Abstract
Dickkopf-1 (DKK1), broadly expressed in myeloma cells but highly restricted in normal tissues, together with its functional roles as an osteoblast formation inhibitor, may be an ideal target for immunotherapy in myeloma. Our previous studies have shown that DKK1 (peptide)–specific CTLs can effectively lyse primary myeloma cells in vitro. The goal of this study was to examine whether DKK1 can be used as a tumor vaccine to elicit DKK1-specific immunity that can control myeloma growth or even eradicate established myeloma in vivo. We used DKK1-DNA vaccine in the murine MOPC-21 myeloma model, and the results clearly showed that active vaccination using the DKK1 vaccine not only was able to protect mice from developing myeloma, but it was also therapeutic against established myeloma. Furthermore, the addition of CpG as an adjuvant, or injection of B7H1-blocking or OX40-agonist Abs, further enhanced the therapeutic effects of the vaccine. Mechanistic studies revealed that DKK1 vaccine elicited a strong DKK1- and tumor-specific CD4+ and CD8+ immune responses, and treatment with B7H1 or OX40 Abs significantly reduced the numbers of IL-10–expressing and Foxp3+ regulatory T cells in vaccinated mice. Thus, our studies provide strong rationale for targeting DKK1 for immunotherapy of myeloma patients.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy that remains incurable in the majority of patients. After treatment with high-dose chemotherapy and autologous stem cell support, complete remission rates of 50% are achieved. However, most patients relapse.1 Additional measures are required after transplantation to eliminate minimal residual disease. Immunotherapy is an appealing option, for this purpose, because chemotherapy and immunotherapy kill tumor cells by different modes of action that are non–cross-resistant. Therefore, these 2 treatment modalities should work synergistically.
Unlike other malignancies, few tumor-associated Ags have been identified in MM. Thus far, the only myeloma Ag tested in clinical trials has been the monoclonal Ig secreted by myeloma cells (idiotype). Immunotherapy, using idiotype-based vaccines, has been explored in myeloma patients by us and others, and the results have been disappointing,2–5 in part because of the weak immunogenicity of idiotype proteins.6,7 Therefore, the identification and use of novel and more potent tumor-associated Ags, especially those shared among patients, is urgently needed to improve the efficacy of immunotherapy for this disease.
Recent studies have shown that Dickkopf-1 (DKK1), a secreted protein and Wnt signaling pathway inhibitor, is highly expressed by the tumor cells of almost all myeloma patients,8 and absent from normal tissues and organs, except placenta and prostate.9–11 Furthermore, myeloma-derived DKK1 may be responsible, at least in part, for suppressed osteoblast formation and bone destruction associated with MM8; inhibiting DKK1 activity increased osteoblast number and bone formation, reduced osteoclast activity, and inhibited myeloma growth in a myeloma animal model.12 Our previous results clearly show that DKK1 is expressed by primary tumor cells from all patients with MM, and DKK1 (peptide)–specific CTLs can effectively lyse primary myeloma cells in vitro.13 Hence, we hypothesize that the broad expression in myeloma but highly restricted expression in normal tissues, together with its functional roles as an osteoblast formation inhibitor and a potential myeloma growth enhancer, make DKK1 an ideal and universal target for immunotherapy in MM. The goal of our studies was to explore whether DKK1 can be used as a tumor vaccine to elicit DKK1-specific immunity that can control myeloma growth or even eradicate established myeloma in vivo. In this study, we chose to use DNA vaccine because it was easy to prepare, less expensive, and had been widely used in a variety of preclinical studies.14–16 Our results demonstrated that the DNA (murine DKK1/defensin-2 fusion) vaccine protected mice from developing myeloma and treated established myeloma in the murine MOPC-21 myeloma model,17 in which murine DKK1 was highly expressed by the myeloma cells. We also elucidated the immunologic mechanism of DKK1-DNA vaccine-mediated antimyeloma responses.
Methods
Myeloma cell lines and mice
Murine myeloma cell line MOPC-21 (ATCC) was maintained in Iscove modified Dulbecco complete medium (Atlanta Biologicals) with 1% penicillin/streptomycin and 2mM l-glutamine at 37°C, in a humidified atmosphere containing 5% CO2.
Animal studies were approved by the Institutional Animal Care and Use Committee of The University of Texas M. D. Anderson Cancer Center. Six- to 8-week-old female Balb/c mice (National Cancer Institute) were used for DNA vaccine and tumor challenge. To study the treatment efficacy of DKK1-DNA vaccine, a murine myeloma model was established, as previously described.17 Briefly, mice were subcutaneously inoculated in the right flank with murine myeloma cell line MOPC-21 cells (1 million cells per mouse) suspended in 100 μL of PBS.
Construction of plasmid for DKK1-DNA vaccine
The vector pCMVE-Chemokine-sFv for DNA vaccine was described previously.18 To construct a plasmid encoding murine DKK1/defensin2 fusion transcript (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), DKK1 cDNA fragment (726 bp) was amplified by RT-PCR, from DKK1-expressing mouse cells with primers 5′-actgcagtcgacaccttgaactcagttctcatcaattc-3′ and 5′-ttagactgtcggtttagtgtctctggc-3′. The cDNA was then subcloned into pCMVE-Chemokine-sFv vector. Vector without DKK1 cDNA insert was used as control. After amplification, plasmids were purified using Maxi-Prep Kit (QIAGEN).
Identifying and synthesizing murine DKK1 peptides and H-2Kd–peptide tetramers
We identified 3 murine DKK1 peptides using peptide binding database described previously.13 Briefly, the sequence of murine DKK1 was reviewed for peptides that could potentially bind to H-2Kd using a peptide-binding database (http://www-bimas.cit.nih.gov/molbio/hla_bind/). After comparing the predictive binding scores, we identified and selected 3 peptides that could potentially bind with H-2Kd molecules (Table 1). Peptides from influenza-A virus HA protein (HA533)19 were used as a control. All peptides and H-2Kd-peptide tetramers were synthesized in the MHC Tetramer Laboratory (Baylor College of Medicine). Purity of synthetic peptides was confirmed to be > 95% by reverse-phase HPLC and mass spectrometry. Synthetic peptides were dissolved in DMSO (Sigma-Aldrich), and stored at −80°C until use.
