Abstract
Mast cells are associated with inflammation and fibrosis. Whether they protect against or contribute to renal fibrosis is unclear. Based on our previous findings that mast cells can express and secrete active renin, and that angiotensin (ANG II) is profibrotic, we hypothesized that mast cells play a critical role in tubulointerstitial fibrosis. We tested this hypothesis in the 14-day unilateral ureteral obstruction (UUO) model in rats and mast cell-deficient (MCD) mice (WBB6F1-W/Wv) and their congenic controls (CC). In the 14-day UUO rat kidney, mast cell number is increased and they express active renin. Stabilizing mast cells in vivo with administration of cromolyn sodium attenuated the development of tubulointerstitial fibrosis, which was confirmed by measuring newly synthesized pepsin-soluble collagen and blind scoring of fixed trichrome-stained kidney sections accompanied by spectral analysis. Fibrosis was absent in UUO kidneys from MCD mice unlike that observed in the CC mice. Losartan treatment reduced the fibrosis in the CC UUO kidneys. The effects of mast cell degranulation and renin release were tested in the isolated, perfused kidney preparation. Mast cell degranulation led to renin-dependent protracted flow recovery. This demonstrates that mast cell renin is active in situ and the ensuing ANG II can modulate intrarenal vascular resistance in the UUO kidney. Collectively, the data demonstrate that mast cells are critical to the development of renal fibrosis in the 14-day UUO kidney. Since renin is present in human kidney mast cells, our work identifies potential targets in the treatment of renal fibrosis.
Keywords: angiotensin; isolated, perfused kidney; tubulointerstitiial fibrosis
in addition to the circulating renin-angiotensin system (RAS), it is believed that many tissues, including kidney and heart, possess their own RAS (36, 39, 47). We previously reported in the heart and lung that mast cells synthesize, store, and release renin capable of cleaving angiotensinogen (Aogen) (48) leading to ANG II formation (32, 54). In an ischemia-reperfusion heart model, release of renin from cardiac mast cells in situ triggers local ANG II formation that promotes excessive release of norepinephrine and arrhythmia (32). In isolated bronchial rings from rat and desensitized guinea pig, local ANG II formation triggered by release of mast cell renin causes bronchoconstriction and airway hypersensitivity (54). These studies demonstrate that a mast cell-dependent activation of a local RAS can lead to pathological complications.
The purpose of the present study was to evaluate the role of mast cells, mast cell renin release, and local ANG II formation in promoting renal fibrosis. We utilized the unilateral ureteral obstruction (UUO) model of renal fibrosis, a well-defined rodent model of hydronephrosis that is marked by an inflammatory cell infiltrate that includes mast cells and tubulointerstitial fibrosis (3, 25, 26). Substantial evidence indicates that intrarenal ANG II contributes to local vasoconstriction in UUO (11) and that transient ischemia induces interstitial fibrosis (14). The role of mast cells in the fibrotic response is unclear, with some evidence pointing to protection against (24, 35) and others demonstrating an active role of mast cells in renal fibrosis (23, 51). We hypothesized that mast cells infiltrating the kidney, as part of an inflammatory response, are pivotal to the development of renal fibrosis and are a source of local renin. In view of the fact that ANG II is profibrotic and causes vasoconstriction, it is proposed that mast cell renin release and the ensuing ANG II trigger excessive collagen deposition and changes in vascular resistance leading to renal fibrosis. To test this hypothesis, we utilized the 14-day UUO model (25, 26) in rats and mice [mast cell-deficient WBB6F1/J-KitW/KitW-v mice (MCD) and their congenic controls (CC)]. The contribution of a mast cell-dependent local RAS to renal fibrosis and to changes in vascular resistance were analyzed. Our findings indicate that mast cells play a critical role in the genesis of tubulointerstitial fibrosis. Our results in the rodent models along with our findings in tissue from human kidney identify novel therapeutic targets in the treatment of renal fibrosis, the major cause of end-stage renal disease.
MATERIALS AND METHODS
In vivo UUO model.
All animal treatments and experiments were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Animals were anesthetized intraperitoneally with a cocktail of ketamine (90 mg/kg) and xylazine (4 mg/kg). Male Sprague-Dawley rats (300–350 g, Charles River) and MCD mice (WBB6F1-W/Wv mice) and their CC (WBB6F1-+/+; 20–25 g, stock number 100410, Jackson Laboratories) underwent right unilateral ureteral ligation with 4-0 silk suture through an abdominal midline incision under sterile conditions. Sham-operated control (CON) animals underwent identical operations, however, with manipulation of the right ureter only. Cromolyn sodium (CR in figures; 24 mg·kg−1·day−1) was administered via osmotic minipump to one group of UUO rats for the duration of the obstruction. For experiments using mice, CC and MCD mice were divided into either losartan-treated or an untreated group. Losartan (0.4 mg/ml) was placed into the drinking water for the animals to imbibe ad libitum. Animals were maintained for up to 14 days.
Human tissue samples were obtained as coded deindentified slides from the pathology archive or unidentified waste tissue specimens from macroscopically normal kidneys and approved by the Institutional Review Board.
Isolated, perfused kidneys.
Isolated, perfused kidney experiments were performed according to the methods of De Mello and Maack (7, 31). At 14 days post-UUO/sham surgery, UUO and CON rat kidneys were isolated and perfused with modified Krebs-Henseleit solution in the open-circuit mode. With a constant flow rate, the mast cell-degranulating agent compound 48/80 (C48/80; 300 μg/ml) was injected into the renal artery to induce mast cell degranulation. To assess the contribution of mast cell renin to the increase in vascular resistance, the selective renin inhibitor BILA2157 (1 μM) (49) was added to the perfusate after 2 min. BILA2157 had no effect on flow rate before the 48/80 injection and was maintained in the perfusate for the remainder of the experiment. Renal effluent was collected every 2 min under a constant mean perfusion pressure of 103 mmHg, and the flow rate was calculated.
Histochemical and immunocytochemical techniques.
Kidneys were fixed in 10% neutral buffered formalin followed by embedding in paraffin. Sections (5–10 μm) were collected and prepared accordingly. Masson's trichrome staining was performed using a kit (Richard-Allen Scientific). Slides of paraffinized tissue sections were deparaffinized, rehydrated, and washed in distilled water. Sections were subsequently stained using components from the trichrome kit and according to the manufacturer's protocol. After dehydrating and clearing in xylene, the sections were mounted with coverslips in Vectamount (Vector Laboratories). Mast cells were detected in fixed tissue using either toluidine blue, the classic metachromatic stain for mast cells (48), or the glycoprotein avidin, conjugated either to the enzyme horseradish peroxidase (HRP; Vector Laboratories) or to the fluorochrome dyes fluorescein or rhodamine (50). Conjugated avidin is a well-defined histochemical method for identifying human and rodent mast cells, binding specifically to mast cell heparin granules (1). Nonspecific staining of biotin or biotin-like molecules was not observed in our tissues as also reported by others (1). For the immunoperoxidase detection of mast cells, formalin-fixed and paraffin-embedded sections of rat and human kidney were exposed to avidin-HRP (1:2,000 for human kidney and 1:500 for CON and UUO kidney sections). NovaRED (Vector Laboratories) was used as the chromogen substrate, according to the manufacturer's instructions. Specificity of the avidin-HRP binding was verified by staining sections with anti-mouse IgG antibody linked to HRP (Cell Signaling), The primary antibodies used against mast cell chymase were mouse anti-human (AbD; 1:50; Serotec) and rabbit anti-mouse (1:100; Abcam). Sections of human and mouse kidney were maintained overnight at 4°C with their respective primary antibodies, rinsed, and incubated with the appropriate HRP-conjugated secondary antibodies (IgG). To colocalize the mast cells with collagen, avidin-HRP-stained sections were then immersed in 10% formalin for 1 h at room temperature. Following a wash in distilled water, sections were next stained with Gomori's trichrome reagents using a dilution of Weigert's hematoxylin at 1:10.
