Abstract
MicroRNAs (miRNA) are short (~22 nt) single stranded RNAs that downregulate gene expression. Although recent studies indicate extensive miRNA changes in response to ischemic brain injury, there is currently little information on the roles of specific miRNAs in this setting. Heat shock proteins (HSP) of the HSP70 family have been extensively studied for their multiple roles in cellular protection, but there is little information on their regulation by miRNAs. We used bioinformatics to identify miR-181 as a possible regulator of several HSP70 family members. We validated GRP78/BIP as a target by dual luciferase assay. In response to stroke in the mouse we find that miR-181 increases in the core, where cells die, but decreases in the penumbra, where cells survive. Increased levels of miR-181a are associated with decreased GRP78 protein levels, but increased levels of mRNA, implicating translational arrest. We manipulated levels of miR-181a using plasmid overexpression of pri-miR-181ab or mimic to increase, and antagomir or inhibitor to reduce levels. Increased miR-181a exacerbated injury both in vitro and in the mouse stroke model. Conversely, reduced levels were associated with reduced injury and increased GRP78 protein levels. Studies in C6 cells show that if GRP78 levels are maintained miR-181a no longer exerts a toxic effect. These data demonstrate that miR-181 levels change in response to stroke and inversely correlate with levels of GRP78. Importantly, reducing or blocking miR-181a protects the brain from stroke.
Keywords: MicroRNA, miR-181a, 78 kDa glucose-regulated protein (GRP78/HSPA5/BiP), stroke, Heat shock protein, Translational regulation
Introduction
Chaperones play a key role in the organization of molecular, organellar, and cellular networks under both physiological and pathological conditions. The heat shock proteins of the 70 kDa molecular weight family (HSP70), including HSP72 (cytosol), GRP75 (mitochondria), and GRP78/BIP (endoplasmic reticulum; ER), are highly evolutionarily conserved and have been extensively studied. Studies, including those from our laboratory, show that all three of these HSP70 family members are protective in animal models of stroke (Hoehn et al., 2001; Kudo et al., 2008; Oida et al., 2008; Ouyang et al., 2011; Rajdev et al., 2000; Xu et al., 2009; Xu et al., 2011). GRP78 has numerous essential functions in the cell including participating in protein folding in the ER, the unfolded protein response, stress induced autophagy, cytoprotection and inhibition of apoptosis, and is central to both physiological and pathological conditions (Ni et al., 2011). While strongly localized to the ER, recent work also demonstrates roles for GRP78 in additional cellular locations including the cytosol, and at the plasma membrane primarily in tumor cells (Ni et al., 2011). Complete loss of GRP78 was shown to be embryonic lethal by day 3.5 (Luo et al., 2006). The ER stress response and GRP78 are also central in ischemia (Aoki et al., 2001; Morimoto et al., 2007; Nakka et al., 2010; Ouyang et al., 2011). For recent reviews of the roles of endoplasmic reticulum (ER) stress in disease see (Lin et al., 2008; Marciniak and Ron, 2006; Rao and Bredesen, 2004; Wang et al., 2009; Yang and Paschen, 2009). Although animal studies to date have not distinguished the injury core compared to the surrounding penumbra when assessing GRP78 in models of focal ischemia, increases in Grp78 mRNA (Nakka et al., 2010) and protein (Morimoto et al., 2007) were reported in cortex and striatum after transient and permanent MCAO, respectively. In contrast, GRP78 protein was found to decrease in the hippocampus following global ischemia (Aoki et al., 2001).
The discovery of microRNAs (miRNAs) has added a new level of posttranscriptional regulatory control to our understanding of the regulation of gene expression. Recently, changes in miRNAs with ischemic brain injury have been identified using miRNA profiling techniques in a rat middle cerebral artery occlusion (MCAO) model (Dharap et al., 2009; Jeyaseelan et al., 2007; Liu et al., 2010) and in forebrain ischemia (Yuan et al., 2010) as well as in stroke patients (Tan et al., 2009). These findings suggest miRNAs are potential biomarkers and therapeutic targets in stroke. While inhibition of protein synthesis has long been recognized as a major feature of ischemia, to date no studies have investigated a possible role for miRNAs in translational inhibition of specific mRNAs, particularly in the regulation of induction of stress response chaperones.
In this study, we find for the first time that miR-181 contributes to translational inhibition of GRP78 and to cerebral ischemic injury.
Materials and Methods
miRNA, pri-miRNA, antagomir, 3′UTRs, and controls
Fluorescent-tagged miRNA transfection control, negative control, miR-181a mimic, and inhibitor were purchased from Thermo Scientific via Dharmacon; catalogue numbers are listed in Table 1. miR-181a antagomir and its negative control (mismatched miR-181a antagomir) were from Alnylam Pharmaceuticals, and sequences are in Table 2. Wild-type mature miR-181a-d and their seed mutant sequences are listed in Table 2. MWX-PGK-IRES-GFP vector was a kind gift from Dr. Chang-Zheng Chen at Stanford University. DNA fragments containing the pri-miR-181ab or pri-miR-181cd hairpin and ~250 nt flanking sequence on each side were cloned downstream of the PGK promoter in MWX-PGK-IRES-GFP to create the plasmids for pri-miR-181 overexpression.
Table 1.
