Abstract
Species of Leucadendron, Leucospermum and Protea (Proteaceae) are in high demand for the international floriculture market due to their brightly coloured and textured flowers or bracts. Fungal pathogens, however, create a serious problem in cultivating flawless blooms. The aim of the present study was to characterise several of these pathogens using morphology, culture characteristics, and DNA sequence data of the rRNA-ITS and LSU genes. In some cases additional genes such as TEF 1-α and CHS were also sequenced. Based on the results of this study, several novel species and genera are described. Brunneosphaerella leaf blight is shown to be caused by three species, namely B. jonkershoekensis on Protea repens, B. nitidae sp. nov. on Protea nitida and B. protearum on a wide host range of Protea spp. (South Africa). Coniothyrium-like species associated with Coniothyrium leaf spot are allocated to other genera, namely Curreya grandicipis on Protea grandiceps, and Microsphaeropsis proteae on P. nitida (South Africa). Diaporthe leucospermi is described on Leucospermum sp. (Australia), and Diplodina microsperma newly reported on Protea sp. (New Zealand). Pyrenophora blight is caused by a novel species, Pyrenophora leucospermi, and not Drechslera biseptata or D. dematoidea as previously reported. Fusicladium proteae is described on Protea sp. (South Africa), Pestalotiopsis protearum on Leucospermum cuneiforme (Zimbabwe), Ramularia vizellae and R. stellenboschensis on Protea spp. (South Africa), and Teratosphaeria capensis on Protea spp. (Portugal, South Africa). Aureobasidium leaf spot is shown to be caused by two species, namely A. proteae comb. nov. on Protea spp. (South Africa), and A. leucospermi sp. nov. on Leucospermum spp. (Indonesia, Portugal, South Africa). Novel genera and species elucidated in this study include Gordonomyces mucovaginatus and Pseudopassalora gouriqua (hyphomycetes), and Xenoconiothyrium catenata (coelomycete), all on Protea spp. (South Africa).
Keywords: biodiversity, cut-flower industry, fungal pathogens, ITS, LSU, phylogeny, systematics
INTRODUCTION
Proteaceae is one of the Southern Hemisphere’s most prominent flowering plant families and is amongst the oldest groups of flowering plants. Records show that they existed in Gondwanaland at least 300 million years ago (Vogts 1982). Today, long after the movement of continental plates caused Gondwanaland to break up these plants still survive. The highest degree of species richness occurs in eastern and western Australia, and the Western Cape of South Africa with the greatest diversity of Proteaceae, occurring in Australia, which has representatives of all seven subfamilies. More than 800 species representing 45 genera are found in Australia, of which 550 species are found mainly in the south-western part of that country (Rebelo 2001). Africa, the second most species-rich continent, has members of only two of the subfamilies (Paterson-Jones 2000). Approximately 330 species (representing 14 genera) are confined to the Cape Floristic Region (CFR), making this area exceptional in its natural diversity of Proteaceae, and also in their associated fungi (Crous et al. 2006a). The Fynbos biome is a major part of the Cape Floral Kingdom and the Proteaceae forms one of the main components of the Cape Floristic Region along with ericoids, restioids and geophytes (Cowling & Richardson 1995).
The South African Proteaceae comprises 14 genera, of which 7 genera are commercially utilised (Vogts 1982, Littlejohn 1999, Paterson-Jones 2000, Rebelo 2001). Three of these, Leucadendron, Leucospermum and Protea are of the greatest commercial value and are grown for their exotic, brightly coloured and textured flowers or bracts, which are in high demand on the world floriculture market (Coetzee & Littlejohn 2001). Fungal pathogens, however, create a serious problem in cultivating flawless blooms. Several groups of fungal pathogens of Proteaceae have in recent years been characterised phylogenetically, e.g. Botryosphaeria stem cankers (Denman et al. 1999, 2000, 2003, Crous et al. 2006b, Marincowitz et al. 2008b), Armillaria and Cylindrocladium root rot (Schoch et al. 1999, Coetzee et al. 2003, Lombard et al. 2010a, b, c), Elsinoë scab disease (Swart et al. 2001) Phomopsis cankers (Mostert et al. 2001a, b), and leaf spots caused by species of Mycosphaerella and Teratosphaeria (Crous et al. 2008, 2009a, b, 2011b). Several other pathogenic fungi on Proteaceae however, have never been studied from a phylogenetic perspective based on DNA analyses, and in light of new knowledge are now suspected to represent species complexes.
The aim of the present study, therefore, was to recollect and culture as many of the fungi associated with diseases of Proteaceae as possible, to facilitate DNA phylogenetic studies and characterisation based on DNA analyses, culture characteristics and morphology.
MATERIALS AND METHODS
Isolates
Leaves and stems of Proteaceae with cankers or leaf spots were chosen for study. Single conidial colonies were established from sporulating conidiomata on Petri dishes containing 2 % malt extract agar (MEA; Crous et al. 2009c) as described earlier (Crous et al. 1991). Excised lesions containing ascomata were soaked in water for approximately 2 h, after which they were placed on the underside of Petri dish lids, with the top half of the dish containing MEA, allowing spores to be deposited on the MEA in the dish above. Ascospore germination patterns were examined after 24 h, and single ascospore cultures established as described by Crous (1998). Colonies were subcultured onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), MEA, and oatmeal agar (OA) (Crous et al. 2009c), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. Reference strains are maintained in the CBS-KNAW Fungal Biodiversity Centre (CBS) Utrecht, The Netherlands (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (Crous et al. 2004b).
Table 1.
Collection details and GenBank accession numbers of isolates for which novel sequences were generated in this study.
| Species | Strain no. 1 | Country | Substrate | Collector(s) | GenBank Accession number
2
|
||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | TEF | CHS | TUB | |||||
| Brunneosphaerella jonkershoekensis | CPC 13902 | South Africa | Leaves of Protea repens | P.W. Crous | JN712439 | JN712503 | JN712571 | JN712609 | – |
| CPC 13905 | South Africa | Leaves of Protea repens | P.W. Crous | GU214623 | GU214394 | JN712572 | JN712610 | – | |
| CPC 13908 | South Africa | Leaves of Protea repens | P.W. Crous | JN712440 | JN712504 | JN712573 | JN712611 | – | |
| CPC 13911; CBS 130594 | South Africa | Leaves of Protea repens | P.W. Crous | JN712441 | JN712505 | JN712574 | JN712612 | – | |
| CPC 15237 | South Africa | Protea nitida | L. Mostert | JN712442 | JN712506 | JN712575 | JN712613 | – | |
| CPC 16850 | South Africa | Protea repens | J.E. Taylor | JN712443 | JN712507 | JN712576 | JN712614 | – | |
| CPC 16851 | South Africa | Protea repens | J.E. Taylor | JN712444 | JN712508 | JN712577 | JN712615 | – | |
| CPC 18297 | South Africa | Living leaves of Protea repens | P.W. Crous | JN712445 | JN712509 | JN712578 | JN712616 | – | |
| CPC 18301 | South Africa | Living leaves of Protea repens | P.W. Crous | JN712446 | JN712510 | JN712579 | JN712617 | – | |
| Brunneosphaerella nitidae | CPC 13914; CBS 130596 | South Africa | Protea nitida | P.W. Crous | GU214624 | GU214395 | JN712580 | JN712618 | – |
| CPC 15231; CBS 130595 | South Africa | Leaf litter of Protea nitida | L. Mostert | GU214625 | GU214396 | JN712581 | JN712619 | – | |
| Brunneosphaerella protearum | CPC 16338; CBS 130597 | South Africa | Leaves of Protea sp. | P.W. Crous | GU214626 | GU214397 | JN712582 | JN712620 | – |
| CPC 16849 | South Africa | Living leaves of Protea magnifica | J.E. Taylor | JN712447 | JN712511 | JN712583 | JN712621 | – | |
| CPC 18308; CBS 130598 | South Africa | Leaves of Protea coronata | P.W. Crous | JN712448 | JN712512 | JN712584 | JN712622 | – | |
| CPC 18328 | South Africa | Leaves of Protea mundii | P.W. Crous | JN712449 | JN712513 | JN712585 | JN712623 | – | |
| Coccomyces proteae | CPC 1727; CBS 111704 | South Africa | Leaves of Protea sp. | S. Denman | JN712450 | JN712514 | – | – | – |
| CPC 1730; CBS 111703 | South Africa | Leaves of Protea sp. | S. Denman | JN712451 | JN712515 | – | – | – | |
| Coniothyrium nitidae | CPC 1476; CBS 111322 | South Africa | Leaves of Protea nitida | S. Denman | JN712452 | JN712516 | – | – | – |
| CPC 1477; CBS 111321 | South Africa | Leaves of Protea nitida | S. Denman | JN712453 | JN712517 | – | – | JN712647 | |
| CPC 1478; CBS 111302 | South Africa | Leaves of Protea nitida | S. Denman | JN712454 | JN712518 | – | – | – | |
| CPC 1532; CBS 111380 | South Africa | Leaves of Protea nitida | S. Denman | JN712455 | JN712519 | – | – | – | |
| Curreya grandicipis | CPC 1852; CBS 114272 | South Africa | Leaves of Protea grandiceps | J.E. Taylor & S. Denman | JN712456 | JN712520 | – | – | – |
| CPC 1853; CBS 111702 | South Africa | Leaves of Protea grandiceps | J.E. Taylor & S. Denman | JN712457 | JN712521 | – | – | – | |
| Curvularia trifolii | CPC 2941; CBS 114135 | Australia | Leucospermum sp. | P.W. Crous | JN712458 | JN712522 | – | – | – |
| CPC 2995; CBS 111997 | Australia | Leucospermum sp. | P.W. Crous | JN712459 | JN712523 | – | – | – | |
| Diaporthe leucospermi | CPC 2956; CBS 111980 | Australia | Leaves of Leucospermum sp. | P.W. Crous & B. Summerell | JN712460 | JN712524 | – | – | – |
| Diplodina microsperma | CPC 2336; CBS 114545 | New Zealand | Leaves of Protea sp. (intercepted specimen of flowers exported to California, USA) | M.A. Abdelshife & M.E. Palm | JN712461 | JN712525 | JN712586 | – | JN712648 |
| Drechslera biseptata | CBS 307.69 | Germany | Lolium multiflorum | U.G. Schlösser | – | JN712526 | – | – | – |
| CBS 599.71 | Netherlands | Leaf of Zea mays | H.A. van Kesteren | – | JN712527 | – | – | – | |
| CBS 205.60 | – | – | W.B. Kendrick | JN712462 | JN712528 | – | JN712624 | – | |
| CBS 306.69 | Germany | Lolium multiflorum | U.G. Schlösser | JN712463 | JN712529 | JN712587 | JN712625 | – | |
| CBS 308.69 | Germany | Lolium sp. | U.G. Schlösser | JN712464 | JN712530 | JN712588 | JN712626 | – | |
| Drechslera dematioidea | CBS 108962 | British Columbia | Overwintered grass | G. Zhang | JN712465 | JN712531 | JN712589 | JN712627 | – |
| CBS 108963 | British Columbia | Overwintered grass | G. Zhang | JN712466 | JN712532 | JN712590 | JN712628 | – | |
| Pyrenophora leucospermi | CPC 1293; CBS 111083 | South Africa | Leaves of Leucospermum cordifolium | L. Swart | JN712467 | JN712533 | JN712591 | JN712629 | – |
| CPC 1294; CBS 111084 | South Africa | Leaves of Leucospermum cordifolium | L. Swart | JN712468 | JN712534 | JN712592 | JN712630 | – | |
| CPC 1295; CBS 111085 | South Africa | Leaves of Leucospermum cordifolium | L. Swart | JN712469 | JN712535 | JN712593 | JN712631 | – | |
| CPC 1296; CBS 111180 | South Africa | Leaves of Leucospermum cordifolium | L. Swart | JN712470 | JN712536 | JN712594 | JN712632 | – | |
| CPC 1297; CBS 111086 | South Africa | Leaves of Leucospermum cordifolium | L. Swart | JN712471 | JN712537 | JN712595 | JN712633 | – | |
| CPC 1298; CBS 111087 | South Africa | Leaves of Leucospermum cordifolium | L. Swart | JN712472 | JN712538 | JN712596 | JN712634 | – | |
| CPC 13777 | Spain: Tenerife | Leucospermum sp. | P.W. Crous | JN712473 | JN712539 | JN712597 | JN712635 | – | |
| CPC 13786 | Spain: Tenerife | Leucospermum sp. | P.W. Crous | JN712474 | JN712540 | JN712598 | JN712636 | – | |
| CPC 16268 | Portugal | Leucadendron succeisus | J.J. Morais | JN712475 | JN712541 | JN712599 | JN712637 | – | |
| CPC 1785; CBS 111505 | South Africa | Leucospermum cordifolium | S. Denman | JN712476 | JN712542 | JN712600 | JN712638 | – | |
| CPC 2195; CBS 111862 | USA | Leucospermum sp., cloud bunk | P.W. Crous | JN712477 | JN712543 | JN712601 | JN712639 | – | |
| CPC 2196; CBS 111863 | USA | Leucospermum sp., cloud bunk | P.W. Crous | JN712478 | JN712544 | JN712602 | JN712640 | – | |
| CPC 2200; CBS 114493 | USA | Leucospermum sp. | P.W. Crous | JN712479 | JN712545 | JN712603 | JN712641 | – | |
| CPC 2836; CBS 114131 | South Africa | Leucospermum sp. | L. Swart | JN712480 | JN712546 | JN712604 | JN712642 | – | |
| CPC 2837; CBS 114033 | South Africa | Leucospermum sp. | L. Swart | JN712481 | JN712547 | JN712605 | JN712643 | – | |
| CPC 2839; CBS 114032 | South Africa | Leucospermum sp. | L. Swart | JN712482 | JN712548 | JN712606 | JN712644 | – | |
| CPC 5215; CBS 115178 | Spain | Leucadendron sp. | S. Denman | JN712483 | JN712549 | JN712607 | JN712645 | – | |
| CPC 5238; CBS 115397 | – | Leucadendron sp. | S. Denman | JN712484 | JN712550 | JN712608 | JN712646 | – | |
| Fusicladium proteae | CPC 18282; CBS 130599 | South Africa | Leaves of Protea sp., in association with Vizella interrupta | P.W. Crous | JN712485 | JN712551 | – | – | – |
| Gordonomyces mucovaginatus | CMW 22212; CBS 127273; CPC 18172 | South Africa | Leaf litter of Leucadendron laureolum | S. Marincowitz | JN712486 | JN712552 | – | – | – |
| Aureobasidium leucospermi | CPC 15081 | Portugal | Leaves of Leucospermum cv. ‘Tango’ | P.W. Crous | JN712487 | JN712553 | – | – | – |
| CPC 15099 | Indonesia | Leucospermum sp. | P.W. Crous | JN712488 | JN712554 | – | – | – | |
| CPC 15180; CBS 130593 | South Africa | Leaves of Leucospermum conocarpodendron | F. Roets | JN712489 | JN712555 | – | – | – | |
| Aureobasidium proteae | CPC 13701 | – | Protea sp., intercepted in the Netherlands | P.W. Crous | JN712490 | JN712556 | – | – | – |
| CPC 2824; CBS 114273 | South Africa | Protea cv. ‘Sylvia’ (Protea eximia × Protea susannae) | S. Denman | JN712491 | JN712557 | – | – | – | |
| CPC 2825; CBS 111973 | South Africa | Protea cv. ‘Sylvia’ (Protea eximia × Protea susannae) | S. Denman | JN712492 | JN712558 | – | – | – | |
| CPC 2826; CBS 111970 | South Africa | Protea cv. ‘Sylvia’ (Protea eximia × Protea susannae) | S. Denman | JN712493 | JN712559 | – | – | – | |
| Leptosphaerulina australis | CPC 3712; CBS 116307 | Kenya | Leaves of Protea sp. | – | JN712494 | JN712560 | – | – | – |
| Microsphaeropsis proteae | CPC 1423; CBS 111303 | South Africa | Leaves of Protea nitida | S. Denman | JN712495 | JN712561 | – | – | – |
| CPC 1424; CBS 111320 | South Africa | Leaves of Protea nitida | S. Denman | JN712496 | JN712562 | – | – | JN712649 | |
| CPC 1425; CBS 111319 | South Africa | Leaves of Protea nitida | S. Denman | JN712497 | JN712563 | – | – | JN712650 | |
| Pestalotiopsis protearum | CPC 1765; CBS 114178 | Zimbabwe | Living leaves of Leucospermum cuneiforme cv. ‘Sunbird’ | L. Swart | JN712498 | JN712564 | – | – | – |
| Pseudopassalora gouriqua | CPC 1811; CBS 101954 | South Africa | Leaves of Protea susanne | L. Dryer | – | JN712565 | – | – | – |
| Ramularia stellenboschensis | CPC 18294; CBS 130600 | South Africa | Leaves of Protea sp. | P.W. Crous | JN712499 | JN712566 | – | – | – |
| Ramularia vizellae | CPC 18283; CBS 130601 | South Africa | Leaves of Protea sp., in association with Vizella interrupta (secondary?) | P.W. Crous | JN712500 | JN712567 | – | – | – |
| Teratosphaeria capensis | CPC 13981 | Portugal | Leaves of Protea repens | M.F. Moura | EU707887 | JN712568 | – | – | – |
| CPC 18299; CBS 130602 | South Africa | Living leaves of Protea sp. | P.W. Crous | JN712501 | JN712569 | – | – | – | |
| Xenoconiothyrium catenata | CMW 22113; CBS 128994 | South Africa | Twig litter of Protea laurifolia | S. Marincowitz | JN712502 | JN712570 | – | – | – |
1 CBS: CBS Fungal Biodiversity Centre, Utrecht, The Netherlands; CMW: Culture collection of FABI, University of Pretoria, South Africa; CPC: Culture collection of P.W. Crous, housed at CBS.