Table 1.
Murine DKK1 and control peptides for H-2Kd molecules
| Name | Peptide position | Peptide sequence | Epitope origin | Predictive binding score* |
|---|---|---|---|---|
| P11 | 11 | RFLAVFTMM | DKK1 11-18 | 414 |
| P15 | 15 | VFTMMALCSL | DKK1 15-24 | 800 |
| P210 | 210 | HFWSKICKPV | DKK1 210-219 | 288 |
| HA533 | 533 | IYSTVASSL | Influenza A HA 533-541 | 2400 |
Estimate of half-time of disassociation and calculated score in arbitrary units.
Mouse vaccination, tumor challenge, and Ab treatment
DNA vaccines were administrated as described in supplemental Figure 1B and C for prophylaxis and therapeutic studies, respectively. For each mouse, 50 μg of DKK1 plasmid was IM injected. Control mice received injections of equal amount of control vector. In some experiments, adjuvant CpG (ODN 1826; 50 μg/mouse TCCATGACGTTCCTGACGTT; InvivoGen) was administrated at the DNA vaccination sites to enhance immune response. Tumor burdens were monitored by tumor sizes, and mice were killed when tumors reached 225 mm2 or when mice became moribund.
In some experiments of therapeutic studies, mice were injected intraperitoneally with anti-B7H1 mAb (200 μg/mouse, M1H5, gift from Prof Miyuki Azuma, Tokyo Medical and Dental University, Tokyo, Japan) or anti-OX40 mAb (200 μg/mouse, OX86; Santa Cruz Biotechnology), once every 3 days for a total of 3 injections. Normal rat IgG (Sigma-Aldrich) was used as a control.
In vivo depletion of lymphocytes
In vivo depletion of lymphocytes, such as CD4+, CD8+ T cells, B cells, or NK cells, was performed as previously described.17,20 Balb/c mice were vaccinated by 3 injections of DKK1-DNA vaccine with CpG, followed by Ab depletion, which was accomplished by 3 IP injections of 100 μg of anti-CD8 mAb (2.43; ATCC), anti-CD4 mAb (GK1.5; ATCC), anti-CD20 mAb (AISB12; e-Bioscience), rabbit anti-asialo-GM1 (Wako Pure Chemical Inc), or normal rat IgG (Sigma-Aldrich), of lymphocytes and inoculation of the tumor cells. Treatments were administered every 3 days for CD4+, CD8+ T-cell or NK-cell depletion, and every week for B-cell depletion. The efficacy of the treatment, evaluated by flow cytometry by using T-, B-, and NK-cell markers, was shown to be > 90%.
Generation of DCs
Dendritic cells (DCs) were generated from murine BM stem cells as described previously.21 Briefly, BM cells were cultured, at a density of 2 × 105 cells/mL in 6-well plates, in RPMI 1640 complete medium supplemented with 20 ng/mL GM-CSF (R&D Systems). At day 8, TNF-α (10 ng/mL) and IL-1β (10 ng/mL; R&D Systems) were added to the immature DCs. After 48 hours of culture, mature DCs were pulsed with DKK1 peptides at a concentration of 100 μg per 106 DCs for 4 hours. DKK1 peptide-pulsed mature DCs were collected and used in the study.
Analysis of tumor-specific immunity
ELISA and intracellular cytokine staining were used to measure the amounts of secreted cytokines and cell expression of the cytokines. In the ELISA for IFN-γ and IL-4, splenocytes were activated overnight (18-20 hours) with syngeneic DCs pulsed with murine DKK1 peptides and supernatants were collected and used. All ELISAs were performed following the product instruction (R&D Systems). Intracellular cytokine staining was performed using the Cytofix/Cytoperm kit (BD Pharmingen). Samples were analyzed using a flow cytometer (FACSCalibur; BD Biosciences). DKK1 peptide-H-2Kd tetramer staining was used to analyze DKK1-specific CD8+ T cells. Staining for cell-surface molecules was performed as previously described.22
Ag-specific T-cell proliferation was determined by CFSE dilution assay. Briefly, splenocytes, or cultured T cells, were labeled with 5μM CFSE for 10 minutes at 37°C. After washing, labeled cells were seeded into 96-well, U-bottom plates and incubated with various stimulatory cells for 7 days. Flow cytometric analysis was used to detect dilution of CFSE, which represents T-cell proliferation.17
Standard 4-hour 51Cr-release assay was performed as previously described.23 Target cells were murine myeloma MOPC-21 cells. Splenocytes of mice from each group were pooled and cultured with irradiated MOPC-21 cells for 5 days. After culture, T cells were harvested and incubated with 51Cr-labeled target cells (104 cells/well) at different E:T cell ratios. After a 4-hour culture, 50% of the supernatants were collected, and radioactivity was measured. Percent-specific lysis was calculated using the following formula: percent-specific lysis = (experimental counts − spontaneous counts)/(maximal counts − spontaneous counts).
Statistical analysis
The Student t test was used to compare data from various experimental groups. A P value < .05 was considered statistically significant. Mean and SD are shown unless indicated otherwise.
