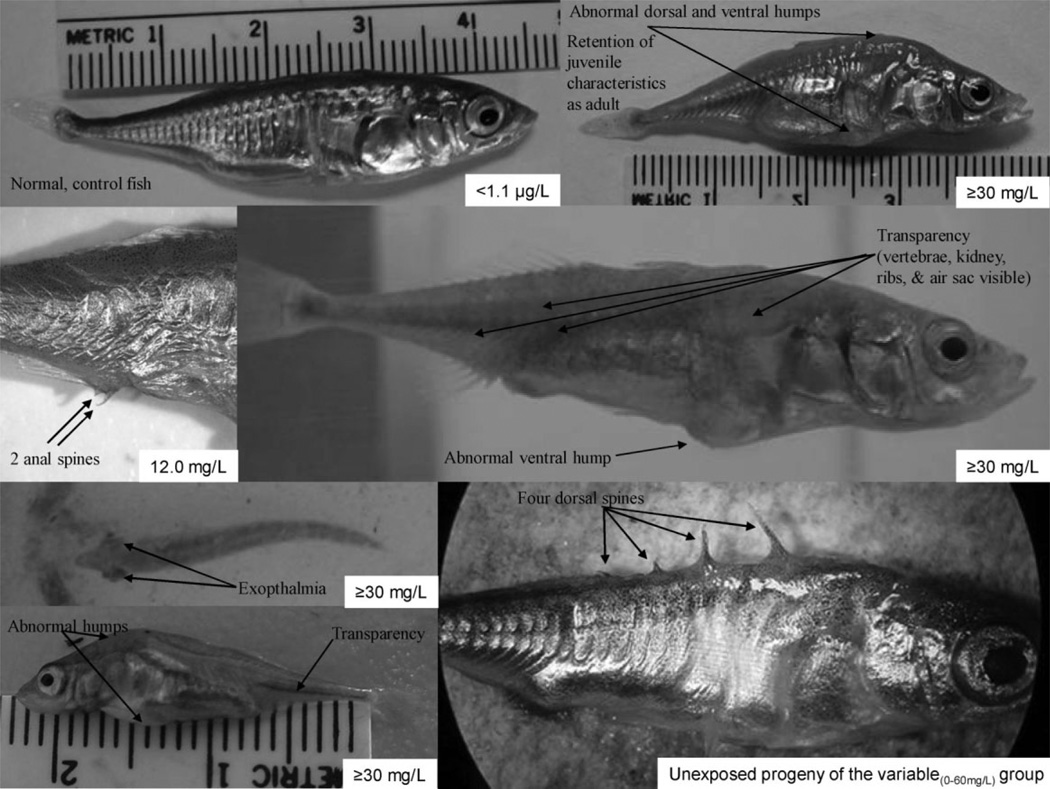
Fig. 4.
Gross developmental abnormalities. Perchlorate clearly disrupted body pigmentation, lateral plate development, body shape, and appeared to express itself in features such as exopthalmia and possibly duplicated spines.
Standardized morphometric results
For G1,2003 fish, after the continuous morphometric characters were transformed to remove effects of differences in SL, statistically significant differences were found in developmental patterns for 24 of 25 continuous characters; all but two of these 24 characters showed highly significant differences (p < 0.0001; Tables 1, 2). The G2,2004 showed differences in seven of 13 traits between groups when the data were transformed to a common SL (Table 2).
Growth rates
Negative control fish reached their maximum SL faster than treated fish, but all fish ultimately reached similar maximum SL at sexual maturity (Fig. 5; ANOVA, F3,100 = 1.418, p = −0.242).
Fig. 5.
Growth of G1,2003. Asterisks represent the degree of significance between each treatment and negative control stickleback (*p < 0.05, **p < 0.01, and ***p < 0.001). Rapid growth among fish in the variable(0–60) and 100 mg/L perchlorate treatments demonstrate a release effect between October 5 and February 24 because densities were standardized to 50 fish per 400-L tank on October 5; prior to that these treatments had higher densities. Yet treated fish in the 30, variable(0–60), and 100 mg/L treatments suffered higher mortality rates and subsequently developed at lower densities than the negative control fish.