2 ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; LSU: partial 28S nrDNA; TEF: partial translation elongation factor 1-alpha gene; CHS: partial chitin synthase gene; TUB: partial beta-tubulin gene.
DNA phylogeny
Genomic DNA was extracted from fungal colonies growing on MEA using the UltraClean™ Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) according to the manufacturer’s protocol. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part (ITS) of the nuclear rDNA operon spanning the 3’ end of the 18S rRNA gene, the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region and the 5’ end of the 28S rRNA gene. The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009a) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon.
For the genera Drechslera and Brunneosphaerella, the partial gene sequences for translation elongation factor 1-α (TEF) were determined using the primers EF1-728F (Carbone & Kohn 1999) and EF1-986R (Carbone & Kohn 1999) or EF-2 (O’Donnell et al. 1998) as described by Crous et al. (2006b) and Bensch et al. (2010). In addition sequences of the chitin synthase (CHS) gene were obtained using the primers CHS-79F and CHS-354R (Carbone & Kohn 1999) following the above amplification protocol. For Diplodina microsperma, TEF was amplified and sequenced as described above; in addition beta-tubulin was amplified and sequenced using the primers T1 (O’Donnell & Cigelnik 1997) and Bt-2b (Glass & Donaldson 1995).
The sequence alignment and subsequent phylogenetic analyses for all the above were carried out using methods described by Crous et al. (2006b). Gaps longer than 10 bases were coded as single events for the phylogenetic analyses; the remaining gaps were treated as ‘fifth state’ data. Sequence data were deposited in GenBank (Table 1) and the alignment and trees in TreeBASE (http://www.treebase.org).
Taxonomy
A minimum of 30 measurements (× 1 000 magnification) were made of conidia and ascospores mounted in lactic acid, with the extremes of spore measurements given in parentheses. Ranges of the dimensions of other characters are given. Colony colours (surface and reverse) were assessed on MEA, OA and PDA at 25 °C in the dark for different periods as stated below, using the colour charts of Rayner (1970).
RESULTS
DNA phylogeny
28S nrDNA generic overview
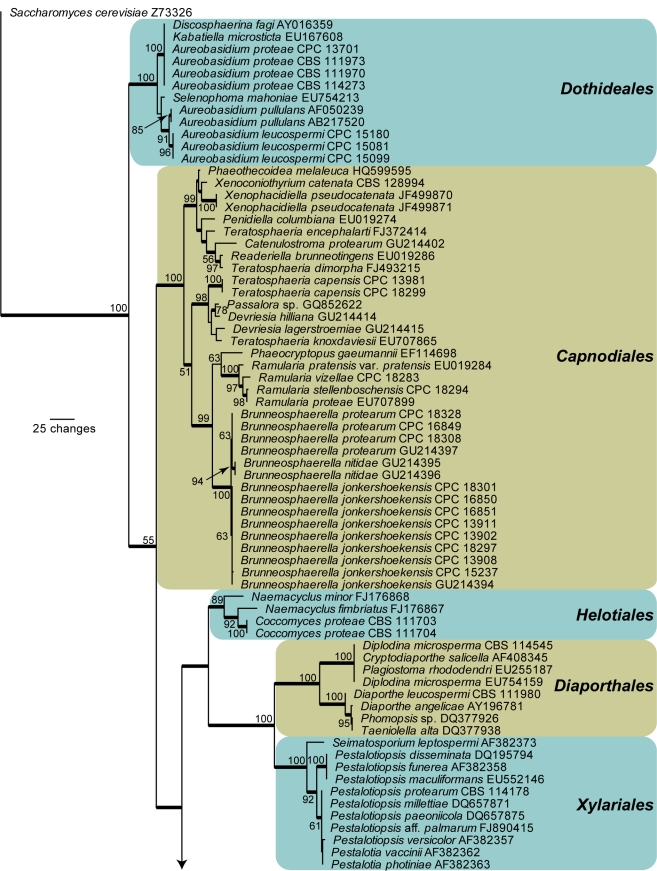
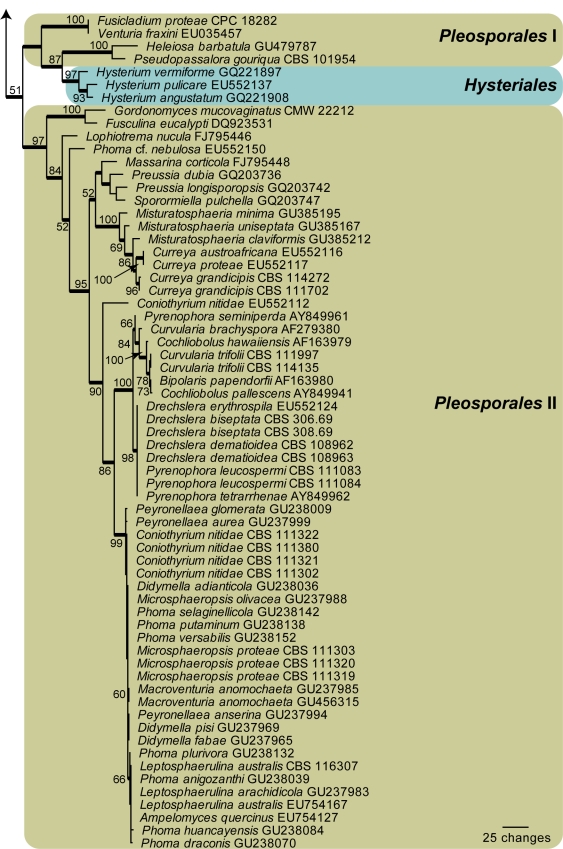
Amplicons of approximately 1 700 bases were obtained ITS (including the first approximately 900 bp of LSU) for the isolates listed in Table 1. The LSU sequences were used to obtain additional sequences from GenBank, which were added to the alignment (Fig. 1) and the ITS to determine species identification (not shown; discussed in species notes where applicable). The manually adjusted LSU alignment contained 136 sequences (including the outgroup sequence) and 759 characters including alignment gaps (available in TreeBASE) were used in the phylogenetic analysis; 326 of these were parsimony-informative, 35 were variable and parsimony-uninformative, and 398 were constant. Neighbour-joining analyses using three substitution models on the sequence alignment yielded trees with identical topologies to one another and support the same clades as obtained from the parsimony analysis. Only the first 1 000 equally most parsimonious trees were saved (TL = 1567 steps; CI = 0.419; RI = 0.911; RC = 0.381). The phylogenetic results obtained (Fig. 1) are discussed where applicable in the descriptive notes below.
Fig. 1.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment. The scale bar shows 25 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Orders are indicated to the right of the tree. Branches present in the strict consensus tree are thickened and the tree was rooted to a sequence of Saccharomyces cerevisiae (GenBank accession Z73326).
Brunneosphaerella combined ITS, TEF and CHS
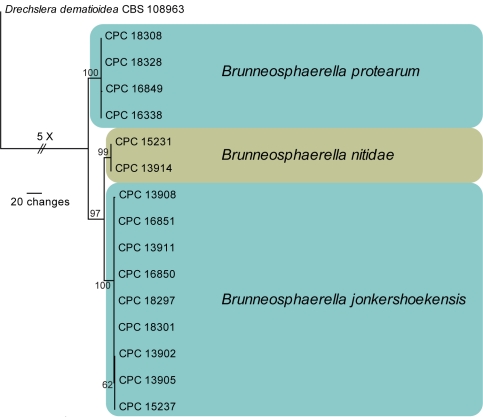
Amplicons of approximately 1 700 bases were obtained ITS (including the first approximately 900 bp of LSU), 560–630 bp for TEF and 300 bp for CHS for the isolates listed in Table 1. The manually adjusted combined alignment contained 16 sequences (including the outgroup sequence) and 1 305 (510, 534 and 261 for ITS, TEF and CHS, respectively) characters including alignment gaps (available in TreeBASE) were used in the phylogenetic analysis; 61 of these were parsimony-informative, 545 were variable and parsimony-uninformative, and 699 were constant. Neighbour-joining analyses using three substitution models on the sequence alignment yielded trees with identical topologies to one another and support the same clades as obtained from the parsimony analysis. The parsimony analysis yielded a single most parsimonious tree (TL = 640 steps; CI = 0.988; RI = 0.962; RC = 0.950). All three species treated can be identified by unique sequence differences in all three sequenced loci (data not shown). The phylogenetic results obtained (Fig. 2) are discussed where applicable in the descriptive notes below. A partition homogeneity test indicated that all three loci were combinable (P value = 0.222).
Fig. 2.
The single most parsimonious tree obtained from a heuristic search with 100 random taxon additions of the combined ITS, TEF and CHS Brunneosphaerella sequence alignment. The scale bar shows 20 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. The tree was rooted to sequences of Drechslera dematioidea (Culture accession CBS 108963).
Pyrenophora combined ITS, TEF and CHS
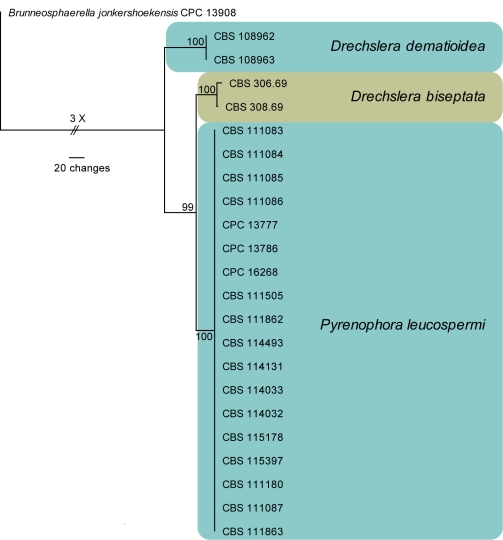
Amplicons of approximately 1 700 bases were obtained ITS (including the first approximately 900 bp of LSU), 660–750 bp for TEF and 300 bp for CHS for the isolates listed in Table 1. The manually adjusted combined alignment contained 23 sequences (including the outgroup sequence) and 1 374 (566, 534 and 274 for ITS, TEF and CHS, respectively) characters including alignment gaps (available in TreeBASE) were used in the phylogenetic analysis; 133 of these were parsimony-informative, 550 were variable and parsimony-uninformative, and 691 were constant. Neighbour-joining analyses using three substitution models on the sequence alignment yielded trees with identical topologies to one another and support the same clades as obtained from the parsimony analysis. The parsimony analysis yielded a single most parsimonious tree (TL = 792 steps; CI = 0.992; RI = 0.972; RC = 0.964). The TEF alignment was found to provide the highest resolution, followed by CHS. On ITS, it was not possible to distinguish between Drechslera biseptata and Pyrenophora leucospermi (data not shown). The phylogenetic results obtained (Fig. 3) are discussed where applicable in the descriptive notes below. A partition homogeneity test indicated that all three loci were combinable (P value = 0.093).
Fig. 3.
The single most parsimonious tree obtained from a heuristic search with 100 random taxon additions of the combined ITS, TEF and CHS Drechslera sequence alignment. The scale bar shows 20 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. The tree was rooted to sequences of Brunneosphaerella jonkershoekensis (Culture accession CPC 13908).
Taxonomy
During the course of the present study several previously described species were either newly collected, or found to represent new species. These taxa are subsequently treated below.
Aureobasidium leaf spot
Although species of the genus Aureobasidium are generally regarded as saprobes, several taxa have the ability to form Kabatiella synanamorphs that generally cause leaf spots, and are considered plant pathogens (Taylor & Crous 2000, Zalar et al. 2008). Aureobasidium is an important pathogen of Proteaceae, and has (as Kabatiella synanamorph) been reported from South African material intercepted by the USDA Animal and Plant Health Inspection Service (APHIS) in the USA (Taylor 2001). Kabatiella proteae has recently been recorded from Protea in Australia (Crous et al. 2000), and a Kabatiella state of a species of Aureobasidium is quite abundant as a leaf spot pathogen on Leucospermum spp. in the Canary Islands and Portugal (P.W. Crous, unpubl. data). In accordance with the Amsterdam Declaration to integrating different morphs of pleomorphic fungi into a single generic name (Hawksworth et al. 2011), preference is given to the older generic name Aureobasidium (1891), rather than the younger, lesser-known Kabatiella (1907).
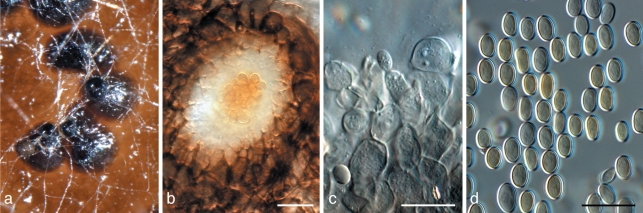
Aureobasidium leucospermi Crous, sp. nov. — MycoBank MB560556; Fig. 4
Fig. 4.
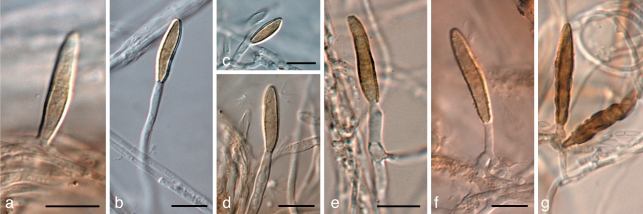
Aureobasidium leucospermi (CBS H-20669). a. Leaf spot with sporulating conidiomata; b, c. conidiogenous cells giving rise to conidia; d. hyaline conidia. — Scale bars = 10 μm.
Aureobasidium proteae morphologice similis, sed conidiis majoribus, 6–15 × 4–8 μm.
Etymology. Named after the host genus on which it occurs, Leucospermum.
Leaf spots subcircular irregular, amphigenous, necrotic, sunken, pale to medium brown with a raised, dark brown margin. Mycelium immersed. Conidiomata acervular to sporodochial, amphigenous, substomatal, subepidermal, pulvinate, dry or crystaline in appearance, pale brown, discrete, 60–100 μm diam. Stroma visible in substomatal cavity, dark brown, mainly consisting of elongated pseudo-parenchymatous cells with large lumina, becoming hyaline, thinner-walled at the apex, 80–130 × 50–100 μm. Conidiogenous cells cylindrical, clavate or globose, integrated, terminal, conidial ontogeny holoblastic, with numerous synchronously produced conidia, 15–30 × 4–11 μm. Conidia in vitro solitary, aseptate, ellipsoidal to spherical, occasionally with a slightly truncate base, hyaline, thin-walled, smooth, (6–)8–11(–15) × 4–5(–8) μm.