Materials bought from companies with catalog numbers
| Name | Catalog Number | Company |
|---|---|---|
| miR-181a Primer | 000480 | Applied Bioscience (Foster City, CA, USA) |
| miR-181b Primer | 001098 | |
| miR-181c Primer | 000482 | |
| miR-181d Primer | 001099 | |
| U6 snRNA Primer | 001973 | |
| miR-181a Mimic | C-310434-05-0005 | Thermo Scientific via Dharmacon (Chicago, IL, USA) |
| miR-181a Inhibitor | C-310434-07-0005 | |
| Positive Control | CP-004500-01-05 | |
| Negative Control | CN-001000-01-05 |
Table 2.
miR-181, miR-181 antagomir and 3′UTR of HSPA5 and their seed mutants or mismatch sequences
| Wild Type (5′ to 3′) | Seed Mutant or Miss Match (5′ to 3′) | |
|---|---|---|
| miR-181a | AACAUUCAACGCUGUCGGUGAGU | AUGUUAGUACGCUGUCGGUGAGU |
| miR-181b | AACAUUCAUUGCUGUCGGUGGGU | AUGUAAGUUUGCUGUCGGUGGGU |
| miR-181c | AACAUUCAACCUGUCGGUGAGU | AAGUGAGUUCCUGUCGGUGAGU |
| miR-181d | AACAUUCAUUGUUGUCGGUGGGU | AUGUAAGUAUGUUGUCGGUGGGU |
| miR-181a | soAsoCoUoCoAoCoCoGoAoCoAoGo | soAsoGoUoCoAoGoCoGoAoGoAoGo |
| Antagomir* | CoGoUoUoGoAoAoUsoGsoUsoUs-chol | CoCoUoUoGoAoUoUsoGsoUsoUs-chol |
| HSPA5 | G-UCUCGAAUGUAA-UU | G-UCUCCUUACAAA-UU |
From Alnylam Pharmaceuticals (Cambridge, MA, USA). oN = 2′-O-Methyl nucleotide; s = phosphorothioate linkage
Dual Luciferase target validation assay
Full length mouse HspA5, HspA9, and HspA1A 3′UTRs were cloned into the Renilla luciferase reporter vector phRL-TK (Promega). The primer sets used to generate specific 3′UTR fragments are shown in Table 3. We generated a mutant 3′UTR of the HspA5 gene with 6 base substitutions. The sequences of wild-type and mutant HspA5 3′UTR segments (nt 86–92 of the mouse sequence) are listed in Table 2. The 6 mutated nucleotides are indicated in bold and underlined. Both wild-type and mutant inserts were confirmed by sequencing.
Table 3.
Primer sets used to generate full length of 3′UTRs of Hsp70 family genes or to measure Grp78 mRNA level
| Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | ||
|---|---|---|---|
| 3′UTR | HSPA1A | TAGAGGCCTCTGCTGGCTCT | TGGCAAGTGATTTTGAAGTTTATTTTC |
| HSPA5 | GTGCACTGATCTGCTAGAGC | CTTAAATTTTAGTTATCTAAAATAAAAGATGG | |
| HSPA9 | TAATAGCAGAAATTTTGAAGCCAGAAG | GGAACCCAGATTTCTTTCCCTA | |
| mRNA | grp78 | GGAAAGAAGGTTACCCATGC | GGAACAGGTCCATGTTCAGC |
| actin | GGCTGTGTTGTCCCTGTAT | CCGCTCATTGCCGATAGTG |
Reverse Transcription Quantitative real-time Polymerase Chain Reaction (RT-qPCR) for miRNA quantitation
Total RNA was isolated with TRIzol® (Invitrogen). Reverse transcription was performed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). Equal amounts of total RNA (200 ng) were reverse-transcribed with 1.3 mM dNTPs (with dTTP), 50 U reverse transcriptase, 10 U RNase inhibitor, and specific miRNA reverse transcriptase primers (Applied Biosystems) at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. PCR reactions were then conducted using the TaqMan® MicroRNA Assay Kit (Applied Biosystems) at 95°C for 10 min, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 min. Each reaction contained 0.75 μl of the RT reaction product, 5 μl TaqMan 2×Universal PCR Master Mix (Applied Biosystems) in a total volume of 10 μl using the 7900HT (Applied Biosystems). Predesigned primer/probes (Applied Biosystems) for miRNAs and mouse U6 were from Applied Biosystems. The expression of miR-181a/b/c/d was normalized using U6 as the internal control. Measurements were normalized to U6 (ΔCt) and comparisons calculated as the inverse log of the ΔΔCT to give the relative fold change for all miRNA levels (Livak and Schmittgen, 2001). Liu et al have validated U6 as not changing in cerebral ischemia (Liu et al., 2010). The PCR experiments were repeated 3 times, each using separate sets of samples.
Reverse Transcription-PCR (RT-PCR) for mRNA quantitation
Total RNA was isolated from the core and penumbra brain regions after MCAO or similar anatomical locations from sham mice using TRIzol® reagent (Invitrogen). Briefly, after quantification 2μg RNA was reverse transcribed into complementary DNA (cDNA) using SuperScript® first-strand synthesis system for RT-PCR (Sigma). PCR was performed using Platinum pfx DNA polymerase kit (Sigma) according to manufacturer’s protocol. PCR was performed in a thermal cycler, PTC-200 (M.J. Research). Each PCR reaction used 0.2 μM primer under conditions: denaturation (94ºC, 15 s), annealing (55 ºC, 30 s), and extension (68 ºC, 1 min) for 30 cycles. The amplified RT-PCR products were separated by 1.2% agarose gel electrophoresis and visualized with ethidium bromide (0.5 μg /ml) staining. The relative band intensity was measured using a gel documentation system (BIO-RAD) and the results were expressed as relative changes in mRNA by densitometry analysis. Primers used for gene expression studies were either designed using online free primer design software (http://www.genscript.com/ssl-bin/app/primer) or used previously reported primer sequences. All primers were purchased from the Protein and Nucleic Acid Facility (Stanford University); sequences are listed in Table 3.
Luciferase reporter assay
The luciferase reporter assay was performed as described by Trujillo et al. (Trujillo et al., 2010). BOSC23 cells were plated at a density of 1.2–1.5 x 104 cells/well in 96-well plates one day before transfection. Cells were co-transfected with 0.25 ng firefly luciferase control reporter plasmid, 0.05ng Renilla luciferase target reporter, and 40 ng miRNA expression vector using Fugene (Roche) according to the manufacturer’s instructions. At 24 hr post-transfection, 100 μl of culture medium was added to each well. Cells were harvested 48 hr post-transfection and assayed using the Dual-Luciferase system (E1960, Promega). Results are expressed as relative luciferase activity by first normalizing to the firefly luciferase transfection control, then to the Renilla/firefly value of the empty control vector and finally to the corresponding seed mutant reporter control.