Sections of formalin-fixed, paraffin-embedded rat, mouse, and human kidneys were immunoscreened for the presence of avidin-positive mast cells and renin. Goat anti-renin antibody (Santa Cruz Biotechnology) was used at a dilution of 1:50–1:100 for rat, mouse, and human kidney sections. Kidney sections from rats were exposed to the pro-renin receptor antibody (1:400 dilution) made against a synthetic peptide (a gift kindly provided by G. Nyguyen of Inserm Unit 833, Experimental Medicine, Collège de France, Paris, France). The secondary antibodies used were Alexa Fluor 488 donkey anti-goat IgG (1:600) or Alexa Fluor 594 donkey anti-rabbit IgG diluted 1:600 (Molecular Probes) in the absence or presence of avidin. To identify avidin-positive mast cells, sections were exposed to avidin conjugated to FITC (1:3,000) or rhodamine diluted 1:1,000 (Vector Laboratories) for 1 h at 37°C. Sections were then washed and immersed in 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes) to stain nuclei, followed by fixation with 4% paraformaldehyde. Washed sections were mounted with coverslips in Vectashield antifading solution (Vector Laboratories) for fluorescence viewing. Mast cells were identified as cells that were triple stained (i.e., renin, FITC-avidin, and DAPI positive). As a negative control, sections were stained with nonimmune rabbit serum (1:400) instead of polyclonal anti-renin antibody.
For all immunofluorescence and histological experiments, tissue sections were examined with an inverted epifluorescence microscope (Nikon Eclipse TE 2000-U) interfaced to an electron multiplying charge-coupled device (Hamamatsu) and processed with Metamorph software (version 6.2; Universal Imaging). Histological preparations were viewed with transmitted light and recorded with a SPOT Insight 2 megapixel color camera (Diagnostic Instruments). All slides were examined by a board-certified renal pathologist (S. V. Seshan).
Quantitation of mast cells in situ.
Paraffin-embedded rat kidneys (CON and UUO) were analyzed for the presence of avidin-positive mast cells. Sections were exposed to avidin-FITC (1:2,500; Vector Laboratories) for 1 h at 37°C. Sections were then washed and immersed in DAPI (Molecular Probes) to stain nuclei, followed by fixation with 4% paraformaldehyde. Washed sections were mounted on coverslips in Vectashield antifading solution (Vector Laboratories) for fluorescent viewing. Mast cells were identified as cells that were double stained (i.e., avidin-FITC and DAPI positive). Avidin-positive mast cells were counted in 10 high-power fields (×400) in each slide and averaged together. Counting was performed by two independent investigators in a blinded fashion. Samples incubated without primary antibody exhibited no staining.
Spectral analysis methodology.
Collagen content was assessed by imaging sections stained with Masson's trichrome. A normalized measure of tissue collagen was taken as the total area of all blue staining, corresponding to tissue collagen, over the total tissue area. Briefly, serial sections from paraffin-embedded kidneys from rats were stained and then imaged with brightfield microscopy using a ×10-objective lens and a color digital camera and its accompanying software. Entire tissue sections were imaged by capturing adjacent, nonoverlapping, fields. For each animal, sections spanning the kidney were imaged and the total area of collagen staining was divided by the total area of tissue imaged. The percent collagen per tissue area, C, is calculated as
where n is the number of slides for a given animal.
Renin activity (ANG I radioimmunoassay).
Renin activity was measured in isolated mast cell lysate (rat kidney and human kidney), as previously reported (32, 48, 54). The detection limit was ∼0.01 pmol (32). Isolated mast cells were lysed in 1 ml of PBS by four cycles of freeze-thaw. The renin-containing lysates were then incubated for 18 h with human angiotensinogen (240 nM). For plasma renin activity, blood was taken from rats by heart puncture at various time points before and during UUO. Lysates and plasma were assayed for renin activity (ANG I formed) in the presence of BILA2157 (100 nM) by use of a GammaCoatPlasma Renin Activity 125I RIA kit (DiaSorin, Stillwater, MN).
Sircol soluble collagen assay.
Kidney homogenates from control and UUO rats were lyophilized and then subjected to overnight incubation in pepsin (dissolved in 0.5 M acetic acid) to extract newly formed collagen. The manufacturer's protocol was followed as outlined in the Sircol Soluble Collagen Assay kit (Accurate Chemical and Scientific). Collagen values were normalized to kidney dry weight.
Isolation of rat and human kidney mast cells.
Mast cells were isolated from macroscopically normal human kidney tissue specimens as previously described (54). In addition, mast cells were isolated from 14-day UUO and CON rat kidneys. Briefly, the rats were anesthetized and the abdominal cavity was opened. Following perfusion of the kidneys with J-MEM buffer (supplemented with HEPES, glutamine, taurine, insulin, and penicillin-streptomycin-amphotericin) for 15 min to remove blood, kidneys were perfused with 1 mg/ml collagenase II (Worthington Biochemicals) for 20 min. After this, the kidney was excised from the animal, minced, homogenized, and cells were pelleted by centrifugation at 770 rpm for 2 min. For isolation of mast cells from human kidney, tissue was placed in ice-cold J-MEM buffer supplemented with 0.5% BSA. After weighing, the tissue was minced in cold buffer and the cell suspension was collected for the isolation procedure. Rat and human cell suspensions were then filtered, pelleted, and washed several times in PBS solution containing 0.5% BSA and 2 mM EDTA. After the final wash, the cell pellet was resuspended in solution containing the rabbit polyclonal anti-FcεRI antibody (1:50, Upstate Cell Signaling) and incubated on a rocking shaker at 4°C for 25 min. Following this, the cells were pelleted (the supernatant discarded) and washed several times in PBS to remove unbound primary antibody. Next, the cell pellet was resuspended and incubated in solution containing goat anti-rabbit IgG colloidal microbeads (1:5; Miltenyi Biotec) for 15 min at 4°C. At the end of 15 min, the cells were pelleted and washed in PBS as described previously. FcεRI-labeled mast cells were isolated from the total cell population by magnetic cell sorting using MACS magnetic separation columns and units (Miltenyi Biotec). Mast cells were resuspended in PBS, and aliquots were used for toluidine blue staining and renin activity assays. Mast cell number was determined in aliquots prepared with toluidine blue and counted with a hemocytometer. The remaining kidney tissue that passed through the column was used for Western blotting.
Western blotting.
Twenty micrograms of isolated rat kidney mast cells and 20 μg of collected kidney tissue were prepared in sample buffer and loaded into Novex Bis-Tris SDS-polyacrylamide minigels (Invitrogen) for electrophoresis as previously described (48). The protein on the gel was transferred to a polyvinylidene difluoride membrane (Invitrogen). Blocking buffer [5% dry milk in Tris-buffered saline and Tween 20 (TBST)] was applied to the membrane for 1 h at room temperature. Polyclonal goat anti-renin antibody (1:100; Santa Cruz Biotechnology) in 5% BSA+TBST was applied to the membrane for 3 h at room temperature. The membrane was then washed with TBST 3× for 5 min each. HRP-conjugated donkey anti-goat IgG. (1:2,000; Santa Cruz Biotechnology) was applied to the membrane for 1 h in 5% dry milk in TBST at room temperature. HRP was developed using chemoluminescence (Millipore) and by exposure to film. Postdevelopment, anti-β-actin-HRP antibody (1:10,000; Alpha Diagnostics International) was applied to the membrane in 5% dry milk in TBST. Protein bands were analyzed using Image J software.
Drugs and chemicals.
C48/80, cromolyn sodium, and toluidine blue were obtained from Sigma-Aldrich. Losartan was obtained from Cayman Chemical (Ann Arbor, MI). BILA2157 was graciously provided by R. Levi, Weill Cornell Medical College. BILA2157 was dissolved in DMSO with further dilutions in the appropriate buffer; at the concentration used, DMSO did not affect mast cell mediator release.
Statistics.
All values were expressed as means ± SE; n corresponds to the number of animals (i.e., kidneys) used. CON refers to kidneys obtained from sham-operated animals. Statistical comparisons among the various experimental conditions were obtained by either Student's paired t-test or ANOVA followed by Dunnett's test. P < 0.05 was considered statistically significant.
RESULTS
UUO leads to an increase in the kidney mast cell population.
