Abstract
Metastasis is a multistage process that requires cancer cells to escape from the primary tumour, survive in the circulation, seed at distant sites and grow. Each of these processes involves rate-limiting steps that are influenced by non-malignant cells of the tumour microenvironment. Many of these cells are derived from the bone marrow, particularly the myeloid lineage, and are recruited by cancer cells to enhance their survival, growth, invasion and dissemination. This Review describes experimental data demonstrating the role of the microenvironment in metastasis, identifies areas for future research and suggests possible new therapeutic avenues.
A variety of stromal cells in the surrounding environment are recruited to tumours, and these not only enhance growth of the primary cancer but also facilitate its metastatic dissemination to distant organs. Cancer cells in an aggressive primary mass are adept at exploiting that particular tissue microenvironment; however, once they leave these favourable surroundings, they must possess traits that will allow them to survive in new environments. In order for a metastasis to occur, the intravasated cancer cell must survive in the circulation, arrive at the target organ (seeding), extravasate into the parenchyma and show persistent growth1. Each of these stages is inefficient and some are rate limiting1,2. For example, senescence or apoptosis of cancer cells at the stage of entry into the metastatic site prevents the spread of the majority of circulating cells2–4. Seeding can occur to multiple organs, but metastatic tumours may grow in only one or a few5. There is also increasing evidence that in some cases cancer cells can lie dormant for many years, and that seeding may occur several years before diagnosis of the primary tumour6–10. In another phenomenon, termed angiogenic dormancy, there is a balance of proliferation and apoptosis that results in micrometastases that do not progress further11,12. The microenvironment clearly suppresses the malignancy of these potentially metastatic cells10, and their re-activation to form a clinically relevant metastasis probably occurs through perturbations in the microenvironment. Nevertheless, despite this evidence for early seeding and dormancy, tumour size and grade are the main predictors of metastasis, and this has been reinforced in recent studies in mouse models13 and by gene expression analysis that linked large tumour size with metastasis-enhancing gene signatures14. It has been hypothesized that this may be due to metastatic re- seeding to primary tumours15. If this is the case, nothing is currently known about the underlying mechanisms. Successful metastatic outgrowth thus depends on the cumulative ability of cancer cells to appropriate distinct microenvironments at each step in the metastatic cascade: the primary tumour, systemic circulation and the final metastatic site. In this Review we discuss instructive, and in some cases dominant, roles for the microenvironment during the process of metastasis, with a particular focus on contributions from bone marrow-derived cells (BMDCs).
Tumour–stroma interactions at the primary site
Tissues contain a plethora of cells that work in concert to effect normal physiology. These cells have positional identity so that their location is defined and their number constrained. Cancers have lost these constraints through mutations in oncogenes and tumour suppressor genes. However, these tumour cells have not lost all their interactions with surrounding non-malignant cells or with the extracellular architecture. Indeed, these interactions are not static: they evolve along with the tumour, in particular through the recruitment of BMDCs. In this section we discuss evidence that the microenvironment can exert inhibitory effects on even aggressive malignant cells. However, during their progression, tumours circumvent these inhibitory signals and instead exploit these surrounding cells to their own ends in processes that result in inappropriate growth, invasion and ultimately metastasis.
Normal tissue homeostasis
Homeostasis in normal tissues requires a tightly controlled balance of cell proliferation and death, which is achieved and maintained through intercellular communication. An important regulator of normal cell behaviour and tissue homeostasis is the surrounding extracellular matrix (ECM). The ECM has many functions, including acting as a physical scaffold, facilitating interactions between different cell types, and providing survival and differentiation signals. Maintaining organ homeostasis can prevent neoplastic transformation in normal tissues by ensuring stable tissue structure, mediated by tight junction proteins and cell adhesion molecules such as β1 integrins and epithelial (E)-cadherin16,17. Insight into the dominance of the microenvironment over epithelial cell behaviour came from some of the earliest pioneering studies in this field. Mintz and colleagues showed that the microenvironment of a mouse blastocyst not only suppressed the tumorigenicity of teratocarcinoma cells, but that those cells were stably reprogrammed, resulting in normal chimeric mice18. Subsequent studies indicated that the embryonic microenvironment is potent in its ability to reprogramme various cancer cells, including metastatic cells, to a less aggressive phenotype19–23. Other groups have demonstrated a particularly important role for stromal fibroblasts in modulating the malignant progression of transformed epithelial cells. For example, co-culture experiments showed that normal fibroblasts prevented the growth of initiated prostatic epithelial cells24, and could even reverse the malignant phenotype of neoplastic epithelial cells25. During early tumour development, however, the protective constraints of the microenvironment are overridden by conditions such as chronic inflammation, and the local tissue microenvironment shifts to a growth-promoting state.
Recruitment of stromal cells to developing tumours
It is now well established that primary tumours are composed of a multitude of stromal cell types in addition to cancerous cells26. Among the stromal cell types that have been implicated in tumour promotion are endothelial cells, which comprise the blood and lymphatic circulatory systems, pericytes, fibroblasts and various BMDCs, including macrophages, neutrophils, mast cells, myeloid cell-derived suppressor cells (MDSCs) and mesenchymal stem cells (MSCs) (FIG. 1; TABLE 1). In recent years, the crucial contribution of BMDCs to malignant progression has become increasingly evident, and will be the central focus of this Review.
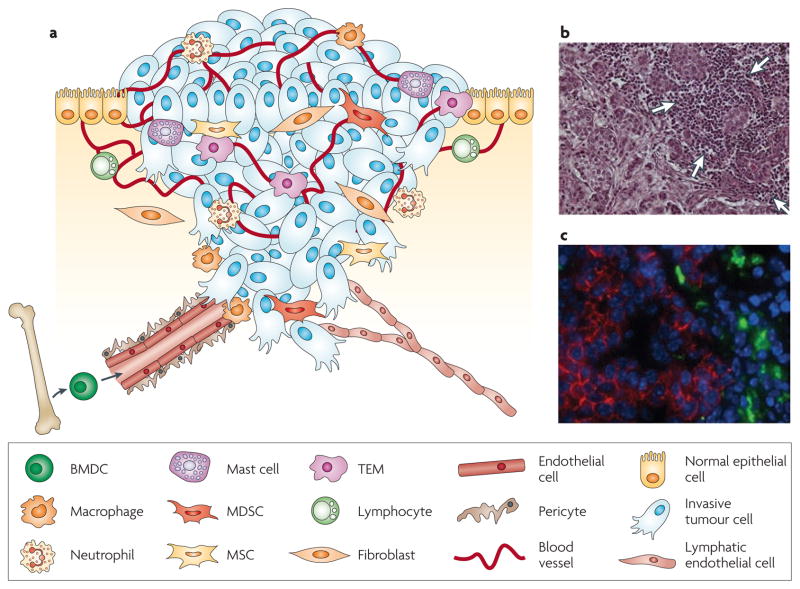
Figure 1. The primary tumour microenvironment.
a | Cancer cells in primary tumours are surrounded by a complex microenvironment comprising numerous cells including endothelial cells of the blood and lymphatic circulation, stromal fibroblasts and a variety of bone marrow-derived cells (BMDCs) including macrophages, myeloid-derived suppressor cells (MDSCs), TIE2-expressing monocytes (TEMs) and mesenchymal stem cells (MSCs). b | Invasive human breast cancer stained with haematoxylin and eosin, in which a prominent infiltration of leukocytes (indicated by white arrows) is evident at the invasive margin. c | Macrophages at the invasive edge of pancreatic islet cancers express cathepsin B (green), which is associated with loss of epithelial cadherin (red) on the neighbouring cancer cells. Cell nuclei are visualized by DAPI (blue). Part c reproduced, with permission, from REF. 151 © (2006) Cold Spring Harbor Laboratory Press.
Table 1.
