Abstract
Background: Abnormal fatty acid metabolism is an important feature in the mechanisms of insulin resistance and β-cell dysfunction. Carnitine palmitoyltransferase-1a (CPT-1a, liver isoform) has a pivotal role in the regulation of mitochondrial fatty acid oxidation. We investigated the role of CPT-1a in the development of impaired glucose tolerance using a mouse model for CPT-1a deficiency when challenged by either a high-carbohydrate (HCD) or a high-fat diet (HFD) for a total duration of up to 46 weeks.
Methods: Insulin sensitivity and glucose tolerance were assessed in heterozygous CPT-1a-deficient (CPT-1a+/−) male mice after being fed either a HCD or a HFD for durations of 28 weeks and 46 weeks. Both glucose and insulin tolerance tests were used to investigate β-cell function and insulin sensitivity. Differences in islet insulin content and hepatic steatosis were evaluated by morphological analysis.
Results: CPT-1a+/− mice were more insulin-sensitive than CPT-1a+/+ mice when fed either HCD or HFD. The increased insulin sensitivity was associated with an increased expression of Cpt-1b (muscle isoform) in liver, as well as increased microvesicular hepatic steatosis compared with CPT-1a+/+ mice. CPT-1a+/− mice were more glucose tolerant than CPT-1a+/+ mice when fed the HCD, but there was no significant difference when fed HFD. Moreover, CPT-1a+/− mice fed HFD or HCD had fewer and smaller pancreatic islets than CPT-1a+/+ mice.
Conclusions: CPT-1a deficiency preserved insulin sensitivity when challenged by long-term feeding of either diet. Furthermore, CPT-1a-deficient mice had distinct phenotypes dependent on the diet fed demonstrating that both diet and genetics collectively have a role in the development of impaired glucose tolerance.
Keywords: carnitine palmitoyltransterase-1a deficiency, mouse model, glucose tolerance, fatty acid oxidation disorders
Introduction
Impaired glucose tolerance is considered to be the earliest identifiable metabolic abnormality in the pathogenesis of Type 2 diabetes.1 Glucose tolerance is thought to be a function of both β-cell responsiveness to increased blood glucose concentration and peripheral tissue responsiveness to insulin.2, 3 Many metabolic phenotypes are influenced by a combination of genotype and environment, particularly the diet.
Aberrant mitochondrial fatty acid oxidation (FAO) may have a pivotal role in the development of impaired glucose tolerance.4, 5, 6 Carnitine palmitoyltransferase-1 (CPT-1) functions as the rate-limiting step of FAO. This enzyme is responsible for converting long-chain acyl-CoAs into long-chain acylcarnitines, a process required for translocation across the mitochondrial membrane into the mitochondrial matrix where β-oxidation occurs. A build-up of fatty acid metabolites, including long-chain fatty acyl-CoAs and diacylglycerols in the cytosol of skeletal muscle and other tissues has been implicated in fatty acid-induced insulin resistance.4 Moreover, studies in rodents have shown that chronic exposure to fatty acids alters β-cell function.7, 8, 9, 10, 11, 12 We have previously demonstrated a range of metabolic phenotypes in mice with inherited deficiencies of other mitochondrial FAO enzymes.13, 14
Our aim was to systematically investigate factors thought to be involved in the development of insulin resistance and Type 2 diabetes by challenging both CPT-1a+/+ or CPT-1a+/− mice with a high-carbohydrate diet (HCD) or a high-fat diet (HFD) diet and evaluate them at an age more similar to human middle age. The rationale behind the HCD challenge was to induce conditions that would promote high levels of malonyl-CoA, a potent CPT-1 inhibitor.15 Our aim in using the HFD was to challenge this impaired CPT-1 system with excess substrate to promote build-up of fatty acids. We hypothesized that both the HCD-induced increase in the CPT-1a inhibitor, malonyl-CoA, as well as the HFD-induced substrate overload, would unmask a phenotype in a mouse model with a genetically compromised state of FAO. Furthermore, we expected to demonstrate that the combination of chronic environmental stresses of diet and the genetic limitation of FAO by decreased CPT-1a activity would lead to one or all of the metabolic abnormalities associated with the development of Type 2 diabetes.
Materials and methods
Animals
Male mice with a heterozygous deficiency in CPT-1a (CPT-1a+/− mice) (129S6/SvEvTac-coisogenic background) were previously described.16 Homozygous CPT-1a-deficient mice are not viable.16 Two separate, age-matched (CPT-1a+/+ and CPT-1a+/−) groups of mice were maintained on either a HFD or HCD for either 28 or 46 weeks. All mice were housed in a pathogen-free facility (12-h light/12-h dark cycle). All experiments were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Diets, body weight and body composition analyses
Mice were fed either a HFD (Teklad diet-TD 97268) containing 43% fat (calorie %) using beef tallow as the major fat source, or a HCD (Teklad diet-TD 97267) that was 14% fat, with starch making up the bulk of calories. Both diets were isocaloric and contained equal amounts of sucrose. Mice (17- to 19-day-olds) were weaned onto their respective diets and fed ad libitum. Body weights were determined weekly using a standard balance. Body composition was determined at the end of the study period using dual-energy X-ray absorptometry, as described previously.17, 18
Serum metabolite assays
Mice were fasted for 20–22 h before collection of serum. Total non-esterified fatty acids were measured by an enzymatic, colorimetric method (‘NEFA-C' reagents, Wako Diagnostics, Richmond, VA, USA). Triglyceride concentrations were measured in 10 μl sera with the Ektachem DT II system (Johnson and Johnson Clinical Diagnostics, Rochester, NY, USA).
