Abstract
Angiotensin (ANG) II-dependent hypertension is characterized by increases in intrarenal ANG II levels, derangement in renal hemodynamics, and augmented tubular sodium reabsorptive capability. Increased nephron expression of renin-angiotensin system components, such as angiotensinogen by proximal tubule cells and renin by collecting duct principal cells, has been associated with an augmented ability of the kidney to form ANG II in hypertensive states. However, the contribution of de novo intrarenal ANG II production to the development and maintenance of ANG II-dependent hypertension remains unclear. The present study was performed to determine the effects of selective intrarenal renin inhibition on whole kidney hemodynamics and renal excretory function in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension in the absence of the confounding influence of associated reductions in mean arterial pressure (MAP). Male Cyp1a1-Ren2 transgenic rats were induced to develop malignant hypertension, anesthetized, and surgically prepared for intrarenal administration of the direct renin inhibitor aliskiren (0.01 mg/kg). Following acute aliskiren treatment, urine flow and sodium excretion increased (10.5 ± 1.1 to 15.9 ± 1.9 μl/min, P < 0.001; 550 ± 160 to 1,370 ± 320 neq/min, P < 0.001, respectively) and ANG II excretion decreased (120 ± 30 to 63 ± 17 fmol/h, P < 0.05). There were no significant changes in MAP, glomerular filtration rate, estimated renal plasma flow, plasma ANG II levels, or protein excretion. The present findings demonstrate that selective renal renin inhibition elicits diuretic and natriuretic responses in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension. Elevated intraluminal ANG II levels likely act to augment tubular reabsorptive function and, thereby, contribute to the elevated blood pressure in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension.
Keywords: kidney, renin-angiotensin system, aliskiren, arterial blood pressure, renal hemodynamics, sodium excretion, urine flow
since the discovery of an augmented intrarenal renin-angiotensin system (RAS) during angiotensin II (ANG II)-dependent hypertension, great interest has surrounded the possible paracrine actions of kidney-derived ANG II on renal hemodynamics and tubular reabsorptive function in hypertensive states (10). Increased tubular ANG II levels can result from uptake from the systemic circulation and from increased conversion of proximal tubule-derived angiotensinogen (AGT) to ANG I by collecting duct (CD) renin (12). ANG II concentrations well below that measured in the urine have been shown to stimulate distal tubular sodium transport (20). However, the relative contribution of de novo intrarenal ANG II production to the development and maintenance of ANG II-dependent hypertension remains unclear.
RAS blockade with the use of pharmacological inhibitors that act at multiple levels of this cascade is widely used to treat hypertension and to prevent associated comorbidities. However, chronic treatment with many of these drugs is known to result in an increase in plasma renin activity (PRA). Aliskiren is the first direct renin inhibitor approved for the treatment of hypertension (3). Aliskiren suppresses the activity of renin to catalyze the conversion of AGT to ANG I (25). While aliskiren was designed to inhibit human renin (IC50 = 0.6 nM), it also effectively inhibits the activity of mouse renin (IC50 = 4.5 nM) and rat renin (IC50 = 80 nM) (3). It is reported to localize within glomeruli, juxtaglomerular cells of the afferent arteriole, intrarenal arteries, vasa recta, distal tubules, and CDs following intravenous administration (4, 5). The compound binds the active site of both soluble and receptor-bound renin and prorenin, thus acting to suppress both PRA and the production of the vasoactive hormone ANG II (4).
The Cyp1a1-Ren2 transgenic rat line [strain name: TGR (Cyp1a1Ren2)] is a high-PRA and high-circulating ANG II model of hypertension. It was generated by insertion of an 11.5-kb fragment of the cytochrome P-450 1a1 (Cyp1a1) promoter, fused to the mouse Ren2 renin gene, into a neutral genomic site on the Y chromosome of the Fischer 344 rat (11). Cyp1a1, which catalyzes the oxidation of a wide range of endogenous lipophilic compounds and xenobiotics, is not constitutively expressed in the rat, but it is highly inducible upon exposure to aryl hydrocarbons, such as indole-3-carbinol (I3C) (1, 15). After Cyp1a1-Ren2 rats ingest I3C in rat chow, I3C can interact with the aryl hydrocarbon receptor, a basic helix-loop-helix-transcription factor, to drive expression of the Cyp1a1-Ren2 transgene and thus stimulate hepatic release of mouse renin into the systemic circulation. This transgenic rat model therefore allows clamping of extrarenal renin gene expression and induction of hypertension consequent to fixed levels of elevated plasma renin and prorenin that are not subject to normal physiological feedback mechanisms. When I3C is withheld, Cyp1a1-Ren2 transgenic rats do not express the Ren2 renin gene and do not spontaneously develop hypertension (11, 18).
Administration of I3C at a dose of 0.3% (wt/wt) induces the development of ANG II-dependent malignant hypertension (9, 17, 30), which is a form of severe hypertension characterized by fibrinoid necrosis and vascular damage in many tissues, including the kidney (7). In rats, this condition manifests as a rapid rise in systemic blood pressure accompanied by a significant loss of body weight, polyuria, polydipsia, piloerection, lethargy, and hunched posturing (22). In addition, Cyp1a1-Ren2 transgenic rats with malignant hypertension exhibit increases in PRA, plasma ANG II levels, and intrarenal ANG II levels (18).
