Abstract
Large granular lymphocyte (LGL) leukemia is a clonal lymphoproliferative disease of mature T and natural killer cells. The etiology of LGL leukemia is unknown. IL-15 is an inflammatory cytokine that stimulates T and natural killer cells and is critical for their survival and proliferation. IL-15 signals through a heterotrimeric receptor that is composed of a private receptor, IL-15Rα and IL-2/IL-15Rβ and γc shared with IL-2. Using a newly developed assay, we demonstrated increased levels of soluble IL-15Rα in the serum of patients with T-LGL leukemia. Furthermore, IL-15Rα mRNA levels were also up-regulated in the PBMCs of these patients. FACS analysis indicated that IL-15Rα was expressed both on monocytes as well as on some CD8+ leukemic cells of the patients. Interestingly, the mRNA levels of IFN-γ, a known inducer of IL-15Rα, were also up-regulated in patients' PBMCs. Moreover, PBMCs of some T-LGL patients proliferated at higher levels in response to exogenously added IL-15 compared with those of normal donors. In summary, our study demonstrated increased expression of IL-15Rα in T-LGL leukemia. It is conceivable that higher IL-15Rα expression may lower IL-15 response threshold in vivo and, therefore, may contribute to the pathogenesis of the disease.
Introduction
IL-15 is a proinflammatory cytokine that stimulates T and natural killer (NK) cell activity and induces the expression of TNF-α, IL-1β, and other inflammatory chemokines.1–4 IL-15 is important for the maintenance of long-lasting, high-avidity T-cell responses directed at invading pathogens by supporting the survival of CD8+ memory T cells. In addition, IL-15 also inhibits IL-2–induced activation-induced cell death.5–7 IL-15 signals through the heterotrimeric IL-15 receptor that includes a private IL-15–specific receptor subunit IL-15Rα, the IL-2/IL-15Rβ subunit (CD122) that is also shared with the IL-2 receptor, and the common γ-chain (γc) receptor that is shared by IL-2, IL-4, IL-7, IL-9, and IL-21.3,8 IL-15 binds to IL-15Rα with high affinity (Kd of ∼ 1 × 10−11M).9 Under physiologic conditions, IL-15 does not act as a secreted molecule but rather is trans-presented on the cell surface of activated monocytes and dendritic cells as part of an immunologic synapse to T and NK cells that express IL-2/IL15Rβ and γc.10
Abnormalities of IL-15 expression have been implicated in many autoimmune disorders, including rheumatoid arthritis,11 psoriasis,12 celiac disease,13 inflammatory bowel disease,14 and multiple sclerosis,15 as well as in select lymphoid malignancies and diseases associated with human T-cell lymphotropic virus 1 infection.16 In celiac disease, abnormal expression of IL-15Rα was found to be associated with abnormal IL-15 expression, and together they contribute to the pathogenesis of the disease.17 This highlights the importance of IL-15Rα for the function of IL-15.
Large granular lymphocyte (LGL) leukemia represents a spectrum of lymphoproliferative diseases that are characterized by abnormal clonal expansions of mature T or NK cells.18 Based on the cellular origin, LGL leukemia can be divided into T-cell LGL leukemia and NK-cell LGL leukemia. The majority of patients with T-cell LGL leukemia have a clinically indolent course. However, a significant fraction will develop neutropenia, anemia, recurrent bacterial infections, autoimmune disorders, or symptomatic splenomegaly.19 Neutropenia, the most common hematologic disorder associated with T-LGL leukemia, is the major reason for these patients seeking medical attention.18 Approximately 70% to 80% patients with T-LGL leukemia develop neutropenia and may be predisposed to infection. Anemia is the second most common hematologic disorder associated with T-LGL leukemia. Another common association is autoimmunity, with rheumatoid arthritis occurring most often.20,21 Rheumatoid arthritis has been reported in approximately one-third of T-LGL leukemia patients. Although it has been suggested that LGL leukemic cells represent cytotoxic T lymphocytes activated by chronic antigenic stimulation, the molecular mechanisms that lead to LGL leukemia are unknown. The wide association of LGL leukemia with hematologic and autoimmune disorders suggests the possibility of a common pathogenesis in LGL leukemia and their hematologic disorders and autoimmune diseases.