Results
DKK1-DNA vaccines protect mice from tumor challenge and rechallenge
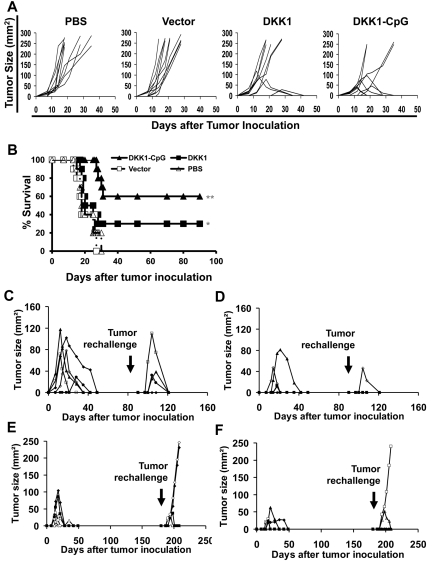
In prophylactic studies, 10 Balb/c mice per group were immunized with the DNA (murine DKK1/defensin-2 fusion) vaccine before tumor challenge. As shown in Figure 1A, all mice receiving PBS injection or vector vaccine developed tumors. However, 3 of 10 of mice receiving DKK1-DNA vaccine survived without tumor burden by the end of the experiment period (day 90). DNA vaccine plus adjuvant CpG generated superior protection against tumor challenge and 6 of 10 of mice survived without tumor burdens. Mice receiving DKK1-DNA vaccine or DKK1-DNA vaccine plus CpG had 30% or 60% survival rates, respectively, by the end of the experiment (Figure 1B, P < .05 for DKK1-DNA vaccine only and P < .01 for DKK1-DNA vaccine plus CpG, compared with PBS control).
Figure 1.
Protective effects of DKK1-DNA vaccines against myeloma. Results shown are measurements of tumor burdens (A) and survival (B) of mice receiving different treatments. Balb/c mice (10 per group) were IM vaccinated thrice with either PBS, vector control, DKK1 DNA, or DKK1 DNA plus CpG, followed by challenge with MOPC-21 myeloma cells. Tumor burdens were measured twice every week. Mice were euthanized when subcutaneous tumors reached 225 mm2 or when mice became moribund. Tumor burdens of tumor-free surviving mice (5 per group) previously treated with DKK1-DNA vaccine (C,E) or vaccine plus CpG (D,F) after rechallenge with the same myeloma cells on days 90 (C-D) and 180 (E-F) since the first tumor injection. Representative results of one of 3 to 4 independent experiments performed are shown. *P < .05; **P < .01, compared with PBS or vector controls.
Next surviving tumor-free mice from the above experiments were rechallenged with the same tumor cells (1 million cells/mouse) at days 90 or 180 since the initial tumor inoculation and followed for tumor development. The data showed that surviving mice receiving prior DKK1-DNA vaccination (Figure 1C,E) or DKK1-DNA vaccination plus CpG (Figure 1D,F) were protected against tumor rechallenge. All surviving mice were protected when tumor rechallenge was given at day 90 after the initial tumor inoculation (Figure 1C,D), while only 3 of 5 and 4 of 5 of mice receiving prior DKK1-DNA vaccine or DKK1-DNA vaccination plus CpG, respectively, were protected when tumor rechallenge was given at day 180 after the initial tumor inoculation (Figure 1E-F). Overall, our data indicate that DKK1-DNA vaccine, especially vaccine plus CpG, protected mice from tumor challenge and rechallenge by generating antitumor immune and memory responses, which gradually faded away in aged mice.
DKK1-DNA vaccines exhibit therapeutic effect on mice with established myeloma
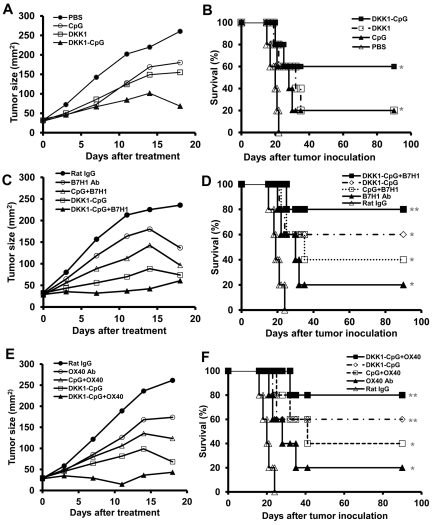
We evaluated the therapeutic efficacy of DKK1-DNA vaccine in mice with established myeloma. In these experiments, DKK1-DNA vaccine plus CpG was used as a standard vaccine. Our previous work has shown that targeting the suppressive tumor microenvironment and breaking immune suppression on effector T cells can enhance the immunogenicity of the vaccines and cure mice with large tumor burden.17 Therefore, we used anti-B7H1 (M5H1) mAb24 to block negative T-cell signaling and anti-OX40 (OX86) mAb25–27 to overcome CD4+ T-cell tolerance in mice receiving DKK1-DNA vaccination. As shown in Figure 2A, mice receiving DKK1-DNA vaccine plus CpG had repressed tumor growth compared with control mice (P < .05). DKK1-DNA vaccine (plus CpG) together with B7H1 (Figure 2C) or OX40 mAbs (Figure 2E) induced more robust tumor growth inhibition (P < .01, compared with PBS control mice). The survival of DKK1-CpG vaccine–, vaccine plus OX40 mAb–, or vaccine plus B7H1 mAb–treated mice were 3 of 5, 4 of 5, and 4 of 5, respectively. Thus, our data showed that DKK1-DNA vaccine has therapeutic effect in the murine myeloma model with established large tumors, and B7H1 blocking or OX40 agonist mAbs can further enhance the therapeutic efficacy of the vaccine.
Figure 2.