Markers and functions of stromal cells in the tumour microenvironment
| Cell type | Cell surface markers* | Functions in the tumor microenvironment | refs |
|---|---|---|---|
| TAM | CD11b+CD14+CD31−CD34− CD45+CD68+CD117−CD133−CD146− CD204+CD206+CCR2+CSF1R+MHC II+ VEGFR1+VEGFR2− (m/h) F4/80+ (m) CD23+CD163+CXCR4+ (h) |
‘Classically activated’ M1 macrophages contribute to tumour rejection through type 1 cytokine production and antigen presentation, whereas ‘alternatively activated’ M2 macrophages enhance angiogenesis and remodelling through type 2 cytokine production TAMs are proposed to share M2 characteristics; their presence in tumours is thought to be tumour promoting, and is associated with poor prognosis |
31, 32, 38, 68, 174, 175 |
| MDSC | CD11b+CD14+/− MHC I+MHC IIlow (m/h) GR1+ CD11b+ (m): can be further subdivided into LY6G+ LY6Clow CD11b+ CD11c+/−CD33+CD34+CD86− (h) |
MDSCs are increased in almost all patients and animal models with cancer. Because of the variation in MDSC gene expression between different tumour microenvironments, it has been challenging to identify a unique set of markers. This has emphasized the importance of showing their ability to suppress T cells as a defining trait | 30, 38, 69 |
| MSC | CD14−CD29+CD31−CD34−CD44+CD45− CD51+CD71+CD73+CD90+CD105+CD133− CD166+CD271+ (m/h) |
MSCs infiltrate various human cancers and have been shown to increase cancer cell dissemination in animal models. MSCs are also immunosuppressive, in part through inhibition of T-cell proliferation | 71, 72, 176, 177 |
| TEM | CD11b+CD14+CD31lowCD34−CD45+ CD117−CD133−TIE2+VEGFR2− (m/h) F4/80+GR1lowSCA1− (m) CD11c+CD13+CD16+CD33+CD62L−CD146− CCR2−CCR5+CSF1R+ (h) |
Monocytes that express the angiopoietin receptor TIE2. TEMs have been implicated in angiogenesis from studies in animal models and have been detected in human tumours and at low frequency in the peripheral blood of cancer patients | 34, 38, 178 |
| Neutrophil | CD11b+CD14lowCD31+CD66B+CXCR2+ (m/h) GR1+VEGFR1+CXCR1− (m) CD15+CXCR1+ (h) |
Levels of neutrophils are increased in patients with colon, gastric and lung cancer. Neutrophil infiltration is further enhanced in the invasive areas of CRCs, and increased numbers are associated with poor prognosis in bronchioalveolar carcinoma. Neutrophils have been implicated in enhancing angiogenesis and metastasis in animal models | 38, 179 |
| Mast cell | CD43+CD117+CD123+CD153+(m/h) CD11b+CD16+CD34+SCA1+ (m) CCR1+CCR3+CCR4+CCR5+CXCR1+CXCR2+ CXCR4+ (h) |
Mast cells are important in generating and maintaining innate and adaptive immune responses. Increased mast cell numbers have been reported in a wide range of tumours and, in some cases, this correlates with poor prognosis. Mast cells have been implicated in angiogenic switching in several animal models | 38, 180 |
| Endothelial cell | CD31+CD34+CD105+CD106+CD144+ (m/h) | Endothelial cells comprise the blood vasculature of the tumour, and increased microvessel density is frequently associated with poor prognosis in a wide range of human cancers | 179, 181 |
| EPC | CD13+CD31lowCD45−CD105+ CD133+CD117+CD146+CD144+ (VE-cadherin: E4G10+ Ab)VEGFR2+ (m/h) |
EPCs contribute to blood vessel formation in various animal models and some patient studies, although the functional importance of EPCs in tumour angiogenesis remains controversial | 123, 124, 182–184 |
| Pericyte | Desmin+/−NG2+/−αSMA+/−PDGFRβ+/−‡ | Pericytes are mural cells that provide physical support and stabilization, as well as pro-survival factors, to blood vessels. Pericytes may also be crucial in limiting metastasis by maintaining vessel integrity | 185, 186 |
| Fibroblast | Vimentin+desmin+αSMA+/−FSP1+FAP+ (m/h) | CAFs are a large component of the stroma, and at later stages of tumour progression are generally tumour promoting, as shown in animal models and studies using patient samples | 24, 187, 188 |
| Platelet | CD41+CD42a-dCD51+CD110+ (m/h) | Platelets are activated and circulate at higher levels in cancer patients, particularly those with advanced malignancy. | 79, 179 |
| CD4+ T cell | CD3+CD4+CD45+ (m/h) | T helper type 1 cells aid CD8+ T cells in tumour rejection, whereas T helper type 2 cells polarize immunity away from an anti-tumour response, and regulatory T cells block CD8+ cell activation and NK cell killing | 29, 30, 179 |
| CD8+ T cells | CD3+CD8+CD45+ (m/h) | Also known as CTLs, these are the effector cells of the adaptive immune system and specifically recognize and destroy cancer cells through perforin- and granzyme-mediated apoptosis | 29, 179 |
| B cell | CD3−CD19+CD20+CD45+ (m/h) CD45RA+ B220+ (m) |
B lymphocytes are important mediators of humoral immunity, but have been shown to promote malignancy in an animal model of squamous cell carcinogenesis | 29, 179, 189 |
| NK cell | CD11b+CD27+§ CD3−CD16+/−CD56+ || CD3−CD335+ NKp46+ (m/h) |
NK cells are effector lymphocytes of the innate immune system that are cytotoxic to cancer cells through the perforin– granzyme pathway NK cells contribute to immunosurveillance of cancer, and low NK-like cytotoxicity in the peripheral blood is associated with increased risk of cancer | 177, 190–192 |
Markers that are shared by mouse and human are indicated (m/h), as are those that are unique to mouse (m) or human (h).
Stromal cells can be identified by the multiple cell surface markers listed, but not necessarily requiring all of the markers to be used simultaneously, as this list compiles markers from multiple studies.
Three subsets in mouse tumours, based on relative levels of expression of these genes. Platelet-derived growth factor-β (PDGFRβ) is considered a marker of pericyte progenitors, rather than mature pericytes.
Three subsets in mouse, based on relative levels of CD11b and CD27 expression.
Two subsets in human, based on relative levels of CD16 and CD56 expression. αSMA, α-smooth muscle actin; Ab, antibody; CAF, cancer-associated fibroblast; CCR, C–C chemokine receptor; CRC, colorectal cancer; CSF1, colony-stimulating factor 1; CTL, CD8+ cytotoxic T cell; CXCR, C-X-C chemokine receptor; EPC, endothelial progenitor cell; FAP, fibroblast-activated protein; FSP, fibroblast-specific protein; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; MSC, mesenchymal stem cell; NK, natural killer; SCA, stem cell antigen; TAM, tumour-associated macrophage; TEM, TIE2-expressing monocyte; VE-cadherin, vascular endothelial cadherin ; VEGFR, vascular endothelial growth factor receptor.
Chronic inflammation and BMDC recruitment
The presence of leukocytes in tumours in the past was generally thought to be a consequence of a failed attempt at cancer cell destruction. However, tumours are not only effective in escaping from immune-mediated rejection, they also modify certain inflammatory cell types to render them tumour promoting rather than tumour suppressive. Furthermore, many of these infiltrating immune cells may not be associated with the detection of cancer cell antigens, but may alternatively be associated with the tissue disruption that is caused by inflammatory agents or be a response to the growth of the tumour as it is established. This is particularly evident in cancers associated with chronic inflammation, where the initial inflammatory response is not resolved, and systemic conditions that promote continued recruitment of bone marrow-derived inflammatory cells to the tumour mass are established instead27. Thus a chronic inflammatory state can quickly set up a cascade of events in which the tumour-promoting effects of immune cells are progressively amplified, often as a by-product of their normal wound-repairing or developmental roles28. However, a complication is that there is no clear association between the presence of any individual adaptive or innate immune cell type and a defined outcome in terms of malignancy or prognosis across a range of different tumour microenvironments. Even within individual cell types, there are opposing functions; for example, CD4+ T cells, macrophages, and natural killer (NK) T cells have either tumour-suppressive or tumour-promoting properties depending on the tissue context and cellular stimuli29,30.
Classification of these immune cells into different cellular states or subtypes has helped provide some insight into their disparate functions (TABLE 1). For example, type 1 CD4+ T cells (TH1) aid CD8+ T cells in tumour rejection, whereas type 2 CD4+ T cells (TH2) and CD4+ T regulatory cells block the activation of CD8+ T cells30. Like CD4+ T cells, macrophages can either impede or promote tumour progression, depending on their functional state (TABLE 1). Several recent studies have found correlations between particular immune cell infiltrates in primary tumours and patient prognosis. For example, infiltration of CD8+ T cells and mature dendritic cells is associated with a favourable prognosis in colorectal cancer and head and neck cancer (reviewed in REF. 31). An extensive macrophage infiltration, however, correlates with poor patient prognosis in >80% of cancers analysed, including breast, thyroid and bladder cancer in which there is a positive association with metastasis (reviewed in REFS 31,32).
The induction of angiogenesis is a crucial early stage in the development and growth of most solid tumours, and is also necessary for haematogenous dissemination of cancer cells. Bone marrow-derived myeloid cells, including macrophages33, TIE2-expressing monocytes (TEMs)34, neutrophils35 and mast cells36,37 have all been shown to contribute to tumour angiogenesis through their production of growth factors, cytokines and pro-teases such as vascular endothelial growth factor A (VEGFA), PROK2 (also known as BV8) and matrix metalloproteinases (MMPs), respectively (reviewed in REF. 38) (BOX 1). Several of these cell types have also been implicated in the later stages of tumour progression, namely invasion and metastasis, and the ability of these BMDCs to enhance tumour malignancy directly and indirectly is discussed below.
Box 1. Proteases in invasion and metastasis.
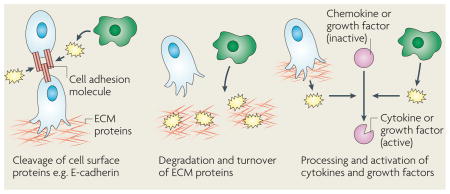
The importance of chemoattractant signalling in cancer cell intravasation has been revealed by the studies that are discussed throughout this Review. However, to physically invade into blood vessels, proteolytic degradation is required. Proteases are often produced by invasive cancer cells, but in many cases bone marrow-derived cells, including macrophages, have been shown to be the major cell type that supplies crucial proteases to the tumour microenvironment. These stromal cell-derived proteases include specific matrix metalloproteinases142,143, cysteine cathepsins144,145 and serine proteases146. There are several possible mechanisms by which these proteases can promote cancer cell invasion and intravasation, as indicated in the figure. Individual proteases cleave cell-adhesion molecules, such as epithelial (E)-cadherin, leading to the disruption of cell–cell junctions145,147. The loosening of cell contacts facilitates cancer cell migration, either as individual cells or in groups, and protease degradation or turnover of proteins in the extracellular matrix (ECM) and basement membrane enables invasive cells to migrate into the surrounding tissue and vasculature. Not only are proteases essential for the degradation of extracellular proteins, they also have more specialized processing roles that are important for cell signalling, such as in the restricted cleavage of pro-domains and subsequent activation of growth factors and cytokines148, which may significantly increase chemoattraction, cell migration and metastasis. These different modes of protease-enhanced invasion are not mutually exclusive; rather, it is likely that they act in concert to promote cancer cell spread. All of these functions are tightly regulated in a cascade of protease interactions, allowing for control and amplification of proteolysis in invasion and metastasis149. Accordingly, when members of some of these protease families are pharmacologically inhibited or genetically ablated, there is a marked reduction in cancer cell invasion142,150,151.
