Abstract
The molecular mechanisms that lead to tubular atrophy, capillary loss, and fibrosis following acute kidney injury are not very clear but may involve cell cycle inhibition by increased expression of cyclin kinase inhibitors. The INK4a/ARF locus encodes overlapping genes for two proteins, a cyclin kinase inhibitor, p16INK4a, and a p53 stabilizer, p19ARF, from independent promoters. To determine if decreased INK4a gene expression results in improved kidney regeneration, INK4a knockout (KO) and wild-type (WT) mice were subjected to ischemia-reperfusion injury (IRI). p16INK4a and p19ARF levels were increased markedly in WT mice at 1–28 days after injury. Kidneys were examined to determine the localization and levels of p16INK4a, apoptosis, cell proliferation, and capillary rarefaction. KO mice displayed decreased tubular cell apoptosis, increased cell proliferation, and lower creatinine levels after injury. KO mice had significantly higher capillary density compared with WT mice at 14–42 days after IRI. Plasma granulocyte colony-stimulating factor (G-CSF) increased after ischemia in both WT and KO mice and was elevated markedly in KO compared with WT mice. KO kidney digests contained higher counts of Gr-1+/Cd11b+ myeloid cells by flow cytometry. KO mice treated with a Gr-1-depleting antibody displayed reduced vascular endothelial growth factor mRNA, plasma G-CSF, and capillary density, and an increase in serum creatinine and medullary myofibroblasts, compared with untreated KO mice 14 days after ischemia. The anti-angiogenic effect of Gr-1 depletion in KO mice was confirmed by Matrigel angiogenesis assays. These results suggest that the absence of p16INK4a and p19ARF following IRI has a protective effect on the kidney through improved epithelial and microvascular repair, in part by enhancing the mobilization of myeloid cells into the kidney.
Keywords: p16, p19, myeloid cells, acute kidney injury
following acute kidney injury (AKI), complete tissue regeneration results in normal long-term kidney function. In many cases of severe injury and in older individuals, however, AKI frequently results in incomplete recovery because of impaired regeneration of damaged tubular epithelium and capillary endothelial cells, leading to tissue fibrosis. Ischemia-reperfusion injury (IRI) is a commonly used model for reversible AKI. Incomplete repair following severe injury is characterized by tubular atrophy, capillary rarefaction, and fibrosis. The molecular pathways that lead to this loss of function following IRI are not well understood but may involve cell cycle inhibition caused by increased expression of proteins, including the cyclin kinase inhibitors (19). Inhibition of cell proliferation and changes in secretory phenotype caused by cell cycle arrest may lead to insufficient and abnormal tubular regeneration and angiogenesis following injury (29, 33).
The INK4a/ARF locus encodes overlapping genes for two proteins, p16INK4a, a cyclin kinase inhibitor, and p19ARF, a p53 stabilizer, which are regulated by distinct promoters. Expression of p16 and p19 in normal young kidney cells is low to undetectable. Studies in rodents and in human kidney biopsies demonstrate that p16 levels increase with age and in glomerular disease in proximal tubular epithelial and interstitial cells and correlate with tubular atrophy and interstitial fibrosis (15–17). Interestingly, the INK4a proteins are the only cell cycle inhibitors shown to increase with age (11). The pathological effects of prolonged proximal tubular cell INK4a gene expression following AKI and the potential regenerative effects of INK4a/ARF inhibition are unknown. Decreased INK4a/ARF expression may promote tissue repair and regeneration through multiple mechanisms, including induction of dedifferentiation and proliferation of mature cells as demonstrated in myotubes (18), prevention of apoptosis, and promotion of adult stem cell self-renewal as demonstrated in the central nervous system and hematopoietic cells (2). INK4a deletion has also been shown to result in increased tumor angiogenesis (6, 8), suggesting another possible mechanism for promoting tissue repair.
Activation of the INK4a/ARF locus by DNA damage or aberrant mitogenic signals prevents the growth of abnormal cells, an important mechanism by which the INK4a/ARF locus acts as a major tumor suppressor. Increased INK4a/ARF expression, however, may come at the expense of impaired tissue regeneration. In the present study, we have used INK4a/ARF knockout (KO) mice to examine the effect of INK4a/ARF deletion on tissue regeneration after IRI. Results demonstrate that KO mice have increased tubular cell proliferation and decreased apoptosis during the early reparative phase following injury and decreased long-term capillary rarefaction that may be the result of increased mobilization of proangiogenic myeloid cells.
MATERIALS AND METHODS
Mice strains.
Homozygous INK4a/ARF KO mice strain B6.129-Cdkn2atm1Rdp maintained on a C57BL/6J background were obtained from the NCI mouse repository (strain 01XB1; Frederick, MD). This strain carries a targeted deletion of exons 2 and 3 of the INK4a/ARF locus, which eliminates both p16 and p19. KO mice were identified by genotyping using PCR primers that amplify exon 2 of the INK4a/ARF locus. The INK4a/ARF genotyping primer sense sequence was 5′-TCAACTACGGTGCAGATTCG-3′, and the antisense sequence was 5′-TCGCACGATGTCTTGATGTC-3′. Wild-type (WT) littermate mice were used as controls for all experiments. All animals were housed in accordance with guidelines from the American Association for Laboratory Animal Care and Research Protocols and were approved by the Institutional Animal Care and Use Committees of New York Medical College.
Quantitative PCR.