Intracerebroventricular infusion of miRNA plasmid and antagomir
All experimental protocols using animals were performed according to protocols approved by the Stanford University Animal Care and Use Committee and in accordance with the NIH guide for the care and use of laboratory animals. Plasmid encoding pri-miR-181ab was injected intracerebroventricularly as we did previously in mice (Han et al., 2009). Adult male CB57/B6 mice (25–30g from Charles River) were anesthetized with 2% isoflurane in 70% N2O balance O2 by facemask and placed in a stereotaxic frame with a mouse head holder. The 26g brain infusion cannula was stereotaxically placed into the left lateral ventricle (bregma: −0.58 mm; dorsoventral: 2.1 mm; lateral: 1.2 mm) via a burr hole as described before (Xiong et al., 2011) and affixed to the skull. One μg of plasmid or antagomir or their controls was mixed with the cationic lipid DOTAP (1:3 μg/μl; Roche). After mixing for 5 seconds and incubating at 37 oC for 15 min, the mixture (total 6μl) was infused into the left lateral cerebral ventricle over 20 min. After that the bone wound was closed with bone wax, anesthesia was discontinued, and mice were returned to their cages.
Transient focal cerebral ischemia
Two days after injection of plasmid or 1 day after injection of antagomir, mice were anesthetized as described above and focal cerebral ischemia was produced by 1 hr of middle cerebral artery occlusion (MCAO) with a silicone-coated 6-0 monofilament followed by reperfusion as described before (Han et al., 2009; Xiong et al., 2011). Sham-operated mice underwent an identical procedure, without inserting the suture. Rectal temperature was maintained at 37 ± 0.5 ºC with a heating pad. Temperature and respiratory rate were monitored continuously and surgical duration was 20 min for all animals for suture placement. After different durations of reperfusion, mouse brains were removed after transcardial perfusion first with ice cold phosphate buffered saline (PBS) then 4% paraformaldehyde in PBS to assess infarct volume, or rapidly after perfusion only with PBS for RT-qPCR or Western blot analysis. To explore possible regional changes of miRNA expression, predetermined regions of brain tissue corresponding approximately to the ischemic core and penumbra were separated by dissection for RT-qPCR and Western blot as previously described (Gao et al., 2008). Mice were randomized to surgery or sham, and mice with no evidence of acute neurological deficit or with evidence of hemorrhage were excluded from analysis.
Measurement of cerebral infarction area
At 24 hr after MCAO, the mice were deeply anesthetized with isoflurane, and brains were harvested rapidly after perfusion with cold phosphate buffered saline and cold 4% paraformaldehyde. Brains were sectioned coronally into 50 micron sections with a vibratome and stained with cresyl violet (EMD Chemicals). Infarction volume was determined using six slices per mouse, analyzed by a blinded observer, and corrected for edema using Adobe Photoshop CS3 as described previously (Han et al., 2009).
Cell cultures and transfection
Primary astrocyte cultures were prepared from postnatal day 1–3 Swiss Webster mice (Charles River) as described previously (Ouyang et al., 2006). Briefly, neocortices were dissected, treated with trypsin, and plated as a single-cell suspension. BOSC 23 and C6 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and grown in Dulbecco’s Modified Eagle Medium (DMEM, #11995, Gibco) supplemented with 10% FBS and 100 μg/ml penicillin/streptomycin. Astrocyte primary cultures in 24-well plates were transfected with pri-miR-181 plasmids or their controls using FuGeneHD (Roche) according to the manufacturer’s instructions. A stably transfected subline overexpressing GRP78 was created by transfecting C6 cells with the pcDNA3-His-Grp78 plasmid (Zeng et al., 2004) using lipofectamine 2000 (Invitrogen) and selection with 500 μg/ml G418 for 2 months. Overexpression of GRP78 was confirmed by immunoblot.
Injury paradigms, assessment of cell death, and live cell imaging
Glucose deprivation (GD) was performed on primary astrocyte cultures and C6 cells as described previously (Ouyang et al., 2011; Ouyang et al., 2006). Cell injury was quantified after GD by microscopic evaluation and cell counting after Hoechst 33342 (5 μM) and propidium iodide (PI, 5 μM) staining. PI stains dead cells but does not cross intact plasma membranes. Hoechst is a cell-permeant nucleic acid stain that labels both live and dead nuclei. Reactive oxygen species (ROS) and mitochondrial membrane potential were monitored using hydroethidine (HEt) and tetramethylrhodamine methyl ester (TMRE) respectively, as previously described (Ouyang et al., 2011) using a Zeiss Axiovert 200M fluorescence microscope. In some experiments, LDH activity was measured for cell injury as previously described (Ouyang et al., 2006). Medium was sampled at the end of the experiments. Cells were frozen/thawed to provide maximum LDH release values. The percent death (% of LDH release) was calculated by dividing the experimental time point by the maximum values x 100.
Immunoblotting
Immunoblotting was performed as previously described (Han et al., 2009). Briefly, equal amounts (50 μg) of protein were loaded and separated on a polyacrylamide gel (Invitrogen), and electrotransferred to Immobilon polyvinylidene fluoride membrane (Millipore Corp.). Membranes were blocked and incubated overnight with primary antibody against GRP78 (1:10, PA1-37806, Affinity BioReagents) and β-actin (1:1000, 926–42210, LiCOR Bioscience), washed and incubated with 1:15000 anti-rabbit antibody (926–32221, LiCOR Bioscience). Immunoreactive bands were visualized using the LICOR Odyssey infrared imaging system according to the manufacturer’s protocol. Densitometric analysis of bands was performed using ImageJ software (NIH). GRP78 band intensity was normalized to β-actin.
Statistics
All data reported represent at least 3 independent experiments for n=3–6 cultures in each experiment; numbers of animals are indicated in figure legends. Data reported are means ± SD. Statistical difference was determined using T-test for comparison of two groups or ANOVA followed by Newman Keuls post test for experiments with >2 groups. P < 0.05 was considered significant.