Several studies have suggested that IL-15 might play a role in the pathogenesis of LGL leukemia.22,23 Furthermore, a phase 1 clinical trial of IL-15 blockade in T-cell LGL leukemia was conducted in our Branch using the Mikβ1 monoclonal antibody (anti-CD122) that blocks IL-15 trans-presentation to cells expressing IL-2/IL-15Rβ and γc.24 However, Mikβ1 does not block IL-15 action on cells that express the heterotrimeric receptor in a cis orientation. In our phase 1 trials, Mikβ1 therapy was not effective in the treatment of patients with monoclonal T-LGL. To gain more insights into the role of IL-15 in the pathogenesis of T-LGL leukemia, we investigated whether abnormal IL-15Rα expression was associated with T-LGL leukemia in the present study. Using a newly developed assay, we found increased serum levels of soluble IL-15Rα (sIL-15Rα) in patients with T-LGL leukemia. Consistent with this, up-regulation of IL-15Rα mRNA expression in the PBMCs of patients with T-LGL leukemia was also demonstrated. FACS analysis showed that IL-15Rα was expressed both on monocytes and CD8+ leukemic cells in certain patients. Furthermore, the mRNA levels of IFN-γ, a known inducer of IL-15Rα, were also up-regulated in patients with T-LGL leukemia. Interestingly, although PBMCs from patients with T-LGL leukemia did not proliferate spontaneously ex vivo, they proliferated in response to exogenously added IL-15. In some patients, and the proliferation was much more robust compared with that of normal donors. In summary, our data demonstrated increased expression of IL-15Rα in T-LGL leukemia. It also suggested that the higher expression of IL-15Rα in T-LGL leukemia may lower the IL-15 response threshold of the cells in vivo, and thereby, may contribute to the pathogenesis of T-LGL leukemia.
Methods
Patients and normal donors
Serum samples were obtained from a total of 43 patients with T-LGL leukemia and 29 healthy controls with their consent on institutional review board-approved protocols in accordance with the Declaration of Helsinki. The diagnosis of T-LGL leukemia was based on clinical and laboratory parameters as described previously25: (1) a peripheral blood smear or bone marrow biopsy/aspirate with morphologic findings consistent with LGL; (2) CD3+, CD8+, usually CD57+ cells in the peripheral blood or bone marrow as measured by flow cytometry; and (3) clonal T-cell receptor rearrangement as determined by southern blotting or PCR. Neutropenia and anemia were graded following the Common Toxicity Criteria Version 3.0. Neutropenia (absolute neutrophil count decreased): grade 0 indicates no abnormality in labs; grade 1, less than lower limits of normal to 1500/mm3; grade 2, 1000 to 1500/mm3; grade 3, 500 to 1000/mm3; and grade 4, less than 500/mm3. Anemia (decreased hemoglobin concentration): grade 0 indicates no abnormality; grade 1, less than lower limits of normal to 10.0 g/dL; grade 2, 8.0 to 10.0 g/dL; grade 3, 6.5 to 8.0 g/dL; and grade 4, less than 6.5 g/dL.
Cell lines and blood cells
The 293T cells were cultured in RPMI 1640 supplemented with 10% FBS. PT18, PT18 IL-15Rα cells were cultured with 10% WEHI-3 conditioned medium as described previously.26 PBMCs were obtained from patients and normal donors by Ficoll-hypaque centrifugation. CD8+ T cells were purified to 90% to 95% purity by positive selection using CD8-conjugated MACS beads (Miltenyi Biotec).
sIL-15Rα-IL-2 chimeric protein production
sIL-15Rα composing the complete extracellular region of IL-15Rα, a pre-prolactin signal peptide, and Kozak sequence were generated by PCR using primers: 5′-ATTGAATTCGCCGCCACCATGGACAGC-3′ (sIL-15Rα sense primer) and 5′-GACCTGCAGAGTGGTGTCGCTGTGGCCC-3′ (sIL-15Rα anti–sense primer). The IL-2 coding region was amplified using primers 5′-AGCCTGCAGATGTACAGGATG CAACTCC-3′ (IL-2 sense primer) and 5′-GATGGATCCTCAAGTTAGTGTTGAGATGATGC-3′ (IL-2 anti–sense primer). The sIL-15Rα-IL-2 coding region was then amplified with a sIL-15Rα sense primer and an IL-2 anti–sense primer and then cloned into EcoR1 and BamH1 sites of pEF-neo. To express the sIL-15Rα-IL-2 chimeric protein, 293T cells were transfected with pEF-neo-sIL15Rα-IL-2 and culture supernatants were collected after transfection.
sIL-15Rα ELISA
Quantification of sIL-15Rα was performed using an indirect ELISA assay.27 sIL-15Rα-IL-2 was used as a standard. To set up the assay, a high absorption plate was coated with the polyclonal goat anti–human IL-15Rα antibody AF247 (R&D Systems). After blocking, samples were added to the plate to allow sIL-15Rα to bind to the plate. After washing, plate-bound sIL-15Rα was then saturated with recombinant human IL-15 (R&D Systems). After washing, the plate-bound IL-15–sIL-15Rα was detected by adding biotin-labeled anti–IL-15 (Genzyme). The assay was then developed by adding streptavidin-conjugated alkaline phosphatase and subsequently the substrate 4-nitrophenyl phosphate disodium salt. Absorption at 405 nm was read on a microplate reader (Molecular Devices). The sensitivity of the assay is approximately 0.3pM.
