Abstract
Risk factors for acute GVHD (AGVHD), overall survival, and transplant-related mortality were evaluated in adults receiving allogeneic hematopoietic cell transplants (1999-2005) from HLA-identical sibling donors (SDs; n = 3191) or unrelated donors (URDs; n = 2370) and reported to the Center for International Blood and Marrow Transplant Research, Minneapolis, MN. To understand the impact of transplant regimen on AGVHD risk, 6 treatment categories were evaluated: (1) myeloablative conditioning (MA) with total body irradiation (TBI) + PBSCs, (2) MA + TBI + BM, (3) MA + nonTBI + PBSCs, (4) MA + nonTBI + BM, (5) reduced intensity conditioning (RIC) + PBSCs, and (6) RIC + BM. The cumulative incidences of grades B-D AGVHD were 39% (95% confidence interval [CI], 37%-41%) in the SD cohort and 59% (95% CI, 57%-61%) in the URD cohort. Patients receiving SD transplants with MA + nonTBI + BM and RIC + PBSCs had significantly lower risks of grades B-D AGVHD than patients in other treatment categories. Those receiving URD transplants with MA + TBI + BM, MA + nonTBI + BM, RIC + BM, or RIC + PBSCs had lower risks of grades B-D AGVHD than those in other treatment categories. The 5-year probabilities of survival were 46% (95% CI, 44%-49%) with SD transplants and 33% (95% CI, 31%-35%) with URD transplants. Conditioning intensity, TBI and graft source have a combined effect on risk of AGVHD that must be considered in deciding on a treatment strategy for individual patients.
Introduction
Acute GVHD (AGVHD) remains a common complication of allogeneic hematopoietic cell transplantation (HCT), with a significant impact on early morbidity and mortality. Over the past few decades, diverse clinical factors were reported to be significantly associated with the incidence and severity of AGVHD.1–13 Most of these factors were evaluated in large studies of recipients undergoing HCT from sibling donors (SDs) or unrelated donors (URDs) after myeloablative conditioning (MA). During the same period of time, there was a substantial increase in the use of reduced intensity conditioning (RIC) regimens. The influence of conditioning regimen and the interaction of conditioning regimen, donor source, and graft type on incidence and severity of AGVHD and on factors predicting the occurrence of AGVHD are not well studied. Identifying pretransplantation and transplant-related clinical predictors is important, because modulation of these factors, if possible, could favorably impact transplantation outcome.
We analyzed the impact of demographic, HCT-related and disease-specific variables on AGVHD, overall survival, and transplant-related mortality after allogeneic HCT among 5561 adults with hematologic malignancies who underwent HCT from 1999 to 2005 and who were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR), Minneapolis, MN.
Methods
CIBMTR
CIBMTR is a research organization formed through an affiliation between the International Bone Marrow Transplant Registry (IBMTR) and the National Marrow Donor Program (NMDP) and is composed of a voluntary working network of more than 450 transplant centers worldwide. Detailed clinical data on consecutive autologous and allogeneic HCTs are reported to a Statistical Center associated with the Division of Hematology and Oncology in the Department of Medicine of the Medical College of Wisconsin (Milwaukee, WI) and with the NMDP Coordinating Center (Minneapolis, MN). Data quality is maintained by on-site audits, computerized checks for errors, and physician review of submitted data. Observational studies conducted by CIBMTR are performed with informed consent in accordance with the Declaration of Helsinki and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the NMDP and Medical College of Wisconsin Institutional review boards.
Study design
This is a retrospective cohort study to analyze the incidence and risk factors for AGVHD, overall survival, and transplant-related mortality. The study population consisted of adults (≥ 20 years old) who received an HLA-identical SD (n = 3191) or URD (n = 2370) HCT with a non-T cell–depleted graft from 1999 to 2005 and were reported to the CIBMTR. SD transplants were reported at 177 centers, and 87 centers reported URD transplants. All unrelated recipient–donor pairs had high-resolution typing available at HLA-A, -B, -C, and DRB1 loci. Subjects with acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML), or myelodysplastic syndromes were included. Both MA and RIC regimens were included, to study the impact of these factors on AGVHD.14 We used the IBMTR grading system (A-D) for classifying AGVHD because it is less reliant on physicians' subjective assessments of performance status and it performs similarly to the Glucksberg system in explaining variability in AGVHD outcomes.15,16
Definitions and study endpoints
Disease status at transplant was classified as early, intermediate, or advanced. Early disease was defined as acute leukemia (AML or ALL) in first complete remission, CML in first chronic phase or myelodysplastic syndromes with refractory anemia or acquired idiopathic sideroblastic anemia. Intermediate stage disease was defined as acute leukemia (AML or ALL) in second or greater complete remission, CML in accelerated phase or second or greater chronic phase. Advanced stage disease was defined as primary induction failure or relapse of acute leukemia (AML or ALL), refractory anemia with excess blasts, or CML in blast crisis. We defined MA regimens as follows: busulfan dose ≥ 9 mg/kg, melphalan dose ≥ 150 mg/m2, or total body irradiation (TBI) dose ≥ 5 Gy (single or fractionated) or > 8 Gy (fractionated).14 Regimens not meeting these criteria were classified as reduced intensity. HLA matching for URD transplants was determined using high-resolution HLA typing. Eight of 8 matched URDs were matched at the allele level at HLA-A, -B, -C, or DRB1 loci; 7/8 matched URD had a single mismatch at either the antigen or allele level; and ≤ 6/8 matched URD had 2 or more mismatches at either the antigen or allele level.
The primary end points of the study were incidences of grades B-D AGVHD, grades C-D AGVHD, overall survival, and transplant-related mortality. Overall survival was estimated from day of HCT. Death from any cause was treated as an event. Transplant-related mortality was defined as death in continuous remission.
Statistical methods
Analyses of recipients of SD and URD transplants were performed separately. Models were designed to study the effects of risk factors on incidence and grade of AGVHD and other clinical outcomes. For discrete variables, number and proportions were calculated. For continuous factors, the median and range are presented. Survival probabilities were calculated using the Kaplan-Meier estimator, and 95% confidence intervals (CIs) were calculated using variance estimated by the Greenwood formula.17 Estimates of AGVHD and transplant-related mortality were calculated using the cumulative incidence function. Estimates of AGVHD were calculated using death as the competing risk, whereas estimates of transplant-related mortality were calculated using disease relapse or progression as the competing risk.18 Multivariate analyses were done to study the association of risk factors with the odds of AGVHD at 100 days using logistic regression. A stepwise selection procedure was performed with P ≤ .05 as the criterion for inclusion in final models. Multivariate analyses of transplant-related mortality were done using the pseudovalue approach of Klein.19–21 A stepwise regression model using a generalized linear model for the pseudovalues was used. Patient-related variables considered were recipient and donor age (10-year increments), sex and Karnofsky performance score. Because conditioning regimen and graft source are often given as “packages,” we created 6 treatment categories to evaluate the risk of AGVHD associated with common current treatment strategies for transplantation, considering conditioning intensity (MA or RIC), use of TBI (TBI and nonTBI) and graft source (bone marrow [BM] or peripheral blood stem cells [PBSCs]) as follows: MA + TBI + PBSCs (category 1), MA + TBI + BM (category 2), MA + nonTBI + PBSCs (category 3), MA + nonTBI + BM (category 4), RIC + PBSCs (category 5), and RIC + BM (category 6). Other transplant-related variables that were analyzed were HLA match in URD HCT (8/8, 7/8, or ≤ 6/8), donor–recipient sex mismatch, parity of female donors, donor–recipient cytomegalovirus (CMV) serology, ABO mismatch, GVHD prophylaxis, AGVHD grade, and year of transplantation. Disease-related variables considered were diagnosis and disease status pretransplant. Interactions were evaluated between treatment category and all significant variables affecting the incidence of AGVHD and were not statistically significant.