Kidneys were removed aseptically, and RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified using a spectrophotometer (Eppendorf, Hauppauge, NY). cDNA was prepared by reverse transcription of 1 μg total RNA using a qScript cDNA synthesis kit (Quanta, Surrey, UK) according to the manufacturer's instructions. Quantitative real-time PCR was performed using PerfeCTa SYBR Green Fastmix Low ROX (Quanta) and the Mx3000 real-time PCR system (Stratagene, La Jolla, CA). Specific primers were designed based on published sequences. The p16 sense sequence was 5′-AACTCTTTCGGTCGTACCCC-3′ and antisense was 5′-GCGTGCTTGAGCTGAAGCTA-3′. The p19 sense sequence was 5′-CTGAACCGCTTTGGCAAGAC-3′ and antisense was 5′-GCCCTCTCTTATCGCCAGAT-3′. The vascular endothelial growth factor (VEGF) sense sequence was 5′-CAGGCTGCTGTAACGATGAA-3′ and antisense was 5′-AATGCTTTCTCCGCTCTGAA-3′. The 18S sense sequence was 5′-TGTCTCAAAGATTAAGCCATGCAT-3′, and antisense was 5′-AACCATAACTGATTTAA-TGAGCCATTC-3′. mRNA expression levels were calculated using the ΔΔCt method and were reported compared with the reference gene 18S.
Antibodies and reagents.
For immunohistochemistry and Western blots, primary antibodies used include: p16 (F-12; Santa Cruz Biotechnology, Santa Cruz, CA), p19 (rat mAb 5-C3–1; Calbiochem, La Jolla, CA), α-smooth muscle actin (SMA) (Ab 5694; Abcam, Cambridge, MA), Ki67 (Ab15580; Abcam), β-tubulin (clone TUB2.1; Sigma, St. Louis, MO), and platelet-derived growth factor (PDGF)-β receptor (clone APB5; eBioscience, San Diego, CA). Donkey anti-rabbit and anti-mouse secondary antibodies used in Western blots were from GE Healthcare (Piscataway, NJ). Alexa Fluor donkey anti-goat and anti-rabbit secondary antibodies were from Invitrogen (Eugene, OR). Antibodies for flow cytometry were fluorescein isothiocyanate (FITC) anti-Gr-1 and PE anti-CD11b (eBioscience). VEGF and granulocyte colony-stimulating factor (G-CSF) enzyme-linked immunosorbent assays (ELISAs) were performed with commercially available kits (Peprotech, Rocky Hill, NJ, and RayBiotech, Norcross, GA) according to the manufacturer's instructions. Tissue lysates were diluted 1:10 in sample buffer for VEGF ELISA, and plasma samples were diluted between 1:1 and 1:100,000 in sample buffer for G-CSF ELISA. Results were reported as means ± SD for three to five samples at each point measured in duplicate. Gr-1 depletion was performed by intraperitoneal injection of 100 μg of anti-mouse Ly-6G (clone RB6–8C5; eBioscience). Control mice were injected intraperitoneally with 100 μg rat IgG (BD Biosciences, Carlsbad, CA).
Ischemia-reperfusion injury.
Eight- to ten-week-old male mice were anesthetized with ketamine and xylazine. Mice were placed on a heating pad at 37°C. The left renal hilum was identified, and the renal vessels were dissected and clamped for 35 min. The vessels of the right kidney were ligated with sutures, and the kidney was subsequently removed. The midline incision was closed with 3–0 silk sutures.
Immunofluorescence.
Kidneys were fixed in 4% paraformaldehyde and frozen in optimum cutting temperature (OCT) medium. Five-micrometer cryostat sections were cut and applied to Probe-on plus slides (Fisher Scientific, Pittsburgh, PA). Slides were treated with PBS 0.2% Triton X-100 and blocked with 10% goat serum for 1 h. Samples were incubated with primary antibody overnight at 4°C. Secondary antibody was applied the following day. Following washes in PBS, slides were incubated with DAPI (Vector Laboratories, Burlingame, CA) for nuclear staining and examined by immunofluorescence using a Nikon Eclipse 1000 microscope equipped with a charge-coupled device camera. Quantification of capillary area was performed using Adobe Photoshop and NIH ImageJ software.
TdT dUTP nick end-labeling staining.
TdT dUTP nick end labeling (TUNEL) staining was performed with a commercially available kit (In Situ Cell Death Detection Kit, TMR Red; Roche, Indianapolis, IN). Following deparaffinization and permeabilization according to the manufacturer's instructions, tissue sections were labeled with TUNEL reaction mixture for 1 h at 37°C. Cells were labeled with Hoechst and examined by immunofluorescence microscopy. Results were reported as mean ± SD TUNEL-positive cells per square millimeter (n = 5 mice/group).
Western blotting.
Cells cultures were washed with PBS and lysed in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, and 0.5% Triton X-100 in PBS) containing Complete Mini protease inhibitor (Roche) and 2 mM phenylmethylsulfonyl fluoride. Protein concentrations were measured using a Bradford assay (Bio-Rad, Hercules, CA). The protein samples were denatured by adding SDS sample buffer and heating at 95°C for 5 min. Twenty to forty micrograms of sample containing β-mercaptoethanol were resolved on 4–20% Tris glycine polyacrylamide gels. Gels were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA) and blocked with 5% nonfat dry milk in 0.1% Tween 20 in PBS. The membranes were then incubated overnight at 4°C with primary antibody in blocking buffer. This was followed by washing and 1 h incubation with an appropriate horseradish peroxidase-conjugated secondary antibody. Protein bands were detected by chemiluminescence using a commercial kit (Thermo Scientific, Rockford, IL) according to the manufacturer's instructions. For quantification of protein levels, autoradiographs were scanned with the Scion Image densitometry program, and results were corrected for variations in the amount of protein loaded on each lane using corresponding β-tubulin levels.