Results
GRP78 is a target of miR-181
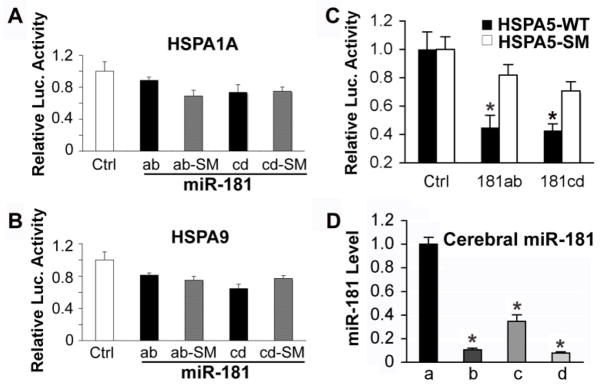
The broadly conserved miRNA family miR-181 is one of many miRNAs which change in the brain in response to ischemia when studied using miRNA profiling techniques (Dharap et al., 2009; Jeyaseelan et al., 2007; Yuan et al., 2010). Using computational miRNA target prediction algorithms TargetScan (http://targetscan.org, Release 5.1) and Microcosm Targets (http://www.ebi.ac.uk/enright-srv/microcosm), we found that miR-181 could potentially target the 3′UTRs of three HSP70 family members, HspA5 (GRP78), HspA1A (HSP72), and HspA9 (GRP75). Interestingly, the sequence identified in the HSPA5 3′UTR that might be targeted by miR-181, nt 86–92 of the mouse 3′UTR, is highly conserved, suggesting a possible critical role in normal cell function. To validate whether miR-181 directly recognizes the 3′UTR of these genes we cotransfected BOSC23 cells with the firefly luciferase control reporter, the Renilla luciferase target reporter containing the 3′UTR of HspA5, HspA1A, or HspA9 (wild type or mutant) and the pri-RNAs (miR-181 or miR-181 seed mutant (SM)). As shown in Fig. 1A and B, none of the miR-181 family members (a to d) had a significant effect on luciferase activity when the 3′ UTRs of HspA1A or HspA9 were present. In contrast, Fig. 1C shows that luciferase activity with the 3′UTR of HspA5 was significantly decreased about 50% compared to the 3′UTR seed mutant control with either pri-miR-181ab or pri-miR-181cd. As reported earlier (Chen et al., 2004), miR-181 is strongly expressed in normal brain. In normal control brain miR-181a was present at the highest level, with miR-181b about 10%, miR-181c about 34%, and miR-181d about 8% of miR-181a levels (Fig. 1D).
Fig. 1.
Hspa5 is the target of miR-181. A, B. Dual luciferase activity assays performed in BOSC23 cells co-transfected with the plasmid containing Renilla luciferase followed by the Hspa1a or Hspa9 3′UTR (WT) and plasmids encoding either pri-miR-181ab or pri-miR-181cd or their seed mutants (SM) demonstrates that miR-181 does not recognize either of these 3′UTRs, as there is no reduction of luciferase activity compared to the SM control. C. The same assay performed with the Hspa5 3′UTR (Hspa5-WT) or its seed mutant (Hspa5-SM) shows that miR-181ab and cd both reduce luciferase activity. *P<0.05, statistically different from the SM group by T-test. Luciferase assays were performed 3 times in triplicate. D. Differing levels of expression of miR-181a, b, c, and d are detected in cortex of normal control brains. N=4 animals, assayed in triplicate, * P<0.01 different than levels of miR-181a by ANOVA and Newman-Keuls post hoc test.
miR-181, GRP78 protein, and Grp78 mRNA change after stroke
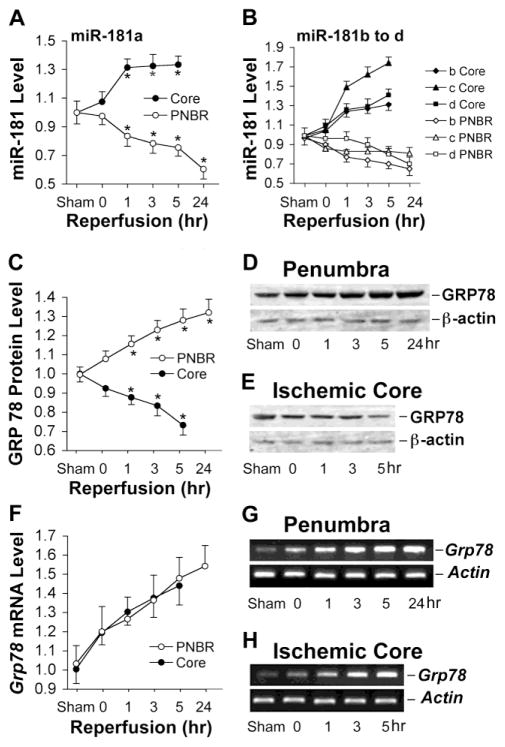
To explore possible regional and spatial changes of miR-181 expression in response to stroke, transient middle cerebral artery occlusion (MCAO) was performed in the mouse. Brain tissue was dissected into ischemic core and ischemic penumbra after 0, 1, 3, 5, and 24 hr reperfusion following 1 hr MCAO. Levels of all miR-181 family members changed, increasing in the core but decreasing in the penumbra (Fig. 2A, B). By 24 hr of reperfusion, the miR-181a level in the penumbra decreased the most of the miR-181 family to 60±8% compared to sham control animals (Fig. 2A). At 24 hr data is only presented for penumbra since cell death has begun to occur in the core making interpretation of miRNA levels difficult. Although all members of the family change in a similar direction in response to ischemia and have identical seed sequences, since miR-181a is the most abundant it is likely to have the largest effect.
Fig. 2.
Expression of miR-181, GRP78 protein, and Grp78 mRNA after transient focal ischemia (MCAO). A. miR-181a expression in ischemic core and penumbra at different durations of reperfusion after 1 hr MCAO in mice shows increased levels in core but decreased levels in the penumbra (PNBR). B. miR-181b, c, and d show similar changes after ischemia, increasing in the core and decreasing in the penumbra. C. GRP78 protein decreases in the ischemic core and increases in the penumbra with increasing durations of reperfusion after MCAO. Quantitation by densitometry of westerns for 4 mice at each timepoint. D, E. Examples of western blots for GRP78 isolated from penumbra and ischemic core with increasing reperfusion time. F. Expression of Grp78 mRNA in ischemic core and penumbra increases with reperfusion time after MCAO. G, H. Examples of RT-PCR products from RNA harvested at increasing times after MCAO. Actin was used as the loading control for westerns and internal control for RT-PCR. N=4 mice/group in all experiments. *P<0.05 by ANOVA and Newman-Keuls post hoc test.