Results
Recipient and donor characteristics are outlined in Table 1. Median follow-up times after SD and URD allogeneic HCT were 40.1 months (range, 3.3-99.2 months) and 40.2 months (range, 4.4-101.7 months), respectively. Transplant-specific parameters are outlined in Table 2. Approximately 20% of patients receiving SD and URD transplants received RIC regimens, and 75% of SD and 54% of URD received PBSCs as their graft source.
Table 1.
Patient characteristics
| Variable | SD |
URD |
||||
|---|---|---|---|---|---|---|
| No. assessable | No. | % | No. assessable | No. | % | |
| Recipient age, y | 3191 | 2370 | ||||
| Median | 42.7 | 44.2 | ||||
| Range | 20-74 | 20-74 | ||||
| 20-29 | 617 | 19 | 447 | 19 | ||
| 30-39 | 741 | 23 | 493 | 21 | ||
| 40-49 | 872 | 27 | 644 | 27 | ||
| 50-59 | 734 | 23 | 587 | 25 | ||
| ≥ 60 | 227 | 7 | 199 | 8 | ||
| Sex | 3191 | 2370 | ||||
| Male | 1796 | 56 | 1309 | 55 | ||
| Female | 1395 | 44 | 1061 | 45 | ||
| Donor age, y | 3191 | 2370 | ||||
| Median | 41.6 | 35.4 | ||||
| Range | < 1-75 | 18.6-60.6 | ||||
| < 10 | 15 | < 1 | ||||
| 10-19 | 163 | 5 | 24 | 1 | ||
| 20-29 | 476 | 15 | 677 | 29 | ||
| 30-39 | 773 | 24 | 888 | 37 | ||
| 40-49 | 844 | 26 | 612 | 26 | ||
| ≥ 50 | 872 | 27 | 169 | 7 | ||
| Missing | 48 | 2 | ||||
| Donor–recipient sex match | ||||||
| Male→male | 1034 | 32 | 854 | 36 | ||
| Male→female | 709 | 22 | 638 | 27 | ||
| Female→male | 756 | 24 | 455 | 19 | ||
| Female→female | 683 | 21 | 423 | 18 | ||
| Unknown | 9 | < 1 | ||||
| KPS | 3191 | 2370 | ||||
| < 80 | 260 | 8 | 216 | 9 | ||
| 80-100 | 2824 | 88 | 1906 | 80 | ||
| Unknown | 107 | 3 | 248 | 10 | ||
| Disease type | 3191 | 2370 | ||||
| AML | 1377 | 43 | 1044 | 44 | ||
| ALL | 516 | 16 | 446 | 19 | ||
| CML | 831 | 26 | 463 | 20 | ||
| MDS | 467 | 15 | 417 | 18 | ||
| Disease status at transplant | 3191 | 2370 | ||||
| Early | 1761 | 55 | 907 | 38 | ||
| Intermediate | 528 | 17 | 568 | 24 | ||
| Advanced | 681 | 21 | 707 | 30 | ||
| Other | 221 | 7 | 188 | 8 | ||
| Recipient race | 3191 | 2370 | ||||
| White | 2239 | 70 | 2063 | 87 | ||
| Other | 952 | 30 | 307 | 13 | ||
| Donor race | 3191 | 2370 | ||||
| White | 2239 | 70 | 1941 | 82 | ||
| Other | 95 | 30 | 429 | 18 | ||
| Recipient–donor CMV status | 3191 | 2370 | ||||
| R−/D− | 624 | 20 | 738 | 31 | ||
| R+/D− | 461 | 14 | 775 | 33 | ||
| R−/D+ | 301 | 9 | 289 | 12 | ||
| R+/D+ | 1656 | 52 | 552 | 22 | ||
| Unknown | 149 | 5 | 46 | 2 | ||
| Donor pregnancy | 3191 | 2370 | ||||
| Male | 1743 | 55 | 1492 | 62 | ||
| Female, no pregnancy | 276 | 9 | 343 | 14 | ||
| Female, > 1 pregnancy | 730 | 23 | 500 | 21 | ||
| Unknown | 442 | 14 | 35 | 1 | ||
KPS indicates Karnofsky performance score; MDS, myelodysplastic syndrome; R, recipient; and D, donor.
Table 2.
Transplant characteristics
| Variable | SD |
URD |
||||
|---|---|---|---|---|---|---|
| No. assessable | No. | % | No. assessable | No. | % | |
| Graft source | 3191 | 2370 | ||||
| Bone marrow | 806 | 25 | 1081 | 46 | ||
| Peripheral blood | 2385 | 75 | 1289 | 54 | ||
| Preparative regimen | 3191 | 2370 | ||||
| Myeloablative | 2463 | 78 | 1882 | 79 | ||
| Reduced intensity | 689 | 22 | 488 | 21 | ||
| Other | 39 | 1 | ||||
| GVHD prophylaxis | 3191 | 2370 | ||||
| CSA + MTX ± other | 2683 | 84 | 1208 | 51 | ||
| Tacrolimus + MTX ± other | 484 | 15 | 1140 | 48 | ||
| Other | 24 | 1 | 22 | 1 | ||
| HLA high-resolution typing, -A, -B, -C, and -DRB1 (URD only) | 2370 | |||||
| 8/8 matched | 1532 | 65 | ||||
| 7/8 | 614 | 26 | ||||
| ≤ 6/8 | 224 | 9 | ||||
| Year of transplant | 3191 | 2370 | ||||
| 1999 | 498 | 16 | 233 | 10 | ||
| 2000 | 478 | 15 | 284 | 12 | ||
| 2001 | 453 | 14 | 266 | 11 | ||
| 2002 | 446 | 14 | 239 | 10 | ||
| 2003 | 350 | 11 | 340 | 14 | ||
| 2004 | 503 | 16 | 486 | 21 | ||
| 2005 | 463 | 15 | 522 | 22 | ||
| AGVHD grades B-D | 3191 | 2370 | ||||
| No | 1952 | 61 | 969 | 41 | ||
| Yes | 1239 | 39 | 1401 | 59 | ||
| AGVHD grades C-D | 3191 | 2370 | ||||
| No | 2692 | 84 | 1618 | 68 | ||
| Yes | 499 | 18 | 752 | 32 | ||
| AGVHD organ | ||||||
| Skin | 3191 | 2370 | ||||
| Stage 0 | 325 | 20 | 264 | 16 | ||
| Stage 1 | 522 | 32 | 313 | 19 | ||
| Stage 2 | 482 | 29 | 479 | 29 | ||
| Stage 3 | 274 | 17 | 502 | 30 | ||
| Stage 4 | 46 | 3 | 9 | 5 | ||
| GI | 3191 | 2370 | ||||
| Stage 0 | 922 | 56 | 820 | 50 | ||
| Stage 1 | 309 | 19 | 392 | 24 | ||
| Stage 2 | 152 | 9 | 123 | 7 | ||
| Stage 3 | 138 | 8 | 134 | 8 | ||
| Stage 4 | 118 | 7 | 176 | 11 | ||
| Liver | 3191 | 2370 | ||||
| Stage 0 | 1121 | 69 | 1160 | 71 | ||
| Stage 1 | 154 | 9 | 112 | 7 | ||
| Stage 2 | 114 | 7 | 127 | 8 | ||
| Stage 3 | 127 | 8 | 112 | 7 | ||
| Stage 4 | 108 | 7 | 117 | 7 | ||
| AGVHD organ involvement combination | ||||||
| Skin + GI + liver | 234 | 15 | 282 | 18 | ||
| Skin + GI | 262 | 17 | 342 | 21 | ||
| Skin + liver | 158 | 10 | 119 | 7 | ||
| GI + liver | 69 | 4 | 50 | 3 | ||
| Skin | 670 | 42 | 641 | 40 | ||
| GI | 152 | 10 | 151 | 9 | ||
| Liver | 42 | 3 | 17 | 1 | ||
Other GVHD prophylaxis include for SD, MTX ± other (no MMF) 16, MMF ± other (no MTX) 1, steroids ± other (no MTX or MMF) 1, MTX + MMF ± other 3, unknown 3; and for URD, MTX ± other (no MMF) 8, MMF ± other (no MTX) 3, MTX + MMF ± other 4, unknown 7.
