Abstract
The majority of mesophilic waterborne species of the black yeast genus Exophiala (Chaetothyriales) belong to a single clade judging from SSU rDNA data. Most taxa are also found to cause cutaneous or disseminated infections in cold-blooded, water animals, occasionally reaching epidemic proportions. Hosts are mainly fish, frogs, toads, turtles or crabs, all sharing smooth, moist or mucous skins and waterborne or amphibian lifestyles; occasionally superficial infections in humans are noted. Cold-blooded animals with strictly terrestrial life styles, such as reptiles and birds are missing. It is concluded that animals with moist skins, i.e. those being waterborne and those possessing sweat glands, are more susceptible to black yeast infection. Melanin and the ability to assimilate alkylbenzenes are purported general virulence factors. Thermotolerance influences the choice of host. Exophiala species in ocean water mostly have maximum growth temperatures below 30 °C, whereas those able to grow until 33(−36) °C are found in shallow waters and occasionally on humans. Tissue responses vary with the phylogenetic position of the host, the lower animals showing poor granulome formation. Species circumscriptions have been determined by multilocus analyses involving partial ITS, TEF1, BT2 and ACT1.
Keywords: amphibian disease, black yeasts, Chaetothyriales, Exophiala, fish disease, lethargic crab disease, pathogenicity, waterborne fungi
INTRODUCTION
Exophiala is an anamorph genus defined by annellidic conidiogenesis producing slimy heads of conidia, and a phylogenetic affiliation to the ascomycete order Chaetothyriales. Where known, teleomorphs belong to Capronia. Nearly all species are characterized and recognisable within the order by their production of budding cells, and the yeast/hypha transition mostly proceeds via torulose hyphae. The Exophiala ecotype of Chaetothyriales is therefore morphologically characteristic, despite its polyphyletic position within the order. Some Exophiala species produce phialidic, catenate or sympodial synanamorphs, reflecting dynamic life cycles.
The genus Exophiala contains numerous potential opportunists or pathogens of immunocompetent humans. The most serious pathogens, eventually leading to disseminated, fatal infections are the neurotrope Exophiala dermatitidis (Sudhadham et al. 2008), the osteotrope E. spinifera (Li et al. 2008), and a species tending to cause disseminated infection, E. asiatica (Li et al. 2009). These species are able to grow at 37–40 °C, which is taken to be one of the main virulence factors in Chaetothyriales, also being expressed in several pathogenic Cladophialophora species (Badali et al. 2008). However, in the last few decades many Exophiala isolates have been recovered that consistently lacked thermotolerance, but nevertheless were involved in animal disease. Infections were particularly found in fish and amphibians, but occasionally also in invertebrates. This indicates that, in addition to thermotolerance, other intrinsic virulence factors enabling animal infection are shared by members of Chaetothyriales.
Among the early reports of fish infections caused by melanized fungi was that of Reichenbach-Klinke (1956). Carmichael (1966) introduced the genus Exophiala with a report of E. salmonis from cerebral lesions in cut-throat trout (Salmo clarkii). Infections by this species repeatedly took epizootic proportions with up to 40 % mortality in fish hatcheries in Calgary, Canada, where the fish were grown in water drawn from underground springs with a temperature of 12–14 °C. Otis et al. (1985) described visceral infections in Atlantic salmon (Salmo salar) after fishes from Canadian hatcheries were transported to an aquaculture centre. Langvad et al. (1985) reported epizootics occurring over several years in farmed Atlantic salmon (Salmo salar) in Norway. Mortalities of up to 50 % were caused by Exophiala psychrophila (Pedersen & Langvad 1989). Infections took place when smolts were transferred to seawater, leading to visceral invasion with a predilection for the kidney. Identical pathologies linked to visceral symptomatology were described by Richards et al. (1978) in Scotland, but ascribed to Exophiala salmonis.
Fijan (1969) reported an epizootic in channel catfish (Ictalurus punctatus) in a private pond. Lesions were cutaneous and visceral, with a predilection for formation in the kidney. The etiologic agent was later described as Exophiala pisciphila (McGinnis & Ajello 1974). Langdon & McDonald (1987) reported this species from fifteen cranial mycoses in Atlantic salmon (Salmo salar) in Australia. Gaskins & Cheung (1986) described Exophiala pisciphila from brain and skin lesions in a smooth dogfish (Mustelis canis); this concerned a single infection in the New York Aquarium. These authors also provided an overview of the 18 species of fish known to have become infected by members of Exophiala up to that time (1986). Reuter et al. (2003) reported an epidemic in captured King George whiting (Sillaginodes puntuta) in Australian seawater tanks due to an unidentified Exophiala species. Kurata et al. (2008) reported ulcerative skin lesions in the Japanese Flounder (Paralichthys olivaceus). Cutaneous ulcers in captive American plaice (Hippoglossoides platessoides) were reported by Strongman et al. (1997). The infections were ascribed to Hormoconis resinae but de Hoog et al. (2009) corrected the identification of the infective organism to Exophiala pisciphila.
Infections by black yeast-like fungi in cold-blooded animals thus appear to be relatively frequent, at least in captive and farmed fish and amphibians. Many of the etiologic agents above have not been ITS-sequenced (or DNA-barcoded sensu Schoch et al. in prep.), which is a prerequisite for correct identification of Exophiala species (Zeng & de Hoog 2007), and the case reports are scattered in medical, veterinary and environmental literature. More infections, of which the etiologic agent has been preserved and was identified by current standards, are listed in the text below. Outbreaks of infections by melanized fungi in farmed fish and aquarium animals may cause severe losses in aquaculture and fishery industries, but due to the scattered nature of reports it is difficult to estimate the magnitude of the problem.
Numerous additional reports of Exophiala infection concern other kinds of cold-blooded animals. A classical study was that of Beneke (1977) on infections in laboratory-housed frogs. Cicmanec et al. (1973), Velázquez & Restrepo (1975) and Bube et al. (1992) reported spontaneous neurological disorders in marine toads (Bufo marinus). Agents were frequently identified as Fonsecaea pedrosoi; our unpublished analyses suggest that, in recent taxonomy, the causal isolates would be more likely to have been Cladophialophora species close to C. devriesii (G.S. de Hoog, unpubl. data). Manharth et al. (2005) described a disseminated infection in a Galapagos tortoise (Geochelone nigra), caused by an Exophiala species, while Joyner et al. (2006) described a subcutaneous inflammatory mass in an eastern box turtle (Terrapene carolina carolina) and Stringer et al. (2009) reported an infection of bone and carapace in Aldabra tortoise (Geochelone gigantea). Elkan & Philpot (1973) described an Exophiala species (as ‘Phialophora’) with septate conidia, thus strongly resembling E. salmonis or E. pisciphila, from a systemic infection in a frog (Phyllobates trinitatis). Nyaoke et al. (2009) described several disseminated infections in weedy and leafy sea dragons (Phyllopteryx taeniolatus and Phycodurus eques, respectively).
Black yeasts also occur in invertebrates. From 1998, an epidemic took place in mangrove crabs (Ucides cordatus) along the Brazilian coast. This epidemic was caused by a hitherto undescribed Exophiala species, while sometimes co-infection was noted with a Cladophialophora species (Boeger et al. 2005), another member of the order Chaetothyriales. Vakali (1993) reported infection in earthworms (Octolasian tyrtaeum) and was able to reproduce the disease by artificial inoculation and recovery of the organism from cocoons. Dover et al. (2007) reported on a large epizootic of mussels (Bathymodiolus brevior) in the Fiji Basin; the etiologic agent was a relative of Capronia moravica.
Until today, only very few human infections caused by fish-associated species have been reported. A rare example is Exophiala pisciphila in a liver transplant recipient presenting skin papules, which eventually drained (Sughayer et al. 1991). However, in the course of our study we encountered numerous cutaneous cases, which will be discussed below. Recent isolation data suggest that Exophiala species may be dispersed via municipal drinking water (Göttlich et al. 2002, Porteous et al. 2003a, b), where they were hypothesized to be stimulated by the presence of amoebae (Cateau et al. 2009). Several black yeasts known to cause superficial infections in humans have been suggested to have an environmental reservoir in bathing facilities (Hamada & Abe 2009, Lian & de Hoog 2010). This finding raises serious questions concerning safety of tap water for the users.
The taxonomy of the psychrophilic, waterborne Exophiala species has not been sufficiently studied. Given the pressing questions on human and animal health mentioned above, a revision of this group is overdue. In the present paper, the phylogeny, taxonomy and ecology of relevant waterborne Exophiala species is analysed in a multi-locus study using the concept of ‘Genealogical Concordance Phylogenetic Species Recognition’ (GCPSR) (Taylor et al. 2000). Sequence analysis was based on the SSU and ITS rDNA, the partial β-tubulin (BT2) and the translation elongation factor 1-α genes (TEF1).
All waterborne Exophiala species were confirmed to belong to the order Chaetothyriales. Another group of black fungi reported to be common in municipal drinking water (Göttlich et al. 2002) was the genus Cadophora, anamorphs of Pyrenopeziza in the Helotiales (Nauta et al. in prep.). In this order another ecological trend was observed, featuring opportunism and pathogenicity to plants rather than to animals. It is the aim of the present paper to describe the chaetothyrialean component of the waterborne black yeast biota.
MATERIAL AND METHODS
Strains and culture conditions
Strains analysed are listed in Table 1. Reference strains were taken from the CBS culture collection, and eventually supplemented with published materials sent upon request. Strains from drinking water had been isolated between October 1998 and September 1999 by a pour-plate method, using 2 657 1.0 mL water samples from 700 sampling points at 29 separate locations in North Rhine-Westphalia, Germany (Göttlich et al. 2002). Sampling locations were ground water wells, waterworks and storage tanks, hydrants in the distribution network, water taps after water meters or elsewhere in house installations. In most cases the water was unchlorinated. Antarctic strains included were derived from an EU-funded Micromat project (www.sciencepoles.org). Arctic strains were collected by N. Gunde-Cimerman (Ljubljana, Slovenia). Strains were maintained on MEA (2 % Malt Extract Agar) or PDA (Potato-Dextrose Agar) (Crous et al. 2009) slants at 4 °C. Prior to analysis, small pieces from mature colonies were suspended in 4.5 mL sterile water to obtain conidial suspensions. Aliquots of 0.5 mL were plated on PDA in culture plates and incubated at 24 °C for 2–4 wk. Sequences derived in this study were lodged at GenBank, the alignment in TreeBASE (www.treebase.org/treebase/index.html), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Table 1.
Strains analysed of mesophilic waterborne Exophiala species.
| Name | CBS | Other reference numbers | Source | Geography | GenBank |
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSU | ITS | TEF1 | BT2 | ACT1 | ||||||
| Capronia coronata | 617.96 (T) | ATCC 56201 | Wood | New Zealand | JN856009 | JF747040 | JN128782 | JN112422 | JN112378 | Müller et al. 1987 |
| E. alcalophila | 520.82 (T) | Soil | Japan, Wako-shi, Hirose | JN856010 | JF747041 | JN128771 | JN112423 | JN112379 | Goto et al. 1981 | |
| 521.82 | Soil | Japan, Wako-shi, Hirose | JF747042 | JN128772 | JN112424 | JN112380 | Goto et al. 1981 | |||
| 118723 | ISO13G | Soil | Brazil | |||||||
| 118722 | ISO13 | Soil | Brazil | JF747043 | ||||||
| 122256 | dH 17077 | Human, skin | Denmark | JF747044 | JN128773 | JN112425 | ||||
| GHP R18 | Soap container washing machine | Germany | ||||||||
| E. angulospora | 120272 | dH 17395, DTO 06.095 nr.6.1 / 2006 | Drinking water tap | The Netherlands, Geldermalsen | JF747045 | JN128781 | JN112427 | JN112382 | ||
| 482.92 (T) | Drinking water | Japan | JN856011 | JF747046 | JN128780 | JN112426 | JN112383 | Iwatsu et al. 1991 | ||
| 109906 | dH 11628, IWW 324 | Drinking water | Germany | JF747047 | JN128777 | JN112428 | ||||
| 109905 | dH 11626, IWW 327 | Drinking water | Germany | JF747048 | JN128778 | JN112429 | ||||
| 121503 | dH 12621, LE 212405 | Fish | Russia | JF747049 | JN128779 | |||||
| dH 13563, VANADIJ-CI | Fish nursery | Russia, Stravropol Kraj | ||||||||
| 119911 | UTHSC 05-3397, dH 16409 | Weedy seadragon | USA, Boston | JF747050 | JN128784 | JN112430 | JN112384 | Nyaoke et al. 2009 | ||
| Frasca 06-4543, UTHSC R-3889 | Lumpfish, skin | USA | ||||||||
| UTHSC R-3890 | Lumpfish, spleen | USA | ||||||||
| UTHSC 07-871 R-3925 | Lumpfish | USA | ||||||||
| 441,92 | Human, nail | The Netherlands | JF747051 | JN128785 | JN112431 | JN112385 | ||||
| 122264 | dH 17026, Saunte 83 | Human, leg | Denmark, Copenhagen | JF747052 | JN128786 | JN112432 | JN112386 | |||
| 146.93 | Tilia wood | Germany | JF747053 | JN128787 | JN112433 | JN112387 | ||||
| dH 18649 | Polluted soil, petrol refinery | Brazil, Paulinia City | ||||||||
| E. aquamarina | 119918 (T) | dH 16401, UTHSC 00-1181 | Leafy seadragon, skin | USA | JN856012 | JF747054 | JN112434 | JN112388 | ||
| 119916 | dH 16404, UTHSC 04-3445 | Leafy seadragon, necrotic tissue | USA | JF747055 | JN112435 | JN112389 | ||||
| 119919 | dH 16403, UTHSC 02-852 | Leafy seadragon, skull | USA | JF747056 | JN112436 | |||||
| 120417 | dH 17512, UTHSC 06-3123 | Leafy seadragon, bone | USA | JF747057 | JN112437 | |||||
| 119917 | dH 16402, UTHSC 02-554 | Leafy seadragon | USA | JF747058 | JN112438 | JN112390 | ||||