Flow cytometry
Anti–CD8 FITC, anti–CD14 FITC, anti–CD122 PE, anti–CD132 PE, anti–CD3 APC, and isotype controls were purchased from BD Biosciences. Biotin-labeled anti–IL-15Rα was obtained from R&D Systems. To stain cell surface markers, the cells were blocked by a human FcγR binding inhibitor (eBioscience) at 4°C for 20 minutes, and the cells were then stained with the FACS developing antibody at 4°C for 30 minutes. After the washing, the cells were analyzed using a FACSCaliber (BD Biosciences).
IFN-α, IFN-β, IFN-γ, and IL-18 ELISA
The serum levels of multitype IFN-α, IFN-β, IFN-γ, and IL-18 were measured using the ELISA kits from R&D System (41105-1, 41410, DIF50, and 7620) following the manufacturer's instructions.
TaqMan real-time quantitative RT-PCR
Total RNA was extracted from PBMCs using the RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions. Reverse transcription reactions were carried out for each sample (250 ng) using the Superscript First-Strand synthesis System (Invitrogen). The TaqMan Universal PCR Master Mix, the human IL-15, IL-15Rα primer/probe, IFN-γ, and the hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) primer/probe sets were purchased from Applied Biosystems. The detection of human IL-15, IL-15Rα, IFN-γ, and HPRT1 was performed using an ABI Prism 7500 sequence detection system (Applied Biosystems) according to the manufacturer's instructions. The copy numbers of IL-15, IL-15Rα, and IFN-γ mRNA were normalized to the copy number of human HPRT1 mRNA.
Proliferation assay
PBMCs from T-LGL leukemia patients or normal donors were isolated by Ficoll-Hypaque density centrifugation. PBMCs were washed with PBS and then resuspended in RPMI 1640 media supplemented with 10% FBS at a concentration of 1 × 106 cells/mL. Cell suspensions (100 μL) were seeded into 96-well microtiter plates in triplicate and cultured for 6 days at 37°C in 5% CO2. Recombinant human IL-15 (200 pg/mL and 20 ng/mL) was added to the plate at the beginning of the culture. Cultures were pulsed with 1 μCi 3H- thymidine 6 hours before harvesting.
Statistical analysis
The difference in the distributions of sIL-15Rα serum levels among different grades of neutropenia and anemia in patients with T-LGL leukemia was tested using the Kruskal-Wallis rank test. P values < .05 were considered statistically significant.
Results
Development of an sIL-15Rα assay
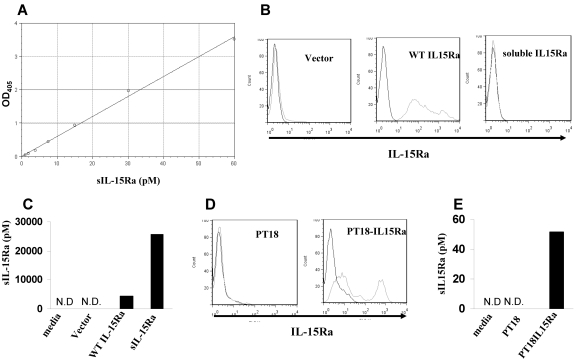
To measure the concentration of sIL-15Rα, an indirect ELISA assay was developed that used the polyclonal anti–IL-15Rα as a capture antibody, IL-15 as a linker, and an anti–IL-15 antibody as the detection antibody. When calibrated using a human sIL-15Rα–IL-2 fusion protein, the assay gave a linear response up to a concentration of 60pM with a sensitivity approximately 0.3pM (Figure 1A). sIL-15Rα can be produced by its shedding from the cell surface as a result of proteolytic cleavage or through alternative splicing. To test whether our assay could detect sIL-15Rα produced by both protein cleavage or alternative splicing, we transfected 293T cells with a cDNA encoding wild-type full-length human IL-15Rα and a cDNA encoding only the extracellular domain of human IL-15Rα. Flow cytometry demonstrated that IL-15Rα was expressed on the cells transfected with wild-type IL-15Rα, but not on the cell surfaces of cells transfected with the extracellular domain of IL-15Rα because of the lack of the anchoring transmembrane domain (Figure 1B). We then analyzed the culture supernatants to measure the concentration of sIL-15Rα. Culture supernatants from both cells transfected with wild-type IL-15Rα and cells transfected with the extracellular domain of IL-15Rα contained high concentrations of sIL-15Rα at 4286pM and 25 628pM, respectively. Control supernatants from cells transfected with an empty vector demonstrated no detectable sIL-15Rα (Figure 1C). This indicated that the assay we developed could detect the natural, cleaved form of sIL-15Rα as well as sIL-15Rα generated by alternative splicing. We also validated the assay using the mouse cell line PT18, which was transfected to stably express human IL-15Rα. Culture supernatants from PT18–IL-15Rα contained 51.8pM sIL-15Rα, whereas supernatants from PT18 contained no detectable sIL-15Rα (Figure 1D-E).