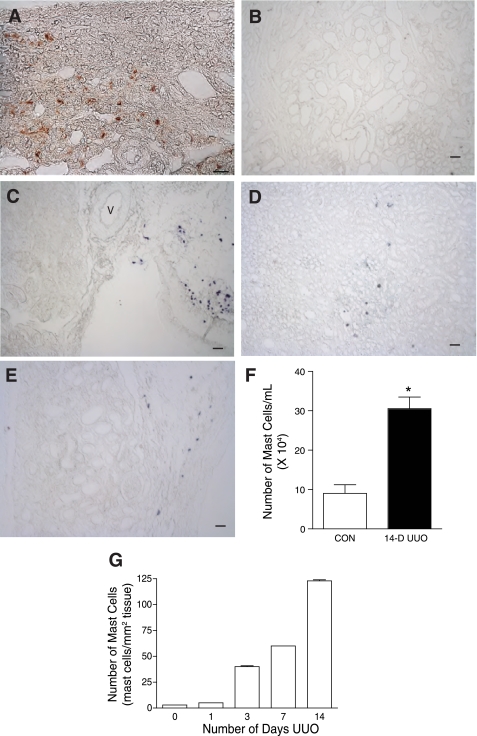
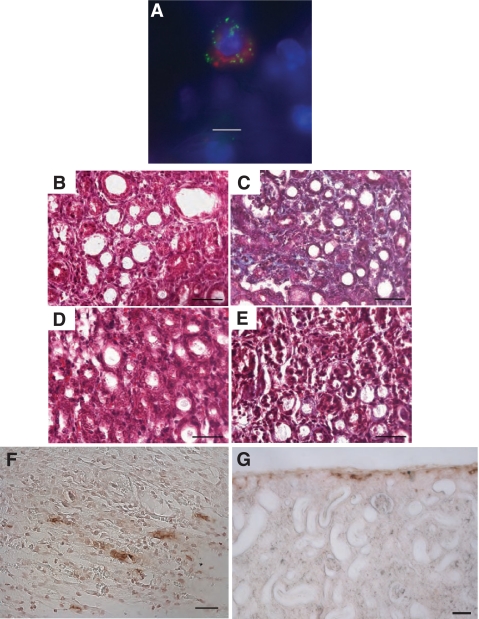
Fixed kidney sections from UUO rats were screened for mast cells (Fig. 1). Figure 1A is an image from a 14-day UUO rat kidney stained with HRP-avidin for detecting mast cells and shows the accumulation of HRP-avidin-labeled mast cells in the kidney parenchyma. Avidin binds selectively to the negatively charged heparin proteoglycans of the mast cell granules (27, 32, 54). At the concentration of avidin used, only mast cells were stained; there was no other tissue staining observed. Avidin specificity was verified by omitting the avidin and running a negative control (anti-mouse IgG linked to HRP) as shown in Fig. 1B. Toluidine blue was also used to stain for mast cells in kidney sections from varying time points: day 0 (sham-operated control) and days 7 and 14 UUO. In the sham-operated controls, the toluidine blue staining was limited to mast cells in the surrounding connective tissue, in close proximity to blood vessels (Fig. 1C). In the UUO kidneys, mast cells were observed within the parenchyma as shown for the 7-day (Fig. 1D) and 14-day (Fig. 1E) time points.
Fig. 1.
14-Day unilateral ureteral obstruction (UUO) leads to an increase in the kidney mast cell population. A: mast cells infiltrate the renal parenchyma in the 14-day UUO kidney. Section shows avidin-horseradish peroxidase (HRP)-labeled mast cells in the peritubular space. Scale bar = 20 μm. B: negative control for avidin-HRP staining. Low-power image is shown of 14-day UUO kidney section stained with nonimmune mouse IgG linked to HRP. Scale bar = 50 μm. C: section from sham-operated control kidney stained with toluidine blue. Mast cells are found primarily in the connective tissue surrounding the kidney. V, vessel. Scale bar = 50 μm. D: section from 7-day UUO kidney stained with toluidine blue. Scale bar = 50 μm. E: section from 14-day UUO kidney stained with toluidine blue. Scale bar = 50 μm. F: the number of avidin-positive mast cells increases with the duration of UUO as determined on consecutive sections of fixed and stained rat kidney (means ± SE). G: a greater number of toluidine-stained mast cells were isolated from UUO kidneys (14 day, n = 3 rats) than from control (CON) kidneys (n = 5 rats). Values are means ± SE. *P < 0.05.
We also evaluated whether the mast cell number is increased in UUO kidneys. Fixed sections of rat kidney were evaluated for avidin-positive mast cells at 0, 1, 3, 7, and 14 days UUO. There was a progressive increase in the number of mast cells with the duration of UUO; the 14-day time point had the greatest number of mast cells (Fig. 1F). This observation was further verified using an alternative technique where mast cells were isolated from kidney homogenates by immunomagnetic selection, stained with toluidine blue, and counted. The isolates from UUO kidneys contained significantly more toluidine blue- positive mast cells than those from CON kidneys (Fig. 1G) [UUO: 305,466 ± 29,659 cells/ml isolate (n = 3) vs. CON: 90,540 ± 21,975 cells/ml isolate (n = 5), P < 0.05]. These two independent analyses show that kidney mast cell number is increased in kidneys subjected to 14 days of UUO.
Mast cells play a role in tubulointerstitial fibrosis in the 14-day UUO kidney.
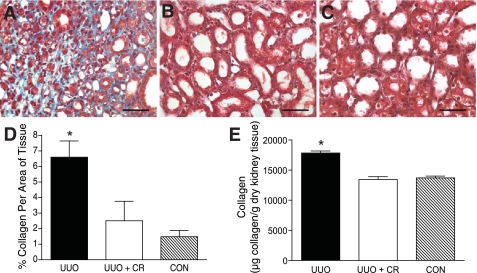
To demonstrate a role for mast cells in renal fibrosis as occurs in UUO, rats were treated with the mast cell stabilizer cromolyn sodium, continuously administered via osmotic minipump over the 14-day time course, to see whether preventing mast cell degranulation alters collagen deposition. Fixed sections of kidney were prepared with Masson's trichrome for staining collagen, with blind scoring by a renal pathologist. UUO kidneys scored 3+ compared with a trace in the UUO kidneys from rats treated with cromolyn sodium and CON kidney (Table 1). Representative sections of Masson's trichrome-stained kidneys are shown in Fig. 2. Extensive collagen staining was observed in the UUO kidneys as shown in Fig. 2A by the blue stain. Treatment with the mast cell stabilizer reduced the fibrosis normally seen in the UUO kidneys as shown by the absence of blue collagen staining (Fig. 2B). The CON kidney section also had minimal blue staining kidney (Fig. 2C).
Table 1.
Fibrosis scoring of fixed sections of rat and mouse kidneys stained with Masson's trichrome
| Condition | n | Score |
|---|---|---|
| Rats | ||
| CON | 4 | Trace |
| UUO | 3 | 3+ |
| UUO+cromolyn sodium | 5 | Trace |
| CC mice | ||
| CON | 3 | Trace |
| UUO | 7 | 2+ to 3+ |
| UUO+losartan | 3 | Trace |
| MCD mice | ||
| CON | 3 | Trace |
| UUO | 3 | Trace |
| UUO+losartan | 3 | Trace |
n, No. of animals; CON, sham-operated control; UUO, unilateral ureteral obstruction; CC, congenic control; MCD, mast cell deficient.
Fig. 2.
Mast cell stabilization reduces tubulointerstitial fibrosis in UUO kidney. A: representative section of 14-day UUO rat kidney stained with trichrome. Fibrosis is seen by the extensive collagen staining (blue). Scale bar = 50 μm. B: representative section of 14-day UUO kidney from rat treated with cromolyn sodium (CR) stained with trichrome. Note the absence of collagen staining. Scale bar = 50 μm. C: representative section of trichrome-stained 14-day kidney from CON rat. Scale bar = 50 μm. D: graph of mean percentage of collagen (±SE) per area of tissue as analyzed by spectral separation in trichrome-stained kidney sections, collagen being represented by blue-stained areas of tissue. UUO kidney (n = 5) displayed significantly more collagen staining (P < 0.05) than either the UUO kidney from rats treated with the mast cell stabilizer (UUO+CR; n = 3) or the kidney analyzed from CON rats (n = 4). E: graph of pepsin-soluble collagen content measured by biochemical assay in homogenates from rat UUO kidneys (14 day, n = 3), 14-day UUO kidney from rats treated with cromolyn sodium (UUO + CR; n = 3), and kidney from CON rats (n = 4). Values are means ± SE. The pepsin-soluble collagen content was significantly greater in UUO kidney homogenate compared with homogenate from UUO+CR or CON rats (*P < 0.05).