| 119921 | dH 16412, UTHSC 05-3314, R-3673 | Weedy seadragon | USA | JF747059 | JN112439 | JN112391 | ||||
| UTHSC R-3685 | Weedy seadragon | USA | ||||||||
| 119912 | dH 16408, UTHSC 05-3142, R-3669 | Winter flounder | USA | JF747060 | JN112440 | JN112392 | ||||
| 119915 | dH 16405, UTHSC 05-32 | Little tunnyfish | USA | JF747061 | JN112441 | |||||
| UAMH 10488 | Lumpfish | Canada | ||||||||
| UTHSC R-4110 | Sandlance, aquarium outbreak | USA | ||||||||
| UTHSC R-4111 | Sandlance | USA | ||||||||
| UTHSC 05-3605 R-3678 | Sandlance | USA | ||||||||
| E. brunnea | 587.66 (T) | ATCC 32288, PRE 43729 | Acacia karoo, litter | South Africa, Potchefstroom | JN856013 | JF747062 | JN128783 | JN112442 | JN112393 | |
| E. cancerae | 120532 | dH 17408, Vicente EXO1 | Mangrove crab | Brazil | JF747063 | JN128746 | JN112443 | |||
| 120420 (T) | dH 17409, Vicente HF 16/08 | Mangrove crab | Brazil | JF747064 | JN128800 | JN112444 | JN112394 | |||
| 119920 | dH 16425, IMI 380731, Cunningham 179/99 | Green toad, liver | Israel | JF747065 | JN128801 | JN112445 | JN112395 | |||
| Det 154M / 2005 | Human | |||||||||
| Det. M154/ 2007 | Human, nail | The Netherlands | ||||||||
| UTHSC 87-269 (EC001), dH 13414 | Human | |||||||||
| UWFP 724 | Human | USA, Washington | Rakeman et al. 2005 | |||||||
| GHP 2409 | Germany | |||||||||
| GHP 2419 | Human, diabetic, skin | Germany | ||||||||
| Det 127/2002 8, dH 12901 | Water | Germany | ||||||||
| Det 127-2 2002, dH 12895 | Water | Germany | ||||||||
| DTO Tm 01.00, dH 12673 | Water | |||||||||
| 117491 | dH 13595, DTO Tm 04.045 M13 | Clean water from cip tank | The Netherlands, Bodegraven | JF747066 | JN128799 | JN112446 | JN112396 | |||
| 115142 | CPC 11044, DQ008139 | Fruit drink | Australia | JF747067 | ||||||
| E. castellanii | 122325 | dH 16683, Saunte 30 | Human, foot | Denmark, Copenhagen | JF747068 | JN128763 | JN112447 | |||
| 122265 | dH 17085, Saunte 142 | Human, hand | Denmark, Copenhagen | JF747069 | JN128749 | JN112448 | ||||
| 158.58 (T) | ATCC 1865, IFM 4702, MUCL 10097 | Human, skin | Sri Lanka | JN856014 | JF747070 | JN128766 | JN112449 | Iwatsu et al. 1984 | ||
| 662.76 | Nematode, cyst | UK | ||||||||
| 110025 | dH 12071, IWW 970 | Drinking water | Germany | JF747072 | JN128770 | |||||
| IWW 778, dH 12065 | Drinking water | Germany | ||||||||
| 109915 | dH 11634, IWW 502 | Drinking water | Germany | JF747073 | JN128764 | JN112450 | JN112397 | |||
| 121496 | dH 12245, IWW 694 | Drinking water | Germany | JF747074 | JN128768 | JN112451 | ||||
| 109812 | dH 12246, IWW 493 | Drinking water | Germany | JF747075 | JN128769 | |||||
| 109914 | dH 11627, IWW 326 | Germany | JF747076 | JN128765 | JN112452 | |||||
| 120913 | dH 17747-2, DTO Tm 06.131 20/10 A | Ice water for cooling | The Netherlands, Oosterwolde | JF747144 | JN128750 | JN112506 | ||||
| E. equina | 109913 | dH 11629, IWW 544 | Drinking water | Germany | JF747145 | JN128817 | JN112507 | |||
| 121501 | dH 12466, det 175-01 | Drinking water | The Netherlands | JF747077 | JN128806 | JN112453 | JN112398 | |||
| 122977 | dH 19905, DTO 57-E4 S | Drinking water | The Netherlands | JF747078 | ||||||
| Det 221 / 2006 | Drinking water | The Netherlands | ||||||||
| 120278 | dH 17390, DTO 06.095-1.3 | Drinking water, after water meter | The Netherlands, Geldermalsen | JF747079 | JN128803 | JN112454 | ||||
| GHP R53 | Drinking water | Germany | ||||||||
| 115143 | CPC 11047 | Bottled water | Australia | JF747080 | Avila de la Calle et al. 2006 | |||||
| 120904 | dH 13558, det 36/2004 h | Water from water machine | The Netherlands, Joure | JF747081 | JN128807 | |||||
| 121513 | dH 14518, DTO M 14A Tm 05.033 | Water system of packaging machine | The Netherlands | JF747082 | ||||||
| 124181 | dH 20175 | Bathroom-flask | The Netherlands | JF747083 | ||||||
| 124180 | dH 20174 | Bathroom-flask | The Netherlands | JF747084 | ||||||
| dH 19902 | Bathroom-flask | The Netherlands | ||||||||
| 124173 | dH 20043 | Bathroom-plate | The Netherlands | JF747085 | ||||||
| Hamada 1238 | Bathroom | Japan, Osaka | ||||||||
| 109789 | dH 12503, Det 239/01 | Human, dialysis | JF747086 | JN128808 | JN112455 | |||||
| 121283 | dH 14520, DTO Tm 05.033 / V85A | Waste water | The Netherlands | JF747087 | JN128809 | JN112456 | ||||
| 120905 | dH 13762, UTHSC 04-526 | Human, ulcer cornea | USA, Falmouth | JF747088 | JN128810 | JN112457 | ||||
| 122267 | dH 17015, Saunte 72 | Human, finger nail | Denmark, Statens Serum Institut | JF747089 | ||||||
| 121285 | dH 13080, Det M-116 / 2003 | Human, skin flakes | The Netherlands | JF747090 | JN128811 | JN112458 | ||||
| 121282 | dH 13350, UTHSC 97-1647 | Human | USA, San Antonio | JF747091 | JN128804 | JN112459 | JN112399 | |||
| 121286 | dH 13330, Det M327 / 2003 | Human, sputum | The Netherlands | JF747092 | JN128812 | JN112460 | ||||
| 120906 | dH 13647, UTHSC 89-386 | Stool | USA | JF747093 | JN128813 | JN112461 | JN112400 | |||
| 119.23 (T of Haplographium debellae-marengoi v. equinum) | Horse | Italy | JN856017 | JF747094 | JN128814 | JN112462 | JN112401 | Pollacci 1923 | ||
| 116009 | dH 13221, F1090 | Galapagos turtle | USA, Chicago, Zoo Aquarium | JF747095 | JN128805 | Manharth et al. 2005 | ||||
| 150.93 | Washed Tilia root | Germany | JF747096 | JN128815 | JN112463 | |||||
| 116922 | DTO Tm 04.136 | Silica gel | The Netherlands | JF747097 | JN128816 | |||||
| DTO Tm 04.114 | Tube of gelly installation | The Netherlands | ||||||||
| 121504 (T) | dH 12647, det M360/2002 Brasch | Tinea on leg of child (18 mo) | Germany, Kiel | JF747098 | JN128820 | JN112464 | JN112402 | |||
| GHP 2426 | Human, diabetic, skin | Germany | ||||||||
| GHP 2411 | Human, diabetic, skin | Germany | ||||||||
| 122263 | dH 17045, Saunte 102 | Human, foot | Denmark, Copenhagen | JF747099 | JN128821 | JN112465 | JN112381 | |||
| 120387 | dH 16674, Saunte 21 | Human, toe nail | Denmark, Copenhagen | JF747100 | JN128822 | JN112466 | JN112403 | |||
| 122270 | dH 16692, Saunte 39 | Human, foot | JF747101 | JN128818 | JN112467 | |||||
| 515.76 | dH 12615 | Soil | Canada | JF747102 | JN128819 | JN112468 | ||||
| 661.76 | Nematode cyst, Heterodera | Germany | JF747103 | JN128823 | JN112469 | JN112404 | ||||
| 160,89 | Washed root, Hordeum | The Netherlands | JF747104 | JN128824 | JN112470 | |||||
| 159.89 | Washed root | The Netherlands | JF747105 | JN128825 | JN112471 | |||||
| Selosse Isolate 1 | Root mycorrhiza, Cephalanthera damasonium | Julou et al. 2005 | ||||||||
| Det 238-1855, dH 12507 | Olea, twig | |||||||||
| 121502 | dH 12489, det 209-01 F | Olea, twig | Italy, Bari | JF747106 | JN128826 | JN112472 | JN112405 | |||
| Ms16Mb14 | Phragmitis australis | Germany, Lake Constance | Neubert et al. 2006 | |||||||
| T of Exophiala tremulae | UAMH 10998 | Populus tremuloides, root | Canada, Alberta | FJ665274 | ||||||
| E. halophila | 123150 | Det 08-017-20 | Salty water | JF747107 | ||||||
| 121512 (T) | dH 13757, UTHSC 03-2191 | Human, skin axilary | USA | JN856015 | JF747108 | JN128774 | JN112473 | |||
| 121499 | dH 12324, Mayser 2151/99 | Human, nail | Germany | JF747109 | JN128775 | JN112474 | JN112406 | |||
| E. lacus | 117497 (T) | dH 13711 | Lake water, 1 m depth | The Netherlands, Loosdrecht | JF747110 | JN128776 | JN112475 | JN112407 | ||
| E. mesophila | 402.95 (T) | Shower joint | Germany | JN856016 | JF747111 | JN128761 | JN112476 | Listemann & Freiesleben 1996 | ||
| 836,95 | dH 16276 | Swimming pool | Germany | JF747112 | JN128752 | JN112477 | ||||
| 119910 | dH 16410, UTHSC R-3282 | Dental waterline | USA | JF747113 | JN128753 | JN112478 | ||||
| 109147 | dH 11838, Matos T-20 | Bathroom | The Netherlands | JF747114 | JN128754 | JN112479 | ||||
| dH 18626 | Bathroom | The Netherlands, Hilversum | ||||||||
| 121498 | dH 12261, M415-10-320/01 | JF747115 | JN128755 | JN112480 | ||||||
| 121509 | dH 13436, UTHSC R-1444 (EC001) | Human, phaeohyphomycotic cyst | JF747116 | JN128762 | ||||||
| 121508 | dH 13400, UTHSC 91-270 (EJ001) | Human, finger | USA | JF747117 | JN128756 | JN112481 | ||||
| 120910 | dH 13763, UTHSC 04-611 | Human, sinus | USA | JF747118 | JN128757 | JN112482 | ||||
| 120907 | dH 13765, UTHSC 04-1300 | Human, hip joint | USA | JF747119 | JN128758 | JN112483 | ||||
| 121507 | dH 13387, UTHSC 96-1493 (EJ001) | Human, hair | USA | JF747120 | JN128759 | JN112484 | ||||
| 121497 | dH 12260, M415-08-966/01 | Human, immunosuppressed, bronchial endoscopy | France, Rouen | JF747121 | JN128760 | |||||
| 121511 | dH 13460, UTHSC 92-1021 (EJ005) | Human, nasal tissue | USA | JF747122 | JN128751 | JN112485 | ||||
| GHP R28 | Germany | |||||||||
| E. opportunistica | 109811 (T) | dH 12243, IWW 720 | Drinking water | Germany | JF747123 | JN128792 | JN112486 | JN112408 | ||
| 122269 | dH 16680, Saunte 27 | Human, nail | Denmark, Copenhagen | JF747124 | JN128795 | JN112487 | JN112409 | |||
| 122268 | dH 16705, Saunte 52 | Human, foot | Denmark, Copenhagen | JF747125 | JN128794 | JN112488 | JN112410 | |||
| 660.76 | dH 16144 | Rhizosphere, Triticum aestivum | West Australia | JF747126 | JN128793 | JN112489 | ||||
| 637.69 | dH 16111 | Polyvinyl alcohol | JF747127 | JN128796 | JN112490 | |||||
| 631.69 | Unknown | The Netherlands | JF747128 | JN128797 | ||||||
| E. pisciphila | 121505 | dH 13077, Det 100 / 2002 | Swimming pool | Germany | JF747129 | JN128790 | JN112491 | |||
| 101610 | dH 11173 | Water pipe | Germany | JF747130 | JN112492 | JN112411 | ||||
| 537.73 (T) | WUC 137 | Catfish | USA | JN856018 | JF747131 | JN128788 | JN112493 | JN112412 | Fijan 1969; McGinnis & Ajello 1974 | |
| 119913 | dH 16407, UTHSC 05-656, 05-460 | Potbelly seahorse | JF747132 | JN112494 | JN112413 | |||||
| 119914 | dH 16406, UTHSC 05-173, 5-317 | Potbelly seahorse | JF747133 | JN128791 | JN112495 | |||||
| 121500 | dH 12328, Mayser 1748/00 | Human, nail | Germany | JF747134 | JN128789 | JN112496 | JN112414 | |||
| E. psychrophila | 191.87 (T) | Salmo salar in fish farm | Norway | JN856019 | JF747135 | JN128798 | JN112497 | Pedersen & Langvad 1989 | ||
| 256.92 | Salmon | Ireland | JF747136 | JN112498 | ||||||
| E. salmonis | 157.67 (T) | BMU 00834 | Trout, brain | Canada | JN856020 | JF747137 | JN128747 | JN112499 | JN112415 | Carmichael 1966 |
| 120274 | dH 17392, DTO 06.095 nr.2.2 | Drinking water tap | The Netherlands, Geldermalsen | JF747138 | JN128802 | JN112500 | JN112416 | |||
| 110371 | dH 12699, det 405-01-8862/01 | Drinking water | The Netherlands | JF747139 | JN128748 | JN112501 | JN112417 | |||
| V. botryosa | Jorg Mayer M 218, dH 11516 | Frog | USA, Rhode Island, Park Zoo | |||||||
| 121506 | dH 13325, Padhye 2004-000517 | Human, wrist skin | Japan | JF747140 | JN112502 | JN112418 | ||||
| 101462 | dH 11373 | Human, skin | JF747141 | JN112503 | JN112419 | |||||
| 102593 | dH 11917, CDC 5937 | Human, disseminated in child (12 y) | China, Jiangsu Province | JF747142 | JN112504 | JN112420 | ||||
| 254.57 (T) | Sansa olive slag | Italy, Toscana, Pisa | JN856021 | JF747143 | JN112505 | JN112421 | Ciferri & Montemartini 1957 | |||
| dH 18628 | Eucalyptus wood treated with creosote 20 y ago | Brazil, Rio Claro | * | |||||||
| dH 18639 | Eucalyptus | Brazil, Rio Claro | * | |||||||
| dH 18642 | Eucalyptus | Brazil, Rio Claro | * | |||||||
| dH 18630 | Eucalyptus | Brazil, Rio Claro | * | |||||||
| dH 18640 | Eucalyptus | Brazil | * | |||||||
Physiology
Fungal strains were cultured in duplicate. Growth was monitored on four different media in culture plates. Growth velocities were measured with subtraction of a baseline defined after 1–3 d, and subsequent periodical measurements of colony diameters during 4 wk. Incubation temperatures were between 4 and 40 °C with 3 °C intervals. Averages of two to three measurements were calculated.
Microscopy
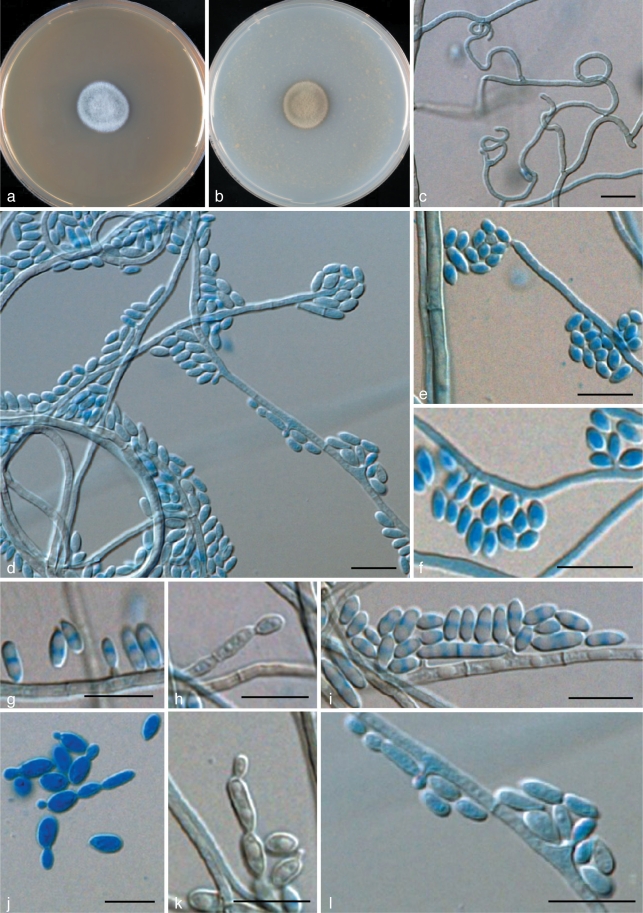
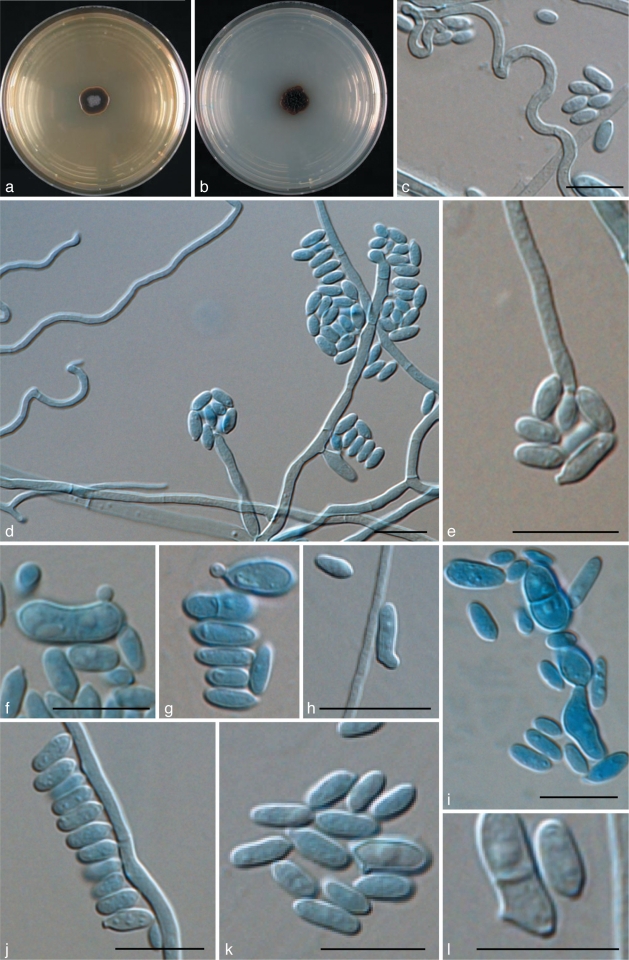
Agar blocks (MEA) of ~1 cm2 were placed on a sterile object glass supported by a V-shaped glass bar and inoculated at the four sides. The block was subsequently covered with a sterile cover slip (~2 cm2). Growth was allowed in a closed glass Petri dish; the bottom was covered with sterile paper filter soaked with 5 mL sterile water to ovoid drying of the culture. The chambers were incubated at room temperature for 5, 10 or 14 d. Slides were made in lactic acid. Permanent slides were sealed with polyvinyl alcohol. Micrographs were taken using a Nikon Eclipse 80i microscope and DS Camera Head DS-Fi1/DS-5m/DS-2Mv/DS-2MBW using NIS-Element freeware package (Nikon Europe, Badhoevedorp, the Netherlands).
DNA extraction
Methods were outlined by Gerrits van den Ende & de Hoog (1999). About 1 cm2 of fungal material from 3–4 wk old cultures was transferred to a 2 mL Eppendorf tube containing about 80 mg of a silica mixture (silica gel H, Merck 7736 / Kieselguhr Celite 545, Machery, 2 : 1, w/w) and 300 μL TES buffer (1.2 g Tris, 0.38 g Na-EDTA and 2 g sodium dodecylsulphate (SDS) in 80 mL ultrapure water, pH 8.0). Cells were ground mechanically with a tight-fit pestle for 1–2 min. Subsequently 200 μL TES was added. After the mixture was vortexed, 10 μL Proteinase K was added and the mixture incubated at 65 °C for 10 min. 140 μL 5 M NaCl and 65 μL 1 % CTAB (cetyltrimethylammonium bromide) were added, and the solution incubated at 65 °C for 30 min. Subsequently 700 μL SEVAG was added and carefully mixed by hand for about 1 min and incubated during 30 min at 0 °C (on ice water). The solution was centrifuged for 10 min at 4 °C at 20,400 g. The upper water phase was transferred to a clean Eppendorf tube to which 225 μL 5 M NH4-acetate were added and gently mixed. The mixture was incubated again on ice water for at least 30 min and centrifuged for 10 min at 4 °C at 20,400 g. The supernatant was transferred to a clean Eppendorf and supplemented with 510 μL isopropanol, mixed carefully and directly centrifuged for 5 min at 20,400 g. The supernatant was decanted and the pellet washed twice with 70 % ethanol. The pellet with DNA was vacuum dried in a DNA Speed Vac (New Brunswick Scientific, Nijmegen, the Netherlands) for 10–15 min at a medium Drying Rate stand. Finally, the DNA was resuspended in 50 μL TE buffer including 1.5 μL RNAse and incubated for 15–30 min at 37 °C. DNA quality was verified by NanoDrop® ND-1000 Spectrophotometer using ND-1000 v. 3.3.0 software (Coleman Technologies, Wilmington, DE, USA). Samples were stored at −20 °C.