Culture characteristics — Colonies flat, spreading, lacking aerial mycelium, with feathery margins, covering the dish in 3 wk. On MEA rosy-buff, reverse buff; on OA surface buff; on PDA sectors of leaden-black, with patches of buff, similar in reverse.
Specimens examined. Indonesia, on leaves of Leucospermum sp., 16 Apr. 2008, P.W. Crous, CPC 15099–15101. – Portugal, on leaves of Leucospermum cv. ‘Tango’, 1 Mar. 2008, P.W. Crous, CPC 15081–15083. – South Africa, Western Cape Province, Stellenbosch, J.S. Marais Garden, on leaves of Leucospermum conocarpodendron, 20 Apr. 2008, F. Roets, holotype CBS H-20669, culture ex-type CPC 15180–15182 = CBS 130593.
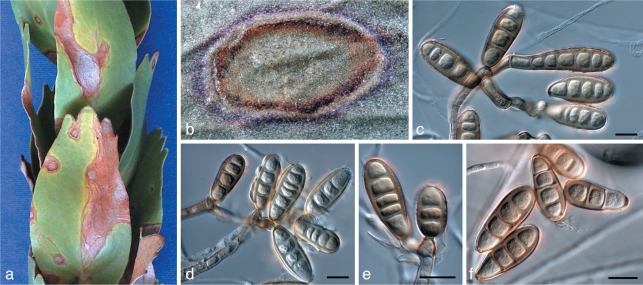
Aureobasidium proteae (Joanne E. Taylor & Crous) Joanne E. Taylor & Crous, comb. nov. — MycoBank MB560557; Fig. 5
Fig. 5.
Aureobasidium proteae (CBS 114273). a. Sporulating conidiomata in leaf tissue; c–e. conidiogenous cells giving rise to conidia; f. hyaline conidia. — Scale bars = 10 μm.
Basionym. Kabatiella proteae Joanne E. Taylor & Crous, Mycol. Res. 104: 619. 2000.
Leaf spots irregular, occurring on the petiole and base of lamina, extending up the leaf, necrotic, sunken, pale to medium brown with a raised, dark brown margin; areas where sporodochia occur often darkened. Mycelium immersed. Conidiomata acervular to sporodochial, amphigenous, substomatal, subepidermal, pulvinate, dry or crystaline in appearance, pale brown, discrete, 60–100 μm diam. Stroma visible in substomatal cavity, dark brown, mainly consisting of elongated pseudo-parenchymatous cells with large lumina, becoming hyaline, thinner-walled at the apex, (80–)101–121(–125) × (50–)72–92(–100) μm. Conidiogenous cells cylindrical, clavate or globose, integrated, terminal, conidial ontogeny holoblastic, with numerous synchronously produced conidia, (13–)16–20(–27) × (4–)6–8(–11) μm. Conidia in vivo solitary, aseptate, ellipsoidal to spherical, occasionally with a slightly truncate base, hyaline, thin-walled, smooth, often with small guttules, (5–)6.5–7.5(–10) × (2–)2.5–3(–3.5) μm; conidia in vitro similar, (4–)6–7(–9) × (2–)2.5–3(–3.5) μm.
Culture characteristics — Colonies with moderate to sparse aerial mycelium on MEA, surface olivaceous-grey, reverse olivaceous-grey to iron-grey, radial striations appearing in the agar and visible from underneath.
Specimens examined. South Africa, Somerset West, Hilly Lands Farm, on a leaf of a Protea cynaroides seedling, 21 July 1998, S. Denman & J.E. Taylor, JT338 (holotype PREM 56192); on leaves of Protea cv. ‘Sylvia’ (P. eximia × P. susannae), 19 July 1999, S. Denman, epitype designated here as CBS H-20668, cultures ex-epitype CPC 2824 = CBS 114273, CPC 2825 = CBS 111973, CPC 2826 = CBS 111970, CPC 2827. – Unknown origin, on leaves of Protea sp., intercepted in the Netherlands, 24 Feb. 2006, P.W. Crous, CPC 13701–13703.
Notes — Leaf spots caused by A. leucospermi appear very similar to those of A. proteae, except that the former is so far only known from species of Leucospermum. The two species are also morphologically similar and phylogenetically closely related (Fig. 1). They can be distinguished morphologically, however, in that conidia of A. leucospermi (6–15 × 4–8 μm) are larger than those of A. proteae (4–9 × 2–3.5 μm).
Brunneosphaerella leaf blight
Crous et al. (2009a) recently introduced the genus Brunneosphaerella to accommodate Leptosphaeria protearum, which is a major leaf spot and blight pathogen of Protea spp. (Knox-Davies et al. 1987). This pathogen occurs naturally on native protea in South Africa and is damaging to commercially cultivated crops there, but it also causes severe losses in other countries where South African proteas are cultivated (Taylor & Crous 2000, Taylor et al. 2001a, b, d, Crous et al. 2004a). Although Brunneosphaerella was recognised as distinct from Leptosphaeria, additional collections and molecular data were required to resolve the species complex represented by isolates identified as B. protearum. Based on their distinct phylogeny (Fig. 2), morphology and host ranges, three species of Brunneosphaerella are now recognised and described below. Although highly similar in ITS, the three Brunneosphaerella species treated in this study can be resolved easily based on their diagnostic TEF and CHS sequences (Fig. 2).
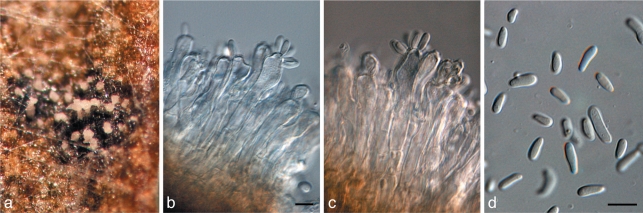
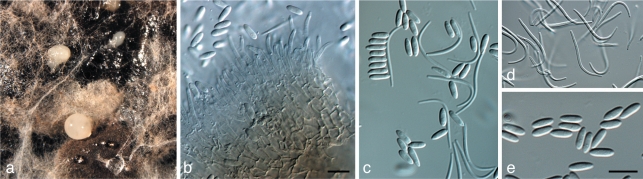
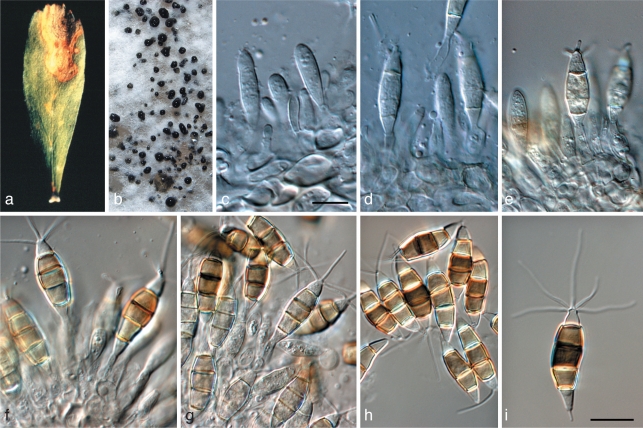
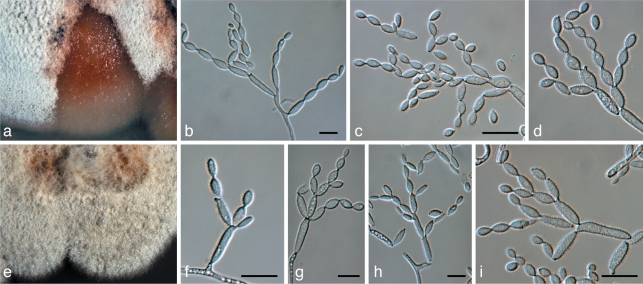
Brunneosphaerella jonkershoekensis (Marinc., M.J. Wingf. & Crous) Crous, Stud. Mycol. 64: 31. 2009 — Fig. 6
Fig. 6.
Brunneosphaerella jonkershoekensis (CBS H-20333). a, b. Leaf spots; c. globose ascomata visible on lesion surface; d. substomatal ascoma with central ostiole; e. vertical section through ascoma wall of textura angularis; f. germinating ascospore; g–i. asci; j. ascospores. — Scale bars = 10 μm.
Basionym. Leptosphaeria jonkershoekensis Marinc., M.J. Wingf. & Crous, in Marincowitz et al., Microfungi occurring on Proteaceae in the fynbos: 62. 2008.
Leaf spots amphigenous, up to 15 mm diam, pale brown, with a raised, red-brown border. Ascomata perithecioid, subepidermal, amphigenous, remaining immersed, obpyriform in section, 180–205 × 160–235 μm, with a papillate ostiole. Peridium 20–30 μm thick, composed of relatively large cells, 11–15 × 2.5–5.5 μm, cells arranged in three strata; outer stratum consisting of 3–5 layers of dark brown, very thick-walled cells; middle stratum transient, consisting a few layers of pale brown, thick-walled, compressed cells; inner stratum consisting of 1–2 layers of thin-walled, very compressed cells. Hamathecium not observed in mature ascomata. Asci bitunicate, inflated cylindrical to clavate, 70–95 × 12–15 μm, ocular chamber dome-shaped, indistinct. Ascospores pale brown, finely verruculose, fusoid to ellipsoid, tapering towards the base, (25–)27–34(–37) × (5–)6–7(–9) μm (av. 31 × 6.7 μm), apical cell the shortest, upper hemispore slightly bigger than lower, at times slightly curved, 3-septate, smooth, guttulate, with each cell containing a large central guttule, prominently constricted at median septum, with globose mucoid caps up to 3 μm diam at each end; ascospores widest in second cell from apex.
Culture characteristics — After 2 mo on OA flat, spreading with moderate to sparse aerial mycelium; surface olivaceous-grey with patches of iron-grey and pale olivaceous-grey and smoke-grey; margins lobate, smooth, reaching 35 mm diam. On PDA flat, spreading, with moderate aerial mycelium and uneven surface and feathery, lobate margins; surface olivaceous-grey with patches of pale olivaceous-grey to smoke-grey; reverse iron-grey, reaching 35 mm diam. On MEA flat, spreading, with sparse aerial mycelium and even, lobate margins; surface dirty white with patches of smoke-grey; reverse iron-grey, reaching 35 mm diam.
Specimens examined. South Africa, Western Cape Province, Jonkershoek Nature Reserve, on leaf litter of Protea repens, 6 June 2000, S. Marincowitz, holotype PREM 59447; Jonkershoek Nature Reserve, S33°59’11.2″ E18°57’14.7″, on leaves of P. repens, 1 Apr. 2007, P.W. Crous, epitype designated here as CBS H-20333, cultures ex-epitype CPC 13902–13907; CBS H-20332, cultures CPC 13908–13910; CBS H-20331, cultures CPC 13911–13913 = CBS 130594; Stellenbosch, J.S. Marais Garden, S33°55’59.3″ E18°52’22.5″, on living leaves of P. repens, 6 May 2010, P.W. Crous, CBS H-20670, culture CPC 18297; CBS H-20671, CPC 18301; Jonkershoek Nature Reserve, on leaves of P. nitida, 12 Apr. 2008, L. Mostert, CBS H-20672, culture CPC 15237; Jonkershoek Nature Reserve, on leaves of P. repens, 28 Jan. 1999, J.E. Taylor, CPC 16850, 16851.
Notes — Brunneosphaerella jonkershoekensis was originally described from leaf litter of Protea repens collected in Jonkershoek (Marincowitz et al. 2008a), but no cultures were available for study until now. Brunneosphaerella jonkershoekensis appears to be a serious pathogen, particularly of P. repens, but is presently only known from the Stellenbosch-Jonkershoek area of the Western Cape Province of South Africa. This species has been largely overlooked and incorrectly identified as B. protearum. Brunneosphaerella jonkershoekensis is morphologically similar to B. protearum, but distinct in having much larger ascospores (Marincowitz et al. 2008a).
Brunneosphaerella nitidae Crous, sp. nov. — MycoBank MB560558; Fig. 7
Fig. 7.
Brunneosphaerella nitidae (CBS H-20334). a, b. Leaf spots; c, d. ascomata visible on lesion surface (note rupture in central ostiole); e, f. germinating ascospores; g–j. asci; k. ascospores. — Scale bars = 10 μm.
Brunneosphaerellae protearum similis, sed ascosporis longioribus et angustioribus, (20–)24–28(–30) × (3–)5–6(–7) μm.
Etymology. Named after the host on which it was collected, Protea nitida.
Leaf spots circular to irregular, discrete to confluent, variable in size, up to 2 cm diam, medium brown, with a raised, red-brown border. Ascomata amphigenous, immersed to semi-immersed, becoming erumpent when mature, black, single, gregarious, 180–300 μm diam; in section, substomatal, subepidermal, pyriform or globose with a papillate, periphysate ostiole, frequently opening by means of irregular rupture when mature. Peridium consisting of three strata of slightly compressed textura angularis, an outer stratum of dark brown, thick-walled cells, becoming paler in the central stratum, and hyaline, thin-walled in the inner stratum, altogether (20–)24.5–30(–40) μm thick. Asci clavate to cylindro-clavate, often curved, tapering to a pedicel, narrowing slightly to a rounded apex with an indistinct ocular chamber, 8-spored, bitunicate with fissitunicate dehiscense, 65–80 × 13–15 μm. Ascospores biseriate, fusiform, broader at the apical end, initially hyaline and 1-septate, becoming yellow-brown and 3-septate at maturity, slightly constricted at median septum, with large central guttule per cell, widest in second cell from the apex, and having terminal globose mucoid caps, 3 μm diam, (20–)24–28(–30) × (3–)5–6(–7) μm (av. 26 × 5.5 μm).
Culture characteristics — On PDA and OA spreading, flat with moderate aerial mycelium; margins lobate, smooth; surface smoke-grey with submerged, iron-grey margin; reverse iron-grey. On MEA surface smoke-grey; reverse iron-grey. Colonies reach 20 mm diam after 2 mo on all three media.
Specimens examined. South Africa, Western Cape Province, Jonkershoek Nature Reserve, on leaf litter of Protea nitida, 12 Apr. 2008, L. Mostert, holotype CBS H-20334, culture ex-type CPC 15231 = CBS 130595; Jonkershoek Nature Reserve, on leaves of P. nitida, 1 Apr. 2007, P.W. Crous, CBS H-20330, culture CPC 13914 = CBS 130596.
Notes — Brunneosphaerella nitidae is only known from the Jonkershoek Valley in South Africa, where it occurs on P. nitida. It is morphologically similar to B. protearum (ascospores av. 24.5 × 6.5 μm), but can be distinguished in that on average it has longer and narrower ascospores (av. 26 × 5.5 μm). In culture (on MEA and PDA) ascomatal initials developed after 2–3 mo that formed a few asci and ascospores, suggesting that this species is homothallic. Additionally, this species can be differentiated from the other species of Brunneosphaerella by comparison of their TEF and CHS sequences.
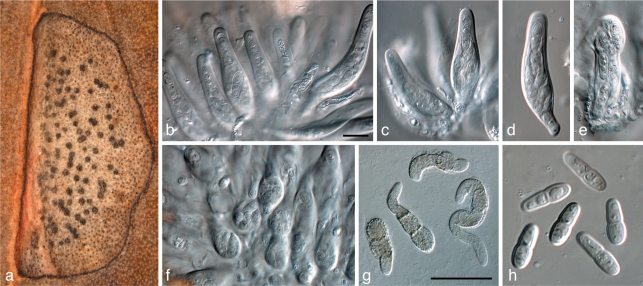
Brunneosphaerella protearum (Syd. & P. Syd.) Crous, Stud. Mycol. 64: 31. 2009 — Fig. 8
Fig. 8.
Brunneosphaerella protearum (CBS H-20335). a, b. Leaf spots; c, d. globose ascomata visible on lesion surface (note rupture in central ostiole); e, f. germinating ascospores; g–j. asci; k. ascospores. — Scale bars = 10 μm.
Anamorph. ‘Coniothyrium’ protearum Joanne E. Taylor & Crous, IMI Descriptions of Fungi and Bacteria No. 1343. 1998.
Basionym. Leptosphaeria protearum Syd. & P. Syd., Ann. Mycol. 10: 441. 1912.