It has previously been demonstrated that systemic administration of the direct renin inhibitor aliskiren decreases mean arterial pressure (MAP) and renal vascular resistance (RVR) while increasing estimated renal plasma flow (RPF) in Cyp1a1-Ren2 transgenic rats with ANG II-dependent malignant hypertension (9). However, little is known about the effects of selective intrarenal administration of aliskiren on whole kidney hemodynamics and excretory function in ANG II-dependent malignant hypertension in the absence of the confounding influence of the associated reductions in arterial blood pressure. In light of the possible paracrine actions of kidney-derived ANG II on the development and maintenance of hypertension, one may attribute the reported beneficial effects of aliskiren to inhibition of de novo intrarenal ANG II generation as well as reduced systemic blood pressure.
The present study was performed to address the hypothesis that de novo intrarenal ANG II generation is elevated in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension, and this increased intrarenal ANG II contributes to the impaired renal hemodynamics and excretory function in ANG II-dependent malignant hypertension. In the present study, intrarenal administration of aliskiren increased urine flow and sodium excretion in hypertensive Cyp1a1-Ren2 rats, indicating that elevated intrarenal ANG II levels contributed to an augmented tubular reabsorptive function in these transgenic rats. To address this issue, additional experiments were performed to evaluate urinary ANG II excretion in hypertensive Cyp1a1-Ren2 rats and to determine the effects of selective intrarenal aliskiren administration on urinary ANG II excretion in these transgenic rats with malignant hypertension.
MATERIALS AND METHODS
The experimental procedures in this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Tulane University Health Sciences Center. Experiments were performed on 12-wk-old Cyp1a1-Ren2 transgenic rats [TGR(Cyp1a1Ren2)] with inducible expression of the mouse Ren2 renin gene (11). All rats used in the present study were bred at Tulane University School of Medicine from stock animals supplied by Harlan UK Limited, Bicester, UK. Two groups of Cyp1a1-Ren2 transgenic rats were maintained on a normal, non-I3C rat diet (diet TD 99414, Harlan-Teklad) for 10 days (noninduced; n = 6–8/group). Two additional groups of Cyp1a1-Ren2 transgenic rats were fed a normal rat diet containing 0.3% I3C (wt/wt; diet TD 05381, Harlan-Teklad) for 10 days to induce ANG II-dependent malignant hypertension (hypertensive; n = 6–7/group). The rats were allowed free access to food and tap water until the day of the experiment. Body weight was measured daily throughout the course of the study.
At the conclusion of the treatment period, animals were surgically prepared for selective intrarenal administration of aliskiren (0.01 mg/kg). The rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and placed on a surgical table thermostatically controlled to maintain body temperature at 37°C. A tracheostomy was performed, and the animals were allowed to breathe humidified air enriched with oxygen (95% O2-5% CO2). The left jugular vein was cannulated with a PE-50 catheter to allow infusion of solutions and additional anesthetic. The rats were infused at a constant rate of 1.2 ml/h with 0.9% saline containing 6% albumin (bovine; Sigma, St. Louis, MO) during surgery and thereafter with 0.9% saline containing 1% albumin, 7.5% polyfructosan (Inutest; Laevosan Geselschaft, Linz, Austria), and 1.5% PAH (Merck Sharp & Dohme, West Point, PA). A PE-50 catheter was inserted into the right femoral artery to allow monitoring of arterial blood pressure and to facilitate collection of blood samples. Blood pressure was monitored with a Statham pressure transducer (model P23DC) and recorded using a computerized data-acquisition system (MP100 System; BIOPAC Systems, Santa Barbara, CA) with the AcqKnowledge Software Package (version 3.7.3, BIOPAC). A PE-10 catheter was inserted into the left femoral artery and advanced to the level of the left renal artery. The abdominal aorta was exposed via a flank incision, and the catheter was advanced into the left renal artery and positioned to allow unobstructed flow of blood into the kidney. The left kidney was then freed from surrounding tissue and placed in a Lucite cup. The left ureter was cannulated to allow timed urine collections to be obtained.
After a 45-min recovery period from surgery, urine was collected during two 30-min control periods, followed by a control blood sample (∼250 μl). Aliskiren (Novartis Pharma AG, Basel, Switzerland) was administered by intra-arterial bolus injection (0.01mg/kg) into the left kidney. This dose of aliskiren was chosen based on preliminary studies where it was shown to be the maximum dose that could be administered directly into the renal artery without significant spillover into the systemic circulation and alteration of arterial blood pressure. After a 15-min equilibration period, urine was collected during two 30-min experimental periods, followed by collection of an additional blood sample (∼250 μl). Aliskiren has a reported average plasma half-life of 23.7 h (29). Therefore, inhibition of intrarenal renin activity is expected to be stable during the 1-h experimental period.
MAP and urine flow were assessed in all transgenic rats (noninduced, n = 14; hypertensive, n = 13) during baseline conditions and following acute intrarenal infusion of aliskiren into the left renal artery. Renal hemodynamics and excretory function were assessed, as described previously (9, 23), in one group of noninduced rats (n = 6) and in one group of hypertensive rats (n = 6) before and after intrarenal aliskiren administration. Urinary protein excretion and urinary and plasma ANG II levels were determined in additional groups of noninduced rats (n = 8) and hypertensive rats (n = 7) during baseline conditions and following acute intrarenal infusion of aliskiren. For these studies, urine and blood samples were collected into a mixed inhibitor solution (final concentration: 5 mmol/l EDTA, 1.25 mmol/l 1,10-phenanthroline, 10 μmol/l PMSF, 20 μmol/l pepstatin, and 20 μmol/l enalapril maleate) to prevent the degradation or further generation of ANG II. At the conclusion of the protocol, the left kidney was excised, blotted dry, and weighed.