Amplification
Fragments of rDNA were amplified using the universal primers V9G and LS266 for rDNA ITS (Gerrits van den Ende & de Hoog 1999), NS1 and NS24 for rDNA SSU or alternative primer combinations (NS1 and Oli16, NS1 and NS8), Ef1-728F and Ef1-986R for TEF1, Bt2a and Bt2b for BT2 and Actfw and Actbw or otherwise combined with EspActbw for ACT1 in a reaction mixture containing 30 μL sterile water, 5 μL PCR buffer 10×, 10 μl dNTP (1 mM), 1 μL of each of the primers (50 pmol/μL, or otherwise for degenerate primers), 1 μL DNA polymerase (1 U/μL) and 1 μL fungal DNA. Thirty-five cycles were performed in a GeneAmp PCR System 9700 (Applied Biosystems), with 5 min delay, and 35 cycles of 94 °C for 45 s (denaturation), 52 °C for 30 s (annealing) and 72 °C for 120 s (extension), with a final delay of 7 min and using the maximum ramp speed for ITS amplification. For SSU the annealing temperature was lowered to 48 °C and for TEF1 amplification raised to 55 °C. Five μL of each PCR product, with 2 μL loading buffer, was electrophoresed in 1 % agarose gels with 0.5 × 10−5 (v/v) ethidium bromide, in TAE 1× buffer (200 ml TAE 50×) (BioRad: 242 g Tris, 57.1 mL acetic acid, 100 mL, 0.5 M EDTA) mixed with 9 800 mL ultrapure water) at 80–100 V for 90 min, and using 5 μL Smart Ladder (Eurogentec, Seraing, Belgium) as marker. Amplicon quality and concentration were estimated on agarose gels (1.0–1.2 %), which were photographed. Amplicons were cleaned using GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences). Sequencing reactions were performed using ITS1 and ITS4 for ITS sequences. BF83, Oli9, BF963, BF1419 and Oli1, BF951, BF1438, NS24 (or Oli3) for SSU sequencing, EF1-728F, EF1-986R for TEF1, Bt2a and Bt2b for BT2, and Actfw and Actbw eventually combined with EspActbw for ACT1, following protocols for DYE-ET terminator cycle sequencing. Reaction mixtures varied with the sample, as follows: 1 μL template DNA (0.1 pmol), 1 μL primer (4 μM), 1 μL sequencing reagent premix, 3 μL dilution buffer completed with 5.5-× μL ultra pure water to 10 μL final volume. Reactions were performed in a GeneAmp in 25 cycles of: 95 °C for 20 s, 50 °C for 15 s, 60 °C for 60 s and stopped with cooling to 4 °C. Samples were purified with Sephadex G-50 Superfine into a 96 wells of a MultiScreen HV plate and recovered in a standard 96-well microtiter plate. This eluting plate is covered with aluminium foil tape (3M Scotch 431, 75 mm) and can directly be loaded on the ABI 3700 machine for sequences reading or stored at –20 °C. Sequences were analysed using SeqMan II software (DNASTAR).
Alignment and phylogenetic reconstruction
For genealogical concordance analysis, four genes ITS, TEF1, BT2 and ACT1 were first analysed separately. Alignment was performed automatically and adjusted iteratively by hand with BioNumerics v. 4.61 (Applied Maths, Kortrijk, Belgium). Topological conflicts were evaluated visually and by using the partition homogeneity test implemented in PAUP v. 4.0b10. ITS and multilocus trees were constructed with maximum likelihood and with 100 bootstrap replicates using RAxML v. 7.2.3 (Stamatakis et al. 2008) as implemented on the Cipres Portal v. 1.10, and edited with MEGA4 software (Tamura et al. 2007).
A phylogenetic approach was used to investigate relationships between 147 waterborne strains of Exophiala and related species (Table 1), with Exophiala castelanii and E. mesophila as outgroups in the SSU tree. For species circumscribed by genealogical concordance, a single strain (usually the ex-type) was selected for sequencing conserved genes. Sequences were compared in a database in BioNumerics containing all described members of Chaetothyriales. The database was regularly updated with recent GenBank and AFTOL submissions. SSU (1 100 comparable sites) trees were generated with the Parsimony option of PAUP after removal of introns.
RESULTS
Phylogeny
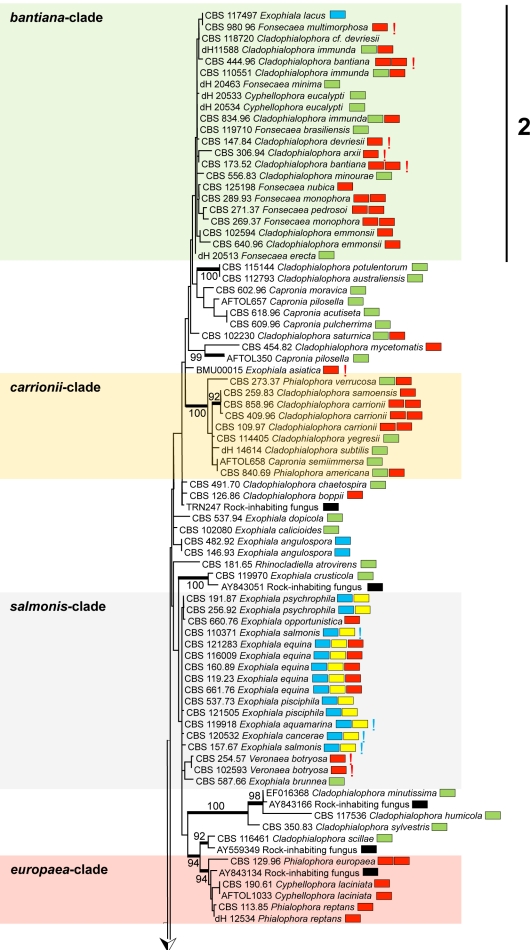
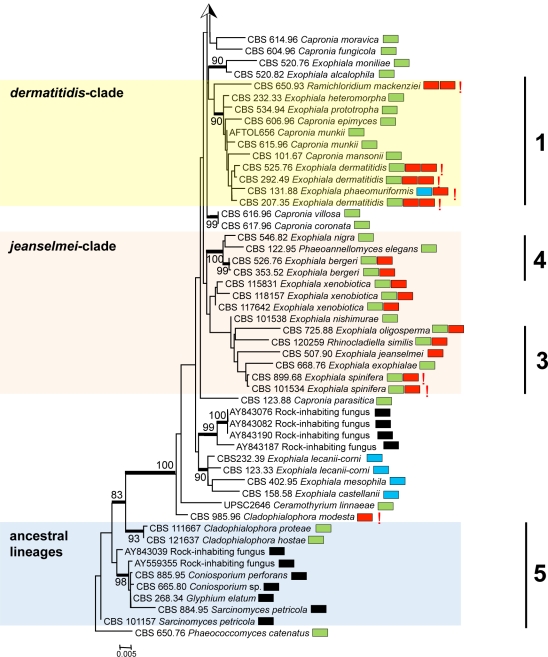
A general tree for a large set of representatives of the order Chaetothyriales was constructed using SSU rDNA data (Fig. 1), with Phaeococcomyces catenatus CBS 650.76 as outgroup. The SSU alignment had 562 distinct patterns; frequency pi(A) = 0.261153, pi(C) = 0.209808, pi(G) = 0.263550, pi(T) = 0.265489. ML was calculated with RAxML v. 7.2.3 with Gamma correction and GTR substitution matrix.
Fig. 1.
Phylogeny of all members of Chaetothyriales described to date, obtained from a ML analysis based on SSU rDNA sequences. Bootstrap support was calculated from 100 replicates; values > 80 % are shown with the branches. Supported branches are drawn in bold. The tree was rooted with Phaeococcomyces catenatus, CBS 650.76. Coloured boxes represent species complexes recognised in this paper. Prevalent species ecologies are summarised in boxes at the right hand side of each strain. Black: rock-inhabiting; blue: waterborne; green: plant-associated; red: invasive in warm-blooded animals; yellow: invasive in cold-blooded animals; red exclamation mark: systemic in warm-blooded animals; blue exclamation mark: systemic in cold-blooded animals; double boxes indicate relative high frequency of the species in more than one category.
Two ancestral lineages, with 98 and 93 % bootstrap support, respectively, contained prevalently rock-inhabiting fungi (Coniosporium spp.) with prevalently isodiametric morphology (group 5 of Haase et al. 1999), plus the plant-inhabiting species Cladophialophora hostae. The remainder of the tree, at rather large distance and with 100 % bootstrap support, is considered to represent the ascomycete family Herpotrichiellaceae. Teleomorphs, when present in this group are Capronia species, except that Ceramothyrium linnaeae is also found in this part of the tree.
Overall 19 bootstrap-supported (i.e. > 80 %, indicated in bold in Fig. 1) branches were present in the Herpotrichiellaceae; the core structure was poorly resolved. Nevertheless, a number of approximate species complexes could be distinguished, some of which corresponded with SSU groups (1–4) previously recognised by Haase et al. (1999). The approximate groups have here been listed as bantiana-clade, carrionii-clade, salmonis-clade, europaea-clade, dermatitidis-clade, jeanselmei-clade, and some ancestral lineages (Fig. 1). Group Haase-1 is recognised below as the dermatitidis-clade, Haase-2 is the bantiana-clade, and Haase-3 and -4 correspond with two clusters, described in more detail below, as the jeanselmei-clade. None of the clades was morphologically homogeneous; the anamorph genera Cladophialophora, Cyphellophora, Exophiala, Fonsecaea and Rhinocladiella are all polyphyletic within the order Chaetothyriales.
When major sources of isolation were plotted on the tree (habitats included rock, plant, water/cold-blooded animal, warm-blooded animal) no meaningful clustering was evident, except for a grouping of waterborne species in a group referred to as the salmonis-clade (Fig. 1). However, in detailed study, different trends become apparent. The bantiana-clade contained thermotolerant systemic pathogens, such as C. bantiana and C. arxii, as well as the Fonsecaea agents of chromoblastomycosis. The bantiana-clade included only a single Exophiala species, E. lacus. This species is known from two strains from water, and has CBS 117497 as the ex-type. The carrionii-clade had 100 % bootstrap support and contained many of the agents of chromoblastomycosis. The dermatitidis-clade (group Haase-1), with 90 % bootstrap support, includes some oligotrophic thermophiles with an invasive, neurotropic ability, in addition to environmental Capronia species. A group of superficial human pathogens clustered around Phialophora europaea (the europaea-clade had 94 % bootstrap support).
The majority of mesophilic, waterborne Exophiala species were strongly clustered. A large clade (seen in Fig. 1 as the salmonis-clade) contained almost exclusively waterborne species; only the drinking water species E. angulospora took an isolated position. The salmonis-clade comprised nine waterborne Exophiala species, E. aquamarina, E. brunnea, E. cancerae, E. equina, E. halophila, E. opportunistica, E. pisciphila, E. psychrophila and E. salmonis. The sympodially reproducing species Veronaea botryosa, a potential agent of disseminated infections in humans, was also found in the same clade at some distance from the Exophiala species.
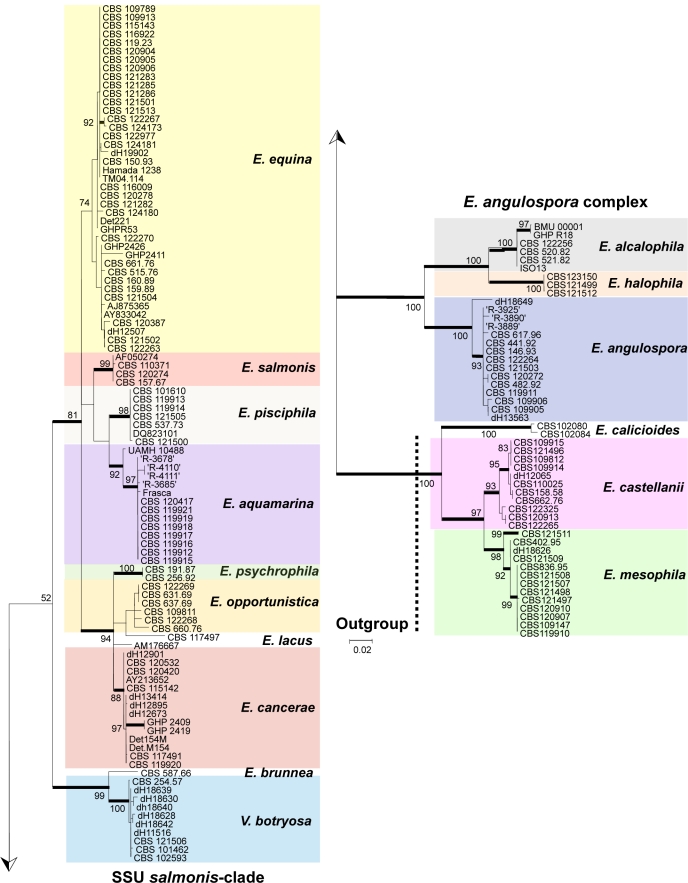
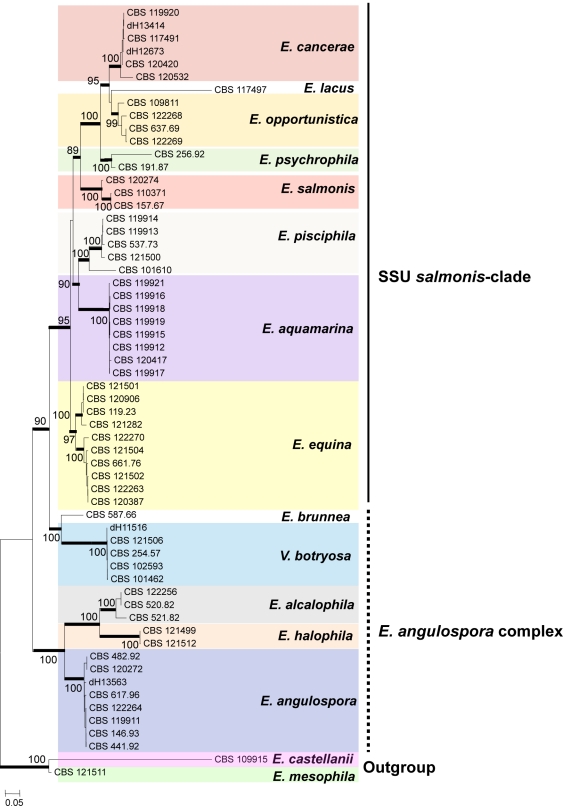
The salmonis-clade was analysed in more detail using ribosomal ITS sequences (Fig. 2) and multilocus data (Fig. 3); Exophiala castellanii and E. mesophila were selected as outgroups for both trees. In the ITS tree (Fig. 2), three groups at high bootstrap support are recognisable: the E. castellanii / E. mesophila outgroup, the E. angulospora complex, and a large cluster (81 % bootstrap support) around the type species of Exophiala, E. salmonis, representing the salmonis-clade as recognised in SSU data. The majority of species in the salmonis-clade were waterborne Exophiala species, containing isolates causing disease on cold-blooded animals such as fish, turtles, crabs, sea horses and frogs. ITS and multilocus analyses distributed these strains over ten clusters (Fig. 2), among which seven have not formally been described as Exophiala species. Veronaea botryosa, with sympodial conidiogenesis very different from the annellidic conidiogenesis of the Exophiala species, is found adjacent to the waterborne species in all genes analyzed (Fig. 1–3). Partition-homogeneity testing, based on heuristic searching of four genes (ITS, TEF1, BT2, ACT1) with 100 replicates and 167 parsimony-informative characters of 2 254 total characters, revealed conflict (significant heterogeneity) among the genes (p = 0.01). In ITS or multilocus trees, the salmonis-clade was composed of 10 distinct groups, mostly with bootstrap support in both trees. These groups were: E. equina (74 % bootstrap in ITS / 100 % in multilocus comparison), E. salmonis (99 ITS / 100 multilocus), E. pisciphila (98 / 100), E. aquamarina (92 / 100), E. psychrophila (100 / 100), E. opportunistica (– / 99), and E. cancerae (88 / 100). Veronaea botryosa and E. brunnea are located separately within the clade, as was also visible in the SSU tree, at 99 % bootstrap support (Fig. 1). The single strain of Exophiala lacus is found in SSU analysis to be a member of the bantiana-clade and consequently it was connected by a long branch to members of the salmonis-clade.
Fig. 2.
Phylogeny of the SSU-based salmonis-clade, obtained from a ML analysis based on ITS rDNA sequences. Bootstrap support was calculated from 100 replicates; values > 80 % are shown with the branches. Supported branches are drawn in bold. The tree was rooted with Exophiala mesophila and E. castellanii.
Fig. 3.
Phylogeny of the SSU-based salmonis-clade, obtained from a ML analysis based on ITS, ACT1, BT2 and TEF1 sequences. Bootstrap support was calculated from 100 replicates; values > 80 % are shown with the branches. Supported branches are drawn in bold. The tree was rooted with Exophiala mesophila and E. castellanii.
Outside the salmonis-clade two clusters containing strains of waterborne species were recognisable in the SSU tree (Fig. 1) and were also found at 100 % bootstrap support in the ITS tree (Fig. 2). Nearly all species within these clusters (E. alcalophila, E. angulospora, E. castellanii, E. halophila and E. mesophila) were strongly supported. Some showed significant intraspecific variability. The cardinal growth temperatures of the species of the salmonis-clade were determined. The maximum growth temperature was found to be 33 °C.
Taxonomy
Exophiala J.W. Carmich., Sabouraudia 5: 122. 1966. — MycoBank MB8233
The genus Exophiala was described as an anamorph taxon by Carmichael (1966), with E. salmonis as its type species. The genus represents an anamorphic element of the order Chaetothyriales. Note that the type species has among its synonyms the name Aureobasidium salmonis. Aureobasidium, however, is a member of the order Dothideales (Schoch et al. 2009). Teleomorphic relations of Exophiala lie in the genus Capronia, typified by Capronia sexdecimspora. Exophiala is the main genus of black yeasts found as opportunists of vertebrates. It is characterised by annellidic conidiogenesis. Some cultures are entirely yeast-like (synanamorph Phaeococcomyces), or form phialidic collarettes (synanamorph Phialophora), sympodial conidiophores (synanamorph Rhinocladiella) or dry conidial chains (synanamorph Cladophialophora). Chlamydospores or sclerotial bodies may also be formed, occasionally leading to entirely meristematic mutants (synanamorph Sarcinomyces). Several of these morphologies are represented in species of the waterborne salmonis-clade (B).