Density effects
To determine whether perchlorate concentration or fish density had a stronger effect on growth, comparisons were made between fish raised at different densities and perchlorate concentrations. Prior to conducting the ANCOVA, preliminary analysis evaluating the homogeneity-of-slopes assumption indicated that the relationship between density (covariate) and SL (dependent variable) differed significantly as a function of the perchlorate concentration (independent variable; F3,224 = 59.67, MSE = 451.43, p < 0.0001, partial χ2 = 0.444). Furthermore, Levene’s homogeneity of variances testing demonstrated that variances were not homogenous (F5,228 = 4.792, p < 0.001). Nevertheless, the ANCOVA was performed to provide insight and was found to be highly significant (F5,227 = 36.89, MSE = 495.46, p < 0.0001). The strength of the relationship between the perchlorate concentration and SL was strong, as assessed by a partial χ2, with perchlorate concentration accounting for 45% of the variance in SL when density of the stickleback was held constant at 253 fish per pool. Since both ANCOVA assumptions were violated, the statistical results were interpreted as being suggestive. However, examination of the four instances when G1,2003 fish were raised in lower perchlorate concentrations and higher densities provided further evidence of perchlorate’s role in growth inhibition. In every instance, fish maintained at higher density and lower perchlorate concentration grew to be larger than those raised in higher perchlorate treatments at lower densities (Table 3).
Table 3.
Effects of perchlorate concentration and fish density on standard length
| Tank | Number of fish | Density (fish / Liter) | Mean standard length (mm) | ||
|---|---|---|---|---|---|
| 15a | 100 | 265 | 0.166 | 16.5 | |
| 4a,b | 0–60 | 291 | 0.182 | 20.5 | |
| 11b | 0–60 | 343 | 0.214 | 18.22 | |
| 12 | 30 | 294 | 0.184 | 24.05 |
Fish raised in treatments with higher perchlorate concentrations and lower densities are smaller than those raised at lower perchlorate concentrations and higher densities, suggesting that perchlorate concentration has a greater effect on growth than does fish density.
Density/Perchlorate concentration comparison 1: Fish from tank 4 exposed to as high as 60 mg/L perchlorate and raised at a density of 0.182 fish/L were larger than fish from tank 15 exposed to 100 mg/L perchlorate and raised at a density of 0.166 fish/L.
Fish in tank 4 were exposed to the same perchlorate treatment as fish in tank 11 [variable(0–60) mg/L treatment], but tank 11 had a higher density of fish and produced smaller fish.
Gross developmental abnormalities
Several gross developmental abnormalities were noted among perchlorate-treated G1,2003 fish (Fig. 4). Ventral and dorsal protrusions developed on 3, 12, and 42% of fish exposed to 30, variable(0–60), and 100 mg/L, respectively, but were absent from negative control fish. Exopthalmia (bulging eyes) was noted in treatments ≥30 mg/L. All fish (2002–2003) exposed to ≥12.0 mg/L perchlorate either lacked lateral plates, or their lateral plates were abnormally small and poorly calcified. Negative control fish had 9 to 10 keel plates on each side of their caudal peduncles, while 50% of 30 mg/L fish, 82% of variable(0–60) mg/L fish, and 95% of 100 mg/L fish completely lacked these plates, and this despite the fact that their wild-caught parents had complete lateral plate development with fully plated keels.
In 2002, fish exposed to ≥1.5 mg/L perchlorate displayed impaired formation of calcified traits and impaired somatic growth [48]. Also in 2002 [48], only stickleback in the highest treatment [variable(0–66) mg/L HTPB] had incomplete formation of the armored ring, developed fewer and smaller lateral plates, completely lacked keel plates, and were transparent. In 2003, subsamples of 27–36 G1,2003 fish per treatment were analyzed for transparency at 35 weeks of age (experimental day 262). None of the 36 negative control fish were transparent, while four of 29 (13.8%), 19 of 25 (76%), and 24 of 26 (92.3%) fish from the 30, variable(0–60), and 100 mg/L treatments, respectively, were transparent. In fact, many treated fish remained transparent at sexual maturity, allowing vertebrae, air sacs, digestive tracts, kidneys, and ribs to be seen externally (Fig. 4). Treated fish failed to produce normal nuptial coloration [45]. Transparency was never observed in wild-caught fish.
DISCUSSION
Chronically exposed stickleback raised in perchlorate-treated water displayed abnormalities that increased in frequency and severity in a dose-dependent manner (Figs. 2, 4) [48]. The G2,2004 stickleback, which were not directly exposed to perchlorate but were the offspring of perchlorate-treated parents (excluding negative control fish), did not show changes in endpoints characteristic of perchlorate exposure, suggesting a lack of perchlorate-related transgenerational effects (Fig. 3). However, one of the seven G2,2004 progeny of fish from the variable(0–60) mg/L experimental group developed an extra dorsal spine (Fig. 4), which is a rare natural event. Whether this was a perchlorate-induced transgenerational effect or a random event is unclear. Because so few fish exposed to higher perchlorate treatments managed to spawn in 2003 [45], there were not enough offspring to draw definitive conclusions about transgenerational effects. Therefore, future research should investigate whether perchlorate induces transgenerational effects in spine duplication, as it may also have done in fish directly exposed to perchlorate (i.e., anal spine duplication, Fig. 4) [48].