Figure 1.
Development of sIL-15Rα ELISA. (A) IL-15Rα ELISA curve using serial dilutions of sIL-15Rα–IL-2 standard. The absorption was measured at 405 nm. (B) The cell surface expression of IL-15Rα in 293T cells transfected with vector only, wild-type IL-15Rα, or extracellular domain of IL-15Rα (sIL-15Rα) as assessed by flow cytometry. (C) sIL-15Rα levels in the supernatants from 293T cells transfected with vector only, wild-type IL-15Rα, and sIL-15Rα measured with the assay. N.D. indicates nondetectable. (D) The cell surface expression of IL-15Rα in a murine mast cell line PT-18 and PT18-IL15Rα by flow cytometry. (E) sIL-15Rα levels in the supernatants from cell lines PT18 and PT18-IL15Rα. N.D. indicates nondetectable.
Increased serum sIL-15Rα levels in patients with T-LGL leukemia
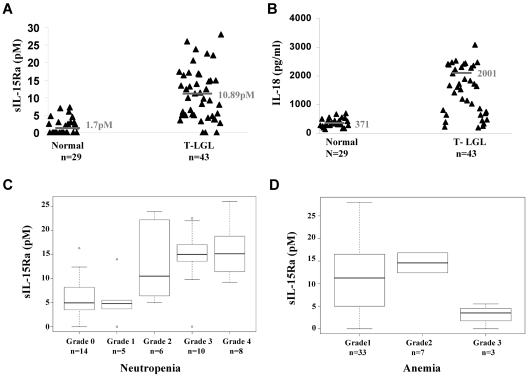
IL-15 has been implicated in the pathogenesis of LGL leukemia.22,23 IL-15Rα, the private receptor for IL-15, has high affinity for IL-15 and trans-presents IL-15 to neighboring cells during an immunologic synapse.10 A significant increase in the concentrations of sIL-15Rα was observed in serum samples collected from patients with T-LGL leukemia compared with those from normal donors (P < .0001; Figure 2A). The mean concentration of serum sIL-15Rα was 10.89pM in patients with T-LGL leukemia compared with a mean of 1.7pM in the serum of healthy donors. Moreover, sIL-15Rα was detected in 40 of 43 patients with T-LGL leukemia, whereas only 13 of 29 normal donors had detectable sIL-15Rα in the serum. The serum level of IL-18, a proinflammatory cytokine that is induced by IL-15,28 was also significantly increased in patients with T-LGL leukemia (Figure 2B). The average serum IL-18 level was 2001 pg/mL in patients with T-LGL leukemia compared with 371 pg/mL in normal donors (P < .0001). Neutropenia is a common clinical finding associated with T-LGL leukemia.18 There was a strong correlation between the sIL-15Rα levels and the severity of neutropenia in the patients examined. Higher levels of sIL-15Rα were usually associated with more severe neutropenia in the patients (P < .001, n = 43; Figure 2C). The average sIL-15Rα levels in patients with Common Toxicity Criteria more than grades 2, 3, and 4 neutropenia were 13.08pM (n = 6), 14.58pM (n = 10), and 15.75pM (n = 8), respectively, whereas the average sIL-15Rα levels in grade 0 (n = 14) and grade 1 (n = 5) neutropenia were 6.19pM and 5.6pM (Figure 2C). There was also an inverse correlation of sIL-15Rα levels with anemia in the patients (P < .05; Figure 2D).
Figure 2.
Increased serum levels of sIL-15Rα in patients with T-LGL leukemia. (A) sIL-15Rα concentrations were determined using the ELISA assay described in “sIL15-Rα ELISA” in serum samples from T-LGL leukemic patients (n = 43). Twenty-nine serum samples from healthy normal donors were included as controls (P < .0001). (B) The serum levels of IL-18 in patients with T-LGL leukemia (n = 43) compared with that of normal donors (n = 29). (C) The distribution sIL-15Rα serum levels in patients with T-LGL leukemia with different grades of neutropenia (P < .001). (D) The distribution sIL-15Rα serum levels in patients with T-LGL leukemia with different grades of anemia (P < .05).
