Abstract
Diaporthe (anamorph = Phomopsis) species are plant pathogens and endophytes on a wide range of hosts including economically important crops. At least four Diaporthe taxa occur on soybean and they are responsible for serious diseases and significant yield losses. Although several studies have extensively described the culture and morphological characters of these pathogens, their taxonomy has not been fully resolved. Diaporthe and Phomopsis isolates were obtained from soybean and other plant hosts throughout Croatia. Phylogenetic relationships were determined through analyses of partial translation elongation factor 1-alpha (EF1-α) gene and ITS nrDNA sequence data. By combining morphological and molecular data, four species could be distinguished on soybeans in Croatia. Diaporthe phaseolorum is described in this study and its synonyms are discussed. Diaporthe phaseolorum var. caulivora is raised to species status and the name Diaporthe caulivora is introduced to accommodate it. A species previously known as Phomopsis sp. 9 from earlier studies on sunflower, grapevine, rooibos and hydrangea is reported for the first time on soybean, and is formally described as Diaporthe novem. The well-known soybean pathogen Phomopsis longicolla was also collected in the present study and was transferred to Diaporthe longicolla comb. nov. The presence of these species on herbaceous hosts raises once more the relevance of weeds as reservoirs for pathogens of economically important plants.
Keywords: anamorph, EF1-α, ITS, mating-types, phylogeny, systematics, taxonomy, teleomorph
INTRODUCTION
Diaporthe species (and their Phomopsis anamorphs) are endophytes and pathogens on a wide range of hosts, and are responsible for several diseases, some of which are of economic importance. On soybean (Glycine max), Diaporthe species cause seed decay, stem blight and stem canker, resulting in significant yield and quality losses (Backman et al. 1985, Fernández et al. 1999). For example, these diseases are responsible for yield losses of up to 62 % in the former Yugoslavia, depending on the pathogen involved (Vidić & Jasnić 1998). Four Diaporthe taxa can be found on soybean: Diaporthe phaseolorum var. sojae, the causal agent of pod and stem blight; D. phaseolorum var. caulivora and D. aspalathi (formerly referred to as D. phaseolorum var. meridionalis), causal agents of Northern and Southern stem cankers, respectively; and Phomopsis longicolla, primarily causing seed decay. Separation of D. phaseolorum into varieties was based on morphological characters including colony appearance, size of stromata, arrangement of perithecia, presence of an anamorph, presence of alpha- and beta-conidia (Morgan-Jones 1985), symptomology and virulence on soybean (Sinclair & Backman 1989). Because of the variability found in morphology, physiology and host relationships in the D. phaseolorum complex, classification at varietal level is considered unsatisfactory (Morgan-Jones 1989).
Formerly, species in Diaporthe (and their Phomopsis anamorphs) were largely identified on host association. However, it is now recognised that the host is of minor importance in the taxonomy of these fungi (Rehner & Uecker 1994, Mostert et al. 2001). On the other hand, morphological characters are not always suitable for species definition because of their inter- and intra-species variability (van der Aa et al. 1990, Santos 2008). Currently, species of Diaporthe are distinguished mainly based on their molecular phylogenies, especially those derived from sequences of the internal transcribed spacer (ITS) of the nuclear ribosomal DNA (nrDNA) (van Niekerk et al. 2005, van Rensburg et al. 2006, Santos & Phillips 2009). Some authors have also used other genomic regions as molecular markers for phylogenetic analysis in Diaporthe and Phomopsis, such as the translation elongation factor 1-alpha (EF1-α) gene (e.g., van Rensburg et al. 2006, Santos et al. 2010).
The aim of this study was to determine the Diaporthe species that are found on soybean in Croatia, and to revise their taxonomic status in terms of molecular and morphological data.
MATERIALS AND METHODS
Isolates and morphology
Strains of Diaporthe and Phomopsis were isolated from stems and seeds of soybean as well as from stems of sunflower (Helianthus annuus), Arctium lappa, Asclepias syriaca and Dipsacus lacinatus from several localities throughout Croatia (Table 1). Small tissue pieces (10–15 mm long) were excised from diseased stems. These pieces, as well as symptomless soybean seeds, were washed with tap water, surface disinfected with 96 % ethanol for 10 s and 1 % sodium hypochlorite (NaOCl) for 1 min, rinsed three times with sterile distilled water, dried on filter paper and then placed on potato-dextrose agar (PDA, Difco Laboratories, Detroit, MI, USA) plates. Some tissue pieces were placed on moist filter paper inside a Petri dish. Petri dishes were kept at 25 °C under a 12 h light/dark regime. Developing mycelium or spores (ascospores or conidia) exuding from fruiting bodies were transferred to fresh PDA plates. Isolates were established by transferring hyphal tips from the edge of the developing mycelium to fresh PDA plates. Morphological characters were studied and recorded as in Santos & Phillips (2009). Reference isolates were deposited in the culture collection at Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands, and nomenclatural novelties in MycoBank (Crous et al. 2004). Mean, standard deviation (S.D.) and 95 % confidence intervals were calculated for asci, ascospores, alpha- and beta-conidia. Minimum and maximum dimensions are given in parentheses. In the case of asci, these values were rounded to the closest half micron.
Table 1.
Croatian isolates of Diaporthe spp. used in this study.
| GenBank accession numbers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Isolate no. 1 | Host | Source of isolation | Origin | Collector | EF1-α 2 | ITS 3 | MAT1-1-1 | MAT1-2-1 |
| D. caulivora | Dip1 | Dipsacus lacinatus | Stem | Bosnjaci | K. Vrandečić | HM347687 | HM347703 | (−) | (+) |
| Dip2 | D. lacinatus | Stem | Bosnjaci | K. Vrandečić | HM347689 | n.d. | (−) | (+) | |
| Dpc1 | |||||||||
| CBS 127268 | Glycine max | Stem | Osijek | K. Vrandečić | HM347691 | HM347712 | (−) | (+) | |
| Dpc4 | G. max | Stem | Klisa | K. Vrandečić | HM347690 | n.d. | (−) | (+) | |
| Dpc11 | G. max | Stem | Klisa | K. Vrandečić | HM347688 | HM347704 | (−) | (+) | |
| D. novem | 3/27-3-1 | G. max | Seed | Slavonija | T. Duvnjak | HM347697 | HM347706 | (−) | (+) |
| 4-27/3-1 | |||||||||
| CBS 127270 | G. max | Seed | Slavonija | T. Duvnjak | HM347696 | HM347709 | (−) | (+) | |
| 4-27/3-2 | G. max | Seed | Slavonija | T. Duvnjak | HM347698 | n.d. | n.d. | n.d. | |
| 5-27/3-1 | |||||||||
| CBS 127269 | G. max | Seed | Slavonija | T. Duvnjak | HM347693 | HM347708 | (+) | (−) | |
| 5/27/3-2 | G. max | Seed | Slavonija | T. Duvnjak | HM347694 | n.d. | (−) | (+) | |
| 5/27/3-3 | |||||||||
| CBS 127271 | G. max | Seed | Slavonija | T. Duvnjak | HM347695 | HM347710 | (+) | (−) | |
| AS1 | Asclepias syriaca | Stem | Slavonija | K. Vrandečić | HM347692 | n.d. | (−) | (+) | |
| D. phaseolorum | Ar2 | Arctium lappa | Stem | Osijek | K. Vrandečić | HM347679 | HM347705 | (+) | (+) |
| PS03 | G. max | Stem | Sopot | K. Vrandečić | HM347670 | HM347702 | (+) | (+) | |
| PS06 | G. max | Stem | Osijek | K. Vrandečić | HM347669 | n.d. | (+) | (+) | |
| PS8 | G. max | Stem | Sopot | K. Vrandečić | HM347671 | HM347701 | (+) | (+) | |
| PSu1 | |||||||||
| CBS 127266 | Helianthus annuus | Stem | Ostrovo | K. Vrandečić | HM347672 | HM347707 | (+) | (+) | |
| PSu5 | H. annuus | Stem | Ostrovo | K. Vrandečić | HM347678 | n.d. | (+) | (+) | |
| PSuB9 | H. annuus | Stem | Bosnjaci | K. Vrandečić | HM347675 | n.d. | (+) | (+) | |
| PSuS3 | H. annuus | Stem | Sapci | K. Vrandečić | HM347676 | n.d. | (+) | (+) | |
| PSuS4 | H. annuus | Stem | Sapci | K. Vrandečić | HM347677 | n.d. | (+) | (+) | |
| Su9 | H. annuus | Stem | Opatovac | K. Vrandečić | HM347673 | GQ149763 | (+) | (+) | |
| Su10 | H. annuus | Stem | Ilok | K. Vrandečić | HM347674 | GQ149764 | (+) | (+) | |
| D. longicolla | 1-2/4-1 | G. max | Seed | Slavonija | T. Duvnjak | HM347680 | n.d. | (+) | (+) |
| 1-2/4-2 | G. max | Seed | Slavonija | T. Duvnjak | HM347681 | n.d. | n.d. | n.d. | |
| 1-2/4-3 | G. max | Seed | Slavonija | T. Duvnjak | HM347682 | HM347711 | (+) | (+) | |
| 3-27/3-2 | G. max | Seed | Slavonija | T. Duvnjak | HM347684 | n.d. | (+) | (+) | |
| Pl1a | G. max | Stem | Sopot | K. Vrandečić | HM347686 | n.d. | (+) | (+) | |
| PL4 | |||||||||
| CBS 127267 | G. max | Stem | Sopot | K. Vrandečić | HM347685 | HM347700 | (+) | (+) | |
| PL7 | G. max | Stem | Karanac | K. Vrandečić | HM347683 | HM347699 | (+) | (+) | |
1 CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. All isolates are deposited in the culture collection housed at Centro de Recursos Microbiológicos, Caparica, Portugal. Ex-type cultures are listed in bold.