To confirm that intrarenal ANG II, regardless of whether it is locally generated or delivered by the systemic circulation, stimulates tubular sodium and water reabsorption, additional studies were performed to determine the effects of selective intrarenal administration of the AT1 receptor antagonist candesartan (AstraZeneza R&D, Mölndal, Sweden) on renal hemodynamics and excretory function. Candesartan was administered by intra-arterial bolus injection (750 ng) in one group of Cyp1at-Ren2 rats (n = 5) that had been fed a normal rat diet containing 0.3% I3C for 10 days to induce ANG II-dependent malignant hypertension and then surgically prepared as described above. MAP, glomerular filtration rate (GFR), estimated RPF, RVR, urine flow, and urinary and fractional sodium excretion were determined during control conditions and following selective intrarenal administration of candesartan. Pilot studies and previous publications (2, 13) indicated that this dose of candesartan is sufficient to block the renal vasoconstrictor effects of ANG II without spillover into the systemic circulation in sufficient amounts to produce significant decreases in MAP.
Urine volume was determined gravimetrically. Sodium concentration in urine and plasma was measured using flame photometry. Urine protein concentrations were measured by a colorimetric assay using a commercially available kit (Bio-Rad, Hercules, CA). Inulin and PAH concentrations in both urine and plasma were measured by standard spectrophotometry. GFR and RPF were estimated from the clearances of inulin and PAH, respectively. Renal blood flow (RBF) was calculated as estimated RPF/(1 − Hct). RVR was determined from the quotient of MAP and calculated RBF.
For urinary and plasma ANG II measurements, samples underwent extraction and radioimmunoassay (RIA) as described previously (27). Briefly, urine collected into a mixed inhibitor solution was centrifuged, and representative 0.25-ml aliquots of the supernatants were diluted in 100% methanol, spun at 4,000 rpm for 10 min to remove any insoluble contaminants, and then evaporated to dryness under vacuum. Blood collected into the same mixed inhibitor solution was centrifuged to isolate plasma. Plasma was applied to phenyl-bonded solid-phase extraction columns (Bond-Elut, Varian) that had been prewashed with 90% methanol followed by water. After sample application, each solid-phase extraction column was washed sequentially with water, hexane, and chloroform. Angiotensin peptides were eluted from the solid-phase extraction column with 90% methanol (8). The eluants were collected and evaporated to dryness under vacuum. All samples were then reconstituted with RIA buffer and incubated with rabbit anti-ANG II antisera (Peninsula Laboratories) and 125I-radiolabeled ANG II (Perkin Elmer Life and Analytical Sciences) for 48 h at 4°C. Bound and free ANG II peptides were separated by dextran-coated charcoal, and the supernatant was counted by a computer-linked gamma counter for 3 min.
Statistical analyses were performed using two-way repeated-measures ANOVA followed by Student-Newman-Keuls test. All statistical analyses were performed using SigmaPlot for Windows (version 11; Systat Software, San Jose, CA). Statistical significance was defined as P < 0.05. All data are expressed as means ± SE.
RESULTS
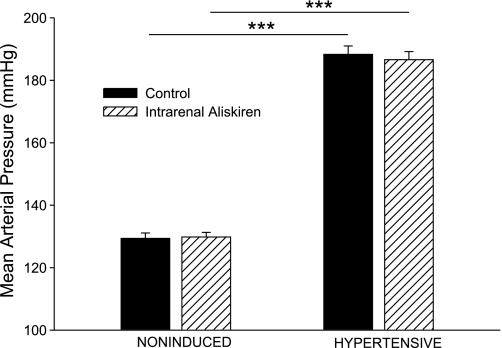
Chronic dietary administration of 0.3% I3C to Cyp1a1-Ren2 rats for 10 days resulted in significant elevations in MAP (188 ± 3 vs. 129 ± 2 mmHg, P < 0.001; Fig. 1) and marked decreases in body weight (321 ± 10 to 256 ± 10 g, P < 0.001). The I3C-induced rats demonstrated severe lethargy, piloerection, and assumption of hunched posture, which are manifestations of malignant hypertension in the rat (7, 11, 17, 18). As shown in Fig. 1, intrarenal aliskiren administration did not change MAP in either hypertensive or noninduced rats.
Fig. 1.
Mean arterial blood pressure in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). ***P < 0.001.