IL-15Rα expression was increased in the PBMCs from patients with T-LGL leukemia
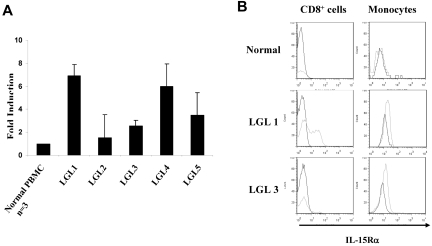
The differences in the serum sIL-15Rα concentrations observed between T-LGL leukemia patients and normal donors suggested that there might be up-regulation of IL-15Rα expression in PBMCs from patients with the leukemia. To examine the mRNA levels of IL-15Rα in the patients, we isolated PBMCs from 5 patients with CD8+ T-LGL leukemia (Tables 1 and 2). TaqMan real-time RT-PCR showed that IL-15Rα mRNA levels were increased 3- to 7-fold in patients with T-LGL compared with those from normal donors (Figure 3A). To define the cellular source of the IL-15Rα expression within PBMCs, we performed a FACS analysis to examine the expression of IL-15Rα in different cell populations. IL-15Rα was detected on the cell surfaces of CD8+ leukemic cells in 2 of 5 patients, whereas IL-15Rα was expressed on monocytes among all 5 patients studied (Figure 3B). These data suggest that both monocytes and some CD8+ leukemic cells from T-LGL patients showed the potential to produce and release sIL-15Rα into the serum.
Table 1.
Characteristics of patients with T-LGL leukemia
| Patient no. | Age, y | Sex | Receiving G-CSF before study | Present treatment | Neutropenia | Anemia | Rheumatoid arthritis | History of infection | Bleeding |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | Male | No | No | Yes | No | Yes | Yes | No |
| 2 | 56 | Male | No | No | Yes | No | Yes | No | No |
| 3 | 74 | Male | No | No | Yes | No | Yes | No | No |
| 4 | 80 | Female | Yes | No | Yes | No | Yes | No | No |
| 5 | 32 | Female | No | No | Yes | Yes | No | Yes | No |
Table 2.
Absolute number of lymphocytes, monocytes, and the percentage and absolute number of LGL cells in the peripheral blood from patients with T-LGL leukemia
| Patient no. | sIL15Rα, pM | No. of lymphocytes per mm3 | No. of monocytes per mm3 | Percentage of LGL cells among lymphocytes | No. of LGL cells per mm3 |
|---|---|---|---|---|---|
| 1 | 9.8 | 11 926 | 536 | 76.4 | 9111 |
| 2 | 9.7 | 3368 | 933 | 21.1 | 711 |
| 3 | 14.7 | 1740 | 590 | 10 | 174 |
| 4 | 20.6 | 14 350 | 300 | 71.4 | 10 246 |
| 5 | 22.2 | 21 112 | 928 | 30.2 | 6367 |
Figure 3.
Increased expression of IL-15Rα in the PBMCs from patients with T-LGL leukemia. (A) TaqMan real-time RT-PCR analysis of IL-15Rα mRNA levels in the PBMCs from patients with T-LGL leukemia. The copy numbers of IL-15Rα mRNA were normalized to the copy number of HPRT1 mRNA. The fold induction was calculated based on the normalized IL-15Rα mRNA copy number in PBMCs from normal donors (n = 3). (B) FACS analysis of the cell surface expression of IL-15Rα on both CD8+ T cells and monocytes in PBMCs from T-LGL leukemic patients compared with those from normal donors. The data from patient LGL1 are representative of those from 2 T-LGL patients who showed IL-15Rα expression on both CD8+ cells and monocytes. The data from patient LGL3 are representative of those from patients that showed IL-15Rα expression on monocytes alone. CD14 was used as the monocyte marker.
IFN-γ expression was up-regulated in the PBMCs from patients with T-LGL leukemia
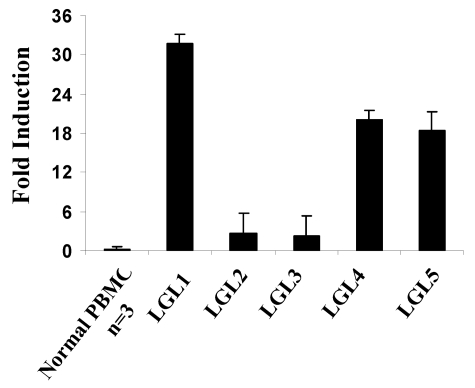
Interferons are known inducers of IL-15Rα.29 To better understand the mechanism of IL-15Rα expression in our leukemia patients, we analyzed the expression of interferons in the patients' PBMCs. We found that IFN-γ mRNA expression was markedly increased in the PBMCs from T-LGL leukemia patients (eg, 32-fold increase in 1 patient; Figure 4). However, the serum levels of IFN-γ in the patients studied were similar to those in normal donors (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The serum levels of IFN-β were also normal, but we found slightly increased multitype IFN-α levels in the serum from patients with T-LGL leukemia (supplemental Figure 1).
Figure 4.
Increased expression of IFN-γ message in patients with T-LGL leukemia. TaqMan real-time RT-PCR analysis of IFN-γ mRNA levels in the PBMCs from T-LGL leukemia patients. The copy numbers of IFN-γ mRNA were normalized to the copy numbers of HPRT1 mRNA. The fold induction was calculated based on the normalized IFN-γ mRNA copy number in PBMCs from normal donors (n = 3).