Size differences by experimental group were apparent among the G2,2004 (Fig. 3), but these did not appear to be perchlorate-related transgenerational effects. Instead, these effects were likely the result of different fish densities among the G2,2004 experimental groups, since fish raised at lower densities grew faster and larger than those at higher densities. The progeny of fish exposed to 30 mg/L perchlorate are statistically similar to negative control fish and the progeny of fish in the variable(0–60) mg/L treatment were larger than both, which is contrary to what would have been expected if parental perchlorate exposure imposed dose-dependent transgenerational effects.
The G1,2003 fish in higher perchlorate treatments experienced higher mortality (percent mortality between October 2003 and May, 2004 was 14, 52, 56, and 74% for Control, 30, 60, and 100 ppm fish, respectively; details in Bernhardt et al. [45]). Therefore, fish in higher perchlorate concentrations were raised at lower densities between 15 and 52 weeks of age. Fish maintained at lower densities typically grow larger and/or faster than those raised at higher densities [51–55] even, as in the present study, when food is not a limiting factor [52]. However, stickleback maintained at higher densities and lower perchlorate concentrations were larger than those raised at lower densities and higher perchlorate concentrations (Table 3). These results support the hypothesis that the concentration of perchlorate had a greater effect on stickleback growth than did fish density, within the respective ranges of the present study. Although assumptions were violated, our ANCOVA analysis also suggests that perchlorate reduced growth rate when the effects of density were held constant. An interaction study specifically designed to analyze these factors would be necessary to definitively tease them apart.
Analyses of size-adjusted data demonstrated that perchlorate also affected the relative size of characters (Tables 1, 2). Therefore, size differences were not due solely to fish from higher perchlorate concentrations being smaller. Rather, perchlorate exposure caused the relative size of most traits (24 out of 25) to be proportionally smaller.
Overall, our findings demonstrate that fish raised in higher perchlorate treatments showed impaired development, slower growth, and smaller characters, with the exception of the G1,2003 3.7 mg/L fish, which were larger than expected (Table 3, Fig. 2). Several characters showed evidence of reduced calcium deposition, and nearly every measured characteristic on fish from higher treatments was smaller than on fish from lower treatments or negative controls. Many characters were absent or had incomplete expression among fish from higher treatments. Thus, the trends noted in the 2002 pilot study [48] were reinforced by the present study.
These findings reflect what have become typical dose-related trends for animals exposed to perchlorate during development. Crane et al. [19] found that 10 and 100 mg/L perchlorate-treated fathead minnows (Pimephales promelas) also had significantly lower wet body mass and standard lengths compared to negative controls and those exposed to 1 mg/L perchlorate. Goleman et al. [16] found that growing Xenopus laevis exposed to 425 mg/L ammonium perchlorate had a reduced snout to vent length after only 16 d of exposure. However, evidence of hormesis, an overcompensation to insult resulting in overdevelopment or overexpression, has occasionally been reported among organisms exposed to approximately 1 mg/L perchlorate [19,21,46,47,56]. Therefore, we cannot rule out the possibility that the larger G1,2003 exposed to 3.7 mg/L perchlorate displayed a hormetic effect, but their lower density almost certainly contributed to their enhanced growth.
A prominent trend in the present study was for calcified traits to be underdeveloped among perchlorate-treated fish (Tables 1, 2, Fig. 4). Dose-dependent effects were revealed with fish in higher treatments having smaller (or missing) calcified traits (Tables 1, 2). Reduced calcium deposition is known to be associated with chronic hypothyroidism among fishes [30]. Crane et al. [19] found that fathead minnows exposed to 10 and 100 mg/L ammonium perchlorate for 28 d had smaller scales than negative controls or those exposed to 1 mg/L. Srivastava [57,58] showed that thyroxine administration stimulates phosphate uptake from aquarium water, which may reflect an intensification of calcification and/or protein synthesis.
Robust calcification of characters such as lateral plates, spines, and the pelvic girdle on threespine stickleback reduce the risk of predation and may help in the acquisition and maintenance of breeding territories [59]. A robust armored ring (including anterior lateral plates, pelvic girdle, pelvic spines, and dorsal spines) poses a formidable defensive barrier to gape-limited predators, such as many piscivorous fish [60].