To confirm that miR-181 acts as a negative regulator of GRP78 translation under ischemic conditions we examined the time course for both GRP78 protein (Fig 2 C-E) and Grp78 mRNA (Fig 2F-H) expression at the same reperfusion time points. A reciprocal expression of miR-181a and GRP78 protein was found in both core and penumbra (Fig. 2C). Following MCAO, Grp78 mRNA was induced in both ischemic core and penumbra (Fig. 2F), while GRP78 protein declined in the core. The increased levels of miR-181a in the ischemic core could cause translational block and reduced levels of GRP78 protein. The differential regional expression of miR-181, GRP78 protein, and Grp78 mRNA indicates that miR-181 may play a role in the development of the ischemic core as well as in protection of the penumbra from further injury after cerebral ischemia. Since miR-181 is regulated in response to ischemia we further investigated its effect on brain cell death.
Changing miR-181a levels regulates cell death in vitro
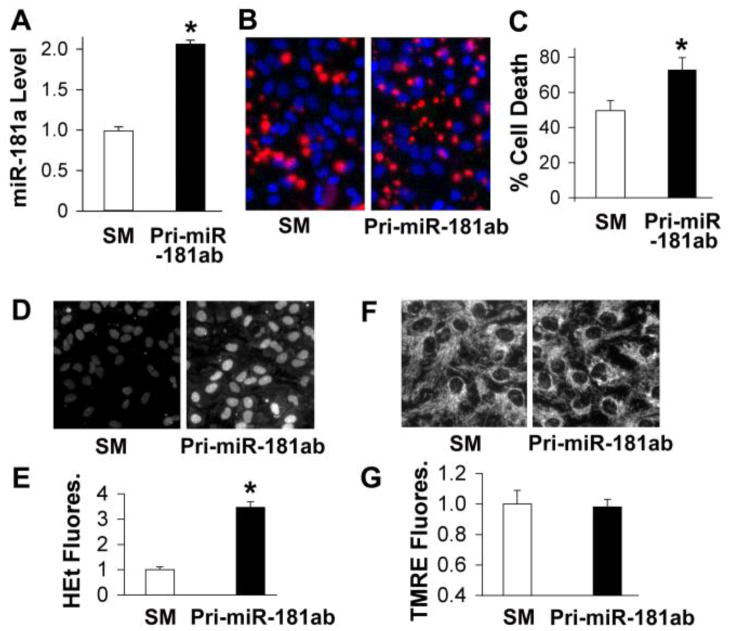
To investigate the functional significance of miR-181a in brain cell death, we used a pri-miR-181ab construct to increase miR-181. RT-qPCR analysis revealed that miR-181a expression was more than 7 fold that of miR-181b in BOSC23 cells, which express negligible amounts of miR-181 under normal conditions (data not shown). Thus the effect of pri-miR-181ab is likely mainly due to miR-181a. Fig. 3 shows the effect of expressing pri-miR-181ab in primary astrocyte cultures. miR-181a levels approximately double after transfection with pri-miR-181ab (Fig. 3A), and glucose deprivation (GD) induced cell death increased significantly compared to cells transfected with the seed mutant construct when assessed by cell counting after propidium iodide staining (Fig. 3B, C). These results support the conclusion that increased miR-181a levels worsen ischemia-like injury. Astrocytes transfected with pri-miR-181ab (Fig. 3D, E) showed markedly increased baseline generation of ROS after transfection. In contrast, pri-miR-181ab (Fig. 3F, G) did not affect mitochondrial membrane potential in unstressed cells through 48 hr after transfection. Our data indicate that although cells produce more ROS, there was no cell death caused by increased miR-181a levels under control conditions.
Fig. 3.
Effect of pri-miR-181ab transfection on reactive oxygen species and ischemia-like astrocyte injury in vitro. A. pri-miR-181ab induced increased levels of miR-181a in astrocytes following transfection. B. pri-miR-181ab overexpression by plasmid transfection aggravates cell injury induced in primary astrocytes by 24 hr glucose deprivation (GD). Representative micrographs of cultures stained with PI and Hoechst dye are shown. C. Quantitation by cell counting is shown for 3 separate sets of cultures. D. Representative micrographs show increased ROS by increased HEt fluorescence in astrocytes after transfection with pri-miR-181a compared to its seed mutant (SM) control in unstressed cells under normal growth conditions. E. Bar graph shows quantitation of HEt fluorescence in transfected cultures under normal growth conditions. F. Representative micrograph shows similar mitochondrial membrane potential by TMRE fluorescence in cultures under normal growth conditions whether transfected with pri-miR-181ab or the seed mutant. G. Bar graph shows quantitation of TMRE fluorescence. All experiments were performed in triplicate using three sets of culture each time. *P<0.01 by T-test.
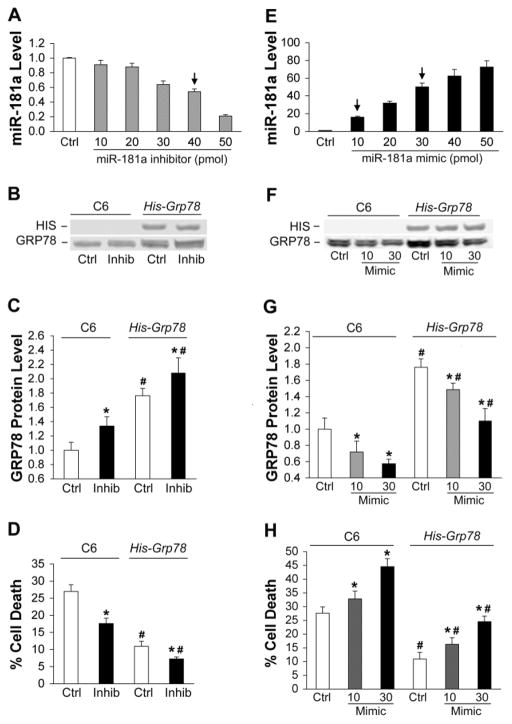
To confirm and extend these observations gain- or loss-of-function was achieved by transfecting C6 glial cells with miR-181a mimic or inhibitor (Fig. 4). We found that high concentrations (50 pmol) of mimic or inhibitor damaged cells after transfection. After assessing the dose response we chose a concentration that caused no damage under normal conditions, but still significantly changed miR-181a levels. The dose-response to transfection with increasing concentrations of miR-181a inhibitor is shown in Fig. 4A; 40 pmol inhibitor was used for subsequent experiments.