Leaf spots circular to irregular, discrete to confluent, variable in size, under conditions favourable to disease symptoms more similar to a blight than a leaf spot, necrotic, sunken with a raised dark brown margin and with conspicuous black ascomata in the dead tissue, 4–30 mm diam. Ascomata amphigenous, immersed to semi-immersed, not erumpent, black, single, gregarious, 180–320 μm diam; in section, substomatal, subepidermal, pyriform or globose with a papillate, periphysate ostiole, immersed in a stroma consisting of deteriorated host mesophyll cells filled with fungal hyphae, (210–)230–264(–288) μm high, (180–)200–255(–300) μm diam. Peridium consisting of three strata of slightly compressed textura angularis, an outer stratum of dark brown, thick-walled cells, becoming paler in the central stratum, and hyaline, thin-walled in the inner stratum, altogether (20–)24.5–37.5(–50) μm thick. Asci clavate to cylindro-clavate, often curved, tapering to a pedicel, narrowing slightly to a rounded apex with an indistinct ocular chamber, 8-spored, bitunicate with fissitunicate dehiscense, (70–)80–87.5(–105) × (13.5–)14.5–16(–21.5) μm. Ascospores biseriate, fusiform, broader at the apical end, initially hyaline and 1-septate, becoming yellow-brown and 3-septate at maturity, slightly constricted at median to supra-median septum, with large central guttule per cell, widest in second cell from the apex, and having globose mucoid caps, 2 μm diam, (20–)23–26(–30) × (5–)6–7(–8) μm (av. 24.5 × 6.5 μm). Conidiomata barely visible and interspersed between ascomata, pycnidial, subepidermal, substomatal, separate, globose to pyriform, occasionally with well-developed papilla, dark brown, < 200 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, smooth, hyaline, doliiform to ampulliform, holoblastic, proliferating 1–2 times percurrently, 4–6 × 3–4 μm. Conidia pale brown to medium brown, thick-walled on maturity, smooth to finely verruculose, eguttulate, ellipsoidal to globose, often truncate at one end, 5–10 × 3–7 μm.
Culture characteristics — On OA spreading, flat with moderate aerial mycelium; margins lobate, smooth; surface smoke-grey with submerged, iron-grey margin. On PDA similar, surface smoke-grey with broad, iron-grey margin; reverse iron-grey. On MEA surface folded, smoke-grey with patches of dirty white and olivaceous-grey and submerged iron-grey margin; reverse iron-grey. Colonies reach 35 mm diam after 2 mo on all three media.
Specimens examined. South Africa, Western Cape Province, Wellington, on leaves of Protea lepidocarpodendron (as P. melaleuca), 22 Feb. 1912, E.M. Doidge, holotype PREM 2061; Kirstenbosch Botanical Garden, on leaves of Protea sp., 13 Jan. 2009, P.W. Crous, epitype designated here as CBS H-20335, culture ex-epitype CPC 16338 = CBS 130597; Kirstenbosch Botanical Garden, on leaves of P. coronata, 8 May 2010, P.W. Crous, CBS H-20673, culture CPC 18308 = CBS 130598; Harold Porter Botanical Garden, Betties Bay, on leaves of P. mundii, 4 May 2010, P.W. Crous, CBS H-20683, culture CPC 18328; Stellenbosch, J.S. Marais Garden, S33°55’59.3″ E18°52’22.5″, on living leaves of P. magnifica, 1 Apr. 1998, J.E. Taylor, culture CPC 16849.
Notes — Brunneosphaerella protearum appears to have a broad host range, and is widely distributed on Protea hosts in South Africa (Crous et al. 2004a). Although highly similar in ITS, the three Brunneosphaerella species treated in this study can be resolved easily based on their diagnostic TEF and CHS gene sequences (Fig. 2).
Coniothyrium leaf spot
Coniothyrium-like species are commonly associated with necrotic spots at the tips or margins of leaves of Proteaceae (Swart et al. 1998). As such, they are regularly intercepted during phytosanitary inspections. Many of these species have no known teleomorph, or are anamorphs of Teratosphaeriaceae, or other species belonging to Dothideomycetes (Schoch et al. 2006, 2009, Crous et al. 2007a, 2009a, b, Zhang et al. 2009). Recent studies have shown that many species of Phoma produce conidia that become dark and thick-walled with age, appearing Coniothyrium-like in morphology (Aveskamp et al. 2009, 2010, de Gruyter et al. 2009, 2010). For that reason the taxonomy of the Coniothyrium-like species occurring on Proteaceae was reevaluated.
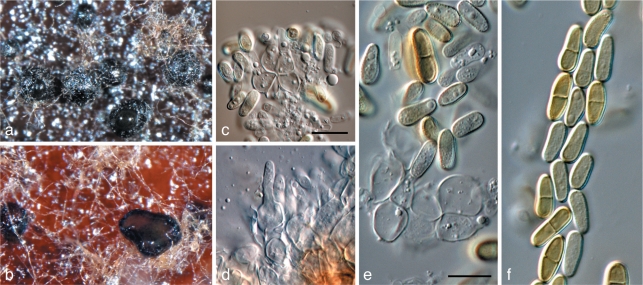
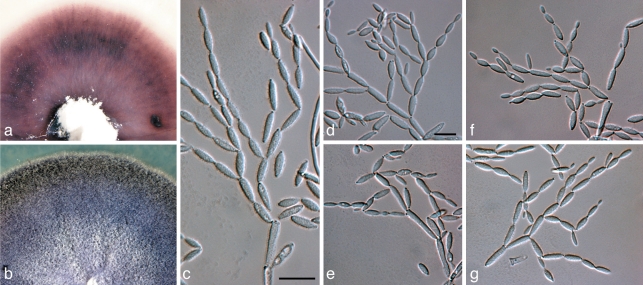
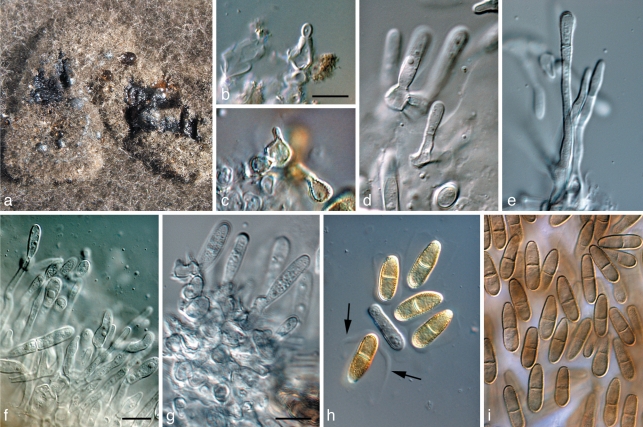
Coniothyrium nitidae Crous & Denman, S. African J. Bot. 64: 138. 1998 — Fig. 9
Fig. 9.
Coniothyrium nitidae (CBS 111322). a, b. Colonies sporulating on MEA; c–e. conidiogenous cells giving rise to conidia; f. pigmented, verruculose, 1-septate conidia. — Scale bars = 10 μm.
Specimen examined. South Africa, Western Cape Province, Hermanus, on leaves of Protea nitida, 29 Aug. 1996, S. Denman, holotype PREM 55346, cultures ex-type CPC 1476 = CBS 111322, CPC 1477 = CBS 111321, CPC 1478 = CBS 111302, CPC 1532 = CBS 111380.
Notes — Coniothyrium nitidae is not a member of Coniothyrium s.str in the Leptosphaeriaceae (Zhang et al. 2009), but clusters in Didymellaceae. However, given the nature of its conidia, becoming 1-septate, dark brown and verruculose with age, it does not fit into any genus within the family as discussed by Aveskamp et al. (2010). We thus retain this species under its current name until this generic complex has been better resolved.
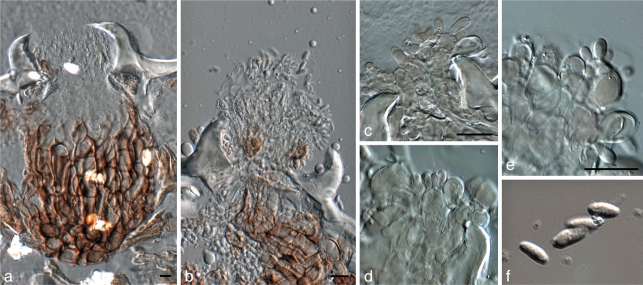
Curreya grandicipis (Joanne E. Taylor & Crous) Joanne E. Taylor & Crous, comb. nov. — MycoBank MB560559; Fig. 10
Fig. 10.
Curreya grandicipis (CBS 114272). a, b. Colonies sporulating on OA; c, d. vertical sections through conidiomata; e. conidiomatal wall of textura angularis; f–i. conidiogenous cells giving rise to conidia; j. pigmented conidia. — Scale bars: b, c = 150 μm, all others = 10 μm.
Basionym. Coniothyrium grandicipis Joanne E. Taylor & Crous, In Crous et al., Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea: 60. 2004.
Specimen examined. South Africa, Western Cape Province, Elgin, on leaves of Protea grandiceps, 20 July 1998, J.E. Taylor & S. Denman, holotype PREM 56616, cultures ex-type CPC 1852 = CBS 114272, CPC 1853 = CBS 111702.
Notes — The type species of Coniothyrium, C. palmarum, is allied to Leptosphaeriaceae (Zhang et al. 2009). The generic type of Curreya is C. conorum (Cucurbitariaceae), which is reported to have a Coniothyrium-like anamorph (von Arx & van der Aa 1983). Marincowitz et al. (2008a) also induced Coniothyrium-like anamorphs for species of Curreya in culture, and according to Fig. 1 it appears that ‘Coniothyrium’ grandicipis is best placed in Curreya, though the distinction between Curreya and Misturatosphaeria (Mugambi & Huhndorf 2009) is less clear at this stage (Fig. 1). Furthermore, von Arx & van der Aa (1983) list several Curreya-like teleomorph genera that have Coniothyrium-like anamorphs, revealing this generic complex to be in need of urgent taxonomic revision.
Microsphaeropsis proteae (Crous & Denman) Crous & Denman, comb. nov. — MycoBank MB560560; Fig. 11
Fig. 11.
Microsphaeropsis proteae (CBS 111303). a. Colonies sporulating on OA; b. central ostiole with oozing conidia; c. conidiogenous cells giving rise to conidia; d. pigmented conidia. — Scale bars = 10 μm.
Basionym. Coniothyrium proteae Crous & Denman, S. African J. Bot. 64: 139. 1998.
Specimen examined. South Africa, Western Cape Province, Hermanus, on leaves of Protea nitida, S. Denman, 29 Aug. 1996, holotype PREM 55347, cultures ex-type CPC 1423 = CBS 111303, CPC 1424 = CBS 111320, CPC 1425 = CBS 111319.
Notes — Coniothyrium proteae produces thin-walled conidia, 5–8 × 3.5–4 μm in vivo, 3–4 × 2–2.5 μm in vitro (Swart et al. 1998), that become brown with age, and phialidic conidiogenous cells that proliferate with periclinal thickening or with percurrent proliferation on conidiogenous cells (in old conidiomata), appearing Coniothyrium-like. Phylogenetically it is closely allied to species in the M. olivacea complex.
Diaporthe and Diplodina leaf spots and cankers
To date Phomopsis saccharata has been the only species of Phomopsis (teleomorph: Diaporthe) described from Proteaceae. The fungus causes a severe canker and dieback disease on Protea repens both in natural and cultivated stands in the Western and Eastern Cape Province of South Africa (Orffer & Knox-Davies 1989). However a number of other records of Diaporthe associated with Proteaceae exist, for example the Phomopsis state of a Diaporthe sp. was recorded in South Africa by Benic (1986) on dead P. repens mistbed cuttings showing basal and tip dieback and necrosis of leaves, as well as on seeds of different species of Proteaceae. A Diaporthe shoot and stem canker of Protea spp. was recorded in Queensland, Australia, and that fungus was reported to enter through wounds and cause sunken lesions which result in death of branches and entire plants (Greenhalgh 1981). Moura & Rodrigues (2001) reported a Diaporthe sp. on stems of Protea cynaroides in Madeira, Portugal. Diaporthe/Phomopsis spp. have also been recorded as endophytes on Proteaceae (Swart et al. 2000, Taylor et al. 2001c), but until now the significance of this occurrence has not been given context on Proteaceae. In accordance with the Amsterdam Declaration for pleomorphic fungi (Hawksworth et al. 2011), preference is given to the older generic name Diaporthe (1870), rather than the younger Phomopsis (1905) (see Crous et al. 2011a).
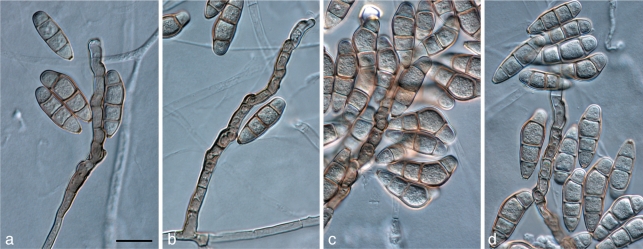
Diaporthe leucospermi Crous & Summerell, sp. nov. — MycoBank MB560561; Fig. 12
Fig. 12.
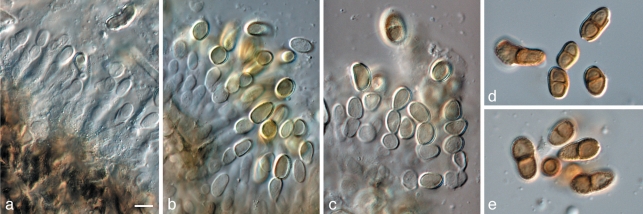
Diaporthe leucospermi (CBS 111980). a. Colonies sporulating on PDA; b. conidiogenous cells giving rise to conidia; c. alpha and beta conidia; d. beta conidia; e. alpha conidia. — Scale bars = 10 μm.
Alpha conidia aseptata, guttulata, ellipsoidea, (6–)7(–8) × (2.5–)3 μm. Beta conidia aseptata, fusiformia, (20–)25–30(–35) × (1–)1.5 μm.
Etymology. Named after the host on which it occurs, Leucospermum.
On OA. Conidiomata pycnidial, dark brown, imbedded, solitary to aggregated, opening via a central ostiole, exuding a creamy white conidial cirrhus; pycnidia up to 300 μm diam; wall consisting of several layers of dark brown textura angularis. Conidiophores lining the inner cavity, subcylindrical, hyaline (though pale brown at base), smooth, reduced to conidiogenous cells, or 1–3-septate, 15–30 × 2–3 μm. Conidiogenous cells phialidic, apical or lateral, hyaline, smooth, subcylindrical with apical taper, 10–15 × 2–3 μm; apex with visible periclinal thickening and flaring collarette, 1 μm long. Alpha conidia hyaline, smooth, aseptate, with two prominent guttules, ellipsoid, tapering to acutely rounded apex and obtuse to truncate base, (6–)7(–8) × (2.5–)3 μm; hilum with flattened scar, 1 μm diam. Beta conidia hyaline, smooth, aseptate, spindle-shaped, prominently hooked in apical part, apex acute, base truncate, (20–)25–30(–35) × (1–)1.5 μm.
Culture characteristics — Colonies spreading, flat, with sparse to moderate aerial mycelium, covering dish in 2 wk; on OA growing with concentric zones, middle olivaceous-grey, with alternating zones of smoke-grey and olivaceous-buff; on PDA pale olivaceous-grey to smoke-grey, reverse olivaceous-grey; on MEA surface dirty white with patches of olivaceous-buff to smoke-grey, reverse olivaceous.
Specimen examined. Australia, New South Wales, the Blue Mountains Botanic Gardens, Mount Tomah, on leaves of Leucospermum sp., Aug. 1999, P.W. Crous & B. Summerell, CBS H-20674 holotype, culture ex-type CPC 2956 = CBS 111980.
Notes — The ITS sequence of this species is 100 % identical to Diaporthe sp. (GenBank GQ250223) from Hydrangea macrophylla in Portugal, Diaporthe sp. (GenBank EU002916) isolated as a fruit endophyte from Coffea arabica in Hawaii and Diaporthe sp. (GenBank GQ250207) from Acer negundo in Portugal. Whether this species represents an endophyte with a broader host range remains to be tested.