To augment the blind scoring technique, Masson's trichrome-stained kidney sections were also analyzed computationally using an algorithm that spectrally separates blue- from red-stained areas of each slide. The greatest percentage of collagen (blue) staining was calculated in the sections from UUO kidney (n = 5), and it was significantly greater (P < 0.05) than that measured in the kidney sections from UUO rats treated with cromolyn sodium [UUO+CR; (n = 3) and CON rats (n = 4)] (Fig. 2D). There was no significant difference in the collagen staining between the kidney sections from UUO rats treated with cromolyn sodium and CON rats.
Kidney homogenates were also analyzed for newly synthesized pepsin-soluble collagen content. The collagen content in the UUO kidney homogenates was significantly greater (P < 0.05) than that measured from the kidneys from rats treated with cromolyn sodium (UUO+CR) and from CON rats (Fig. 2E).
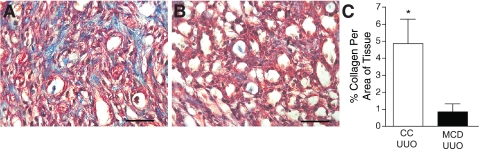
Seeking further evidence that mast cells are essential to the development of tubulointerstitial fibrosis in UUO over the 14-day time point, we analyzed the fibrotic response in UUO kidneys harvested from MCD mice and their CC. Representative sections of Masson's trichrome-stained UUO kidneys from MCD and CC mice are shown in Fig. 3. Fibrosis, as shown by excessive collagen staining, was absent in the UUO kidney from MCD mice (Fig. 3B), whereas it was observed in sections from the CC UUO kidneys (Fig. 3A). Blind scoring of the coded slides for fibrosis yielded the following results: 14-day UUO kidney from MCD mice scored only a trace for fibrosis (n = 3) while the CC kidneys averaged 2+ to 3+ (n = 4) (Table 1). There was no fibrosis in CON CC and MCD kidneys (not shown). Based on spectral separation, the percent area of mouse kidney that was positive for collagen was significantly greater in UUO kidneys from CC mice compared with UUO kidneys from MCD mice (P < 0.05) (Fig. 3C), consistent with the blind scoring. These results, along with our findings in UUO kidneys from rats treated with a mast cell stabilizer, demonstrate that mast cells play a significant role in the development of renal fibrosis in the 14-day UUO model.
Fig. 3.
Mast cell-deficient (MCD) mice do not develop tubulointerstitial fibrosis with 14-day UUO. A: representative section of Masson's trichrome-stained 14-day UUO kidney from a congenic control (CC) mouse. Collagen is stained blue. Scale bar = 50 μm. B: representative section of Masson's trichrome-stained 14-day UUO kidney from a MCD mouse. Note the lack of collagen (blue) staining. Scale bar = 50 μm. C: graph of mean percentage of collagen (±SE) per area of tissue as analyzed by spectral separation in trichrome-stained sections of UUO kidneys from CC (n = 4) and MCD (n = 3) mice. UUO kidneys from CC mice have significantly more (*P < 0.05) collagen-stained areas than UUO kidneys from MCD mice.
Rat kidney mast cells express renin.
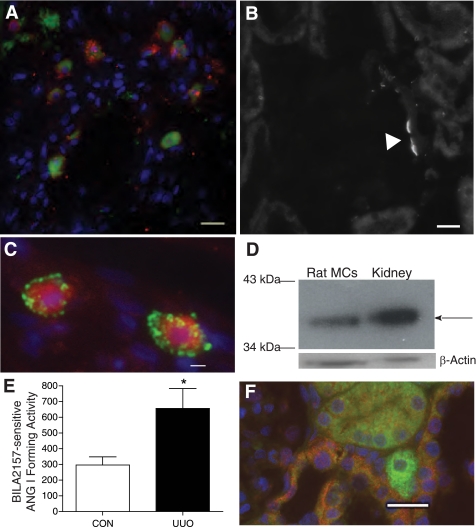
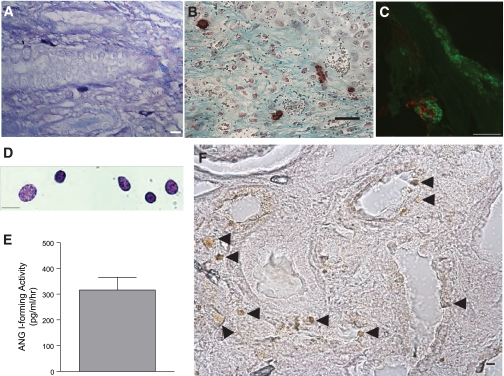
We next explored whether kidney mast cells express renin. We first verified that control kidney mast cells residing in the kidney connective tissue constitutively express renin, as we previously demonstrated in rat heart and lung, respectively (48, 54). Kidney sections from sham-operated control kidneys were immunoscreened with a polyclonal anti-renin antibody (48) and colabeled with avidin. Figure 4A shows immunopositive renin staining (red) in mast cells colabeled with avidin (green). Figure 4B is a representative section of rat kidney stained with the anti-renin antibody; it shows the vascular pole of a glomerulus immunostained with the antibody. The renin expressed in connective tissue control kidney mast cells is active as evidenced from the ANG I-forming activity measured in the lysates from mast cells immunomagnetically isolated from sham-operated control rat kidneys (CON) (Fig. 4E). Figure 4C demonstrates the immunostaining of avidin-labeled mast cells (red) in UUO rat kidneys with granules containing renin (green). The immunostaining of mast cells from control and UUO kidneys show that renin is contained in granules that are different from avidin-labeled heparin granules. We also observe this in mouse (Fig. 5) and human (see Fig. 7) kidney mast cells. Western blotting was next performed on the rat kidney mast cells immunomagnetically isolated from rat kidneys and the remaining kidney tissue (i.e., that which did not bind to the magnetic column). Western blot analysis of isolated rat mast cell lysates and rat kidney tissue probed with the anti-renin antibody showed a 35-kDa band for renin (Fig. 4D), typical for the rat (34). β-Actin expression is also shown as a loading control. The abundance of renin protein was much greater in rat kidney homogenate effluent than in the isolated mast cell lysates.
Fig. 4.
Rat kidney mast cells express renin. A: control kidney mast cells constitutively express renin. Shown is staining of connective tissue mast cells in rat kidney with FITC-conjugated avidin (green) and a polyclonal anti-renin antibody conjugated to Alexa Fluor 594 (red). The staining is not colocalized. Scale bar = 10 μm. B: the polyclonal anti-renin antibody exhibits specific binding to rat kidney at the vascular pole of the glomerulus. Scale bar = 10 μm. C: mast cells are immunopositive for renin. Shown is staining of mast cells in UUO rat kidney (14 day) with rhodamine-conjugated avidin (red) and a FITC-conjugated polyclonal anti-renin antibody (green). The staining is not colocalized. Scale bar = 10 μm. D: Western blot probing rat kidney mast cells (20 μg/lane) and rat kidney tissue for renin with the polyclonal anti-renin antibody. The arrow shows the ≈35-kDa band for renin. β-Actin expression was used as a loading control. E: ANG I formed (BILA2157-sensitive renin activity in pg·ml−1·min−1) as measured in lysates from mast cells isolated from 14-day UUO and CON kidneys [657 ± 126 pg·ml−1·min−1 UUO (n = 3) vs. 297 ± 51 pg·ml−1·min−1 CON (n = 3)]. Values are means ± SE. *P < 0.05. F: (pro)renin receptor immunoexpression in UUO rat kidney. Shown are representative images of kidney sections incubated with anti-(pro)renin receptor antibody conjugated to Alexa Fluor 594 (red) and avidin-FITC (green) to identify mast cells. (Pro)renin receptor expression is seen in the distal tubules (red) but is not expressed in the avidin-labeled degranulating mast cell (green) shown in the image. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). Scale bar = 20 μm.