Tumour-associated macrophages
Macrophages can be considered the prototypical BMDC type capable of modifying cancer cell behaviour and have been shown to promote tumour angiogenesis, invasion, intravasation and metastasis in animal models39,40. Macrophages are inherently plastic cells, and this adaptability may be exploited by the tumour to elicit distinct functions at different stages of tumour progression. Although macrophage classification schemes (TABLE 1) have been useful in terms of assigning potential functions to tumour-associated macrophages (TAMs), little is known about the complexity of individual macrophage activities and their associated molecular profiles in cancer. In particular, the factors controlling the balance between tumour-suppressing and tumour-promoting activities of macrophages, and how that equilibrium changes over the course of tumour progression, are not known. We propose that multiple subpopulations of TAMs exist within a tumour, which probably change temporally during tumour development and geographically on the basis of their location within the tumour microenvironment. For example, TAMs recruited to hypoxic areas are likely to then be usurped to promote tumour angiogenesis41, whereas TAMs at the tumour–stroma interface play an active part in invasion and angiogenesis42. Transcriptome profiling of freshly isolated TAMs also suggests that they are similar to those that are involved in development43.
Most of the studies that have demonstrated a crucial role for macrophages in regulating tumour progression have either used genetic manipulation, such as Csf1op/op mice, which are deficient in the macrophage growth factor colony stimulating factor 1 (CSF1), or pharmacological depletion, such as clodronate-liposome treatment, which causes selective killing of macrophages. These approaches resulted in a substantial decrease in macrophage infiltration into the tumour, which led to inhibition of tumour angiogenesis, tumour growth and metastasis in different animal models33,44–47. Although these experiments have been crucial in demonstrating pro-tumorigenic functions for macrophages in tumours, there are undoubtedly heterogenous consequences from broadly depleting all macrophage populations. An alternative is to ablate subpopulations or individual factors produced by macrophages. This strategy was recently used for myeloid cell-specific deletion of vascular endothelial growth factor A (VEGFA) in the MMTV–PyMT breast cancer model. This was associated with decreased tumour angiogenesis, as might have been expected, but surprisingly accelerated tumour progression48. Whether lung metastasis was also increased in these mice was not reported; however, this study raises some concerns for the therapeutic targeting of stromal factors, as discussed below.
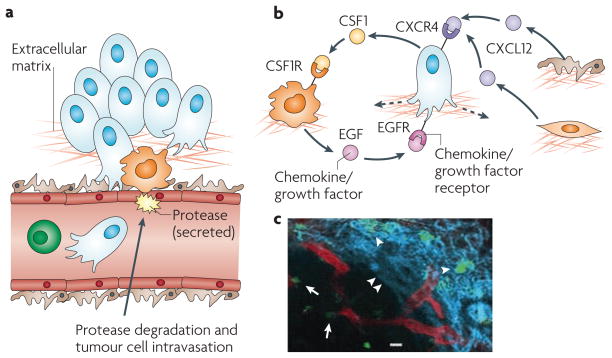
One of the mechanisms involved in TAM-enhancement of cancer cell invasion involves a paracrine loop in which epidermal growth factor (EGF) produced by TAMs increases the invasiveness and migration of neighbouring breast cancer cells that express the EGF receptor (EGFR). Cancer cells in turn express CSF1, which acts as a potent chemoattractant and chemokinetic molecule for CSF1R-expressing TAMs (FIG. 2). This reciprocal cross-talk can be blocked by either EGFR or CSF1R antagonists, resulting in a decrease in migration and invasion of both cancer cells and macrophages49,50. Tumour cell invasion can be initiated by other factors, such as neuregulin 1 (also known as heregulin) in a mouse model of breast cancer caused by the mammary epithelium-restricted expression of the oncoprotein ERBB2 or stromal cell-derived factor 1 (SDF1, also known as CXCL12) in the MMTV–PyMT model. However, in each case the motor for tumour cell migration requires macrophages and their reciprocal EGF and CSF1 signalling with tumour cells. As SDF1 can be synthesized by pericytes, fibroblasts or tumour cells, these data suggest that particular microenvironmental cues can determine the stimulation of tumour invasion and indicates the potential for crosstalk between multiple stromal cells and the tumour51 (FIG. 2). Thus it is possible that ‘invasive niches’ exist within the primary tumour, in which the proximity of cancer cells, macrophages and the endothelium establishes paracrine signalling loops that lead to enhanced intravasation and dissemination of cancer cells (FIG. 2). Indeed, clusters of these three different cell types, termed the tumour microenvironment of metastasis (TMEM), are found in human breast cancer, and their increased density is associated with the development of distant organ metastasis52.
Figure 2. The invasive microenvironment.
a | Cancer cell intravasation into the blood circulation preferentially occurs in close proximity to perivascular macrophages. Disruption of endothelial cell contacts and degradation of the vascular basement membrane is required for cancer cell intravasation, which is mediated by proteases supplied from the cancer cells, macrophages or both. b | Cancer cell migration is controlled through a paracrine loop involving colony-stimulating factor 1 (CSF1), epidermal growth factor (EGF) and their receptors, which are differentially expressed on carcinoma cells and macrophages, resulting in movement of cancer cells towards macrophages (dashed arrow). Additional paracrine loops exist between cancer cells expressing C-X-C chemokine receptor 4 (CXCR4) and stromal cells, such as fibroblasts and pericytes, producing the cognate ligand stromal cell-derived factor 1 (SDF1, also known as CXCL12), which contribute to directional cancer cell migration. c | Tumour-associated macrophages (green) can be visualized in mammary tumours in living animals, in proximity to blood vessels (red), as indicated by arrows, and migrating along collagen fibres (blue, visualized by second harmonic resonance) as indicated by arrowheads. Part c reproduced, with permission, from REF. 54 © (2007) American Association for Cancer Research.
The identification of this paracrine loop in mammary cancers raises the question of whether different sets or the same set of growth factors or chemokines and their cognate receptors control the interplay between stromal and cancer cell invasion in other tumour microenvironments. In a mouse model of colorectal cancer — in which, in a process termed collective invasion, cancer cells invade as protruding sheets of cells rather than as single cells — immature myeloid cells (iMCs) without definitive macrophage characteristics (CD11b+ (also known as integrin αM)CD34+F4/80− (also known as EMR1)GR1−) are recruited by tumour-produced C–C chemokine 9 (CCL9) signalling through CCR1 on the iMCs. In turn the iMCs promote collective tumour cell invasion, at least in part through expression of MMP2 and MMP9 at the invasive front53.
TAMs also directly promote the process of cancer cell intravasation into the blood circulation, as shown by multiphoton intravital imaging of the breast microenvironment54. This major technological advance, which incorporated transgenic animals expressing fluorescently tagged cancer cells, endothelial cells and macrophages, allowed the visualization of dynamic interactions between these cell types at the point of intravasation. Wyckoff and colleagues found that the association of cancer cells with TAMs throughout the tumour significantly increased their motility, an effect that was further amplified when cancer cells were found in close proximity to perivascular TAMs. Moreover, cancer cells were observed to invade blood vessels only where perivascular TAMs were located. The functional importance of this interaction for intravasation was demonstrated in Csf1op/op PyMT mice, which have a reduction in macrophage infiltration, and a concomitant decrease in circulating cancer cells. In addition, the EGF–CSF paracrine loop was also shown to be important for intravasation, as blocking either signalling pathway led to a significant reduction in the number of blood-borne cancer cells54. Cancer cells and macrophages also use collagen fibres as tram lines to rapidly travel through the stroma. Many of these fibres are tethered to blood vessels, resulting in cancer cells accumulating at these vessels55. The density of these fibres is regulated by macrophages, at least during development56.
Myeloid-derived suppressor cells
Another BMDC type, which may share a common progenitor with TAMs, is the MDSC57. MDSCs suppress the adaptive immune response by blocking the functions of CD4+ and CD8+ T cells, in part through arginase and nitric oxide production, by expanding the regulatory T cell pool, and by inhibiting NK cell activation58,59. MDSCs are a heterogenous collection of immature myeloid cells at different stages of differentiation that are broadly defined as CD11b+GR1+ cells in mice, with a wider range of markers in humans (TABLE 1). MDSC levels are increased in the bone marrow, blood and spleen of cancer patients and tumour-bearing mice, and their accumulation is associated with tumour growth and malignant progression30.
Although MDSC expansion is extensively described in cancer patients and mouse models, the mechanisms leading to this increase are incompletely understood. Chronic inflammation has been shown to induce MDSC accumulation in the 4T1 metastatic mammary cancer model60,61, and the pro-inflammatory S100 proteins and prostaglandin E2 have recently been identified as two principal effectors62,63. S100A9-deficient mice mount efficient anti-tumour immune responses and reject implanted colorectal cancer cells. Crucially, this effect was reversed by administration of wild-type MDSCs from tumour-bearing mice64. Intriguingly, the S100A8 and S100A9 chemokines have also been implicated in preparing the pre-metastatic niche65,66, as discussed later, suggesting a predominant role for this family in directing systemic changes that promote metastasis. Disruption of transforming growth factor-β (TGFβ) signalling, through Tgfbr2 deletion, was also shown to increase MDSC homing to tumours in a spontaneous mammary cancer model, an effect that was mediated through the SDF1–CXCR4 and CXCL5–CXCR2 (also known as IL8RB) chemokine axes67.
An immunosuppressive state favours primary tumour development, but whether there is a direct role for MDSCs in increasing tumour cell metastasis, rather than just an indirect correlation, is only beginning to be elucidated. One example of a connection was suggested in Tgfbr2-deficient mice, in which MDSCs (identified by GR1 expression) were concentrated at the invasive tumour margin67. CXCR2 or CXCR4 antagonists reduced lung metastasis in this model, an effect that was attributed to MDSC depletion but that could also result from the pleiotropic targets of these chemokine receptors. A general strategy that many groups have used to suggest a functional role for MDSCs in tumour progression and metastasis has involved GR1-specific antibodies, which not only target CD11b+GR1+ cells but also GR1+ granulocytes and a significant proportion of monocytes68, thus complicating the interpretation of these results. Although identification of specific MDSC markers, at least in mouse models69 (TABLE 1), should facilitate their selective depletion in the future, it is possible that broad targeting of MDSCs along with other myeloid cell types could still be beneficial in eliciting potent anticancer effects.