Diplodina microsperma (Johnst.) B. Sutton, Mycol. Pap. 141: 69. 1977 — Fig. 13
Fig. 13.
Diplodina microsperma (CBS 114545). a. Colonies sporulating on sterile pine needles; b, c. conidiogenous cells giving rise to conidia; d. conidiomata forming on OA; e. conidiogenous cells giving rise to conidia; f. 0–1-septate conidia. — Scale bars = 10 μm.
Basionym. Stilbospora microsperma Johnst., in Johnston, A Flora of Berwick-upon-Tweed 2: 192. 1831.
= Sphaeria apiculata Wallr., Fl. Crypt. Germ. 2: 778. 1833.
≡ Metasphaeria apiculata (Wallr.) Sacc., Syll. Fung. 2: 166. 1883.
≡ Gnomonia apiculata (Wallr.) G. Winter, Rabenh., Kryptog.- Fl., ed. 2, vol. 1, 2: 589. 1887.
≡ Diaporthe spina Fuckel var. apiculata (Wallr.) Rehm, Ann. Mycol. 7: 404. 1909.
≡ Cryptodiaporthe apiculata (Wallr.) Petr., Ann. Mycol. 19: 177. 1921.
≡ Plagiostoma apiculatum (Wallr.) L.C. Mejía, Stud. Mycol. 68: 219. 2011.
Associated with leaf spots, and initially suspected to represent a species of Diaporthe. Conidiomata brown, multilocular, up to 400 μm diam, globose to depressed, immersed on MEA, superficial on PNA, opening by means of irregular rupture. Conidiophores lining the cavity, base pale brown, becoming hyaline towards apex, smooth, densely aggregated, irregularly branched, 1–3-septate, subcylindrical, 10–25 × 2–3 μm. Conidiogenous cells phialidic, pale brown to hyaline, smooth, terminal or lateral, doliiform to ampulliform, tapering towards a truncate apex with visible periclinal thickening, 5–12 × 2–2.5 μm. Conidia hyaline, smooth, guttulate, thick-walled when mature, fusiform, straight to curved, medianly 1-septate, apex acutely rounded, base truncate, (10–)12–14(–17) × (2–)3–3.5(–4) μm.
Culture characteristics — Colonies on MEA and OA covering the plate within 1 mo; colonies on MEA with abundant aerial mycelium, cream to dirty white with patches of sienna and umber; similar on OA, but also with patches of olivaceous-grey.
Specimen examined. New Zealand, on leaves of Protea sp., intercepted specimen of flowers exported to California, USA, LA143956, carrier UA 842, 11 Mar. 1999, M.A. Abdelshife & M.E. Palm, CBS H-20675, culture CPC 2336 = CBS 114545.
Notes — The genus Diplodina has Plagiostoma (= Cryptodiaporthe) teleomorphs (Mejía et al. 2011). The ITS, partial beta-tubulin, and TEF sequences of the isolate from Protea is identical to Diplodina microsperma (= Plagiostoma apiculatum) from Salix dasyclados in France (Sogonov et al. 2008, Mejía et al. 2011) (GenBank ITS: GU367068, Cryptodiaporthe apiculata) and Salix sitchensis from Washington, USA (GenBank ITS: GU367066, as Cryptodiaporthe apiculata; GenBank TUB: GU367009, as Cryptodiaporthe apiculata; GenBank TEF: GU353991, as Cryptodiaporthe apiculata). The ITS is also identical to Cryptodiaporthe salicella from Salix sp. in Austria (GenBank ITS: DQ323529), but differs on TUB (GenBank GU367008; identities = 718/725 (99 %), Gaps = 6/725 (1 %)) and TEF (GenBank EU221916; very little identity). Diplodina microsperma is a pathogen of Salix, thus it is unusual to find this species occurring on Proteaceae. Although conidia of the present isolate appear somewhat smaller than that known for D. microsperma (Sogonov et al. 2008, Mejía et al. 2011), more collections are required, and cross pathogenicity trials will need to be conducted to determine if this is simply a chance occurrence, or a real pathogen of Proteaceae.
Fusicladium leaf spot
Fusicladium proteae Crous, sp. nov. — MycoBank MB560562; Fig. 14
Fig. 14.
Fusicladium proteae (CBS H-20677). a–d. Conidiophores with conidiogenous cells giving rise to conidia; e. 1-septate conidia. — Scale bar = 10 μm.
Conidiophora solitaria, erecta, subcylindrica, recta vel curvata, 1–6-septata, 20–70 × 3–4 μm. Cellulae conidiogenae integratae, terminales, 15–40 × (3–)4–5 μm, cicatricibus conidialibus in parte apicali, marginaliter fuscatis et incrassatis, 1.5–2 μm diam. Conidia solitaria, obpyriformia, inequaliter 1-septatis, (13–)17–22(–30) × 4(–5) μm.
Etymology. Named after the host genus on which it was collected, Protea.
Mycelium consisting of smooth to finely verruculose, medium brown, septate, branched, 2.5–4 μm diam hyphae. Conidiophores medium brown, smooth, solitary, erect, subcylindrical, straight to curved, or once geniculate, 1–6-septate, 20–70 × 3–4 μm. Conidiogenous cells integrated, terminal on medium brown, smooth, subcylindrical, straight to geniculate-sinuous, 15–40 × (3–)4–5 μm; scars aggregated in apical part, darkened and thickened along the rim, 1.5–2 μm diam. Conidia solitary, pale to medium brown, smooth, guttulate, obpyriform, widest at obconically truncate base, that tapers abruptly to a darkened, thickened hilum, 1.5–2.5 μm; unequally 1-septate, with septum in lower third of the conidium (6–9 μm from base), tapering to an acutely rounded apex, (13–)17–22(–30) × 4(–5) μm.
Culture characteristics — Colonies flat, spreading, with moderate aerial mycelium (on PDA and MEA, sparse on OA), with even, lobate margins, reaching 40 mm diam after 2 mo. On OA olivaceous-grey with patches of smoke-grey; on PDA smoke-grey with patches of olivaceous-grey; on MEA surface with patches of smoke-grey and iron-grey, reverse iron-grey with smoke-grey in outer region.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, on leaves of Protea sp., in association with Vizella interrupta, 5 May 2010, P.W. Crous, holotype CBS H-20677, culture ex-type CBS 130599 = CPC 18282.
Notes — No other species of Fusicladium has thus far been reported from Proteaceae (Schubert et al. 2003). DNA sequence data (ITS) of Fusicladium proteae are not identical to any other species of Fusicladium presently in GenBank. Although the genus Fusicladium (1851) is older than its teleomorph, Venturia (1882), the latter is far more commonly used, and a proposal will have to be prepared to conserve Venturia over Fusicladium.
Pestalotiopsis leaf spot
Two species of Pestalotiopsis have been described from Proteaceae. Pestalotiopsis montellicoides was isolated from Protea cynaroides leaves from South Africa (Mordue 1986), and a Pestalotiopsis sp., the anamorph of Pestalosphaeria leucospermi, was described from living leaves of a Leucospermum sp. in New Zealand (Samuels et al. 1987). In Zimbabwe, a species of Pestalotiopsis was recorded causing leaf spots on several Protea and Leucospermum hosts (Swart et al. 1999), and has subsequently been intercepted at quarantine inspection points (Taylor 2001). This species is named as new below. The anamorph genus Pestalotiopsis (1949) is more commonly used, and older than the teleomorph genus Pestalosphaeria (1975), and hence has precedence.
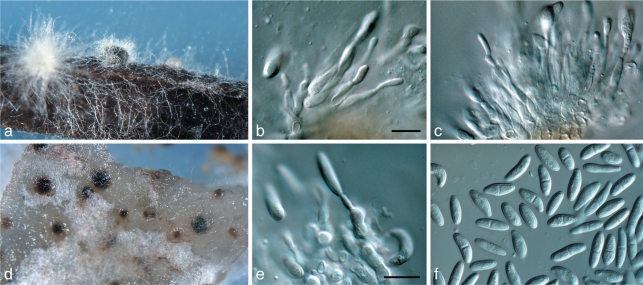
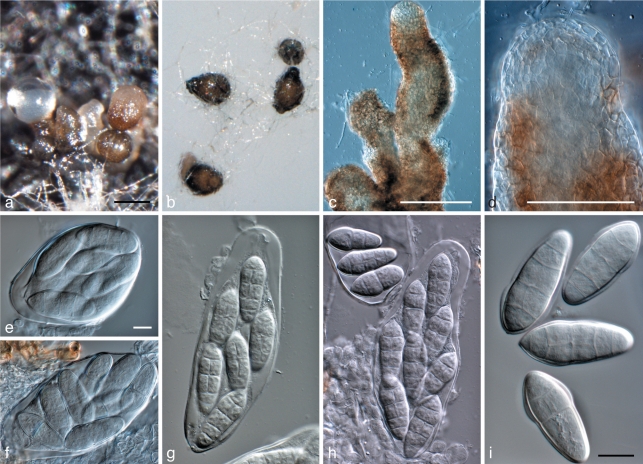
Pestalotiopsis protearum Crous & L. Swart, sp. nov. — MycoBank MB560563; Fig. 15
Fig. 15.
Pestalotiopsis protearum (CBS 114178). a. Leaf spot on Leucospermum cuneiforme; b. colony sporulating on PDA; c–g. conidiogenous cells giving rise to conidia; h, i. appendaged conidia. — Scale bars = 10 μm.
Pestalotiopsis montellicoidis similis, sed conidiis minoribus, in medio tricellularibus, cellulis (14–)16–17(–18) × (6.5–)8–9(–10) μm.
Etymology. Named after its occurrence on Proteaceae.
Leaf spots irregular, necrotic, associated with leaf margins or causing tip dieback; slightly sunken, pale brown with red-brown margins that are mostly raised and distinct, rarely diffuse, 2–35 mm diam. Conidiomata amphigenous, pycnidioid to acervular, immersed, becoming erumpent, unilocular, dark brown to black, dehiscing by irregular splits in the apical wall and the overlying host tissue, scattered, (100–)195–240(–400) μm; in section pyriform or conical, with applanate base, intra-epidermal in origin (125–)138–165(–180) μm wide, and (125–)138–165(–180) μm diam. Peridium comprising two strata of textura angularis, an outer stratum of pale brown, thick-walled cells becoming hyaline in the inner layer, apical and lateral walls composed of slightly compressed, thinner-walled cells; basal wall (13–)17–21(–23) μm, apical wall (7–)11–17(–19) μm thick. Conidiophores peripheral, reduced to conidiogenous cells, invested in mucus. Conidiogenous cells discrete, ampulliform, hyaline, smooth, (4–)5.5– 6.5(–8) × (2–)4–5(–6) μm; conidiogenesis initially holoblastic, with up to two enteroblastic, percurrent proliferations to produce additional conidia at slightly higher levels. Conidia ellipsoidal to obovoid, 4-euseptate, the second and third septa often darkened and indistinct, cells unequal, without constrictions at the septa, versicoloured, bearing appendages; basal cell obconic with a truncate base, bearing minute marginal frills, hyaline below, thin-walled, (3.5–)5–6(–7.5) × 4–4.5(–5) μm; second cell from base subcylindrical, pale brown, verruculose, (4–)5–5.5(–6) μm, third and fourth cells doliiform to subcylindrical, dark red-brown, verruculose, (4–)5–6(–7) μm and (4–)5.5–6(–7) μm long, respectively, combined dimensions of 3 median cells (14–)16–17(–18) × (6.5–)8–9(–10) μm; apical cell subconical, hyaline, collapsed at maturity, thin-walled, smooth, (3–)3.5–4.5(–6) × (3–)3.5–4(–5) μm; 2–4 appendages arising apically, tubular, branched or not, straight to flexuous, tip rounded, (15–)26–32(–43) μm long; basal appendage occasionally absent, filiform, flexuous, slender, centric, (2–)4.5–6(–9) μm. Conidia in vitro ellipsoidal to obovoid, 4(–6)-euseptate, the second and third septa often darkened and indistinct, cells unequal, without constrictions at the septa, versicoloured, bearing appendages; basal cell obconic with a truncate base, bearing minute marginal frills, hyaline below, thin-walled, (4–)5–6(–8) × 4–4.5(–5) μm; second cell subcylindrical, pale brown, faintly verruculose, third and fourth cells doliiform to subcylindrical, medium brown, verruculose, combined lengths of median cells (14.5–)16–17(–19) × (6–)7–7.5(–8) μm [length of second cell from base (5–)5.5–6(–7) μm; central cell, (4–)5–5.5(–7) μm; fourth cell, (4.5–)5–5.5(–6) μm] apical cell subconical, hyaline, collapsed at maturity, thin-walled, smooth, (3.5–)4–4.5(–5) × 3.5–4(–5) μm; appendages tubular, branched, straight to flexuous; (1–)2–4(–5) appendages arising apically, tip rounded but often absent due to frequent breakage of appendage, (10–)15– 17(–22) μm long; basal appendage occasionally absent, filiform, flexuous, slender, centric, (2–)3–3.5(–5) μm.
Culture characteristics — Colonies circular with undulate margins; mycelium of medium density, woolly, with white aerial mycelium, white in reverse; 69 mm diam after 7 d at 25 °C. Conidiomata fertile after 12 d at 25 °C under white light, with conidiomata developing over the entire surface of the colony and producing black, wet spore masses.
Specimen examined. Zimbabwe, Harare, Aveley Farm, on living leaves of Leucospermum cuneiforme cv. ‘Sunbird’, 6 Mar. 1998, L. Swart, holotype PREM 56186, culture ex-type CPC 1765 = CBS 114178.
Notes — Pestalotiopsis montellicoides differs from P. protearum from Zimbabwe in the larger dimensions of the conidia of the former, specifically the size of three median cells (26–35 × 7.5–10.5 μm). The Pestalotiopsis anamorph of Pestalosphaeria leucospermi from Leucospermum (Samuels et al. 1987) however, has conidia of similar dimensions to P. protearum, but the conidia of the former have concolorous median cells and more cylindrical conidiogenous cells (11–18 × 2–2.5 μm). In culture, Pestalosphaeria leucospermi becomes green-yellow (also noted for some Zimbabwean isolates, e.g. CPC 1783), and produces conidiomata in distinct concentric rings all over the surface of the colony. The combination of these features indicates that the collections in the present study represent a distinct species. When compared to the Pestalotiopsis species in the key provided by Nag Raj (1993), the species from Zimbabwe does not correspond to any previously described species, although it is most similar in dimensions and morphology to P. macrospora. In a recent study of Pestalotiopsis and allied genera (Jeewon 2002), several isolates from Proteaceae were included and identified as follows: Pestalotiopsis longisetula, Leucospermum sp., (CPC 1771) (Sicily); Pestalotiopsis aquatica, Leucospermum sp., (JT 615) (USA, Hawaii); Pestalotiopsis leucothoës, Telopea sp., (JT 551) (USA, Hawaii); Pestalotiopsis sydowiana, endophyte from P. neriifolia, (JT 258) (South Africa); Pestalotiopsis theae, endophyte from Protea neriifolia, (JT 258) (South Africa); Pestalotiopsis vismiae, Leucospermum sp., (JT 694) (USA, Hawaii). Attempts to use the ITS sequences of these isolates in a blast search of NCBIs GenBank nucleotide database were inconclusive with regard to species identification as several species with near identical sequences were obtained. Also, studying the GenBank ITS sequences of strains associated with the obtained species names did not yield a conclusive barcode representative of each of those species. The genus Pestalotiopsis requires major revision, incorporating type studies with multi-locus DNA sequence data and cultures.
Pyrenophora blight
Two Drechslera morphs of Pyrenophora species have been reported from Proteaceae, namely D. biseptata and D. dematioidea. Shoemaker (1998) placed D. biseptata and D. dematioidea in a new genus, Marielliottia, based on their unusual conidial morphology, while a phylogenetic study by Zhang & Berbee (2001) concluded that Marielliottia was best retained in Drechslera. In accordance with the Amsterdam Declaration for pleomorphic fungi (Hawksworth et al. 2011), preference is given to the older generic name Pyrenophora (1849), rather than the younger Drechslera (1930).