Fig. 4.
Effect of miR-181a mimic and inhibitor on C6 glia with or without GRP78 overexpression. A. Dose-response of miR-181a levels to transfection with increasing amounts of miR-181a inhibitor in C6 cultures. N=3. The arrow indicates the concentration used for B, C, and D of this figure. B. Representative blot shows miR-181a inhibitor increases GRP78 protein expression both in parental C6 cells and in the C6 line that stably overexpresses His-Grp78 under the control of the CMV promoter and lacking the native 3′UTR. C. Bar graph showing quantitation of significant changes in GRP78 protein expression with miR-181a inhibitor. D. miR-181a inhibitor reduces injury induced by 8 hr glucose deprivation (GD) in C6 cultures and His-Grp78 overexpressing cultures. E. Dose-response of miR-181a levels after transfection with increasing amounts of mimic in C6 cultures. N=3. The arrows indicate the concentrations used for F, G, and H of this figure. F. Representative blot shows miR-181a mimic decreases GRP78 protein expression in both C6 and C6- His-Grp78 overexpressing cells. G. Bar graph shows quantitation of GRP78 protein expression after transfection with miR-181a mimic. Protein levels in Ctrl C6 cells and His-Grp78 overexpressing cells with 30 pmol mimic were not statistically different. N=3. H. miR-181a mimic aggravates injury induced by 8 hr GD in both C6 and His-GRP78 overexpressing C6 cells. Cell death in Ctrl C6 cells and His-Grp78 overexpressing cells with 30 pmol mimic were not statistically different. All experiments were performed 3 times in triplicate. *P<0.01 compared with same cell line control (Ctrl) and #P<0.01 compared with the same dose of miR-181 inhibitor or mimic in C6 group by ANOVA and Newman-Keuls post hoc test.
Because microRNAs commonly have multiple targets we wished to test whether GRP78 was the important target in the case of ischemic injury. To do this we created a C6 subline stably expressing his tagged Grp78 under the control of the CMV promoter and lacking the Grp78 3′UTR and therefore not regulated by miR-181, rather than try to use cells lacking GRP78 since it is an essential protein and knockout is early embryonic lethal (Luo et al., 2006). We assessed changes in protein levels of GRP78 in both normal and GRP78 overexpressing C6 cells after transfection with inhibitor (Fig 4B, C) to decrease levels of miR-181a. Only the overexpressing line shows a HIS immunoreactive band (Fig 4B) and baseline levels of GRP78 are elevated in the overexpressing cell line (Fig 4 B, C). In both cases there is an increase of GRP78 after transfection with miR-181a inhibitor (Fig 4C), though the percent increase is higher in the normal C6 cells (34%) vs the overexpressing cells (18%), the absolute increases are similar. The effect of reducing miR-181a levels was to increase cell survival of 8 hr GD (Fig. 4D).
To further confirm the importance of GRP78 in the effect of miR-181 on cell survival we compared the GRP78 overexpressing C6 cell line with the parent C6 line but now increased miR-181a levels by transfection with mimic. The dose-response achieved with miR-181a mimic transfection is shown in Fig. 4E; 10 pmol and 30 pmol were used for subsequent experiments. As shown in Fig. 4F and G, miR-181a mimic decreases GRP78 protein in a dose dependent manner and aggravates cell damage in both control and GRP78 over-expressed cells (Fig. 4H). Importantly, when miR-181a mimic at 30 pmol reduces GRP78 protein levels in the GRP78 over-expressing cells (Fig. 4G, last bar) to approximately the level in the C6 control group (not statistically different, Fig 4G) in the absence of mimic (Fig. 4G, first bar), cell injury in the two groups is also similar (Fig. 4H, first and last bars), despite a greater than 40 fold difference in levels of miR-181a. Thus GRP78 is a major target of miR-181a in determining cell survival or death from stress.
Effect of miR-181a overexpression and inhibition on transient focal cerebral ischemic injury in vivo
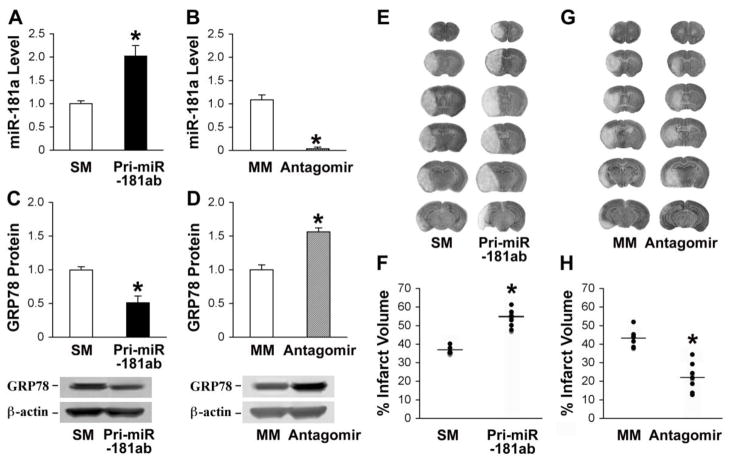
To evaluate the biological role of miR-181 in ischemic brain injury in vivo we altered brain levels by injecting either pri-miR-181ab plasmid or miR-181a antagomir by intracerebroventricular (ICV) infusion. Two days after administration of pri-miR-181ab, about 2 fold overexpression was observed (Fig. 5A), and one day after administration of the antagomir nearly complete knockdown of miR-181a levels (Fig. 5B) in the brain were confirmed by quantitative RT-PCR analysis. In contrast, the plasmid encoding pri-miR-181ab with seed mutations or mutant miR-181a antagomir had no effect on the expression level of miR-181a compared with the saline control (data not shown). The expression levels of GRP78 protein were inversely correlated with the expression of miR-181a (Fig. 5C, D). Overexpression of miR-181a aggravated cerebral ischemic damage and increased the infarct size at 24 hr of reperfusion after MCAO (Fig. 5E, F) and knockdown of miR-181a effectively attenuated ischemic injury (Fig. 5G, H). These data demonstrate that inhibition of cerebral miR-181a provides neuroprotection from ischemia, likely via increasing levels of GRP78. Since neither the antagomir nor the Pri-miR-181ab used in this experiment were targeted to a specific cell type, the changes in injury likely reflect changes in miR-181ab levels in more than one cell type.