Therapeutic effects of DKK1-DNA vaccines against established myeloma. Balb/c mice (5 per group) were inoculated subcutaneously with MOPC-21 tumor cells, and when the tumors reached 25 mm2, received different treatments at a 3-day interval. Tumor burdens were measured twice every week. Mice were euthanized when subcutaneous tumors reached 225 mm2 or when mice became moribund. Shown are tumor burdens (A,C,E) and survival (B,D,F) of mice receiving 3 injections of PBS, CpG, DKK1-DNA vaccine (DKK1), or DKK1 DNA vaccine plus CpG (DKK1-CpG; A-B); or DKK1-DNA vaccine plus CpG (DKK1-CpG), rat IgG, B7H1 mAb, CpG plus B7H1 mAb (CpG+B7H1), or DKK1-DNA vaccine plus CpG (DKK1-CpG) in combination with B7H1 mAb (DKK1-CpG+B7H1; C-D); or DKK1-DNA vaccine plus CpG (DKK1-CpG), rat IgG, OX40 mAb, CpG plus OX40 mAb (CpG+OX40), or DKK1-DNA vaccine plus CpG (DKK1-CpG) in combination with OX40 mAb (DKK1-CpG+OX40; E-F). Representative results of 1 of 2 independent experiments are shown. *P < .05; **P < .01, compared with PBS controls.
DKK1-DNA vaccines induce CD4+ and CD8+ T-cell responses against tumor cells
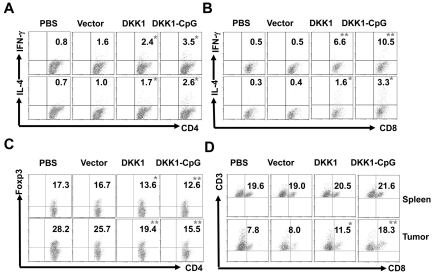
To demonstrate DKK1-DNA vaccine-induced antimyeloma immunity, we assessed whether CD4+ and CD8+ T cells were activated after DNA vaccine and tumor inoculation. Mice were vaccinated thrice followed by tumor injection, and 2 weeks after tumor injection, mice were killed, and splenocytes were isolated and cultured ex vivo with irradiated myeloma cells for 5 days. Intracellular cytokine staining showed that DKK1 DNA-vaccinated mice had more tumor-specific IFN-γ– and IL-4–expressing CD4+ (P < .05 for both IFN-γ and IL-4; Figure 3A) and CD8+ (P < .01 for IFN-γ and P < .05 for IL-4; Figure 3B) T cells compared with PBS-injected or vector-vaccinated mice. DNA vaccination plus CpG induced more potent tumor-specific CD4+ (P < .01) and CD8+ (P < .001) T-cell responses than DNA vaccination alone. In addition, DKK1 DNA-vaccinated mice had stronger tumor-specific CD8+ T-cell response than CD4+ T-cell response because significantly more IFN-γ (P < .05) and IL-4 (P < .05) expressing CD8+ T cells were detected. These findings suggest a crucial role of tumor-specific CD8+ T cells in DKK1-DNA vaccine-induced antitumor immunity.
Figure 3.
DKK1-DNA vaccines induce CD4+ and CD8+ T-cell responses. Mice (5 per group) were vaccinated thrice with PBS, vector control, DKK1 DNA, or DKK1 DNA plus CpG, followed by challenge with MOPC-21 cells. T cells were isolated from spleens and tumors after 2 weeks of tumor challenge, restimulated by the irradiated tumor cells, and analyzed by flow cytometry. Shown are percentages of IFN-γ– and IL-4–expressing CD4+ (A) and CD8+ (B) T cells in the spleens of mice receiving different treatments. Also shown are the percentages of CD4+Foxp3+ Treg cells (C) and CD3+ and CD8+ T cells (D) in the spleens (top panels) and tumors (bottom panels) of mice receiving different treatments. Representative results of 1 of 2 independent experiments performed are shown. *P < .05; **P < .01, compared with PBS controls.
Activation and subsets of T cells in tumors and mouse spleens were examined. Flow cytometric analysis showed that the percentages of CD4+Foxp3+ regulatory T-cell (Treg) were reduced in the spleens and tumors of vaccinated mice compared with control mice (P < .05 in spleens and P < .01 in tumors in DNA vaccine alone, and P < .01 in both in DNA vaccine plus CpG; Figure 3C), and more CD3+CD8+ T cells were detected in the tumors but not spleens of vaccinated mice (P < .05), especially in mice vaccinated together with CpG (P < .01; Figure 3D). Overall, these data showed that strong tumor-specific CD4+ and CD8+ T-cell responses were elicited following DKK1-DNA vaccination, and tumor-specific CD8+ CTLs might play an important role in vaccine-induced antimyeloma immunity.
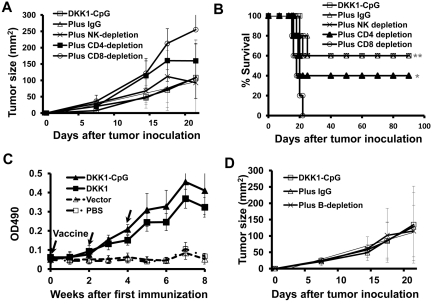
Next, we examined the role of CD4+ and CD8+ T cells, and NK cells in DKK1-DNA vaccine-induced antimyeloma immunity in vivo by using Ab depletion assay. As shown in Figure 4A, mice receiving DKK1 vaccine plus CpG had repressed tumor growth, which was abrogated in mice depleted of CD4+ (P < .05, compared with control) or CD8+ (P < .01) T cells but not NK cells. Importantly, CD8+ T-cell depletion led to a total loss of the vaccine-induced protection. Figure 4B shows the survival data of the mice. Thus, this data confirm the importance of vaccine-induced CD4+ and, especially, CD8+ T-cell responses in antitumor immunity in the mouse model.
Figure 4.
Role of cellular and humoral immunities in DKK1-DNA vaccine–induced antitumor response. Mice (5 per group) were vaccinated thrice with DKK1-DNA vaccine plus CpG, followed by in vivo depletion of CD4+ or CD8+ T cells, or NK cells, or B cells and then tumor challenge. Tumor burdens were measured twice each week. Mice were euthanized when subcutaneous tumors reached 225 mm2 or when mice became moribund. Shown are tumor burdens (A) and survival (B) of mice receiving different treatments. (C) Titers of DKK1-specific Abs in mice receiving different treatment, and (D) tumor burdens of DKK1-DNA plus CpG-vaccinated mice treated with IgG or CD20 mAb to deplete B cells. Representative results of 1 of 2 independent experiments performed are shown. *P < .05; **P < .01, compared with controls.