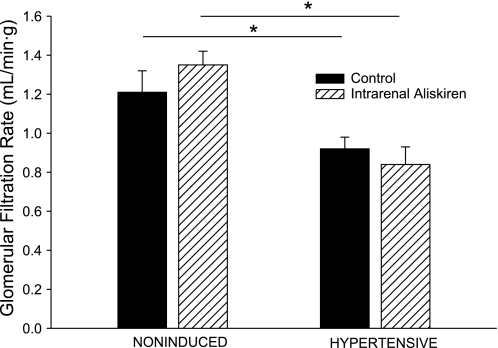
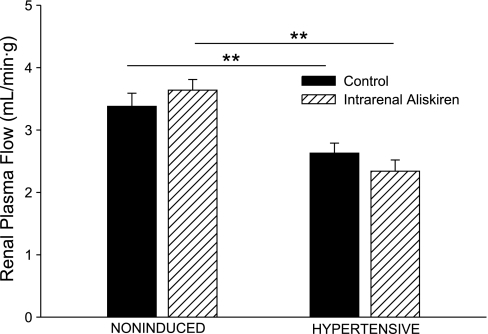
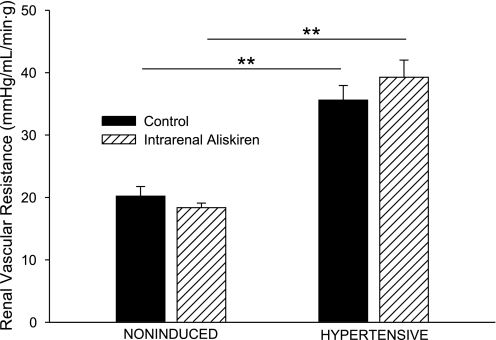
Baseline estimated RPF and GFR in the hypertensive rats were significantly lower than the corresponding values in the noninduced rats (2.63 ± 0.16 vs. 3.38 ± 0.21 ml·min−1·g−1, P < 0.01, and 0.92 ± 0.06 vs. 1.21 ± 0.11 ml·min−1·g−1, P < 0.05, respectively; Figs. 2 and 3). Intrarenal aliskiren administration did not change estimated RPF or GFR in either group of Cyp1a1-Ren2 transgenic rats. As shown in Fig. 4, baseline RVR was greater in the hypertensive rats than that in the noninduced rats (35.6 ± 2.3 vs. 20.2 ± 1.6 mmHg·ml−1·min·g, P < 0.01). Aliskiren administration did not alter RVR in either group of Cyp1a1-Ren2 transgenic rats.
Fig. 2.
Glomerular filtration rate in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). *P < 0.05.
Fig. 3.
Renal plasma flow in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). **P < 0.01.
Fig. 4.
Renal vascular resistance in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). **P < 0.01.
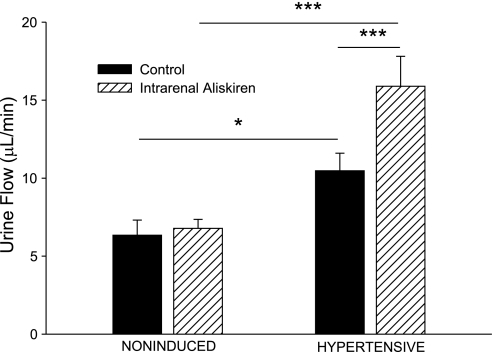
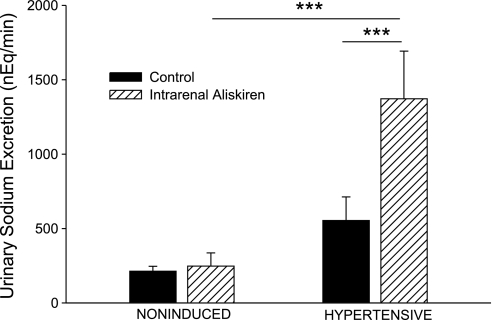
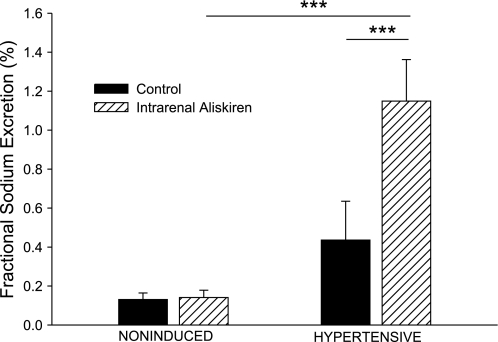
As shown in Fig. 5, the basal value for urine flow in hypertensive rats was higher than in noninduced rats (10.5 ± 1.1 vs. 6.3 ± 1.0 μl/min, P < 0.05). Intrarenal aliskiren increased urine flow in hypertensive Cyp1a1-Ren2 rats (10.5 ± 1.1 to 15.9 ± 1.9 μl/min, P < 0.001). Baseline urinary sodium excretion was not significantly different between the two groups (Fig. 6). Intrarenal aliskiren increased absolute sodium excretion (550 ± 160 to 1,370 ± 320 neq/min, P < 0.001) and fractional sodium excretion (0.44 ± 0.12 to 1.15 ± 0.21%, P < 0.001) in hypertensive Cyp1a1-Ren2 rats (Figs. 6 and 7).
Fig. 5.
Urine flow in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). *P < 0.05, ***P < 0.001.
Fig. 6.
Urinary sodium excretion in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). ***P < 0.001.
Fig. 7.
Fractional sodium excretion in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). ***P < 0.001.