Fig. 5.
Effect of miR-181 up- or down-regulation on miR-181a, GRP78, and infarction after focal ischemia. A. Mice injected ICV with pri-miR-181ab plasmid show elevated brain levels of miR-181a by RT-qPCR. B. Mice pretreated with miR-181a antagomir show reduced levels of miR-181a in the brain. C. GRP78 protein levels are decreased in the brains of mice pretreated with pri-miR-181ab plasmid. D. GRP78 protein levels increased in brains pretreated with miR-181a antagomir. Representative immunoblots are shown under the graphs. N=4 mice in each group for A to D. E. Representative cresyl violet-stained coronal sections demonstrate an increased infarct size in a representative miR-181a overexpressing brain compared with the brain of a miR-181 seed mutation (SM)-injected animal also subjected to MCAO. F. The bar graph shows quantitation of infarct size by cresyl violet staining for the group of mice. G. Representative cresyl violet stained brains of miR-181a antagomir transfected or mismatch (MM) miR-181a-antagomir-injected animals. H: The graph shows quantification of the infarct size. N=7 mice/group for E to H. *P<0.01 compared to SM or MM control by T-test.
Discussion
The major finding of this report is that a brain-enriched miRNA, miR-181, regulates GRP78 expression and outcome from cerebral ischemia. For the first time we report that GRP78 is a physiological target of miR-181, with expression reduced due to translational repression rather than mRNA degradation.
Transient translational arrest is appreciated to be a stereotypical response to ischemia and a variety of cell stresses, including the heat shock response and the unfolded protein response which may also occur in the setting of ischemia. Transient translational arrest limits the increase in unfolded/misfolded proteins and allows for rapid induction of a stress response with selective translation of stress proteins before synthesis of constitutive proteins resumes. Several biochemical pathways and sequestration of ribosomes are well studied components of ischemic translational arrest. Prior studies have focused on biochemical mechanisms of transient translational arrest, particularly phosphorylation of eukaryotic Initiation Factor 2 α (eIF2α ), and sequestration of ribosomes in persistent translational arrest (DeGracia and Hu, 2007; DeGracia et al., 2008). However, whether miRNAs participate in translational arrest of specific proteins or aspects of the stress response following ischemia has not yet been studied.
This study provides evidence for a novel transcript-specific mechanism of suppression of protein synthesis and regulation of the stress response following ischemia, in addition to the previously described general mechanisms of translational arrest. It provides novel insight into the long standing observation that cells destined to die fail to produce heat shock proteins, as is well documented for HSP72 (Kinouchi et al., 1993a; Kinouchi et al., 1993b; Vass et al., 1988), while cells that survive make new heat shock proteins. We report here that the ischemic core shows increased levels of miR-181, while the penumbra, which can survive, shows decreased levels of miR-181, and this correlates with a failure to elevate levels of GRP78 protein in the core, while GRP78 is successfully induced in the penumbra. Interestingly, a recent post-mortem study of brains of stroke victims found a relatively early increase in GRP78 in the penumbra (Duan et al., 2010), consistent with our observations in mice.
The miR-181 family, especially miR-181a and miR-181b, are enriched in brain (Miska et al., 2004) and their aberrant expression has been associated with brain diseases. hsa-miR-181a and hsa-miR-181b are reduced in human gliomas and glioma cell lines, and expression was negatively correlated with tumor grade (Shi et al., 2008). Conversely, miR-181a sensitized human malignant glioma cells to radiation (Chen et al., 2010). Our current results suggest that low levels of miR-181 could also be associated with increased levels of chaperones, particularly GRP78, a common adaptation to chronic stress in tumor cells with inhibition of apoptosis.
A few studies have assessed changes in miRNA profiles in different models of cerebral ischemia. After 20 min of global ischemia in rat, Yuan and colleagues (Yuan et al., 2010) reported that hippocampal miR-181a was upregulated at 30 min of reperfusion with a further increase after 24 hours while miR-181d decreased more than 5 fold. As total hippocampi were used, these results reflect changes both in the CA1 region that will die, and the rest of the hippocampus that generally survives this type of injury. No change in brain miR-181 was found after permanent focal ischemia by Liu et al. (Liu et al., 2010) or transient focal ischemia between 3 hours and 3 days reperfusion (Dharap et al., 2009), while Jeyaseelan et al. (Jeyaseelan et al., 2008) detected a decrease in miR-181a, b, and c at 48 hours reperfusion only. All of these studies were in rat, while our experiments were in mice. In addition, we separated the ischemic hemisphere into core and penumbra, where opposite changes were observed, while these prior studies did not separate these regions. Our results show that at early reperfusion miR-181 increases in the injured area destined to die, but decreases in the area that is potentially salvageable. Future studies should also investigate the expression of miR-181 in individual cell types within the brain after ischemic insults.