Thermophilic species of Exophiala have been described from systemic infections in humans, e.g. E. dermatitidis and E. spinifera. In contrast, numerous species of the salmonis-clade (B) are mesophilic or psychrotolerant, and are regularly found as opportunists on cold-blooded vertebrates. Many such cases have been reported in the literature, and these cases have been attributed to a diversity of Exophiala species. However, the identification of etiologic agents in many of these cases must be questioned, since the morphological characters used have proven not to be reliable for species identification. This unreliability is attributable to variable morphologies within species and to overlapping characters among them. Therefore in the present paper we will only reference the case reports where voucher strains have been preserved and sequenced.
Exophiala alcalophila Goto & Sugiy., in Goto et al., Trans. Mycol. Soc. Japan 22: 430. 1981. — MycoBank MB110200
= Phaeococcomyces alcalophilus Goto & Sugiy., Trans. Mycol. Soc. Japan 22: 432. 1981.
Description of CBS 520.82 after 2 wk incubation on MEA, 24 °C.
Colonies restricted, appearing slimy, smooth, soft, jet-black, convex with sharp margin. Initial growth with budding cells, later (after 1 mo) becoming slightly floccose at the centre and remaining slimy at the margin. Reverse brownish black; a rust brown pigment exuded into the agar. Budding cells abundant, smooth- and thin-walled, 1-celled, (sub)spherical to broadly ellipsoidal, 4–8 × 3–6 μm. Germinating cells present, (sub)spherical, 7–9 μm diam. Aerial hyphae smooth-walled, irregularly branched, 1.5–3.0 μm wide. Conidiogenous cells arising from undifferentiated hyphae, terminal or intercalary, with short annellated zones, mostly without discernible annellations; occasionally conidia produced apically in more or less sympodial order. Conidia hyaline, thin- and smooth-walled, 1-celled, spherical, ellipsoidal to slightly reniform, occasionally with truncate base, 3–7 × 2–5 μm, aggregated in slimy heads.
Cardinal temperatures — Minimum 4–9 °C, optimum 24–27 °C, maximum 36–40 °C.
Specimens examined. Japan, Hirose, Wako-shi, Saitama pref., 23 Apr. 1978, K. Horikoshi, from soil, holotype specimen of Exophiala alcalophila, CBS H-19960, culture ex-type IAM 12519 = CBS 520.82; Hirose, Wako-shi, Saitama pref., 23 Apr. 1978, K. Horikoshi, from soil, ex-type culture of Phaeococcomyces alcalophilus, IAM 12520 = CBS 521.82.
Additional material examined. Table 1.
Notes — The species was originally isolated with two morphotypes: one hyphal, and the other purely consisting of budding cells, for which reason the fungus was introduced under two generic names, Exophiala and Phaeococcomyces. This underlines the strongly dimorphic character of the species. Under the growth conditions applied in the present study, the yeast-like phase was pronounced during the first week, while hyphae later became more prevalent. The preponderance of either morphotype is unstable. Yamada et al. (1989) demonstrated that while E. salmonis had a coenzyme Q 10(H2) ubiquinone system, E. alcalophila had Co-Q 10.
The original cultures were derived from soil on a minimal medium at a pH of 10.4 (Goto et al. 1981). Another strain (GHP R-18; Table 1) came from the soap container of a laundry machine. Nishimura et al. (1987) isolated the fungus repeatedly from bath water. Strain CBS 122256 was isolated from mildly symptomatic human skin in Denmark, but without precise clinical information. Lian & de Hoog (2010) recently noticed a possible link between cutaneous infection and the presence of potentially causal black yeast-like fungi in low-nutrient indoor water systems rich in soap, such as bathrooms. The authors repeatedly isolated several black yeast-like species from bathrooms, which until then had only been known from human skin and nails. They suggested that maceration of skin may provide a portal of entry during hygienic procedures for these fungi having moderate invasive capacity. Exophiala alcalophila apparently belongs to the same ecological group, although at low virulence.
Exophiala angulospora Iwatsu, Udagawa & Takase, Mycotaxon 4: 322. 1991. — MycoBank MB355245
Teleomorph. Capronia coronata Samuels, Trans. Brit. Mycol. Soc. 88: 65. 1967.
Description of CBS 482.92 after 2 wk incubation on MEA, 24 °C.
Colonies restricted, centrally mucous, velvety towards the outside, greyish green to olivaceous black. Germinating cells present, 6–10 × 2.4–4.0 μm. Hyphae pale olivaceous, smooth-walled, 1.5–3.0 μm wide. Budding cells present. Conidiogenous cells intercalary, lateral and terminal and then 1-celled, flask-shaped, 6–16 × 2.5–3.0 μm; conidia produced from a single short annellated zone per cell. Conidia aggregating in slimy heads, 1-celled, smooth- and thin-walled, subhyaline or pale olivaceous, mostly more or less triangular with rounded ends, 2.5–4.0 × 2–3 μm.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 24–27 °C, maximum 30–33 °C. No growth at 37 °C.
Specimens examined. Japan, Yokohama-shi, 18 Apr. 1989, K. Arai, from drinking well water, ex-type culture anamorph, CBS 482.92 = NHL 3101. – New Zealand, Westland County, Nemona State Forest, 5 May 1983, G.J. Samuels, from decorticated wood, ex-type culture teleomorph, CBS 617.96 = ATCC 56201 = PDD 35308.
Additional material examined. Table 1.
Notes — The species was originally repeatedly isolated from cold, low-nutrient drinking water (Iwatsu et al. 1991). The majority of strains sequenced originated from cold water, such as drinking water, aquaria and fish nurseries (Table 1). A teleomorph, Capronia coronata, originating from decorticated wood, was found to be identical in ITS sequence data. Nyaoke et al. (2009) noted a disseminated infection by CBS 119911 in a seawater-dependent weedy sea dragon (Phyllopteryx taeniolatus) in the New England Aquarium in Boston, USA, and also repeatedly in the marine lumpfish (Cyclopterus lumpus). Strain CBS 121503 was isolated from living freshwater fish Scenodus leucichtys in a nursery in Stravropol Kraj near Kislovodsk, southern Russia (V.A. Mel’nik, pers. comm.). Human isolates such as CBS 441.92 and CBS 122264 originated from skin and nail samples, but no proof of infection is available (Saunte et al. 2011). The species has been isolated once from hydrocarbon-polluted soil (Table 1).
In conclusion it appears that this fungus inhabits cold waters worldwide, where it has an invasive potential with fatal dissemination in cold-blooded vertebrates. Human infections have not been proven and may have concerned colonization only. This pathology is likely to be determined by its relatively low maximum growth temperature.
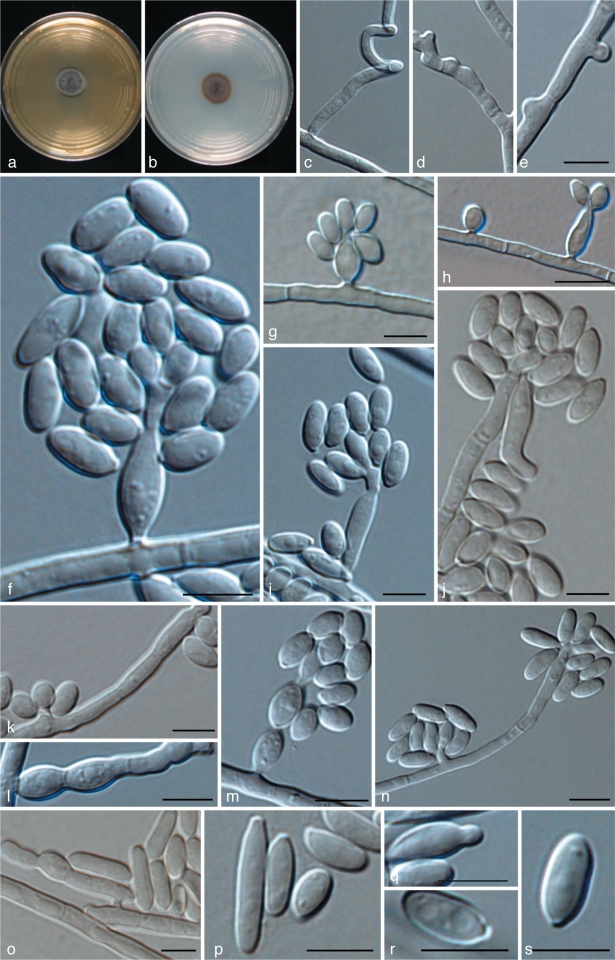
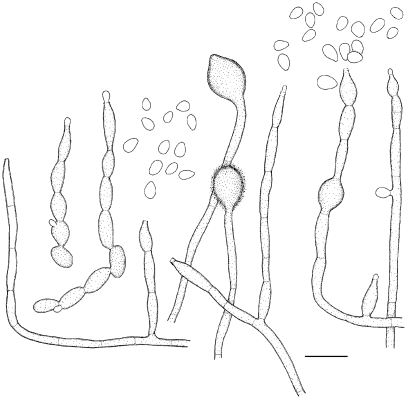
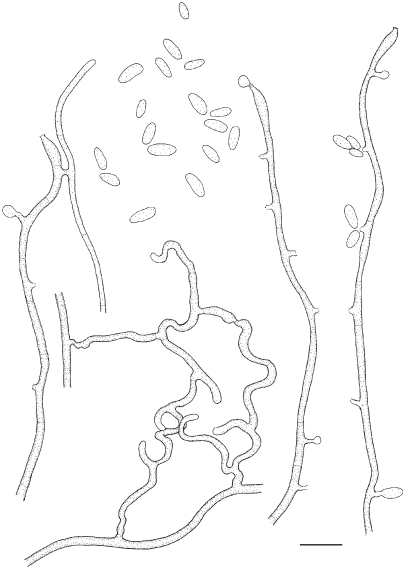
Exophiala aquamarina de Hoog, Vicente, Najafzadeh, Harrak, Badali, Seyedmousavi & Nyaoke, sp. nov. — MycoBank MB515716; Fig. 4, 5
Fig. 4.
Exophiala aquamarina, CBS 119918. a. Colony on MEA; b. colony on PDA; c, d. spirally twisted hyphae; e–n. conidial apparatus with conidia; f–j. annellidic conidiogeneses with sympodial conidiophores; o. anastomosis between discrete cells; p–s. conidia; q. budding cells. — Scale bars = 10 μm.
Fig. 5.
Exophiala aquamarina, CBS 119918. Conidial apparatus and conidia. — Scale bar = 10 μm.
Coloniae in agaro maltoso dicto 24 °C lente crescentes, velutinae, griseae; reversum olivaceo-nigrum. In agaro PDA pigmentum brunneum absens. Hyphae leves, dilute olivaeo-brunneae, intervallis regularibus septatae, nonnumquam spiralis. Conidiophora vix distinguenda, ramose vel simplicia, terminalia vel intercalaria; conidium annellidorum vel sympodiorum producens. Conidia late ellipsoidea, levia, 6.7–19.2 × 4.0–4.8 μm. Temperatura maxima crescentiae 36 °C. Teleomorphe ignota.
Etymology. Name refers to sea water, the environment of most of the hosts of this species.
Description of CBS 119918 after 2 wk incubation on MEA, 27 °C.
Colonies restricted, olivaceous black, velvety with aerial mycelium at the centre. Reverse olivaceous black. No diffusible pigment produced. Conidiogenous cells flask-shaped, with short annellated zones, sometimes with sympodial conidiogenesis. Spirally twisted hyphae present. Conidia ellipsoidal to cylindrical, 6.7–19.2 × 4.0–4.8 μm. Yeast cells rarely present.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 24–30 °C, maximum 33–36 °C. No growth at 37 °C.
Specimen examined. USA, Boston, New England Aquarium, S. Frasca, from skin of leafy sea dragon (Phycodures eques), holotype CBS H-19950, ex-type culture CBS 119918 = UTHSC 00-1181 = dH 16401.
Additional material examined. Table 1.
Notes — The species repeatedly caused disseminated infections in several species of fish in the New England Aquarium in Boston, Mass., USA, and in the Adventure Aquarium in Camden, N.J., USA (Nyaoke et al. 2009), particularly in leafy sea dragon (Phycodurus eques) and in weedy sea dragon (Phyllopteryx taebiolatus), but also in winter flounder (Pseudopleuronectes americanus) and little tunnyfish (Euthynnus alletteratus). Necrotic skin lesions were observed with mild inflammatory response, with invasion of blood vessels, and infection of skull and bone, but no brain involvement. Massive amounts of hyphae were observed in tissue. Infections took place over a 5-year period (Nyaoke et al. 2009). Exophiala aquamarina is so far restricted to fish, but is not host specific.
Exophiala brunnea Papendorf, Trans. Brit. Mycol. Soc. 52: 487. 1969. — MycoBank MB330806
Description of CBS 587.66 after 2 wk incubation on MEA, 24 °C.
Colonies developing slowly, with mouse-grey aerial mycelium at the centre; peripheral area depressed, dark olivaceous; reverse greenish black. Hyphae poorly branched, smooth-walled, pale olive-brown, 1–3 μm diam. Budding cells absent. Conidiogenous cells lateral, slightly differentiated from vegetative hyphae, frequently with 1–2 septa, simple or branched, variable in shape, flask-shaped, ovoidal to elongate, pale brown, 8–350 μm long. Annellated zones inconspicuous or occasionally finely fimbriate, 6–20 × 2–4 μm, often inserted on intercalary cells of hyphae and conidiophores. Conidia forming a coherent mass, broadly ellipsoidal or ovoidal, with a broad truncate hilum, continuous or occasionally with a median septum and then slightly constricted, smooth-walled, pale brown, 4.5–10 × 2–3 μm.
Cardinal temperatures — Minimum 4–9 °C, optimum 21–24 °C, maximum 30–33 °C. No growth at 37 °C.
Specimen examined. South Africa, Potschefstroom, from leaf litter of Acacia karroo, specimens CBS H-12618, CBS H-19966, ex-type culture CBS 587.66.
Notes — The species is known from a single strain isolated from topsoil (leaf litter) of an Acacia karroo community (Leguminosae-Mimosoideae). The species was synonymised with Exophiala salmonis on morphological grounds (de Hoog 1977), but was later shown to be distinct based on molecular data.
Exophiala calicioides (Fr.) G. Okada & Seifert, Stud. Mycol. 45: 184. 2000. — MycoBank MB464794
Synonyms listed in Okada et al. (2000).
Notes — This species has fully been described and illustrated by Okada et al. (1998, 2000). It is morphologically remarkable by being synnematous. The fungus resides in rotten wood, eventually in association with bark beetles.
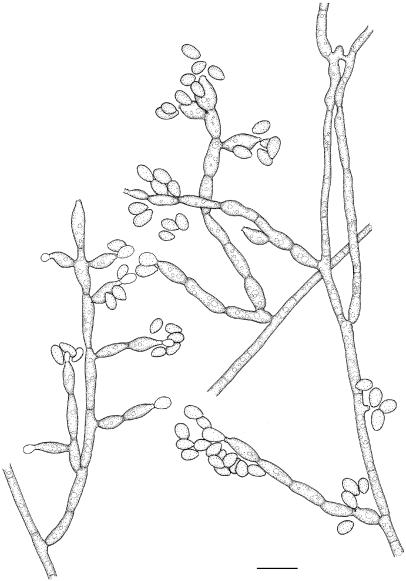
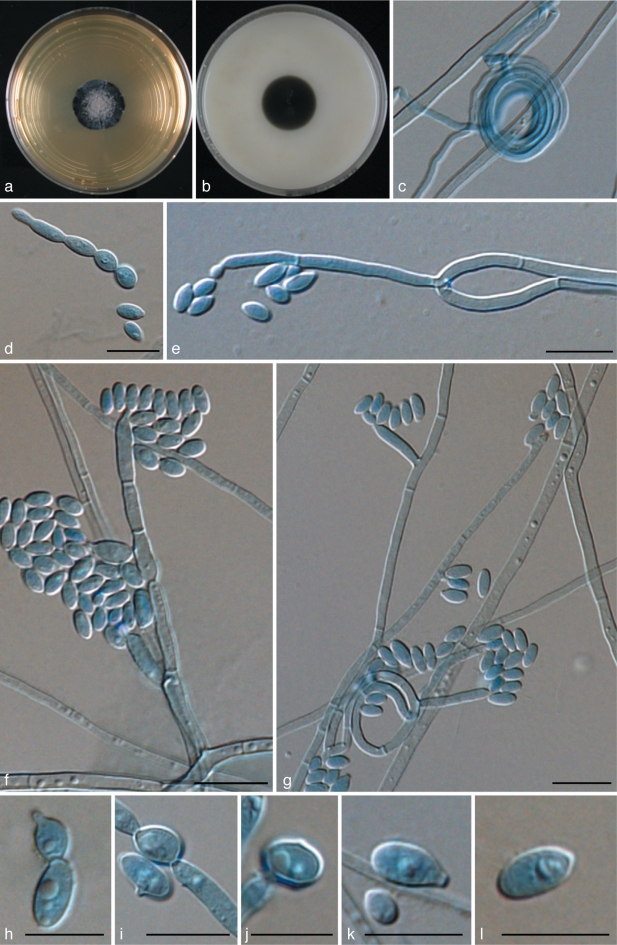
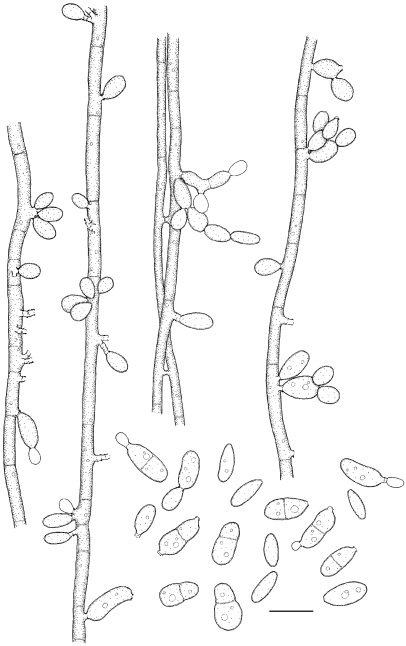
Exophiala cancerae de Hoog, Vicente, Najafzadeh, Harrak, Badali, Seyedmousavi & Boeger, sp. nov. — MycoBank MB515720; Fig. 6, 7
Fig. 6.