Glucose tolerance tests
The oral glucose tolerance test outcomes of interest included basal (fasted) serum glucose concentration, glucose and insulin concentrations at individual time points following glucose ingestion. From these data, the rate of decline of blood glucose was determined.
At 20 h before performing oral glucose tolerance test, animals were food-deprived with water overnight. After the mice were weighed, a baseline blood sample was obtained by tail bleed, and additional blood samples were obtained at 15, 30, 60 and 120 min following oral glucose administration at 2.0 mg per g body weight.
Insulinogenic index calculations were made to assess differences in first-phase insulin secretion. The formula used is ((insulin) 30 min−(insulin) 0 min)/((glucose) 30 min−(glucose) 0 min).19 This represents the change in insulin level at 30 min after glucose administration divided by the change in glucose level 30 min after the oral dose of glucose.
Insulin tolerance tests
Mice were deprived of food for 4 h, baseline blood samples were collected and recombinant human insulin (Humulin R, 100 U ml−1 stock) was injected intraperitoneally (0.1 ml per 10 g body weight). Before injection, insulin was diluted in saline to a concentration of 0.075 U ml−1 (1:1330). Additional blood samples were obtained at 10, 20 and 30 min, following insulin injection. Outcomes of interest included the final (30 min) glucose concentration, and the rate of decline of glucose, which was calculated from the slope of the descending points after insulin injection. The rate of decline of blood glucose was determined to be a reflection of the degree of insulin sensitivity.
Blood glucose concentrations were measured with a blood glucose meter (Accu-chek; Roche Diagnostics, Indianapolis, IN, USA), and serum insulin levels were measured with a rat sensitive immunoassay kit (LINCO Research, St Charles, MO, USA) with a 1:10 dilution of mouse serum used as per the manufacturer's instructions.
RNA extraction and real-time quantitative RT-PCR analysis
Total RNA was extracted from heart, liver and skeletal muscle (soleus and gastrocnemius) using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA). First-strand cDNA was generated from 5 μg of RNA in a 20 μl volume reaction containing both oligo-(dT) and random hexamers (Invitrogen First-Strand Synthesis Kit). Real-time quantitative RT-PCR (QRT-PCR) was carried out in a 20 μl reaction volume containing 10 μl supermix UDG (Invitrogen Life Technologies Inc.), 2 μl cDNA, 0.2 μ LUX Cpt-1a and 1b, primer forward, 0.2 μ LUX Cpt-1a and 1b, primer reverse (FAM-labeled, Invitrogen Life Technologies), 0.1 μ 18S RNA primer forward, 0.1 μ 18S RNA primer reverse (JOE-labeled, Invitrogen, Life Technologies). Cycling conditions included incubation at 50 °C for 2 min, a 2 min 95° C denaturing step, followed by 44 cycles of 95 °C denaturation, 45 s annealing time at 58 °C and a 30 s extension step at 72 °C. A melting curve analysis was completed following amplification from 55–95 °C to assure product identification and homogeneity.
Each sample was normalized to the housekeeping gene actin to compensate for any differences in starting quantity of total RNA. The relative quantification for Cpt-1b and Cpt-1a mRNA in CPT-1a+/− compared with CPT-1a+/+ was calculated using the ΔΔCt=2 exp−(ΔCt sample (CPT-1a+/−)−ΔCt reference (CPT-1a+/+))20 where Ct is the cycle number when threshold fluorescence is significantly above background. The fold-change is then established by comparing CPT-1a+/− sample (relative quantification=calculated ΔΔCt) against CPT-1a+/+ reference (relative quantification=1).
Histology and morphometric analyses of liver and pancreas
Liver and pancreas were collected from mice fasted for 24 h who had been fed a HCD or HFD for 46 weeks, processed routinely for paraffin sectioning and stained with hematoxylin and eosin. Hepatocellular fatty change was assessed by subjective scoring. The evaluator examined the slides without knowledge of genotype or diet classification. Microvesicular and macrovesicular fatty changes, each were scored 0 for normal/absent, 1 for mild, 2 for moderate and 3 for severe, using increments of 0.5 for intermediate degrees. Estimated distribution of each type of change was scored 1 for up to 1/4 of the section, 2 for 1/4 to 1/2, 3 for 1/2 to 3/4 or 4 for 3/4 to all of the section. Severity scores for microvesicular and macrovesicular fatty changes were determined by multiplying the scores of severity and distribution and were summed for a total severity score. Size and numbers of islets in immunohistochemically stained sections of pancreas were determined by histomorphometry. Images of each section were made at × 1 with a Nikon Super CoolScan 5000ED scanner (Melville, NY, USA) at 4000 pixels per inch. For each section, the total area of pancreas and the area of each immunostained islet were determined with Image-Pro Plus 5.1 image analysis software (Media Cybernetics, Silver Spring, MD, USA), and average islet area in mm2, number of islets per mm2 pancreas, and the ratio of total islet area to pancreas area were calculated.
Immunohistochemistry
Paraffin sections of pancreas were stained for insulin according to a modification of a previously reported method.21 Briefly, endogenous peroxides were blocked with 0.3% hydrogen peroxide in phosphate-buffered saline for 30 min. Background staining was decreased by blocking with 1% bovine serum albumin in phosphate-buffered saline for 1 h. Anti-pig insulin (Sigma-Aldrich, St Louis, MO, USA) developed in guinea pig, was used as the primary antibody at 1:9000 dilution and incubated for 2 h in a humidity chamber at room temperature. Anti-guinea pig IgG (whole molecule) peroxidase conjugate (Sigma-Aldrich) at 1:1000 dilution was used for the secondary antibody and incubated for 30 min at room temperature in a humidity chamber. True blue peroxidase substrate (Kierkegaard and Perry Laboratories, Gaithersburg, MD, USA) was applied for 10 min and washed in water for 3 min. Sections were counterstained with Nuclear Fast Red (Vector Labs, Burlingame, CA, USA) for 10 min followed with a 3 min wash in water, then air-dried and coverslipped with Permount (Fisher Chemicals, Fairlawn, NJ, USA).