CSA indicates cyclosporine; MTX, methotrexate; GI, gastrointestinal; and MMF, mycophenolate mofetil.
AGVHD risk factors
Tables 3 and 4 summarize the multivariate analyses of risk factors associated with incidence of AGVHD in SD and URD cohorts for grades B-D and C-D AGVHD, respectively.
Table 3.
Multivariate analyses of risk factors for grades B-D AGVHD
| Variable | SD |
URD |
||||||
|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | P | n | OR | 95% CI | P | |
| Treatment category | < .0001 | .0006 | ||||||
| 1: MA + TBI + PBSC | 709 | 1.00 | 534 | 1.00 | ||||
| 2: MA + TBI + BM | 245 | 0.94 | 0.70-1.27 | .69 | 733 | 0.71 | 0.56-0.91 | .006 |
| 3: MA + nonTBI + PBSC | 1017 | 0.97 | 0.80-1.18 | .78 | 350 | 0.87 | 0.65-1.16 | .34 |
| 4: MA + nonTBI + BM | 492 | 0.56 | 0.44-0.71 | < .0001 | 265 | 0.55 | 0.40-0.75 | .0001 |
| 5: RIC + PBSC | 622 | 0.70 | 0.56-0.88 | .002 | 405 | 0.75 | 0.57-1.00 | .05 |
| 6: RIC + BM | 67 | 0.90 | 0.54-1.49 | .67 | 83 | 0.47 | 0.29-0.76 | .002 |
| GVHD prophylaxis | .0001 | .03 | ||||||
| CSA + MTX ± other | 2645 | 1.00 | 1208 | 1.00 | ||||
| Tacrolimus ± MTX ± other | 484 | 0.65 | 0.53-0.80 | < .0001 | 1140 | 0.79 | 0.67-0.94 | .008 |
| Other | 23 | 0.54 | 0.21-1.39 | .20 | 22 | 1.04 | 0.44-2.46 | .94 |
| Disease | .008 | |||||||
| ALL | 446 | 1.00 | ||||||
| AML | 1044 | 1.02 | 0.81-1.30 | .85 | ||||
| CML | 463 | 1.51 | 1.14-1.99 | .004 | ||||
| MDS | 417 | 1.12 | 0.83-1.49 | .46 | ||||
| HLA match | .03 | |||||||
| 8/8 matched | 1532 | 1.00 | ||||||
| 7/8 matched | 614 | 1.27 | 1.05-1.54 | .02 | ||||
| ≤ 6/8 matched | 224 | 1.26 | 0.94-1.68 | .13 | ||||
Pairwise comparisons significant for the treatment categories were as follows for SD, treatment category 2 versus treatment category 4 (P = .003), treatment category 3 versus treatment category 4 (P < .0001), treatment category 3 versus treatment category 5 (P = .003); and for URD, treatment category 3 versus treatment category 4 (P = .006), and treatment category 3 versus treatment category 6 (P = .01).
CSA indicates cyclosporine; MTX, methotrexate; MDS, myelodysplastic syndrome; and MA, myeloablative.
Table 4.
Multivariate analyses of risk factors for grades C-D AGVHD
| Variable | SD |
URD |
||||||
|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | P | n | OR | 95% CI | P | |
| Treatment category | .03 | .04 | ||||||
| 1: MA+TBI+PBSC | 709 | 1.0 | 534 | 1.00 | ||||
| 2: MA + TBI + BM | 245 | 0.90 | 0.62-1.32 | .60 | 733 | 0.79 | 0.62-1.00 | .05 |
| 3: MA + nonTBI + PBSC | 1017 | 1.03 | 0.81-1.32 | .79 | 350 | 0.92 | 0.68-1.23 | .56 |
| 4: MA + nonTBI + BM | 492 | 0.61 | 0.44-0.85 | .003 | 265 | 0.67 | 0.48-0.93 | .02 |
| 5: RIC + PBSC | 622 | 1.00 | 0.76-1.31 | .98 | 405 | 0.85 | 0.64-1.12 | .25 |
| 6: RIC + BM | 67 | 0.88 | 0.46-1.70 | .71 | 83 | 0.48 | 0.28-0.83 | .009 |
| GVHD prophylaxis | .0003 | |||||||
| CSA + MTX ± other | 2645 | 1.00 | ||||||
| Tacrolimus ± MTX ± other | 484 | 0.56 | 0.42-0.75 | < .0001 | ||||
| Other | 23 | 0.59 | 0.17-1.99 | .39 | ||||
| Disease status at transplant | .01 | |||||||
| Early | 1750 | 1.00 | ||||||
| Intermediate | 520 | 1.02 | 0.78-1.32 | .90 | ||||
| Advanced | 666 | 1.32 | 1.05-1.66 | .02 | ||||
| Unknown | 216 | 1.55 | 1.10-2.18 | .01 | ||||
| Sex mismatch (D/R) | .009 | |||||||
| M/M, M/F, F/F | 2397 | 1.00 | ||||||
| F/M | 746 | 1.37 | 1.12-1.69 | .002 | ||||
| Missing | 9 | 0.62 | 0.08-5.06 | .66 | ||||
| Disease | .003 | |||||||
| ALL | 446 | 1.00 | ||||||
| AML | 1044 | 1.01 | 0.78-1.29 | .97 | ||||
| CML | 463 | 1.52 | 1.14-2.01 | .004 | ||||
| MDS | 417 | 1.25 | 0.93-1.70 | .15 | ||||
| HLA match | .0006 | |||||||
| 8/8 matched | 1532 | 1.00 | ||||||
| 7/8 matched | 614 | 1.36 | 1.12-1.66 | .002 | ||||
| ≤ 6/8 matched | 224 | 1.55 | 1.16-2.08 | .003 | ||||
Pairwise comparisons significant for the treatment categories were as follows for SD, none; and for URD, treatment category 3 versus treatment category 6 (P = .02).