PBMCs from some T-LGL leukemia patients proliferated at a higher rate in response to IL-15 ex vivo
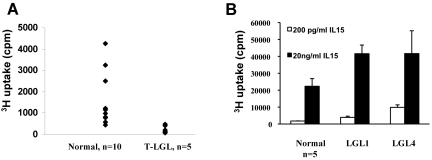
Higher levels of IL-15Rα expression in the T-LGL leukemia patients led us to suspect that there might be an IL-15 autocrine/paracrine loop in the PBMCs of T-LGL patients. To test this, we cultured PBMCs from T-LGL patients as well as normal donors ex vivo without addition of mitogens and antigens. Surprisingly, although IL-15Rα was expressed in T-LGL leukemia patients, PBMCs from the patients did not proliferate spontaneously without addition of cytokines, antigens, or mitogens (Figure 5A). Moreover, the 3H-thymidine uptake was significantly lower than that of normal PBMCs. This is probably because of lack of cytokine (eg, IL-2, IL-15) expression as we could not detect IL-2/IL-15 mRNA in the PBMCs (data not shown). We then asked whether the PBMCs from T-LGL leukemia patients could respond to exogenously added IL-15 ex vivo. We tested the proliferation responses of T-LGL PBMCs to both 200 pg/mL and 20 ng/mL IL-15, which activate high affinity and intermediate affinity receptors, respectively. The patients' PMBCs proliferated slightly to 200 pg/mL IL-15, and the proliferation was more robust in response to 20 ng/mL IL-15 (data not shown). Moreover, in 2 patients where IL-15Rα was expressed on the leukemic cells, the proliferative responses to IL-15 were significantly higher compared with those of normal PBMCs (n = 5, P < .05; Figure 5B). When cultured with 200 pg/mL IL-15, the patients' PBMCs proliferated at 3921 cpm and 9841 cpm compared with the 1771 cpm observed with normal donors (n = 5). When cultured at 20 ng/mL, the patients' proliferation responses based on 3H-thymidine uptake were 41 580 cpm and 41 668 cpm compared with 22 323 cpm for normal donors (n = 5). These data suggest that the PBMCs from T-LGL patients had a lower response threshold to IL-15 ex vivo.
Figure 5.
PBMCs from some T-LGL leukemia patients had higher response to exogenously added IL-15 compared with those of normal donors. (A) Six-day spontaneous proliferation of PBMCs from T-LGL leukemic patients and normal donors ex vivo without addition of antigens or mitogens. (B) The proliferation of PBMCs from select T-LGL leukemia patients (LGL1 and LGL4) in response to exogenously added IL-15. Two concentrations of IL-15 (200 pg/mL and 20 ng/mL) were used in the assay. The proliferation of normal donors represented data from 5 normal donors.
Discussion
IL-15 is a pleiotropic cytokine that is required for the growth and homeostasis of CD8+ T cells, TCR γδ+ intraepithelial lymphocytes, NKT cells, and NK cells.29–31 IL-15 signals through a heterotrimeric receptor composed of a high-affinity subunit, IL-15Rα, and the intermediate affinity receptor signaling complex, IL-2/IL-15Rβ-γc. IL-15Rα is expressed on multiple cell types, including activated dendritic cells and monocytes, as well as some nonlymphoid cells and even nonhematopoietic cells.32 The expression of IL-15Rα by monocytes and dendritic cells is essential for the function of IL-15 in priming NK cells and maintaining CD8+ memory T cells.10,33 Given the importance of IL-15Rα in IL-15 signaling, we hypothesized that there might be differences in sIL-15Rα levels in physiologic and pathologic conditions, and this might be an indicator of abnormal IL-15 signaling. Two mechanisms have been reported for the generation of sIL-15Rα. One is spontaneous or active shedding of IL-15Rα from cell surfaces as a result of enzymatic cleavage of the protein. This process is dependent on the TNF-α-converting enzyme ADAM 17. Another mechanism is alternative splicing, which results in soluble forms of IL-15Rα. In the present study, we developed an assay to measure the sIL-15Rα levels in serum or plasma. The assay is very sensitive and gave a linear response when tested with recombinant sIL-15Rα in the range of 0.9 to 60pM (Figure 1A). The assay was able to detect both the natural shed form of sIL-15Rα as well as an alternatively spliced form of sIL-15Rα (Figure 1C). One advantage of this assay is that it does not use radioactive materials in contrast to other assays.34 Moreover, it can be used to measure the total sIL-15Rα level as well as sIL-15Rα bound with IL-15 using a modification of the assay procedures (data not shown).