Statistics
All data were expressed as mean values with s.e.m. Student's t-tests and two-way analysis of variance were used to analyze effects of genotype and diet. Liver macrovesicular and microvesicular fat scores were analyzed using the Kruskal–Wallis test, the rank-sum test with Tukey's probability correction for multiple comparisons, and analysis of variance of ranks. P-values < 0.05 were considered significant.
Results
Clinical markers for insulin resistance, impaired glucose tolerance and Type 2 diabetes
We evaluated clinical markers of obesity, insulin resistance and Type 2 diabetes such as body weight, body fat and liver fatty change in CPT-1a+/− mice as compared with the CPT-1a+/+ mice fed either HCD or HFD.
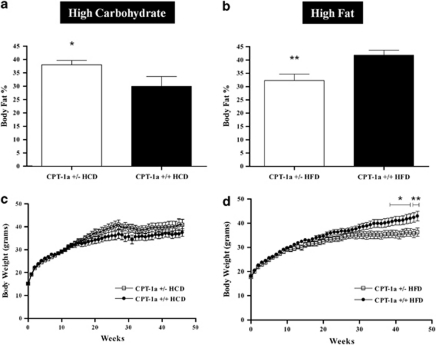
Using dual-energy X-ray absorptometry analysis, body fat was significantly greater in CPT-1a+/− mice fed HCD for 46 weeks than for CPT-1a+/+ mice (P=0.0241) (Figure 1a), although there were no significant differences in body weight of CPT-1a+/− mice (Figure 1c) as compared with CPT-1a+/+ mice.
Figure 1.
Effect of diet and CPT-1a deficiency on body weight and body-fat percentage over 46 weeks. (a) Body fat percentages in CPT-1a+/− mice (n=17) fed a HCD as compared with CPT-1a+/+ mice (n=16). (b) Body fat percentages in CPT-1a+/− mice fed a HFD (n=16) as compared with CPT-1a+/+ mice (n=19). (c) Body weights for CPT-1a+/− (n=19) versus CPT1a+/+ (n=17) mice fed HCD for 46 weeks. (d) Body weights for CPT-1a+/− (n=18) versus CPT1a+/+ (n=20) mice fed HFD for 46 weeks reached significant differences during the final 8 weeks (*P<0.05, **P<0.01).
In contrast to the findings on the HCD, body fat was significantly greater in CPT-1a+/+ mice fed HFD for 46 weeks compared with CPT-1a+/− mutants (P=0.00019) (Figure 1b). The body weights of the oldest CPT-1a+/− mice were significantly lower than CPT-1a+/+ mice eating HFD (Figure 1d).
Fasting serum glucose, insulin, triglycerides and free fatty acids were evaluated in mice challenged with either a HCD or HFD for either 28 or 46 weeks (Table 1). CPT-1a+/− mice fed HFD had significantly higher serum glucose values at 28 weeks; however, there were no other significant differences in fasting glucose values as compared with CPT-1a+/+ mice fed either diet. Fasting insulin levels were significantly lower (28 weeks P=0.01; 46 weeks P=0.03) in HFD-fed CPT-1a+/− mice as compared with CPT-1a+/+ mice, suggesting a state of increased insulin sensitivity. In contrast, there were no significant differences in fasting insulin values among either group fed the HCD. There were no significant differences in fasting serum triglycerides or free fatty acids between CPT-1a+/+ and +/− mice fed either diet.
Table 1. Serum metabolites and insulin concentrations.
| Fasted CPT-1a+/− | Fed CPT-1a+/− | Fasted CPT-1a+/+ | Fed CPT-1a+/+ | ||||
|---|---|---|---|---|---|---|---|
| Serum glucose mmol l−1 | |||||||
| HFD, 28 weeks | n=8 | 3.66±0.54* | n=10 | 3.00±0.61* | P=0.03 | ||
| HFD, 46 weeks | n=9 | 3.27±1.3 | n=9 | 3.11±0.83 | |||
| HCD, 28 weeks | n=9 | 3.22±0.88 | n=10 | 3.83±2.72 | |||
| HCD, 46 weeks | n=9 | 2.83±0.67 | n=10 | 2.78±0.61 | |||
| Serum insulin pmol l−1 | |||||||
| HFD, 28 weeks | n=13 | 36.1±10* | n=11 | 63.7±33* | P=0.01 | ||
| HFD, 46 weeks | n=9 | 32.7±13* | n=8 | 63.7±36* | P=0.03 | ||
| HCD, 28 weeks | n=10 | 27.5±14 | n=10 | 75.7±75 | |||
| HCD, 46 weeks | n=9 | 27.5±1.7 | n=9 | 36.1±15 | |||
| Triglycerides mmol l−1 | |||||||
| SRD, 24–32 weeks | n=7 | 1.59±0.73 | 1.19±0.30* | n=7 | 1.56±0.41 | 1.90±0.55* | P=0.01 |
| HFD, 46 weeks | n=10 | 1.65±0.32 | n=13 | 1.58±0.43 | |||
| HCD, 46 weeks | n=12 | 1.76±0.63 | n=11 | 1.63±0.25 | |||
| Serum free fatty acids mmol l−1 | |||||||
| SRD, 24–32 weeks | n=7 | 1.6±0.38 | 0.90±0.2* | n=7 | 1.6±0.30 | 1.3±0.20* | P=0.018 |
| HFD, 46 weeks | n=10 | 0.82±0.23 | n=13 | 0.98±0.24 | |||
| HCD, 46 weeks | n=12 | 1.2±0.040 | n=11 | 0.91±0.24 | |||
Abbreviations: CPT, carnitine palmitoyltransferase; HCD, high-carbohydrate diet; HFD, high-fat diet; SRD, standard rodent diet.