CSA indicates cyclosporine; MTX, methotrexate; D, donor; R, recipient; M, male; F, female; MDS, myelodysplastic syndrome; and MA, myeloablative.
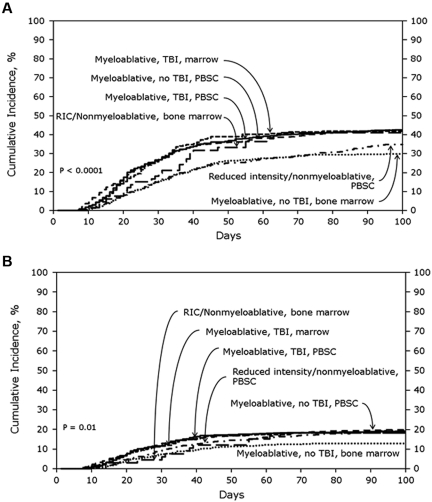
SD cohort
In the SD cohort, the cumulative incidences of AGVHD grades B-D and grades C-D at 100 days were 39% (95% CI, 37%-41%) and 16% (95% CI, 14%-17%), respectively. Figure 1A shows the cumulative incidences of grades B-D AGVHD stratified by transplant treatment category. In multivariate analyses, using MA + TBI + PBSCs as the reference category, lower risks of grades B-D AGVHD were seen with MA + nonTBI + BM (odds ratio [OR], 0.56; 95% CI, 0.44%-0.71%; P < .0001) and RIC + PBSCs (OR, 0.70; 95% CI, 0.56%-0.88%; P = .002). Tacrolimus plus methotrexate-based AGVHD prophylaxis (OR, 0.65; 95% CI, 0.53%-0.80%; P < .0001) also was associated with significantly lower odds of grades B-D AGVHD. No other patient, disease, or treatment factors were associated with grades B-D AGVHD after SD transplantation. Figure 1B shows the cumulative incidences of grades C-D AGVHD stratified by transplant treatment category. MA + nonTBI + BM (OR, 0.61; 95% CI, 0.44%-0.85%; P = .003; Figure 1B) and tacrolimus plus methotrexate-based AGVHD prophylaxis (OR, 0.56; 95% CI, 0.42%-0.75%; P < .0001) also were associated with significantly lower odds of grades C-D AGVHD. Advanced disease status and transplantation from female donors to male recipients were associated with significantly higher odds of grades C-D AGVHD.
Figure 1.
Cumulative incidence of AGVHD grades in SD cohorts. (A) Cumulative incidence of AGVHD grades B-D in SD cohort stratified by treatment category. (B) Cumulative incidence of AGVHD grades C-D in SD cohort stratified by treatment category.
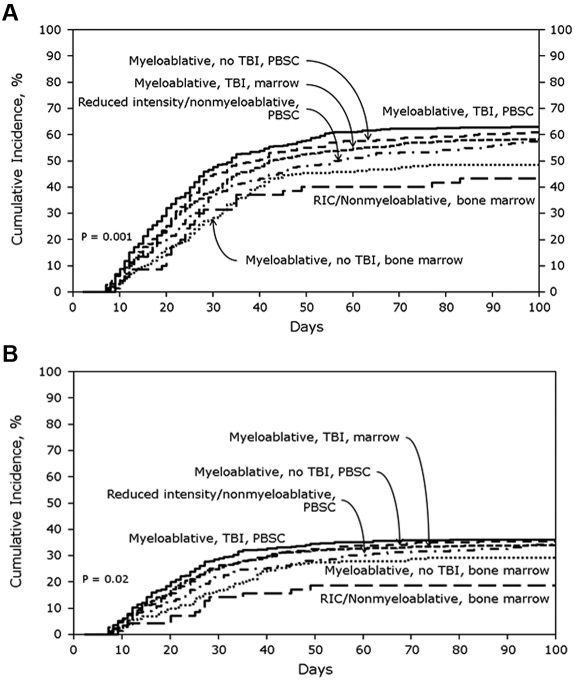
URD cohort
In the URD cohort, the cumulative incidences of AGVHD grades B-D and C-D at 100 days were 59% (95% CI, 57%-61%) and 32% (95% CI, 30%-34%), respectively. Figure 2A shows the cumulative incidences of grades B-D AGVHD stratified by transplant treatment category. In multivariate analysis, MA + TBI + BM (OR, 0.71; 95% CI, 0.56%-0.91%; P = .006), MA + nonTBI + BM (OR, 0.55; 95% CI, 0.40%-0.75%; P = .0001) and RIC + BM (OR, 0.47; 95% CI, 0.29%-0.76%; P = .002) were significantly associated with lower odds of grades B-D AGVHD compared with MA + TBI + PBSCs. Tacrolimus plus methotrexate-based GVHD prophylaxis (OR, 0.79, 95% CI, 0.67%-0.94%; P = .008) also was associated with lower odds of grades B-D AGVHD. Patients with CML had significantly higher odds of grades B-D AGVHD (OR, 1.51; 95% CI, 1.14%-1.99%; P = .004), as did recipients of transplants from a 7/8 HLA-mismatched donor (OR, 1.27; 95% CI, 1.05%-1.54%; P = .02).
Figure 2.
Cumulative incidence of AGVHD grades in URD cohorts. (A) Cumulative incidence of AGVHD grades B-D in URD cohort stratified by treatment category. (B) Cumulative incidence of AGVHD grades C-D in URD cohort stratified by treatment category.
Figure 2B shows the cumulative incidences of grades C-D AGVHD after URD transplantation stratified by transplant treatment category. MA + TBI + BM (OR, 0.79; 95% CI, 0.62%-1.0%; P = .05), MA + nonTBI + BM (OR, 0.67; 95% CI, 0.48%-0.93%; P = .02), and RIC + BM (OR, 0.48; 95% CI, 0.28%-0.83%; P = .009) were all associated with significantly lower odds of grades C-D AGVHD compared with MA + TBI + PBSCs. Type of AGVHD prophylaxis was not significantly associated with grade C-D AGVHD risk. However, patients with CML had significantly higher odds of grades C-D AGVHD (OR, 1.52; 95% CI, 1.14%-2.01%; P = .004) as did recipients of transplants from 7/8 HLA-mismatched donors (OR, 1.36; 95% CI, 1.12%-1.66%; P = .002) or ≤ 6/8 HLA-mismatched donors (OR, 1.55; 95% CI, 1.16%-2.08%; P = .003).
Transplant-related mortality and overall survival risk factors
Multivariate analyses of risk factors associated with transplant-related mortality and overall survival in SD and URD cohorts are outlined in Tables 5 and 6, respectively.
Table 5.