DKK1-DNA vaccine induces anti-DKK1 Ab production
In addition to cellular immunity, we also observed anti-DKK1 Ab production after the DNA vaccine. Mouse serum was collected every week and tested for anti-DKK1 Abs by ELISA. Figure 4C shows that anti-DKK1 Abs were induced by DKK1-DNA vaccination.
To evaluate the role of the Abs or B cells in DKK1-DNA vaccine-induced antitumor responses in vivo, we depleted B cells by using anti-CD20 mAb. Our preliminary studies showed that > 90% of B cells were depleted by the treatment and anti-DKK1 Abs were undetectable in mice depleted of B cells (data not shown). As shown in Figure 4D, there were no significant differences in myeloma development in the 3 groups. In all groups, 3 of 5 mice were protected from developing myeloma (data not shown). These results demonstrate that anti-DKK1 Abs or B cells may not play a role in controlling the growth of myeloma cells in vivo.
DKK1-DNA vaccine induces DKK1-specific CTL response
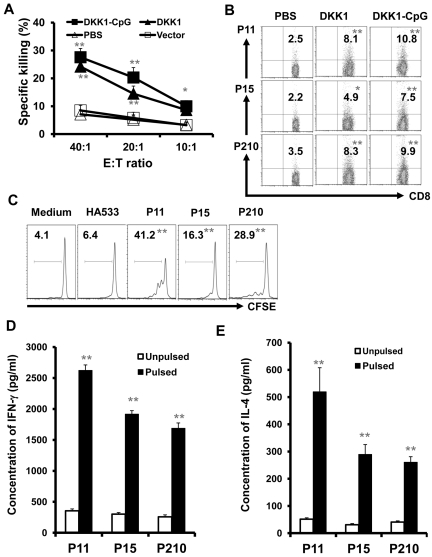
Our data showed that DKK1-DNA vaccine–induced CD8+ T-cell activation and depletion of CD8+ T cells significantly affected vaccine-induced antitumor activity. To examine CTL response, we first detected cytotoxicity of splenocytes in mice after DNA vaccine. As shown in Figure 5A, splenocytes from DKK1 DNA–vaccinated mice efficiently killed the myeloma cells in vitro. No tumor-specific CTL activity was detected in control mice. To confirm the specificity of the T cells from immunized mice, 3 DKK1 peptides, potentially binding to H-2Kd molecules, were used (Table 1). By using DKK1 peptide-tetramer staining and CFSE dilution assay, DKK1-specific CD8+ T cells were detected in mice receiving DKK1-DNA vaccination. As shown by a representative staining using 3 different DKK1 peptide tetramers depicted in Figure 5B, DKK1-DNA vaccination, especially vaccination plus CpG, stimulated DKK1 peptide-specific CD8+ T-cell response in the mice.
Figure 5.
Induction of DKK1- and tumor-specific CD8+ CTLs in DKK1-DNA vaccinated mice. Mice (5 per group) were vaccinated thrice with PBS, vector control, DKK1 DNA, or DKK1 DNA plus CpG, followed by challenge with MOPC-21 cells. T cells were isolated from spleens 2 weeks after tumor challenge. (A) Cytotoxicity of T cells against MOPC-21 cells from mice receiving different treatments. (B) Percentages of murine DKK1 peptide-tetramer–positive CD8+ T cells from mice receiving different treatments. (A-B) Splenic T cells were restimulated in vitro with irradiated tumor cells for 5 days before assays. (C) Percentages of proliferative T cells analyzed by CSFE dilution assay. Splenic T cells from DKK1-CpG vaccinated mice were labeled with CFSE and restimulated by syngeneic DCs pulsed with or without DKK1 peptides (P11, P15, or P210) or a control influenza–A peptide (HA533) for 7 days. Concentrations of IFN-γ (D) or IL-4 (E) secreted by splenic T cells from DKK1-CpG–vaccinated mice after overnight restimulation by syngeneic DCs pulsed without or with DKK1 peptides P11, P15, or P210. Secreted cytokines in culture supernatants were measured by ELISA. Representative results of 1 of 2 independent experiments performed are shown. *P < .05; **P < .01, compared with controls.
Second, we used CFSE dilution assay to examine DKK1 peptide-specific T cells from mice receiving DKK1-DNA vaccination. As shown in Figure 5C, DKK1-specific CD8+ T cells proliferated in response to syngeneic DCs pulsed, but not unpulsed, with DKK1 peptides P11, P15, and P210 (P < .01, compared with unpulsed DC) but not with a control influenza-A peptide HA533.
Third, we examined cytokines secreted by T cells from DKK1-DNA–vaccinated mice. Figure 5D and E show that IFN-γ and IL-4 secretion was significantly increased in splenic T cells restimulated with DKK1 peptide-pulsed DCs, but not with unpulsed DCs. Taken together, these data demonstrate that DKK1-specific T cells and CD8+ CTLs were generated after DKK1-DNA vaccination.
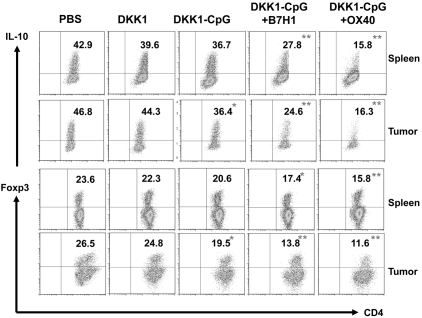
B7H1 or OX40 Abs down-regulate regulator T cells in vivo
We showed previously in this study that anti-B7H1 or OX40 mAbs enhanced the therapeutic efficacy of DKK1-DNA vaccine, and studies were performed to elucidate the underlying immunologic mechanism. As shown in Figure 6, the percentages of IL-10–secreting or Foxp3+ Tregs in the spleens and tumors were significantly decreased in mice vaccinated with DKK1-DNA plus CpG in combination with anti-B7H1 or anti-OX40 Abs (P < .01, compared with mice receiving DKK1 DNA alone). These results indicate that the beneficial effects of anti-B7H1 or OX40 mAbs were derived from their ability to reduce the numbers of Tregs in tumor-bearing mice.