Matrigel assay.
Eight- to ten-week-old WT and INK4a/ARF KO male mice were anesthetized with ketamine and xylazine. Three hundred microliters of growth factor-reduced Matrigel (BD Biosciences) diluted to 10 mg/ml with minimal essential medium were injected subcutaneously. Mice were injected with 100 μg of intravenous 500,000 mol wt dextran (Invitrogen) 5 min before death. Matrigel plugs were removed after 10 days and fixed in 4% paraformaldehyde. Plugs were embedded in paraffin for hematoxylin and eosin staining or OCT for cryostat sections for immunofluorescence staining. For Gr-1 depletion, KO mice were injected intrperitoneally with 100 μg of anti-mouse Ly-6G 1 and 4 days after plug implantation.
Aortic ring angiogenesis assay.
Thoracic aortas were removed from WT and INK4a/ARF KO mice using sterile technique. Surrounding connective tissue was removed completely followed by washes in EGM-2 medium (Lonza, Walkersville, MD). One-millimeter slices were placed between two layers of growth factor-reduced Matrigel (10 mg/ml) and incubated with EGM-2 medium. Capillary outgrowth was measured after 2 wk using Metamorph software.
Flow cytometry.
Kidneys were removed aseptically and digested using a modification of the method of Vielhauer et al. (30). The minced kidney was treated with 1 mg/ml collagenase in Hanks' balanced salt solution (HBSS) for 20 min at 37°C with shaking. After being washed with HBSS, the kidney digest was treated with HBSS with 2 mM EDTA for 20 min at 37°C with shaking. The kidneys were centrifuged at 250 g for 5 min. The supernatant was reserved and kept on ice. The pellet was resuspended in 1 mg/ml collagenase in HBSS and incubated for 20 min at 37°C with shaking. These cells were passed through a 20-gauge needle three times. The digest was then placed on ice for 5 min, after which the supernatant was collected and combined with the previously reserved supernatant. The cells were washed in HBSS and passed through a 40-μm filter. The cells were resuspended in 36% Percoll and overlaid upon 70% Percoll. A Percoll gradient was formed upon centrifugation at 1,000 g for 30 min. The cells from the interface of the two Percoll concentrations were resuspended in 100 μl of PBS with 2% FBS and 0.01% sodium azide and incubated with primary antibodies at 4°C for 45 min. Ten microliters of propidium iodide were added for the final 15 min of the antibody incubation. The cells were washed and then fixed with 4% paraformaldehyde for 10 min, washed, and resuspended in PBS with 2% FBS and 0.01% sodium azide.
Flow cytometry was performed using a MACSQuant cytometer (Miltenyi, Bergisch Gladbach, Germany). Dead cells were excluded on the basis of staining positive for propidium iodide. Results are reported as means ± SE.
Statistical analysis.
Data were collected from three to six independent experiments and presented as means ± SD. A Student's t-test was used to compare two series of data. A P value of <0.05 was considered significant.
RESULTS
INK4a/ARF gene expression is increased in tubular epithelial cells following IRI.
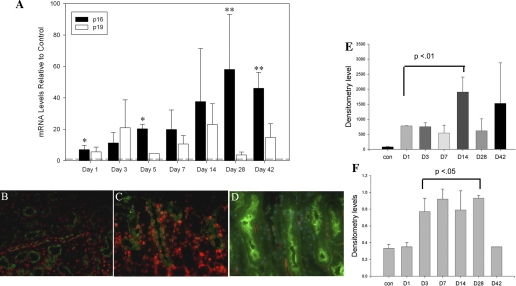
INK4a/ARF proteins are expressed at low levels in young adult organs, including the kidney, and increase with age (11). Previous work from our laboratory and others showed that p16 is detected in interstitial fibroblasts, glomerular mesangial cells, and collecting duct epithelial cells in the normal mouse kidney (14, 46). As part of the DNA damage response program, INK4a/ARF gene expression is increased following cell injury (3). This suggested a possible role in controlling repair following kidney injury. To determine if p16 and p19 expression is increased following acute injury, we examined RNA and protein levels at various time points following IRI. qRT-PCR showed a marked and persistent increase in p16 and p19 levels within 1 day following IRI with contralateral nephrectomy in 2- to 3-mo-old mice. One week following IRI, p16 mRNA levels were increased up to 35-fold (Fig. 1A). Levels peaked at 100-fold compared with sham-operated mice 28 days following IRI and were still elevated significantly at 42 days, a time when kidney regeneration is usually complete. qPCR using proximal tubules isolated from these mice as previously described (32) demonstrated a 10- to 100-fold increase in p16 and p19 levels compared with total kidney RNA (data not shown), consistent with localization in this tubule segment. By immunofluorescence, increased p16 expression was detected in proximal tubular nuclei in damaged areas of the outer medulla beginning at 1 day following ischemia (Fig. 1, B–D). p19 levels showed greater variability by qPCR, peaking at 3 days following IRI followed by a second, nonsignificant increase at day 14. By Western blot analysis, p16 (Fig. 1E) and p19 (Fig. 1F) levels were increased significantly by 1–3 days following IRI and returned to baseline levels by 42 days.