2 EF1-α: translation elongation factor 1-alpha.
3 ITS: internal transcribed spacer.
(−): Negative PCR result; (+): Positive PCR result; n.d.: not determined.
DNA extraction and amplification
Genomic DNA was extracted and part of the EF1-α gene was amplified for all isolates (Table 1) as described in Santos et al. (2010). The ITS-D1/D2 nrDNA region of a subset of isolates was amplified following the methods described by Santos & Phillips (2009). PCR diagnosis of mating-types (MAT1-1 and MAT1-2) was done using primers MAT1-1-1FW, MAT1-1-1RV, MAT1-2-1FW and MAT1-2-1RV following the protocol of Santos et al. (2010). All PCR products were visualised under ultraviolet light in agarose gels stained with GelRed™ Nucleic Acid Gel Stain (Biotium, Inc., Hayward, CA, USA) at a final concentration of 0.25×.
Sequence analysis
Amplicon purification and sequencing, sequence editing and dataset assembling followed the protocols of Santos et al. (2010). Phylogenetic trees were inferred in MrBayes v. 3.0b4 (Ronquist & Huelsenbeck 2003) by Bayesian analysis and in PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2002) by Maximum Parsimony (MP) using the heuristic search option with random addition of sequences (1 000 replications), tree bisection-reconnection (TBR) and MULTREES options ON. Bayesian analysis was performed as described in Phillips et al. (2007), setting burn-in at 2 000 generations. Bootstrap support values with 1 000 replications (Felsenstein 1985) were calculated for branches in the MP trees using MULTREES option OFF and 10 random sequence additions in each of 1 000 pseudoreplicates. Sequences obtained from GenBank are listed by their taxon names followed by accession numbers in the trees (Fig. 1, 2), while newly generated sequences are listed by their isolate number. Newly generated sequences have been deposited in GenBank (Table 1) and alignments and phylogenies in TreeBASE (Study S10556; Matrices M5715/M5716 = EF1-α, M5718/M5719 = ITS).
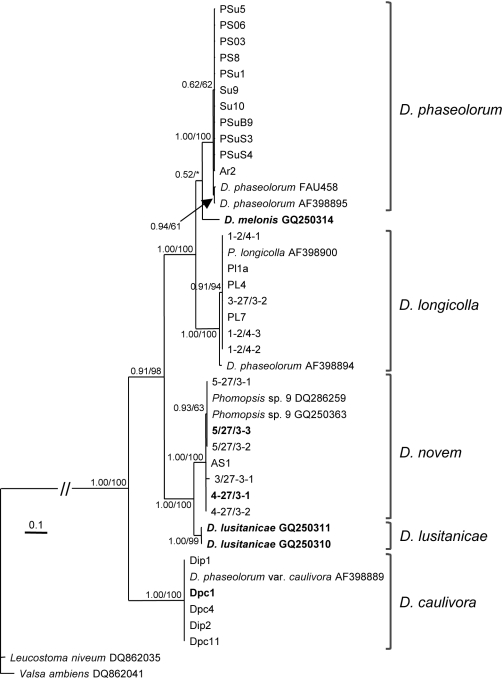
Fig. 1.
Phylogenetic tree resulting from a Bayesian analysis of 387 characters of EF1-α gene (including coded indels). Newly generated sequences are listed by their isolate number. Ex-type cultures are in bold. Bayesian posterior probabilities followed by MP bootstrap values with 1 000 replications are shown at the nodes. The tree was rooted to Leucostoma niveum (DQ862035) and Valsa ambiens (DQ862041). Scale bar represents 0.1 substitutions per site. Phylogeny deposited in TreeBASE (S10556, M5715/M5716).
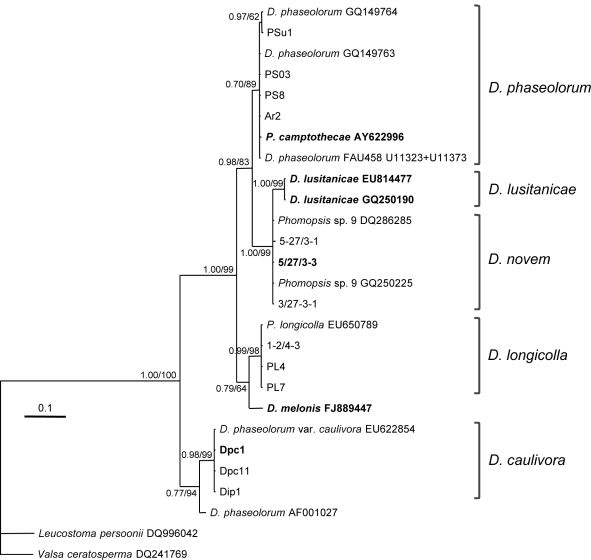
Fig. 2.
Phylogenetic tree resulting from a Bayesian analysis of 443 characters of the ITS nrDNA region (including coded indels). Newly generated sequences are listed by their isolate number. Ex-type cultures are in bold. Bayesian posterior probabilities followed by MP bootstrap values with 1 000 replications are shown at the nodes. The tree was rooted to Leucostoma persoonii (DQ996042) and Valsa ceratosperma (DQ241769). Scale bar represents 0.1 substitutions per site. Phylogeny deposited in TreeBASE (S10556, M5718/M5719).
RESULTS
EF1-α phylogeny
Since Santos et al. (2010) suggested that EF1-α is a better phylogenetic marker in Diaporthe and Phomopsis than ITS, we started by sequencing part of the EF1-α gene, spanning an entire intron and partial sequence of the flanking exons, for all isolates under study. BLAST searches were done to select closely related sequences from GenBank. The sequence of Diaporthe phaseolorum isolate FAU458 was not available from GenBank and was retrieved from TreeBASE study S1506 (van Rensburg et al. 2006). The alignment consisted of 42 taxa (including two outgroups) and 387 characters (including alignment gaps and indel coding). The phylogenetic analysis was done by Bayesian analysis and Maximum Parsimony (MP). Of the 387 characters, 285 were parsimony informative and included in the MP analysis resulting in two equally parsimonious trees. The Bayesian analysis phylogram (Fig. 1) showed a similar topology and clades as the MP phylogenetic trees. Bayesian posterior probabilities followed by MP bootstrap values are shown at the nodes.
Five clades were formed, corresponding to four different species found mainly on soybean, but including other hosts (sunflower, Arctium lappa, Asclepias syriaca and Dipsacus lacinatus) and Diaporthe lusitanicae from Foeniculum vulgare (sequences retrieved from GenBank). The first four clades consist of D. phaseolorum, D. longicolla, D. novem and D. lusitanicae, respectively. All these clades are supported by Bayesian posterior probabilities of 1.00. Diaporthe phaseolorum, D. longicolla and D. novem are supported by MP bootstrap values of 100 %, while D. lusitanicae is supported by a MP bootstrap value of 99 %. The fifth clade consists of D. caulivora with Bayesian posterior probability of 1.00 and MP bootstrap support of 100 %. Although closely related to the taxa found on soybean, D. melonis, represented by its ex-isotype isolate, is shown to be a distinct species.
ITS phylogeny
Since there are many Diaporthe species for which only ITS sequences are available, we decided to further confirm the identity of the species found on soybean by means of ITS sequencing. Therefore, representative isolates of each EF1-α clade were selected for another phylogenetic analysis based on ITS sequences. The ITS nrDNA region of these isolates was sequenced and BLAST searches were done to select closely related sequences. An alignment comprising 27 taxa (including two outgroups) and 443 characters (including alignment gaps and indel coding) and spanning ITS1 and ITS2 complete sequences as well as 18S and 28S partial sequences was included in a phylogenetic analysis using Bayesian analysis and Maximum Parsimony (MP). The sequence of the 5.8S nrDNA gene of D. phaseolorum isolate FAU458 was not available and this region was excluded from all isolates in the analysis. Of the 443 characters, 101 were parsimony informative and included in the MP analysis resulting in a single tree. The Bayesian analysis phylogram (Fig. 2) showed largely the same topology and clades as the MP analysis. Bayesian posterior probabilities followed by MP bootstrap values are shown at the nodes.