Previous experiments have shown that both pectoral fin and lateral plate formation are linked with swimming performance in stickleback [61,62]. In fact, Bergstrom [63] used resident freshwater stickleback to demonstrate that the number of lateral plates was negatively correlated with swimming velocity and attributed these findings, in part, to less drag among fish with fewer lateral plates. Thus, when all else is equal, one might expect to find enhanced swimming performance among stickleback with fewer and smaller lateral plates. However, stickleback exposed to higher perchlorate concentrations performed worse in swimming trials than those exposed to a lower perchlorate concentration or negative controls [64]. These findings suggest that lateral plate reductions are not linked to the impaired swimming performance noted among perchlorate treated fish. Poor swimming performance may have been associated with abnormalities in their shape (Fig. 4), suboptimal energy metabolism, or muscular formation associated with altered TH levels [65], or even reduced size of the pectoral fins (Table 2).
Taylor and McPhail [66] compared resident freshwater stickleback with smaller pectoral fins to anadromous stickleback with larger fins and found that resident freshwater morphotypes were only able to maintain swimming speeds of five mean body lengths per second for 12–24 minutes before failing to resist the current. Longer pectoral fins provide more thrust per stroke [67], and allow stickleback to achieve greater velocity with fewer fin beats. Therefore, fish with smaller pectoral fins have to expend more energy than fish with larger pectoral fins in order to achieve the same velocity. Together, abnormally small fins and reduced armor development would affect key components of fitness, including vulnerability to predation, migratory success, and parental care behavior [64].
Perchlorate-exposed stickleback also displayed impaired silvering and remained transparent at phenotypic maturity, with organs and vertebrae clearly visible (Fig. 4). Although a greater percentage of stickleback in the variable(0–60) mg/L treatment had opaque flesh, compared to the 30 and 100 mg/L treatments, the concentration of the variable(0–60) mg/L treatment was less than the 30 mg/L treatment until the fry were approximately five weeks of age (July 30, 2003), long after the point that integumentary silvering normally occurs. Therefore, the proportion of fish with normal flesh (opaque and silvery) actually decreased in a dose-dependent manner (Fig. 2).
Transparency may be linked to impaired thyroid function. Silvering of teleosts normally occurs when thyroxine and/or TSH stimulates integumentary purine deposition, specifically guanine and hypoxanthine [31,33,68,69]. Goitrogens, such as thiourea [69] and perchlorate [19,43], are known to inhibit thyroxine production, thereby preventing integumentary silvering. Crane et al. [19] showed that fathead minnows exposed to 10 and 100 mg/L ammonium perchlorate for 28 d retained the transparency typical of their larval form. Mukhi et al. [44] demonstrated that zebrafish treated with 100 and 250 mg/L perchlorate exhibited reduced integumentary silvering. Both of these studies [19,43] also demonstrated that perchlorate exposure interfered with thyroid function as indicated by follicular hypertrophy and hyperplasia, colloidal depletion, and altered T4 levels.
Thyroxine has been shown to affect the pigmentation of different species in different ways or not at all [19,30,33,44,70–72], and perchlorate could alter pigmentation by other means. The transparency of our perchlorate-treated stickleback as well as the scarcity of pigmentation on the testes of genotypic male stickleback exposed to perchlorate [45] suggests that perchlorate may inhibit melanogenesis or may be melanocytotoxic. This is a topic worthy of study to determine if the mechanism by which perchlorate disrupts pigmentation is of concern to human health.
LaRoche et al. [30] found that retarded gonadal development among radiothyroidectomized fish was attributed to a chronic state of hypothyroidism. Although retarded gonadal development might be expected among fish with impaired thyroid function, the intersex gonads in genotypic female stickleback produced by perchlorate exposure [45] suggests interference with developmental processes beyond the control of thyroid hormones. In fact, we found that perchlorate-treated genotypic male stickleback developed markedly enlarged testes in a dose-dependent manner [45]. Other studies have also shown that perchlorate has direct effects on animal tissues that appear to be extrathyroidal, such as renal flow, muscular function, and maintenance of bone [73–75].