Fig. 1.
p16 and p19 levels increase after ischemia-reperfusion injury (IRI) in tubular epithelial cells. A: qPCR for p16 and p19 mRNA levels at indicated time points following IRI. Results are reported as means ± SE from 4–6 mice/time point. *P = 0.01 and **P = 0.005. Immunofluorescence for p16 (red) and PNA lectin (green) labeling of distal tubules at 1 day after IRI in wild-type (WT) (B), 3 days after IRI in WT (C), and 3 days after IRI in INK4a/ARF knockout (KO) (D) mice. Original magnification = ×400. E: densitometry analysis of p16 (E) and p19 (F) on Western blotting of protein samples from various time points in WT mice after IRI. D, day. Results normalized vs. β-tubulin densitometry values; n = 2–3 samples/time point. P values calculated relative to control mice.
INK4a/ARF deletion results in improved kidney regeneration and decreased capillary rarefaction following IRI.
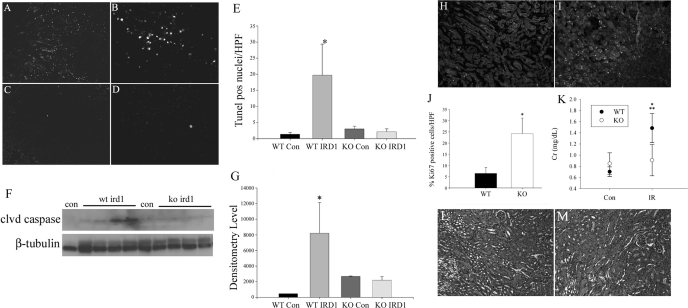
The early and persistent increase in p16 and p19 levels as a result of IRI suggested that increased INK4a/ARF gene expression may be at least partly responsible for incomplete kidney regeneration and the development of capillary rarefaction and fibrosis. To examine this possibility, we compared: 1) tubular cell apoptosis and proliferation following injury, 2) functional recovery by creatinine assays, and 3) the expression of VEGF and capillary density in WT and INK4a KO mice (22) following IRI. Compared with WT mice, KO mice had a 10-fold decrease in outer medullary tubular cell apoptosis by TUNEL staining (Fig. 2, A–E) and no change in cleaved caspase-3 expression 1 day following IRI by Western blot (Fig. 2, F and G). Tubular cell proliferation in KO mice was increased fivefold compared with WT mice (Fig. 2, H–J). These differences were associated with a 20% decrease in creatinine in KO mice (Fig. 2K) during the early recovery period (days 2–5) following IRI.
Fig. 2.
INK4a/ARF KO mice experience decreased apoptosis and increased cell proliferation compared with WT after IRI. Apoptosis assays by TdT dUTP nick end-labeling (TUNEL) staining of WT (A and B) and INK4a KO (C and D) with quantification of positive nuclei 1 day following IRI (E, *P < 0.001) and cleaved caspase levels by Western analysis (F and G, *P = 0.01). Original magnification = ×100. Tubular epithelial cell proliferation quantified by Ki67 staining of WT (H) and KO (I) kidneys from 5 mice at 3 days after IRI (J, *P < 0.05). Original magnification = ×100. Creatinine assay (K) comparing mean values from controls and 2–5 days after IRI in both WT and KO, with 3–6 samples/group (*P < 0.05, WT 2–5 days vs. KO 2–5 days; **P < 0.05, WT 2–5 days vs. WT control). HPF, high-power field; Con, control; IRD1, ischemia-reperfusion day 1; Cr, creatinine; IR, ischemia-reperfusion.
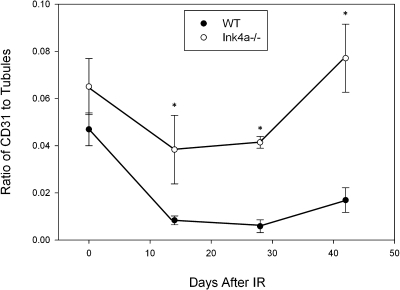
To determine if decreased INK4a/ARF expression can ameliorate capillary rarefaction following IRI, we examined capillary density in WT vs. KO mice. Cortical and medullary peritubular capillary density, determined by the area of CD31 staining as a percentage of tubular area, decreased by ∼80% at 28 days following IRI before recovering to a 60% decrease by day 42, whereas capillary area decreased by only 30% at 28 days in K0 mice before recovering to baseline levels by day 42 (Fig. 3).
Fig. 3.
Ink4a/ARF deletion results in attenuation of capillary loss after ischemia. Ratio of CD31 to tubular area in control mice and 14, 28, and 42 days after IRI. Results are reported as means ± SE from 3–5 mice for each time point. P values compare WT and KO samples at each time point. *P < 0.05.
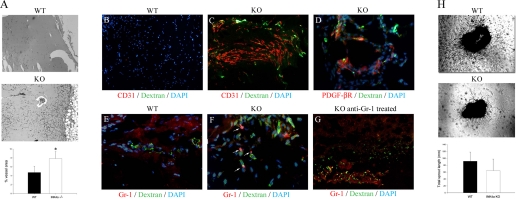
To determine if INK4a/ARF KO results in increased angiogenesis, vessel formation in subcutaneous Matrigel plugs was compared between WT and KO mice. INK4a/ARF KO mice demonstrated a twofold increase in vessel formation compared with WT mice as shown in Fig. 4A. The structural organization of the Matrigel vessels was examined by intravenous injection of FITC dextran before plug removal and immunofluorescence labeling for capillary markers. As shown in Fig. 4, Matrigel plugs from KO mice displayed higher levels of CD31 and FITC dextran signal (Fig. 4C) compared with WT mice (Fig. 4B), suggesting the formation of perfused vessel-like structures. FITC dextran-perfused vessels in KO mice were also closely associated with PDGF-β receptor-positive cells (Fig. 4D), suggesting that these vessels had pericyte coverage characteristic of stable capillary tubes.