Although the phylogenetic relationships between D. novem, D. phaseolorum and D. longicolla are depicted differently in this tree, the same clades can be distinguished as in the EF1-α tree (Fig. 1). The first clade consists of D. phaseolorum with Bayesian posterior probability of 0.70 and MP bootstrap support of 89 %. The ex-type isolate of Phomopsis camptothecae clustered together with this species. The second clade consists of D. lusitanicae (Bayesian posterior probability of 1.00 and MP bootstrap support of 99 %) and the third clade consists of D. novem. The separation between D. lusitanicae and D. novem is not completely evident in this tree, since a Bayesian posterior probability of 1.00 and a MP bootstrap value of 99 % support a single branch that includes both species. However, in the MP tree, a further dichotomic subdivision of this branch is observed, thus unambiguously separating them (not shown). Diaporthe lusitanicae and D. novem are, in this phylogenetic method, supported by bootstrap values of 99 and 64 %, respectively. The fourth clade consists of D. longicolla with Bayesian posterior probability of 0.99 and MP bootstrap support of 98 %. Finally, the fifth clade consists of D. caulivora (Bayesian posterior probability of 0.98 and MP bootstrap support of 99 %). As in Fig. 1, D. melonis clusters as a distinct species based on ITS sequences.
Mating-type diagnosis
Santos et al. (2010) developed degenerate primers for the mating-type diagnosis of Diaporthe and Phomopsis isolates by PCR. This approach was used in the present study to narrow down the number of crossings between isolates necessary to induce teleomorphs in culture. The PCR diagnosis revealed that all D. phaseolorum isolates under study have both MAT1-1-1 and MAT1-2-1 genes (Kanematsu et al. 2007) and are therefore homothallic. This is in accordance with the observation that D. phaseolorum isolates are self-fertile. Diaporthe novem was also shown to have both mating-types but in separate isolates, thus indicating that this is a heterothallic species. This implied that the teleomorph of D. novem could only be observed when isolates of opposite mating-types were crossed in culture. Diaporthe caulivora is also shown to be self-fertile. However, only the MAT1-2 locus could be detected in all isolates. Finally, the PCRs revealed that the tested D. longicolla isolates possess both mating-types, as in the case of D. phaseolorum.
TAXONOMY
Diaporthe caulivora (Athow & Caldwell) J.M. Santos, Vrandečić & A.J.L. Phillips, comb. & stat. nov. — MycoBank MB518520; Fig. 3
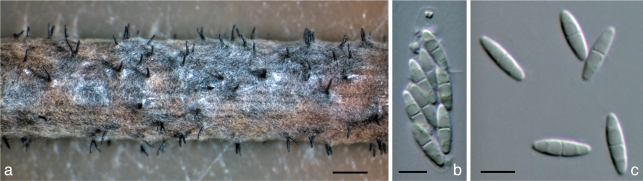
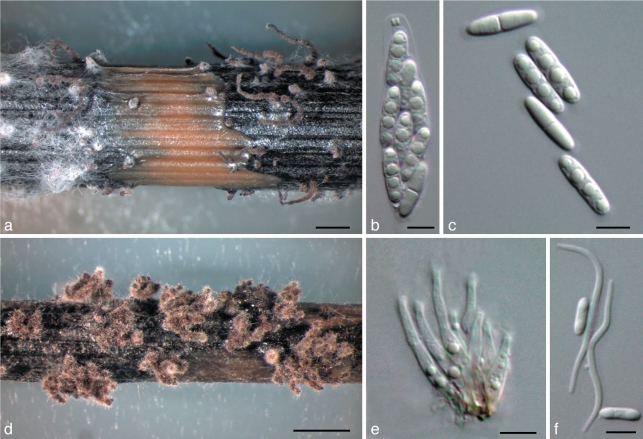
Fig. 3.
Diaporthe caulivora. a. Perithecia on F. vulgare stem in culture (CBS H-20461); b. ascus with 8 ascospores (Dpc1); c. ascospores (Dpc1). — Scale bars: a = 1 mm; b, c = 5 μm. Ex-type culture is in bold.
Basionym. Diaporthe phaseolorum var. caulivora Athow & Caldwell, Phytopathology 44: 323. 1954.
Perithecia on Foeniculum vulgare stems in culture, globose, 230–310 μm in its widest diam, single or clustered in groups of 2–3 perithecia. Black, smooth, straight necks, 330–520 μm. Asci unitunicate, (24.0–)29.5–31.0(–35.0) × (6.5–)8.0–9.0 (–10.5) μm, mean ± S.D. = 30.2 ± 2.1 × 8.3 ± 1.0 μm (n = 20), ellipsoid, widest at centre and rounded towards the apices, with conspicuous refractive apical ring, 8-spored. Ascospores barely biseriate, hyaline, smooth, (8.2–)8.9–9.2(–10.1) × (2.2–)2.4–2.5(–2.9) μm, mean ± S.D. = 9.0 ± 0.5 × 2.5 ± 0.1 μm (n = 50), ellipsoid to fusoid, medianly septate, non constricted, 4-guttules, 2 in each cell, central ones widest, although normally this characteristic is not easily seen. Anamorph not seen.
Sexuality — Homothallic.
Known hosts — Abutilon theophrasti (Vrandečić et al. 2005), Dipsacus lacinatus and Glycine max (Dunleavey 1955, Hobbs et al. 1981, Jasnić & Vidić 1981, Miller & Roy 1982, Krausz & Fortnum 1983, Grand 1985, Kulik & Thomison 1985, Whitney & Bowers 1985, Ginns 1986, Hirrel & Kirkpatrick 1986, Black et al. 1996, Zhang et al. 1997, Pioli et al. 2001, Bradley & Li 2006, Costamilan et al. 2008).
Distribution — Argentina (Pioli et al. 2001), Brazil (Costamilan et al. 2008), Canada (Ginns 1986), Croatia (Vrandečić et al. 2005), Italy (Zhang et al. 1997), USA – Arkansas (Hirrel & Kirkpatrick 1986), Iowa (Dunleavey 1955), Louisiana (Black et al. 1996), Maryland (Kulik & Thomison 1985), Michigan and Ohio (Hobbs et al. 1981), Mississippi (Miller & Roy 1982), North Carolina (Grand 1985), North Dakota (Bradley & Li 2006), South Carolina (Krausz & Fortnum 1983) and Texas (Whitney & Bowers 1985) – and former Yugoslavia (Jasnić & Vidić 1981).
Specimens examined. See Table 1 for isolates studied. Croatia, Osijek, on soybean stem, Sept. 2005, K. Vrandečić, NEOTYPE proposed herein CBS H-20461 (perithecia on Foeniculum vulgare stems in culture), culture ex-neotype Dpc1 = CBS 127268.
Notes — All attempts to induce anamorphic sporulation in culture were unsuccessful. Although only the MAT1-2 mating-type could be detected by PCR in all isolates studied, fertile perithecia formed in pure cultures. Therefore, this species is considered to be homothallic. This species is similar to D. phaseolorum but differs in having slightly wider perithecia with longer necks and shorter asci. The most distinctive character that separates these species is the production of conidiomata: while D. phaseolorum regularly produces pycnidia with alpha- and beta-conidia, this is reported to be extremely rare in D. caulivora. This pathogen is the cause of Northern stem canker of soybean. Kulik (1984) could not locate the type specimen of D. phaseolorum var. caulivora, and our search for the type was also unsuccessful. Therefore we propose CBS H-20461 as neotype.
Diaporthe longicolla (Hobbs) J.M. Santos, Vrandečić & A.J.L. Phillips, comb. nov. — MycoBank MB563213; Fig. 4
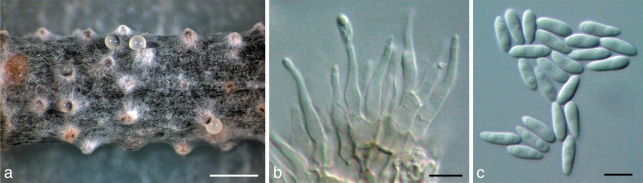
Fig. 4.
Diaporthe longicolla. a. Pycnidia on F. vulgare stem in culture (CBS H-20460); b. conidiophores (PL4); c. alpha-conidia (PL4). — Scale bars: a = 1 mm; b, c = 5 μm.
Phomopsis longicolla Hobbs, Mycologia 77: 542. 1985.
Conidiomata on Foeniculum vulgare stems in culture pycnidial, cone-shaped, 390–540 μm diam, mostly solitary. Pycnidial necks prominent, 290–790 μm long. Conidiophores subcylindrical to cylindrical, hyaline, smooth, 1–3-celled, simple or most commonly branched, 6.8–12.3 × 2.1–5.2 μm. Conidiogenous cells phialidic, cylindrical, tapering towards the apex, periclinal thickening present but not conspicuous. Collarette not seen. Conidiogenous cells terminal, attached to the apex of conidiophores, 8.4–14.2 × 1.6–2.8 μm, and lateral, produced from the main axis of conidiophore but showing a lateral prolongation, 3.7–5.6 × 1.1–1.5 μm. Alpha-conidia unicellular, ellipsoid to ovoid (5.9–)6.9–7.2(–8.1) × (1.8–)2.1–2.2(–2.4) μm, mean ± S.D. = 7.1 ± 0.6 × 2.1 ± 0.1 μm (n = 50), hyaline, biguttulate, exuding from the pycnidial ostiole in a yellowish, translucent drop. Beta-conidia not seen.