Multivariate analyses of risk factors for transplant-related mortality
| Variable | SD |
URD |
||||||
|---|---|---|---|---|---|---|---|---|
| n | RR | 95% CI | P | n | RR | 95% CI | P | |
| Treatment category | .005 | .0006 | ||||||
| 1: MA + TBI + PBSC | 709 | 1.00 | 534 | 1.00 | ||||
| 2: MA + TBI + BM | 245 | 0.84 | 0.62-1.12 | .23 | 733 | 1.16 | 0.97-1.39 | .11 |
| 3: MA + nonTBI + PBSC | 1017 | 1.16 | 0.96-1.40 | .13 | 350 | .94 | 0.75-1.18 | .61 |
| 4: MA + nonTBI + BM | 492 | 0.82 | 0.65-1.05 | .11 | 265 | 0.90 | 0.70-1.16 | .41 |
| 5: RIC + PBSC | 622 | 0.87 | 0.69-1.09 | .22 | 405 | 0.74 | 0.58-0.94 | .01 |
| 6: RIC + BM | 67 | 0.67 | 0.37-1.20 | .18 | 83 | 0.88 | 0.59-1.33 | .55 |
| GVHD prophylaxis | < .0001 | |||||||
| CSA + MTX ± other | 2645 | 1.00 | ||||||
| Tacrolimus ± MTX ± other | 484 | 0.60 | 0.49-0.75 | < .0001 | ||||
| Other | 23 | 1.43 | 0.71-2.88 | .32 | ||||
| Recipient age, y | < .0001 | .0005 | ||||||
| 20-39 | 1342 | 1.00 | 940 | 1.00 | ||||
| 40-49 | 866 | 1.42 | 1.19-1.70 | < .0001 | 644 | 1.27 | 1.08-1.50 | .004 |
| 50+ | 944 | 2.05 | 1.73-2.44 | < .0001 | 786 | 1.38 | 1.16-1.65 | .0003 |
| Donor age, y | ||||||||
| 18-29 | 710 | 1.00 | ||||||
| ≥ 30 | 1669 | 1.33 | 1.14-1.54 | .0003 | ||||
| KPS | < .0001 | .0006 | ||||||
| < 80 | 256 | 1.00 | 216 | 1.00 | ||||
| 80-100 | 2789 | 0.61 | 0.47-0.77 | < .0001 | 1906 | 0.72 | 0.58-0.91 | .005 |
| Unknown | 107 | 0.82 | 0.54-1.24 | .35 | 248 | 0.54 | 0.39-0.74 | .0001 |
| Disease | < .0001 | |||||||
| ALL | 505 | 1.00- | ||||||
| AML | 1362 | 0.61 | 0.49-0.75 | < .0001 | ||||
| CML | 828 | 0.67 | 0.53-0.84 | .0006 | ||||
| MDS | 457 | 0.62 | 0.46-0.82 | .001 | ||||
| Disease status at transplant | < .0001 | < .0001 | ||||||
| Early | 1750 | 1.00 | 907 | 1.00 | ||||
| Intermediate | 520 | 1.30 | 1.07-1.57 | .007 | 568 | 1.12 | 0.94-1.34 | .21 |
| Advanced | 666 | 1.67 | 1.38-2.02 | < .0001 | 707 | 1.63 | 1.38-1.92 | < .0001 |
| Unknown | 216 | 1.50 | 1.10-2.07 | .01 | 188 | 1.71 | 1.33-2.20 | < .0001 |
| Sex mismatch (D/R) | .0003 | .02 | ||||||
| M/M, M/F, F/F | 2397 | 1.00 | 1947 | 1.00 | ||||
| F/M | 746 | 1.35 | 1.16-1.57 | <0.0001 | 423 | 1.21 | 1.03-1.42 | .02 |
| Missing | 9 | 0.55 | 0.08-3.93 | .55 | ||||
| R/D CMV serostatus | < .0001 | |||||||
| NN | 738 | 1.00 | ||||||
| NP/PN/PP | 1586 | 1.36 | 1.17-1.59 | < .0001 | ||||
| Missing | 46 | 2.26 | 1.35-3.77 | .002 | ||||
| ABO match | .01 | |||||||
| Match | 988 | 1.00 | ||||||
| Mismatch | 1370 | 1.18 | 1.03-1.35 | .02 | ||||
| Missing | 12 | 0.37 | 0.11-1.27 | .11 | ||||
| HLA match | < .0001 | |||||||
| 8/8 matched | 1532 | 1.00 | ||||||
| 7/8 matched | 614 | 1.44 | 1.24-1.68 | < .0001 | ||||
| ≤ 6/8 matched | 224 | 2.07 | 1.69-2.53 | < .0001 | ||||
Pairwise comparisons significant for the treatment categories were as follows for SD, treatment category 2 versus treatment category 3 (P = .03), treatment category 3 versus treatment category 4 (P = .002), treatment category 3 versus treatment category 5 (P = .006); and for URD, treatment category 2 versus treatment category 4 (P = .04), treatment category 2 versus treatment category 5 (P = .0001).
RR indicates relative risk; KPS, Karnofsky performance score; MDS, myelodysplastic syndrome; D, donor; R, recipient; M, male; F, female; N, negative; P, positive; CSA, cyclosporine; MTX, methotrexate; and MA, myeloablative.
Table 6.
Multivariate analyses of risk factors for overall survival
| Variable | SD |
URD |
||||||
|---|---|---|---|---|---|---|---|---|
| n | RR | 95% CI | P | n | RR | 95% CI | P | |
| Treatment category | .02 | |||||||
| 1: MA + TBI + PBSC | 709 | 1.00 | ||||||
| 2: MA + TBI + BM | 245 | 0.79 | 0.63-1.00 | .05 | ||||
| 3: MA + nonTBI + PBSC | 1017 | 1.02 | 0.88-1.18 | .81 | ||||
| 4: MA + nonTBI + BM | 492 | 0.78 | 0.65-0.94 | .01 | ||||
| 5: RIC + PBSC | 622 | 0.97 | 0.83-1.14 | .73 | ||||
| 6: RIC + BM | 67 | 0.85 | 0.57-1.26 | .42 | ||||
| GVHD prophylaxis | .04 | |||||||
| CSA + MTX ± other | 2645 | 1.00 | ||||||
| Tacrolimus ± MTX ± other | 484 | 0.83 | 0.72-0.96 | .01 | ||||
| Other | 23 | 1.07 | 0.6-1.90 | .82 | ||||
| Recipient age, y | < .0001 | .0002 | ||||||
| 20-39 | 1342 | 1.00 | 940 | 1.00 | ||||
| 40-49 | 866 | 1.28 | 1.12-1.46 | .0004 | 644 | 1.24 | 1.09-1.42 | .001 |
| 50+ | 944 | 1.59 | 1.39-1.82 | < .0001 | 786 | 1.29 | 1.13-1.46 | .0001 |
| Donor age, y | ||||||||
| 18-29 | 710 | 1.00 | ||||||
| ≥ 30 | 1669 | 1.19 | 1.07-1.34 | .002 | ||||
| KPS | < .0001 | < .0001 | ||||||
| < 80 | 256 | 1.00 | 216 | 1.00 | ||||
| 80-100 | 2789 | 0.52 | 0.45-0.61 | < .0001 | 1906 | 0.68 | 0.58-0.81 | < .0001 |
| Unknown | 107 | 0.64 | 0.47-0.86 | .004 | 248 | 0.59 | 0.47-0.74 | < .0001 |
| Disease | < .0001 | .02 | ||||||
| ALL | 505 | 1.00 | 446 | 1.00 | ||||
| AML | 1362 | 0.68 | 0.59-0.80 | < .0001 | 1044 | 0.87 | 0.75-1.00 | .05 |
| CML | 828 | 0.58 | 0.48-0.70 | < .0001 | 463 | 0.81 | 0.68-0.97 | .02 |
| MDS | 457 | 0.53 | 0.43-0.66 | < .0001 | 417 | 0.74 | 0.60-0.91 | .005 |
| Disease status at transplant | < .0001 | < .0001 | ||||||
| Early | 1750 | 1.00 | 907 | 1.00 | ||||
| Intermediate | 520 | 1.51 | 1.31-1.75 | < .0001 | 568 | 1.28 | 1.11-1.47 | .0007 |
| Advanced | 666 | 2.23 | 1.95-2.55 | < .0001 | 707 | 2.15 | 1.88-2.46 | < .0001 |
| Unknown | 216 | 1.56 | 1.22-2.00 | .0004 | 188 | 1.74 | 1.35-2.24 | < .0001 |
| Sex mismatch (D/R) | .0008 | |||||||
| M/M, M/F, F/F | 2397 | 1.00 | ||||||
| F/M | 746 | 1.25 | 1.11-1.40 | .0002 | ||||
| Missing | 9 | 0.69 | 0.17-2.78 | .60 | ||||
| R/D CMV serostatus | .003 | |||||||
| NN | 738 | 1.00 | ||||||
| NP/PN/PP | 1586 | 1.19 | 1.06-1.33 | .003 | ||||
| Missing | 46 | 1.57 | 1.03-2.37 | .03 | ||||