Fig. 5.
Losartan treatment curtails renal fibrosis in UUO kidney from CC mice. A: mast cells in UUO kidneys from CC mice immunoexpress renin. Shown is a representative section of fixed 14-day UUO kidney from CC mouse showing an avidin-labeled mast cell [conjugated to rhodamine (red)] costained with a polyclonal anti-renin antibody (green). The staining is not colocalized. B: representative section of Masson's trichrome-stained 14-day UUO kidney from a CC mouse treated with losartan. Note the lack of collagen staining (blue). Scale bar = 50 μm. C: representative section of Masson's trichrome-stained 14-day UUO kidney from a CC mouse. Collagen is stained blue. Scale bar = 50 μm. D: representative section of Masson's trichrome-stained 14-day UUO kidney from a MCD mouse treated with losartan. Scale bar = 50 μm. E: representative section of Masson's trichrome-stained 14-day UUO kidney from a MCD mouse. Scale bar = 50 μm. F: representative section of UUO kidney from a CC mouse stained with an anti-chymase antibody using an immunoperoxidase technique. Chymase-positive mast cells were frequently observed in medulla. Scale bar = 20 μm. G: representative section of UUO kidney from a CC mouse treated with losartan and stained with an anti-chymase antibody. Scale bar = 50 μm.
Fig. 7.
Human kidney mast cells express active renin. A: fixed section of archived sample of fibrotic human kidney stained with toluidine blue. The mast cells are peritubular. Scale bar = 10 μm. B: fixed section of archived sample of fibrotic human kidney costained with avidin-HRP for identifying mast cells (brown) and Gomori's trichrome for fibrotic regions (blue). Scale bar = 25 μm. C: fixed section of archived fibrotic human kidney showing a mast cell costained with goat anti-renin antibody conjugated to Alexa Fluor 594 (red) and avidin-FITC (green). Scale bar = 10 μm. D: mast cells immunomagnetically isolated from normal kidney and stained with toluidine blue. Scale bar = 10 μm. E: measurement of ANG I-forming activity (in pg·ml−1·h−1) in the lysate from isolated human kidney mast cells (±SE; n = 3). F: fixed section of archived fibrotic human kidney stained with an anti-chymase antibody using an immunoperoxidase technique. Scale bar = 50 μm.
Mast cell lysates were analyzed for renin-dependent ANG I-forming activity. ANG I activity was measured in the absence and presence of the specific renin inhibitor BILA2157 (100 nM; IC50 = 1 nM) (49). BILA-sensitive ANG I-forming activity was present in mast cell lysates from control and UUO kidneys, but it was significantly greater in lysates from mast cells isolated from UUO kidneys [657 ± 126 pg·ml−1·min−1 UUO (n = 3) and 297 ± 51 pg·ml−1·min−1 CON (n = 3), P < 0.05] (Fig. 4E).
It has been shown that (pro)renin/renin is able to bind to the (pro)renin receptor (40). The possibility that mast cell renin originates from the circulation and is taken up by mast cell surface receptors was considered. While plasma renin activity was not elevated during the 14-day UUO time course [2.9 ± 0.4 ng·h−1·ml−1 UUO (n = 12) vs. 3.5 ± 0.8 ng·h−1·ml−1 CON (n = 4)], we followed up by screening fixed sections of UUO kidney for (pro)renin receptor expression. Immunofluorescence staining of UUO kidney with the anti-(pro)renin receptor antibody (red) showed staining of the distal tubules as previously reported (40). We were unable to detect (pro)renin receptor staining in kidney mast cells, as shown by the representative section showing a degranulating mast cell (green) in the peritubular region (Fig. 4F).
Having determined that mast cells do not express the (pro)renin receptor and that plasma renin levels are normal following UUO, we next evaluated sections of 14-day UUO kidney from CC mice for expression of mast cell renin. As in the rat (and human), the kidney mast cells are immunopositive for renin (Fig. 5A), raising the likelihood that release of mast cell renin and the ensuing ANG II contribute to the fibrotic response in the 14-day UUO kidney. We therefore treated mice (CC and MCD), who have undergone 14-day UUO, with losartan, the ANG II receptor blocker. Treatment with losartan curtails renal fibrosis (Fig. 5B), scoring a trace for fibrosis (Table 1) compared with untreated UUO kidney from CC mice (Fig. 5C and Table 1). UUO kidneys from losartan-treated and untreated MCD mice scored s trace (n = 3) for fibrosis (Fig. 5, D and E, respectively, and Table 1).
Mast cells also express chymase, a serine protease that converts ANG I to ANG II via an ACE-independent pathway (53). A recent study demonstrated that in patients with IgA nephropathy, the number of chymase-positive mast cells increased in parallel with tubulointerstitial fibrosis but was reduced with ANG II receptor blocker treatment (28). We therefore screened CC UUO mouse kidneys for mast cell chymase expression. While we did not quantify the difference in mast cell chymase expression between the UUO kidneys from vehicle- and losartan-treated mice, chymase-positive mast cells appeared more abundantly in kidney sections from vehicle-treated mice, being predominant in the renal medulla (Fig. 5F) compared with kidneys from losartan-treated mice, where they were fewer in number and found at the margins of the kidney (Fig. 5G). This assessment is in line with that observed in the renal biopsy samples from patients with IgA nephropathy (28). These results support the hypothesis that mast cells and the ensuing local RAS contribute to tubulointerstitial fibrosis in the 14-day UUO kidney.
Mast cell degranulation leads to renin-dependent increases in vascular resistance in the isolated, perfused kidney.
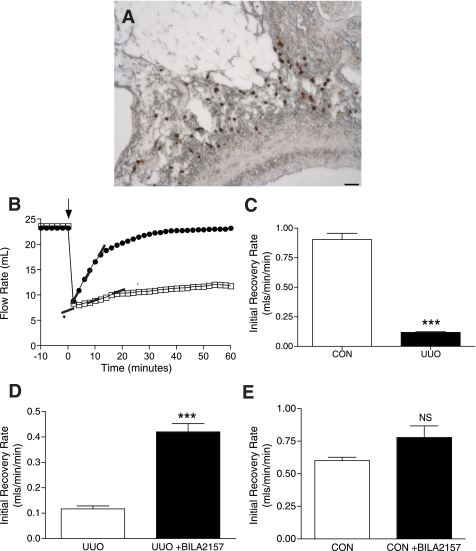
Since ANG II is a potent vasoconstricting agent, we hypothesized that release of mast cell renin upon mast cell degranulation could lead to measurable changes in renal vascular resistance. It was reasoned that this could be significant in the UUO kidney, where we find an increase in the mast cell population. Utilizing the isolated, perfused kidney preparation, flow rate, an indicator of changes in vascular resistance, was monitored in response to chemically induced mast cell degranulation. The mast cell-degranulating agent C48/80 was injected into the renal artery. Mast cells are closely associated with the renal vasculature, as shown in Fig. 6A. The contribution of mast cell renin to changes in flow rate was evaluated using BILA2157, the highly selective renin inhibitor (49). Baseline flow rates were similar in the UUO and CON isolated, perfused kidneys [24 ± 1 ml/min UUO (n = 4) vs. 24 ± 1 ml/min CON (n = 4)]. Mast cell degranulation led to an acute increase in vascular resistance as reflected by a significant reduction in flow rate due to a combination of mediators released from the mast cells, the magnitude of which was also similar between UUO and CON kidneys (7.0 ± 1 ml/min UUO vs. 6.5 ± 0.6 ml/min CON). Representative traces of the flow rate responses to mast cell degranulation in a CON (○) and a UUO kidney (□) are shown in Fig. 6B. C48/80 was injected (arrow), and a decrease in flow rate immediately followed in both kidney preparations. The initial recovery rate back to baseline was significantly prolonged in the UUO kidney compared with the CON kidney with the calculated slopes indicated by the dashed lines, being 0.13 ml·min−1·min−1 in the UUO kidney and 0.85 ml·min−1·min−1 in the CON kidney. For all of the isolated, perfused kidneys studied, the mean initial recovery rates were significantly less in UUO kidneys [0.12 ± 0.004 ml·min−1·min−1 UUO (n = 4) compared with 0.91 ± 0.05 ml·min−1·min−1 CON (n = 4), P < 0.001] (Fig. 6C).