Fig. 4.
Matrigel plugs in KO mice show increased angiogenesis compared with WT mice, which is counteracted by administration of Gr-1-depleting antibodies. A: WT and KO Matrigel sections showing increased vessel growth in KO mice. Original magnification = ×40. *P < 0.05. Matrigel plugs from WT (B) and KO (C) mice injected with fluorescein isothiocyanate (FITC) dextran and stained for CD31, demonstrating increased angiogenesis and vessel perfusion in KO mice in vivo. Original magnification = ×100. D: immunohistochemistry for platelet-derived growth factor (PDGF)-β receptor after dextran injection showing pericyte aggregation around functional capillaries. Original magnification = ×400. Matrigel plugs stained for Gr-1 from WT mice (E) (×100) and KO mice (F) (×400) demonstrating Gr-1+ cells in the KO (arrowheads). G: Gr-1-stained Matrigel plug from a KO mice treated with anti-Gr-1 antibodies. Original magnification = ×100. H: capillary sprouting from 1-mm aortic ring sections from WT and KO mice embedded in growth factor-reduced Matrigel grown in vitro. Original magnification = ×40.
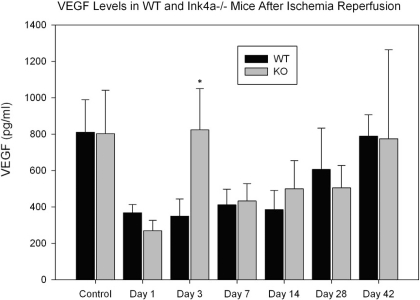
Unlike other organs, kidney VEGF levels are decreased following IRI during the early repair period from 1 to 7 days following ischemia and may result in capillary rarefaction. Administration of VEGF during the early regenerative phase resulted in attenuation of capillary loss and long-term functional protection (12). To determine if decreased INK4a expression following IRI is associated with increased VEGF levels, VEGF expression in WT and KO mice was compared by ELISA (Fig. 5) of kidney lysates. VEGF levels were decreased significantly in WT mice from 1 to 14 days following IRI, as previously reported, with partial recovery by 28 days. KO mice demonstrated a 70% reduction at 1 day postischemia followed by a fourfold elevation at day 3 compared with WT mice.
Fig. 5.
Decreased vascular endothelial growth factor (VEGF) levels in kidneys following IRI. Enzyme-linked immunosorbent assay (ELISA) for VEGF in kidney lysates from WT and INK4a/ARF KO mice at indicated time points (n = 4 to 6 for each group). *P < 0.05.
Increased G-CSF levels result in mobilization of angiogenic myeloid cells in INK4a/ARF KO mice.
Following IRI, kidneys display a biphasic inflammatory response consisting of chemotactic and both inflammatory and anti-inflammatory cytokines. While the initial cytokine peak is produced predominately by influx of leukocytes, during the reparative phase 1–2 wk later, surviving tubular epithelial cells also produce growth factors and cytokines that may coordinate both epithelial and microvascular repair (29). Preliminary results from our laboratory using a Luminex multiplex ELISA assay show that INK4a/ARF KO mice have a highly exaggerated early (1–3 days following ischemia) inflammatory response following injury. This consists of 100-fold increased production of inflammatory cytokines IL-6 and IL-8 following injury that are detected in both kidney and blood and a 100-fold decrease in the production of the anti-inflammatory cytokine IL-10 compared with WT mice (data not shown).
Gr-1+/CD11b+ myeloid cells (a heterogeneous mixture of myeloid progenitors, monocytes, and neutrophils) are mobilized by G-CSF and have been shown to have an important role in angiogenesis following ischemia and in tumors (10, 34) by secretion of angiogenic factors and matrix metallopeptidases that may increase VEGF bioavailability. Because of G-CSF's regenerative and angiogenic effects, we chose to study this factor and recruitment of Gr-1+/CD11b+ myeloid cells in more detail.
Measurement of plasma G-CSF levels following IRI by ELISA demonstrated marked elevations 1 day following ischemia in both WT and KO mice. At later time points, an 18-fold increase in KO compared with WT mice at days 7 and 14 was detected (Table 1).
Table 1.
Plasma G-CSF levels in WT and KO mice after IRI
| WT, pg/ml | KO, pg/ml | |
|---|---|---|
| Control | 564 ± 114 | 246 ± 20 |
| IR D1 | 125,012 ± 56,024 | 139,144 ± 56,990 |
| IR D3 | 4,247 ± 131 | 6,422 ± 1,442 |
| IR D7 | 179 ± 5 | 3,295 ± 141* |
| IR D14 | 307 ± 94 | 5,657 ± 1,029** |
Values are means ± SE. G-CSF, granulocyte macrophage colony-stimulating factor; WT, wild-type; KO, knockout; IRI, ischemia reperfusion injury; IR, ischemia-reperfusion; D, day. *P = 0.003 and **P = 0.02.
The increased G-CSF levels in KO mice at later time points following ischemia suggested that increased mobilization of bone marrow-derived cells, including proangiogenic Gr-1+/CD11b+ myeloid cells, may be responsible for the decreased capillary loss following IRI. By flow cytometry of kidney digests, KO mice demonstrate a ninefold increase in this cell type at 14 days following IRI (Table 2) compared with WT mice.
Table 2.