| ABO match | .02 | |||||||
| Match | 988 | 1.00 | ||||||
| Mismatch | 1370 | 1.17 | 1.05-1.30 | .004 | ||||
| Missing | 12 | 1.00 | 0.48-2.12 | .99 | ||||
| HLA match | < .0001 | |||||||
| 8/8 matched | 1532 | 1.00 | ||||||
| 7/8 matched | 614 | 1.23 | 1.09-1.38 | .0006 | ||||
| ≤ 6/8 matched | 224 | 1.68 | 1.42-1.98 | < .0001 | ||||
Pairwise comparisons significant for the treatment categories were as follows for SD, treatment category 2 versus treatment category 3 (P = .03), treatment category 3 versus treatment category 4 (P = .003) and treatment category 4 versus treatment category 5 (P = .03); and for URD, none.
RR, relative risk; KPS, Karnofsky performance score; MDS, myelodysplastic syndrome; D, donor; R, recipient; M, male; F, female; N, negative; P, positive; CSA, cyclosporine; MTX, methotrexate; and MA, myeloablative.
SD cohort
The cumulative incidences of transplant-related mortality at 1, 3, and 5 years were 21% (95% CI, 20%-23%), 28% (95% CI, 27%-30%), and 31% (95% CI, 29%-33%), respectively. Treatment strategy was significantly associated with transplant-related mortality (overall P value, .005; Table 5). Transplant-related mortality risk did not differ by treatment strategy when comparing each of the 5 strategies to the reference group. However, when evaluating pairwise comparisons, 3 comparisons were significantly different (MA + nonTBI + PBSCs vs MA + TBI + BM [P = .03], MA + nonTBI + PBSCs vs MA + non TBI + BM [P = .002], and MA + non TBI + PBSCs vs RIC + PBSCs [P = .006]). GVHD prophylaxis with tacrolimus plus methotrexate was associated with a lower risk of transplant-related mortality as was a diagnosis other than ALL and a higher Karnofsky performance score at transplantation. Older recipient but not donor age was associated with a significant increase in the risk of transplant-related mortality as was intermediate or advanced disease status at transplantation and use of a female donor for a male recipient.
The probabilities of overall survival at 1, 3, and 5 years after SD transplantation were 64% (95% CI, 62%-66%), 51% (95% CI, 49%-53%), and 46% (95% CI, 44%-49%), respectively. MA + TBI + BM and MA + nonTBI + BM were associated with higher probabilities of survival compared with MA + TBI + PBSCs as was tacrolimus- plus methotrexate-based GVHD prophylaxis. Diseases other than ALL and Karnofsky performance score ≥ 80 also were associated with better survival. Older recipient age, intermediate or advanced disease status, and transplantation from a female donor to a male recipient were associated with a significantly worse survival.
URD cohort
The cumulative incidences of transplant-related mortality at 1, 3, and 5 years were 31% (95% CI, 30%-33%), 37% (95% CI, 35%-39%), and 40% (95% CI, 37%-42%), respectively. RIC + PBSCs was associated with a lower risk of transplant-related mortality compared with MA + TBI + PBSCs. GVHD prophylaxis was not associated with transplant-related mortality. Older recipient age, older donor age, transplantation from a female donor to a male recipient, transplantation from an HLA-mismatched donor, serologic evidence of prior CMV infection in recipient or donor, presence of ABO mismatch, and advanced disease status at transplant were all associated with a significantly increased risk of transplant-related mortality. Higher Karnofsky performance score was associated with less transplant-related mortality.
Probabilities of overall survival at 1, 3, and 5 years were 51% (95% CI, 49%-53%), 38% (95% CI, 35%-40%), and 33% (95% CI, 31%-35%), respectively. Neither treatment category nor GVHD prophylaxis regimen correlated with survival after URD transplantation. Older recipient age, older donor age, intermediate or advanced disease status, transplantation from an HLA-mismatched or ABO mismatched donor, and CMV seropositivity in donor or recipient were associated with significantly lower survival rates. Diseases other than ALL and higher Karnofsky performance score were associated with better survival.
Table 7 summarizes all the risk factors associated with AGVHD (grades B-D and grades C-D), transplant-related mortality, and overall survival for the SD and URD cohorts.
Table 7.
Summary of risk factors for AGVHD, transplantation-related mortality, and overall mortality for SD and URD cohorts
| Variable* | AGVHD grades B-D |
AGVHD grades C-D |
Transplantation-related mortality |
Overall mortality |
||||
|---|---|---|---|---|---|---|---|---|
| SD | URD | SD | URD | SD | URD | SD | URD | |
| MA + TBI + PBSC | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| MA + TBI + BM | — | ↓ | — | ↓ | — | — | ↓ | — |
| MA + nonTBI + PBSC | — | — | — | — | — | — | ↓ | — |
| MA + nonTBI + BM | ↓ | ↓ | ↓ | ↓ | — | — | — | — |
| RIC + PBSC | ↓ | ↓ | — | — | — | ↓ | — | — |
| RIC + BM | — | ↓ | — | ↓ | — | — | — | — |
| Tacrolimus-based GVHD prophylaxis | ↓ | ↓ | ↓ | — | ↓ | — | ↓ | — |
| Older recipient age | — | — | — | — | ↑ | ↑ | ↑ | ↑ |
| Older donor age | — | — | — | — | — | ↑ | — | ↑ |
| Higher KPS | — | — | — | — | ↓ | ↓ | ↓ | ↓ |
| Disease type other than ALL | — | ↑ | — | ↑ | ↓ | — | ↓ | ↓ |
| Disease status other than early | — | — | ↑ | — | ↑ | ↑ | ↑ | ↑ |
| Female donor for male recipient | — | ↑ | — | ↑ | ↑ | ↑ | — | |
| CMV other than NN | — | — | — | — | — | ↑ | — | ↑ |
| ABO mismatch | — | — | — | — | — | ↑ | — | ↑ |
| HLA mismatch | NA | ↑ | — | ↑ | NA | ↑ | NA | ↑ |
NN indicates negative/negative serostatus; MA, myeloablative; —, no association; Ref, reference; NA, not applicable; ↑, increased risk/odd of association with outcome; and ↓, decreased risk/odd of association with outcome.