Fig. 6.
Mast cell renin release increases vascular resistance in isolated, perfused UUO rat kidney. A: mast cells, labeled with avidin-HRP (brown), are closely apposed to renal artery in rat kidney. Scale bar = 50 μm. B: UUO kidney (14 day) has a slower initial rate of flow recovery after an acute increase in vascular resistance compared with CON kidney as shown in the representative traces of the flow rate response [UUO (□) and CON (○)]. Initial rates of flow recovery, as indicated by the dashed lines, are 0.13 ml·min−1·min−1 for UUO kidney and 0.85 ml·min−1·min−1 for CON kidney. C: initial flow recovery rate is significantly impaired in UUO compared with CON kidneys [0.12 ± 0.01 ml·min−1·min−1 UUO (n = 4) vs. 0.91 ± 0.05 ml·min−1·min−1 CON (n = 4)]. Values are means ± SE. ***P < 0.001. D: BILA2157-treated UUO kidney shows an improved initial rate of flow recovery compared with untreated UUO kidney [0.42 ± 0.03 ml·min−1·min−1 +BILA2157 (n = 4) vs. 0.12 ± 0.01 −BILA2157 (n = 4)]. Values are means ± SE. ***P < 0.001. E: BILA2157 treatment has no effect on the initial rate of flow recovery in CON kidney [0.78 ± 0.09 ml·min−1·min−1 +BILA2157 (n = 5) vs. 0.06 ± 0.03 −BILA2157 (n = 5)]. Values are means ± SE.
It is hypothesized that a component of the prolonged recovery rate in the UUO kidneys is due to mast cell renin-dependent ANG II vasoconstriction. Therefore, the protocol was performed in the presence of the renin inhibitor BILA2157 added to the perfusate. The presence of BILA2157 in the perfusate had a significant effect on the initial rate of recovery in the UUO kidneys, increasing it more than three times [0.42 ± 0.03 ml·min−1·min−1 +BILA2157 (n = 4) vs. 0.12 ± 0.01 UUO (n = 4), P < 0.0.001] (Fig. 6D). However, the renin inhibitor did not make a significant difference to the initial rate of recovery in the CON kidneys [0.78 ± 0.09 ml·min−1·min−1 +BILA2157 (n = 5) vs. 0.60 ± 0.03 ml·min−1·min−1 CON (n = 5)] (Fig. 6E).
Human kidney mast cells express renin.
The relevance of these findings to the human kidney was next investigated. Archived sections of fixed human kidneys from patients diagnosed with renal fibrosis were screened for mast cells as shown in the toluidine blue-stained section showing mast cells closely apposed to a tubule (Fig. 7A). Mast cells are also associated with fibrotic regions of the kidney (Fig. 7B) and are immunopositive for renin (Fig. 7C) and chymase (Fig. 7F). The renin localization in the kidney mast cells was discrete from the heparin-rich granules labeled with avidin. Mast cells were next immunomagnetically isolated from macroscopically normal human kidney waste tissue specimens. Aliquots of the isolated cells were stained with toluidine blue to establish mast cell identify (Fig. 7D). To ascertain that the renin protein immunoexpressed in the human kidney mast cells is active, i.e., capable of cleaving Aogen, lysates from the isolated mast cells were assayed for ANG I activity by RIA. ANG I activity was measured in the mast cell lysates in the absence and presence of BILA2157 (Fig. 7E). There was significant ANG I-forming activity in human kidney mast cell lysates (316 ± 49 pg·ml−1·min−1), and 50% of the ANG I-forming activity was BILA2157 sensitive. These results are consistent with our previous findings in the human mastocytyoma cell line HMC-1 and freshly isolated human lung mast cells showing active BILA-sensitive ANG I-forming activity (48, 54).
DISCUSSION
The RAS is a key player in the progression of chronic renal failure, characterized by tubulointerstitial fibrosis, a common pathomorphological feature (57). Studies show that the pathogenesis of tubulointerstitial fibrosis of obstructive nephropathy is mediated, in part, by increased intrarenal levels of ANG II (22). The identification of tissue-specific RAS, whereby locally generated ANG II acts on resident receptors, has gained considerable attention, especially in the heart (19, 32), brain (52), eye (55), testis (42) lung (54), and kidney (57). Micropuncture and microdialysis experiments demonstrate that proximal tubular and interstitial fluid contains ANG II concentrations in the nanomolar ranges compared with the picomoles in the systemic circulation kidney (57), arguing in favor of a local kidney RAS. Renin mRNA and immunoreactive protein have been detected in proximal and connecting tubules as well as in cells of the human and mouse collecting duct (36, 39). Proximal tubule cells can produce Aogen (38). Renin, Aogen, ANG I, angiotensin-converting enzyme (ACE), and ANG II, have been found in renal interstitial fluid (37, 38, 47). Our results demonstrate that kidney mast cells represent an additional intrarenal source of renin, the rate-limiting enzyme in the RAS cascade. In the normal kidney, mast cells are localized to the connective tissue around vascular bundles and are scarce in the parenchyma (43). In inflammatory kidney diseases, mast cells appear in greater number and infiltrate the kidney (2). In diabetic nephropathy (44), IgA nephropathy (9, 10), chronic glomerulonephritis (12), and chronic pyelonephritis (43), mast cells are found in the renal interstitium (2, 10, 41, 43) and peritubular regions (2, 6). Our results demonstrate that mast cells increase in number and infiltrate the kidney in 14-day UUO (Fig. 1). Mature mast cells are noncirculating, yet they have the capacity to proliferate and migrate within tissues (15). Mast cells can either reenter the cell cycle to undergo proliferation locally (15, 16) or come from circulating hematopoietic stem cells (16). The origin of the increased mast cell population we observed in UUO kidney was not determined in the present study.
A correlation between mast cell number and degree of interstitial fibrosis has suggested that mast cells play a pathogenic role in renal fibrosis (43). Our results confirm this in that treatment with the mast cell stabilizer cromolyn sodium reduced collagen deposition and tubulointerstitial fibrosis in the 14-day obstructed kidney (Fig. 2). While the primary target of this cromolyn sodium is mast cells, we cannot rule out that this mast cell stabilizer is also acting on other cells infiltrating the UUO kidney, like monocytes and macrophages (26), that could also contribute to the fibrotic response. Our results from the mast cell-deficient genetic mouse model strengthened our evidence that indeed mast cells are necessary for the development of renal fibrosis in UUO (Fig. 3).
While the results of our study are consistent with other studies demonstrating that mast cells contribute to renal fibrosis (23), they differ from other studies showing that mast cells protect against renal fibrosis (24, 35). Using the same MCD mouse strain that we utilized in our experiments, Kim et al. (24) showed that with 3-wk UUO the kidneys with the mast cells from congenic control mice had less fibrosis compared with the kidneys from the MCD mice. Another study using MCD rats found that mast cells protect against interstitial fibrosis in the puromycin aminonucleoside-nephrosis model of chronic glomerular disease (35). The difference in our findings from those reported by Kim et al. (24) and Miyazawa et al. (35) may reflect the different time frames used to evaluate tubulointerstitial fibrosis. Renal fibrosis is a dynamic and complicated process characterized by initial interstitial inflammation, tubular cell activation, and transformation of epithelial cells. Renal fibrosis is characterized by both excessive accumulation and deposition of extracellular matrix components (30), leading to collagen deposition, and the upregulation of matrix-degrading enzymes which could diminish tubulointerstitial fibrosis (30). Mast cells have the capacity to secrete mediators and enzymes that could either trigger collagen deposition or degrade excessive extracellular matrix. The dual response of mast cells, harmful and protective, may reflect their capacity to alter their phenotype as a function of the microenvironment (16) and account for their different roles. Therefore, evaluating the contribution of mast cells to renal fibrosis may be dependent upon the stage of the fibrotic disease.