Exophiala cancerae, CBS 120420. a. Colony on MEA; b. colony on PDA; c, d. spirally twisted hyphae; e, f. short, erect, cylindrical, multi-celled conidiophores; h, i. apical and intercalary chlamydospores; j. budding cells; k, l. intercalary conidiogenous cells; m. hyphae and conidia with anastomoses; n–p. conidia. — Scale bars = 10 μm.
Fig. 7.
Exophiala cancerae, CBS 120420. Conidial apparatus, conidia and torulose hyphae. — Scale bar = 10 μm.
Coloniae in agaro maltoso dicto 25 °C lente crescentes, primum leves, deinde velutinae, griseo-olivaceae; reversum olivaceo-nigrum. In agaro PDA pigmentum brunneum absens. Hyphae leves, dilute olivaeo-brunneae, intervallis regularibus septatae. Cellulae zymoideum quasi absens. Conidiophora vix distinguenda, ramose vel simplicia, terminalia vel intercalaria. Annelloconidia nonnumquam septata, late ellipsoidea vel cylindrea, levia, 4.9–8.0 × 2.7–4.8 μm. Temperatura maxima crescentiae 33 °C. Teleomorphe ignota.
Etymology. Named after the crab, an arthropod host of this species.
Description of CBS 120420 after 2 wk incubation on MEA, 24 °C.
Colonies moderately expanding, circular, initially (on day 3) flat, olivaceous black, slimy with velvety, olivaceous grey centre and flat margin, later (on day 14) becoming velvety, dark olivaceous grey. Reverse olivaceous black, without diffusible pigment. Yeast cells nearly absent. Conidiophores short, erect, brown, cylindrical, multi-celled, poorly differentiated. Conidia 0–1-septate, subhyaline to pale brown, obovoidal to cylindrical, 4.9–8.0 × 2.7–4.8 μm.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 24–27 °C, maximum 30–33 °C. No growth at 37 °C.
Specimen examined. Brazil, Pernambuco State, Goiana City, from diseased Mangrove crab (Ucides cordatus), W. Boeger, holotype CBS-H 20382, ex-type culture CBS 120420 = dH 17409 = Boeger HF16/8.
Additional material examined. Table 1.
Notes — The ex-type strain was isolated from direct culture from tissue of moribund mangrove crabs (Ucides cordatus, Brachyura: Ocypodidae) with Lethargic Crab Disease (LCD). Since 1997 this systemic disease has caused extensive epizootic mortality of crabs along the Brazilian coast (Boeger et al. 2005). The histopathology of crabs in diverse stages of development of the disease shows that the most commonly affected tissues are the epidermis, connective tissues, heart and hepatopancreas. The fungus disseminates hematogenously. Despite the large scale of outbreak, locally causing 50 % of crabs to become diseased, we were unable to isolate E. cancerae from the environment (Boeger et al. 2007). The worldwide occurrence of the species (Table 1) suggests that it may have been present in Brazil prior to the beginning of the epizootic in 1997. Changes in host or in environmental conditions, rather than emergence of a virulent fungal genotype, are thus likely. This is underlined by the fact that also a second black yeast-like fungus was involved in LCD (Boeger et al. 2007). This was a Cladophialophora species close to C. devriesii, which was first described from a fatal disseminated infection in a human at the Caribbean Grand Cayman Island (Gonzalez et al. 1984).
Strain CBS 119920 was derived from the liver of a green toad (Pseudepidalea viridis) in Israel, which was euthanised with clinical signs of systemic mycosis (A. Cunningham, pers. comm.). Additional strains were isolated on separate occasions from water in Germany (Table 1). The species was once isolated from clean water from a CIP (Clean-in-Place) tank (CBS 11749) in the Netherlands and once from fruit drink (CBS 115142) in Australia.
In humans, a skin infection was observed in a patient with diabetes in Germany (GHP 2419; G. Haase, pers. comm.); further human cases concern mild skin and nail infections. Despite its maximum growth temperature of 33 °C, the species possesses intrinsic virulence factors. These may be expressed particularly in external tissues of the extremities patients with reduced blood circulation, e.g. to underlying diabetes.
GenBank contains a number of accessions under the name Exophiala salmonis, which are now reidentified as E. cancerae: a clinical strain in the USA (AY213652; Rakeman et al. 2005), a strain from a bathroom in Japan (AB 456581; Hamada & Abe 2009) and an isolate from a healthy stem of corn in Australia (AM176667, Molnar & Prillinger unpubl. data).
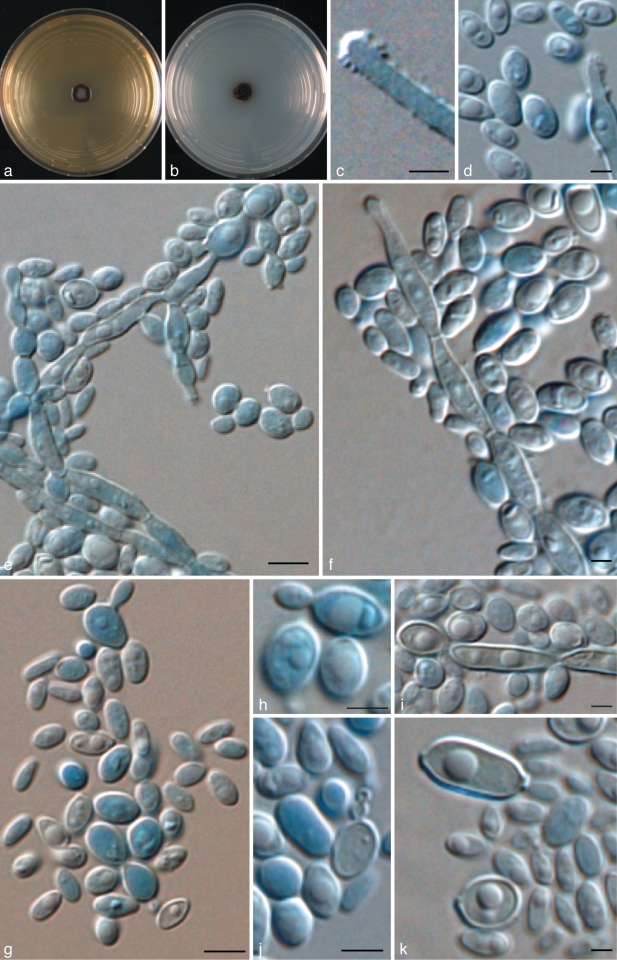
Exophiala equina (Pollacci) de Hoog, Vicente, Najafzadeh, Harrak, Badali & Seyedmousavi, comb. & stat. nov. — MycoBank MB515717; Fig. 8, 9
Fig. 8.
Exophiala equina, CBS 121504. Conidial apparatus and conidia. — Scale bar = 10 μm.
Fig. 9.
Exophiala equina, CBS 119.23. a. Colony on MEA; b. colony on PDA; c. spirally twisted hyphae; d. tolurose hyphae; e, g. conidiophore with single conidiogenous cell; f. conidiogenous subcylindrical cells flask shaped with ellipsoidal conidia; h. budding cell; i, j. chlamydospore; k, l. ellipsoidal conidia. — Scale bars = 10 μm.
Basionym. Haplographium debellae-marengoi Pollacci var. equinum Pollacci, Revta Biol. 5: 370. 1923.
= Exophiala tremulae W. Wang, Fungal Planet 70, Persoonia 26: 113. 2011.
Description of CBS 119.23 after 2 wk incubation on MEA, 27 °C.
Colonies restricted, circular, initially (on day 5) flat, olivaceous black, slimy with velvety, olivaceous grey centre and flat margin, later (on day 15) becoming umbonate, felty, greyish black, with velvety, grey centre. Reverse greyish black. No diffusible pigment produced. Yeast cells, when present, consisting of subspherical cells producing conidia. Conidiogenous cells flask-shaped, intercalary or terminal. Conidia ellipsoidal, 2.4–3.3 × 4.8–5.2 μm, with discernible scars. Chlamydospores ellipsoidal, up to 10 μm long and 5 μm wide; spirally twisted hyphae present.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 24–30 °C, maximum 33–36 °C. No growth at 37 °C.
Specimen examined. Italy, Pavia, Dec. 1923, G. Pollacci, subcutaneous infection of a horse, CBS H-19957, ex-type culture CBS 119.23 = dH 15335.
Additional material examined. Table 1.
Notes — The ex-type strain was isolated as the etiologic agent of a subcutaneous infection of the lower leg of a horse (Pollacci 1923). The majority of remaining strains sequenced of this species, however (Table 1), originated from different kinds of cold water, primarily drinking water but also from the cooling system of a packaging machine, the tubing of a instrument using silica gel and from washings of Tilia roots. One isolate came from a bathroom. Strain CBS 115143 was isolated from bottled spring water destined for human consumption in Australia (Avila de la Calle et al. 2006, Crous et al. 2007), while CBS 109789 came from dialysis tubing. Strain CBS 116009 caused a systemic infection in a Galapagos giant tortoise (Geochelone nigra), reported by Manharth et al. (2005). The tortoise presented with ocular lesions, but upon necropsy a widespread granulomatous inflammation was noted, probably resulting from hematogenous dissemination. Exophiala tremulae (from roots of Populus tremuloides seedings in Canada), is identical based on DNA sequence data, and represents a recent synonym (Crous et al. 2011).
Although the species is unable to grow at 37 °C, some superficial infections in humans were noted, particularly from skin of the extremities. Among the infections was a corneal ulcer and an onychomycosis (Table 1). The case caused by CBS 121504 concerned a 1 yr old child with circular, tinea-like lesions from which the fungus was isolated together with Candida guilliermondii. The lesion was successfully treated with ciclipirox. Exophiala equina was judged to be a secondary invader (J. Brasch, pers. comm.). The species was also noted on the skin of patients with diabetes (G. Haase & P. Mayser, pers. comm.), which had a relatively low body temperature due to impaired circulation. This condition allows invasion by species that are unable to grow at temperatures above 37 °C, particularly when infection takes place on the extremities. Further isolates were recovered from skin flakes, stool and sputum. A transmission route from bathing facilities, as hypothesized for other black yeasts (Lian & de Hoog 2010), seems probable for this species as well. The occurrence of the fungus in bottled water is of concern.
Additional strains sequenced originated from soil (CBS 515.76), from washed roots (CBS 160.89, CBS 159.89) and from a twig of Olea sp. (CBS 121502). Neubert et al. (2006) extracted DNA (GenBank AJ875365) from wetland reed in Germany and encountered the same species. All these environments have relatively low temperatures in common. Human infection seems to be coincidental.
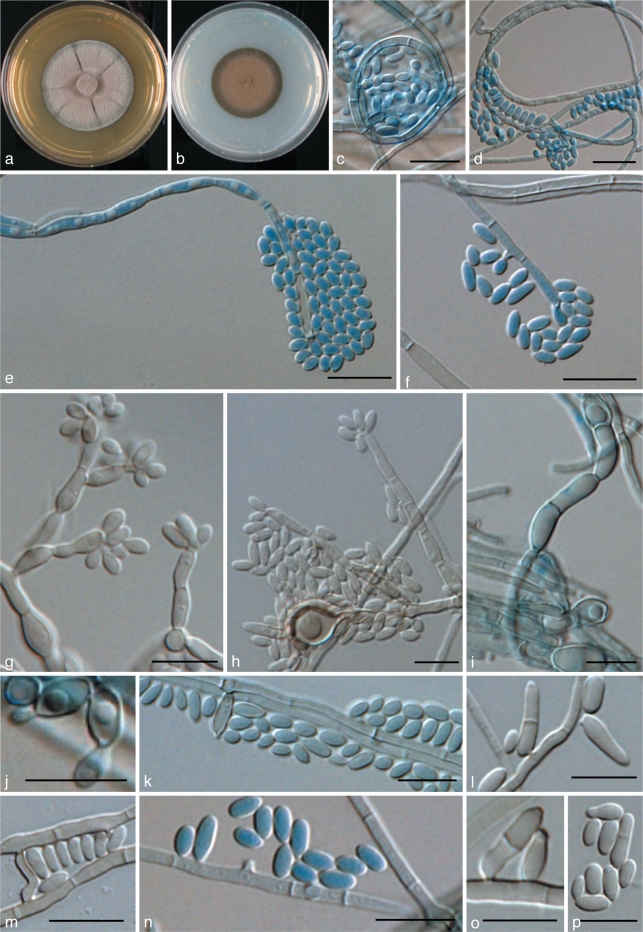
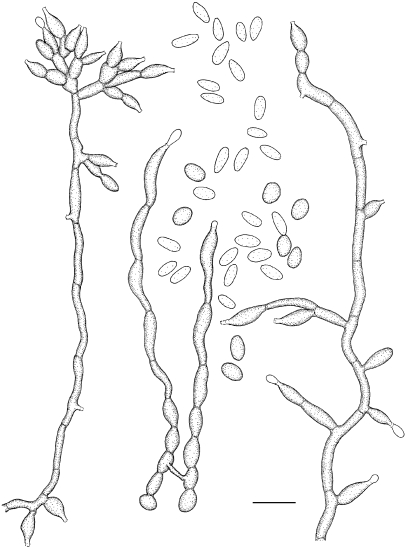
Exophiala halophila de Hoog, Vicente, Najafzadeh, Harrak, Badali & Seyedmousavi, sp. nov. — MycoBank MB515715; Fig. 10, 11
Fig. 10.
Exophiala halophila, CBS 121512. a. Colony on MEA; b. colony on PDA; c–e. conidial apparatus with conidia; f–k. budding cells and conidia; i. tolurose hyphae. — Scale bars = 10 μm.
Fig. 11.
Exophiala halophila, CBS 121512. Conidial apparatus, conidia and anastomosing torulose hyphae. — Scale bar = 10 μm.
Coloniae in agaro maltoso dicto 24 °C lente crescentes, primum leves et zymoideae, deinde elevatae, velutinae, griseae; reversum olivaceo-nigrum. In agaro PDA pigmentum brunneum exudens. Hyphae leves, dilute olivaeo-brunneae, intervallis regularibus septatae; mycelium torulosum proferentes. Conidiophora vix distinguenda, ramose vel simplicia, terminalia vel intercalaria; nonnumquam cellulae gemmantes etiam conidiogenae. Annelloconidia haud septata, late ellipsoidea, levia, 1.9–2.5 × 3.0–5.2 μm. Temperatura maxima crescentiae 33 °C. Teleomorphe ignota.
Etymology. Named after one of the sources of isolation of this species.
Description of CBS 121512 after 2 wk incubation on MEA, 24 °C.
Colonies restricted, compact, circular, olivaceous brown, initially (on day 5) moist and slimy, later (on day 14) becoming brownish grey, velvety at the centre. Reverse olivaceous black. Brown pigment produced on PDA. Yeast cells abundant. Torulose hyphae present. Conidiogenous cells intercalary in undifferentiated hyphae, or discrete, then flask-shaped, lateral or terminal, with short annellated zones. Conidia subhyaline, ellipsoidal to subcylindrical 1.9–2.5 × 3.0–5.2 μm.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 24–27 °C, maximum 30–33 °C. No growth at 37 °C.
Specimen examined. USA, Texas, San Antonio, D.A. Sutton, from human skin, holotype CBS H-19967, ex-type culture CBS 121512 = UTHSC 03-2191 = dH 13757.
Additional material examined. Table 1.
Notes — Only three isolates of this species are available. The ex-type strain was isolated from asymptomatic human skin. The nearest phylogenetic neighbour is E. alcalophila at a minimum distance of 8.4 % (ITS), and thus the novelty of the new species is unambiguous (Fig. 3). The species has been isolated from human skin of the armpit, and from human nails, as well as from salt water. Strains were tolerant of 2.5, 5 and 10 % MgCl2 and of 2.5 and 5 % NaCl2.
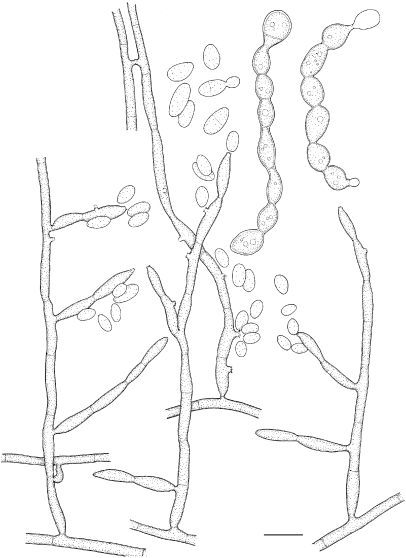
Exophiala lacus de Hoog, Vicente, Najafzadeh, Harrak, Badali & Seyedmousavi, sp. nov. — MycoBank MB515721; Fig. 12, 13
Fig. 12.
Exophiala lacus, CBS 117497. Hyphae with intercalary conidiogenous cells; anastomoses leading network-like hyphae. — Scale bar = 10 μm.
Fig. 13.
Exophiala lacus, CBS 117497. a. Colony on MEA; b. colony on PDA; c. spirally twisted hyphae; d, e. short, erect, cylindrical, multi-celled conidiophores; f, g. hyphae with conidial heads; h. chlamydospores; i, j. budding cells; k. conidia alongside hyphae; l. conidia. — Scale bars = 10 μm.