CONCLUSION
Every threespine stickleback that was chronically exposed to ≥12.0 mg/L of sodium perchlorate displayed morphological abnormalities at phenotypic maturity (Tables 1, 2, Figs. 2, 4) [48]. Aberrant patterns were primarily associated with reproduction, locomotion, calcification, pigmentation, antipredatory structures, and vision. Some genotypic female fish exposed to ≥30 mg/L perchlorate became masculinized and developed intersex gonads, showing both structural and functional hermaphroditism [45]. Many fish exposed to ≥30 mg/L perchlorate failed to become silver and lacked lateral plates, allowing vertebrae and internal organs to be visible. The abnormalities noted among these fish illustrate the profound morphological changes induced by chronic exposure to perchlorate. However, impaired stickleback that reproduced in water without detectable levels of perchlorate (<1.1 µg/L) produced offspring without the suite of abnormalities noted among perchlorate-treated fish. This suggests that surviving populations may be able to recover following remediation of perchlorate-contaminated sites. However, perchlorate may impose selective forces that alter the genetic and phenotypic composition of the surviving population.
Acknowledgement
We thank Jeff Jones, Christoff Furin, Anjali Karve, Heidi Weigner and Rajit Patankar for their assistance in the laboratory. This work was supported in part by the U.S. Air Force Space and Missile Systems Center and The Aerospace Corporation. Additional support came from the Alaska INBRE Program, grant 5P20RR016466 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and from a grant from the National Institute of Environmental Health Sciences (NIEHS), grant 1R01ES017039-01A1.
Footnotes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR, NIEHS, or NIH. Fish were collected under Alaska Department of Fish and Game permits SF-2002-002 and SF-2003-019, and all research protocols were approved by the UAA Institutional Animal Care and Use Committee.
REFERENCES
- 1.Greer MA, Goodman G, Pleus RC, Greer SE. Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Ecotoxicol Environ Saf. 2002;110:927–937. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urbansky ET, Brown SK, Magnuson ML, Kelty CL. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ Pollut. 2001;112:299–302. doi: 10.1016/s0269-7491(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 3.Ericksen GE. Washington DC: U.S. Government Printing Office; 1981. Geology and origin of the Chilean nitrate deposits. U.S. Geological Survey Professional Paper 1188. [Google Scholar]
- 4.Ericksen GE. The Chilean nitrate deposits. Am Sci. 1983;71:366–374. [Google Scholar]
- 5.Ericksen GE, Hosterman JW, St. Amand P. Chemistry, mineralogy and origin of the clay-hill nitrate deposits, Amargosa River Valley, Death Valley region, California, U.S.A. Chem Geol. 1988;67:85–102. [Google Scholar]
- 6.Rajagopalan S, Anderson TA, Fahlquist L, Rainwater KA, Ridley Moira, Jackson WA. Widespread presence of naturally occurring perchlorate in high plains of Texas and New Mexico. Environ Sci Technol. 2006;40:3156–3162. doi: 10.1021/es052155i. [DOI] [PubMed] [Google Scholar]
- 7.Rao B, Anderson TA, Orris GJ, Rainwater KA, Rajagopalan S, Sandvig RM, Scanlon BR, Stonestrom DA, Walvoord MA, Jackson WA. Widespread natural perchlorate in unsaturated zones of the Southwest United States. Environ Sci Technol. 2007;41:4522–4528. doi: 10.1021/es062853i. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Environmental Protection Agency. Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization. NCEA-1-0503. Washington, DC: 2002. [Google Scholar]
- 9.Smith PN, Theodorakis CW, Anderson TA, Kendall RJ. Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas. Ecotoxicology. 2001;10:305–313. doi: 10.1023/a:1016715502717. [DOI] [PubMed] [Google Scholar]
- 10.Backus SM, Klawuun P, Brown S, D’sa I, Sharp S, Surette C, Williams DJ. Determination of perchlorate in selected surface waters in the Great Lakes Basin by HPLC/MS/MS. Chemosphere. 2005;61:834–843. doi: 10.1016/j.chemosphere.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 11.Motzer WE. Perchlorate: problems, detection, and solutions. Environ Forensics. 2001;2:301–311. [Google Scholar]