Fasting glucose and insulin for CPT-1a+/− and CPT-1a+/+ fed HFD or HCD for 28 weeks or 46 weeks. Fasting and fed (SRD only) free fatty acids and triglycerides for mice fed SRD for 24–32 weeks, HFD and HCD for 28 or 46 weeks.
Glucose tolerance
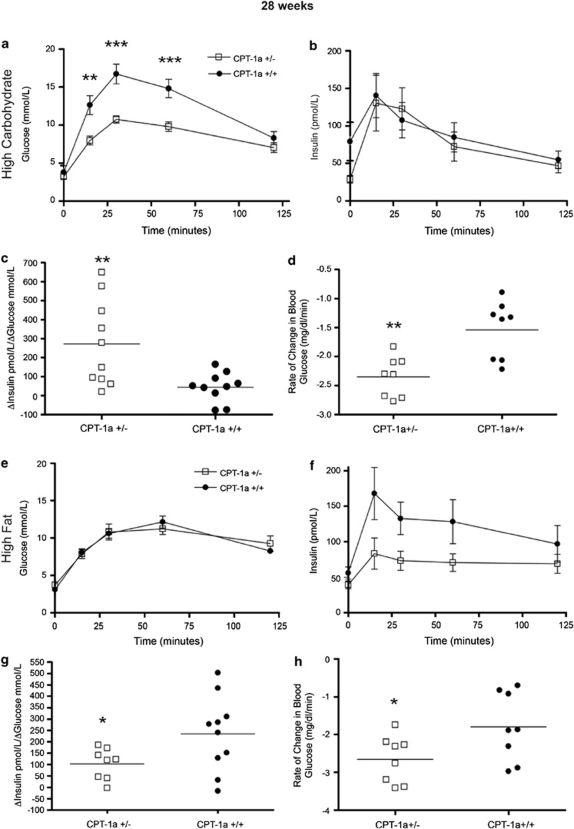
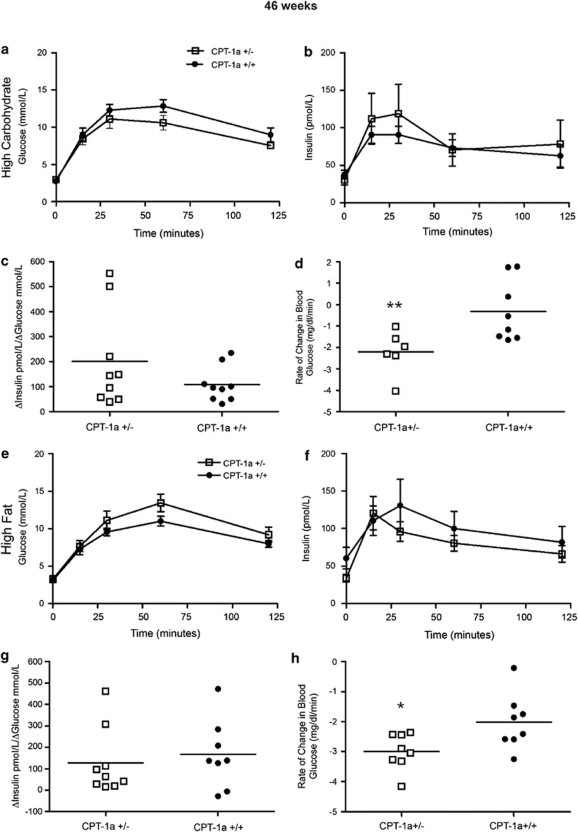
The 28-weeks-HCD group of CPT-1a+/− mice were more glucose tolerant than CPT-1a+/+ mice, as measured by oral glucose tolerance test (28 weeks, HCD); however, these marked differences were lost by 46 weeks (Figures 2a and 3a). Insulin levels, measured throughout oral glucose tolerance tests, were not significantly different between genotypes (Figures 2b and 3b). However, in the 28-weeks groups, CPT-1a+/− mice had significantly higher average first-phase insulin responses to a glucose load, based on insulinogenic index values (Figure 2c), indicating heightened β-cell responsiveness (28-weeks-HCD P=0.0038). In addition, in the 46-weeks diet-duration groups there was a trend toward higher average first-phase insulin responses to a glucose load (Figure 3c), however, this difference did not reach significance.
Figure 2.
Effects of diet on glucose and insulin tolerance in CPT-1a+/− mice after 28 weeks. (a–c) Glucose levels (**P<0.01, ***P<0.001), insulin levels and insulinogenic indexes (**P=0.0038) throughout glucose tolerance test in CPT-1a+/− (n=10) versus CPT-1a+/+ (n=10) mice fed HCD. (d) Insulin tolerance test. Slopes for the rate of decline of glucose (**P<0.002) in response to an intraperitoneal injection of insulin for CPT-1a+/− (n=8) and CPT-1a+/+ (n=8) mice fed HCD. (e–g) Glucose levels, insulin levels and insulinogenic indexes (*P=0.026) throughout glucose tolerance test in CPT-1a+/− (n=8) versus CPT-1a+/+ (n=11) mice fed HFD. (h) Insulin tolerance test. Slopes for the rate of decline of glucose (*P<0.0439) in response to an intraperitoneal injection of insulin for CPT-1a+/− (n=8) and CPT-1a+/+ (n=8) mice fed HFD.