In spite of published reports, the present study revealed the disease to be mainly associated with a single species, described here as Pyrenophora leucospermi. This pathogen causes a severe blight of current-season leaves, stems, and flower heads, particularly during growth flushes. The disease is characterised by discrete leaf lesions and slowly expanding stem cankers that result in death of young shoot tips, or as moderately expanding lesions that are restricted to a few leaves or to leaf tips only. The leaf lesions are yellow, grey or brown and irregularly shaped with distinct red margins, which rapidly enlarge, often covering the entire leaf (von Broembsen, 1986, 1989, Forsberg 1993). The presence of Neofusicoccum spp., which act as important secondary pathogens of the stem cankers caused by P. leucospermi, results in significantly larger lesions than with either alone.
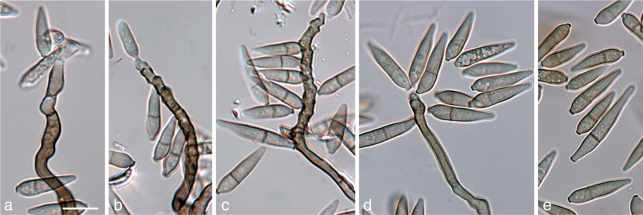
Pyrenophora leucospermi Crous & L. Swart, sp. nov. — MycoBank MB560564; Fig. 16
Fig. 16.
Pyrenophora leucospermi (CBS 111083). a. Leaf spot on Leucospermum cordifolium; b. close-up of leaf spot with red-brown margin; c–e. conidiogenous cells giving rise to conidia; f. conidia. — Scale bars = 10 μm.
Drechslerae dematoideae similis, sed conidiis 3–4(–7)-septatis.
Etymology. Named after its occurrence on Leucospermum.
Conidiophores on host material erect, medium yellow-brown, base slightly enlarged, 7–9(–15) μm, stalk nearly straight, septa approximately 15 μm apart, scars few (1–5), close and dark brown, apex truncate, 22–135 × 4–8 μm. Conidiogenous cells tretic, integrated, terminal, proliferating sympodially, cylindrical, cicatrised. Conidia mostly obovoid to nearly cylindrical, finely verruculose, if 4-septate, widest at the third cell from the base, with hemi-spherical apex, hemi-ellipsoidal base, pale to medium yellow-brown, but the basal cell paler brown, 3–4(–7)-septate, slightly constricted at the basal septum, with non-protruding scar, (1.5–)3 μm wide, (27–)30–38(–50) × (10–)11–13(–15) μm on SNA.
Culture characteristics — Colonies on MEA after 1 mo at 25 °C in the dark spreading, flat, with patches of scarlet and olivaceous-grey (surface), and iron-grey in reverse. On OA iron-grey with olivaceous-grey aerial mycelium.
Specimens examined. South Africa, Western Cape Province, Stellenbosch, Elsenburg Farm, on leaves of Leucospermum cordifolium, 7 Mar. 1996, L. Swart, CBS H-20676 holotype, cultures ex-type CPC 1293 = CBS 111083, CPC 1294 = CBS 111084, CPC 1295 = CBS 111085, CPC 1296 = CBS 111080, CPC 1297 = CBS 111086, CPC 1298 = CBS 111087.
Notes — Pyrenophora blight is the most important disease of commercially cultivated Leucospermum, particularly the cultivars developed from Lsp. cordifolium. Some Leucadendron and Mimetes spp. are also susceptible to blight (von Broembsen 1989). Although ‘D.’ biseptata is reported as the main cause of disease of Leucospermum in the southern states of Australia and Queensland, both ‘D.’ biseptata and ‘D.’ dematoidea are reported to cause blight on Leucospermum in Hawaii (Boesewinkel 1986, Forsberg 1993). A different Pyrenophora sp. that causes leaf spot and blight was reported from the North Island of New Zealand (Soteros 1986), while Shoemaker (1998) also found material of the Bipolaris anamorph of Cochliobolus australiensis to be present in Pyrenophora-like lesions, and concluded that more species were involved in this disease complex. In spite of several collections investigated in the present study (Table 1), no authentic cultures of ‘D.’ biseptata or ‘D.’ dematoidea were obtained from symptomatic Proteaceae in South Africa (Fig. 2). Furthermore, the species commonly associated with Pyrenophora blight in South Africa, which was also collected in Europe, appeared to represent a novel species, described here as P. leucospermi (Fig. 2). Pyrenophora leucospermi can be distinguished from ‘D.’ biseptata and ‘D.’ dematoidea by having conidia that are 3–4(–7)-septate, and by differences in the TEF region (Fig. 2). Furthermore, collections from leaf spots of Leucospermum spp. in Australia (CBS 111997, 114135), resulted in Curvularia trifolii (CPC 2941 = CBS 114135 and CPC 2995 = CBS 111997; Fig. 17) being newly reported from Proteaceae in the present study.
Fig. 17.
Curvularia trifolii (CBS 111997). a–d. Conidiogenous cells giving rise to conidia on SNA. — Scale bar = 10 μm.
Ramularia leaf spot
Species of Ramularia represent anamorphs of Mycosphaerella (Mycosphaerellaceae) (Verkley et al. 2004, Crous et al. 2009b, Koike et al. 2011), and most are assumed to be host specific (Braun 1998). Presently only a single species, R. proteae, has been reported associated with a leaf spot disease on P. longifolia in Tasmania (Crous et al. 2000). The newly described species below represent the first species of Ramularia described from Proteaceae in South Africa. The generic name Ramularia (1833) predates that of Mycosphaerella (1884), and should be used for this genus, as the latter is a confused concept that has been incorrectly applied to numerous genera (Crous et al. 2009b).
Ramularia stellenboschensis Crous, sp. nov. — MycoBank MB560565; Fig. 18
Fig. 18.
Ramularia stellenboschensis (CBS H-20678). a. Colony sporulating on OA; b. colony sporulating on PDA; c–g. branched conidial chains formed on SNA. — Scale bars = 10 μm.
Ramulariae proteae morphologice similis, sed conidiis subcylindraceis et majoribus, (12–)15–17(–20) × (2–)2.5–3(–3.5) μm.
Etymology. Named after the town of Stellenbosch, where this fungus was collected.
On SNA: Mycelium consisting of septate, branched, smooth, hyaline, 1.5–2 μm diam hyphae. Conidiophores solitary on hyphae, erect, smooth, hyaline, subcylindrical, straight to flexuous, terminal or intercalary on hyphae, reduced to conidiogenous cells, or up to 3-septate, 3–60 × 2–2.5 μm. Conidiogenous cells terminal or lateral, subcylindrical, smooth, hyaline, 3–20 × 1.5–2 μm, smooth, hyaline; scars thickened, darkened, refractive, 0.5–1 μm diam. Ramoconidia 0(–1)-septate, hyaline, smooth to finely verruculose, subcylindrical, (12–)15–17(–20) × (2–)2.5–3(–3.5) μm. Intercalary conidia in branched chains of up to 6, hyaline, smooth to finely verruculose, subcylindrical to narrowly ellipsoid-fusoid, (6–)7–9(–10) × (2–)2.5(–3) μm. Terminal conidia narrowly ellipsoid-fusoid, hyaline, smooth to finely verruculose, aseptate, 5–6(–7) × (1.5–)2 μm; hila thickened, darkened, refractive, 0.5–1 μm diam.
Culture characteristics — Colonies erumpent, spreading, surface folded, with sparse aerial mycelium, and smooth, lobate margins. On PDA surface pale vinaceous-grey (centre), vinaceous-grey in outer region, purplish grey underneath. On MEA surface pale vinaceous-grey, reverse iron-grey. On OA white in centre due to profuse sporulation, vinaceous-grey in outer region. Colonies reach 10–15 mm diam after 2 wk at 25 °C on all media tested.
Specimen examined. South Africa, Western Cape Province, Stellenbosch, J.S. Marais Botanical Garden, on leaves of Protea sp., associated with leaf spots of Vizella interrupta, 6 May 2010, P.W. Crous, holotype CBS H-20678, cultures ex-type CPC 18294 = CBS 130600.
Ramularia vizellae Crous, sp. nov. — MycoBank MB560566; Fig. 19
Fig. 19.
Ramularia vizellae (CBS H-20679). a. Colony sporulating on OA; e. colony sporulating on MEA; b–d, f–i. branched conidial chains formed on SNA. — Scale bars = 10 μm.
Ramulariae pratensis var. pratensis morphologice similis, sed conidiis minoribus et subcylindraceis-obclavatis vel ellipsoideis.
Etymology. Named after the fungal genus Vizella, from whose lesions it was isolated as potential secondary coloniser.
On SNA: Mycelium consisting of septate, branched, smooth, hyaline, 1.5–2.5 μm diam hyphae. Conidiophores solitary, erect on hyphae, terminal and lateral, smooth, hyaline, 1–4-septate, or reduced to conidiogenous cells, 2–100 × 1.5–2 μm, cylindrical, straight to curved. Conidiogenous cells smooth, hyaline, terminal and lateral on conidiophores, 2–25 × 1.5–2 μm; scars thickened, darkened, refractive, 0.5–1 μm diam. Ramoconidia subcylindrical to obclavate or ellipsoid, 0(–1)-septate, hyaline, smooth to finely verruculose, (8–)10–12(–23) × (2.5–)3–3.5(–5) μm, with 1–3 apical loci. Intercalary conidia hyaline, smooth, aseptate, ellipsoid, smooth to finely verruculose, (5–)6–7 × (2.5–)3(–3.5) μm. Terminal conidia in branched chains of up to 6, hyaline, smooth, aseptate, ellipsoid, smooth to finely verruculose, 4–5(–5.5) × (2–)3(–3.5) μm; hila thickened, darkened, refractive, 0.5–1 μm diam.
Culture characteristics — Colonies erumpent, spreading, surface folded, with moderate aerial mycelium; margins smooth, lobate. On PDA surface dirty cream, reverse apricot. On MEA surface pale olivaceous-grey, with patches of scarlet; reverse olivaceous-grey with patches of apricot. On OA surface pale olivaceous-grey to dirty cream, with patches of apricot. Colonies reach 12–15 mm diam after 2 wk on all media tested.
Specimen examined. South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, on leaves of Protea sp., in association with Vizella interrupta (secondary?), 2 May 2010, P.W. Crous, holotype CBS H-20679, cultures ex-type CPC 18283 = CBS 130601.
Notes — Ramularia vizellae, obtained from leaves of Protea sp. infected with Vizella interrupta, appears to be genetically related to Ramularia pratensis var. pratensis (on Polygonaceae; Braun 1998). This could have been a chance encounter on the Protea leaf, as no sporulation was observed on the leaf itself. Morphologically, however, conidia of R. pratensis var. pratensis are somewhat larger, ellipsoid-ovoid, subcylindrical-fusoid, (6–)8–25(–35) × (1.5–)2–4(–5) μm. The ITS sequence of R. vizellae is not identical to that of R. pratensis var. pratensis (GenBank EU019284.2; Identities = 564/583 (97 %), Gaps = 6/583 (1 %)).
The three species of Ramularia occurring on Proteaceae can easily be distinguished based on conidial size, with conidia of R. proteae being the smallest, subcylindrical-fusoid, 5–8(–10) × 1–1.5(–2) μm, while the subcylindrical conidia of R. stellenboschensis are larger, (12–)15–17(–20) × (2–)2.5–3(–3.5) μm. Finally, conidia of R. vizellae are more subcylindrical to obclavate or ellipsoid in shape, (8–)10–12(–23) × (2.5–)3–3.5(–5) μm.
Teratosphaeria leaf spot
Crous et al. (2004a) listed 13 species of Mycosphaerella (incl. Teratosphaeria) and 18 associated anamorph species occurring on Proteaceae. Since this date, however, numerous additional species have been described from this host family (Crous et al. 2007a, 2008, 2009b). Species of Teratosphaeria are commonly associated with leaf spots and blotches on Proteaceae, though not much is known about their host specificity (Crous & Groenewald 2005).
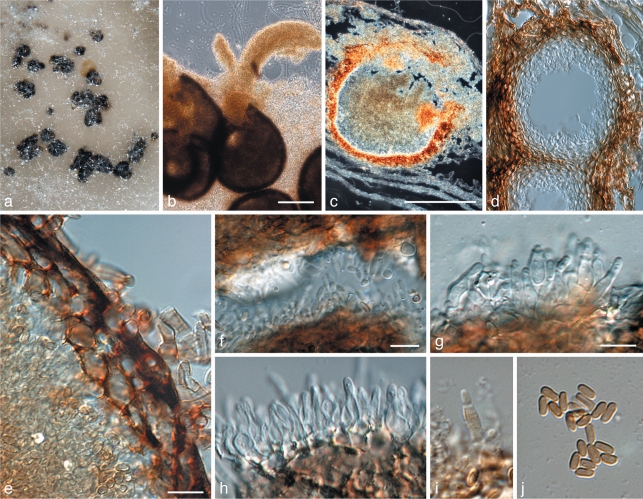
Teratosphaeria capensis Crous, sp. nov. — MycoBank MB560567; Fig. 20
Fig. 20.
Teratosphaeria capensis (CBS H-20680). a. Leaf spot of Brunneosphaerella protearum (prominent, large, semi-erumpent ascomata), surrounded by inconspicuous, substomatal ascomata of T. capensis; b–f. asci; g. germinating ascospores on MEA; h. ascospores. — Scale bars = 10 μm.
Teratosphaeriae bellulae similis, sed ascosporis sine vagina gelatinosa, ascosporis quidem in asco brunnescentibus.
Etymology. Named after the Cape Province, South Africa, where this species was collected.
Leaf spots amphigenous, subcircular to circular, up to 2.5 cm diam, medium brown with a raised, dark brown border. Ascomata amphigenous, black, immersed, substomatal, up to 100 μm diam, along the margins of leaf spots, with Brunneosphaerella protearum ascomata in the centre (which is probably the primary pathogen). This suggests that T. capensis was either endophytic or opportunistic. The ascomatal wall consists of 2–3 layers of medium brown textura angularis. Ostiole central, 5–10 μm diam, lined with hyaline, 0–1-septate periphyses, up to 10 μm long, 2 μm diam. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid, straight to curved, 8-spored, 35–50 × 8–12 μm. Ascospores tri- to multiseriate, overlapping, hyaline, prominently guttulate, thick-walled, straight, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, prominently constricted at the septum, tapering towards both ends, but more prominently towards the lower end, (8–)10–11(–13) × 3.5–4 μm. Germinating ascospores on MEA become brown, verruculose and distorted, with one to several germ tubes that grow at irregular angles to the long axis of the spore, which becomes up to 8 μm wide.
Cultural characteristics — Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margins, reaching 15 mm diam after 1 mo at 25 °C in the dark on all media tested. On MEA olivaceous-grey, reverse iron-grey; on OA olivaceous-grey; on PDA olivaceous-grey, reverse iron-grey; colonies sporulating on MEA after 2 mo, homothallic, ascospores 10–16 × 3–5 μm.
Specimens examined. Portugal, on leaves of P. repens, 1 Jan. 2007, M.F. Moura, CPC 13981. – South Africa, Western Cape Province, Stellenbosch, J.S. Marais Garden, S33°55’59.3″ E18°52’22.5″, on living leaves of Protea sp., 6 May 2010, P.W. Crous, holotype CBS H-20680, culture ex-type CPC 18299 = CBS 130602.
Notes — Teratosphaeria capensis generally is found on necrotic leaf spots of Brunneosphaerella protearum in South Africa. However, it was the sole pathogen associated with necrotic leaf spots of a P. repens in Portugal (CPC 13981) (Crous et al. 2008). The varying niche ecology demonstrated by this species suggests that it is adaptive to its host in different environments. Since P. repens was brought to Portugal this fungus may well have been introduced as an endophyte. Morphologically T. capensis is similar to several species occurring on Proteaceae based on its ascospore dimensions and germination patterns, and would be difficult to distinguish without DNA sequence data. Presently this is the only species of Teratosphaeria known to be homothallic and form fertile ascomata in culture.
ADDENDUM
Several species were encountered during this study that appeared to be saprobic rather than plant pathogenic. These taxa are thus treated in the addendum below.
Coccomyces proteae Marinc., M.J. Wingf. & Crous, in Marincowitz et al., Microfungi occurring on Proteaceae in the fynbos: 32. 2008.