Quantification of Gr-1+ and CD11b+ cells in WT, KO mice, and anti-Gr-1-treated-KO mice following IRI using flow cytometry
| WT, % | KO, % | KO Anti-Gr-1, % | |
|---|---|---|---|
| Gr-1+/CD11b+ | 0.071 ± 0.036 | 0.643 ± 0.086* | 0.181 ± 0.081† |
| CD11b+ total | 1.70 ± 0.77 | 4.96 ± 0.75** | 2.86 ± 1.04 |
Values are means ± SE.
P = 0.0008 vs. WT.
P = 0.03 vs. WT.
P = 0.01 vs. KO.
Depletion of Gr-1+ cells after IRI reverses the angiogenic phenotype of INK4a/ARF KO mice.
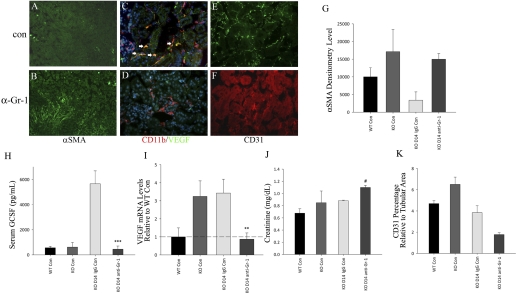
To determine if increased numbers of infiltrating Gr-1+/CDllb+ cells are responsible for the preservation of capillary density in KO mice, KO mice were treated with a Gr-1-depleting antibody at 1, 5, and 9 days following IRI and killed at 14 days. A 3.5-fold depletion of Gr-1+/Cd11b+ cells was confirmed by fluorescence-activated cell sorter analysis of kidney digests at 14 days (Table 2). As shown in Fig. 6, Gr-1-depleted KO mice displayed absence of VEGF expression in infiltrating CD11b+ cells (Fig. 6D) compared with control IgG-treated KO mice (Fig. 6C). This was associated with an 80% reduction in kidney VEGF mRNA expression (Fig. 6I) and a 10-fold reduction in serum G-CSF (Fig. 6H) compared with control mice. The marked reduction in these angiogenic factors was associated with a 60% reduction in capillary density (Fig. 6, E, F, and K), a fivefold increase in medullary myofibroblast α-SMA expression (Fig. 6, A, B, and G), and a 30% increase in creatinine 14 days following IRI (Fig. 6J).
Fig. 6.
Comparison of WT control, KO control, and INK4a/ARF KO mice treated with control IgG or Gr-1-depleting antibody for 2 wk following IRI (n = 4–6 each group). A–F: immunofluorescence using antibodies as described. Arrowheads indicate CD11b/VEGF double-positive cells. Original magnification = ×400. G: α-smooth muscle actin (SMA) Western blot densitometry. H: ELISA for plasma granulocyte colony-stimulating factor (G-CSF) levels. I: VEGF mRNA levels by qPCR. J: plasma creatinine. K: capillary area relative to tubular area, measured by CD31 staining. **P = 0.01, ***P = 0.02, and #P = 0.05. All P values compare KO control IgG vs. KO anti-Gr-1-treated mice at 14 days.
The role of Gr-1+ cells in angiogenesis was also examined by Matrigel plug assays in WT and KO mice. Vessel-like structures with dextran perfusion were associated with increased numbers of Gr-1+ cells in KO mice (Fig. 4F) compared with WT mice (Fig. 4E). Treatment of KO mice with a Gr-1+-depleting antibody following plug implantation resulted in decreased capillary formation at 7 days (Fig. 4G). To determine if KO mice display increased in vitro angiogenesis in the absence of circulating factors, aortic ring angiogenesis assays were compared between WT and KO mice. As shown in Fig. 4H, no significant difference in capillary sprouting was seen between mice at 10 days following ring implantation in Matrigel. Together, these results suggest that the angiogenic effect of INK4a/ARF deletion following AKI may be because of increased G-CSF production and resulting infiltration of angiogenic Gr-1+/CDllb+ neutrophils or myeloid progenitor cells.
DISCUSSION
The INK4a/ARF locus is activated by DNA damage and has an important role in preventing aberrant cell proliferation. In addition to its important function as a tumor suppressor as demonstrated in KO mice (22), INK4a/ARF gene expression increases with age and following chronic kidney injury and has been shown to induce cell senescence by its downstream mediators Rb, p21, and p53. In this study, we demonstrate that INK4a/ARF gene expression is also increased for up to 42 days after AKI. Significant variability in p16 and p19 expression was noted by both qPCR and Western blot at 28 and 42 days and likely correlates with the amount of residual kidney injury and fibrosis as a result of varying degrees of injury following surgery. Knockout of the INK4a/ARF locus results in increased tubular cell proliferation, decreased apoptosis, and decreased capillary rarefaction. The mechanisms by which such capillary preservation occurs may involve increased mobilization of proangiogenic myeloid cells in KO mice.
Decreased INK4a and ARF gene expression has been hypothesized to promote tissue regeneration by increasing stem cell self-renewal (2, 13, 35), decreasing senescence-associated secretory phenotypic changes, including increased production of fibrosis-inducing factors by fibroblasts and epithelial cells (3), and induction of somatic cell dedifferentiation and proliferation following injury (18). Results from this study suggest that the regenerative effect of INK4a/ARF deletion may also be the result of increased angiogenesis. Forty two days following IRI, KO mice demonstrated preserved peritubular capillary density in contrast to a 60% reduction in WT mice. This decrease in capillary density in WT mice is larger than the 30–50% decrease following IRI reported in a previous study (1), a difference that is likely the result of different methods used to measure capillary area.