Figure 3.
Effects of diet on glucose and insulin tolerance in CPT-1a+/− mice after 46 weeks. (a–c) Glucose levels, insulin levels and insulinogenic indexes throughout glucose tolerance test in CPT-1a+/− (n=11) versus CPT-1a+/+ (n=10) mice fed HCD. (d) Insulin tolerance test. Slopes for the rate of decline of glucose (**P=0.0088) in response to an intraperitoneal injection of insulin for CPT-1a+/− (n=6) and CPT-1a+/+ (n=9) mice fed HCD. (e–g) Glucose levels, insulin levels and insulinogenic indexes throughout glucose tolerance test in male CPT-1a+/− (n=9) versus CPT-1a+/+ (n=9) mice fed HFD. (h) Insulin tolerance test. Slopes for the rate of decline of glucose (*P=0.0129) in response to an intraperitoneal injection of insulin for CPT-1a+/− (n=8) and CPT-1a+/+ (n=8) mice fed HFD.
In the 28- and 46-weeks-HFD group, there was no significant difference in glucose tolerance between CPT-1a+/− and CPT-1a+/+ mice (Figures 2e and 3e). In addition, first-phase insulin secretion, expressed by the insulinogenic index, was significantly lower in CPT-1a+/− mice (P=0.0256) fed the HFD for 28 weeks (Figure 2g). However, first-phase insulin secretion in the longer HFD duration was not significantly different (Figure 3f) between CPT-1a+/− and CPT-1a+/+ mice.
In summary, when fed the HCD for 28 weeks, the CPT-1a+/− mice were more glucose tolerant. Moreover, these mice showed higher estimated first-phase insulin secretion, which may partially explain their improved glucose tolerance. In contrast, CPT-1a+/− mice fed the HFD were no less glucose tolerant than CPT-1a+/+ mice.
Insulin tolerance
HCD-fed CPT-1a+/− mice were significantly more insulin-sensitive (28-weeks-HCD P<0.002, 46-weeks-HCD P<0.0088) (Figures 2d and 3d) than were CPT-1a+/+ controls as was found by their steeper rate of decline of blood glucose levels in response to insulin. In fact, the standard protocol had to be modified because the CPT-1a+/− mice became hypoglycemic. In addition, HFD-fed CPT-1a+/− mice were significantly more insulin-sensitive (28-weeks-HFD P=0.0439 and 46-weeks-HFD P=0.0129) (Figures 2h and 3h) than CPT-1a+/+ controls as shown by their steeper rate of decline (negative slope) of blood glucose levels in response to insulin.
Morphometric analysis of β-cells within islets
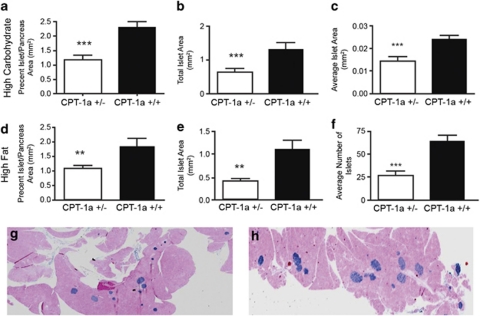
CPT-1a+/− mice fed HCD for 46 weeks had lower percent islet area/pancreas area (P⩽0.001), lower total islet area (P⩽0.001) and lower average islet area (P⩽0.001) (Figures 4a–c and g). CPT-1a+/− mice fed HFD for 46 weeks, also had lower percent islet area/pancreas area (P=0.006), lower total islet area (P<0.005) and lower total islet number (P=0.001) (Figures 4d–f and h). In summary, CPT-1a+/− mice fed either of the diet had significantly fewer insulin positive cells. The only differences between the two diets were that the CPT-1a+/− mice fed HCD had lower average islet area, indicating that their islets were smaller overall, whereas HFD-fed CPT-1a+/− mice had a lower number of islets overall.
Figure 4.
Islet morphology in mice fed a HCD versus a HFD. Differences in (a) % islet to pancreas area (***P⩽0.001), (b) total islet area (***P⩽0.001) and mean islet area (c) (***P⩽0.001) in CPT-1a+/− (n=7) versus CPT-1a+/+ (n=9) mice fed a HCD. There were no significant differences in average islet number (data not shown). Differences in (d) % islet to pancreas area (**P=0.006), (e) total islet area (**P<0.005) and (f) average islet number (***P=0.001) in mice fed a HFD. There were no significant differences in mean islet area (data not shown). CPT-1a+/− mice (n=10) and CPT-1a+/+ mice (n=7). Representative images of mouse islet number and size in CPT 1a+/− (g) and CPT-1a+/+ (h) mouse pancreas sections from mice fed the HCD.
Hepatic steatosis
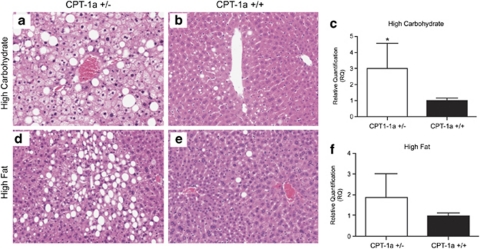
CPT-1a+/− mice fed either of the challenge diets demonstrated significantly higher total (macro plus microvesicular combined) fatty change (P=0.01) scores than CPT-1a+/+ controls (Table 2 and Figures 5a and c). Microvesicular fat scores in mice fed either of the diets were significantly higher than macrovesicular fat scores, independent of genotype (P=0.003) (Table 2). Microvesicular fat scores were significantly higher in CPT-1a+/− mice when fed either diet as compared with CPT-1a+/+ mice (P=0.03) (Figures 5a–d, Table 2). Microvesicular liver steatosis is common in β-oxidation disorders.