- 12.Urbansky ET. Perchlorate chemistry: implications for analysis and remediation. Bioremediat J. 1998;2:81–95. [Google Scholar]
- 13.Flowers TC, Hunt JR. Long term release of perchlorate as a potential source of groundwater contamination. In: Urbansky ET, editor. Perchlorate in the Environment. New York, NY, USA: Kluwer Academic/Plenum; 2000. pp. 177–188. [Google Scholar]
- 14.Plummer LN, Bohlke JK, Doughten MW. Perchlorate in Pleistocene and Holocene groundwater in North-Central New Mexico. Environ Sci Technol. 2006;40:1757–1763. doi: 10.1021/es051739h. [DOI] [PubMed] [Google Scholar]
- 15.Goleman WL, Carr JA, Anderson TA. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environ Toxicol Chem. 2001;21:590–597. [PubMed] [Google Scholar]
- 16.Goleman WL, Urquidi LJ, Anderson TA, Smith EE, Kendall RJ, Carr JA. Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis. Environ Toxicol Chem. 2002;21:424–430. [PubMed] [Google Scholar]
- 17.Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. In utero and lactational exposure of Long-Evans rats to ammonium perchlorate (AP) disrupts ovarian follicle maturation. Reprod Toxicol. 2004;19:155–161. doi: 10.1016/j.reprotox.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Patiño R, Wainscott MR, Cruz-Li EI, Balakrishnan S, McMurry C, Blazer VS, Anderson TA. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ Toxicol Chem. 2003;22:1115–1121. [PubMed] [Google Scholar]
- 19.Crane HM, Pickford DB, Hutchinson TH, Brown JA. Effects of ammonium perchlorate on thyroid function in developing fathead minnows, Pimephales promelas. Environ Health Perspect. 2005;113:396–401. doi: 10.1289/ehp.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miranda LA, Pisano A, Casco V. Ultrastructural study on the thyroid glands of Bufo arenarum larvae kept in potassium perchlorate solution. Biocell. 1996;20:147–153. [PubMed] [Google Scholar]
- 21.McNabb FMA, Jang DA, Larsen CT. Does thyroid function in developing birds adapt to sustained ammonium perchlorate exposure? Toxicol Sci. 2004;82:106–113. doi: 10.1093/toxsci/kfh247. [DOI] [PubMed] [Google Scholar]
- 22.Yu KO, Narayanan L, Mattie DR, Godfrey RJ, Todd PN, Sterner TR, Mahle DA, Lumpkin MH, Fisher JW. The pharmacokinetics of perchlorate and its effect on the hypothalamus-pituitary-thyroid axis in the male rat. Toxicol Appl Pharmacol. 2002;182:148–159. doi: 10.1006/taap.2002.9432. [DOI] [PubMed] [Google Scholar]
- 23.Choksi NY, Jahnke GD, Hilaire C St, Shelby M. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res. 2003;68:479–491. doi: 10.1002/bdrb.10045. [DOI] [PubMed] [Google Scholar]
- 24.Stanbury JB, Wyngaarden JB. Effects of perchlorate on the human thyroid gland. Metabolism. 1952;1:533–539. [PubMed] [Google Scholar]
- 25.Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998;60:89–105. [PubMed] [Google Scholar]
- 26.Brabant G, Bergman P, Kirsch CM, Kohrle J, Hesch RD, von Zer Muhlen A. Early adaptation of thyrotropin and thyroglobulin secretion to experimentally decreased iodine supply in man. Metabolism. 1992;41:1093–1096. doi: 10.1016/0026-0495(92)90291-h. [DOI] [PubMed] [Google Scholar]
- 27.York RG, Brown WR, Girard MF, Dollarhide JS. Two-generation reproduction study of ammonium perchlorate in drinking water in rats evaluates thyroid toxicity. Int J Toxicol. 2001;20:183–197. doi: 10.1080/109158101750408019. [DOI] [PubMed] [Google Scholar]
- 28.Bradford CM, Rinchard J, Carr JA, Theodorakis C. Perchlorate affects thyroid function in Eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environ Sci Technol. 2005;39:5190–5195. doi: 10.1021/es0484505. [DOI] [PubMed] [Google Scholar]
- 29.Capen CC. Toxic responses of the endocrine system. In: Klaasen CD, editor. Casarett & Doull’s Toxicology. The Basic Science of Poisons. 6th ed. New York, NY, USA: McGraw Hill; 2001. pp. 711–759. [Google Scholar]
- 30.La Roche G, Woodall AN, Johnson CL, Halver JE. Thyroid function in the rainbow trout (Salmo gairdnerii Rich.). II. Effects of thyroidectomy on the development of young fish. Gen Comp Endocrinol. 1966;6:249–266. doi: 10.1016/s0016-6480(66)80013-8. [DOI] [PubMed] [Google Scholar]