Sexuality — Asexual.
Known hosts — Abutilon theophrasti (Vrandečić et al. 2004), Ambrosia trifida, Euphorbia maculata, Rumex crispus and Xanthium strumarium (Roy et al. 1997), Arachis hypogaea (Sanogo & Etarock 2009), Aster exilis, Caperonia palustris, Desmanthus illinoensis, Eclipta prostrata, Euphorbia nutans, Ipomoea lacunose, Polygonum aviculare and Sida spinosa (Mengistu & Reddy 2005), Chamaesyce nutans (Mengistu et al. 2007) and Glycine max (Hobbs et al. 1985, Vidić et al. 1996, Zhang et al. 1997, Holevas et al. 2000, Ash et al. 2010).
Distribution — Australia (Ash et al. 2010), Croatia (Vrandečić et al. 2004), Greece (Holevas et al. 2000), New Mexico (Sanogo & Etarock 2009), USA – Arkansas and Missouri (Zhang et al. 1997) and Illinois, Iowa, Mississippi and Ohio (Hobbs et al. 1985) – and former Yugoslavia (Vidić et al. 1996).
Specimens examined. See Table 1 for isolates studied. Croatia, Sopot, on soybean stem, Sept. 2005, K. Vrandečić, Herb. CBS H-20460 (pycnidia on Foeniculum vulgare stems in culture), isolate PL4 = CBS 127267.
Notes — Holotype on seed of Glycine max, Wooster, Ohio, USA (TWH P74 = BPI 358745). Ex-type culture deposited in ATCC (ATCC 60325) (Hobbs 1985). Although all of the isolates we studied possessed both mating-types, as determined by PCR diagnosis, no fertile perithecia formed under any conditions. Therefore, this species is considered to be purely anamorphic. This species differs from D. novem mainly in having solitary pycnidia producing only alpha-conidia. It differs from D. phaseolorum in having larger pycnidia and longer conidiophores and conidiogenous cells, as well as in producing only alpha-conidia. This pathogen is the main cause of seed decay of soybean.
Diaporthe novem J.M. Santos, Vrandečić & A.J.L. Phillips, sp. nov. — MycoBank MB518521; Fig. 5
Fig. 5.
Diaporthe novem. a. Perithecia on F. vulgare stem in culture (CBS-H 20463); b. ascus with 8 ascospores (4-27/3-1 × 5/27/3-3); c. ascospores (4-27/3-1 × 5/27/3-3); d. pycnidia on F. vulgare stem in culture (CBS H-20462); e. conidiophores (5-27/3-1); f. alpha- and beta-conidia (5-27/3-1). — Scale bars: a, d = 1 mm; b, c, e, f = 5 μm. Ex-type cultures are in bold.
Anamorph. Phomopsis sp. 9 (van Rensburg et al. 2006).
Peritheciis in agar cum Foeniculum vulgare stipes: globosis, 320–440 μm diam, separatus, collo apicali erumpenti usque 2250 μm longo praeditis. Ascis unitunicatis, 38.5–43 × 6.5–10.5 μm, clavulatis, hyalinis, octosporis, cum annulis apicalibus distingentibus, refractis. Ascosporis 8.6–13.1 × 2.1–3.6 μm, uniseriatis vel biseriatis, hyalinis, bicellularibus, cylindricis, ad septum constrictis, 4-guttulatis. Anamorphosis in agar cum Foeniculum vulgare stipes: conidiomata eustromatica, ad pycnidia, tubineus, 310–580 μm diam. Conidiophoris cylindricis, 5.3–10.4 × 1.9–3.2 μm. Cellulae conidiogenae hyalinae, phialidicae, enteroblasticae, terminus, 8.9–16 × 1.7–2.7 μm, et lateralis, 4–7.3 × 1.2–1.7 μm. Alpha conidiis hyalinis, ovatis vel cylindricis, unicellularibus, 6.3–8.9 × 1.9–2.5 μm. Beta conidiis hyalinis, filiformis, unicellularibus, 26.4–37.7 × 1–1.3 μm.
Etymology. Latin for nine, the name by which this species has been known since 2006 (van Rensburg et al. 2006), namely Phomopsis sp. 9.
Perithecia on Foeniculum vulgare stems in culture, globose, 320–440 μm in its widest diam, scattered within black ectostromatic areas. Black, long necks, 1600–2250 μm, hairy, filiform and frequently branched. Asci unitunicate, (38.5–)40.5–41.5 (–43.0) × (6.5–)8.5–9.0(–10.5) μm, mean ± S.D. = 40.8 ± 1.2 × 8.8 ± 1.0 μm (n = 20), clavate, with visible refractive apical ring, 8-spored. Ascospores randomly arranged, from loosely uniseriate to biseriate, hyaline, smooth, (8.6–)10.6–11.1(–13.1) × (2.1–)2.8–2.9(–3.6) μm, mean ± S.D. = 10.8 ± 0.9 × 2.8 ± 0.3 μm (n = 50), cylindrical, medianly septate, sometimes constricted at the septum, normally 4-guttulate, sometimes eguttulate, when 4-guttulate, the 2 central guttules, closer to the septum, are the largest. Conidiomata formed on Foeniculum vulgare stems in culture pycnidial, cone-shaped, 310–580 μm diam. Pycnidial necks hairy, 380–710 μm long, arranged in clusters. Yellowish translucent conidial drop exuded from the ostiole. Conidiophores cylindrical, hyaline, smooth, uni- to bicellular, 5.3–10.4 × 1.9–3.2 μm. Conidiogenous cells phialidic, clavate to filiform, tapering towards the apex, periclinal thickening present. Collarette not seen. Conidiogenous cells terminal, attached to the apex of conidiophores, 8.9–16.0 × 1.7–2.7 μm, and lateral, belonging to the main axis of conidiophore but showing a lateral prolongation, 4.0–7.3 × 1.2–1.7 μm. Alpha-conidia unicellular, oval to cylindrical, with obtuse ends, (6.3–)7.3–7.5(–8.9) × (1.9–)2.1–2.2(–2.5) μm, mean ± S.D. = 7.4 ± 0.6 × 2.2 ± 0.1 μm (n = 100), hyaline, biguttulate. Beta-conidia hyaline, aseptate, filiform, curved, eguttulate, with rounded ends, (26.4–)32.0–33.3(–37.7) × (1.0–)1.1(–1.3) μm, mean ± S.D. = 32.6 ± 2.4 × 1.1 ± 0.1 μm (n = 50).
Sexuality — Heterothallic.
Known hosts — Asclepias syriaca, Aspalathus linearis (van Rensburg et al. 2006), Glycine max, Helianthus annuus (Rekab et al. 2004), Hydrangea macrophylla (Santos et al. 2010) and Vitis vinifera (van Niekerk et al. 2005).
Distribution — Croatia, Italy (Rekab et al. 2004), Portugal (Santos et al. 2010) and South Africa (van Niekerk et al. 2005, van Rensburg et al. 2006).
Specimens examined. See Table 1 for isolates studied. Croatia, Slavonija, on soybean seed, Sept. 2008, T. Duvnjak, holotype CBS H-20463 (pycnidia and perithecia on Foeniculum vulgare stems in culture obtained from cross between CBS 127270 and CBS 127271), cultures ex-type 4-27/3-1 = CBS 127270 (MAT1-2) and 5/27/3-3 = CBS 127271 (MAT1-1); Herb. CBS H-20462 (isolate 5-27/3-1 = CBS 127269).
Notes — Fertile perithecia formed in culture only after crossing isolates with opposite mating-types, as diagnosed by PCR. Therefore, this species is heterothallic. This species is similar to D. chailletii but has much longer perithecial necks, shorter and wider asci, and cylindrical ascospores in contrast to the fusoid-ellipsoid ascospores of D. chailletii.
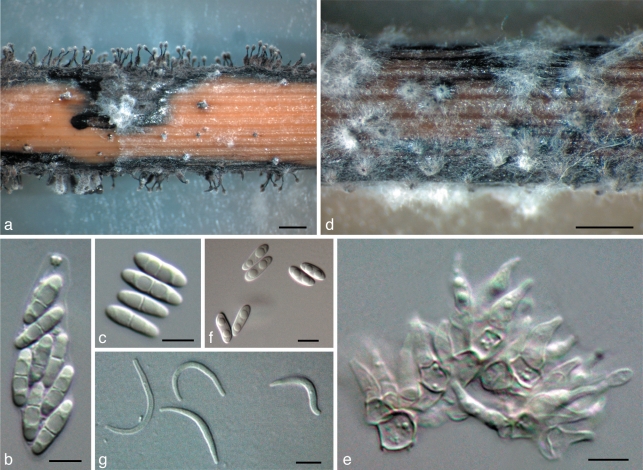
Diaporthe phaseolorum (Cooke & Ellis) Sacc., Syll. Fung. 1: 692. 1882 — Fig. 6
Fig. 6.