Intrarenal candesartan administration increased urine flow (11.1 ± 1.6 to 17.5 ± 3.6 μl/min, P < 0.05), absolute sodium excretion (420 ± 240 to 1,590 ± 500 neq/min, P < 0.01), and fractional sodium excretion (0.33 ± 0.21 to 0.88 ± 0.33%, P < 0.01), without significantly affecting MAP (182 ± 5 to 176 ± 4 mmHg, not significant). Candesartan also increased GFR (1.11 ± 0.13 to 1.33 ± 0.08 ml·min−1·g−1, P < 0.01) and estimated RPF (2.82 ± 0.32 to 3.56 ± 0.43 ml·min−1·g−1, P < 0.05) and decreased RVR (33 ± 3 to 25 ± 3 mmHg·ml−1·min·g, P < 0.05).
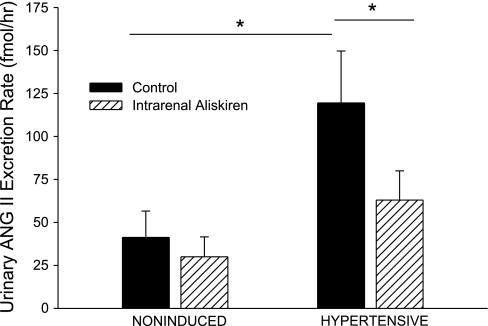
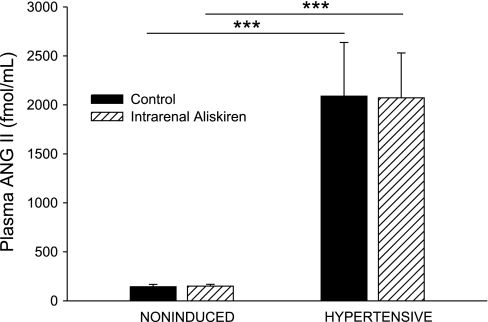
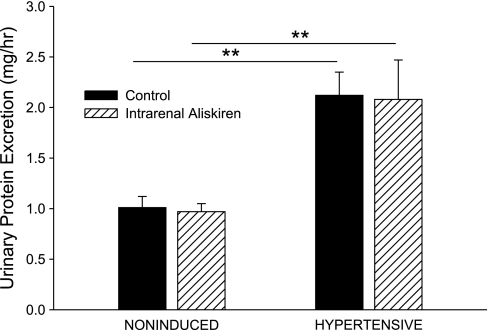
Compared with noninduced control rats, hypertensive rats had increased urinary ANG II excretion rates (120 ± 30 vs. 41 ± 15 fmol/h, P < 0.05; Fig. 8), increased plasma ANG II concentrations (2,090 ± 550 vs. 140 ± 20 fmol/ml, P < 0.001; Fig. 9), and increased urinary protein excretion rates (2.12 ± 0.23 vs. 1.01 ± 0.11 mg/h, P < 0.01; Fig. 10). Selective intrarenal renin inhibition decreased urinary ANG II excretion rate (120 ± 30 to 63 ± 17 fmol/h, P < 0.05; Fig. 8) in hypertensive rats. Plasma ANG II levels and urinary protein excretion rate remained unchanged following intrarenal aliskiren administration. Intrarenal renin inhibition had no effect on urinary ANG II excretion, plasma ANG II levels, or urinary protein excretion in noninduced rats.
Fig. 8.
Urinary ANG II excretion rate in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). *P < 0.05.
Fig. 9.
Plasma ANG II concentration in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). ***P < 0.001.
Fig. 10.
Urinary protein excretion rate in noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions (filled bars) and after intrarenal bolus injection of aliskiren (hatched bars). **P < 0.01.
DISCUSSION
The present study was performed to evaluate the blood pressure-independent effects of acute selective intrarenal direct renin inhibition on renal hemodynamics and excretory function in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Induction of the Ren2 renin gene by dietary administration of 0.3% I3C for 10 days resulted in the development of hypertension with concurrent decrease in body weight, extreme lethargy, assumption of a hunched posture, and piloerection, all of which are clinical manifestations of malignant hypertension in the rat (7, 11, 17, 18). In transgenic rats with malignant hypertension, intrarenal administration of the direct renin inhibitor aliskiren resulted in significant increases in urine flow and urinary sodium excretion with a significant decrease in urinary ANG II excretion rate (∼50%). The absence of a significant decrease in MAP suggests that there was little or no spillover of the drug into the systemic circulation. Furthermore, plasma ANG II concentrations were unchanged following aliskiren administration, demonstrating that aliskiren had little effect on PRA when administered directly into the kidney via the renal artery. These findings demonstrate that selective renal renin inhibition decreases intraluminal ANG II generation by substantially blocking intrarenal renin activity, resulting in diuretic and natriuretic effects in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension. The elevated intraluminal ANG II levels in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension likely act to augment tubular reabsorptive function and, thereby, contribute to the elevated blood pressure.