Table 2. Liver steatosis in mice fed high-carbohydrate diet or high fat diet for 46 weeks.
| CPT-1a+/+ (HCD) | CPT-1a+/− (HCD) | CPT-1a+/+ (HFD) | CPT-1a+/− (HFD) | |
|---|---|---|---|---|
| Macrovesicular fat | 3.5±3.9 | 4.7±3.3 | 2±2.2 | 1.9±1.5 |
| Microvesicular fat | 2.4±4.2 | 10.1±10* | 2.3±3.6 | 6.5±7.9* |
| Total | 5.9±7.6 | 14.8±9.6* | 4.3±4.9 | 8.5±7.3* |
Abbreviations: CPT, carnitine palmitoyltransferase; HCD, high-carbohydrate diet; HFD, high-fat diet.
CPT-1a+/− (n=22), CPT-1a+/+ (n=21) mice fed either diet demonstrated significantly higher total (macro plus microvesicular combined) fatty change (P=0.01) as compared with CPT-1a+/+ controls. Microvesicular fat was significantly higher in CPT-1a+/− mice when fed either diet as compared with CPT-1a+/+ mice (P=0.03). Microvesicular fat was significantly greater than macrovesicular fat in mice fed the HCD, independent of genotype (P=0.003).
Figure 5.
Liver steatosis and Cpt-1b expression in CPT-1a+/− and CPT-1a+/+ mice. Representative images of micro- and macrovesicular fat in liver from (a) CPT 1a+/− mouse and (b) CPT-1a+/+ mice fed HCD. Representative images of micro- and macro vesicular fat in liver from (d) CPT 1a+/− and (e) CPT-1a+/+ mice fed HFD. Quantitative real-time PCR analysis of Cpt-1b expression in liver as measured by QRT-PCR in (c) HFC-fed CPT-1a+/− mice as compared with controls (P<0.05) and (f) HFD-fed mice.
Analysis of steady state mRNA levels by northern blot
Northern blot results showed a striking Cpt-1b mRNA expression in liver of CPT-1a+/− mice as compared with CPT-1a+/+ mice fed a HCD (data not shown). Cpt-1a expression was lower in CPT-1a+/− mice as was expected because of the targeted mutation (data not shown). To further evaluate Cpt-1a and b mRNA expression, we used the more quantitative and sensitive method of quantitative real-time PCR (QRT-PCR).
QRT-PCR analysis of steady-state mRNA levels
CPT-1a+/− mice fed HCD showed significantly higher Cpt-1b expression in liver as measured by QRT-PCR than CPT-1a+/+ controls (P<0.05). (Figure 5c). HFD-fed CPT-1a+/− mice showed a trend toward higher Cpt-1b expression in liver as measured by QRT-PCR than CPT-1a+/+ controls, although the differences were not significant (Figure 5f).
In conclusion of the results section, Table 3 summarizes and provides a contrast of the overall results reported here.
Table 3. Overall summary of results contrasting the CPT-1a+/− mice and their CPT-1a+/+ controls fed either the high carbohydrate or high fat diets.
| High-carbohydrate diet CPT1a+/− versus CPT-1a+/+ mice | High-fat diet CPT1a+/− versus CPT-1a+/+ mice | |
|---|---|---|
| Glucose tolerance | More glucose tolerant | No significant difference |
| Insulin secretion | Higher first-phase insulin secretion | Lower first phase at 28 weeks No significant difference at 46 weeks |
| Insulin tolerance | More insulin-sensitive | More insulin-sensitive |
| Hepatic steatosis | Increased microvesicular fat | Increased microvesicular fat |
| Aberrant hepatic CPT-1b expression | Increased | No significant difference, trend is elevated |
| Pancreatic islet morphology | Smaller islets | Fewer islets |
| Body weight | No significant difference | Slightly lower |
| Body fat | Higher | Lower |
| Fasting insulin | No significant difference | Lower |
Abbreviation: CPT, carnitine palmitoyltransferase
Discussion
We hypothesized that the long-term effects of a high-calorie diet coupled with a deficiency in mitochondrial FAO (CPT-1a deficiency) would lead to both insulin resistance and β-cell dysfunction, pivotal features in development of Type 2 diabetes. This initial hypothesis was based on previous studies using CPT-1 inhibitors,5, 6, 12 results from earlier studies in our group showing the effects of another FAO deficiency, long-chain acyl-CoA dehydrogenase,13 and other studies associating excess fatty acids with development of insulin resistance and β-cell dysfunction.4, 7, 10
We metabolically challenged the CPT-1a+/− mice with diets either high in fat (HFD) or high in carbohydrates (HCD) to promote for conditions favorable to the generation of malonyl-CoA, a potent inhibitor of CPT-1 activity. Post transcriptionally, CPT-1b (muscle isoform) is regulated solely by changes in malonyl-CoA levels, whereas CPT-1a, the hepatic isoform (CPT-1a) is regulated by both concentrations of and changes in sensitivity to malonyl-CoA.15, 22 Thus, CPT-1a is unique in that it responds to physiological states such as starvation, insulin deficiency and hypo- and hyperthyroidism by changing its sensitivity to malonyl CoA.22
Evidence supporting our initial hypothesis included a study showing that rats fed a high-fat or low-fat diet with etomoxir (a CPT-1 inhibitor) were significantly less insulin-sensitive than untreated rats.5 However, our most striking and consistent finding was that the CPT-1a+/− mice were more insulin-sensitive than CPT-1a+/+ mice, when fed either a HCD or HFD, as shown by both insulin tolerance and fasting insulin levels. These results are contrary to our initial hypothesis. We considered the possibility that our results could be explained by the increased Cpt-1b expression in the liver in CPT-1a+/− mice. This mechanism may be similar to that described for overexpression of Cpt-1b in muscle, resulting in improved insulin sensitivity in mice challenged with a HFD.23 We speculate that CPT-1b expressed in liver is out of its normal malonyl-CoA regulatory milieu and would be constitutively more active than the usual CPT-1a. This is likely because, CPT-1a is more sensitive to hormonal changes, which increase or decrease its sensitivity to malonyl-CoA in liver, unlike Cpt1b, which is regulated solely by changes in the concentrations of malonyl-CoA. The increased insulin sensitivity in the CPT-1 +/− mice, especially given the HFD challenge, was more similar to our previous results in the very long-chain acyl-CoA dehydrogenase-deficient mouse model.14