Diaporthe phaseolorum. a. Perithecia on F. vulgare stem in culture (CBS-H 20459); b. ascus with 8 ascospores (PSu1); c. ascospores (PSu1); d. pycnidia on F. vulgare stem in culture (PS03); e. conidiophores (PSu1); f. alpha-conidia (PS03); g. beta-conidia (PS03). — Scale bars: a, d = 1 mm; b, c, e, f, g = 5 μm.
Basionym. Sphaeria phaseolorum Cooke & Ellis, Grevillea 6: 93. 1878.
= Diaporthe phaseolorum var. phaseolorum (Cooke & Ellis) Sacc., Syll. Fung. 1: 692. 1882.
= Diaporthe phaseolorum var. sojae (Lehman) Wehm., The genus Diaporthe Nitschke and its segregates: 47. 1933.
≡ Diaporthe sojae Lehman, Ann. Mo. Bot. Gdn. 10: 128. 1923.
= Diaporthe phaseolorum var. batatae (Harter & Field) Wehm., The genus Diaporthe Nitschke and its segregates: 48. 1933.
≡ Diaporthe batatas Harter & E.C. Field, Phytopathology 2: 121. 1912.
= Septomazzantia phaseolorum (Cooke & Ellis) Lar.N. Vassiljeva, Nizshie Rasteniya, Griby i Mokhoobraznye Dalnego Vostoka Rossii 4: 44. 1998.
Anamorph. Phomopsis phaseoli (Desm.) Sacc., Nuovo Giorn. Bot. Ital., n.s. 22: 47. 1915.
Basionym. Phoma phaseoli Desm., Ann. Sci. Nat., Ser. 3, 6: 247. 1836.
= Phoma subcircinata Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 45: 158. 1893.
= Phomopsis batatas (Ellis & Halst.) Harter & E.C. Field, Phytopathology 2: 121. 1912.
≡ Phoma batatas Ellis & Halst., New Jersey Agric. Coll. Exp. Sta. Bull. 76: 25. 1890.
= Phomopsis phaseoli var. phaseoli (Desm.) Sacc., Nuovo Giorn. Bot. Ital., n.s. 22: 47. 1915.
≡ Phomopsis phaseoli (Desm.) Grove, Bull. Misc. Inform. Kew 60. 1917.
= Phomopsis phaseoli Petch, Ann. Roy. Bot. Gard. (Peradeniya) 7: 311. 1922.
= Phomopsis sojae Lehman, J. Elisha Mitchell Sci. Soc. 38: 13. 1922.
= Phomopsis glycines Petr., Ann. Mycol. 34: 240. 1936.
= Phomopsis camptothecae C.Q. Chang, Z.D. Jiang & P.K. Chi, Mycosystema 24: 145. 2005.
Perithecia on Foeniculum vulgare stems in culture, black, globose, 230–270 μm in its widest diam, scattered in high numbers within black ectostromatic areas. Necks, 210–430 μm long, filiform, with hairy and dilated tip. Asci unitunicate, (32.5–)34.5–36.0(–38.0) × (6.5–)7.5–8.5(–9.5) μm, mean ± S.D. = 35.1 ± 1.8 × 8.0 ± 0.9 μm (n = 20), ellipsoid, with refractive apical ring, 8-spored. Ascospores randomly arranged within the ascus, rarely biseriate, hyaline, smooth, (8.0–)9.3–9.7 (–11.3) × (2.0–)2.4–2.6(–3.0) μm, mean ± S.D. = 9.5 ± 0.7 × 2.5 ± 0.2 μm (n = 50), ellipsoid, medianly septate, not constricted, apical cell sometimes slightly wider, each cell biguttulate, the guttules closest to the septum being the largest. Conidiomata on Foeniculum vulgare stems in culture pycnidial, globose to subglobose, 140–170 × 150–340 μm. Conidiophores cylindrical, hyaline, smooth, unicellular, 2.5–6.0 × 2.2–5.8 μm, bearing 1–2 conidiogenous cells. Conidiogenous cells phialidic, clavate, tapering towards the apex, 6.0–8.8 × 2.0–3.8 μm. Collarette not seen. Alpha-conidia unicellular, ovoid, (5.4–)6.7–7.0(–8.9) × (1.8–)2.4(–3.1) μm, mean ± S.D. = 6.9 ± 0.7 × 2.4 ± 0.2 μm (n = 100), hyaline, biguttulate, conidiogenous scar sometimes visible. Beta-conidia hyaline, aseptate, filiform, bent, eguttulate, wider at the centre and thinner towards the ends, (13.3–)17.1–17.8(–22.5) × (0.9–)1.2(–1.5) μm, mean ± S.D. = 17.4 ± 2.0 × 1.2 ± 0.1 μm (n = 100).
Sexuality — Homothallic.
Known hosts — Abutilon theophrasti (Hepperly et al. 1980), Allium cepa, Allium sativum, Arachis hypogaea, Capsicum frutescens, Hibiscus esculentus, Lespedeza sp., Lupinus hirsutus, Lycopersicon esculentum, Phaseolus vulgaris, Strophostyles helvola and Vigna sinensis (Hanlin 1963), Amaranthus spinosus and Leonorus sibiricus (Cerkauskas et al. 1983), Arctium lappa, Camptotheca acuminata (Chang et al. 2005), Capsicum annuum (Pennycook 1989), Glycine max (Petch 1922, Preston 1945, Gilman 1949, Johnson & Kilpatrick 1953, Gerdemann 1954, Peregrine & Siddiqi 1972, Kmetz et al. 1974, Gorter 1977, Ersek 1978, Ebbels & Allen 1979, Bernaux 1981, Kozireva et al. 1982, Alfieri et al. 1984, Jasnić & Vidić 1985, Richardson 1990, Dahal et al. 1992, Zhang et al. 1997), Helianthus annuus (Vrandečić et al. 2009), Ipomoea batatas, Phaseolus lunatus, Phaseolus sp., Solanum tuberosum and Zea mays (Wehmeyer 1933), Kandelia candel (Cheng et al. 2008) and Stokesia laevis (Sogonov et al. 2008).
Distribution — Argentina, Brazil, Canada, Italy, Puerto Rico, Senegal and Taiwan (Richardson 1990), Bermuda, China, Colombia, Cuba, Egypt, Guyana, India, Israel, Japan, New Zealand, Nigeria, Sierra Leone and Zaire (Punithalingam & Holliday 1972), Croatia (Vrandečić et al. 2009), France (Bernaux 1981), Hungary (Ersek 1978), Korea (Zhang et al. 1997), Malawi (Peregrine & Siddiqi 1972), Nepal (Dahal et al. 1992), Russia (Kozireva et al. 1982), South Africa (Gorter 1977), Sri Lanka (Petch 1922), Tanzania (Ebbels & Allen 1979), USA – Arkansas (Zhang et al. 1997), Florida (Alfieri et al. 1984), Georgia (Hanlin 1963), Illinois (Gerdemann 1954), Indiana, Louisiana, New Jersey and North Carolina (Wehmeyer 1933), Iowa (Gilman 1949), Kentucky and Minnesota (Richardson 1990), Mississippi (Johnson & Kilpatrick 1953), Ohio (Kmetz et al. 1974) and Oklahoma (Preston 1945) – and former Yugoslavia (Jasnić & Vidić 1985).
Specimens examined. See Table 1 for isolates studied.
Sphaeria phaseolorum: USA, New Jersey, Newfield, on old bean stalks, 4 Aug. 1877, J.B. Ellis, isotype Fungi of New Jersey 2651, NY00875162; on old bean vines, July 1888, J.B. Ellis, North American Fungi 188, NY00875150.
Diaporthe phaseolorum: Croatia, Ostrovo, on Helianthus annuus stem, Aug. 2006, K. Vrandečić, Herb. CBS H-20459 (pycnidia and perithecia on Foeniculum vulgare stems in culture), isolate PSu1 = CBS 127266. – USA, New Jersey, Newfield, on old bean vines, July 1896, J.B. Ellis, Ellis & Everhart Fungi Columbiani 1044, CM1278 in NY; on old bean vines, Oct. 1890, J.B. Ellis, NY00875160; on dead stems of Phaseolus, Aug. 1890, J.B. Ellis, NY00875161; Baptist Hill, Conway, Massachusetts, on old stems of Solanum dulcamara, Mar. 1979, ME Barr, MEBB6539 in NY. – Venezuela, trail from El Rincon east along Rio Media to peak of Palo de Agua, Edo. Sucre, on unidentified wood, July 1972, KP Dumont, RF Cain, GJ Samuels, G. Morillo, det. ME Barr Biglow, Dumont-VE 5079 in NY; vicinity of El Arado, c. 12 km SW of Macarao, Edo. Miranda, on unidentified composite stem, July 1972, KP Dumont, RF Cain, GJ Samuels, G. Morillo, Dumont-VE 6319 in NY.