Mesenchymal stem cells
Another cell type that resides predominantly in the bone marrow, although is not of haematopoietic origin, is the MSC (TABLE 1). MSCs are multipotent cells that differentiate into osteoblasts, chondrocytes, adipocytes and other cells of mesenchymal origin70. MSCs can be found in large numbers in primary tumours and, based on this recruitment, MSCs have been proposed as a cellular vehicle to deliver anticancer drugs into the tumour71. Recently, however, Karnoub et al. investigated whether the presence of MSCs at the primary site might actually modulate the behaviour of neighbouring cancer cells72. They found that co-mingling of human MSCs with weakly metastatic breast cancer cells significantly increased the dissemination of the cancer cells to the lung from a subcutaneous xenograft, an effect that was not observed with other mesenchymal cells, such as normal fibroblasts. Interestingly, the inductive effects of MSCs on cancer cells were mediated exclusively at the primary site, apparently priming them for dissemination to the lung. This was controlled, at least in part, through a paracrine loop involving MSC-supplied CCL5 and its receptor, CCR5, on the breast cancer cells. However, the stimulatory effects of MSCs were short lived so it is perhaps not surprising that, although exposure to MSCs increased the number of metastases, there was no obvious increase in subsequent metastatic outgrowth. These experiments point to yet another stromal cell that can communicate with cancer cells to change their phenotype, albeit transiently in this study, but it will be important to identify the signals released by the cancer cells that recruit MSCs to the primary tumour as a potential step to targeting these cells therapeutically. It will also be essential to demonstrate functionally that MSCs are bone fide stem cells, as this may have implications for their potential to adopt distinct differentiation fates and roles in different tumour microenvironments.
Breaking away: cancer cell dissemination
Once cancer cells intravasate into blood or lymphatic vessels, they must navigate an entirely different microenvironment. As the haematogenous circulation is considered the major route for metastatic dissemination and is the most studied, we focus on factors influencing cancer cell survival in the blood. However the lymphatic system also seems to allow metastatic cell spread, which is considered further in BOX 2.
Box 2. Lymph nodes and bone marrow in cancer cell dissemination.
When cancer cells leave the primary tumour they may not immediately home to sites of future metastasis, but rather disseminate to other microenvironments, namely the lymph nodes and bone marrow. Although the presence of tumour cells in regional lymph nodes has long been one of the criteria used in determining the stage and prognosis of many common cancers, it remains controversial as to whether tumour cells actually metastasize to other organs from the lymph nodes or whether the presence of tumour cells in draining lymphatics simply reflects their intrinsic invasiveness152. The process of cancer cell invasion into lymphatic vessels is different from that required for entry into the blood circulation, as there are no inter-endothelial cell tight junctions, pericytes, nor an intact basement membrane to traverse153, and so it has been considered more of a passive mechanism. Lymph nodes have been proposed to function as bridgeheads, or intermediate way stations, in which cancer cells are filtered and concentrated in a restricted space that causes them to aggregate, and consequently increases their survival154. The lymph node may thus provide a supportive environment in which cancer cells acquire additional mutations that increase their metastatic propensity. Similarly, it has been suggested that the bone marrow microenvironment may serve a similar function to select and enrich for cancer cells that subsequently develop the capacity to home to other organs155,156. The bone marrow, in particular, may provide a uniquely supportive stromal environment, given the crucial importance of bone marrow niches in haematopoietic stem cell maintenance157. These are attractive hypotheses in that they could provide an explanation for several genomic studies showing limited similarities between primary tumours and disseminated cells158,159. However, to date there is little experimental evidence supporting a requisite role for transitional stromal microenvironments in the metastatic cascade.
Cancer cell survival in the systemic circulatory environment
Tens of thousands of cancer cells can be shed into the circulation every day, yet less than 0.01% of these will survive to produce metastases1,73. Numerous challenges, including mechanical destruction caused by the shear force of the blood circulation and surveillance by immune cells, particularly NK cells, contribute to making the blood a particularly hostile environment for a potential metastatic cell. Cancer cells, however, are adept at enhancing their chances of survival, in part by using platelets as a shield (FIG. 3). Tumour cells express tissue factor, the receptor for coagulation factors VIIa and X, which serves as the main initiator of coagulation, and has an important role in supporting thrombin-mediated proteolysis and the formation of tumour cell-associated microthrombi74,75. The platelet aggregate increases cancer cell survival through protection from NK cell-mediated lysis76,77, but also through an independent signalling mechanism coupled to circulating prothrombin77. The fibrin clots may also reduce shear forces that can destroy individual circulating cancer cells, and facilitate the slowing, arrest and adhesion of cancer cells, thus increasing their ability to extravasate at a secondary site. As might be expected, high platelet count is associated with decreased survival in a wide range of cancers including breast, colorectal and lung cancer78. Conversely, treatment with a variety of anti-coagulants has been shown to decrease metastasis in experimental models and in patients79. Whether innate immune cells such as macrophages have a similarly protective role in cancer cell survival has not been extensively investigated, but could be important to address given the intimate relationship that exists between macrophages and cancer cells at the site of intravasation (FIG. 2). Another, albeit controversial, hypothesis is that cancer cells might actually fuse with macrophages and thus acquire myeloid cell traits that could be beneficial for their survival in the circulation and homing to target organs80.
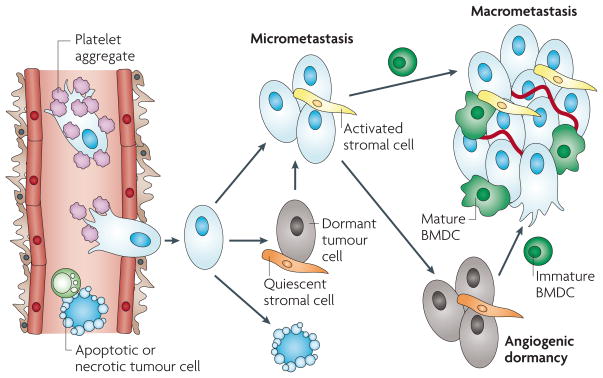
Figure 3. The fate of tumour cells in the metastatic microenvironment.
Following cancer cell intravasation, a series of rate-limiting steps affect the ability of these cells to establish secondary tumours in the metastatic site. At each step, the tumour cells can meet several different fates (death, dormancy or survival), which can be modulated by microenvironmental factors, including shielding by platelet aggregates in the circulation, the activation of resident stromal cells, and the recruitment and differentiation of bone marrow-derived cells (BMDCs).
The microenvironment at metastatic sites
The metastatic site offers new challenges for circulating tumour cells. In a hospitable tissue they must lodge, survive, extravasate, become established and grow before they have clinical relevance. Each stage is inefficient and often rate limiting. Indeed, even after lodgement and escape into the parenchyma many metastatic cells become quiescent with only a few reactivated later to develop into tumours (FIG. 3). The local microenvironment has a major role in every step in metastasis and, as in the primary tumour site, the recruitment of bone marrow derived cells is crucial to the outcome of the final fate of the metastatic cell.
Tissue tropism
The appreciation of interactions between metastatic tumour cells and the microenvironment was evident from the earliest studies in this field, and most clearly enunciated by Paget in his ‘seed and soil’ hypothesis81. In its modern context82,83 this would state that malignant cancer cells (seed) gradually acquire mutations in oncogenes or tumour suppressor genes that confer the ability to egress from the tissue of origin, survive in the haematogenous or lymphatic circulation, and prosper in a distant site (soil). This soil would have characteristics that allow the metastatic cell to adhere and proliferate, whereas other sites would be hostile10. In humans, certain tumours metastasize to preferred organ sites. For example, breast cancers metastasize to lung, liver, bone and brain; melanoma to liver, brain and skin; prostate cancer to bone; and lung cancer to bone, liver and brain (BOX 3). By contrast, some sites such as muscle are rarely if ever sites of metastasis. Conversely, other tumours such as glioma do not metastasize but instead migrate along white matter tracks and invade in that fashion, pancreatic tumours show perineural invasion, and others such as ovarian tumours are restricted to the peritoneal cavity and it is unclear whether this is true metastasis or local tissue invasion. Some others such as head and neck cancers only spread to regional lymph nodes. The molecular bases of these differences are poorly if at all understood, but probably have a microenvironmental component.
Box 3. Bone metastasis: a specialized microenvironment.