Specimens examined. South Africa, Western Cape Province, Jonkershoek Nature Reserve, on leaf litter of Protea nitida, S. Marincowitz, 6 June 2000, holotype PREM 59439; KwaZulu-Natal, Drakensberg Mountains, on leaves of Protea sp., 21 Mar. 1998, S. Denman, epitype designated here as CBS H-20681, cultures ex-epitype CBS 111703 = CPC 1730, CBS 111704 = CPC 1727.
Notes — Both collections of this fungus have been from leaf litter, and thus far nothing is known about its potential role as pathogen of Proteaceae, though this seems unlikely.
Gordonomyces Crous & Marinc., gen. nov. — MycoBank MB560568
Phaeophleosporae morphologice similis, sed conidiomatibus erumpentibus, pariete externo laevi et nigro, conidiis juvenilibus cum vagina mucosa.
Type species. Gordonomyces mucovaginatus Crous & Marinc.
Etymology. Named after Gordon’s Bay, a harbour town in the Western Cape Province of South Africa, which was in turn named after Robert Jacob Gordon (1743–1795), the Dutch explorer of Scottish descent.
Conidiomata up to 600 μm diam, globose, separate to aggregated in clusters, black, outer wall smooth; wall of textura angularis, up to 60 μm thick, outer region of 3–8 layers of dark brown cells, inner region of 3–7 layers of thick-walled, hyaline cells. Paraphyses intermingled among conidiogenous cells, cylindrical, branched below, septate; at times becoming fertile. Conidiogenous cells subcylindrical to ampulliform, mostly reduced to single cells, with apical percurrent proliferation. Conidia medium to golden-brown, verruculose, obovoid to semi-clavate, medianly 1-euseptate, apex obtuse, base subtruncate, conidia covered in mucoid sheath, 2–3 μm wide.
Gordonomyces mucovaginatus Crous & Marinc., sp. nov. — MycoBank MB560559; Fig. 21
Fig. 21.
Gordonomyces mucovaginatus (PREM 59592). a. Colony sporulating on PDA; b–d, g. conidiogenous cells giving rise to conidia; e, f. paraphyses; h. conidia with mucoid appendages (arrows); i. mature conidia. — Scale bars = 10 μm.
Conidia (12–)13–15(–17) × (4–)4.5–5 μm, mediovel aureobrunnea, verruculosa, ovoidea, in medio 1-euseptata, cum vagina mucosa, 2–3 μm lata.
Etymology. Named after the prominent mucoid sheath that surrounds the apices of its conidia.
Sporulating on PDA. Conidiomata up to 600 μm diam, globose, erumpent on agar, separate to aggregated in clusters, black, outer wall smooth; conidiomata filled with a brown-black mucoid mass containing numerous conidia; wall of textura angularis, up to 60 μm thick, outer region of 3–8 layers of dark brown cells, inner region of 3–7 layers of thick-walled, hyaline cells. Paraphyses intermingled among conidiogenous cells, cylindrical, branched below, 1–6-septate, 30–70 × 3–4 μm, with obtuse ends, but at times becoming fertile, giving rise to conidia, terminal and lateral, by means of inconspicuous percurrent proliferation. Conidiogenous cells subcylindrical to ampulliform, mostly reduced to single cells, with apical percurrent proliferation, 5–15 × 3.5–4 μm. Conidia medium to golden-brown, verruculose, obovoid to semi-clavate, medianly 1-euseptate, apex obtuse, base subtruncate, conidia covered in mucoid sheath, 2–3 μm wide, (12–)13–15(–17) × (4–)4.5–5 μm.
Culture characteristics — Colonies on PDA spreading, with smooth, lobed margins, and moderate aerial mycelium; surface sepia (middle), fuscous-black in outer region and underneath, up to 30 mm diam after 2 wk at 25 °C. On OA spreading, flat, with smooth margin, lacking aerial mycelium, rosy-buff, up to 40 μm diam after 2 wk. On MEA spreading, with smooth, lobed margin and moderate aerial mycelium; surface iron-grey with patches of white; reverse fuscous-black; colonies reaching 40 mm diam after 2 wk.
Specimen examined. South Africa, Western Cape Province, Gordon’s Bay, on leaf litter of Leucadendron laureolum, 26 June 2000, S. Marincowitz, holotype PREM 59592, culture ex-type CMW 22212 = CBS 127273 = CPC 18172.
Notes — Gordonomyces is Phaeophleospora-Kirramyces-like in morphology (Crous et al. 1997, Andjic et al. 2007), but distinct in having erumpent conidiomata with a smooth, black outer wall, and juvenile conidia that are encased in a mucoid sheath.
Leptosphaerulina australis McAlpine, in McAlpine, Fungus diseases of stone-fruit trees in Australia and their treatment: 103. 1902 — Fig. 22
Fig. 22.
Leptosphaerulina australis (CBS 116307). a. Colony sporulating on PDA; b. ascomata forming on SNA; c, d. ascomata showing ostiolar region; e–h; asci; i. muriformly septate, hyaline ascospores with mucoid sheath. — Scale bars: a, c = 200 μm, d = 30 μm, all others = 10 μm.
For synonyms see MycoBank.
On SNA: Ascomata pseudothecial, solitary to aggregated in clusters, brown, superficial on agar medium, obpyriform to subglobose, 100–150 × 150–200 μm; ostiole central, up to 30 μm diam; outer wall covered with short, brown hyphal setae, 5–15 × 3–5 μm, with obtuse ends. Asci 100–120 × 35–45 μm, 8-spored, hyaline, obovoid, bitunicate with strongly developed apical chamber, 5–7 × 2–3 μm. Ascospores multiseriate in asci, hyaline, smooth, with mucoid sheath, 4 transverse septa, and 2–3 vertical, and 1–2 oblique septa, constricted at second vertical septum from apex, ellipsoid to obovoid, tapering from middle of upper part of ascospore (widest point) to an acutely rounded apex, base obtusely rounded; hamathecial tissue dissolving among asci, and pseudoparaphyses not observed, (32–)33–27(–40) × (12–)13–14(–15) μm.
Culture characteristics — Colonies spreading, flat, with sparse aerial mycelium and smooth, lobate margins, reaching 22 mm diam after 1 wk at 25 °C. On MEA centre dirty white, outer region sienna, reverse sienna; on PDA centre dirty white, outer region olivaceous-grey, reverse iron-grey; on OA centre dirty white, outer region olivaceous-grey to iron-grey.
Specimen examined. Kenya, on leaves of Protea sp., 1999, culture CPC 3712 = CBS 116307.
Notes — The ITS sequence data and morphology of this isolate matches that of other isolates of the species. Leptosphaerulina australis was isolated from leaf spots of a Protea sp. collected in Kenya, and as such is the first record from Proteaceae. Although species of Leptosphaerulina are well-known plant pathogens (Graham & Luttrell 1961), the pathogenicity status of L. australis is uncertain (Mitkowski & Browning 2004). Notwithstanding this, the fungus has been intercepted on exported agricultural produce, namely on leaf spots of Brassica oleracea var. capitata from China (Hyun et al. 2005), and could be relevant to trade in agricultural produce.
Pseudopassalora Crous, gen. nov. — MycoBank MB560570
Passalorae similis, sed hyphis et cellulis conidiogenis hyalinis, conidiis vetustis verrucosis.
Type species. Pseudopassalora gouriqua Crous.
Etymology. Named after its morphological similarity to Passalora.
Mycelium consisting of smooth, hyaline, septate, branched hyphae. Conidiophores solitary on hyphae, micronematous, reduced to conidiogenous loci, or erect, cylindrical, straight to flexuous, hyaline, smooth, 1–2-septate. Conidiogenous cells integrated, reduced to loci on hyphae, or terminal on short conidiophores, cylindrical, hyaline, smooth, containing a solitary terminal, truncate locus, or at times polyblastic with 1–3 phialidic loci that are thickened along the rim. Conidia solitary, fusoid-ellipsoidal, brown, verruculose to warty, widest in the middle of the upper third, tapering to a subobtose apex, aseptate or medianly 1-septate; base truncate, at times slightly thickened along the rim.
Pseudopassalora gouriqua Crous, sp. nov. — MycoBank MB560571; Fig. 23
Fig. 23.
Pseudopassalora gouriqua (CBS 101954). a–g. Conidiogenous cells on superficial hyphae giving rise to brown, warty, 1-septate conidia. — Scale bars = 10 μm.
Conidia solitaria, fusoida-ellipsoidea, brunnea, verruculosa vel verrucosa, aseptata vel in medio 1-septata, (14–)20–25(–35) × (3–)3.5–4(–4.5) μm; basi truncata, interdum marginaliter leviter incrassata.
Etymology. Named after the location where it was collected. The word ‘Gouriqua’ stems from the Khoi-Khoi, who lived in this area about five hundred years ago.
Mycelium consisting of smooth, hyaline, septate, branched, 2–3 μm diam hyphae. Conidiophores solitary on hyphae, micronematous, reduced to conidiogenous loci, 1 × 1.5 μm, or erect, cylindrical, straight to flexuous, hyaline, smooth, 1–2-septate, 10–35 × 2–3 μm. Conidiogenous cells integrated, reduced to loci on hyphae, or terminal on short conidiophores, cylindrical, hyaline, smooth, 1–20 × (1.5–)2–3 μm; conidiogenous cells containing a solitary terminal, truncate locus, 1.5–3 μm diam, or at times conidiogenous cell polyblastic with 1–3 loci that are thickened along the rim, 0.5–1 μm diam. Conidia solitary, fusoid-ellipsoidal, brown, verruculose to warty, widest in the middle of the upper third, tapering to a subobtose apex, aseptate or medianly 1-septate, (14–)20–25(–35) × (3–)3.5–4(–4.5) μm; base truncate, at times slightly thickened along the rim.
Culture characteristics — Colonies flat, spreading, with sparse aerial mycelium, and even, smooth margins, reaching 20 mm diam after 2 mo on all media tested. On OA sepia, with margins concolorous with agar medium; on PDA surface cinnamon, reverse honey; on MEA surface dirty white, with patches of greyish sepia, cinnamon and honey, reverse cinnamon.
Specimen examined. South Africa, Western Cape Province, Mossel Bay, Gouriqua, Rein’s Coastal Nature Reserve, on leaves of Protea susannae, 1 Apr. 1998, L. Dryer, holotype CBS H-20682, cultures ex-type CBS 101954 = CPC 1811.
Notes — Pseudopassalora resembles Retroconis, but is distinct in producing solitary conidia (Seifert et al. 2011). It is some-what reminiscent of Fusicladium (Venturia) (Beck et al. 2005, Crous et al. 2007b), but clusters apart from this genus, and has somewhat thickened loci, and hyaline hyphae. It also resembles Passalora in having somewhat thickened loci, but is again distinct in having hyaline hyphae and conidiogenous cells, and conidia that become warty with age.
Presently very little is known of the ecology of P. gouriqua. Although occurring on leaves of P. susanne, it was at the time of isolation suspected to be a possible saprobe.
Xenoconiothyrium Crous & Marinc., gen. nov. — MycoBank MB560572
Coniothyrio morphologice simile, sed conidiis primo brevicatenatis, cum poris conspicuis ad extremum singulum vel ad extrema.
Type species. Xenoconiothyrium catenata Crous & Marinc.
Etymology. Mature conidia resembling that of the genus Coniothyrium, but distinct when young, thus (Xeno-) Coniothyrium.
Mycelium consisting of septate, branched, olivaceous-brown to brown, thick-walled, warty hyphae, covered in a mucoid layer, giving rise to pycnidial conidiomata. Conidiophores reduced to phialidic conidiogenous cells that line the cavity, ampulliform, hyaline, tapering to an abrupt apex. Conidia initially in short chains 3(–4), attached via a small locus, eventually breaking free, with minute loci visible on conidial ends, ellipsoid to subcylindrical, thick-walled, initially hyaline, smooth, becoming verruculose, dark brown, 0(–1)-septate, not or constricted at septum, apex obtuse, widest at septum, tapering abruptly to truncate hilum (when viewed directly from above, it appears to have a central pore, which can be basal, or present at both conidial ends); not thickened nor darkened. Spermatia at times formed in same conidioma, hyaline, bacilliform.
Xenoconiothyrium catenata Crous & Marinc., sp. nov. — Myco-Bank MB560573; Fig. 24
Fig. 24.
Xenoconiothyrium catenata (CBS 128994). a, b. Conidiogenous cells giving rise to conidial chains; c. conidia forming in short chains; d, e. mature, brown, verruculose, 1-septate conidia with basal and/or apical attachment loci (apical, basal, or both). — Scale bar = 10 μm.
Conidia ellipsoidea-subcylindracea, crassitunicata, primo hyaline, laevia, deinde verrucolosa, atrobrunnea, 0(–1)-septata, (7–)8–10(–12) × (4–)5 μm, primo brevicatenata, 3(–4), adherentia per locum parvum, ad 0.5 μm diam.
Etymology. Named after its conidia, which occur in chains.
Mycelium consisting of septate, branched, olivaceous-brown to brown, thick-walled, warty, 3–5 μm diam hyphae, covered in a mucoid layer, giving rise to pycnidial conidiomata up to 250 μm diam. Conidiophores reduced to phialidic conidiogenous cells that line the cavity, ampulliform, hyaline, 8–15 × 5–6 μm, tapering to an abrupt apex, locus 0.5 μm diam. Conidia (7–)8–10(–12) × (4–)5 μm, initially in short chains, 3(–4), attached via a small locus up to 0.5 μm diam, eventually breaking free, with minute loci visible on conidial ends, ellipsoid to subcylindrical, thick-walled (1 μm diam), initially hyaline, smooth, becoming verruculose, dark brown, 0(–1)-septate, not or constricted at septum, apex obtuse, widest at septum, tapering abruptly to truncate hilum (when viewed directly from above, it appears to have a central pore, which can be basal, or present at both conidial ends), 0.5 μm diam; not thickened nor darkened. Spermatia at times formed in same conidioma, hyaline, bacilliform, 3–7 × 3–4 μm.
Culture characteristics — Colonies spreading, erumpent with sparse aerial mycelium and even margin; surface folded, reaching 25 mm diam after 2 mo on all media tested; on MEA surface olivaceous-grey, reverse iron-grey; on OA surface iron-grey.
Specimen examined. South Africa, Western Cape Province, Helderberg Nature Reserve, on twig litter of Protea laurifolia, 14 Aug. 2000, S. Marincowitz, holotype PREM 59486, culture ex-type CMW 22113 = CBS 128994.
Notes — Based on the dimensions of its septate, brown conidia, this fungus was originally identified as Coniothyrium septatum by Marincowitz et al. (2008a). It is distinct from the genus Coniothyrium, however, in that conidia occur in short chains when young, and have pores visible at one or either end. Based on its LSU sequence it is closest to Xenophacidiella pseudocatenata (GenBank JF499870; Crous & Groenewald 2011) and Phaeothecoidea melaleuca (GenBank HQ599595; Crous et al. 2010) in Teratosphaeriaceae. It was originally collected from twig litter of Protea laurifolia, and nothing is thus known about its potential role as pathogen of Proteaceae.
DISCUSSION
The Cape Floristic Region (CFR) is located in the south-western part of South Africa, and includes 42 % of the vascular plants occurring in the Southern African subcontinent (Goldblatt & Manning 2000, Rouget et al. 2003). The CFR comprises components of fynbos and five other biomes (Cowling & Holms 1992). As a shrub land, the fynbos has four different plant growth forms. These are tall Protea shrubs with large leaves (proteoids), heath-like shrubs (ericoids), wiry reed-like plants (restioids) and bulbous herbs (geophytes) (Cowling & Richardson 1995, Rebelo 2001).
Very little is known, however, about the fungi occurring in the CFR, and recent studies have highlighted the unusual diversity that occur in saprobic (Schubert et al. 2007, Visagie et al. 2009, Bensch et al. 2010), and plant pathogenic fungi (Crous et al. 2009a, 2011b). Mycological exploration in South Africa commenced in the late 1700s, and thus later than plant collections, which began in the 1600s (Eicker & Baxter 1999, Rong & Baxter 2006). For more than a century, both collections and studies on fungi were largely conducted by European visiting scientists. It was only in the late 1800s that the first resident mycologists were appointed in South Africa. A third generation mycologist, Dr Ethel M. Doidge, compiled the list of fungi and lichens collected in South Africa to the end of 1945. In this compilation, about 30 fungi are listed associated with members of Proteaceae (Doidge 1950), while based on the known plant biodiversity of South Africa, Crous et al. (2006a) estimated the potential fungal biodiversity as approximately 200 000 species, many of which would occur on Proteaceae.