Despite high Cpt-1b expression, we also found, however, that microvesicular fatty change was significantly greater in CPT-1a+/− livers as compared with diet-induced liver steatosis in CPT-1a+/+ mice. However, CPT-1a+/− mice having greater amounts of microvesicular liver fat may not be inconsistent with them being more insulin-sensitive. The appearance of microvesicular fat is likely a consequence of the FAO deficiency24, 25, 26 that is unrelated to their heightened insulin sensitivity.27, 28, 29 This is further supported by a previous study using CPT-1 inhibitor treatment in mice, showing severe microvesicular fatty liver in the absence of insulin resistance.27 Furthermore, studies using mice with acyl-CoA:diacylglycerol acyltransferase deficiency also demonstrated a dissociation between hepatic steatosis and insulin resistance.30
It may also be important to note that differences in glucose and insulin tolerance, as well as fasting insulin levels between the two genotypes and diets may also be explained by different contributions of either hepatic or peripheral sensitivity. As described previously by others,3, 22, 31 in the pre-diabetic state, insulin resistance could occur separately and/or with different degrees in liver versus skeletal muscles, and realized as either impaired fasting glucose or as impaired glucose tolerance.
We measured size of islet by immunostaining β-cells for insulin. We expected to see either hyperplasia or hypertrophy as a result of excessive secretion from a predicted fatty acid build-up, or alternatively, a β-cell loss as a result of long-term excess fatty acid exposure. We found that HFD-fed CPT-1a+/− mice had lower percent islet to pancreas area, indicating that they did not have as many insulin-containing β-cells as did the CPT-1a+/+ mice. The lower number of β-cells could have been a result of apoptosis or reduction in the production of insulin granules within the β-cells.32, 33 We did not find degenerative lesions of islets in any of the groups. A fatty acid-induced decreased β-cell function would explain the decreased glucose tolerance found in HFD-fed CPT-1a+/− mice. In support of these conclusions, a recent study demonstrating that overexpression of SREBP-1c in β-cells, which resulted in β-cell triglyceride build-up, caused the onset of impaired glucose tolerance, which these investigators attributed partially to the reduction in number and size of islets.33
CPT-1a+/− mice fed the HCD were also found to have lower percent islet area/pancreas area, lower total islet area and lower average islet area. In contrast to CPT-1a+/− mice fed the HFD, these mice were more glucose tolerant and had higher first-phase insulin secretion, indicating that the lower islet area either did not have an effect on their glucose tolerance because of their heightened insulin sensitivity, or that the lower islet area may have been a result of their heightened insulin sensitivity. We interpret the global reduction in islet mass as a response to the overall improved insulin sensitivity as reflected in the CPT-1a+/− mice fed either diet.
In conclusion, CPT-1a+/− mice were found to be more insulin-sensitive when fed either a HCD or a HFD, which we speculate may be explained by increased CPT-1b activity in liver. Findings of lower fasting insulin levels in CPT-1a+/− mice fed HFD for either 28 or 46 weeks further supported the finding of increased insulin sensitivity from insulin tolerance testing. CPT-1a+/− mice were more glucose tolerant when fed the HCD but less glucose tolerant when fed the HFD. We conclude that this difference was due to an impaired insulin response to glucose as a result of both the HFD and the FAO deficiency. This was further supported by the results indicating that CPT-1a+/− mice fed HFD had lower percent islet/pancreas area, lower total islet area, lower number of islets and lower average number of islets per mm2 of pancreas. In contrast, the CPT-1a+/− mice fed HCD were also found to have lower percent islet/pancreas area, lower total islet area and lower average number of islets per mm2 of pancreas; however, they were still more glucose tolerant, and this may be attributed to their greater insulin sensitivity.
Thus, the interaction of the CPT-1a deficiency with either HCD or HFD includes increased insulin sensitivity when fed either diet along with enhanced β-cell responsiveness in those fed the HCD, whereas, the HFD appeared to be detrimental to β-cell response. There was a decrease in islet cell mass in mutants fed either diet that corresponded with their increased insulin sensitivity. These findings indicate that it is the interaction between genotype and diet that determines metabolic phenotype rather than diet or genotype alone. Therefore, full consideration of the genetic determinants of disease risk should be considered in the context of the individual genotype along with environment and in this case the diet.
Acknowledgments
We are grateful to the late Jane Hosmer and Alvin Almodovar for providing excellent assistance. The project was supported by the following grants: RO1RR02599 (PAW), UL1RR025777 (BAG: UAB-Center of Clinical and Translational Science), P30DK56336 (TRN, BAG, PAW: Nutrition and Obesity Research Center) and P60DK079626 (BAG: UAB-Diabetes Research and Training Center); all are components of the National Institutes of Health, and contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources, the National Institute for Diabetes, Digestive and Kidney Disease or the NIH.