Normal bone density is dynamically regulated by the coordinated actions of osteoblasts that lay down bone, and osteoclasts that degrade bone160. Metastatic lesions in bone are usually classified as osteolytic or osteoblastic160,161. However, this is probably too rigid a classification, as approximately 25% of particular cancers cause the opposite type of lesion162. The osteoclastic lineage is regulated by colony-stimulating factor 1 (CSF1) acting on osteoclast–myeloid progenitors in the bone marrow that further differentiate into mature osteoclasts under the influence of receptor activator of NF-κB ligand (RANKL, also known as TNFSF11) expressed on osteoblasts,160,163,164. CSF1 is also produced by osteoblasts and the action of RANKL is inhibited by the soluble RANKL receptor osteoprotegerin (also known as TNFRSF11B), which is synthesized by the same cell, thus ensuring tight regulation of the lineage164,165. Breast cancer metastases cause osteolytic lesions by stimulating the formation and activity of osteoclasts166. They produce CSF1 (REF. 167) as well as parathyroid hormone-related protein (PTHRP) and tumour necrosis factor-α, which activate RANKL and inhibit osteoprotegerin synthesis, thereby increasing the number and activity of osteoclasts160,162,167,168. Inhibition of RANKL in a melanoma model of bone metastasis dramatically reduced metastatic growth and bone disruption without affecting metastasis to other organs168. Bone matrix is rich in sequestered growth factors and its degradation releases these factors, including insulin-like growth factor 1, transforming growth factor-β (TGFβ) and bone morphogenetic proteins that can act upon the metastatic cells, enhancing their survival and growth while further stimulating PTHRP synthesis162,163. This creates a positive feedback cycle of increased bone loss and increased growth of the metastatic cells162. Selecting for breast cancer cells that home to bone revealed a gene expression signature that defined this tropism, which included upregulation of C-X-C chemokine receptor 4 (CXCR4), osteopontin, matrix metalloproteinase 1 and interleukin 11 (IL-11) transcripts169. Bone marrow stroma is a rich source of stromal cell-derived factor 1 (SDF1, also known as CXCL12) that may contribute to this tropism by signalling through CXCR4 (REF. 89) and, once the metastatic cells arrive, IL-11 synthesis would activate osteoclasts162 and matrix metalloproteinase 1 activity would result in further release of growth factors causing a similar positive feedback mechanism for the promotion of metastatic growth.
A similar mechanism appears to operate in neuroblastoma, a cancer that also preferentially metastasizes to the bone170. Although some neuroblastomas secrete RANKL, many do not, and in this case IL-6 synthesized by MSCs, responding to neuroblastoma-produced factors, appears to be the major culprit170. IL-6 is a potent activator of osteoclastic activity and its inhibition limits neuroblastoma metastasis in experimental models170. This bone marrow-derived stromal IL-6 also directly stimulates the survival and proliferation of IL-6R+ neuroblastoma metastases, suggesting a mechanism for the preferred growth of these tumours in the bone171.
In contrast to osteolytic lesions, prostate cancer predominantly results in osteoblastic metastases in which there is a significant amount of disorganized bone deposition due to local activation of osteoblastic activity160. Several osteoblastic factors are synthesized by prostatic metastatic cells including endothelin 1, TGFβ2, fibroblast growth factors and the bone morphogenetic proteins, all of which influence bone physiology162,172,173. In addition, these cells produce proteases such as urokinase-type plasminogen activator or prostate-specific antigen that might liberate growth factors bound to bone matrix to increase the proliferation of metastatic cells162.
To address the mechanisms underlying tissue tropism associated with haematogenous spread, several investigators took parental cancer cells and selected tissue-specific homing variants through serial passage in mice83–85. More recent versions of these experiments have used the power of transcriptome profiling to identify cohorts of genes of which expression confers both enhanced metastatic potential and altered tissue tropism86,87. These data, reviewed in another article in this Focus issue88, show that the ‘seed’ can have a preferred site for growth that is encoded by genetic alterations in the tumour cell itself.
The tropism of metastatic cells for specific organs may be mediated by chemokines, the local expression of these chemoattractants might guide cognate chemokine receptor-expressing tumour cells to specific destinations, as a result of locally induced chemotaxis and invasion of tumour cells89,90. For example, signalling through the chemokine receptors CXCR4 and CCR7 expressed by breast cancer cells mediates actin polymerization and pseudopod formation that contributes to a chemotactic and invasive response89. The specificity of homing was achieved through expression of the cognate ligands, SDF1 and CCL21, respectively, in the sites of metastasis, but not other organs. These chemokine responses are important, as neutralizing antibodies to either SDF1 or CXCR4 impaired breast cancer metastasis in an experimental metastasis model89. Of all the chemokine receptors, the most prevalently overexpressed in human tumours is CXCR4, which correlates with poor prognosis91. CXCR4 signalling is also essential for ERBB2-induced breast cancer metastasis92. Additional chemokine receptors are important for tumour cell homing to other organs; for instance, expression of CCR10 in melanoma cells, in addition to CXCR4 and CCR7, confers a tropism to the skin89. Another interesting example is the neural tropism and spread of pancreatic ductal adenocarcinomas, whereby the expression of CX3CR1 by pancreatic tumour cells and its reciprocal ligand, CX3CL1, on peripheral neurons promotes metastasis93.
The data discussed above have been interpreted as evidence for a positive selection of the microenvironment causing metastatic homing. However, an alternative and persuasive view, based upon intravital imaging, is of a passive role of the microenvironment in homing, at least for haematogenous metastases1. This suggests that the predominant sites of metastases simply reflect the first pass of the cells in the circulation and their entrapment in local capillaries. Thus, breast cancer cells spread predominantly to lung, lymph nodes and bone, whereas colon cancer cells travel to the liver through the hepatic–portal circulation. Tissue tropism in this view is not due to active homing, but instead to the ability of a small portion of the many cells that become lodged in various tissues to survive, invade and grow in a particular environment (soil) either by chance or because they have appropriate mutations. Support for this comes from the studies by Massagué and colleagues in which gene signatures have been identified whose expression confers the ability to grow in particular tissues87. The local environment may therefore provide appropriate tropic factors that enable a particular tumour cell, which expresses the relevant cohort of genes, to prosper. Indeed, the effect of chemokines on tissue tropism described above can be interpreted as their ability to induce migration and invasion of the tumour cells1 in particular sites, and also the capacity of some chemokines, such as GROA (also known as CXCL1) to promote tumour cell proliferation94.
Although still not definitive, the current data suggests that dissemination and homing depends on prevailing circulatory patterns, whereas the initial survival of tumour cells and their subsequent growth at the distant site is the rate-limiting step. Survival in the circulation and at the landing site is essential in all models of dissemination (FIG. 3). Substantial evidence implicates platelets in this process, as discussed above. Platelets are also a potent source of SDF1 that may affect the migration of CXCR4-expressing tumour cells and the recruitment of BMDCs to sites of metastasis95. Furthermore, platelets express many pro- and anti-angiogenic factors96 that could influence the establishment and growth of micrometastases.
Adhesion and invasion in the establishment of metastasis
Whereas passive entrapment may have part of the role in metastatic seeding, active adhesion and invasion is essential for the subsequent establishment and persistent growth97. Little is known of the early steps in this process. In some cases, proliferation occurs within the blood vessels and these eventually rupture as the metastatic tumour grows bigger98. In others, cancer cells extravasate first and then proliferate. Integrins expressed on the cancer cell surface are important in this process. Integrins adhere to many extracellular matrix molecules, including laminin and fibronectin, with specificity dictated by their α and β chain combinations99. For example, α3β1 integrin is required for adhesion to laminin during pulmonary metastasis100, whereas blocking αvβ1 integrin inhibited metastasis through suppression of expression of the protease urokinase plasminogen activator (uPA)11. The data for αvβ1 integrin are consistent with the inhibition of metastasis associated with uPA deficiency in the MMTV–PyMT model of breast cancer101. Early experiments by Liotta and colleagues showed that co-injection of tumour cells with fibronectin in experimental metastasis models increased tumour cell adhesion and metastasis102. This potentiation is consistent with the pre-metastatic niche concept discussed below, whereby BMDCs have been suggested to increase fibronectin deposition at homing sites for metastatic cells103.
Interactions between the primary tumour and meta-static sites
Metastatic seeding of circulating tumour cells has been shown in some cases to be enhanced by the primary tumour, whose secreted products create an environment that favours establishment of metastases at unique distant sites, termed pre-metastatic niches103–105. For example, melanoma cells induce these niches in multiple sites compared with Lewis lung carcinoma (LLC), which only induces a niche in the lung and liver, following a pattern consistent with the relative tropism of these two tumour types. Strikingly, LLC cells can be redirected to multiple and different metastatic sites by repeated intraperitoneal injection of conditioned media from the melanoma cells103. These niches, covered in detail in an Opinion piece in this Focus issue106, are populated by BMDCs of the myeloid lineage and are abundant in fibronectin produced by activated fibroblasts. This fibronectin deposition, together with other ECM molecules, may also be increased by the secretion of enzymes such as lysyl oxidase from primary tumours acting on these distant fibroblasts107,108.
Several other molecules have been identified as important in the creation of the niche, including the chemoattractants S100A8 and S100A9, and the metalloproteinase MMP9, which liberates VEGFA locally and promotes myeloid cell recruitment through VEGFR1 signalling65,109. Inhibition of VEGFR1 signalling blocked the metastatic enhancement by the primary tumour, suggesting the importance of myeloid cell recruitment103, although VEGFR1 is also expressed on endothelial cells. Furthermore, Weinberg and colleagues110 have shown that certain tumours, termed instigators, can mobilize bone marrow precursors through an osteopontin- mediated but unknown mechanism, to home to secondary metastatic sites where they promote the growth of weakly malignant cells such that they form macro-metastases. This mechanism requires seeded metastatic cells and long periods of incubation, and so it is functionally distinct from the pre-metastatic niche concept. Nevertheless, both processes highlight the importance of recruitment of BMDCs to metastatic sites.
Despite the persuasiveness of these experiments there have been other studies that appear to contradict them. In these conflicting studies, the presence of a primary tumour inhibits metastasis, and removal of the tumour promotes metastatic growth111. This is consistent with clinical observations of a burst of metastasis following surgical removal of the primary tumours in some patients111,112. These types of data led to the identification of anti-angiogenic molecules, mostly fragments of the ECM such as angiostatin, a fragment of plasminogen113, secreted from primary tumours114,115. The transition of these molecules into clinical practice has been complicated, suggesting difficulties in quality control and different mechanisms of action dependent on context113, but it seems important to reconcile these disparate data in more complex models of metastatic disease and in clinical practice.