Figure 6.
Effects of B7H1-blocking and OX40-agonist mAbs on CD4+ IL-10–expressing and Foxp3+ Treg in vaccinated mice. Mice (5 per group) were inoculated with MOPC-21 tumor cells, and when subcutaneous tumors reached 25 mm2, received 3 injections (every 3 days) of PBS, DKK1 DNA (DKK1), DKK1 DNA plus CpG (DKK1-CpG), DKK1 DNA plus CpG in combination with anti-B7H1 mAbs (DKK1-CpG+ B7H1) or DKK1 DNA plus CpG in combination with anti-OX40 mAbs (DKK1-CpG+ OX40). Seven days after the third treatment, T cells were isolated from spleens and tumors, restimulated by irradiated tumor cells for 5 days, and analyzed with flow cytometry. Shown are percentages of CD4+ T cells expressing IL-10 or FoxP3 (Tregs) in the spleens and tumors of mice receiving different treatments as indicated. Representative results of 1 of 2 independent experiments are shown. *P < .05; **P < .01, compared with DKK1 vaccine only.
Discussion
The goal of this study was to examine whether DKK1 can be used as a tumor vaccine to elicit DKK1-specific immunity that can control myeloma growth or even eradicate established myeloma in vivo. By using the murine MOPC-21 myeloma mouse model and murine DKK1-DNA (murine DKK1/defensin-2 fusion) vaccine, the results showed that active immunization with the vaccine efficiently protected mice from developing myeloma and treated mice against established tumor. The vaccination generated memory immune responses that protected surviving mice from tumor rechallenge. We also showed that mice receiving DKK1-DNA vaccine, plus CpG, as adjuvant provided better protective and therapeutic effects against myeloma than vaccine alone, and the addition of B7H1-blocking or OX40-agonist mAbs further improved the therapeutic efficacy of the vaccine. Immunologic studies revealed that the vaccine elicited strong DKK1- and myeloma-specific CD4+ and CD8+ IFN-γ T-cell and CTL responses, and depletion of CD4+ and especially CD8+ T cells in vivo largerly abrogated antitumor effects of the vaccine. Interestingly, we also observed a DKK1-specific, IL-4–secreting Th2 response in vaccinated mice. Previous studies showed that both idiotype-specific Th1 and Th2 responses had antitumor effects in plasmacytoma mouse model28,29, while our recent study clearly showed that idiotype-specific Th2 cells promoted myeloma cell growth.30 In vivo injections of CpG or B7H1-blocking or OX40-agonist mAbs potentiated the therapeutic efficacy of the vaccine by increasing the frequency of DKK1- and myeloma-specific effector (IFN-γ–secreting and CTL) T cells and by reduceing the numbers of IL-10–secreting and Foxp3+ Treg cells in vaccinated mice. Thus, these findings strongly suggest that DKK1 can be used as a tumor vaccine for active immunotherapy of MM and provide a rationale and platform for future clinical application of DKK1-based immunotherapy in myeloma patients.
Active immunotherapy, using tumor-associated Ags (vaccination), is considered a potentially promising cancer immunotherapy approach.31 Among different forms of tumor vaccines, DNA vaccine strategy allows broad usage.14–16 Studies have shown that antitumor immunity can be triggered by targeting Ag delivery to APCs by chemokine receptor-mediated binding, uptake, and processing of tumor Ag for more efficient presentation to T cells. These fusion vaccines have been shown to elicit much more potent antitumor effects, compared with the unfused counterpart, in several mouse tumor models.18,32 The chemokine motif in the fusion has been shown to be essential for breaking immune tolerance of tumor Ag since vaccination of plasmid DNA that encodes the fusion elicited potent Ag-specific immune responses, while their nonfused counterparts did not.32 Moreover, the chemokine needs to be physically linked with the Ag as the antitumor immunity was not detected in mice coinjected with the nonfusion Ag and the chemokine adjuvant.18,32 Among the panel of chemokine receptor ligands tested, murine defensin-2 has consistently been shown to generate strong Ag-specific immunity when used in the context of a DNA fusion vaccine.18,32 Although initially tested for its ability to interact with CC chemokine receptor 6,18 defensin-2 has been shown to play a role in activating APCs via TLR4.32 Thus, its dual capacity to target APCs and to induce DC maturation makes defensin-2 an attractive candidate for chemokine fusion vaccines.18,32 Therefore, we designed the expression plasmid encoding DKK1 cDNA-defensin-2 fusion gene for this study. As expected, murine DKK1/defensin-2 fusion DNA vaccine was efficient at eliciting antitumor immunity in vivo. However, for future clinical application, DKK1 vaccines could be given in the forms of DNA (plasmid), recombinant protein, or (short and long) peptides. Future work may be needed to examine which form of the vaccines works best.