The authors declare no conflict of interest.
Footnotes
Author contributions
LRN: managed and performed a majority of the experiments, analyzed data, lead writer of the manuscript. LT: performed experiments, analyzed data, reviewed the manuscript. DAH: performed experiments, analyzed data, reviewed the manuscript. TRS: performed morphological studies and analyzed data, wrote parts of the manuscript. BAG: performed analyte analyses, reviewed the manuscript. TRN: performed body composition analyses, reviewed the manuscript. PAW: oversaw the entire project, reviewed all data, major role in writing manuscript and obtained primary funding for project.
References
- Costa A, Conget I, Gomis R. Impaired glucose tolerance: is there a case for pharmacologic intervention. Treat Endocrinol. 2002;1:205–210. doi: 10.2165/00024677-200201040-00001. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin action and insulin secretion predict the development of impaired glucose tolerance. Diabetologia. 1996;39:1201–1207. doi: 10.1007/BF02658507. [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001;50:123–130. doi: 10.2337/diabetes.50.1.123. [DOI] [PubMed] [Google Scholar]
- Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest. 1998;101:1094–1101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins RL, Chester MW, Daniels MB, McGarry JD, Stein DT. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes. 1998;47:1613–1618. doi: 10.2337/diabetes.47.10.1613. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Yaney G, McGarry JD, Weir G. Fatty acids and beta-cell function. Diabetologia. 1997;40:B21–6. doi: 10.1007/BF03168182. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–138. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtihet M, Welsh N, Berggren PO, Cook GA, Sjoholm A. Glibenclamide inhibits islet carnitine palmitoyltransferase 1 activity, leading to PKC-dependent insulin exocytosis. Am J Physiol Endocrinol Metab. 2003;285:E438–E446. doi: 10.1152/ajpendo.00057.2003. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, et al. Mitochondrial dysfunction due to long-chain acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA. 2007;104:17075–17080. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Christianson J, Liu Z-X, Tian L, Cheol Soo C, Neschen S, et al. Resistance to high-fat diet-induced obesity and insulin resistance in mice with very long-chain acyl-CoA dehydrogenase deficiency. Cell Metab. 2010;11:1–10. doi: 10.1016/j.cmet.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaoui M, Cohen I, Esnous C, Lenoir V, Sournac M, Girard J, et al. Modulation of the hepatic malonyl-CoA-carnitine palmitoyltransferase 1A partnership creates a metabolic switch allowing oxidation of de novo fatty acids. Biochem J. 2009;420:429–438. doi: 10.1042/BJ20081932. [DOI] [PubMed] [Google Scholar]
- Nyman LR, Cox KB, Hoppel CL, Kerner J, Barnoski BL, Hamm DA, et al. Homozygous carnitine palmitoyltransferase 1a (liver isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2005;86:179–187. doi: 10.1016/j.ymgme.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- Goetzman ES, Tian L, Nagy TR, Gower BA, Schoeb TR, Elgavish A, et al. HIV protease inhibitor ritonavir induces lipoatrophy in male mice. AIDS Res Hum Retroviruses. 2003;19:1141–1150. doi: 10.1089/088922203771881248. [DOI] [PubMed] [Google Scholar]
- Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Stoehr JP, Nadler ST, Schueler KL, Rabaglia ME, Yandell BS, Metz SA, et al. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes. 2000;49:1946–1954. doi: 10.2337/diabetes.49.11.1946. [DOI] [PubMed] [Google Scholar]
- Bonnefont JP, Demaugre F, Prip-Buus C, Saudubray JM, Brivet M, Abadi N, et al. Carnitine palmitoyltransferase deficiencies. Mol Genet Metab. 1999;68:424–440. doi: 10.1006/mgme.1999.2938. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, et al. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–208. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]
- Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- Fraser JL, Antonioli DA, Chopra S, Wang HH. Prevalence and nonspecificity of microvesicular fatty change in the liver. Mod Pathol. 1995;8:65–70. [PubMed] [Google Scholar]
- Grefhorst A, Hoekstra J, Derks TG, Ouwens DM, Baller JF, Havinga R, et al. Acute hepatic steatosis in mice by blocking beta-oxidation does not reduce insulin sensitivity of very-low-density lipoprotein production. Am J Physiol Gastrointest Liver Physiol. 2005;289:G592–G598. doi: 10.1152/ajpgi.00063.2005. [DOI] [PubMed] [Google Scholar]
- Deschamps D, Fisch C, Fromenty B, Berson A, Degott C, Pessayre D. Inhibition by salicylic acid of the activation and thus oxidation of long chain fatty acids. Possible role in the development of Reye's syndrome. J Pharmacol Exp Ther. 1991;259:894–904. [PubMed] [Google Scholar]
- Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Faerch K, Brons C, Alibegovic AC, Vaag A. The disposition index: adjustment for peripheral vs hepatic insulin sensitivity. J Physiol. 2010;588:759–764. doi: 10.1113/jphysiol.2009.184028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollheimer LC, Kemptner DM, Kagerbauer SM, Kestler TM, Wrede CE, Buettner R. Intracellular depletion of insulin: a comparative study with palmitate, oleate and elaidate in INS-1 cells. Eur J Endocrinol. 2003;148:481–486. doi: 10.1530/eje.0.1480481. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Motomura K, Kato T, Yoshikawa T, Nakagawa Y, Yahagi N, et al. Transgenic mice overexpressing nuclear SREBP-1c in pancreatic beta-cells. Diabetes. 2005;54:492–499. doi: 10.2337/diabetes.54.2.492. [DOI] [PubMed] [Google Scholar]