Using our assay, we found that the sIL-15Rα levels were significantly increased in patients with T-LGL leukemia over that found in normal healthy donors (Figure 2A). Moreover, higher sIL-15Rα levels were associated with more severe neutropenia (P < .001; Figure 2C). The mechanism for neutropenia in patients with T-LGL leukemia is unknown; however, increased expression of Fas ligand (FasL) on LGL and in the serum has been thought to play a role.35,36 The FasL gene is constitutively expressed in T-LGL leukemia, and increased soluble FasL induces neutrophil apoptosis.34 Interestingly, in preclinical studies, the administration of high-dose IL-15 to rhesus macaques was also associated with a neutropenia.37,38 In our study, the neutropenia appeared to be the result of redistribution of neutrophils from the peripheral blood into the tissues.37 IL-15 has been shown to induce neutrophil migration by triggering a cascade of cytokine and chemokine expression initiated through IL-18.28 In patients with T-LGL leukemia, the serum levels of IL-18 were dramatically increased (P < .0001; Figure 2B). It is possible that the sIL-15Rα levels in T-LGL leukemia not only reflected the level of leukemic cell expansions and neutropenia through FasL expression but also may contribute to neutrophil migration together with IL-15 by inducing cytokine/chemokine expression.
Consistent with the sIL-15Rα ELISA data, the IL-15Rα mRNA expression in the PBMCs from patients with T-LGL leukemia were also up-regulated (Figure 3A). Furthermore, the cellular origins of IL-15Rα expression in the PBMCs of patients with T-LGL leukemia appeared to be heterogeneous. By FACS analysis, all 5 patients expressed IL-15Rα on monocytes, whereas only 2 of 5 patients expressed IL-15Rα on CD8+ leukemic cells. Interestingly, the IFN-γ mRNA levels were also up-regulated in the PBMCs of patients with T-LGL leukemia (Figure 4). IFN-γ induces IL-15Rα expression in dendritic cells and monocytes.29 This observation could potentially contribute to IL-15Rα up-regulation in the PBMCs of patients with T-LGL leukemia.
Although IL-15 has been suggested to be a survival factor for LGL leukemic cells,22,23 we were unable to detect IL-15 mRNA in the PBMCs of patients with the leukemia (data not shown). Furthermore, the patients' PBMCs did not spontaneously proliferate ex vivo, suggesting a lack of autocrine/paracrine cytokine loop (Figure 5A). However, both CD8+ T cells and monocytes from LGL leukemia patients expressed IL-2/IL-15Rβ and γc (data not shown). Although dendritic cells and monocytes are the major sources of IL-15, it has been reported that nonhematopoietic cells, such as skeletal muscle39 and astrocytes,40 produce IL-15 in vivo. With increased expression of IL-15Rα on CD8+ T cells and monocytes in select patients with T-LGL leukemia, it is conceivable that the cells might respond more effectively to very low concentrations of IL-15 in the microenvironment where IL-15 is being made. Indeed, in 2 patients where IL-15Rα was expressed on the leukemic cells, when we cultured their PBMCs with 200 pg/mL IL-15, a concentration that only activates the high affinity IL-15 receptor, they proliferated more robustly compared with those of normal donors. More robust proliferation was also observed when we cultured patients' PBMCs with 20 ng/mL IL-15 that activates the intermediate affinity IL-15 receptor in these 2 patients. This observation supported the view that there was an expression of high-affinity IL-15 receptors in the leukemic cells of these patients. It also suggested that higher IL-15Rα expression in some patients with T-LGL leukemia may decrease the IL-15 response threshold that is needed to activate downstream signaling in leukemic cells.
Collectively, our data indicate that sIL-15Rα is increased in the serum of patients with T-LGL leukemia and that IL-15Rα expression was up-regulated in the patients' PBMCs as well. Up-regulation of IL-15Rα may decrease the IL-15 response threshold of the cells in the patients, an alteration that may contribute to the pathogenesis of the disease.
Supplementary Material
Acknowledgments
This work was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
The sIL15Rα assay is under the patent applications 61/241,265 and 61/242,595 filed by National Cancer Institute, National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.C. designed and performed research, collected, analyzed, and interpreted data, and wrote the paper; M.P. performed research; J.H.S. performed statistical analysis; J.C.M. and J.E.J. provided patient care and collected patient samples; R.B. provided critical insights in research design; and T.A.W. designed research and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas A. Waldmann, National Institutes of Health, 10 Center Dr, Bldg 10, Rm 4N115, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.