Notes — All isolates studied possessed both mating-types as determined by PCR diagnosis. Fertile perithecia formed in pure cultures and therefore this species is homothallic. Although similar to D. caulivora, these species show some morphological differences (see above). Another similar species is D. aspalathi, as indicated by van Rensburg et al. (2006), which has wider perithecia, longer and cylindrical asci, longer ascospores and only produces fusoid alpha-conidia. This pathogen has a very wide host range, but on soybean it causes pod and stem blight.
Key to Diaporthe species on soybean
1. Self-infertile 2
1. Self-fertile 3
2. Teleomorph produced in crosses of opposite mating-types D. novem
2. Both mating-types present but teleomorph never produced D. longicolla
3. Anamorph rarely seen in culture D. caulivora
3. Anamorph and teleomorph form readily in culture 4
4. Asci 34.5–36 × 7.5–8.5 μm, ascospores 9.3–9.7 × 2.4–2.6 μm D. phaseolorum
4. Asci 52–55 × 7–8 μm, ascospores 13–15 × 3 μm D. aspalathi
DISCUSSION
Phylogenetic analyses based on EF1-α and ITS sequence data revealed four species of Diaporthe on soybean in Croatia. Three of these (D. phaseolorum, D. caulivora and D. longicolla) are well-known from soybean, while D. novem has thus far never been recorded on this host. Not only were the species phylogenetically distinct, but they could also be distinguished based on their morphology, mating behaviour and on the disease that they cause.
The species of Diaporthe occurring on soybean has been the subject of a considerable amount of research and discussion. Much of the discussion has been aimed at the subdivision of D. phaseolorum into varieties or formae speciales. Although there have been arguments that classification at varietal level is not satisfactory (Morgan-Jones 1989), this system has persisted. The phylogenetic information that we supply here, together with the morphological differences and pathology of the varieties on soybean (Backman et al. 1985) adds further weight to the arguments against varietal separation, and we have proposed certain taxonomic changes. One of the varieties (D. phaseolorum var. meridionalis) has already been shown to be phylogenetically distinct and was elevated by van Rensburg et al. (2006) to species status. The name D. aspalathi was newly introduced since D. meridionalis was already occupied.
One factor that has hampered research on D. phaseolorum complex is the lack of isolates directly linked to the type specimens. We followed the precedent set by van Rensburg et al. (2006) and regarded isolate FAU458 as a reference strain of D. phaseolorum. All of the isolates that we studied that clustered phylogenetically with FAU458 correlated entirely with the morphology of the isotype of Sphaeria phaseolorum. Furthermore, all of these isolates were homothallic and readily formed the teleomorph in culture, which is a recognised feature of D. phaseolorum. For these reasons we are confident that these isolates represent D. phaseolorum.
In 1933, Wehmeyer noticed that D. phaseolorum var. phaseolorum on Phaseolus spp., D. phaseolorum var. sojae on soybean and D. phaseolorum var. batatae on sweet potato were morphologically highly similar and probably the same taxon. Later, for the same reason and after a thorough search of the literature, together with morphological studies of various specimens and isolates, Kulik (1984) placed all of these pathogens under the same name (D. phaseolorum), rejecting their separation into different varieties. Their anamorphs, Phomopsis phaseoli, P. sojae and P. batatas, respectively, were placed under P. phaseoli. In fact, the amount of variation in the size of perithecia, ascospores, pycnidia, alpha- and beta-conidia reported within each taxon at least equals the variation between them (Kulik 1984). Almost at the same time, Hobbs et al. (1985) considered the type specimen of Phomopsis glycines to be morphologically the same as P. sojae, and included this binomial to the list of synonyms of D. phaseolorum.
The ITS sequence of the ex-type isolate of Phomopsis camptothecae clusters perfectly with D. phaseolorum in the ITS phylogram (Fig. 2). According to Chang et al. (2005) the type specimen of this species is deposited in the Mycological Herbarium of South China Agricultural University (SCHM), Guangzhou. An extensive search for the herbarium specimens was done but unfortunately none could be found. However, the original description of this fungus corresponds in all ways with the anamorph of D. phaseolorum. Since P. camptothecae is morphologically and phylogenetically indistinguishable from the anamorph of D. phaseolorum it was considered to be a synonym.
Kulik (1984) was not sure about the differences between the stem canker pathogenic agent (D. phaseolorum var. caulivora) and all the other varieties of D. phaseolorum. Although he placed this variety as a synonym of D. phaseolorum, he suggested that it should be called D. phaseolorum f. sp. caulivora until further studies confirm its true identity. In the present work, we have shown that D. phaseolorum var. caulivora is a distinct species (D. caulivora) that also occurs on soybean in Croatia. We started the identification of the isolates of this species by studying their morphology. Since Kulik (1984) had reported an unfruitful search for the type specimen of D. phaseolorum var. caulivora, we based our preliminary identification on the original description given by Athow & Caldwell (1954) and other authors (e.g., Kulik 1984). Our next approach was similar to the one followed by van Rensburg et al. (2006) to describe D. aspalathi. In that study, the ITS and EF1-α of isolates identified as D. phaseolorum var. meridionalis were sequenced and shown to be too distinct from the reference isolate of D. phaseolorum (FAU458) to consider them as a mere variety. In our work, we showed that both ITS and EF1-α support a clear separation of D. phaseolorum var. caulivora from the same reference isolate of D. phaseolorum, and thus named it D. caulivora.
Although several different media and incubation conditions were tried to induce anamorphic sporulation of D. caulivora, none of these attempts was successful. This taxon is renowned for the rarity of its conidial state (e.g., Welch & Gilman 1948, Punithalingam & Holliday 1972, Kmetz 1975, Kulik 1984). Nevertheless, Kulik (1984) compiled and compared a series of anamorphic characters given by other authors and that work can thus be considered a good reference for the anamorphic morphology of D. caulivora. Only MAT1-2 mating-type could be detected in the isolates that we studied. Since this species is self-fertile, it is likely that the MAT1-1-1 primers simply failed to amplify this gene in this species. A similar situation was previously found for D. viticola isolates (Santos et al. 2010).
A third species found on soybean in Croatia was D. longicolla, which is another well-known soybean pathogen (Morgan-Jones 1985, Sinclair & Backman 1989). The holotype of this taxon (Phomopsis longicolla) is housed in BPI (TWH P74 = BPI 358745) while the ex-type culture is deposited in ATCC (ATCC 60325). Our isolates were phylogenetically identical to others identified as such. Moreover, the morphology of our isolates correlated entirely with the original description of this species as given by Hobbs et al. (1985). The absence of beta-conidia in the isolates used in the present study correlates with the scarcity of this type of spores as reported by Hobbs et al. (1985). Since this is clearly a species of Diaporthe, a new combination as D. longicolla was introduced.
The PCR mating-type diagnosis showed that both mating-types exist in D. longicolla and both are present in the genome of a single isolate. However, no teleomorph was seen in pure cultures. The inability for isolates to form their sexual state, together with the fact that the teleomorph of this species has never been found in nature indicates that this might be a purely anamorphic species. The existence of different mating-types in asexual ascomycetes is well documented (Coppin et al. 1997, Arie et al. 2000, Pöggeler 2001, Groenewald et al. 2006) and other asexual Phomopsis species are known to possess both mating-types, such as Phomopsis viticola (Santos et al. 2010). Nevertheless, in D. longicolla both mating-types are present in the same isolate, which is in contrast to P. viticola where the different mating-types are present in separate isolates (Santos et al. 2010).
The fourth species found on soybean in Croatia clustered in both phylogenetic trees with isolates identified as Phomopsis sp. 9. This is an unidentified species that was reported recently on Aspalathus linearis (van Rensburg et al. 2006) and Hydrangea macrophylla (Santos et al. 2010). ITS sequence comparison revealed that this species has also been found on Helianthus annuus (Rekab et al. 2004) and Vitis vinifera (van Niekerk et al. 2005) under the name of D. helianthi. Van Rensburg et al. (2006) declined to apply a name to this species because they had only one isolate that did not sporulate. In the present study, not only did our isolates produce fertile pycnidia but they also formed perithecia when isolates of opposite mating-types were crossed in culture. This is the first time that the teleomorph of this species has been observed and the name D. novem is applied to it. To our knowledge, this is also the first report of this species on soybean. Moreover, all of our isolates, except AS1, were from soybean seeds collected during September 2008 in Slavonija County in Croatia. This can thus be considered a consistent isolation of this species from soybean in Croatia. Pathogenicity studies would help to clarify if this species represents a threat or if it is a weak pathogen on this host. It is important to note that this fungus was always isolated from symptomless seeds and never from diseased stems. Future isolations of Diaporthe and Phomopsis from soybean, especially in Croatia, will confirm whether this is an emergent pathogen on this host.