Despite the markedly elevated arterial blood pressure and urinary ANG II excretion rate, the control values for both absolute and fractional sodium excretion in the hypertensive rats were not significantly different from those observed in the noninduced rats. This suggests that the kidneys of Cyp1a1-Ren2 rats with malignant hypertension exhibit an impaired natriuretic response to elevated arterial blood pressure and can maintain normal rates of sodium excretion only at hypertensive pressures. Intrarenal renin inhibition significantly increased absolute and fractional sodium excretion and decreased urinary ANG II excretion rate in these rats, despite exposure of the kidney to the high-circulating levels of ANG II. This suggests that de novo ANG II production in the kidney contributes to the impaired sodium excretory function of the hypertensive Cyp1a1-Ren2 rat and, thus, to the maintenance of an elevated arterial blood pressure. In this regard, impaired sodium excretory function, mediated by de novo intrarenal ANG II generation, would attenuate the diuretic and natriuretic response to the ANG II-mediated elevation in arterial blood pressure and prevent the kidney from maintaining normal rates of sodium excretion except at elevated arterial pressures. In this manner, the impaired sodium excretory function observed in the present study would likely contribute to the hypertension in Cyp1a1-Ren2 rats. The finding that intrarenal aliskiren did not increase urinary sodium excretion in noninduced rats suggests that endogenous rat renin is not susceptible to inhibition with the direct renin inhibitor, aliskiren, at the administered dose. While basal levels of de novo ANG II production in the kidney of noninduced rats may contribute to baseline sodium reabsorption, the dose of aliskiren used in the present study was clearly insufficient to inhibit tubular sodium and water reabsorption and to elicit diuresis and natriuresis in the noninduced rats.
The basal values for urine flow in the hypertensive rats were higher than those seen in the noninduced rats. We previously demonstrated that Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension exhibit polyuria and polydipsia (22), which are clinical manifestations of malignant hypertension in the rat. ANG II stimulates thirst, causing the rats to drink more water which in turn results in increased urine flow. This, together with the pressure diuretic actions of the elevated arterial pressure under anesthesia, may have contributed to the elevated basal urine flow in the hypertensive rats. Intrarenal administration of aliskiren significantly increased urine flow in hypertensive transgenic rats, suggesting that intrarenal ANG II generation contributes to an augmented fluid reabsorption in ANG II-dependent malignant hypertension. Given the long half-life of aliskiren (t1/2 = 23.7 h) (29), the sustained elevations in urine flow and urinary sodium excretion, and the persistent decrease in urinary ANG II excretion rate for 1 h following intrarenal administration of aliskiren, the dose of aliskiren used in the study appears to elicit prolonged and substantial inhibition of intrarenal renin activity in hypertensive Cyp1a1-Ren2 transgenic rats.
The diuretic and natriuretic effects of renin inhibition may be localized to one or more specific segments of the nephron. Aliskiren has been detected within glomeruli, juxtaglomerular cells of the afferent arteriole, intrarenal arteries, vasa recta, distal tubules, and CDs (4). In the absence of an increase in renal hemodynamics, it is possible that the aliskiren-mediated increases in fluid and sodium excretion occurred secondary to a reduction in the distal nephron reabsorptive function. In this regard, we and others provided evidence for an intact renal RAS with identification of RAS components within the distal tubular lumen and distal tubular cells (6, 16, 19). Furthermore, it was recently reported that upregulation of renin and the soluble pro(renin) receptor (PRR) in the CD of hypertensive Cyp1a1-Ren2 rats may contribute to an elevated formation of ANG II in the distal nephron in this model of ANG II-dependent malignant hypertension (26). Further studies are required to address this issue.
Identification of PRR, a new player in the RAS, has revealed a possible ANG II-independent function for renin and prorenin. Binding of renin or prorenin to its receptor stimulates extracellular signal-regulated kinase (ERK) 1/2 signaling, which may have effects on kidney function and on the development of fibrotic kidney injury (3). Aliskiren has not been shown to inhibit binding of renin or prorenin to its receptor, nor has it been shown to alter the activation of ERK 1/2 (3). The apparent beneficial effects of aliskiren over angiotensin-converting enzyme inhibitors in the setting of fibrotic kidney injury may be attributable to the reported reduction in PRR expression in glomeruli, tubules, and cortical vessels following chronic aliskiren administration (3). However, the present study does not allow assessment of the effects of intrarenal aliskiren administration independent of its effect to inhibit renin activity and thus intrarenal ANG II levels.
Hypertensive transgenic rats exhibited significantly decreased values for GFR and estimated RPF and significantly elevated RVR compared with noninduced transgenic rats. These findings are consistent with our previous observations that GFR and estimated RPF are maintained within or slightly below the normal range and that RVR is significantly elevated in Cyp1a1-Ren2 transgenic rats with malignant hypertension (16, 18, 21, 23). In a previous study, acute systemic aliskiren administration increased estimated RPF and decreased MAP and RVR in transgenic rats with malignant hypertension (9). However, in the present study, acute intrarenal aliskiren administration did not significantly change MAP, estimated RPF, GFR, or RVR. One possible explanation for this observation is that the tubular epithelial cells are more responsive to ANG II and thus are more responsive to aliskiren-mediated inhibition of intrarenal ANG II. Another explanation is that high-circulating ANG II levels, which were unchanged following selective intrarenal renin inhibition, acted to maintain the vasoconstriction of pre- and postglomerular vascular resistance elements. In this regard, in the present study, selective intrarenal administration of the ANG II type 1 receptor (AT1R) antagonist candesartan resulted in increases in GFR and estimated RPF, in addition to increases in urine flow and in urinary sodium excretion, in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension in the absence of changes in MAP. Furthermore, previous studies showed similar increases in GFR and RBF in the absence of changes in MAP following acute intrarenal administration of candesartan into the nonclipped kidneys of Goldblatt hypertensive rats (2) and into the kidneys of prehypertensive TG(mRen2)27 transgenic rats (13). These studies demonstrate that indeed intrarenal ANG II, regardless of whether it is circulating or locally generated, stimulates tubular sodium and water reabsorption and exerts significant vasoconstrictor effects on the renal vasculature. Therefore, the urine flow and sodium excretion responses to intrarenal aliskiren represented the inhibition of locally generated ANG II to stimulate salt and water reabsorption. Intrarenal aliskiren administration reduced de novo intrarenal ANG II formation, but it did not affect the AT1R-mediated activity of ANG II taken up by the kidney from the systemic circulation. Such maintained renal delivery of ANG II appeared sufficient to exert persistent and pronounced vasoconstrictor effects and, thereby, prevent intrarenal aliskiren administration from altering GFR, estimated RPF, or RVR.