Coloniae in agaro maltoso dicto 25 °C lente crescentes, velutinae, griseo-olivaceae; reversum olivaceo-nigrum. In agaro PDA pigmentum brunneum absens. Hyphae leves, dilute olivaeo-brunneae, intervallis regularibus septatae. Mycelium torulosum quasi absens. Conidiophora vix distinguenda, brevia, simplicia, preferentia intercalaria. Annelloconidia nonnumquam septata, late ellipsoidea, levia, 1.8–2.6 × 3.1–9.6 μm. Chlamydosporum praesens, maximum 5.2 × 2.6 μm. Temperatura maxima crescentiae 30 °C. Teleomorphe ignota.
Etymology. Named after the lake environment where the species was isolated.
Description of CBS 117497 after 2 wk incubation on MEA, 24 °C.
Colonies moderately expanding, circular, velvety with olivaceous grey aerial mycelium. Reverse olivaceous black, without diffusible pigment. Yeast cells, when present, consisting of subspherical cells producing conidia. Conidiophores short, erect, cylindrical, multi-celled, poorly differentiated with submerged hyphae with conidial heads. Conidia arising alongside the hyphae; conidia 0–1-septate, ellipsoidal to cylindrical, 1.8–2.6 × 3.0–9.6 μm. Chlamydospores ellipsoidal, up to 5.2 × 2.6 μm; spirally twisted hyphae present.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 21–24 °C, maximum 27–30 °C. No growth at 37 °C.
Specimen examined. The Netherlands, Loosdrecht, M.J. Harrak, from fresh-water lake (1 m depth), holotype CBS-H 20407, ex-type culture CBS 117497 = dH 13711.
Notes — Only a single strain of this species is available. It was isolated from a shallow freshwater lake, the lake bottom consisting of a few metres of plant material. The lake is unpolluted, being fed by seepage water from sandy hills.
Exophiala opportunistica de Hoog, Vicente, Najafzadeh, Harrak, Badali & Seyedmousavi, sp. nov. — MycoBank MB515719; Fig. 14, 15
Fig. 14.
Exophiala opportunistica, CBS 109811. Hyphae with mostly intercalary conidiogenous cells with extended annellated zones, and 1–2-celled conidia. — Scale bar = 10 μm.
Fig. 15.
Exophiala opportunistica, CBS 109811. a. Colony on MEA; b. colony on PDA; c. spirally twisted hyphae; d, e. erect cylindrical multi-celled conidiophores; f–l. yeast cells and conidia; i. torulose hyphae. — Scale bars = 10 μm.
Coloniae in agaro maltoso dicto 25 °C lente crescentes, velutinae, griseo-olivaceae; reversum olivaceo-nigrum. In agaro PDA pigmentum brunneum absens. Hyphae leves, dilute olivaeo-brunneae, dense septatae. Mycelium torulosum quasi absens. Conidiophora vix distinguenda, ramose vel simplicia, terminalia vel intercalaria. Annelloconidia nonnumquam septata, late ellipsoidea, levia, 2.4–2.9 × 1.1–1.2 μm. Temperatura maxima crescentiae 30 °C. Teleomorphe ignota.
Etymology. Named after the apparent ability of the species to grow on human nails.
Description of CBS 109811 after 2 wk incubation on MEA, 17 °C.
Colonies restricted, olivaceous grey, velvety with floccose margin and with grey aerial mycelium at the centre. Reverse olivaceous black, without diffusible pigment. Yeast cells, torulose hyphae and spirally twisted hyphae present. Hyphae rather wide, profusely septate and strongly anastomosing. Conidia arising alongside the hyphae or on broadly ellipsoidal, poorly differentiated conidiophores; conidia (0–1)-septate, (sub)hyaline, obovoidal to ellipsoidal, 2.4–2.9 × 1.1–1.2 μm.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 21–24 °C, maximum 27–30 °C. No growth at 37 °C.
Specimen examined. Germany, Duisburg, from drinking water, E. Göttlich, holotype CBS-H 20383, ex-type culture CBS 109811 = dH 12243 = IWW 720.
Additional material examined. Table 1.
Notes — The original strain was derived from drinking water and also from rhizosphere of Triticum aestivum in Western Australia (CBS 660.76). One strain, CBS 637.69 originated from polyvinyl alcohol. We recently also noted presence of E. opportunistica on human nail and foot lesions in Denmark (Table 1).
Exophiala pisciphila McGinnis & Ajello (as ‘pisciphilus’), Mycologia 66: 518. 1974. — MycoBank MB314043
Teleomorph. Unknown.
Description of CBS 537.73 after 2 wk incubation on MEA, 24 °C.
Colonies moderately expanding, dry, floccose, olivaceous black. Yeast cells absent. Conidiogenous cells flask-shaped, mostly in loose clusters or branched systems, with inconspicuous annellated zones. Conidia 0(–1)-septate, (sub)hyaline, ellipsoidal, 6–8 × 2.5–4.0 μm.
Cardinal temperatures — Minimum ≤ 4–9 °C, optimum 24–30 °C, maximum 30–33 °C. No growth at 37 °C.
Specimen examined. USA, Alabama, privately owned freshwater pond, April 1969, N. Fijan, from systemic mycosis in channel catfish (Ictalurus punctatus), ex-type culture CBS 537.73, dried culture CBS H-7135.
Additional material examined. Table 1.
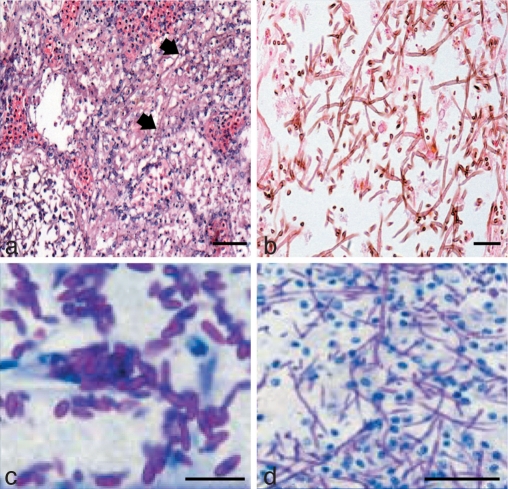
Notes — This species was one of the first Exophiala species described as causing epizootics in cold-blooded vertebrates (Fijan 1969). Eighty percent of a sample of freshwater channel catfish (Ictalurus punctatus) in a pond had cutaneous ulcers. These were 2–15 mm diam and up to 5 mm deep, without inflamed margins. Numerous nodules were found in visceral organs. Hematogenous dissemination led to hemorrhagic peritonitis with purulent exudates. The isolated fungus killed the fish within 13 days after intraperitoneal inoculation, but no neurotropism was noted.
The species was later reported as an opportunistic invader in the marine coastal smooth dogfish (Mustelus canis) in the New York Aquarium (Gaskins & Cheung 1986), but the identity of this now unavailable strain cannot be confirmed by sequencing. Strains causing an epizootic in the captive marine plaice (Pleuronectes platessa), published as Hormoconis resinae (Strongman et al. 1977) were re-identified as Exophiala pisciphila (de Hoog et al. 2009). The fish were maintained in tanks with a continuous flow of pre-filtered seawater. Lesions were mainly cutaneous. The epizootic was thought to have been promoted by a relative high maintenance temperature of up to 15.2 °C. Several isolates originated from disseminated infections in marine potbelly seahorses (Hippocampus abdominalis; Nyaoke et al. 2009). Additional strains sequenced (Table 1) originated from a swimming pool (CBS 121505) and from a water pipe (CBS 101610). The fungus thus occurs on fish living in freshwater as well as seawater, with a low degree of host specificity. In humans, a skin infection in an immunosuppressed patient from Brazil was reported by Sughayer et al. (1991), without sequence confirmation. We also recorded an isolate from the nail of a human patient in Germany (P. Mayser, unpubl. data; Table 1). Yamada et al. (1989) reported a Coenzyme Q10(H2) system in the species.
Exophiala psychrophila O.A. Pedersen & Langvad, Mycol. Res. 92: 153. 1989. — MycoBank MB135481
Description of CBS 191.87 after 2 wk incubation on MEA, 24 °C.
Colonies initially yeast-like and black, gradually becoming effuse and dome-shaped. After 14 d at 18 °C colonies have a dark centre containing the bulk of the conidial mass surrounded by a felt of mouse grey mycelium. Hyphae pale brown, septate, sparingly branched, hyphae 1–3 μm wide. Moniliform cells very common, 3–6 × 5–15 μm, chains consisting of two to several hundred cells, 4–12 being the most common. Conidiogenous cells with several enteroblastic proliferations at the apices with 2.0–3.5 μm diam. Conidia holoblastic, aseptate, varying in shape from spherical to oblong, sometimes tapered, 1.5–2.5 × 3–6 μm, tending to accumulate in slimy balls at apex of conidiogenous cells. Lipid globules may sometimes give the false impression that the conidia are septate. Conidia may be produced from discrete conidiogenous cells, directly from hyphae, from moniliform cells and from conidia. Yeast-like cells also produce conidia.
Cardinal temperatures — Minimum 0 °C (growth present after 6 mo at 0 °C); optimum 17–21 °C; maximum 23 °C.
Specimen examined. Norway, Mar. 1987, F. Langvad, from Atlantic salmon smolt (Salmo salar), ex-type culture, CBS 191.87 = dH15499 = CBS H-20009 = MBSPEC1293.
Notes — The species was originally isolated from farmed Atlantic salmon smolts (Salmo salar) in a farm in Western Norway. The disease led to very high mortality for four years with correspondingly great economic losses for the farmer (Langvad et al. 1985). Strains from salmon in other countries also became available for this study (Table 1).
Exophiala salmonis J.W. Carmich., Sabouraudia 5: 120. 1966. — MycoBank MB119468
Description of CBS 157.67 after 2 wk incubation on MEA, 24 °C.
Colonies moderately expanding, dry, depressed, hairy, olivaceous black. Yeast cells nearly absent. Conidiogenous cells poorly differentiated, intercalary or flask-shaped; annellated zones short, inconspicuous. Conidia 0–3-septate, subhyaline to pale brown, ellipsoidal to short cylindrical, 5.5–8.5 × 2.0–3.5 μm.
Cardinal temperatures — Minimum ≤ 4 °C, optimum 18–24 °C, maximum 30–33 °C. No growth at 37 °C.
Specimens examined. Canada, Alberta, Calgary, Alberta hatchery; cerebral mycetoma of fingerling trout (Salmo clarkii), J.W. Carmichael, specimens CBS H-12617, CBS H-7136, ex-type cultures CBS 157.67 = BMU 00834 = ATCC 16986 = IHEM 3405 = IMI 124165 = MUCL 10078 = UAMH 34 = VKM F-3000.
Additional material examined. Table 1.
Notes — This is the generic type species. Although isolates have frequently been reported under this name, only very few confirmed isolates are available. The species was originally reported as causing three epidemic episodes of cerebral mycetoma in freshwater fingerling trout cod (Maccullochella macquariensis) at the Provincial Government Fish Hatchery in Calgary, Alberta, Canada (Carmichael 1966). The hatchery drew its water from an underground spring which has a temperature range of 12–14 °C and sometimes had a high nitrate content. We sequenced two further isolates of E. salmonis, both from fresh water in The Netherlands. Otis et al. (1985) ascribed an infection in captive Atlantic salmon (Salmo salar) in the USA to the same fungus, but no isolates were available for sequencing. The authors observed interesting parallels to the Canadian case. Both mycoses were systemic in nature and occurred only in captive or hatchery-raised fishes. It appeared that the fishes were debilitated and hence predisposed to infection by opportunistic pathogens. The Atlantic salmon in this study had not been fed properly in captivity, and had just undergone an unsuccessful spawning period. Infections by cestodes (tapeworm, Proteocephalidea) could have weakened the fish, and provided a route of entry for the fungus with subsequent hematogenous spread to the kidney. Madan et al. (2006) reported on subcutaneous nodules in elbows and knees of a 64 yr old male under cyclophosphamide and prednisolone therapy for non-Hodgkin lymphoma ascribed to E. salmonis, but as no sequence data are available this report has to be regarded as doubtful.
Yamada et al. (1989) demonstrated the presence of a coenzyme Q10(H2) system in E. salmonis.
Veronaea botryosa Cif. & A.M. Corte, Atti Ist. Bot. Lab. Crittog. Univ. Pavia, Ser. 5, 15: 68. 1958. — MycoBank MB307734
Description of CBS 254.57 after 2 wk incubation on MEA, 24 °C.
Colonies growing rapidly, velvety to lanose, greyish brown or blackish brown. Conidiophores erect, straight or flexuose, unbranched or occasionally loosely branched, sometimes geniculate, smooth-walled, olivaceous brown, up to 250 μm long, 2–4 μm wide. Conidiogenous cells terminal or lateral, often becoming intercalary, cylindrical in the apical part with numerous flat scars. Conidia smooth-walled or slightly verrucose, sometimes cylindrical, rounded at the apex and truncate at the base, pale brown, usually 1-septate, 5–12 × 3–4 μm.
Cardinal temperatures — Minimum 4–9 °C, optimum 24–30 °C, maximum 33–36 °C. No growth at 37 °C.
Specimen examined. Italy, from sansa olive slag, specimen CBS H-19962, ex-type culture CBS 254.57 = IMI 070233 = MUCL 9821.
Additional material examined. Table 1.
Notes — This sympodial species, which is morphologically very different from the annellidic genus Exophiala (Arzanlou et al. 2007), is found amidst the waterborne species in the salmonis-clade. The ex-type strain was isolated from sansa olive slag (olive presscake) in Italy (Ciferri & Montemartini 1957), a substrate rich in phenolic compounds. Additional environmental strains were isolated on separate occasions from Eucalyptus wood treated with creosote in Brazil (Table 1).
Otherwise the species is known to cause moderately severe to highly mutilating human infections. Matsushita et al. (2003) published a chronic disseminated mycosis of a 12-year-old child in China (CBS 102593). The patient did not have any known immune disorder. A deep skin lesion in a 37-year-old male patient from the Philippines (CBS 101462) was reported by Medina et al. (1998). Further cases have been reported from China (Nishimura et al. 1989), Libya (Ayadi et al. 1995), the USA (Sutton et al. 2004) and France (Foulet et al. 1999), mostly in immunocompromised patients. Several of these cases have not been verified by sequencing, but the species is morphologically sufficiently stable and characteristic for reliable classical identification. Strain CBS 121506 was isolated from a lesion on the hand of a female patient and represents the first case in Japan caused by this fungus.
In contrast to most Exophiala members of the salmonis-clade, combining waterborne ecology with an ability to invade cold-blooded animals and possibly cool human body sites, Veronaea botryosa has a strong predilection for human hosts, causing chronic, deep infections. The above-mentioned disseminated case in a Chinese adolescent, which developed over a six-year period (Matsushita et al. 2003), was a particularly impressive example.
DISCUSSION
Although the black yeast teleomorph genus Capronia was described in 1883 (Saccardo 1883), with the type species C. sexdecimspora even dating back to 1871 (Cooke 1871), its anamorph genus Exophiala was described only quite recently (Carmichael 1966). Exophiala-like anamorphs were occasionally encountered in older literature, demonstrated by the invalidly described genera Foxia (Castellani 1908) and Nadsoniella (Issatchenko 1914). In the present paper it was shown that Haplographium debellae-marengoi var. equinum (Pollacci 1923) could be reidentified as an Exophiala species. Exophiala species are difficult to recover from the environment as they grow very slowly. They are frequently overlooked. They show a remarkable association with monoaromatic hydrocarbons and may have a competitive advantage when these compounds are present (Prenafeta-Boldú et al. 2006). Many species require dedicated isolation methods (Prenafeta-Boldú et al. 2006, Sudhadham et al. 2008, Vicente et al. 2008, Zhao et al. 2010). When isolated, they are very difficult to diagnose based on morphology. This has hampered the accumulation of knowledge and understanding of these organisms.
A pathogenic potential towards immunocompetent animals is observed in nearly all major clades of the Chaetothyriales. The lowest, ancestral clade (Fig. 1) with uncertain affiliation contains predominantly rock-inhabiting species, with occasional taxa involved in mild cutaneous disease. The derived clades are considered to belong to the Herpotrichiellaceae (teleomorph genus Capronia; Fig. 1) and are pathogen-rich (Ruibal et al. 2008). Pathogenicity is particularly observed in fish, amphibians and humans, while infections in marine invertebrates are also known; in mammals, particularly humans are susceptible. Infections in reptiles and birds are absent. All chaetothyrialean fungi are obligatorily melanized, and thus melanin does not explain these predilections. Reptiles and birds are terrestrial animals with thick or protected, dry skin-lacking sweat glands. Infections in furred mammals are also rare; the majority of mammal infections are observed in humans, who, as a species, have thin, predominantly nearly hairless skin provided with sweat glands for cooling.
If we compare this preponderance of potentially invasive species with another fungal order with numerous invasive species, the Onygenales, containing the dermatophytes and classical systemic pathogens, an entirely different picture emerges. Ancient onygenalean species are classified in Chrysosporium and are particularly found on the skin of snakes (Bertelsen et al. 2005), chameleons (Paré et al. 2006), crocodiles (Thomas et al. 2002) and other reptiles. The most derived species are anthrophophilic dermatophytes, which went through repeated host shifts from domesticated animals (Gräser et al. 2006). In the Onygenales we witness a consistent pathogenic evolution, paralleling the phylogeny of the host, whereas in Chaetothyriales all types of opportunism are scattered over the order. Chaetothyrialean opportunism tends, however, to manifest in consistent patterns within species, and thus is not a random process.
Waterborne animals are infected by Chaetothyrialean fungi much more frequently than terrestrial animals (Table 3). Host body temperatures can be correlated with maximum growth temperatures of invasive species in each of the clades. Roughly speaking, clades with species able to grow at temperatures well over 36–37 °C (bantiana, dermatitidis and jeanselmei clades) may cause systemic or disseminated infections in humans, while those with a maximum around 36–37 °C (carrionii and europaea clades) cause (sub)cutaneous and superficial infections. Species of the salmonis clade have maxima at 27–33 °C, exceptionally 36 °C, and cause superficial, barely invasive infections at most (Li et al. 2009, Saunte et al. 2011).