Besides the species described in this paper, D. aspalathi (formerly named D. phaseolorum var. meridionalis) has also been reported on soybean. This species was first described as causing soybean stem canker in the South-eastern USA (Fernández & Hanlin 1996) and the disease was named Southern soybean stem canker. It was later established as the main causal agent of canker and die-back of rooibos (A. linearis) in South Africa (van Rensburg et al. 2006). Diaporthe aspalathi was not found on soybean in Croatia during the present study. Although this species seems to be geographically restricted, phytosanitary measures designed to control this pathogen should not be neglected.
It is important to note that the species found on soybean in Croatia are also present on several herbaceous hosts such as Arctium lappa, Asclepias syriaca and Dipsacus lacinatus (Table 1). Diaporthe phaseolorum was also found on Helianthus annuus, and this has already been reported in Croatia (Vrandečić et al. 2009). This observation raises once more the relevance of weeds as reservoir hosts for pathogens of economically important plants.
Acknowledgments
This work was financed by the European Regional Development Fund and Fundação para a Ciência e a Tecnologia (FCT) under the project PTDC/AGR-AAM/67064/2006 and J. Santos was supported by a research grant within the project. A. Phillips was supported by grant number SFRH/BCC/15810/2006 from FCT.
REFERENCES
- Aa HA van der, Noordeloos ME, Gruyter J.1990. Species concepts in some larger genera of the Coelomycetes. Studies in Mycology 32: 3– 19 [Google Scholar]
- Alfieri SA, Jr, Langdon KR, Wehlburg C, Kimbrough JW.1984. Index of plant diseases in Florida (revised). Florida Department of Agriculture and Consumer Services, Division of Plant Industry Bulletin 11: 1– 389 [Google Scholar]
- Arie T, Kaneko I, Yoshida T, Noguchi M, Nomura Y, Yamaguchi I.2000. Mating type genes from asexual phytopathogenic ascomycetes Fusarium oxysporum and Alternaria alternata. Molecular Plant-Microbe Interactions 13: 1330– 1339 [DOI] [PubMed] [Google Scholar]
- Ash GJ, Stodart B, Sakuanrungsirikul S, Anschaw E, Crump N, Hailstones D, Harper JDI.2010. Genetic characterization of a novel Phomopsis sp., a putative biocontrol agent for Carthamus lanatus. Mycologia 102: 54– 61 [DOI] [PubMed] [Google Scholar]
- Athow KL, Caldwell RM.1954. A comparative study of Diaporthe stem canker and pod stem blight of soybean. Phytopathology 44: 319– 325 [Google Scholar]
- Backman PA, Weaver DB, Morgan-Jones G.1985. Soybean stem canker: an emerging disease problem. Plant Disease 69: 641– 647 [Google Scholar]
- Bernaux P.1981. Mycelium progress of Phomopsis sojae inside the soybean stem. Agronomie 1: 783– 786 [Google Scholar]
- Black BD, Padgett GB, Russin JS, Griffin JL, Snow JP, Gerggren GT., Jr1996. Potential weed hosts for Diaporthe phaseolorum var. caulivora, causal agent for soybean stem canker. Plant Disease 80: 763– 765 [Google Scholar]
- Bradley CA, Li S.2006. First report of northern stem canker of soybean caused by Diaporthe phaseolorum var. caulivora in North Dakota. Plant Disease 90: 687 [DOI] [PubMed] [Google Scholar]
- Cerkauskas RF, Dhingra OD, Sinclair JB, Asmus G.1983. Amarahthus spinosus, Leonotis nepetaefolia, and Leonorus sibiricus: New hosts for Phomopsis spp. in Brazil. Plant Disease 67: 821– 824 [Google Scholar]
- Chang C-Q, Xi P-G, Xiang M-M, Jiang Z-D, Chi P-K.2005. New species of Phomopsis on woody plants in Hunan province. Mycosystema 2: 145– 154 [Google Scholar]
- Cheng Z, Tang W, Xu S, Sun S, Huang B, Yan X, Chen Q, Lin Y.2008. First report of an endophyte (Diaporthe phaseolorum var. sojae) from Kandelia candel. Journal of Forestry Research 19: 277– 282 [Google Scholar]
- Cooke MC, Ellis JB.1878. New Jersey fungi. Grevillea 6: 81– 96 [Google Scholar]
- Coppin E, Debuchy R, Arnaise S, Picard M.1997. Mating types and sexual development in filamentous ascomycetes. Microbiology and Molecular Biology Reviews 61: 411– 428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costamilan LM, Yorinori JT, Almeida AMR, Seixas CDS, Binneck E, Araújo MR, Carbonari JA.2008. First report of Diaporthe phaseolorum var. caulivora infecting soybean plants in Brazil. Tropical Plant Pathology 33: 381– 385 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G.2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19– 22 [Google Scholar]
- Dahal G, Amatya P, Manandhar H.1992. Plant diseases in Nepal. Review of Plant Pathology 71: 797– 807 [Google Scholar]
- Dunleavey J.1955. Soybean diseases in Iowa in 1954. Plant Disease Reporter 39: 169– 170 [Google Scholar]
- Ebbels DL, Allen DJ.1979. A supplementary and annotated list of plant diseases, pathogens and associated fungi in Tanzania. Phytopathology Papers 22: 1– 89 [Google Scholar]
- Ersek T.1978. Pod and stem blight of soybeans in Hungary. Acta Phytopathologica Academiae Scientiarum Hungaricae 13: 365– 367 [Google Scholar]
- Felsenstein J.1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 6: 227– 242 [DOI] [PubMed] [Google Scholar]
- Fernández FA, Hanlin RT.1996. Morphological and RAPD analyses of Diaporthe phaseolorum from soybean. Mycologia 88: 425– 440 [Google Scholar]
- Fernández FA, Phillips DV, Russin JS, Rupe JC.1999. Stem canker. In: Hartman GL, Sinclair JB, Rupe JC. (eds), Compendium of soybean diseases. 4th edn American Phytopathological Society Press, Saint Paul, Minnesota, USA: 33– 35 [Google Scholar]
- Gerdemann JW.1954. The association of Diaporthe phaseolorum var. sojae with root and basal stem rot of soybean. Plant Disease Reporter 38: 742– 743 [Google Scholar]
- Gilman JC.1949. Second supplementary list of parasitic fungi from Iowa. Iowa State College Journal of Science 23: 261– 272 [PubMed] [Google Scholar]
- Ginns JH.1986. Compendium of plant disease and decay fungi in Canada 1960–1980. Canadian Government Publishing Centre, Ottawa, Ontario, Canada: [Google Scholar]
- Gorter GJMA.1977. Index of plant pathogens and the diseases they cause in cultivated plants in South Africa. Republic of South Africa, Department of Agricultural Technical Services, Plant Protection Research Institute Scientific Bulletin 392: 1– 177 [Google Scholar]
- Grand LF.1985. North Carolina plant disease index. North Carolina Agricultural Research Service Technical Bulletin 240: 1– 157 [Google Scholar]
- Groenewald M, Groenewald JZ, Harrington TC, Abeln ECA, Crous PW.2006. Mating type gene analysis in apparently asexual Cercospora species is suggestive of cryptic sex. Fungal Genetics and Biology 43: 813– 825 [DOI] [PubMed] [Google Scholar]
- Hanlin RT.1963. A revision of the Ascomycetes of Georgia. Georgia Agricultural Experiment Station Mimeo Series n.s. 175: 1– 65 [Google Scholar]
- Hepperly PR, Kirkpatrick BL, Sinclair JB.1980. Abutilon theophrasti: wild host for three fungal parasites of soybean. Phytopatology 70: 307– 310 [Google Scholar]
- Hirrel MC, Kirkpatrick TL.1986. First report of stem canker on soybeans in Arkansas. Plant Disease 70: 78 [Google Scholar]
- Hobbs TW, Schmitthenner AF, Ellett CW, Hite RE.1981. Top dieback of soybean caused by Diaporthe phaseolorum var. caulivora. Plant Disease 65: 618– 620 [Google Scholar]
- Hobbs TW, Schmitthenner AF, Kuter GA.1985. A new Phomopsis species from soybean. Mycologia 77: 535– 544 [Google Scholar]
- Holevas CD, Chitzanidis A, Pappas AC, Tzamos EC, Elena K, et al. 2000. Disease agents of cultivated plants observed in Greece from 1981 to 1990. Benaki Phytopathology Institute, Kiphissia, Athens 19: 1– 96 [Google Scholar]
- Jasnić SM, Vidić M.1981. Crna pegavost stabla nova bolest soje u Jugoslaviji. Glasnik Zaštita Bilja 2: 44– 46 [Google Scholar]
- Jasnić SM, Vidić M.1985. Occurrence of soybean diseases in Yugoslavia. Eurosoya 3: 43– 46 [Google Scholar]
- Johnson HW, Kilpatrick RA.1953. Soybean diseases in Mississippi in 1951–52. Plant Disease Reporter 37: 154– 155 [Google Scholar]