Cytokines, growth factors and metastases
The molecular mechanisms of these pro-metastatic functions of the microenvironment remain poorly understood. Several growth factors and chemokines have been implicated in increasing metastasis. Primary tumour-secreted VEGFA, TGFβ and tumour necrosis factor-α (TNFα) induce expression of S100A8 and S100A9 by lung endothelium and myeloid cells, which increased the invasion and adhesion of circulatory tumour cells, and also of myeloid cells, into the metastatic niche65,66. TNFα has been shown in several studies to increase metastasis. It can upregulate several adhesion molecules on endothelial cells that promote tumour cell adhesion and migration116,117, including E-selectin, P-selectin and VCAM1 (vascular cell adhesion protein 1). TNFα also protects against NK cell attack118. These metastasis-promoting actions of TNFα appear to be mediated mostly through effects on stromal and inflammatory cells. TNFα signals through the transcription factor nuclear factor-κB (NF-κB), and signalling through this molecule in resident macrophages created an inflammatory microenvironment that enhanced LLC cell metastasis119. Although the sources of TNFα were not identified in the studies above, it is noteworthy that macrophages are the most potent producers of this molecule. Indeed, Karin and colleagues recently showed that conditioned media from LLC cells, of which the main active component was the ECM protein versican, was a robust inducer of TNFα production in macrophages through activation of Toll-like receptor 2 (TLR2) and TLR6. Consequently, deletion of Tnf or Tlr2 in the host significantly reduced experimental lung metastasis120.
Persistent growth of metastases
After seeding, persistent growth of the metastatic tumour requires the establishment of a vasculature that is achieved through the production of angiogenic growth factors such as VEGFA, and the recruitment and proliferation of endothelial cells and their accompanying support cells such as pericytes. Bone marrow-derived endothelial cell precursors are recruited to metastases by VEGFA signalling through VEGFR2 (REF. 121). These endothelial progenitor cells (EPCs) are recruited to macrometastases but not to micrometastases and are incorporated into the endothelial wall122. Tumour-associated EPCs express the inhibitor of differentiation 1 (ID1) transcription factor and suppression of ID1 blocks the angiogenic switch and formation of macrometastases, showing that the ID1+ EPCs were necessary for the initiation of angiogenesis122. EPCs themselves express a variety of angiogenic molecules, suggesting that their recruitment further potentiates local angiogenesis and subsequent metastatic tumour growth. However, other studies, albeit disputed123, using parabiotic mice have called into question the recruitment of EPCs to tumours, owing to the complete absence of EPCs in the parabiotic twin, which had no adverse effect on tumour growth and metastasis124.
Folkman and colleagues suggested that dormant metastases fail to grow because of the lack of vascularization; a phenomenon termed angiogenic dormancy12. Consequently, acquisition of an angiogenic switch that produces a dense vasculature can initiate the onset of growth125. In this scenario, additional oncogenic mutations or alterations in the microenvironment enable the onset of angiogenesis. It seems unlikely that mutations occur in the non-proliferating quiescent tumour cells125. However, changes in the microenvironment due to inflammation or ageing could enable tumour cells to begin to proliferate and initiate angiogenesis126. Senescent fibroblasts produce cytokines, particularly inflammatory ones, and proteases that can promote epithelial cell proliferation and might activate the dormant cells and trigger angiogenesis. Such factors are not produced by ‘young’ fibroblasts, providing a link between ageing, inflammation and cancer127,128. Further, induction of an inflammatory response through activation of macrophages in the lung increased metastases in an experimental metastasis model using LLC cells119. Hyperoxic injury to the lung and allergen-induced pulmonary inflammation also increased metastasis129,130. In primary tumours, macrophages regulate the angiogenic switch through their production of a wide range of angiogenic factors including VEGFA33. However, despite their abundance in metastases, whether they are actively involved in angiogenesis at metastatic sites is unknown. In addition, the phenomenon of primary tumour instigation of slow-growing metastases through the recruitment of BMDCs might also be a mechanism for the induction of angiogenesis110. All these data suggest that alterations in the microenvironment might reawaken dormant metastases or promote the growth of slow-growing ones (FIG. 3).
Conclusions and perspectives
It is evident from the data presented in this Review that primary tumours and their metastatic off-shoots are complex ecologies consisting of numerous cell types. Many of these are derived from the bone marrow but there are also abundant resident cells. In analysing these tumours, by necessity, experiments are usually focused upon a single cell type or a single gene product within a cell. However, it is naive to think that individual cell types function in isolation in a complex system. Thus, a major area for advances in understanding the role of the microenvironment must incorporate a systems biology approach in order to model these complex interactions and their evolution over time.
It is also apparent that cancer is a systemic disease with the tumour affecting multiple systems in the host that can result in spread of cancer cells and enhancement of metastasis. It is important to realize that this usually involves mobilization of BMDCs, which home to the tumour sites and dramatically alter their ecology, generally but not always in favour of the tumour. In addition, these changes modify immune responses in the tumour-bearing animals, often with a profound immunosuppression against new antigens expressed in the tumour cells themselves. It remains obscure how the mobilization of BMDCs occurs, how they are trafficked from bone marrow into the blood and back as well as to different tissues, and what regulates the final differentiation and function of BMDCs at these sites. Several molecules have been shown to be important, including SDF1, VEGFA, KIT ligand and osteopontin as discussed above, but the major points of regulation still remain to be elucidated. Nevertheless this is an area of research that needs to be expanded, along with detailed phenotyping of the cells that BMDCs differentiate into, as this may be the key to controlling metastatic spread. These approaches will be significantly enhanced by new imaging techniques that can track cells and their interactions in vivo in both humans and mice. These include intravital imaging using multi-photon microscopy of cells fluorescently labelled either intrinsically or by barcodes of identifying antibodies55; nanoparticles that attach to the cell surface through tags or other innovative means; enhanced magnetic resonance imaging techniques using targeted nanoiron probes; positron emission tomography; and spectroscopic methods coupled with magnetic resonance imaging or microscopy131 to individually identify cell types based on intrinsic fluorescence in vivo — a technique that might even be applicable to human tumours.
The goal of all these experiments is to create sufficient biological insight to reverse the tumour-enhancing effects of the microenvironment with the ideal of recreating a suppressive microenvironment that can fully revert the malignant phenotype to normal (or at least a controlled phenotype), as was found in the pioneering experiments of reprogramming malignant tumour cells in normal environments that are described above. This raises the issue of whether stromal alterations are always reversible, or whether there is a point after which these changes cannot be reversed. These questions apply both to modifications conferred on the stroma by the cancer cells and to those conferred on cancer cells by the stroma. Although it is probably optimistic to think that all changes can be fully reversed, various strategies have been used to target stromal cells, particularly in the primary tumour132,133, several of which have therapeutic efficacy. A wide range of agents are already available, at least in animal models, to block the functions of stromal cells discussed in this Review, including EGFR and CSF1R antagonists, VEGFA and Vegfr inhibitors, TNFα inhibitors, S100 antibodies, protease inhibitors, anticoagulants and chemokine inhibitors, including CXCR4 antagonists. It is likely that these will need to be used in combination to attack the metastatic microenvironment in its diversity and to undermine its robustness. Moreover, it is unlikely that any of these strategies will work alone without the incorporation of a direct attack on the tumour cell itself.
An emerging concept in anticancer therapy involves the mobilization of several types of BMDC following treatment with traditional chemotherapeutics or targeted therapies, which may contribute either to a lack of response or acquired drug resistance. For example, EPCs are rapidly mobilized through SDF1 and VEGFR2 signalling in response to certain chemotherapies, including the taxanes and 5-fluorouracil. Resistance to these drugs can be overcome in part through anti-VEGFR2 blocking antibodies, or by genetic ablation of EPCs in ID1 and ID3-deficient mice134,135. BMDCs can also confer an inherent refractoriness to therapy, as in the case of MDSCs, which are recruited to certain tumour models in response to anti-VEGFA treatment136. However, when the anti-VEGFA antibody was combined with an anti-GR1 antibody, tumour growth was effectively reduced. These examples indicate that it will be important to inhibit mobilized BMDCs alongside drugs that target cancer cells, and this synergy may in fact explain some of the therapeutic efficacy observed in certain combination trials in mice and humans to date.
Among the stromal cell types important for metastatic dissemination and outgrowth, drugs that target endothelial cells are the most clinically advanced. Several Vegf antagonists have been approved by the US Food and Drug Administration137 that increase survival in patients with metastatic breast cancer138 and metastatic colorectal cancer139 when combined with chemotherapy. The translational promise of this first targeted therapy against the tumour microenvironment, however, is somewhat tempered by emerging instances of resistance to anti-angiogenic therapy140, and examples of stromal cell targeting in animal models that unexpectedly resulted in increased invasion48 or metastasis141. These data reinforce the importance of fully understanding the intricacy of cellular interactions in the tumour microenvironment, using approaches discussed in this Review, in order to isolate the cancer cells from their multiple support networks and effectively destroy them.
At a glance.
The tumour microenvironment has a major role in modulating the metastatic capacity of most cancers. Seminal experiments indicated that certain microenvironments can suppress malignancy. However, in most tumours these restraints are overcome such that the tumour now exploits the supporting cells to increase metastatic potential.
Primary and metastatic tumours cause systemic perturbations that often involve mobilizing bone marrow-derived cells that home to the tumour and promote tumour progression, malignant cell escape and survival, and growth at the secondary site.
Primary tumours recruit macrophages to their microenvironment and these cells increase metastatic potential by increasing tumour cell migration, invasion and intravasation. They also increase angiogenesis and thereby increase the targets for metastatic cell escape.
Myeloid cell-derived suppressor cells suppress immune responses to newly displayed tumour antigens and promote the metastatic potential of the tumour.
Mesenchymal stem cells can differentiate into many different cell types and are recruited to primary tumours where they enhance metastasis.
Tumour cells are protected in their travels through the circulation, particularly by platelets. These platelets together with the tumour cells activate the clotting system such that microthrombi form that help tumour cells lodge in target tissues.