See individual tables of multivariate analyses for reference groups.
Discussion
The impact of various components of a transplant (namely, regimen intensity, graft source, use of TBI) has historically been studied as distinct entities. Patients receive a preparative regimen as a package and needs to be analyzed as such. These 3 variables can be modified to optimize transplant outcome. Because of the correlation and potential differential effects of graft types with type of conditioning regimen, we created 6 categories to characterize the treatment strategies used in these patients. Using this approach, we report the impact of risk factors on AGVHD incidence and severity, survival, and transplant-related mortality in a large cohort of patients receiving transplants from SD or URD. Most prior studies were not only restricted to transplants done with MA regimens but also focused on one disease, donor, or graft type. In contrast to prior studies of GVHD prognostic factors, ∼ 25% of the transplants in this cohort were done with RIC. We also looked for interactions between treatment category and all significant variables affecting the incidence of AGVHD, and no significant interactions were noted.
Table 7 summarizes the association of all analyzed risk factors with grades B-D AGVHD, grades C-D AGVHD, transplant-related mortality, and overall mortality for the SD and URD cohorts. Some risk factors are differentially associated with outcomes of SD versus URD transplantation. Although many of these risk factors are nonmodifiable, graft source, use of TBI, regimen intensity, and GVHD prophylaxis are therapeutic choices.
In our study, all patients were diagnosed with acute GVHD before day 100. This study cohort included patients during the time period before National Institutes of Health consensus conference (1999-2005).22 Because late acute GVHD was not captured in this era, some patients diagnosed with acute GVHD after day 100 would have been reported as CGVHD. Hence, to ensure accurate data, we have restricted the data regarding acute GVHD at day 100.
Effect of graft source (PBSCs vs marrow) on HCT outcomes has been evaluated in several studies and has been most consistently associated with chronic GVHD.2,23–33 Increased risk of AGVHD with use of PBSCs as a graft source has been less frequently reported.29,31 Our study did not evaluate the graft source independently, but it did evaluate this risk when this was given as a package associated with type of conditioning and use of TBI. The use of PBSCs + TBI + MA conditioning in HLA-identical sibling donor transplant and PBSCT + MA conditioning in URD transplants was associated with a higher risk of acute GVHD, indicating that consideration toward modification of the “regimen packages” used will probably lead to lower incidence. Further comparative studies testing components of treatment packages in more homogenous cohorts are needed.
Multiple studies have analyzed the impact of regimen intensity on both acute and chronic GVHD.34–36 In this study, consistent with prior reports, the risk of AGVHD was significantly lower with RIC.
We studied the impact of another modifiable factor, namely, GVHD prophylaxis. Calcineurin inhibitor (cyclosporine or tacrolimus) plus methotrexate remains the standard for GVHD prophylaxis after HCT with MA regimens. A randomized phase 3 study in URD BM HCT showed that tacrolimus-based prophylaxis is associated with lower cumulative incidence of grades 2-4 AGVHD than cyclosporine-based prophylaxis, without any impact on survival.37 Similarly, tacrolimus based GVHD prophylaxis was associated with lower incidence of grade 2-4 AGVHD without any difference in disease-free or overall survival in patients with nonadvanced disease after SD BM SCT.38 In the current study, tacrolimus plus methotrexate prophylaxis was associated with lower odds of grades B-D and grades C-D AGVHD in both the SD and the URD cohort. Tacrolimus plus methotrexate was associated with significantly lower transplant-related mortality and higher survival in the SD cohort but not in the URD cohort. The exact mechanism of the beneficial effect of tacrolimus compared with cyclosporine, and the differential impact on survival in SD and URD group is not clear. It is known that tacrolimus, allows for proliferation of human T-regulatory cells, in contrast to cyclosporine, which inhibits T-regulatory cells.39,40 It can be hypothesized that tacrolimus based GVHD prophylaxis facilitates tolerance.
We performed comprehensive analyses of other nonmodifiable factors that have previously been identified to influence AGVHD. A prior study identified female donor–male recipient, parity of female donors, and older recipient age as significant risk factors for AGVHD in recipients of SD transplants for severe aplastic anemia and leukemia.4 In our study, recipient and donor age and sex mismatch between donor and recipient were not significantly correlated with grades B-D AGVHD. However, transplants from female donors to male recipients were associated with higher odds of grades C-D AGVHD, worse survival, and increased transplant-related mortality in the SD and URD cohorts. Older recipient age also correlated with transplant-related mortality and overall mortality after both SD and URD transplants. An influence of donor age on these endpoints was seen only in URD transplants, similar to a previous study.41 The influence of donor age on outcomes of SD transplants is difficult to study because recipient and donor age are highly correlated. Although Karnofsky performance score (≥ 80) was not associated with AGVHD incidence, contrary to a previous IBMTR study, higher Karnofsky performance score (≥ 80) was associated with lower transplant-related mortality and better survival.29
CML was associated with a higher risk of grades B-D and C-D AGVHD than other diseases only after URD transplants. This contrasts with a prior IBMTR SD study of patients receiving non-RIC regimens where CML diagnosis correlated with a higher odds ratio of grade 2-4 and 3-4 AGVHD.29 Diagnosis of ALL was associated with worse survival after both URD and SD transplants. Advanced disease state at transplantation correlated with worse survival and increased transplant-related mortality after SD and URD transplants. There was no significant correlation between CMV serologic status and AGVHD, survival, or transplant-related mortality in the SD cohort. In the URD cohort, CMV seropositivity in donor or recipient correlated with worse survival and more transplant-related mortality, but it had no effect on AGVHD incidence. This is consistent with some prior studies,42,43 but contrasts with a study that analyzed a cohort of URD transplants from 1987 to 1999. In that study, CMV serologic status did not correlate with overall survival or transplant-related mortality.41 Similarly, ABO-mismatch correlated with worse survival and increased transplant-related mortality in the URD cohort in our analysis.
Although differences in outcomes between URD and SD HCT are decreasing, some studies show that outcomes continue to be inferior after URD transplantation especially with greater degrees of HLA mismatch.44 In another study of patients with standard-risk hematologic malignancy undergoing HCT with MA conditioning using TBI, outcome after 10/10 HLA allele–matched URD HCT was similar to outcome after HLA-identical SD HCT.43 In our study, we did not compare SD to URD transplantation. Within the URD cohort, HLA mismatch (< 8/8) was associated with higher odds of grades B-D AGVHD, grades C-D AGVHD, transplant-related mortality, and all cause mortality.
In summary, we report a comprehensive analysis of both modifiable and nonmodifiable risk factors for AGVHD, survival and transplant-related mortality after both SD and URD transplants. Our study population reflects current practices: only 20% to 25% of transplants were for CML, and there were a substantial proportion of RIC transplants. Several of our findings in this study differ from older studies and may reflect changes in outcomes and prognostic factors from introduction of newer treatment strategies. In fact, the predominant determinants of AGVHD risk in this study were the intensity of conditioning, type of graft and GVHD prophylaxis regimen. We were able to study these variables as a package and show the differential impact of these treatment groups. These results can help guide a more rational selection of treatment strategies to optimize outcomes after HCT.