- Kanematsu S, Adachi Y, Ito T.2007. Mating-type loci of heterothallic Diaporthe spp.: homologous genes are present in opposite mating-types. Current Genetics 52: 11– 22 [DOI] [PubMed] [Google Scholar]
- Kmetz KT.1975. Soybean seed decay: studies on disease cycles, effects of cultural practices on disease severity and differentiation of the pathogens Phomopsis sp., Diaporthe phaseolorum var. sojae, and D. phaseolorum var. caulivora. PhD thesis, Ohio State University, Columbus, USA: [Google Scholar]
- Kmetz KT, Ellett CW, Schmitthenner AF.1974. Isolation of seedborne Diaporthe phaseolorum and Phomopsis from immature soybean plants. Plant Disease Reporter 58: 978– 982 [Google Scholar]
- Kozireva EP, Primakovskaja MA, Skripka OV.1982. Bolezni soji. Zaštita Rastenij 11: 38– 39 [Google Scholar]
- Krausz JP, Fortnum BA.1983. An epiphytotic of Diaporthe stem canker of soybean in South Carolina. Plant Disease 67: 1128– 1129 [Google Scholar]
- Kulik MM.1984. Symptomless infection, persistence, and production of pycnidia in host and non-host plants by Phomopsis batatae, Phomopsis phaseoli, and Phomopsis sojae, and the taxonomic implications. Mycologia 76: 274– 291 [Google Scholar]
- Kulik MM, Thomison PR.1985. First report of soybean stem canker in Maryland. Plant Disease 69: 811 [Google Scholar]
- Mengistu A, Castlebury LA, Smith JR, Rossman AY, Reddy KN.2007. Isolates of Diaporthe-Phomopsis from weeds and their effect on soybean. Canadian Journal of Plant Pathology 29: 283– 289 [Google Scholar]
- Mengistu A, Reddy KN.2005. Detection of phomopsis spp. on weed hosts and its pathogenicity on soybean. Seed Technology 27: 97– 100 [Google Scholar]
- Miller WA, Roy KW.1982. Mycoflora of soybean leaves, pods, and seeds in Mississippi. Canadian Journal of Botany 60: 2716– 2723 [Google Scholar]
- Morgan-Jones G.1985. The Diaporthe/Phomopsis complex of soybean: morphology. In: Proceedings of the Conference on Diaporthe/Phomopsis Disease Complex of Soybean: 1–7 United States Department of Agriculture, Beltsville, Maryland, USA: [Google Scholar]
- Morgan-Jones G.1989. The Diaporthe/Phomopsis complex: taxonomic considerations. In: Pascale A. (ed), Proceedings of the world soybean research conference IV: 1699–1706. Orientación Gráfica, Buenos Aires, Argentina: [Google Scholar]
- Mostert L, Crous PW, Kang JC, Phillips AJL.2001. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93: 146– 167 [Google Scholar]
- Niekerk JM van, Groenewald JZ, Farr DF, Fourie PH, Halleen F, Crous PW.2005. Reassessment of Phomopsis species on grapevine. Australasian Plant Pathology 34: 27– 39 [Google Scholar]
- Pennycook SR.1989. Plant diseases recorded in New Zealand. Vol. 3 Plant Disease Division, D.S.I.R., Auckland, New Zealand [Google Scholar]
- Peregrine WTH, Siddiqi MA.1972. A revised and annotated list of plant diseases in Malawi. Phytopathology Papers 16: 1– 51 [Google Scholar]
- Petch T.1922. Additions to Ceylon fungi II. Annals of the Royal Botanic Gardens Peradeniya 7: 279– 322 [Google Scholar]
- Phillips AJL, Crous PW, Alves A.2007. Diplodia seriata, the anamorph of “Botryosphaeria” obtusa. Fungal Diversity 25: 141– 155 [Google Scholar]
- Pioli R, Morandi EN, Bisaro V.2001. First report of soybean stem canker caused by Diaporthe phaseolorum var. caulivora in Argentina. Plant Disease 85: 95. [DOI] [PubMed] [Google Scholar]
- Pöggeler S.2001. Mating-type genes for classical strain improvements of ascomycetes. Applied Microbiology and Biotechnology 56: 589– 601 [DOI] [PubMed] [Google Scholar]
- Preston DA.1945. Host index of Oklahoma plant diseases. Oklahoma Agricultural College Agricultural Experiment Station Technical Bulletin 21: 1– 168 [Google Scholar]
- Punithalingam E, Holliday P.1972. Diaporthe phaseolorum. CMI Descriptions of pathogenic bacteria and fungi, no. 336. Commonwealth Mycological Institute, Kew, England [Google Scholar]
- Rehner SA, Uecker FA.1994. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycetes Phomopsis. Canadian Journal of Botany 72: 1666– 1674 [Google Scholar]
- Rekab D, Sorbo G del, Reggio C, Zoina A, Firrao G.2004. Polymorphisms in nuclear rDNA and mtDNA reveal the polyphyletic nature of isolates of Phomopsis pathogenic to sunflower and a tight monophyletic clade of defined geographic origin. Mycological Research 108: 393– 402 [DOI] [PubMed] [Google Scholar]
- Rensburg JCJ van, Lamprecht SC, Groenewald JZ, Castlebury LA, Crous PW.2006. Characterisation of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Studies in Mycology 55: 65– 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MJ.1990. An annotated list of seed-borne diseases. 4th edn International Seed Testing Association, Zurich, Switzerland [Google Scholar]
- Ronquist F, Huelsenbeck JP.2003. MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572– 1574 [DOI] [PubMed] [Google Scholar]
- Roy KW, Ratnayake S, McLean K.1997. Colonization of weeds by Phomopsis longicolla. Canadian Journal of Plant Pathology 19: 193– 196 [Google Scholar]
- Sanogo S, Etarock BF.2009. First report of Phomopsis longicolla causing stem blight of valencia peanut in New Mexico. Plant Disease 93: 965. [DOI] [PubMed] [Google Scholar]
- Santos JM.2008. Resolução do complexo de espécies de Phomopsis e dos seus teleomorfos Diaporthe no hospedeiro Foeniculum vulgare. MSc thesis, Departamento de Biologia Vegetal, Universidade de Lisboa, Portugal [Google Scholar]
- Santos JM, Correia VG, Phillips AJL.2010. Primers for mating-type diagnosis in Diaporthe and Phomopsis: their use in teleomorph induction in vitro and biological species definition. Fungal Biology 114: 255– 270 [DOI] [PubMed] [Google Scholar]
- Santos JM, Phillips AJL.2009. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Diversity 34: 111– 125 [Google Scholar]
- Sinclair JB, Backman PA.1989. Compendium of soybean diseases, 3rd edn American Phytopathological Society Press, Saint Paul, Minnesota, USA [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, Mejia LC, White JF.2008. Leaf-inhabiting genera of the Gnomonianceae, Diaporthales. Studies in Mycology 62: 1– 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL.2002. PAUP 4.0b10: Phylogenetic Analysis Using Parsimony. Sinauer Associates, Sunderland [Google Scholar]
- Vidić M, Jasnić S.1998. Bolesti soje. In: Hrustić M, Vidić M, Jocković Đ. (eds), Soja. Institut za ratarstvo i povrtarstvo, Novi Sad, Serbia: 277– 338 [Google Scholar]
- Vidić M, Jasnić S, Stojšin V.1996. Cultural and morphological characteristic of Phomopsis sojae and Phomopsis longicolla originating from soybean. Zaštita Bilja 215: 37– 44 [Google Scholar]
- Vrandečić K, Ćosić J, Jurković D, Duvnjak T, Riccioni L.2009. First report of Diaporthe phaseolorum on sunflower (Helianthus annuus) in Croatia. Plant Disease 93: 1074 [DOI] [PubMed] [Google Scholar]
- Vrandečić K, Ćosić J, Riccioni L, Duvnjak T, Jurković D.2004. Phomopsis longicolla - new pathogen on Abutilon theophrasti in Croatia. Plant Pathology 53: 251 [Google Scholar]
- Vrandečić K, Ćosić J, Riccioni L, Duvnjak T, Jurković D.2005. Isolation of Diaporthe phaseolorum var. caulivora from Abutilon theophrasti in Croatia. Plant Pathology 54: 576 [Google Scholar]
- Wehmeyer LE.1933. The genus Diaporthe Nitschke and its segregates. University of Michigan Studies Scientific Series 9: 1– 349 [Google Scholar]
- Welch AW, Gilman JC.1948. Hetero and homothallic types of Diaporthe of soybeans. Phytopathology 38: 628– 637 [Google Scholar]
- Whitney NG, Bowers GR., Jr1985. Stem canker of soybean in Texas. Plant Disease 69: 361 [Google Scholar]
- Zhang AW, Hartman GL, Riccioni L, Chen WD, Ma RZ, Pedersen WL.1997. Using PCR to distinguish Diaporthe phaseolorum and Phomopsis longicolla from other soybean fungal pathogens and to detect them in soybean tissues. Plant Disease 81: 1143– 1149 [DOI] [PubMed] [Google Scholar]