The formation of metastases has many rate-limiting steps including survival in the distant organ, extravasation and the establishment of persistent growth. Microenvironmental cues are important at all steps and the recruitment of a variety of bone marrow-derived cells including endothelial progenitors and myeloid cell-derived cells is crucial for these processes.
Acknowledgments
We apologize to the many authors whose work we could not cite owing to space constraints. Research in the authors’ laboratories is supported by the National Cancer Institute (J.A.J. and J.W.P.), the Emerald Foundation, the Sidney Kimmel Foundation and the Rita Allen Foundation (J.A.J.). J.A.J. is a Geoffrey Beene Junior Faculty Chair and J.W.P. is the Louis Goldstein Swan Chair in Women’s Cancer Research.
Glossary
- Extracellular matrix (ECM)
The matrix laid down by cells upon which they adhere and move. It consists of many components including laminin and fibronectin, which can influence tumour cell behaviour. ECM is also a rich source of growth factors that can be released upon proteolytic degradation and which in many cases increase metastasis
- Myeloid cell-derived suppressor cell (MDSC)
MDSCs are immature cells of the myeloid lineage that suppress T-cell responses to tumours and also enhance metastasis in the MMTV–PyMT model
- Mesenchymal stem cell (MSC)
MSCs are multipotent cells that differentiate into osteoblasts, chondrocytes adipocytes, and other cells of mesenchymal origin that can be recruited to tumours and increase metastasis
- Tumour-associated macrophage (TAM)
TAMs are cells recruited to the tumour microenvironment where they are educated to perform tasks that enhance metastasis, such as stimulating tumour cell migration, invasion and intravasation
- MMTV–PyMT breast cancer model
Mammary cancers are induced in mice by the mammary-restricted expression of the polyoma virus middle T oncoprotein from the mouse mammary tumour virus (MMTV) long terminal repeat promoter. This model progresses through stereotypical stages of tumour progression and metastasizes to the lung, reminiscent of those seen in human breast cancer
- Immature myeloid cells (iMC)
Myeloid cells without definitive macrophage characteristics (CD11b+CD34+F4/80−GR1−) that stimulate collective tumour cell invasion in mouse models of colon cancer
- Multiphoton intravital imaging
Visual imaging of tumours in live animals using infrared light and the quantum phenomena of two low energy photons when focused together having sufficient energy to elicit a fluorescent event. This enables imaging in real time of fluorescently tagged cells inside tumours
- Second harmonic resonance
A quantum mechanical phenomena that enables the visualization by multi-photon microscopy of repeating structures such as collagen I in the blue channel. This enables visualization of the tumour microenvironment in real time in live animals
- Pre-metastatic niche
A proposed environment induced by the primary tumour in secondary organs that enhances metastatic cell seeding and that is populated by bone marrow-derived cells
- Experimental metastasis model
This is a xenograft model of metastasis in which malignant cells are introduced into experimental animals usually by intravenous injection but also through the spleen or heart. It is usually used to study lung metastases
- Toll-like receptors
A class of receptors expressed particularly by myeloid cells that recognize foreign substances and have evolved to be a pattern recognition system to detect invading pathogens. Their activation triggers, among others, the NF-κB signalling pathway, which is involved in metastasis in several tumour types
- Endothelial progenitor cell (EPC)
EPCs are bone marrow-derived cells that are recruited to the nascent tumour vasculature, and that have been shown to be important for angiogenesis and metastasis in certain animal cancer models
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
Tgfbr2
UniProtKB: http://www.uniprot.org
CCL21 | CCL5 | CCL9 | CCR1 | CCR10 | CCR5 | CCR7 | CSF1 | CSF1R | CX3CR1 | CXCL5 | CXCR4 | EGF | EGFR | EMR1 | ERBB2 | fibronectin | GROA | ID1 | IL8RB | integrin αM | KIT ligand | MMP2 | MMP9 | osteopontin | osteoprotegerin | plasminogen | PROK2 | PTHRP | RANKL | S100A8 | S100A9 | SDF1 | tissue factor | TLR2 | TLR6 | TNFα | uPA | VCAM1 | VEGFA | VEGFR1 | versican
FURTHER INFORMATION
Johanna Joyce’s webpage: http://www.mskcc.org/mskcc/html/52336.cfm
Jeffrey Pollard’s webpage: http://www.aecom.yu.edu/home/faculty/profile.asp?id=3865
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Johanna A. Joyce, Email: joycej@mskcc.org.
Jeffrey W. Pollard, Email: pollard@aecom.yu.edu.
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. A seminal review summarizing the authors’ research on the various routes for metastasis. [DOI] [PubMed] [Google Scholar]
- 2.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nature Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 3.Kim JW, et al. Rapid apoptosis in the pulmonary vasculature distinguishes non-metastatic from metastatic melanoma cells. Cancer Lett. 2004;213:203–212. doi: 10.1016/j.canlet.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald IC, Groom AC, Chambers AF. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. Bioessays. 2002;24:885–893. doi: 10.1002/bies.10156. [DOI] [PubMed] [Google Scholar]
- 5.Husemann Y, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Cameron MD, et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–2546. [PubMed] [Google Scholar]
- 7.Engel J, et al. The process of metastasisation for breast cancer. Eur J Cancer. 2003;39:1794–1806. doi: 10.1016/s0959-8049(03)00422-2. [DOI] [PubMed] [Google Scholar]
- 8.Goodison S, et al. Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clin Cancer Res. 2003;9:3808–3814. [PubMed] [Google Scholar]
- 9.Brackstone M, Townson JL, Chambers AF. Tumour dormancy in breast cancer: an update. Breast Cancer Res. 2007;9:208. doi: 10.1186/bcr1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki M, Mose ES, Montel V, Tarin D. Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. Am J Pathol. 2006;169:673–681. doi: 10.2353/ajpath.2006.060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nature Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Med. 1995;1:149–153. doi: 10.1038/nm0295-149. Early concepts of the presence of dormant metastatic cells and the requirement for an angiogenic switch to re-awaken them. [DOI] [PubMed] [Google Scholar]
- 13.Kouros-Mehr H, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minn AJ, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci USA. 2007;104:6740–6745. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton L, Massague J. Is cancer a disease of self-seeding? Nature Med. 2006;12:875–878. doi: 10.1038/nm0806-875. [DOI] [PubMed] [Google Scholar]
- 16.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. This seminal paper showed that malignant cancer cells could be reprogrammed by the embryonic blastocyst microenvironment, remarkably resulting in normal mosaic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce GB, Pantazis CG, Caldwell JE, Wells RS. Specificity of the control of tumor formation by the blastocyst. Cancer Res. 1982;42:1082–1087. [PubMed] [Google Scholar]
- 20.Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 21.Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. A three-dimensional model to study the epigenetic effects induced by the microenvironment of human embryonic stem cells. Stem Cells. 2006;24:501–505. doi: 10.1634/stemcells.2005-0459. [DOI] [PubMed] [Google Scholar]
- 22.Gerschenson M, Graves K, Carson SD, Wells RS, Pierce GB. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci USA. 1986;83:7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrix MJ, et al. Reprogramming metastatic tumour cells with embryonic microenvironments. Nature Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 24.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi N, Cunha GR. Mesenchyme-induced changes in the neoplastic characteristics of the Dunning prostatic adenocarcinoma. Cancer Res. 1991;51:4924–4930. [PubMed] [Google Scholar]
- 26.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 29.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 30.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 32.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 33.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 34.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coussens LM, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soucek L, et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nature Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 39.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 40.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 42.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ojalvo LS, King W, Cox D, Pollard JW. High density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. References 33 and 44 used the MMTV–PyMT mammary cancer model to show that macrophage depletion, in a CSF1-deficient background, substantially decreased tumour angiogenesis, invasion and lung metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiraoka K, et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Sci. 2008;99:1595–1602. doi: 10.1111/j.1349-7006.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeisberger SM, et al. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miselis NR, Wu ZJ, Van Rooijen N, Kane AB. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7:788–799. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 48.Stockmann C, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 50.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez L, et al. The EGF/CSF-1 paracrine invasion loop can be triggered by heregulin beta 1 and CXCL12. Cancer Res. doi: 10.1158/0008-5472.CAN-08-2871. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson BD, et al. Tumor microenvironment of metastasis (TMEM) in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-08-2179. (in the press). This study found that analysis of invasive carcinoma cells, macrophages and endothelial cells in combination (the TMEM) could be used as a prognostic marker for breast cancer metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitamura T, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nature Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 54.Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. References 49 and 54 identified a paracrine loop involving the differential expression of EGF and CSF1, and their receptors, on macrophages and cancer cells, which is an important mediator of cancer invasion and intravasation. [DOI] [PubMed] [Google Scholar]
- 55.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nature Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 56.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 57.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 60.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 61.Bunt SK, et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 63.Sinha P, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng P, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 66.Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nature Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. References 65 and 66 identify the inflammatory chemoattractants S100A8 and S100A9 as mediators of myeloid cell recruitment to the pre-metastatic niche in the lung through the serum amyloid A3–TLR4 signalling cascade. [DOI] [PubMed] [Google Scholar]
- 67.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. An important paper linking myeloid cell-derived suppressor cells with metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gordon S. Alternative activation of macrophages. Nature Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 69.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 71.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 72.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. This study showed that mesenchymal stem cells, when mixed with weakly metastatic breast cancer cells, substantially enhance their metastatic capability, in part through CCL5–CCR5 signalling. [DOI] [PubMed] [Google Scholar]
- 73.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- 74.Im JH, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64:8613–8619. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 75.Palumbo JS. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin Thromb Hemost. 2008;34:154–160. doi: 10.1055/s-2008-1079255. [DOI] [PubMed] [Google Scholar]
- 76.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. [PubMed] [Google Scholar]
- 77.Palumbo JS, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and -independent mechanisms. Blood. 2007;110:133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet–cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143:819–826. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3:425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 80.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nature Rev Cancer. 2008;8:377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 81.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 82.Fokas E, Engenhart-Cabillic R, Daniilidis K, Rose F, An HX. Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev. 2007;26:705–715. doi: 10.1007/s10555-007-9088-5. [DOI] [PubMed] [Google Scholar]