Mast cells release a number of proinflammatory and profibrotic mediators which can promote fibrosis in the UUO kidney. ANG II is a known fibrogenic agent in the kidney (22) and is known to play a significant role in UUO (24). Furthermore, mice genetically deficient in ANG II develop less renal fibrosis in obstruction (8, 13). Our previous findings in the lung and heart have demonstrated that mast cells synthesize and release catalytically active renin (32, 54). Kidney mast cells were therefore screened for renin expression and activity (Figs. 4 and 7). Our findings demonstrate that kidney mast cells express active renin that can cleave Aogen to form ANG I (Figs. 4E and 7E). These results demonstrate that mast cells may serve as an additional source of renin in the kidney. Mast cells are also known to store and release cathepsin D and chymase, two proteases involved in the formation of ANG II. Mast cell-derived cathepsin D cleaves Aogen to ANG I, although at a rate 105 times slower than renin and at a much lower pH (i.e., pH optima at 3.9 and 6.8 for cathepsin D and renin, respectively) (18). Chymase converts ANG I to ANG II independently of ACE. It cleaves the phenylalanine-histidine peptide bond in ANG I to generate ANG II. While not all mast cells produce chymase (28), we demonstrate that chymase-positive mast cells are present in the UUO mouse kidney (Fig. 5, F and G) and human kidney (Fig. 7F).
To rule out a systemic counterpart to a local kidney RAS, we measured plasma renin activity levels over the 14-day time course of UUO. The mean rat plasma renin activity during the course of UUO was not significantly different from the controls, in agreement with that reported by others (22). The possibility exists that an acute elevation in plasma renin levels at the initial postsurgical period may have been cleared from the circulation by binding to surface receptors, like the pro(renin) receptor. Our immunoscreen of fixed sections from UUO kidneys showed that the mast cells were not immunopositive for the pro(renin) receptor (Fig. 4F). Our data support that kidney mast cells represent an additional source of active renin.
UUO is known to cause changes in renal hemodynamics (22) with vasoconstriction of the ipsilateral kidney (4). Our studies demonstrate that in the isolated, perfused 14-day UUO kidney, mast cell degranulation leads to sustained vasoconstriction. In that our results show that the mast cell population is increased in obstructed kidney and that the mast cells express renin, experiments were performed with the isolated, perfused kidney preparation, open-circuit mode (7, 31, 46) to determine whether mast cell renin contributes to increased renal vascular resistance. Since the isolated and perfused kidney preparation represents a dilated state (7, 31, 46), we cannot evaluate the contribution of mast cell mediators to decreased vascular resistance. Chemically induced mast cell degranulation led to an acute increase in vascular resistance as reflected by a significant reduction in flow rate that was due to a combination of mediators released from the mast cells. Our results show that in UUO kidneys, mast cell degranulation leads to prolonged flow rate recovery back to baseline compared with CON kidneys and that renin (ANG II) plays a role in this prolonged recovery (Fig. 6). This is consistent with our finding that mast cell renin is active.
Mast cells release a variety of mediators, some of which are vasodilatory and others of which are vasoconstrictive. It is known that infusion of histamine stimulates release of renin from the juxtaglomerular apparatus via histamine H2 receptor activation (46). It is therefore feasible that histamine released from mast cells could also stimulate renin release from mast cells in an autocrine fashion in that mast cells express the histamine H4 receptor (KD = 136 nM for rat histamine H4 receptor) (29).
The pathogenesis of renal fibrosis is a progressive and complicated process involving multiple molecular pathways and cellular targets (56). Among the many factors involved in this process, transforming growth factor (TGF-β) plays a major role (8, 30) as demonstrated by Isaka et al. (21), who blocked tubulointerstitial fibrosis in UUO kidneys with TGF-β1 antisense oligodeoxynucleotides. ANG II is known to stimulate production of TGF-β in UUO (22). The relationship between ANG II and TGF-β has been documented in vivo in genetically defined mouse models of Marfan syndrome (17). Studies show that selected manifestations of Marfan syndrome reflect excessive signaling by TGF-β (5). Systemic antagonism of TGF-β through administration of a TGF-β-neutralizing antibody or treatment with losartan prevented aortic aneurysm (17) and normalized muscle architecture, repair, and function in Marfan-like fibrillin-1-deficient mice (5).
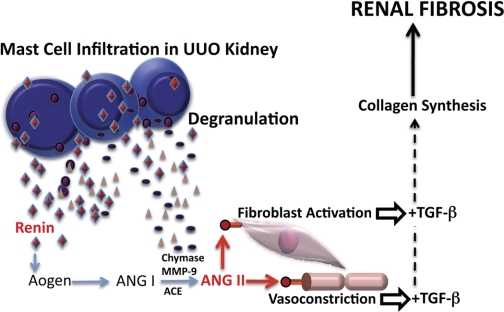
Based on the results from our study and those of others, we propose the following model linking mast cells, mast cell renin, mast cell chymase, and local ANG II formation to increases in TGF-β and collagen synthesis, leading to renal fibrosis (Fig. 8). It describes a paracrine pathway and the initiation of a local intrarenal RAS due to mast cell infiltration as part of an inflammatory response in the obstructed kidney. It is hypothesized that activation of this mast cell-dependent local RAS pathway contributes to excessive collagen deposition and vasoconstriction. According to our scheme, degranulation of infiltrating mast cells releases renin into the surrounding tubulointerstitial space, making it available to Aogen. The cleavage of Aogen by renin results in ANG I formation. ANG I is further cleaved to ANG II either by ACE or mast cell-derived chymase (20, 53) or MMP-9 (33). Our model predicts that the local production of ANG II, triggered by mast cell degranulation, exerts multiple effects via activation of AT1R, such as vasoconstriction and fibroblast stimulation (45). It is speculated that ANG II activation of AT1R on neighboring fibroblasts stimulates TGF-β production. The ischemia caused by ANG II-induced vasoconstriction may also increase TGF-β production (8). Accordingly, mast cell degranulation with the concomitant release of renin, triggering the local production of ANG II, is a critical component in the development of tubulointerstital fibrosis resulting from the stimulation of TGF-β and the concomitant increase in collagen production.
Fig. 8.
Model depicting the role of mast cell renin and the ensuing ANG II in increased levels of transforming growth factor (TGF)-β and renal fibrosis.
In conclusion, this study identifies a novel therapeutic target in early-onset renal disease. In that the RAS is associated with fibrogenesis in the obstructed kidney (22, 26), our finding that mast cells express and secrete active renin, representing a previously unknown pool of this enzyme in the kidney, may represent a new approach in the management of end-stage renal disease. In addition, screening urine for mast cell mediators in patients susceptible to renal fibrosis may have prognostic implications before the onset of histologically discernible tubulointerstitial fibrosis.
GRANTS
This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK060726 (R. B. Silver) and in part by the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust and the Robert J. Kahn Foundation (D. Felsen and D. P. Poppas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.V., A.C.R., N.O., R.M., J.A.B., R.E., T.K., J.C., and D.F. performed experiments; A.V., A.C.R., N.O., R.M., J.A.B., R.E., T.K., D.F., S.V.S., D.P.P., T.M., and R.B.S. analyzed data; A.V., A.C.R., N.O., R.M., J.A.B., R.E., T.K., D.F., S.V.S., D.P.P., T.M., and R.B.S. interpreted results of experiments; A.V., A.C.R., N.O., R.M., J.A.B., R.E., and R.B.S. prepared figures; A.V., A.C.R., N.O., R.M., J.A.B., R.E., T.K., J.C., D.F., S.V.S., D.P.P., T.M., and R.B.S. approved final version of manuscript; D.F., D.P.P., T.M., and R.B.S. provided conception and design of research; R.B.S. drafted manuscript; R.B.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We acknowledge Karl Migally for help with the computational evaluation of collagen staining and Marin Schlossberg for manuscript preparation. We are grateful to A. Weinstein and R. Levi for a critical reading of the manuscript.