Acknowledgments
The CIBMTR is supported by Public Health Service grant/cooperative agreement U24-CA76518 from the National Cancer Institute (NCI); the National Heart, Lung, and Blood Institute (NHLBI); and the National Institute of Allergy and Infectious Diseases (NIAID); grant/cooperative agreement 5U01HL069294 from NHLBI and NCI; contract HHSH234200637015C with Health Resources and Services Administration; grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos Inc; Amgen Inc; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children's Leukemia Research Association; Fresenius-Biotech North America Inc; Gamida Cell Teva Joint Venture Ltd; Genentech Inc; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Optum Healthcare Solutions Inc; Otsuka America Pharmaceutical Inc; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix Inc; Swedish Orphan Biovitrum; THERAKOS Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.J., M.A., N.C., P.L.M., and T.H. designed the study and wrote the manuscript; M.A., M.D.H., and M.-J.Z. analyzed data and provided statistical input; and M.E.D.F., C.S.C., A.U.-I., S.Z.P., J.H.A., B.J.B., C.B., J.-Y.C., M.C., R.P.G., V.G., S.J.L., M.L., D.J.W., and M.M.H. reviewed and provided critical input for the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madan Jagasia, Hematology and Stem Cell Transplantation Section, Vanderbilt University Medical Center, 3973 The Vanderbilt Clinic, Nashville, TN 37232-5505; e-mail: madan.jagasia@vanderbilt.edu.
References
- 1.Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29(2):79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 2.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood. 2000;95(12):3702–3709. [PubMed] [Google Scholar]
- 3.Eisner MD, August CS. Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone Marrow Transplant. 1995;15(5):663–668. [PubMed] [Google Scholar]
- 4.Gale RP, Bortin MM, van Bekkum DW, et al. Risk factors for acute graft-versus-host disease. Br J Haematol. 1987;67(4):397–406. doi: 10.1111/j.1365-2141.1987.tb06160.x. [DOI] [PubMed] [Google Scholar]
- 5.Hägglund H, Bostrom L, Remberger M, Ljungman P, Nilsson B, Ringden O. Risk factors for acute graft-versus-host disease in 291 consecutive HLA-identical bone marrow transplant recipients. Bone Marrow Transplant. 1995;16(6):747–753. [PubMed] [Google Scholar]
- 6.Jacobsen N, Badsberg JH, Lonnqvist B, et al. Graft-versus-leukaemia activity associated with CMV-seropositive donor, post-transplant CMV infection, young donor age and chronic graft-versus-host disease in bone marrow allograft recipients. The Nordic Bone Marrow Transplantation Group. Bone Marrow Transplant. 1990;5(6):413–418. [PubMed] [Google Scholar]
- 7.Michallet M, Perrin MC, Belhabri A, et al. Impact of cyclosporine and methylprednisolone dose used for prophylaxis and therapy of graft-versus-host disease on survival and relapse after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;23(2):145–150. doi: 10.1038/sj.bmt.1701529. [DOI] [PubMed] [Google Scholar]
- 8.Nash RA, Pepe MS, Storb R, et al. Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992;80(7):1838–1845. [PubMed] [Google Scholar]
- 9.Oh H, Loberiza FR, Jr, Zhang MJ, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105(4):1408–1416. doi: 10.1182/blood-2004-06-2385. [DOI] [PubMed] [Google Scholar]
- 10.Przepiorka D, Shapiro S, Schwinghammer TL, et al. Cyclosporine and methylprednisolone after allogeneic marrow transplantation: association between low cyclosporine concentration and risk of acute graft-versus-host disease. Bone Marrow Transplant. 1991;7(6):461–465. [PubMed] [Google Scholar]
- 11.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308(6):302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- 12.Wojnar J, Giebel S, Krawczyk-Kulis M, et al. Acute graft-versus-host disease. The incidence and risk factors. Ann Transplant. 2006;11(1):16–23. [PubMed] [Google Scholar]
- 13.Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ. Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N Engl J Med. 1988;319(2):65–70. doi: 10.1056/NEJM198807143190201. [DOI] [PubMed] [Google Scholar]
- 14.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana-Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106(4):1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26(24):4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 20.Klein JP, Andersen PK. Regression modeling of competing risks data based on pseudovalues of the cumulative incidence function. Biometrics. 2005;61(1):223–229. doi: 10.1111/j.0006-341X.2005.031209.x. [DOI] [PubMed] [Google Scholar]
- 21.Andersen P, Klein J, Rosthoj S. Generalized linear models for correlated pseudo observations, with application to multi-state models [abstract]. Biometrika. 2003;90:15–27. [Google Scholar]
- 22.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344(3):175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 24.Vigorito AC, Azevedo WM, Marques JF, et al. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone Marrow Transplant. 1998;22(12):1145–1151. doi: 10.1038/sj.bmt.1701510. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz N, Bacigalupo A, Hasenclever D, et al. Allogeneic bone marrow transplantation vs filgrastim-mobilised peripheral blood progenitor cell transplantation in patients with early leukaemia: first results of a randomised multicentre trial of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21(10):995–1003. doi: 10.1038/sj.bmt.1701234. [DOI] [PubMed] [Google Scholar]
- 26.Blaise D, Kuentz M, Fortanier C, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 2000;18(3):537–546. doi: 10.1200/JCO.2000.18.3.537. [DOI] [PubMed] [Google Scholar]
- 27.Powles R, Mehta J, Kulkarni S, et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet. 2000;355(9211):1231–1237. doi: 10.1016/S0140-6736(00)02090-0. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz N, Eapen M, Horowitz MM, et al. Long-term outcome of patients given transplants of mobilized blood or bone marrow: a report from the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. Blood. 2006;108(13):4288–4290. doi: 10.1182/blood-2006-05-024042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn T, McCarthy PL, Jr, Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26(35):5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrezenmeier H, Passweg JR, Marsh JC, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110(4):1397–1400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13(12):1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23(22):5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remberger M, Ringden O, Blau IW, et al. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98(6):1739–1745. doi: 10.1182/blood.v98.6.1739. [DOI] [PubMed] [Google Scholar]
- 34.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10(3):178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102(2):756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 36.Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104(4):961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 37.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 38.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 39.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107(3):1018–1023. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 40.Kogina K, Shoda H, Yamaguchi Y, et al. Tacrolimus differentially regulates the proliferation of conventional and regulatory CD4(+) T cells. Mol Cells. 2009;28(2):125–130. doi: 10.1007/s10059-009-0114-z. [DOI] [PubMed] [Google Scholar]
- 41.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 42.Meijer E, Dekker AW, Rozenberg-Arska M, Weersink AJ, Verdonck LF. Influence of cytomegalovirus seropositivity on outcome after T cell-depleted bone marrow transplantation: contrasting results between recipients of grafts from related and unrelated donors. Clin Infect Dis. 2002;35(6):703–712. doi: 10.1086/342332. [DOI] [PubMed] [Google Scholar]
- 43.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24(36):5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 44.Arora M, Weisdorf DJ, Spellman SR, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27(10):1644–1652. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]