- 83.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 84.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 85.Langley RR, Fidler IJ. Tumor cell–organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 86.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nature Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 88.Nguyen DX, Bos PD, Massagué J. Metastasis: from infiltration to colonization. Nature Rev Cancer. (in the press) [Google Scholar]
- 89.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. This research revealed that chemokine signalling loops are important in directing tissue-specific metastasis of breast cancer cells. [DOI] [PubMed] [Google Scholar]
- 90.Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14:181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–213. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 92.Li YM, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 93.Marchesi F, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 94.Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15:49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 95.Jin DK, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nature Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massberg S, et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25:305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 98.Al-Mehdi AB, et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nature Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 99.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, et al. Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J Cell Biol. 2004;164:935–941. doi: 10.1083/jcb.200309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Almholt K, et al. Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice. Int J Cancer. 2005;113:525–532. doi: 10.1002/ijc.20631. [DOI] [PubMed] [Google Scholar]
- 102.Liotta LA, Rao CN, Wewer UM. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- 103.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. This study first defined the concept of the pre-metastatic niche and identified VEGFR1+ bone marrow-derived progenitors as key cells in establishing the niche, a process mediated in part through integrin α4 and fibronectin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rafii S, Lyden D. S100 chemokines mediate bookmarking of premetastatic niches. Nature Cell Biol. 2006;8:1321–1323. doi: 10.1038/ncb1206-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–574. doi: 10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nature Rev Cancer. doi: 10.1038/nrc2621. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 108.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hiratsuka S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 110.McAllister SS, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. This paper showed that human breast carcinomas instigate the growth of otherwise indolent cancer cells, through a phenomenon involving BMDC recruitment and osteopontin signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demicheli R, Retsky MW, Hrushesky WJ, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nature Clin Pract Oncol. 2007;4:699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- 112.Demicheli R, Retsky MW, Swartzendruber DE, Bonadonna G. Proposal for a new model of breast cancer metastatic development. Ann Oncol. 1997;8:1075–1080. doi: 10.1023/a:1008263116022. [DOI] [PubMed] [Google Scholar]
- 113.Cao Y, Xue L. Angiostatin. Semin Thromb Hemost. 2004;30:83–93. doi: 10.1055/s-2004-822973. [DOI] [PubMed] [Google Scholar]
- 114.O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nature Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 115.O’Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science. 1999;285:1926–1928. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 116.Stoelcker B, Hafner M, Orosz P, Nieswandt B, Mannel DN. Role of adhesion molecules and platelets in TNF-induced adhesion of tumor cells to endothelial cells: implications for experimental metastasis. J Inflamm. 1995;46:155–167. [PubMed] [Google Scholar]
- 117.Mannel DN, Orosz P, Hafner M, Falk W. Mechanisms involved in metastasis enhanced by inflammatory mediators. Circ Shock. 1994;44:9–13. [PubMed] [Google Scholar]
- 118.Hafner M, Orosz P, Kruger A, Mannel DN. TNF promotes metastasis by impairing natural killer cell activity. Int J Cancer. 1996;66:388–392. doi: 10.1002/(SICI)1097-0215(19960503)66:3<388::AID-IJC20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 119.Stathopoulos GT, et al. Host nuclear factor-κB activation potentiates lung cancer metastasis. Mol Cancer Res. 2008;6:364–371. doi: 10.1158/1541-7786.MCR-07-0309. [DOI] [PubMed] [Google Scholar]
- 120.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–107. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nature Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 122.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 123.Kerbel RS, et al. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci USA. 2008;105:E54. doi: 10.1073/pnas.0804876105. author reply E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Purhonen S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 126.Schwartsburd PM. Age-promoted creation of a pro-cancer microenvironment by inflammation: pathogenesis of dyscoordinated feedback control. Mech Ageing Dev. 2004;125:581–590. doi: 10.1016/j.mad.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 127.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 129.Adamson IY, Young L, Orr FW. Tumor metastasis after hyperoxic injury and repair of the pulmonary endothelium. Lab Invest. 1987;57:71–77. [PubMed] [Google Scholar]
- 130.Taranova AG, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 2008;68:8582–8589. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nature Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 132.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 133.Gadea BB, Joyce JA. Tumour-host interactions: implications for developing anti-cancer therapies. Expert Rev Mol Med. 2006;8:1–32. doi: 10.1017/S1462399406000172. [DOI] [PubMed] [Google Scholar]
- 134.Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 135.Shaked Y, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. References 134 and 135 demonstrate that mobilization of endothelial progenitor cells contributes to chemotherapy resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. This study showed that another BMDC type, CD11b+GR1+ cells, confer refractoriness to anti-VEGFA treatment in cancer models. [DOI] [PubMed] [Google Scholar]
- 137.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 138.Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 139.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 140.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xian X, et al. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 143.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–573. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 144.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nature Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 145.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–64. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 146.Laufs S, Schumacher J, Allgayer H. Urokinase-receptor (u-PAR): an essential player in multiple games of cancer: a review on its role in tumor progression, invasion, metastasis, proliferation/dormancy, clinical outcome and minimal residual disease. Cell Cycle. 2006;5:1760–1771. doi: 10.4161/cc.5.16.2994. [DOI] [PubMed] [Google Scholar]
- 147.Masterson J, O’Dea S. Posttranslational truncation of E-cadherin and significance for tumour progression. Cells Tissues Organs. 2007;185:175–179. doi: 10.1159/000101318. [DOI] [PubMed] [Google Scholar]
- 148.Van Damme J, Struyf S, Opdenakker G. Chemokine-protease interactions in cancer. Semin Cancer Biol. 2004;14:201–208. doi: 10.1016/j.semcancer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 149.Mason SD, Joyce JA. In: Cancer Metastasis: Biologic Basis and Therapeutics. Welsh DR, Lyden DC, editors. Cambridge Univ. Press; Cambridge, UK: 2009. (in the press) [Google Scholar]
- 150.Joyce JA, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5:443–453. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 151.Gocheva V, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. References 150 and 151 show that cysteine cathepsin proteases promote cancer invasion, in part through cleavage of the cell adhesion molecule E-cadherin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Das S, Skobe M. Lymphatic vessel activation in cancer. Ann NY Acad Sci. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- 153.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 154.Sleeman JP. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res. 2000;157:55–81. doi: 10.1007/978-3-642-57151-0_6. [DOI] [PubMed] [Google Scholar]
- 155.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nature Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 156.Klein CA, Holzel D. Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle. 2006;5:1788–1798. doi: 10.4161/cc.5.16.3097. [DOI] [PubMed] [Google Scholar]
- 157.Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Klein CA, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002;360:683–689. doi: 10.1016/S0140-6736(02)09838-0. [DOI] [PubMed] [Google Scholar]
- 159.Schmidt-Kittler O, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. 2003;100:7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 161.Mundy GR, Edwards JR. The osteoclast — not always guilty. Cell Metab. 2007;6:157–159. doi: 10.1016/j.cmet.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 162.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 163.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 164.Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann NY Acad Sci. 2008;1143:123–150. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]
- 165.Felix R, Hofstetter W, Wetterwald A, Cecchini MG, Fleisch H. Role of colony-stimulating factor-1 in bone metabolism. J Cell Biochem. 1994;55:340–349. doi: 10.1002/jcb.240550311. [DOI] [PubMed] [Google Scholar]
- 166.Kakonen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97:834–839. doi: 10.1002/cncr.11132. [DOI] [PubMed] [Google Scholar]
- 167.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–162. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 168.Jones DH, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 169.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 170.Sohara Y, Shimada H, DeClerck YA. Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer Lett. 2005;228:203–209. doi: 10.1016/j.canlet.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 171.Ara T, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bagnato A, Rosano L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008;40:1443–1451. doi: 10.1016/j.biocel.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 173.Valta MP, et al. FGF-8 is involved in bone metastasis of prostate cancer. Int J Cancer. 2008;123:22–31. doi: 10.1002/ijc.23422. [DOI] [PubMed] [Google Scholar]
- 174.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 175.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lodie TA, et al. Systematic analysis of reportedly distinct populations of multipotent bone marrow-derived stem cells reveals a lack of distinction. Tissue Eng. 2002;8:739–751. doi: 10.1089/10763270260424105. [DOI] [PubMed] [Google Scholar]
- 177.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 178.Venneri MA, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 179.Janeway C, Travers P, Walport M, Shlomchik M. Immunobiology: the immune system in health and disease. 6. Garland Science Publishing; New York: 2005. [Google Scholar]
- 180.Ribatti D, Crivellato E, Roccaro AM, Ria R, Vacca A. Mast cell contribution to angiogenesis related to tumour progression. Clin Exp Allergy. 2004;34:1660–1664. doi: 10.1111/j.1365-2222.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- 181.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 182.Bertolini F, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 183.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nature Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 184.Nolan DJ, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nature Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gerhardt H, Semb H. Pericytes: gatekeepers in tumour cell metastasis? J Mol Med. 2008;86:135–144. doi: 10.1007/s00109-007-0258-2. References 24 and 186, found that fibroblasts isolated from human prostate or breast cancer dramatically increase tumour cell growth in tissue recombination experiments. [DOI] [PubMed] [Google Scholar]
- 187.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 188.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 189.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 190.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 191.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 192.Walzer T, Jaeger S, Chaix J, Vivier E. Natural killer cells: from CD3−NKp46+ to post-genomics meta-analyses. Curr Opin Immunol. 2007;19:365–372. doi: 10.1016/j.coi.2007.04.004. [DOI] [PubMed] [Google Scholar]