The role of INK4a/ARF gene expression in inhibiting angiogenesis has not been clear. Recent studies using p16 and p19 KO mice have demonstrated that loss of p16 results in increased tumor angiogenesis by contributing to increased VEGF levels, an effect that can be reversed by exogenous expression of p16 (6, 7, 27). The INK4a/ARF locus may also regulate angiogenesis by controlling growth and differentiation of pericytes. p19 was demonstrated to reduce pericyte PDGF-β receptor expression and proliferation in the developing mouse retina (26), resulting in loss of capillaries and blindness. p19 also inhibits NF-κB and c-myc activity, factors that have been implicated in promoting angiogenesis through their proinflammatory activity and may also inhibit angiogenesis by nucleolar sequestration of hypoxia-inducible factor-1α and control of VEGF mRNA translation (9, 20, 21, 23, 26). Our results demonstrate that INK4a KO mice have a twofold increase in vessel formation by Matrigel plug angiogenesis assays compared with WT mice. The formation of organized, perfused vessels in Matrigel plug assays without addition of growth factors such as fibroblast growth factor 2 or VEGF usually occurs at very low levels (28), suggesting that KO mice have increased angiogenic potential, a characteristic that may add to the tumor-promoting effect of INK4a/ARF deletion. Aortic ring assays from KO mice, however, show decreased capillary sprouting compared with WT mice, suggesting the possibility that circulating factors may be responsible for the observed increased angiogenesis and decreased capillary rarefaction in KO mice. Previous work from our laboratory demonstrated that KO mice have a highly exaggerated inflammatory response following kidney injury by unilateral ureteral obstruction (32), suggesting that the observed differences in KO mice using the reversible injury model of IRI may be because of increased reparative cytokine or chemokine production. Immunoassays for inflammatory cytokines showed >100-fold increased kidney IL-8 levels and an ∼18-fold increase in G-CSF levels at 7–14 days following IRI. These results suggested that increased infiltration of proangiogenic circulating cells may have an important role in the observed effects.
G-CSF promotes tissue regeneration by both recruitment of bone marrow-derived cells and direct anti-apoptotic effects on tubular epithelial cells (14). In a mouse model of hind limb ischemia, G-CSF increased VEGF production by Gr-1+ neutrophils, increased systemic VEGF levels, and improved angiogenesis. This was associated with an increase in VEGF-expressing Gr-1+ cells detected in ischemic muscle (10).
Gr-1+ cells are defined by their recognition by monoclonal antibodies recognizing the Ly-6G antigen. Gr-1+/CD11b+ cells are composed of a heterogeneous population of cells including neutrophils, monocytes, and myeloid progenitors. This group of cells contains subsets of cells that have been found to have important functions in tumor progression, including myeloid-derived suppressor cells that mediate immune suppression and cells mediating VEGF-independent angiogenesis (25). Gr-1+/CD11b+ cells are increased in tumors refractory to anti-VEGF therapy and are able to increase tumor growth and angiogenesis when implanted in tumors or mobilized by G-CSF (5). The role of the INK4a/ARF locus in the angiogenic function of these cells remains unknown. Depletion of Gr-1+ cells resulted in decreased capillary density and increased medullary α-SMA expression, a change that may be the result of increased endothelial-myofibroblast transition. Together, these results suggest that the increased angiogenesis observed in Matrigel assays and decreased capillary rarefaction in KO mice following injury may be because of an increased proangiogenic effect of circulating myeloid cells. The literature presents conflicting evidence as to the mechanism by which these circulating Gr-1+/CD11b+ myeloid cells promote angiogenesis. In an hind limb ischemia model, increased angiogenesis was shown to be at least partly due to these cells' capacity to differentiate into vascular cells (10). Gr-1+/CD11b+ cells have also been found to incorporate into tumor endothelium and acquire endothelial markers (34). On the other hand, Gr-1+/CD11b+ treatment has also been found to promote angiogenesis without direct incorporation into capillary walls (4, 24), presumably through paracrine effects, including production of VEGF. Additional experiments, including examination of the effect of bone marrow transplant from KO donor mice into WT recipients, are necessary to determine if the observed effects are because of changes in myeloid cell phenotype due to INK4a deletion or to changes in the kidney microenvironment that promote increased myeloid cell infiltration and angiogenic function.
The INK4a/ARF locus has been shown to have a critical role in promoting myeloid cell differentiation and prevention of leukemia (31), suggesting that KO mice may have relatively immature granulocytes with increased angiogenic capabilities. Our results add an additional possible mechanism for the tumor suppressor function of this gene. Additional experiments examining the role of Gr-1+/CD11b+ cells in tumors in INK4a/ARF KO mice may help to support this possibility.
Results from this study using INK4a/ARF deletion do not differentiate between the effects of p16 and p19 and are complicated by the pleiotropic effects of knockout of this gene. Future experiments using selective p16 and p19 KO mice and cell-specific INK4a/ARF knockouts in tubular epithelial cells, infiltrating leukocytes, and endothelial cells will be necessary to provide definitive evidence of which cell types mediate the angiogenic effect of INK4a/ARF deletion.
The present study examines the effect of INK4a/ARF deletion on kidney regeneration and repair. INK4a/ARF levels increase with aging in the kidney and other organs (11). Results from this study suggest the possibility that the decreased tissue regeneration and capillary loss following injury associated with aging may the result of increased INK4a/ARF gene expression. Future studies using INK4a/ARF-overexpressing mice or conditional knockout of INK4a in aging mice can be performed to examine this possibility.