Our results also suggest that, in addition to developing effective vaccines, other forms of immune manipulations, such as adjuvants and Abs specific for T-cell costimulatory molecules, are also required for achieving better antitumor responses. CpGs have been shown to interact with TLR9 in APCs and in B cells, where they induce the expression of costimulatory molecules such as CD80 and CD86, MHC class II molecules, and proinflammatory cytokines.33 Many studies support an effective role for TLR ligands in the induction of antitumor immune responses.34 The “danger model” of Matzinger suggests that tumor Ags presented in the context of bacterial signals, may be viewed by the immune system as dangerous, leading to tumor elimination.35 Indeed, reports from Speiser et al36 and Kochenderfer et al37 have shown that the addition of CpG to tumor Ags (in these cases, a melanoma peptide) stimulates the expansion and function of peptide-specific CD8+ T cells in humans and mice. Our previous study showed that gp96 vaccines in combination with CpG eradiated all intermediate tumors in the mice,17 suggesting that CpG may be a better adjuvant for immunotherapy in tumor vaccines. Our current studies support the notion that CpG has a stimulatory effect on APCs, which leads to enhanced Ag presentation and, in turn, better stimulation of an antitumor immune response induced by DKK1-DNA vaccine.
Recently, molecular homologs of B7-like ligands, B7H1 (also termed PDL1) and B7DC (also termed PDL2), have been identified. PD-1, their receptor, is a 55-kDa type 1 transmembrane protein belonging to the CD28/CTLA-4 family.38 These B7 family members have been shown to down-regulate T-cell activation through PD-1.39,40 Cross-linking of PD-1 with B7H1 or B7DC results in decreased IFN-γ, IL-10, IL-4, and IL-2 secretion.39,40 Thus, B7H1 or B7DC lead to diminished immune responses by regulating T-cell activation. Unlike the response to foreign pathogens, which elicit potent danger signals, the immune response to tumor Ags is usually less robust and the tumor environment can be immunosuppressive, leading to Ag-specific T-cell tolerance.41 On the other hand, signaling through OX40 in vivo leads to a series of proinflammatory events that can overcome CD4+ T-cell tolerance.27,42 Agonist Abs and a soluble OX40L–immunoglobulin fusion protein have been injected in vivo to test their ability to enhance immune responses in tumor-bearing mice.43–45 Ligation of OX40 in vivo in tumor-bearing mice enhanced antitumor immunity, leading to tumor-free survival in mouse models of melanoma, sarcoma, colon cancer, breast cancer, and glioma.43–47 In addition, a recent study has shown that OX40 signals might contribute to the deletion and homeostasis of CD4+CD25+ Treg cells. OX40-deficient mice have fewer Treg cells in the periphery as infants than do wild-type mice, and Treg cells from OX40-deficient mice have a marked reduction in suppressive effect on CD3-stimulated T-cell proliferation.26 These observations indicate that OX40 signaling might affect the roles of Treg cells because mouse Treg cells constitutively express OX40.26 To break the immunosuppression and achieve better therapeutic efficacy of DKK1-DNA vaccines, B7H1-blocking and OX40-agonist Abs were used as combination treatment with DNA vaccine. Results demonstrated that the Abs could decrease IL-10 expression in CD4+ T cells and reduce the numbers of CD4+Foxp3+ Tregs, leading to much better antitumor immunity induced by the vaccine.
Recent studies showed that anti-DKK1 Abs may have therapeutic potentials for the treatment of cancers including MM.48,49 However, as DKK1 is a secreted protein by tumor cells, it is unlikely that anti-DKK1 Abs have direct antitumor effects in vivo. Rather, anti-DKK1 Abs could inhibit the bone destructive process in MM.50 There are advantages with active DKK1 vaccination. First, it can induce DKK1-specific T-cell responses including CTLs that can directly kill myeloma cells. Second, DKK1 vaccines can also elicit anti-DKK1 Abs, which could inhibit myeloma-related bone disease in patients.
In conclusion, our study suggests that DKK1-DNA vaccine can be used as a universal tumor vaccine for immunotherapy of patients with MM. This is supported by our results that DKK1-DNA vaccines provided strong protection against mice from developing myeloma and was effective at treating established myeloma in the mouse model. Furthermore, by using CpG, B7H1 blocking or OX40 agonist Abs, the therapeutic efficacy of DKK1-DNA vaccines can be greatly improved to eradicate tumors developed in the mice. Thus, this study is the first preclinical study to provide strong and direct evidence to support the application of DKK1-based immunotherapy in MM.
Supplementary Material
Acknowledgments
The authors thank Angelique Harkins for providing editorial assistance.
This work was supported by National Cancer Institute R01 CA138402, R01 CA138398, and P50 CA142509; Leukemia & Lymphoma Society translational research grants; the Multiple Myeloma Research Foundation, the Commonwealth Foundation for Cancer Research; and the Center for Targeted Therapy of The University of Texas M. D. Anderson Cancer Center.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.Q., J. Hou, and Q.Y. initiated the work, designed the experiments, and wrote the manuscript; J.Q., Y.Z., C.Z., L.W., H.Q., and S.H. performed the experiments and statistical analyses; and H.L., Y.L., J. He, J.Y., S.N., and L.W.K. provided samples and critical suggestions to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qing Yi, MD, PhD, Department of Lymphoma/Myeloma, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0903, Houston, TX 77030; e-mail: qyi@mdanderson.org; or Jian Hou, MD, Department of Hematology, Shanghai Chang Zheng Hospital, Shanghai 200003, People's Republic of China; e-mail: houjian@medmail.com.cn.