We previously demonstrated that renal pathological changes occur during the development of malignant hypertension in Cyp1a1-Ren2 rats, such as myointimal hyperplasia and tubular dilation, inflammation and cellular proliferation in the cortical vessels and tubulointerstitium, and pronounced glomerulosclerosis, contributing to the development of proteinuria (7). These changes may contribute to the impaired renal hemodynamic function in hypertensive transgenic rats. Acute intrarenal aliskiren administration had no effect on urinary protein excretion, and it is unlikely that renal vascular and glomerular injury was reversed by intrarenal renin inhibition in the time frame of the present study.
In addition to significant renal pathological changes, other groups have reported the development of cardiac hypertrophy in Cyp1a1-Ren2 transgenic rats induced with either low-dose I3C (0.125% wt/wt) (24) or high-dose I3C (0.3% wt/wt) (28). ANG II-dependent hypertension is historically associated with cardiac fibrosis and end-organ injury (14), but surprisingly, cardiac fibrosis was not observed following low-dose I3C administration in this transgenic rat model (24). Little information is available regarding the effects of high-dose I3C (0.3% wt/wt) on cardiac fibrosis, which was the dose utilized in the present study. Significant tubulointerstitial fibrosis has been reported in the kidneys of Cyp1a1-Ren2 transgenic rats with malignant hypertension (7), making it unlikely that I3C exerts any intrinsic antifbrotic effects. The lack of cardiac fibrosis in Cyp1a1-Ren2 rats maintained on a low-dose I3C diet may be explained by the absence of additional profibrotic factors induced by the severely elevated ANG II levels that occur in Cyp1a1-Ren2 rats induced to develop malignant hypertension with 0.3% I3C (24). To the extent that this is the case, transgenic rats with ANG II-dependent malignant hypertension would be expected to develop significant cardiac fibrosis and end-organ damage. Neither cardiac fibrosis nor end-organ damage was investigated in the present study, and we cannot exclude the possibility that these conditions would play a role in the maintenance of the hypertensive phenotype in this model.
It has been reported that aliskiren is able to bind both circulating and receptor-bound prorenin to decrease ANG I and II generation (4). Prorenin protein levels are augmented in renal inner medullary tissues in hypertensive Cyp1a1-Ren2 transgenic rats (26). Thus, it is possible that augmented inner medullary prorenin levels contributed to the hypertensive phenotype in Cyp1a1-Ren2 transgenic rats and that the renal functional responses to acute aliskiren administration observed in the present study occurred, in part, as a consequence of inhibition of intrarenal prorenin. However, the present study does not allow determination of the relative contribution of intrarenal renin and prorenin inhibition to the renal functional responses to acute aliskiren administration. Furthermore, the present study does not allow assessment of the degree to which intrarenal aliskiren inhibited intrarenally generated prorenin/renin vs. prorenin/renin derived from the systemic circulation. Additional studies are required to address these issues. Regardless of the degree to which aliskiren inhibited renin derived from either source, the results of the present study clearly demonstrate that the dose of aliskiren employed in the present study elicited substantial and selective blockade of intrarenal renin activity and intrarenal ANG II generation in the absence of changes in circulating ANG II levels.
In summary, the present data demonstrate that acute intrarenal renin inhibition with aliskiren increases urine flow and urinary sodium excretion while decreasing urinary ANG II excretion rate in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Acute aliskiren administration did not significantly affect urinary protein excretion rate, demonstrating that the decrease in urinary ANG II excretion rate was a specific effect of intrarenal renin inhibition and cannot be explained by a general decrease in total urinary protein excretion. The absence of an improvement in GFR or RPF indicates that high-circulating levels of ANG II are sufficient to maintain vasoconstriction of pre- and postglomerular vascular elements and thus maintain reduced renal hemodynamic function in Cyp1a1-Ren2 transgenic rats. The diuretic and natriuretic effects of intrarenal aliskiren suggest that de novo intrarenal ANG II generation significantly contributes to enhanced sodium and water reabsorption and thus to the maintenance of hypertension in this model of ANG II-dependent malignant hypertension.
GRANTS
This study was supported by the Tulane COBRE in Hypertension and Renal Biology (NCRR 2P20RR017659), National Heart, Lung, and Blood Institute Grant HL26371, American Heart Association Greater Southeast Affiliate Predoctoral Fellowship (11PRE7720020), and a grant from Novartis Pharmaceuticals (CSPP100A-US21T).