- 31.Premdas FH, Eales JG. The influence of TSH and ACTH on purine and pteridine deposition in the skin of rainbow trout (Salmo gairdneri) Can J Zool. 1976;54:576–581. doi: 10.1139/z76-067. [DOI] [PubMed] [Google Scholar]
- 32.Hurlburt ME. Role of the thyroid gland in ovarian maturation of the goldfish Carassius auratus L. Can J Zool. 1977;55:1906–1913. doi: 10.1139/z77-244. [DOI] [PubMed] [Google Scholar]
- 33.Leatherland JF. Environmental physiology of the teleostean thyroid gland: a review. Environ Biol Fish. 1982;7:83–110. [Google Scholar]
- 34.Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev Fish Biol Fish. 1996;6:165–200. [Google Scholar]
- 35.Koumoundouros G, Gagliardi F, Divanach P, Boglione C, Cataudella S, Kentouri M. Normal and abnormal osteological development of caudal fin in Sparus aurata L. fry. Aquaculture. 1997;149:215–226. [Google Scholar]
- 36.Manzon RG, Youson JH. The effects of exogenous thyroxine (T-4) or triiodothyronine (T-3) in the presence and absence of potassium perchlorate, on the incidence of metamorphosis and on serum T-4 and T-3 concentrations in larval sea lampreys (Petromyzon marinus L.) Gen Comp Endocrinol. 1997;102:211–220. doi: 10.1006/gcen.1996.6867. [DOI] [PubMed] [Google Scholar]
- 37.Bentley PJ. Comparative Vertebrate Endocrinology. 3rd ed. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- 38.Castonguay M, Cyr DG. Effects of temperature on spontaneous and thyroxine-stimulated locomotor activity of Atlantic cod. J Fish Biol. 1998;53:303–315. [Google Scholar]
- 39.Janz DM, Weber LP. The endocrine system. In: Ostrander GK, editor. The Laboratory Fish. San Diego, CA, USA: Academic; 2000. pp. 415–440. [Google Scholar]
- 40.Power DM, Llewellyn L, Faustino M, Nowell MA, Bjornsson BT, Einarsdottir IE, Canario AVM, Sweeney GE. Thyroid hormones in growth and development of fish. Comp Biochem Physiol C. 2001;130:447–459. doi: 10.1016/s1532-0456(01)00271-x. [DOI] [PubMed] [Google Scholar]
- 41.Brown SB, Adams BA, Cyr DG, Eales JG. Contaminant effects on the teleosts fish thyroid. Environ Toxicol Chem. 2004;23:1680–1701. doi: 10.1897/03-242. [DOI] [PubMed] [Google Scholar]
- 42.Blanton ML, Specker JL. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit Rev Toxicol. 2007;37:97–115. doi: 10.1080/10408440601123529. [DOI] [PubMed] [Google Scholar]
- 43.Smith LS. Endocrine system. In: Smith LS, editor. Introduction to Fish Physiology. Neptune, NJ, USA: T.F.H. Publications; 1982. pp. 232–255. [Google Scholar]
- 44.Mukhi S, Torres L, Patiño R. Effects of larval-juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. Gen Comp Endocrinol. 2007;150:486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Bernhardt RR, von Hippel FA, Cresko WA. Perchlorate induces hermaphroditism in threespine sticklebacks. Environ Toxicol Chem. 2006;25:2087–2096. doi: 10.1897/05-454r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhi S, Patiño R. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol Sci. 2007;96:246–254. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- 47.Park JW, Rinchard J, Liu F, Anderson TA, Kendall RJ, Theodorakis CW. The thyroid endocrine disruptor perchlorate affects reproduction, growth, and survival of mosquitofish. Ecotoxicol Environ Saf. 2006;63:343–352. doi: 10.1016/j.ecoenv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Bernhardt RR. PhD thesis. Fairbanks, AK, USA: University of Alaska; 2008. The effects of perchlorate on a model vertebrate species: the threespine stickleback. [Google Scholar]
- 49.Bell MA, Orti G, Walker JA, Koenings JP. Evolution of pelvic reduction in threespine stickleback fish: a test of competing hypotheses. Evolution. 1993;47:906–914. doi: 10.1111/j.1558-5646.1993.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 50.von Hippel FA, Weigner H. Sympatric anadromous-resident pairs of threespine stickleback species in young lakes and streams at Bering Glacier, Alaska. Behaviour. 2004;141:1441–1464. [Google Scholar]
- 51.Ewing RD, Ewing SK. Review of the effects of rearing density on the survival to adulthood for Pacific salmon. Prog Fish Culturist. 1995;57:1–25. [Google Scholar]