Table 3.
Characteristics of species in the salmonis-clade: maximum growth temperature, main ecology, ability to cause infection in animals.
| Salmonis-clade | Maximum growth temperature (°C) | Main environmental habitat | Infections in animal kingdom | |||||
|---|---|---|---|---|---|---|---|---|
| Invertebrate | Vertebrates | |||||||
| Fish | Amphibian | Reptile | Bird | Mammal | ||||
| E. angulospora | 30–33 | Cold drinking water, sea water aquaria, fish nurseries | – | Sea dragon, marine lumpfish, unspecified fish | – | – | – | Human skin (colonizer?) |
| E. halophila | 30–33 | Salty water | – | – | – | – | – | Human skin (colonizer?) |
| E. alcalophila | 36–40 | Alkaline, bath waste water | – | – | – | – | – | Human skin (colonizer?) |
| E. pisciphila | 30–33 | Fresh water, seawater | – | Channel catfish, captive marine plaice, dog fish | Frog | – | – | Human nail (colonizer?) |
| E. aquamarina | 33–36 | Seawater, aquarium | – | Leafy & weedy sea dragon, winter flounder, tunnyfish, lumpfish | – | – | – | – |
| E. equina | 33–36 | Drinking water, waste water | – | – | – | Galapagos turtle | – | Horse; cutaneous in diabetic human |
| E. salmonis | 30–33 | Cold water (12–14 °C) | – | Trout cod, atlantic salmon | Frog | – | – | – |
| E. opportunistica | 27–30 | Drinking water? | – | – | – | – | – | Human skin and nail (colonizer?) |
| E. psychrophila | 24–27 | Cold water (12–14 °C) | – | Atlantic salmon | – | – | – | – |
| E. cancerae | 30–33 | Mangrove at Brazilian coast | Mangrove crab | – | Green toad | – | – | Human skin (colonizer?), cutaneous in diabetic human |
| E. calicioides | × | Otten wood, soil | – | – | – | – | – | – |
| V. botryosa | 33–36 | Sansa olive slag, treated wood | – | – | – | – | – | Disseminated and fatal in human |
Animal hosts vary greatly in their immune responses to fungal infections. The invertebrate Crustaceans have poorly developed innate immunity and no adaptive immunity. In invasive infection, no or primitive granulomas are formed, and hyphae grow densely and regularly (Fig. 16a, b; Boeger et al. 2005). Primitive fish such as sea horses, with poorly developed innate as well as adaptive immunity, show similar histopathology (Fig. 16c, d; Nyaoke et al. 2009). True granulomas, with lymphocytes, granulocytes and giant cells are observed only in higher animals, which are equipped with fully developed innate and adaptive cellular immunity.
Fig. 16.
Examples of histopathology of Exophiala species. – a, b. Disseminated phaeohyphomycosis by E. aquamarina in weedy seadragons (Phyllopteryx taeniolatus); a. abundant brown fungal hyphae (arrows) coursing through necrotic tubules, interstitium, and sinusoids in renal parenchyma. Stained with H & E. Reproduced from Nyaoke et al. 2009; b. fungal hyphae, kidney; weedy seadragon. Hyphae are slender, filamentous, and septate with occasional right-angled branches. Fontana-Masson staining; walls of hyphae stain brown, indicative of melanin. Reproduced from Nyaoke et al. 2009. – c. Light micrograph of transverse section of a gill of lethargic mangrove crab (Ucides cordatus) lamella with numerous conidia of E. cancerae in lacunae. Stained with PAS. Reproduced from Boeger et al. 2005. – d. Light micrograph of cardiac tissue of lethargic mangrove crab (Ucides cordatus) parasitized by hyphae of E. cancerae. Stained with PAS and counter-stained with H & E. Reproduced from Boeger et al. 2005. — Scale bars: a, d = 50 μm; b = 25 μm; c = 15 μm.
Several clades contain Exophiala species that are relatively common in low-nutrient aquatic environments, ranging from municipal drinking water and Arctic ocean water to hot springs and swimming pools. In all mentioned environments strains are encountered that cause infections in vertebrates, rarely in invertebrates. This apparent intrinsic pathogenic potency is generally recognised for thermophilic and mesophilic Exophiala species that can be isolated from moist indoor environments. The best-known example is E. dermatitidis from Turkish steam baths. It causes neurotropic disseminated infections in humans (Hiruma et al. 1993, Chang et al. 2000). Mesophilic species such as E. oligosperma are also regularly encountered in human infections (de Hoog et al. 2009). Among the medical problems caused by E. oligosperma was a pseudoepidemic due to clinical use of contaminated hospital water (Nucci et al. 2001, isolates identified as E. jeanselmei).
Another factor discussed as a potential virulence factor is the ability to assimilate monoaromatic hydrocarbons by opening the benzene ring (Rustler et al. 2008). At this moment, insufficient data are available on physiological profiles of black yeasts in connection with the large diversity of alkylbenzenes. Nevertheless the relatively frequent infections of amphibians (e.g. Cicmanec et al. 1973, Elkan & Philpot 1973, Beneke 1977, Bube et al. 1992) that produce aromatic toxins in their skin such as epibatidine and bufotenin, is remarkable. A central role of alkylbenzene assimilation in the development of chaetothyrialean phylogeny seems highly probable. In summary, it may be stated that in Chaetothyriales the presence of melanin and the ability to assimilate alkylbenzenes generally enhance infection thermotolerance and physical skin properties determine host preferences, and cellular immunity determines the type of tissue response.
As noted above, the majority of preponderantly waterborne species are clustered in a single clade within the Chaetothyriales, the salmonis-clade, with the exception of scattered species elsewhere in the phylogenetic tree (Fig. 1). Most of the waterborne Exophiala species are meso- or even psychrophilic (Table 3). Generally, a correlation is observed between the maximum growth temperature and the natural habitat of the host. Fungi with maximum growth temperatures below 33 °C are found causing diseases in animals in cold waters, such as the deep sea or the polar oceans. Examples are epizootics of Exophiala psychrophila in Atlantic salmon (Pedersen & Langvad 1989) and Exophiala salmonis in trout (Carmichael 1966). This is a remarkable feature, since elsewhere in the fungal Kingdom, species lacking thermotolerance tend to be saprobes without infectious abilities. Human infections caused by these non-thermotolerant fungi are rare. Some species with maximum growth temperatures around 33 °C, exceptionally up to 36 °C may cause zoonotics in cold-blooded animals living in shallow tidal zones in the subtropics. A striking example is the emerging Lethargic Crab Disease in crabs inhabiting mangrove areas along the east coast of Brazil (Boeger et al. 2005, 2007). Species of this group may be seen on human skin, although their role in pathology has not unambiguously been proven (Saunte et al. 2011; Table 1). Lian & de Hoog (2010) have shown that several members of Chaetothyriales known from superficial infections in humans are commonly isolated from bathrooms when appropriate isolation methods are applied.
The infection pattern of members of Chaetothyriales suggests the existence of intrinsic factors enhancing vertebrate invasion, but infection is probably not a prime factor in the natural habitat of these fungi. The different degrees and types of virulence to particular hosts is striking. The main susceptible groups are amphibians, fishes and humans, while other groups are significantly underrepresented; reptiles and birds are missing (Table 3). This may largely be due to the preponderant life styles of these hosts. While amphibians and fish are waterborne and have thin, mucous skins, reptiles and bird are predominantly terrestrial and are protected by thick, dry, water-repellent skin and, in birds, an envelope of water-repelling feathers. Most mammals have a hairy pelt which is difficult to penetrate, whereas the soft, naked skin of humans is more vulnerable to black yeast infection, particularly after maceration. Reports of Exophiala species infections in domestic and wild animals are very rare (Helms & McLeod 2000, Kano et al. 2000). Of note, black yeast infections in invertebrates all are recorded from moist animals, such as mussels (van Dover et al. 2007), earthworms (Vakali 1993) and mangrove crabs (Boeger et al. 2005), while terrestrial insects are never affected.
Besides integumental barriers, immune responses may be involved in host defence, since relatively few systemic infections in mammals other than humans are known. The immune response to waterborne Exophiala species varies with the host. The relative importance of specific innate versus adaptive defence mechanisms differs with the evolutionary position of metazoans (Muller & Muller 2003; Table 2). The involvement of phagocytic cells in engulfing foreign cells has been documented in virtually all metazoan organisms. Phagocytic cells possess a limited capacity to discriminate self from non-self, which is partly due to their use of a limited diversity of cell surface lectins for recognition. Although there is no evidence to suggest that invertebrate lectins and vertebrate immunoglobulins are homologous structures, sufficient diversity exists within lectins of certain species to indicate that these types of molecules and their cellular expression on phagocytes might serve as a primitive and universal recognition mechanism (Bosch et al. 2009). The presence of lymphocytes and circulating antibodies has been documented in all extant vertebrate species. However, the existence of induced, specific reactions homologous to the immune repertoire of vertebrates has not been clearly established in invertebrates (Frank 2002). All true vertebrates possess cells clearly recognisable as lymphocytes that can carry out T-cell functions, and show the capacity of B-cells to synthesize and secrete immunoglobulins. True lymph nodes are not present in vertebrate species more primitive than mammals, but birds possess aggregates of lymphoid tissue probably serving a similar function (Davison et al. 2008).
Table 2.
Different pathogenic potentials in clades of Chaetothyriales: maximum growth temperature, synthesis of melanin and different levels of development of the immune system of potential hosts.
| Clades of Chaetothyriales | Maximum growth temperature (°C) | Synthesis of melanin | Maximum disease in invertebrates | Maximum disease in vertebrates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crustacean | Fish | Amphibian | Reptile | Bird | Mammal | |||||||||
| Level of Immunity | ||||||||||||||
| Innate | Adaptive | Innate | Adaptive | Innate | Adaptive | Innate | Adaptive | Innate | Adaptive | Innate | Adaptive | |||
| + | − | ++ | + | ++ | + | +++ | +++ | ++++ | ++++ | +++++ | +++++ | |||
| bantiana-clade | 37–40 | + | − | − | − | − | − | Disseminated | ||||||
| dermatitidis-clade | 36–42 | + | − | − | − | − | − | Disseminated | ||||||
| jeanselmei-clade | 36–38 | + | − | − | − | − | − | Disseminated | ||||||
| carrionii-clade | 36–37 | + | − | − | − | − | − | Subcutaneous | ||||||
| europaea-clade | 37 | + | − | − | − | − | − | Superficial, cutaneous | ||||||
| salmonis-clade | 27–33(36) | + | Disseminated | Disseminated | Disseminated | − | − | Superficial | ||||||
| rock-clade | ? | + | − | − | − | − | − | Superficial | ||||||
Humans possess five major classes or isotypes of immunoglobulin: IgG, IgM, IgA, IgE and IgD. The IgM molecule is the first immunoglobulin to appear in ontogeny, and the first to appear in the phylogeny of the immune system (Davis & Hamilton 1998). Immunoglobulins of cyclostomes, sharks, rays and many teleost fishes consist of IgM polymers only. Some lungfish (Dipnoi) have a low-molecular-weight non-IgM immunoglobulin termed IgN (Magnadóttir 2006). Birds possess IgM and IgA immunoglobulins, but also possess a non-IgM immunoglobulin similar to that found in amphibians as their major immunoglobulin class. This immunoglobulin has been termed IgY (Davison et al. 2008). IgG immunoglobulins containing gamma chains and homologous to those of humans and other derived mammals are found only in the three subclasses of living mammals, namely Eutherians, Metatherians (Marsupials) and Monotremes (for example, the Echidna).
Although the precise nature of the precursors of elements of the immune system in evolution remains to be determined, the genetic and cellular events which lead to the ability of specific immune recognition, diversification, and reactivity are likely to have occurred early in vertebrate evolution (Abbas & Lichtman 2005). Nevertheless, different responses to comparable types of infection are known. For example, absence of advanced immune responses, such as granuloma formation or significant inflammatory response, is observed in fishes (Cooper & Alder 2006) and primitive metazoans (Du Pasquier 2001). This might be the result of inadequate or deficient immunological machinery. This is observed, for example, in the Lethargic Crab Disease (Boeger et al. 2005) and in systemic infections in seahorses (Nyaoke et al. 2009), where internal organs are homogeneously infected by black yeasts.
What is the nature of virulence in Chaetothyriales? The wide distribution of invasive abilities over members of the order is remarkable. Infections are generally regarded as being opportunistic, i.e. coincidental, not forming an essential part of the natural life cycle of the organism, and not conveying any evolutionary advantage because fitness is not increased. In this hypothesis, pathogenicity does not emerge over the course of evolutionary time. Pathogenicity is defined ecologically as a fitness advantage that is derived when an animal host is regularly used in any part of the fungal life cycle (de Hoog et al. 2009). If there is no self-reinforcing evolution in the infective ability of Chaetothyriales, one may wonder why opportunism is such a consistent feature throughout the order.
To our knowledge, infection is never caused by ingestion of one of the waterborne Exophiala species with food or water. Matos et al. (2003) and Hiruma et al. (1993) suggested an ingestive route of infection for the more virulent, systemic species E. dermatitidis, but this species has not been found in municipal water distribution networks. Therefore we consider the health risk of municipal water to be very low. In addition, since no pulmonary cases are known by any of the species treated, a route of infection through aerosols during showering or running tap water is unlikely. The main health risk of these fungi probably is cutaneous inoculation after moistening and abrasion of the skin, as suggested by Lian & de Hoog (2010).
Acknowledgments
Elke Göttlich, Ditte Saunte Linhardt, Gerhard Haase, Peter Mayser, Jörg Brasch, Akinyi Nyaoke, Ann Manharth, Scott Weber, Deanna Sutton and Andrew Cunningham are gratefully acknowledged for providing strains for study. Bert Gerrits van de Ende, Katia Cruz, Jenny Miteva and Margit Sieberer are thanked for technical assistance. Steph Menken is thanked for useful comments on the manuscript. The work of Vania A. Vicente was supported by the Brazilian Government fellowship from Coordenação de Pessoal de Nível Superior (CAPES) and the work of Mohammad Javad Najafzadeh by the Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. We thank Richard Summerbell for careful editing of the script.