Prior induction of GRP78 with a pharmacological inducer was shown to reduce neuronal loss in both forebrain (Oida et al., 2008) and focal cerebral ischemia (Kudo et al., 2008). We recently reported that overexpressing GRP78 protects astrocytes against ischemic injury, reducing oxidative stress and maintaining mitochondrial function (Ouyang et al., 2011). Increased Grp78 mRNA was reported in cortex and striatum from 0 to 24 hr of reperfusion after 2 hr MCAO (Nakka et al., 2010). While our results confirm an increase in Grp78 mRNA, we found this in both ischemic core and penumbra after MCAO, while GRP78 protein levels decrease in the ischemic core, consistent with translational block in this area destined to die. The continuing increase of miR-181 in the area could contribute to this specific translational block. In contrast, the penumbra showed induction of both Grp78 mRNA and GRP78 protein. This area that can survive the ischemic insult also had decreased miR-181 levels. Our data indicate that downregulation of miR-181 might represent an important adaptive mechanism to facilitate ischemic recovery by increasing protein levels of GRP78. A key regulator of the unfolded protein response, GRP78 also has important functions in development, cancer, metabolic, and neurological disorders (Lin et al., 2008; Marciniak and Ron, 2006; Rao and Bredesen, 2004; Wang et al., 2009; Yang and Paschen, 2009). This report now adds miR-181 as an additional point of regulation for GRP78, in addition to the well-studied mechanisms that comprise the unfolded protein response to ER stress.
Conclusions
Our data demonstrates that miR-181 contributes to cerebral ischemic injury. Knockdown of endogenous miR-181 provides protection against ischemia/reperfusion-induced brain cell death by targeting GRP78, a well-studied and important molecular chaperone and cytoprotective protein. Identifying this miRNA as a critical player in the regulation of ischemic brain cell death in vitro and in vivo expands our understanding of the function of miRNAs and also identifies a novel direction for the development of future treatment strategies to reduce the impact of ischemic injury by manipulating miRNA levels.
Highlights.
Reducing miR-181a protects the brain from stroke and neural cells from ischemic stress
miR-181 increases in the core but decreases in the penumbra after MCAO
Reciprocal expression of miR-181a and GRP78 protein was found
GRP78, an essential ER protein, is a target of miR-181
mir181 causes translational arrest of GRP78 mRNA after ischemia
Acknowledgments
This work was supported in part by NIH grants NS053898 and GM49831 to RGG and T32 GM089626 to REW. The authors would like to thank Chang-Zheng Chen for plasmid and technical consulting, Dong-Xue He for technical assistance with brain sectioning and analysis of infarcts, and William Magruder for help preparing the manuscript. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki M, et al. Hypothermic treatment restores glucose regulated protein 78 (GRP78) expression in ischemic brain. Mol Brain Res. 2001;95:117–28. doi: 10.1016/s0169-328x(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Chen CZ, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chen G, et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Hu BR. Irreversible translation arrest in the reperfused brain. J Cereb Blood Flow Metab. 2007;27:875–893. doi: 10.1038/sj.jcbfm.9600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia DJ, et al. Translation arrest and ribonomics in post-ischemic brain: layers and layers of players. Journal of Neurochemistry. 2008;106:2288–2301. doi: 10.1111/j.1471-4159.2008.05561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, et al. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–87. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan SR, et al. Ischemia induces endoplasmic reticulum stress and cell apoptosis in human brain. Neurosci Lett. 2010;475:132–5. doi: 10.1016/j.neulet.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Gao X, et al. The Akt signaling pathway contributes to postconditioning’s protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–55. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han RQ, et al. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth Analg. 2009;108:280–7. doi: 10.1213/ane.0b013e318187ba6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn B, et al. Overexpression of HSP72 After Induction of Experimental Stroke Protects Neurons From Ischemic Damage. J Cereb Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, et al. MicroRNAs as therapeutic targets in human diseases. Expert Opin Ther Targets. 2007;11:1119–29. doi: 10.1517/14728222.11.8.1119. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, et al. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–66. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, et al. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993a;13:105–15. doi: 10.1038/jcbfm.1993.13. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, et al. Induction of heat shock hsp70 mRNA and HSP70 kDa protein in neurons in the ‘penumbra’ following focal cerebral ischemia in the rat. Brain Res. 1993b;619:334–8. doi: 10.1016/0006-8993(93)91630-b. [DOI] [PubMed] [Google Scholar]
- Kudo T, et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–75. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- Lin JH, et al. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annual Review of Pathology: Mechanisms of Disease. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DZ, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo S, et al. GRP78/BiP Is Required for Cell Proliferation and Protecting the Inner Cell Mass from Apoptosis during Early Mouse Embryonic Development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Ron D. Endoplasmic Reticulum Stress Signaling in Disease. Physiological Reviews. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- Miska EA, et al. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto N, et al. Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience. 2007;147:957–967. doi: 10.1016/j.neuroscience.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Nakka V, et al. Endoplasmic Reticulum Stress Plays Critical Role in Brain Damage After Cerebral Ischemia/Reperfusion in Rats. Neurotoxicity Research. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- Ni M, et al. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochemical Journal. 2011;434:181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oida Y, et al. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 2008;1208:217–24. doi: 10.1016/j.brainres.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, et al. Overexpressing GRP78 influences Ca(2+) handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion. 2011;11:279–86. doi: 10.1016/j.mito.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, et al. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11:180–6. doi: 10.1379/CSC-182R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–91. [PubMed] [Google Scholar]
- Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Current Opinion in Cell Biology. 2004;16:653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, et al. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–93. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Tan KS, et al. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo RD, et al. The potential functions of primary microRNAs in target recognition and repression. EMBO J. 2010;29:3272–3285. doi: 10.1038/emboj.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass K, et al. Localization of 70-kDa stress protein induction in gerbil brain after ischemia. Acta Neuropathol. 1988;77:128–35. doi: 10.1007/BF00687422. [DOI] [PubMed] [Google Scholar]
- Wang M, et al. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, et al. Increased brain injury and worsened neurological outcome in IL-4 Knockout mice following transient focal cerebral ischemia. Stroke. 2011 doi: 10.1161/STROKEAHA.110.593772. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29:365–74. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al. Heat shock protein 72 (Hsp72) improves long term recovery after focal cerebral ischemia in mice. Neuroscience Letters. 2011;488:279–282. doi: 10.1016/j.neulet.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Paschen W. The endoplasmic reticulum and neurological diseases. Experimental Neurology. 2009;219:376–381. doi: 10.1016/j.expneurol.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Yuan Y, et al. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J Clin Neurosci. 2010;17:774–8. doi: 10.1016/j.jocn.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Zeng L, et al. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 2004;23:950–958. doi: 10.1038/sj.emboj.7600106. [DOI] [PMC free article] [PubMed] [Google Scholar]