- 52.Rose KA, Cowan JH, Jr, Winemiller KO, Myers RA, Hilborn R. Compensatory density dependence in fish populations: importance, controversy, understanding and prognosis. Fish Fish. 2001;2:293–327. [Google Scholar]
- 53.Ellis T, North B, Scott AP, Bromage NR, Porter M, Gadd D. The relationships between density and welfare in farmed rainbow trout. J Fish Biol. 2002;61:493–531. [Google Scholar]
- 54.Bolasina S, Tagawa M, Yamashita Y, Tanaka M. Effect of stocking density on growth, digestive enzyme activity and cortisol level in larvae and juveniles of Japanese flounder, Paralichthys olivaceus. Aquaculture. 2006;259:432–443. [Google Scholar]
- 55.Huntingford FA, Adams C, Braithwaite VA, Kadri S, Pottinger TG, Sandoe P, Turnbull JF. Review paper: current issues in fish welfare. J Fish Biol. 2006;68:332–372. [Google Scholar]
- 56.Siglin JC, Mattie DM, Dodd DE, Hildebrandt PK, Baker WH. A 90-day drinking water toxicity study in rats of the environmental contaminant ammonium perchlorate. Toxicol Sci. 2000;57:61–74. doi: 10.1093/toxsci/57.1.61. [DOI] [PubMed] [Google Scholar]
- 57.Srivastava PN. Influence of thyroid on radiophosphorous metabolism in fish. Nature. 1960;188:512–513. [Google Scholar]
- 58.Srivastava PN. Thyroidal control of radiophosphorus metabolism in yearling salmon, Salmo salar, L. Can J Zool. 1960;38:249–256. doi: 10.1038/185621a0. [DOI] [PubMed] [Google Scholar]
- 59.Wootton RJ. The Biology of Sticklebacks. New York, NY, USA: Academic; 1976. [Google Scholar]
- 60.Reimchen TE. Predators and morphological evolution in threespine stickleback. In: Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. New York, NY, USA: Oxford University Press; 1994. pp. 240–276. [Google Scholar]
- 61.Schlichting H. Boundary Layer Theory. New York, NY, USA: McGraw-Hill; 1960. [Google Scholar]
- 62.Webb PW. Fast-start resistance of trout. J Exp Biol. 1982;96:93–106. [Google Scholar]
- 63.Bergstrom CA. Fast-start swimming performance and reduction in lateral plate numbering threespine stickleback. Can J Zool. 2002;80:207–213. [Google Scholar]
- 64.Bernhardt RR, von Hippel FA. Chronic perchlorate exposure disrupts stickleback reproductive behavior. Behaviour. 2008;145:527–559. doi: 10.1163/156853908792451511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marieb EN. The endocrine system. In: Marieb EN, editor. Human Anatomy and Physiology. 6th ed. San Francisco, CA, USA: Pearson Benjamin Cummings; 2004. pp. 603–643. [Google Scholar]
- 66.Taylor EB, McPhail JD. Prolonged and burst swimming in anadromous and freshwater threespine stickleback, Gasterosteus aculeatus. Can J Zool. 1985;64:416–420. [Google Scholar]
- 67.Blake RW. Influence of pectoral fin shape on thrust and drag in labriform locomotion. J Zool Lond. 1981;194:53–66. [Google Scholar]
- 68.Dales S, Hoar WS. Effects of thyroxine and thiourea on the early development of chum salmon (Oncorhynchus keta) Can J Zool. 1954;32:244–251. [Google Scholar]
- 69.Chua D, Eales JG. Thyroid function and dermal purines in the brook trout Salvelinus fontinalis (Mitchill) Can J Zool. 1971;49:1557–1561. [Google Scholar]
- 70.Fortune PY. The effect of induced hypothyroidism on the dermal melanophores of teleosts. Proc Zool Soc Lond. 1960;135:55–64. [Google Scholar]
- 71.Wright MR, Lerner AB. Action of thyroxine analogues on frog melanocytes. Nature. 1960;185:169–170. doi: 10.1038/185169a0. [DOI] [PubMed] [Google Scholar]
- 72.Leatherland JF, Beckolay R, McLean RS, Brown D. Pituitary control of pigmentation in xanthic, moor, and white Carassius auratus L. Can J Zool. 1977;55:456–464. doi: 10.1139/z77-061. [DOI] [PubMed] [Google Scholar]
- 73.Yen MH, Chow SY, Chang LR. Perchlorate and renal excretory functions. Chin J Physiol. 1973;21:205–214. [PubMed] [Google Scholar]
- 74.Moonga BS, Datta HK, Bevis PJ, Huang CL, MacIntyre I, Zaidi M. Correlates of osteoclast function in the presence of perchlorate ions in the rat. Exp Physiol. 1991;76:923–933. doi: 10.1113/expphysiol.1991.sp003554. [DOI] [PubMed] [Google Scholar]
- 75.Huang CL. The influence of perchlorate ions on complex charging transients in amphibian striated muscle. J Physiol. 1998;506:699–714. doi: 10.1111/j.1469-7793.1998.699bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]