References
- 1.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89(3):789–793. [PubMed] [Google Scholar]
- 2.Bergenbrant S, Yi Q, Osterborg A, et al. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996;92(4):840–846. doi: 10.1046/j.1365-2141.1996.419959.x. [DOI] [PubMed] [Google Scholar]
- 3.Osterborg A, Yi Q, Henriksson L, et al. Idiotype immunization combined with granulocyte-macrophage colony-stimulating factor in myeloma patients induced type I, major histocompatibility complex-restricted, CD8- and CD4-specific T-cell responses. Blood. 1998;91(7):2459–2466. [PubMed] [Google Scholar]
- 4.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma–a feasibility study. Blood. 1999;93(7):2411–2419. [PubMed] [Google Scholar]
- 5.Titzer S, Christensen O, Manzke O, et al. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects. Br J Haematol. 2000;108(4):805–816. doi: 10.1046/j.1365-2141.2000.01958.x. [DOI] [PubMed] [Google Scholar]
- 6.Yi Q, Desikan R, Barlogie B, Munshi N. Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol. 2002;117(2):297–305. doi: 10.1046/j.1365-2141.2002.03411.x. [DOI] [PubMed] [Google Scholar]
- 7.Yi Q. Dendritic cell-based immunotherapy in multiple myeloma. LeukLymphoma. 2003;44(12):2031–2038. doi: 10.1080/1042819031000116599. [DOI] [PubMed] [Google Scholar]
- 8.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 9.Krupnik VE, Sharp JD, Jiang C, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238(2):301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 10.Kohn MJ, Kaneko KJ, DePamphilis ML. DkkL1 (Soggy), a Dickkopf family member, localizes to the acrosome during mammalian spermatogenesis. Mol Reprod Dev. 2005;71(4):516–522. doi: 10.1002/mrd.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65(17):7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 12.Yaccoby S, Ling W, Saha R, et al. Inhibition of DKK1 activity is associated with increased osteoblast numbers and bone formation, reduced osteoclast activity and inhibition of tumor growth in the SCID-rab model for primary myeloma [abstract]. Blood(ASH Annual Meeting Abstracts) 2005;106(11):189a. Abstract 638. [Google Scholar]
- 13.Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110(5):1587–1594. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredriksen AB, Bogen B. Chemokine-idiotype fusion DNA vaccines are potentiated by bivalency and xenogeneic sequences. Blood. 2007;110(6):1797–1805. doi: 10.1182/blood-2006-06-032938. [DOI] [PubMed] [Google Scholar]
- 15.Qin H, Cha SC, Neelapu SS, et al. Vaccine site inflammation potentiates idiotype DNA vaccine-induced therapeutic T cell-, and not B cell-, dependent antilymphoma immunity. Blood. 2009;114(19):4142–4149. doi: 10.1182/blood-2009-05-219683. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly JJ, Friedman A, Martinez D, et al. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1(6):583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 17.Qian J, Hong S, Wang S, et al. Myeloma cell line-derived, pooled heat shock proteins as a universal vaccine for immunotherapy of multiple myeloma. Blood. 2009;114(18):3880–3889. doi: 10.1182/blood-2009-06-227355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biragyn A, Surenhu M, Yang D, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167(11):6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 19.Masaki H, Tamura M, Kurane I. Induction of cytotoxic T lymphocytes of heterogeneous specificities by immunization with a single peptide derived from influenza A virus. Viral Immunol. 2000;13(1):73–81. doi: 10.1089/vim.2000.13.73. [DOI] [PubMed] [Google Scholar]
- 20.Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 207(8):1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 22.Xie J, Qian J, Wang S, Freeman ME, 3rd, Epstein J, Yi Q. Novel and detrimental effects of lipopolysaccharide on in vitro generation of immature dendritic cells: involvement of mitogen-activated protein kinase p38. J Immunol. 2003;171(9):4792–4800. doi: 10.4049/jimmunol.171.9.4792. [DOI] [PubMed] [Google Scholar]
- 23.Qian J, Wang S, Yang J, et al. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005;11(24 Pt 1):8808–8815. doi: 10.1158/1078-0432.CCR-05-1553. [DOI] [PubMed] [Google Scholar]
- 24.Kanai T, Totsuka T, Uraushihara K, et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol. 2003;171(8):4156–4163. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]
- 25.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4(6):420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 26.Takeda I, Ine S, Killeen N, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172(6):3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 27.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7(8):907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 28.Lauritzsen GF, Bogen B. Idiotype-specific, major histocompatibility complex restricted T cells are of both Th1 and Th2 type. Scand J Immunol. 1991;33(6):647–656. doi: 10.1111/j.1365-3083.1991.tb02537.x. [DOI] [PubMed] [Google Scholar]
- 29.Lauritzsen GF, Weiss S, Bogen B. Anti-tumour activity of idiotype-specific, MHC-restricted Th1 and Th2 clones in vitro and in vivo. Scand J Immunol. 1993;37(1):77–85. doi: 10.1111/j.1365-3083.1993.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 30.Hong S, Qian J, Yang J, Li H, Kwak LW, Yi Q. Roles of idiotype-specific t cells in myeloma cell growth and survival: Th1 and CTL cells are tumoricidal while Th2 cells promote tumor growth. Cancer Res. 2008;68(20):8456–8464. doi: 10.1158/0008-5472.CAN-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. J Biomed Biotechnol. 2010;pii:596432. doi: 10.1155/2010/596432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 33.Jahrsdorfer B, Hartmann G, Racila E, et al. CpG DNA increases primary malignant B cell expression of costimulatory molecules and target antigens. J LeukocyteBiol. 2001;69(1):81–88. [PubMed] [Google Scholar]
- 34.Li J, Song W, Czerwinski DK, et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179(4):2493–2500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 35.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 36.Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115(3):739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kochenderfer JN, Simpson JL, Chien CD, Gress RE. Vaccination regimens incorporating CpG-containing oligodeoxynucleotides and IL-2 generate antigen-specific antitumor immunity from T-cell populations undergoing homeostatic peripheral expansion after BMT. Blood. 2007;110(1):450–460. doi: 10.1182/blood-2006-11-057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 41.Staveley-O'Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164(1):107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164(4):2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 44.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60(19):5514–5521. [PubMed] [Google Scholar]
- 45.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6(4):528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 46.Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. 2001;167(11):6669–6677. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- 47.Morris A, Vetto JT, Ramstad T, et al. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67(1):71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- 48.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109(5):2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato N, Yamabuki T, Takano A, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 70(13):5326–5336. doi: 10.1158/0008-5472.CAN-09-3879. [DOI] [PubMed] [Google Scholar]
- 50.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.