DISCLOSURES
The renin inhibitor aliskiren was kindly provided by Novartis Pharmaceuticals Corporation, and the study was funded, in part, by a grant from Novartis Pharmaceuticals Corporation (CSPP100A-US21T).
AUTHOR CONTRIBUTIONS
Author contributions: C.G.H. and K.D.M. conception and design of research; C.G.H. performed experiments; C.G.H. and K.D.M. analyzed data; C.G.H. and K.D.M. interpreted results of experiments; C.G.H. and K.D.M. prepared figures; C.G.H. and K.D.M. drafted manuscript; C.G.H. and K.D.M. edited and revised manuscript; C.G.H. and K.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dale M. Seth and Porcha D. Davis for excellent technical assistance. We also thank Dr. L. Gabriel Navar for helpful comments and suggestions, and Dr. Barb Mickelson, Harlan-Teklad, for help with the design and production of the I3C-containting rat diet.
REFERENCES
- 1. Campbell SJ, Carlotti F, Hall PA, Clark AJ, Wolf CR. Regulation of the CYP1A1 promoter in transgenic mice: an exquisitely sensitive on-off system for cell specific gene regulation. J Cell Sci 109: 2619–2625, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension 33: 102–107, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension 52: 130–136, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Feldman DL. New insights into the renoprotective actions of the renin inhibitor aliskiren in experimental renal disease. Hypertens Res 33: 279–287, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Feldman DL, Schuetz H, Persohn E, Muller DN. Light microscopy autoradiographic localization of the renin inhibitor aliskiren in rat kidneys (Abstract). Hypertension 56: e109, 2010 [Google Scholar]
- 6. Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol 295: F772–F779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graciano ML, Mouton CR, Patterson ME, Seth DM, Mullins JJ, Mitchell KD. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 292: F1858–F1866, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension 20: 763–767, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Howard CG, Mullins JJ, Mitchell KD. Direct renin inhibition with aliskiren normalizes blood pressure in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Med Sci 341: 383–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol 143: 117–130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGrath I, Kotelevtsev Y, Mullins JJ. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem 276: 36727–36733, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Kopkan L, Kramer HJ, Husková Z, Vanourková Z, Skaroupková P, Thurmová M, Cervenka L. The role of intrarenal angiotensin II in the development of hypertension in Ren-2 transgenic rats. J Hypertens 23: 1531–1539, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Lijnen PJ, Petrov VV. Role of intracardiac renin-angiotensin-aldosterone system in extracellular matrix remodeling. Methods Find Exp Clin Pharmacol 25: 541–564, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst 54: 985–988, 1975 [PubMed] [Google Scholar]
- 16. Milani CJ, Kobori H, Mullins JJ, Mitchell KD. Enhanced urinary angiotensinogen excretion in Cyp1a1-Ren2 transgenic rats with inducible Ang II-dependent malignant hypertension. Am J Med Sci 340: 389–394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell KD, Mullins JJ. Enhanced tubuloglomerular feedback in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 289: F1210–F1216, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst 7: 74–86, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol 17: 412–422, 1997 [PubMed] [Google Scholar]
- 20. Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Opay AL, Mouton CR, Mullins JJ, Mitchell KD. Cyclooxygenase-2 inhibition normalizes arterial blood pressure in CYP1A1-REN2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 291: F612–F618, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Ortiz RM, Graciano ML, Mullins JJ, Mitchell KD. Aldosterone receptor antagonism alleviates proteinuria, but not malignant hypertension, in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol 293: F1584–F1591, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Patterson ME, Mouton CR, Mullins JJ, Mitchell KD. Interactive effects of superoxide anion and nitric oxide on blood pressure and renal hemodynamics in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol 289: F754–F759, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Peters J, Schlüter T, Riegel T, Peters BS, Beineke A, Maschke U, Hosten N, Mullins JJ, Rettig R. Lack of cardiac fibrosis in a new model of high prorenin hyperaldosteronism. Am J Physiol Heart Circ Physiol 297: H1845–H1852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pilz B, Shagdarsuren E, Wellner M, Fiebeler A, Dechend R, Gratze P, Meiners S, Feldman DL, Webb RL, Garrelds IM, Jan Danser AH, Luft FC, Müller DN. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats. Hypertension 46: 569–576, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Prieto MC, Williams DE, Liu L, Kavanagh KL, Mullins JJ, Mitchell KD. Enhancement of renin and prorenin receptor in the collecting duct of Cyp1a1-Ren2 rats contribute to development and progression of malignant hypertension. Am J Physiol Renal Physiol 300: F581–F588, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiontensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 296: F1067–F1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanourková Z, Kramer HJ, Husková Z, Vanecková I, Opocenský M, Chábová VC, Tesar V, Skaroupková P, Thumová M, Dohnalová M, Mullins JJ, Cervenka L. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens 24: 2465–2472, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Verdecchia P, Angeli F, Mazzotta G, Gentile G, Reboldi G. The renin angiotensin system in the development of cardiovascular disease: role of aliskiren in risk reduction. Vasc Health Risk Manag 4: 971–981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams DE, Prieto MC, Mullins JJ, Navar LG, Mitchell KD. AT1 receptor blockade prevents the increase in blood pressure and the augmentation of intrarenal ANG II levels in hypertensive Cyp1a1-Ren2 transgenic rats fed with a high-salt diet. Am J Med Sci 339: 356–361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]