The first fungus described from Proteaceae in the Western Cape was Pseudocercospora protearum (as Cercospora protearum) (Cooke 1883). After many decades of work, the plant pathogenic fungi associated with the family, especially on Protea, Leucospermum and Leucadendron was published (Crous et al. 2004a), as well as the common saprobic fungi occurring on twig and leaf litter (Marincowitz et al. 2008a).
We now have a fragmentary amount of information about fungi in the fynbos, which clearly shows that a unique, endemic mycoflora exists. Nevertheless, the fact that the present study can add an additional 12 species and three genera to the number of known taxa occurring on Proteaceae, many of them obvious and clearly plant pathogenic, underlines the fact that more attention needs to be directed towards studying the fungal biodiversity of the CFR. This region not only represents an important part of natural resource-based ecotourism, that also generates thousands of jobs for the local community, many involved with the sale and export of cut-flowers, but is also a key biogeographic region that is the focus of intensive study to better understand evolutionary processes. Adding a mycological component to these studies will greatly benefit our understanding of evolutionary processes in fungi.
Acknowledgments
The University of Stellenbosch is thanked for financial support to P.W. Crous during a recent collecting trip to the fynbos region in South Africa. Prof. dr U. Braun (Martin-Luther-Univ., Halle, Germany) is thanked for providing the Latin diagnoses. We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Andjic V, Barber PA, Carnegie AJ, Hardy GEStJ, Wingfield MJ, Burgess TI.2007. Phylogenetic reassessment supports accommodation of Phaeophleospora and Colletogloeopsis from eucalypts in Kirramyces. Mycological Research 111: 1184– 1198 [DOI] [PubMed] [Google Scholar]
- Arx JA von, Aa HA van der.1983. Notes on Curreya (Ascomycetes, Dothideales). Sydowia 36: 1– 5 [Google Scholar]
- Aveskamp M, Gruyter H de, Woudenberg J, Verkley G, Crous PW.2010. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65: 1– 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp MM, Verkley GJM, Gruyter J de, Murace MA, Perelló A, et al. 2009. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101: 363– 382 [DOI] [PubMed] [Google Scholar]
- Beck A, Ritschel A, Schubert K, Braun U, Triebel D.2005. Phylogenetic relationships of the anamorphic genus Fusicladium s.lat. as inferred by ITS nrDNA data. Mycological Progress 4: 111– 116 [Google Scholar]
- Benic LM.1986. Pathological problems associated with propagation material in nurseries in South Africa. Acta Horticulturae 185: 229– 237 [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, et al. 2010. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1– 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesewinkel HJ.1986. New plant disease records from New Zealand. Australasian Plant Pathology 15: 18– 21 [Google Scholar]
- Braun U.1998. A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 2 IHW-Verlag, Eching [Google Scholar]
- Broembsen SL von.1986. Blight of pincushions (Leucospermum spp.) caused by Drechslera dematioidea. Plant Disease 70: 33– 36 [Google Scholar]
- Broembsen SL von.1989. Handbook of diseases of cut-flower Proteas. International Protea Association, Victoria, Australia [Google Scholar]
- Carbone I, Kohn LM.1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553– 556 [Google Scholar]
- Coetzee JH, Littlejohn GM.2001. Protea: A floriculture crop from the Cape Floristic Kingdom. Horticultural Reviews 26: 1– 48 [Google Scholar]
- Coetzee MPA, Wingfield BD, Roux J, Crous PW, Denman S, Wingfield MJ.2003. Discovery of two northern hemisphere Armillaria species on Proteaceae in South Africa. Plant Pathology 52: 604– 612 [Google Scholar]
- Cooke MC.1883. Some exotic fungi. Grevillea 12: 37– 39 [Google Scholar]
- Cowling RM, Holms PM.1992. Flora and vegetation. In: Cowling RM. (ed), The ecology of fynbos: Nutrients, fire and diversity: 23–61. Oxford University Press, Cape Town, South Africa [Google Scholar]
- Cowling RM, Richardson D.1995. Fynbos: South Africa’s unique floral kingdom. Fernwood Press, Vlaeberg, South Africa [Google Scholar]
- Crous PW.1998. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1– 170 [Google Scholar]
- Crous PW, Braun U, Groenewald JZ.2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1– 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME.2004a. Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1–228. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands [Google Scholar]
- Crous PW, Ferreira FA, Sutton BC.1997. A comparison of the fungal genera Phaeophleospora and Kirramyces (coelomycetes). South African Journal of Botany 63: 111– 115 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G.2004b. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19– 22 [Google Scholar]
- Crous PW, Groenewald JZ.2005. Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463– 470 [Google Scholar]
- Crous PW, Groenewald JZ.2011. Why everlastings don’t last. Persoonia 26: 70– 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG.2010. Phaeothecoidea melaleuca. Fungal Planet 61. Persoonia 25: 142– 143 [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, Edwards J, Seifert KA, et al. 2011a. Fungal Planet Description Sheets: 69–91. Persoonia 26: 108– 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Rong IH, Wood A, Lee S, Glen H, et al. 2006a. How many species of fungi are there at the tip of Africa? Studies in Mycology 55: 13– 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al. 2009a. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17– 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schubert K, Braun U, Hoog GS de, Hocking AD, et al. 2007b. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology 58: 185– 217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al. 2006b. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235– 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, et al. 2009b. Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99– 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ.2008. Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20: 59– 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Taylor JE, Bullock S.2000. Fungi occurring on Proteaceae in Australia: selected foliicolous species. Australasian Plant Pathology 29: 267– 278 [Google Scholar]
- Crous PW, Tanaka K, Summerell BA, Groenewald JZ.2011b. Additions to the Mycosphaerella complex. IMA Fungus 2: 49– 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds). 2009c. Fungal Biodiversity. CBS Laboratory Manual Series 1: 1–269. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands [Google Scholar]
- Crous PW, Wingfield MJ, Park RF.1991. Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628– 632 [Google Scholar]
- Denman S, Crous PW, Groenewald JG, Slippers B, Wingfield BD, Wingfield MJ.2003. Circumscription of Botryosphaeria species associated with Proteaceae based on morphology and DNA sequence data. Mycologia 95: 294– 307 [PubMed] [Google Scholar]
- Denman S, Crous PW, Taylor JE, Kang J-C, Pascoe I, Wingfield MJ.2000. An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Studies in Mycology 45: 129– 140 [Google Scholar]
- Denman S, Crous PW, Wingfield MJ.1999. A taxonomic reassessment of Phyllachora proteae, a leaf pathogen of Proteaceae. Mycologia 91: 510– 516 [Google Scholar]
- Doidge EM.1950. The South African fungi and lichens to the end of 1945. Bothalia 5: 1– 1094 (www.cbs.knaw.nl/publications/mycoheritage.aspx). [Google Scholar]
- Eicker A, Baxter AP.1999. An historical overview of southern African systematic mycology. Transactions of the Royal Society of South Africa 54: 5– 19 [Google Scholar]
- Forsberg L.1993. Protea diseases and their control. Queensland Government, Department of Primary Industries, Brisbane, Australia [Google Scholar]
- Glass NL, Donaldson G.1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323– 1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt P, Manning J.2000. Cape plants. A conspectus of the Cape flora of South Africa, Strelitzia 9. National Botanical Institute of South Africa, Pretoria, South Africa [Google Scholar]
- Graham JH, Luttrell ES.1961. Species of Leptosphaerulina on forage plants. Phytopathology 51: 680– 693 [Google Scholar]
- Greenhalgh FC.1981. Diseases of proteaceous plants. In: Mathews P. (ed), The growing and marketing of proteas: 30–39. Report of the first international conference of protea growers, 4–8 October, Melbourne, Victoria, Australia. [Google Scholar]
- Gruyter J de, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW.2009. Molecular phylogeny of Phoma and allied anamorph genera: Towards a reclassification of the Phoma complex. Mycological Research 113: 508– 519 [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW.2010. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066– 1081 [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, Seifert KA, Taylor JW, Wingfield MJ, et al. 2011. The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105– 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG.1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183– 189 [DOI] [PubMed] [Google Scholar]
- Hyun I-H, Heo N-Y, Chang S-Y, Heo J-Y, Mel’nik V.2005. Identification of three fungi newly intercepted from importing plants in Korea. Mycrobiology 33: 243– 244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewon R.2002. Pestalotiopsis taxonomy: molecular phylogenetics, species nomenclature and teleomorph relationships. PhD dissertation, University of Hong Kong, Hong Kong [Google Scholar]
- Knox-Davies PS, Wyk PS van, Marasas WFO.1987. Diseases of Protea, Leucospermum and Leucadendron recorded in South Africa. Phytophylactica 19: 327– 337 [Google Scholar]
- Koike SK, Baameur A, Groenewald JZ, Crous PW.2011. Cercosporoid leaf pathogens from whorled milkweed and spineless safflower in California. IMA Fungus 2: 7– 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn G.1999. Cape fynbos products for cultivation. In: Fynbos Cultivation: 2.1–2.28. Agricultural Research Council, South Africa [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ.2010a. Species concepts in Calonectria (Cylindrocladium). Studies in Mycology 66: 1– 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ.2010b. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology 66: 15– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ.2010c. Phylogeny and systematics of the genus Calonectria. Studies in Mycology 66: 31– 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ.2008a. Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7: 1–166. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands [Google Scholar]
- Marincowitz S, Groenewald JZ, Wingfield MJ, Crous PW.2008b. Species of Botryosphaeriaceae occurring on Proteaceae. Persoonia 21: 111– 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF., Jr.2011. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Studies in Mycology 68: 211– 235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitkowski NA, Browning M.2004. Leptosphaerulina australis associated with intensively managed stands of Poa annua and Agrostis palustris. Canadian Journal of Plant Pathology 26: 193– 198 [Google Scholar]
- Mordue JEM.1986. Another unusual species of Pestalotiopsis: P. montellicoides sp. nov. Transactions of the British Mycological Society 86: 665– 668 [Google Scholar]
- Mostert L, Crous PW, Kang J-C, Phillips AJL.2001a. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93: 145– 166 [Google Scholar]
- Mostert L, Kang J-C, Crous PW, Denman S.2001b. Phomopsis saccharata sp. nov., causing a canker and die-back disease of Protea repens in South Africa. Sydowia 53: 227– 235 [Google Scholar]
- Moura MF, Rodrigues PF.2001. Fungal diseases on Proteas identified in Madeira Island. Acta Horticulturae 545: 265– 268 [Google Scholar]
- Mugambi GK, Huhndorf SM.2009. Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae re-circumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Studies in Mycology 64: 103– 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag Raj TR.1993. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publ., Waterloo, Ontario [Google Scholar]
- O’Donnell K, Cigelnik E.1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103– 116 [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC.1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America 95: 2044– 2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orffer S, Knox-Davies PS.1989. A canker and die-back disease of Protea repens. Phytophylactica 21: 189– 194 [Google Scholar]
- Paterson-Jones C.2000. The Protea family in Southern Africa. Struik Publishers (Pty) Ltd., Cape Town, South Africa [Google Scholar]
- Rayner RW.1970. A mycological colour chart. CMI and British Mycological Society; Kew, Surrey, England [Google Scholar]
- Rebelo T.2001. SASOL Proteas: A field guide to the Proteas of Southern Africa. Fernwood Press, Vlaeberg, South Africa [Google Scholar]
- Rong IH, Baxter AP.2006. The South African national collection of fungi: celebrating a centenary 1905–2005. Studies in Mycology 55: 1– 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget M, Richardson DM, Cowling RM.2003. The current configuration of protected areas in the Cape Floristic Region, South Africa – reservation bias and representation of biodiversity patterns and processes. Biological Conservation 112: 129– 145 [Google Scholar]
- Samuels GJ, Müller E, Petrini O.1987. Studies in the Amphisphaeriaceae (sensu lato) 3. New species of Monographella and Pestalosphaeria, and two new genera. Mycotaxon 28: 473– 499 [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1– 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, Wingfield MJ.1999. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia 91: 286– 298 [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW.2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1043– 1054 [DOI] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, et al. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105– 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K, Ritschel A, Braun U.2003. A monograph of Fusicladium s. lat. (hyphomycetes). Schlechtendalia 9: 1– 132 [Google Scholar]
- Seifert KA, Morgan-Jones G, Gams W, Kendrick B.2011. The genera of Hyphomycetes. CBS Biodiversity Series 9: 1–997. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands [Google Scholar]
- Shoemaker RA.1998. Marielliottia, a new genus of cereal and grass parasites segregated from Drechslera. Canadian Journal of Botany 76: 1558– 1569 [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, Mejía LC, White JF.2008. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1– 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soteros JJ.1986. New fungal disease in Leucospermum. Review of Plant Pathology 65: 616 [Google Scholar]
- Swart L, Crous PW, Denman S, Palm ME.1998. Fungi occurring on Proteaceae. I. South African Journal of Botany 64: 137– 145 [Google Scholar]
- Swart L, Crous PW, Kang J-C, Mchau GRA, Pascoe IA, Palm ME.2001. Differentiation of species of Elsinoë associated with scab disease of Proteaceae based on morphology, symptomatology, and ITS sequence phylogeny. Mycologia 93: 365– 379 [Google Scholar]
- Swart L, Crous PW, Petrini O, Taylor JE.2000. Fungal endophytes of Proteaceae, with particular emphasis on Botryosphaeria proteae. Mycoscience 41: 123– 127 [Google Scholar]
- Swart L, Taylor JE, Crous PW, Percival K.1999. Pestalotiopsis leaf spot disease of Proteaceae in Zimbabwe. South African Journal of Botany 65: 239– 242 [Google Scholar]
- Taylor JE.2001. Proteaceae pathogens: the significance of their distribution in relation to recent changes in phytosanitary regulations. Acta Horticulturae 545: 253– 264 [Google Scholar]
- Taylor JE, Crous PW.2000. Fungi occurring on Proteaceae. New anamorphs for Teratosphaeria, Mycosphaerella and Lembosia, and other fungi associated with leaf spots and cankers of Proteaceous hosts. Mycological Research 104: 618– 636 [Google Scholar]
- Taylor JE, Crous PW, Palm ME.2001a. Foliar and stem fungal pathogens of Proteaceae in Hawaii. Mycotaxon 78: 449– 490 [Google Scholar]
- Taylor JE, Crous PW, Swart L.2001b. Foliicolous and caulicolous fungi associated with Proteaceae cultivated in California. Mycotaxon 78: 75– 103 [Google Scholar]
- Taylor JE, Denman S, Crous PW.2001c. Endophytes isolated from three species of Protea in a nature reserve in the Western Cape, South Africa. Sydowia 53: 247– 260 [Google Scholar]
- Taylor JE, Lee S, Crous PW.2001d. Biodiversity in the Cape Floral Kingdom: Fungi occurring on Proteaceae. Mycological Research 105: 1480– 1484 [Google Scholar]
- Verkley GJM, Crous PW, Groenewald JZ, Braun U, Aptroot A.2004. Mycosphaerella punctiformis revisited: morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycological Research 108: 1271– 1282 [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M.1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238– 4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Roets F, Jacobs K.2009. A new species of Penicillium, P. ramulosum sp. nov., from the natural environment. Mycologia 101: 888– 895 [DOI] [PubMed] [Google Scholar]
- Vogts M.1982. South Africa’s Proteaceae: know them and grow them. Struik, Cape Town, South Africa [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor SB.1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego, California, USA [Google Scholar]
- Zalar P, Gostinčar C, Hoog GS de, Uršič V, Sudhadham M, Gunde-Cimerman N.2008. Redefinition of Aureobasidium pullulans and its varieties. Studies in Mycology 61: 21– 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Berbee ML.2001. Pyrenophora phylogenetics inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 93: 1048– 1063 [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, Gruyter J de, et al. 2009. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85– 102 [DOI] [PMC free article] [PubMed] [Google Scholar]