REFERENCES
- Abbas AK, Lichtman AH.2005. Cellular and molecular immunology. Saunders Co. [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW.2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57– 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila de la Calle A, Groenewald JZ, Trapero A, Crous PW.2006. Characterization and epitypification of Pseudocercospora cladosporioides, the causal organism of Cercospora leaf spot of olives. Mycological Research 106: 881– 888 [DOI] [PubMed] [Google Scholar]
- Ayadi A, Huerre MR, Bièvre C de.1995. Phaeohyphomycosis caused by Veronaea botryosa. Lancet 346: 1703– 1704 [DOI] [PubMed] [Google Scholar]
- Badali H, Gueidan C, Najafzadeh MJ, Bonifaz A, Gerrits van den Ende AHG, Hoog GS de.2008. Biodiversity of Cladophialophora. Studies in Mycology 61: 175– 191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneke ES.1977. Dematiaceous fungi in laboratory-housed frogs. Proceedings of the IV International Conference on Mycoses: ‘The black and white yeasts’. Pan American Health Organization Scientific Publication 356: 101– 108 [Google Scholar]
- Bertelsen MF, Crawshaw GJ, Sigler L, Smith DA.2005. Fatal cutaneous mycosis in tentacled snakes (Erpeton tentaculatum) caused by the Chrysosporium anamorph of Nannizziopsis vriesii. Journal of Zoo and Wildlife Medicine 36: 82– 87 [DOI] [PubMed] [Google Scholar]
- Boeger WA, Pie RM, Ostrensky A, Patella L.2005. Lethargic crab disease: multidisciplinary evidence supports a mycotic etiology. Memorias Instituto Oswaldo Cruz, Rio de Janeiro 100: 161– 167 [DOI] [PubMed] [Google Scholar]
- Boeger WA, Pie RM, Vicente V, Ostrensky A, Hungria D, Castilho GG.2007. Histopathology of the mangrove land crab Ucides cordatus (Ocypodidae) affected by lethargic crab disease. Diseases of Aquatic Organisms 78: 73– 81 [DOI] [PubMed] [Google Scholar]
- Bosch TCG, Augustin R, Anton-Erxleben F, Fraune S, Hemmrich G, Zill H, et al. 2009. Uncovering the evolutionary history of innate immunity: the simple metazoan hydra uses epithelial cells for host defence. Developmental and Comparative Immunology 33: 559– 569 [DOI] [PubMed] [Google Scholar]
- Bube A, Burkhardt E, Weiß R.1992. Spontaneous chromomycosis in the marine toad (Bufo marinus). Journal of Comparative Pathology 106: 73– 77 [DOI] [PubMed] [Google Scholar]
- Carmichael JW.1966. Cerebral mycetoma of trout due to a Phialophora-like fungus. Sabouraudia 6: 120– 123 [DOI] [PubMed] [Google Scholar]
- Castellani A.1908. Tropical dermatomycoses. The Journal of Tropical Medicine and Hygiene 17: 261– 267 [Google Scholar]
- Cateau E, Mergey T, Kauffmann-Lacroix C, Rodier MH.2009. Relationships between free living amoebae and Exophiala dermatitidis: a preliminary study. Medical Mycology 47: 115– 118 [DOI] [PubMed] [Google Scholar]
- Chang CL, Kim DS, Park DJ, Kim HJ, Lee CH, Shin JH.2000. Acute cerebral phaeohyphomycosis due to Wangiella dermatitidis accompanied by cerebrospinal fluid eosinophilia. Journal of Clinical Microbiology 38: 1965– 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicmanec JL, Ringler DH, Beneke ES.1973. Spontaneous occurrence and experimental transmission of the fungus Fonsecaea pedrosoi, in the marine toad, Bufo marinus. Laboratory Animal Science 23: 43– 47 [PubMed] [Google Scholar]
- Ciferri R, Montemartini A.1957. Sui generi Muchmoria Sacc. e Veronaea n. gen. (Dematiaceae, Didymosporae). Atti dell’Istituto Botanica Laboratorio Crittogamia dell’Università di Pavia, Sér. 5, 15: 67– 72 [Google Scholar]
- Cooke MC.1871. Handbook of British Fungi 2. Macmillan, London, UK [Google Scholar]
- Cooper MD, Alder MN.2006. The evolution of adaptive immune systems. Cell 124: 815– 822 [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G.2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19– 22 [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, Edwards J, Seifert KA, et al. 2011. Fungal Planet description sheets: 69–91. Persoonia 26: 108– 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schubert K, Braun U, Hoog GS de, Hocking AD, et al. 2007. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology 58: 185– 217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkleij GJM, Groenewald JZ, Samson RA. (eds). 2009. Fungal Biodiversity. CBS Laboratory Manual Series. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands [Google Scholar]
- Davis WC, Hamilton MJ.1998. Comparison of the unique characteristics of the immune system in different species of mammals. Veterinary Immunology and Immunopathology 63: 7– 13 [DOI] [PubMed] [Google Scholar]
- Davison F, Kaspers B, Schat KA.2008. Avian immunology. Academic, New York: . [Google Scholar]
- Dover CL van, Ward ME, Scott JL, Underdown J, Anderson B, et al. 2007. A fungal epizootic in mussels at a deep-sea hydrothermal vent. Marine Ecology 28: 54– 62 [Google Scholar]
- Elkan E, Philpot CM.1973. Mycotic infections in frogs due to a Phialophora-like fungus. Sabouraudia 11: 99– 105 [DOI] [PubMed] [Google Scholar]
- Fijan N.1969. Systemic mycosis in channel catfish. Bulletin of the Wildlife Disease Association 5: 109– 110 [DOI] [PubMed] [Google Scholar]
- Foulet F, Duvoux C, Bièvre C de, Hézode C, Bretagne S.1999. Cutaneous phaeohyphomycosis caused by Veronaea botryosa in a liver transplant recipient successfully treated with itraconazole. Clinical Infectious Disease 29: 689– 690 [DOI] [PubMed] [Google Scholar]
- Frank SA.2002. Immunology and evolution of infectious disease. Princeton University Press; [PubMed] [Google Scholar]
- Gaskins JE, Cheung PJ.1986. Exophiala pisciphila, a study of its development. Mycopathologia 93: 173– 184 [DOI] [PubMed] [Google Scholar]
- Gerrits van den Ende AHG, Hoog GS de.1999. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Studies in Mycology 43: 151– 162 [Google Scholar]
- Gonzalez MS, Alfonso B, Seckinger D.1984. Subcutaneous phaeohyphomycosis caused by Cladosporium devriesii, sp. nov. Sabouraudia 22: 427– 432 [PubMed] [Google Scholar]
- Goto S, Aono R, Sugiyama J, Horikoshi K.1981. Exophiala alcalophila, a new black, yeast-like hyphomycete with an accompanying Phaeococcomyces alcalophilus morph, and its physiological characteristics. Transactions of the Mycological Society of Japan 22: 429– 439 [Google Scholar]
- Göttlich E, Lubbe W van der, Lange B, Fiedler S, Melchert I, et al. 2002. Fungal flora in groundwater-derived public drinking water. International Journal of Hygiene and Environmental Health 205: 269– 279 [DOI] [PubMed] [Google Scholar]
- Gräser Y, Hoog GS de, Summerbell RC.2006. Dermatophytes: recognizing species of clonal fungi. Medical Mycology 44: 199– 209 [DOI] [PubMed] [Google Scholar]
- Haase G, Sonntag L, Melzer-Krick B, Hoog GS de.1999. Phylogenetic inference by SSU gene analysis of members of the Herpotrichiellaceae, with special reference to human pathogenic species. Studies in Mycology 43: 80– 97 [Google Scholar]
- Hamada N, Abe N.2009. Physiological characteristics of 13 common fungal species in bathrooms. Mycoscience 50: 421– 429 [Google Scholar]
- Helms SR, McLeod CG.2000. Systemic Exophiala jeanselmei infection in a cat. Journal of the American Veterinary Medical Association 217: 1858– 1861 [DOI] [PubMed] [Google Scholar]
- Hiruma M, Kawada A, Ohata H, Ohnishi Y, Takahashi H, et al. 1993. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 36: 1– 7 [DOI] [PubMed] [Google Scholar]
- Hoog GS de.1977. The black yeasts and allied hyphomycetes. Studies in Mycology 15: 1– 144 [Google Scholar]
- Hoog GS de, Guarro J, Figueras MJ, Gené J.2009. Atlas of clinical fungi. 3rd CD-ROM ed CBS-KNAW Fungal Biodiversity Centre, Utrecht: / Universitat Rovira I Virgili, Reus [Google Scholar]
- Issatschenko BL.1914. O rozovykh I chernykh proffach. Trudy Murmanskoĭ Nauchno-Promyslovoĭ Ekspeditsii 1906. Goda (Petrograd): 264– 275 [Google Scholar]
- Iwatsu T, Nishimura K, Miyaji M.1984. Exophiala castellanii sp. nov. Mycotaxon 20: 307– 314 [Google Scholar]
- Iwatsu T, Udagawa SI, Takase T.1991. A new species of Exophiala recovered from drinking water. Mycotaxon 41: 321– 328 [Google Scholar]
- Joyner PH, Shreve AA, Spahr J, Fountain AL, Sleeman JM.2006. Phaeohyphomycosis in a free-living eastern box turtle (Terrapene carolina carolina). Journal of Wildlife Diseases 42: 883– 888 [DOI] [PubMed] [Google Scholar]
- Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse A.2005. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytologist 166: 639– 653 [DOI] [PubMed] [Google Scholar]
- Kano R, Kusuda M, Nakamura Y.2000. First isolation of Exophiala dermatitidis from a dog: identification by molecular analysis. Veterinary Microbiology 76: 201– 205 [DOI] [PubMed] [Google Scholar]
- Kurata O, Munchan C, Wada S, Hatai K, Miyoshi Y, Fukuda Y.2008. Novel Exophiala infection involving ulcerative skin lesions in Japanese Flounder Paralichthys olivaceus. Fish Pathology 43: 35– 44 [Google Scholar]
- Langdon JS, McDonald WL.1987. Cranial Exophiala pisciphila in Salmo salar in Australia. Bulletin of the European Association of Fish Pathologists 35: 117– 119 [Google Scholar]
- Langvad F, Pedersen O, Engjom K.1985. A fungal disease caused by Exophiala sp. nov. in farmed Atlantic salmon in Western Norway. In: Ellis AE. (ed), Fish and shellfish pathology. Academic Publishers, London [Google Scholar]
- Li DM, Hoog GS de, Lindhardt Saunte DM, Gerrits van den Ende AHG, Chen XR.2008. Coniosporium epidermidis sp. nov., a new species from human skin. Studies in Mycology 61: 131– 136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DM, Li RY, Hoog GS de, Wang YX, Wang DL.2009. Exophiala asiatica, a new species from a fatal case in China. Medical Mycology 47: 101– 109 [DOI] [PubMed] [Google Scholar]
- Lian X, Hoog GS de.2010. Indoor wet cells harbour melanized agents of cutaneous infection. Medical Mycology 48: 622– 628 [DOI] [PubMed] [Google Scholar]
- Listemann H, Freiesleben H.1996. Exophiala mesophila spec. nov. Mycoses 39: 1– 3 [DOI] [PubMed] [Google Scholar]
- Madan V, Bisset D, Harris P, Howard S, Beck MH.2006. Phaeohyphomycosis caused by Exophiala salmonis. British Journal of Dermatology 154: 1082– 1084 [DOI] [PubMed] [Google Scholar]
- Magnadóttir B.2006. Innate immunity of fish (overview). Fish and Shellfish Immunology 20: 137– 151 [DOI] [PubMed] [Google Scholar]
- Manharth A, Lemberger K, Mylniczenko N, Pinkerton M, Pessier AP, et al. 2005. Disseminated phaeohyphomycosis due to Exophiala species in a Galapagos tortoise, Geochelone nigra. Journal of Herpetological Medicine and Surgery 15: 20– 26 [Google Scholar]
- Matos T, Haase G, Gerrits van den Ende AHG, Hoog GS de.2003. Molecular diversity of oligotrophic and neurotropic members of the black yeast genus Exophiala, with accent on E. dermatitidis. Antonie van Leeuwenhoek 83: 293– 303 [DOI] [PubMed] [Google Scholar]
- Matsushita A, Jilong L, Hiruma M, Kobayashi M, Matsumoto T, et al. 2003. Subcutaneous phaeohyphomycosis caused by Veronaea botryosa in the People’s Republic of China. Journal of Clinical Microbiology 41: 2219– 2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MR, Ajello L.1974. A new species of Exophiala isolated from channel catfish. Mycopathologia 66: 518– 520 [PubMed] [Google Scholar]
- Medina AL, Redondo JAD, Nebrida LM.1998Two unusual cases of mycoses in the Philippines. Proceedings of the 4th China Japan International Congress of Mycology: 88 [Google Scholar]
- Müller E, Petrini O, Fischer PJ, Samuels GJ, Rossman AY.1987. Taxonomy and anamorphs of the Herpotrichiellaceae with notes on generic synonymy. Transactions of the British Mycological Society 88: 63– 74 [Google Scholar]
- Muller WG, Muller IM.2003. Origin of the metazoan immune system: identification of the molecules and their functions in sponges. Integrative and Comparative Biology 43: 281– 292 [DOI] [PubMed] [Google Scholar]
- Neubert K, Mendgen K, Brinkmann H, Wirsel SG.2006. Only a few fungal species dominate highly diverse mycofloras associated with the common reed. Applied and Environmental Microbiology 72: 1118– 1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Miyaji M, Taguchi H, Tanaka R.1987. Fungi in bathwater and sludge of bathroom drainpipes. I. Frequent isolation of Exophiala species. Mycopathologia 97: 17– 23 [DOI] [PubMed] [Google Scholar]
- Nishimura K, Miyaji M, Taguchi H, Wang DL, Li RY, Meng ZH.1989. An ecological study on pathogenic dematiaceous fungi in China. Proceedings of the 4th International Symposium of the Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University: 17– 20 [Google Scholar]
- Nucci M, Akiti T, Barreiros G, Silveira F, Revankar SG, Sutton DA, Patterson TF.2001. Nosocomial fungemia due to Exophiala jeanselmei var. jeanselmei and a Rhinocladiella species: newly described causes of bloodstream infection. Journal of Clinical Microbiology 39: 514– 518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyaoke A, Weber ES, Innis C, Stremme D, Dowd C, et al. 2009. Disseminated phaeohyphomycosis in weedy, Phyllopteryx taeniolatus, and leafy, Phycodurus eques, seadragons caused by species of Exophiala, including a novel species. Journal of Veterinary Diagnostic Investigation 21: 69– 79 [DOI] [PubMed] [Google Scholar]
- Okada G, Jacobs K, Kirisits T, Louis-Seize GW, Seifert KA, et al. 2000. Epitypification of Graphium penicillioides Corda, with comments on the phylogeny and taxonomy of graphium-like synnematous fungi. Studies in Mycology 45: 169– 188 [Google Scholar]
- Okada G, Seifert KA, Takematsu A, Yamaoka Y, Miyazaki S, Tabaki K.1998. A molecular phylogenetic reappraisal of the Graphium complex based on 18S rDNA sequences. Canadian Journal of Botany 76: 1495– 1506 [Google Scholar]
- Otis EJ, Wolke RE, Blazer VS.1985. Infection of Exophiala salmonis in Atlantic salmon (Salmo salar L). Journal of Wildlife Diseases 21: 61– 64 [DOI] [PubMed] [Google Scholar]
- Paré JA, Coyle KA, Sigler L, Maas AK, Mitchell RL.2006. Pathogenicity of the Chrysosporium anamorph of Nannizziopsis vriesii for veiled chameleons (Chamaeleo calyptratus). Medical Mycology 44: 25– 31 [DOI] [PubMed] [Google Scholar]
- Pasquier L du.2001. The immune system of invertebrates and vertebrates. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 129: 1– 15 [DOI] [PubMed] [Google Scholar]
- Pedersen OA, Langvad F.1989. Exophiala psychrophila sp. nov., a pathogenic species of the black yeasts isolated from Atlantic salmon. Mycological Research 92: 153– 156 [Google Scholar]
- Pollacci G.1923. Miceti del corpo umano e degli animali. Atti dell’Istituto Botanico Università di Pavia 18: 1– 9 [Google Scholar]
- Porteous NB, Grooters AM, Redding SW, Thompson EH, Rinaldi MG, et al. 2003a. Identification of Exophiala mesophila isolated from treated dental unit waterlines. Journal of Clinical Microbiology 41: 3885– 3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous NB, Redding SW, Thompson EH, Grooters AM, Hoog GS de, Sutton DA.2003b. Isolation of an unusual fungus in treated dental unit waterlines. Journal of the American Dental Association 134: 853– 858 [DOI] [PubMed] [Google Scholar]
- Prenafeta-Boldú FX, Summerbell R, Hoog GS de.2006. Fungi growing on aromatic hydrocarbons: biotechnology’s unexpected encounter with biohazard? FEMS Microbiological Reviews 30: 109– 130 [DOI] [PubMed] [Google Scholar]
- Rakeman JL, Bui U, LaFe K, Ching Chen Y, Honeycutt RJ, Cookson BT.2005. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. Journal of Clinical Microbiology 43: 3324– 3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach-Klinke H-H.1956. Ueber einige bisher unbekannte Hyphomyceten bei verschiedenen Süsswasser- und Meeresfischen. Mycopathologia Mycologia Applicata 7: 333– 347 [DOI] [PubMed] [Google Scholar]
- Reuter RE, Hutchinson W, Ham J, Davis S.2003. Exophiala sp. infection in captured King George whiting (Sillaginodes punctata). Bulletin of the European Association of Fish Pathologists 23: 128– 134 [Google Scholar]
- Richards RH, Holliman A, Helgason S.1978. Exophiala salmonis infection in Atlantic salmon Salmo salar L. Journal of Fish Diseases 1: 357– 368 [Google Scholar]
- Ruibal C, Platas G, Bills GF.2008. High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 21: 93– 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustler S, Chmura A, Sheldon RA, Stolz A.2008. Characterisation of the substrate specificity of the nitrile hydrolyzing system of the acidotolerant black yeast Exophiala oligosperma R1. Studies in Mycology 61: 165– 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. Sylloge Fungorum (Abellini) 1883;2:288. [Google Scholar]
- Saunte DM, Tarazooie B, Arendrup MC, Hoog GS de.2011Melanized fungi in skin and nail: it probably matters. Mycoses. doi:10.1111/j.1439-0507.2011.02055.x. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EW, Burgess TI, et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 62: 1– 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont JA.2008. Rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75: 758– 771 [DOI] [PubMed] [Google Scholar]
- Stringer EM, Garner MM, Proudfoot JS, Ramer JC, Bowman MR, et al. 2009. Phaeohyphomycosis of the Carapace in an Aldabra tortoise (Geochelone gigantea). Journal of Zoo and Wildlife Medicine 40: 160– 167 [DOI] [PubMed] [Google Scholar]
- Strongman DB, Morrison CM, McClelland G.1997. Lesions in the musculature of captive American plaice Hippoglossoides plaessoides caused by the fungus Hormoconis resinae (Deuteromycetes). Diseases of Aquatic Organisms 28: 107– 113 [Google Scholar]
- Sudhadham M, Prakitsin S, Sivichai S, Chaiwat R, Menken SBJ, et al. 2008. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Studies in Mycology 61: 145– 155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sughayer M, DeGirolami PC, Khettry U, Korzeniowski D, Grumney A, et al. 1991. Human infection caused by Exophiala pisciphila: case report and review. Reviews of Infectious Diseases 13: 379– 382 [DOI] [PubMed] [Google Scholar]
- Sutton DA, Rinaldi MG, Kielhofner M.2004. First U.S. report of subcutaneous phaeohyphomycosis caused by Veronaea botryosa in a heart transplant recipient and review of the literature. Journal of Clinical Microbiology 42: 2843– 2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S.2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596– 1599 [DOI] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21– 32 [DOI] [PubMed] [Google Scholar]
- Thomas AD, Sigler L, Peucker S, Norton JH, Nielan A.2002. Chrysosporium anamorph of Nannizziopsis vriesii associated with fatal cutaneous mycoses in the salt-water crocodile (Crocodylus porosus). Medical Mycology 40: 143– 151 [DOI] [PubMed] [Google Scholar]
- Vakali NG.1993. Exophiala jeanselmei, a pathogen of earthworm species. Journal of Medical and Veterinary Mycology 31: 343– 346 [Google Scholar]
- Velázquez LF, Restrepo A.1975. Chromomycosis in the toad (Bufo marinus) and a comparison of the etiologic agent with fungi causing human chromomycosis. Sabouraudia 13: 1– 9 [DOI] [PubMed] [Google Scholar]
- Vicente VA, Attili-Angelis D, Pie MR, Queiroz-Telles F, Cruz LM, Najafzadeh MJ, Hoog GS de, Pizzirani-Kleine AR.2008. Environmental isolation of black yeast-like fungi involved in human infection. Studies in Mycology 61: 137– 144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Sugihara K, Eijk GW van, Roeijmans HJ, Hoog GS de.1989. Coenzyme Q systems in ascomycetous black yeasts. Antonie van Leeuwenhoek 56: 349– 356 [DOI] [PubMed] [Google Scholar]
- Zeng JS, Hoog GS de.2007. Exophiala spinifera and its allies: diagnostics from morphology to DNA barcoding. Medical Mycology 46: 193– 208 [DOI] [PubMed] [Google Scholar]
- Zhao J, Zeng J, Hoog GS de, Attili-Angelis D, Prenafeta-Boldú FX.2010. Isolation of black yeasts by enrichment on atmospheres of monoaromatic hydrocarbons. Microbial Ecology 60: 149– 156 [DOI] [PMC free article] [PubMed] [Google Scholar]