In summary, results from this study demonstrate that INK4a/ARF locus expression is increased in tubular epithelial cells for a prolonged period following IRI. INK4a/ARF gene deletion results in improved kidney regeneration based on evidence of increased tubular cell proliferation and decreased apoptosis immediately following injury. Following the initial reparative phase, INK4a KO mice also display a marked decrease in capillary rarefaction compared with WT mice, a difference that may be mediated by increased plasma G-CSF levels and increased kidney infiltration with angiogenic myeloid cells.
GRANTS
This research was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-067881 (M. D. Plotkin).
DISCLOSURES
No conflicts of interests, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Carl Hamby for help with FACS analysis and Dr. Jun Chen for help with aortic ring assays.
REFERENCES
- 1. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, Hulsman D, Leung C, Arsenijevic Y, Marino S, van Lohuizen M. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev 19: 1438–1443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Dudley AC, Udagawa T, Melero-Martin JM, Shih SC, Curatolo A, Moses MA, Klagsbrun M. Bone marrow is a reservoir for proangiogenic myelomonocytic cells but not endothelial cells in spontaneous tumors. Blood 116: 3367–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrara N. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr Opin Hematol 17: 219–224, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Gibson SL, Boquoi A, Chen T, Sharpless NE, Brensinger C, Enders GH. p16(Ink4a) inhibits histologic progression and angiogenic signaling in min colon tumors. Cancer Biol Ther 4: 1389–1394, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibson SL, Dai CY, Lee HW, DePinho RA, Gee MS, Lee WM, Furth EE, Brensinger C, Enders GH. Inhibition of colon tumor progression and angiogenesis by the Ink4a/Arf locus. Cancer Res 63: 742–746, 2003 [PubMed] [Google Scholar]
- 8. Harada H, Nakagawa K, Iwata S, Saito M, Kumon Y, Sakaki S, Sato K, Hamada K. Restoration of wild-type p16 down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human gliomas. Cancer Res 59: 3783–3789, 1999 [PubMed] [Google Scholar]
- 9. Kawagishi H, Nakamura H, Maruyama M, Mizutani S, Sugimoto K, Takagi M, Sugimoto M. ARF suppresses tumor angiogenesis through translational control of VEGFA mRNA. Cancer Res 70: 4749–4758 [DOI] [PubMed] [Google Scholar]
- 10. Kim JA, March K, Chae HD, Johnstone B, Park SJ, Cook T, Merfeld-Clauss S, Broxmeyer HE. Muscle-derived Gr1(dim)CD11b(+) cells enhance neovascularization in an ischemic hind limb mouse model. Blood 116: 1623–1626, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis JL, Chinswangwatanakul W, Zheng B, Marley SB, Nguyen DX, Cross NC, Banerji L, Glassford J, Thomas NS, Goldman JM, Lam EW, Gordon MY. The influence of INK4 proteins on growth and self-renewal kinetics of hematopoietic progenitor cells. Blood 97: 2604–2610, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Wu J, Shou Z, He Q, Zhang P, Han F, Li H, Chen J. Pretreatment with granulocyte colony-stimulating factor attenuated renal ischaemia and reperfusion injury via activation of PI3/Akt signal pathway. Nephrology (Carlton) 13: 508–516, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int 63: 2134–2143, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65: 510–520, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Melk A, Schmidt BM, Vongwiwatana A, Rayner DC, Halloran PF. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5: 1375–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Pajcini KV, Corbel SY, Sage J, Pomerantz JH, Blau HM. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell 7: 198–213, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qi Y, Gregory MA, Li Z, Brousal JP, West K, Hann SR. p19ARF directly and differentially controls the functions of c-Myc independently of p53. Nature 431: 712–717, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Rocha S, Campbell KJ, Perkins ND. p53- and Mdm2-independent repression of NF-kappa B transactivation by the ARF tumor suppressor. Mol Cell 12: 15–25, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85: 27–37, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Sherr CJ, Bertwistle D, WDENB, Kuo ML, Sugimoto M, Tago K, Williams RT, Zindy F, Roussel MF. p53-Dependent and -independent functions of the Arf tumor suppressor. Cold Spring Harb Symp Quant Biol 70: 129–137, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 25: 911–920, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA 106: 6742–6747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silva RL, Thornton JD, Martin AC, Rehg JE, Bertwistle D, Zindy F, Skapek SX. Arf-dependent regulation of Pdgf signaling in perivascular cells in the developing mouse eye. Embo J 24: 2803–2814, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takeuchi H, Ozawa S, Shih CH, Ando N, Kitagawa Y, Ueda M, Kitajima M. Loss of p16INK4a expression is associated with vascular endothelial growth factor expression in squamous cell carcinoma of the esophagus. Int J Cancer 109: 483–490, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development 135: 523–532, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vielhauer V, Anders HJ, Perez de Lema G, Luckow B, Schlondorff D, Mack M. Phenotyping renal leukocyte subsets by four-color flow cytometry: characterization of chemokine receptor expression (Abstract). Nephron Exp Nephrol 93: e63, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Williams RT, Sherr CJ. The INK4-ARF (CDKN2A/B) locus in hematopoiesis and BCR-ABL-induced leukemias. Cold Spring Harb Symp Quant Biol 73: 461–467, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Wolstein JM, Lee DH, Michaud J, Buot V, Stefanchik B, Plotkin M. INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am J Physiol Renal Physiol 299: F1486–F1495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6: 409–421, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Zhang HW, Ding J, Jin JL, Guo J, Liu JN, Karaplis A, Goltzman D, Miao D. Defects in mesenchymal stem cell self-renewal and cell fate determination lead to an osteopenic phenotype in Bmi-1 null mice. J Bone Miner Res 25: 640–652, 2010 [DOI] [PubMed] [Google Scholar]