References
- 1.Bamford RN, Grant AJ, Burton JD, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91(11):4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA, Dubios S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14(2):105–110. [PubMed] [Google Scholar]
- 5.Marks-Konczalik J, Dubios S, Losi JM, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97(21):11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Sun S, Hwang I, et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 7.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288(5466):675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 8.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 9.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14(15):3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois S, Mariner J, Waldmann TA, et al. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17(5):537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 11.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3(2):189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 12.Rückert R, Asadullah K, Seifert M, et al. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J Immunol. 2000;165(4):2240–2250. doi: 10.4049/jimmunol.165.4.2240. [DOI] [PubMed] [Google Scholar]
- 13.Mention JJ, Ben Ahmed M, Bègue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125(3):730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Geboes K, Colpaert S, et al. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol. 2000;164(7):3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 15.Kivisäkk P, Matusevicius D, He B, et al. IL-15 mRNA expression is up-regulated in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis (MS). Clin Exp Immunol. 1998;111(1):193–197. doi: 10.1046/j.1365-2249.1998.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azimi N, Nagai M, Jacobson S, Waldmann TA. IL-15 plays a major role in the persistence of Tax-specific CD8 cells in HAM/TSP patients. Proc Natl Acad Sci U S A. 2001;98(25):14559–14564. doi: 10.1073/pnas.251540598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardo D, Garrote J, Allegretti Y, et al. Higher constitutive IL15R alpha expression and lower IL-15 response threshold in coeliac disease patients. Clin Exp Immunol. 2008;154(1):64–73. doi: 10.1111/j.1365-2249.2008.03743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokol L, Loughran TP., Jr Large granular lymphocyte leukemia. Curr Hematol Malig Rep. 2007;2(4):278–282. doi: 10.1007/s11899-007-0038-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Shah MV, Loughran TP., Jr The root of many evils: indolent large granular lymphocyte leukaemia and associated disorders. Hematol Oncol. 2010;28(3):105–117. doi: 10.1002/hon.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamy T, Loughran TP., Jr Large granular lymphocyte leukemia. Cancer Control. 1998;5(1):25–33. doi: 10.1177/107327489800500103. [DOI] [PubMed] [Google Scholar]
- 21.Loughran TP., Jr Clonal diseases of large granular lymphocytes. Blood. 1993;82(1):1–14. [PubMed] [Google Scholar]
- 22.Zhang R, Shah MV, Yang J, et al. Network model of survival signaling in large granular lymphocytes leukemia. Proc Natl Acad Sci U S A. 2008;105(42):16308–16313. doi: 10.1073/pnas.0806447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zambello R, Facco M, Trentin L, et al. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood. 1997;89(1):201–211. [PubMed] [Google Scholar]
- 24.Morris JC, Janik J, White JD, et al. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc Natl Acad Sci U S A. 2006;103(2):401–406. doi: 10.1073/pnas.0509575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semenzato G, Zambello R, Starkebaum G, Oshimi K, Loughran TP. The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood. 1997;89(1):256–260. [PubMed] [Google Scholar]
- 26.Tagaya Y, Burton JD, Miyamoto Y, Waldmann TA. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 1996;15(18):4928–4939. [PMC free article] [PubMed] [Google Scholar]
- 27.Crowcher JR. The ELISA Guidebook. Totowa, NJ: Humana Press; 2001. p. 149. [Google Scholar]
- 28.Verri WA, Jr, Cunha TM, Ferreira SH, et al. IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol. 2007;37(12):3373–3380. doi: 10.1002/eji.200737488. [DOI] [PubMed] [Google Scholar]
- 29.Dubois S, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci U S A. 2005;102(24):8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodolce JP, Boone D, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 31.Cooper MA, Bush JE, Fehniger TA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 32.Schluns KS, Nowak EC, Cabrera-Hernandez A, et al. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101(15):5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koka R, Burkett P, Chien M, et al. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173(6):3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 34.Mortier E, Bernard J, Plet A, Jacques Y. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol. 2004;173(3):1681–1688. doi: 10.4049/jimmunol.173.3.1681. [DOI] [PubMed] [Google Scholar]
- 35.Liu JH, Wei S, Lamy T, et al. Chronic neutropenia mediated by fas ligand. Blood. 2000;95(10):3219–3222. [PubMed] [Google Scholar]
- 36.Perzova R, Loughran TP., Jr Constitutive expression of Fas ligand in large granular lymphocyte leukaemia. Br J Haematol. 1997;97(1):123–126. doi: 10.1046/j.1365-2141.1997.d01-2113.x. [DOI] [PubMed] [Google Scholar]
- 37.Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in Rhesus macaques. Blood. 2011;117(18):4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger C, Berger M, Hackman RC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114(12):2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugiura T, Kawagchi Y, Harigai M, et al. Increased CD40 expression on muscle cells of polymyositis and dermatomyositis: role of CD40-CD40 ligand interaction in IL-6, IL-8, IL-15, and monocyte chemoattractant protein-1 production. J Immunol. 2000;164(12):6593–6600. doi: 10.4049/jimmunol.164.12.6593. [DOI] [PubMed] [Google Scholar]
- 40.Saikali P, Antel JP, Pittet CL, Newcombe J, Arbour N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol. 2010;185(10):5693–5703. doi: 10.4049/jimmunol.1002188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.