REFERENCES
- 1. Bergstresser PR, Tigelaar RE, Tharp MD. Conjugated avidin identifies cutaneous rodent and human mast cells. J Invest Dermatol 83: 214–218, 1984 [DOI] [PubMed] [Google Scholar]
- 2. Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y. Mast cells and inflammatory kidney disease. Immunol Rev 217: 79–95, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Chevalier RL, Thornhill BA, Gomez RA. EDRF modulates renal hemodynamics during unilateral ureteral obstruction in the rat. Kidney Int 42: 400–406, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med 13: 204–210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colvin RB, Dvorak AM, Dvorak HF. Mast cells in the cortical tubular epithelium and interstitium in human renal disease. Hum Pathol 5: 315–326, 1974 [DOI] [PubMed] [Google Scholar]
- 7. De Mello G, Maack T. Nephron function of the isolated perfused rat kidney. Am J Physiol 231: 1699–1707, 1976 [DOI] [PubMed] [Google Scholar]
- 8. Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Ehara T, Shigematsu H. Contribution of mast cells to the tubulointerstitial lesions in IgA nephritis. Kidney Int 54: 1675–1683, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Ehara T, Shigematsu H. Mast cells in the kidney. Nephrology (Carlton) 8: 130–138, 2003 [DOI] [PubMed] [Google Scholar]
- 11. el-Dahr SS, Gee J, Dipp S, Hanss BG, Vari RC, Chao J. Upregulation of renin-angiotensin system and downregulation of kallikrein in obstructive nephropathy. Am J Physiol Renal Fluid Electrolyte Physiol 264: F874–F881, 1993 [DOI] [PubMed] [Google Scholar]
- 12. El-Koraie AF, Baddour NM, Adam AG, El Kashef EH, El Nahas AM. Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int 60: 167–172, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Fern RJ, Yesko CM, Thornhill BA, Kim HS, Smithies O, Chevalier RL. Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J Clin Invest 103: 39–46, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furuichi K, Gao JL, Murphy PM. Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am J Pathol 169: 372–387, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galli SJ. New concepts about the mast cell. N Engl J Med 328: 257–265, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 23: 749–786, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hackenthal E, Hackenthal R, Hilgenfeldt U. Isorenin, pseudorenin, cathepsin D and renin. A comparative enzymatic study of angiotensin-forming enzymes. Biochim Biophys Acta 522: 574–588, 1978 [DOI] [PubMed] [Google Scholar]
- 19. Hirsch AT, Opsahl JA, Lunzer MM, Katz SA. Active renin and angiotensinogen in cardiac interstitial fluid after myocardial infarction. Am J Physiol Heart Circ Physiol 276: H1818–H1826, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Ibels LS, Gyory AZ. IgA nephropathy: analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine (Baltimore) 73: 79–102, 1994 [PubMed] [Google Scholar]
- 21. Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M. Transforming growth factor-beta 1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int 58: 1885–1892, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Ishidoya S, Morrissey J, McCracken R, Reyes A, Klahr S. Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int 47: 1285–1294, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Jones SE, Kelly DJ, Cox AJ, Zhang Y, Gow RM, Gilbert RE. Mast cell infiltration and chemokine expression in progressive renal disease. Kidney Int 64: 906–913, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Kim DH, Moon SO, Jung YJ, Lee AS, Kang KP, Lee TH, Lee S, Chai OH, Song CH, Jang KY, Sung MJ, Zhang X, Park SK, Kim W. Mast cells decrease renal fibrosis in unilateral ureteral obstruction. Kidney Int 75: 1031–1038, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Klahr S, Ishidoya S, Morrissey J. Role of angiotensin II in the tubulointerstitial fibrosis of obstructive nephropathy. Am J Kidney Dis 26: 141–146, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kokkonen JO, Kovanen PT. Low-density-lipoprotein binding by mast-cell granules. Demonstration of binding of apolipoprotein B to heparin proteoglycan of exocytosed granules. Biochem J 241: 583–589, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konishi Y, Morikawa T, Okada N, Maeda I, Kitabayashi C, Yoshioka K, Okumura M, Nishiyama A, Ueda M, Takai S, Miyazaki M, Imanishi M. Evidence for abundant presence of chymase-positive mast cells in the kidneys of patients with immunoglobulin A nephropathy: effect of combination therapy with prednisolone and angiotensin II receptor blocker valsartan. Hypertens Res 31: 1517–1524, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Liu C, Wilson SJ, Kuei C, Lovenberg TW. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther 299: 121–130, 2001 [PubMed] [Google Scholar]
- 30. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Maack T. Physiological evaluation of the isolated perfused rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 238: F71–F78, 1980 [DOI] [PubMed] [Google Scholar]
- 32. Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T, Silver RB, Levi R. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 116: 1063–1070, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin MM, Buckenberger JA, Jiang J, Malana GE, Knoell DL, Feldman DS, Elton TS. TGF-β1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK, JNK, and PI3K signaling pathways. Am J Physiol Lung Cell Mol Physiol 293: L790–L799, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Matoba T, Murakami K, Inagami T. Rat renin: purification and characterization. Biochim Biophys Acta 526: 560–571, 1978 [DOI] [PubMed] [Google Scholar]
- 35. Miyazawa S, Hotta O, Doi N, Natori Y, Nishikawa K. Role of mast cells in the development of renal fibrosis: use of mast cell-deficient rats. Kidney Int 65: 2228–2237, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navar LG. The intrarenal renin-angiotensin system in hypertension. Kidney Int 65: 1522–1532, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Navar LG, Harrison-Bernard LM, Imig JD, Wang CT, Cervenka L, Mitchell KD. Intrarenal angiotensin II generation and renal effects of AT1 receptor blockade. J Am Soc Nephrol 10, Suppl 12: S266–S272, 1999 [PubMed] [Google Scholar]
- 38. Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol 10, Suppl 11: S189–S195, 1999 [PubMed] [Google Scholar]
- 39. Navar LG, Nishiyama A. Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pavone-Macaluso M. Tissue mast cells in renal diseases. Acta Pathol Microbiol Scand 50: 337–346, 1960 [DOI] [PubMed] [Google Scholar]
- 42. Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept 43: 1–20, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Roberts IS, Brenchley PE. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol 53: 858–862, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruger BM, Hasan Q, Greenhill NS, Davis PF, Dunbar PR, Neale TJ. Mast cells and type VIII collagen in human diabetic nephropathy. Diabetologia 39: 1215–1222, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Ruiz-Ortega M, Egido J. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int 52: 1497–1510, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Schwertschlag U, Hackenthal E. Histamine stimulates renin release from the isolated perfused rat kidney. Naunyn Schmiedebergs Arch Pharmacol 319: 239–242, 1982 [DOI] [PubMed] [Google Scholar]
- 47. Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest 86: 1352–1357, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R. Mast cells: a unique source of renin. Proc Natl Acad Sci USA 101: 13607–13612, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simoneau B, Lavallee P, Anderson PC, Bailey M, Bantle G, Berthiaume S, Chabot C, Fazal G, Halmos T, Ogilvie WW, Poupart MA, Thavonekham B, Xin Z, Thibeault D, Bolger G, Panzenbeck M, Winquist R, Jung GL. Discovery of non-peptidic P2-P3 butanediamide renin inhibitors with high oral efficacy. Bioorg Med Chem 7: 489–508, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Tharp MD, Seelig LL, Jr, Tigelaar RE, Bergstresser PR. Conjugated avidin binds to mast cell granules. J Histochem Cytochem 33: 27–32, 1985 [DOI] [PubMed] [Google Scholar]
- 51. Timoshanko JR, Kitching R, Semple TJ, Tipping PG, Holdsworth SR. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J Am Soc Nephrol 17: 150–159, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Unger T, Badoer E, Ganten D, Lang RE, Rettig R. Brain angiotensin: pathways and pharmacology. Circulation 77: I40–I54, 1988 [PubMed] [Google Scholar]
- 53. Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem 265: 22348–22357, 1990 [PubMed] [Google Scholar]
- 54. Veerappan A, Reid AC, Estephan R, O'Connor N, Thadani-Mulero M, Salazar-Rodriguez M, Levi R, Silver RB. Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci USA 105: 1315–1320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, Schalekamp MA, Ganten D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol 80: 159–163, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wen JG, Frokiaer J, Jorgensen TM, Djurhuus JC. Obstructive nephropathy: an update of the experimental research. Urol Res 27: 29–39, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Wolf G, Butzmann U, Wenzel UO. The renin-angiotensin system and progression of renal disease: from hemodynamics to cell biology. Nephron Physiol 93: P3–P13, 2003 [DOI] [PubMed] [Google Scholar]