Abstract
Augmentation of intrarenal angiotensinogen (AGT) synthesis, secretion, and excretion is associated with the development of hypertension, renal oxidative stress, and tissue injury during ANG II-dependent hypertension. High salt (HS) exacerbates hypertension and kidney injury, but the mechanisms remain unclear. In this study, we determined the consequences of HS intake alone compared with chronic ANG II infusion and combined HS plus ANG II on the stimulation of urinary AGT (uAGT), renal oxidative stress, and renal injury markers. Sprague-Dawley rats were subjected to 1) a normal-salt diet [NS, n = 5]; 2) HS diet [8% NaCl, n = 5]; 3) ANG II infusion in NS rats [ANG II 80 ng/min, n = 5]; 4) ANG II infusion in HS rats [ANG II+HS, n = 5]; and 5) ANG II infusion in HS rats treated with ANG II type 1 receptor blocker (ARB) [ANG II+HS+ARB, n = 5] for 14 days. Rats fed a HS diet alone did not show changes in systolic blood pressure (SBP), proteinuria, cell proliferation, or uAGT excretion although they did exhibit mesangial expansion, collagen deposition, and had increased NADPH oxidase activity accompanied by increased peroxynitrite formation in the kidneys. Compared with ANG II rats, the combination of ANG II infusion and a HS diet led to exacerbation in SBP (175 ± 10 vs. 221 ± 8 mmHg; P < 0.05), proteinuria (46 ± 7 vs. 127 ± 7 mg/day; P < 0.05), and uAGT (1,109 ± 70 vs.. 7,200 ± 614 ng/day; P < 0.05) associated with greater collagen deposition, mesangial expansion, interstitial cell proliferation, and macrophage infiltration. In both ANG II groups, the O2− levels were increased due to increased NADPH oxidase activity without concomitant increases in peroxynitrite formation. The responses in ANG II rats were prevented or ameliorated by ARB treatment. The results indicate that HS independently stimulates ROS formation, which may synergize with the effect of ANG II to limit peroxynitrite formation, leading to exacerbation of uAGT and greater injury during ANG II salt hypertension.
Keywords: renin-angiotensin system, reactive oxygen species, kidney injury, macrophage infiltration, dihydroethidium staining
the enhancement of intrarenal angiotensin II (ANG II) content contributing to hypertension and kidney damage is supported by the presence of all components of the renin-angiotensin system (RAS) in the kidney (27–29). During ANG II-dependent hypertension, intrarenal RAS activation is characterized by increased tissue levels of ANG II due to an augmented ANG II type 1 receptor (AT1R) binding with concomitant internalization of circulating ANG II (5, 40) and by de novo formation of ANG II (30). In response to chronic ANG II infusions, there are 1) enhancement of angiotensinogen (AGT) synthesis and secretion by the proximal tubule cells (12), 2) increased urinary AGT (uAGT) excretion (13, 14), 3) stimulation of renin synthesis and secretion by the principal cells of the connecting tubules and collecting ducts (31–33), and 4) increased angiotensin-converting enzyme (ACE) activity along the nephron (1, 17, 33), which support further ANG II generation in distal nephron segments (31, 33).
The association of high salt (HS) consumption with cardiovascular and renal diseases including hypertension is well recognized. However, the mechanisms by which HS is translated into greater hypertension and renal injury remain unclear. While HS suppresses the RAS in normotensive individuals (2, 9), excessive salt intake exerts deleterious effects causing renal injury in hypertensive subjects through pressure-independent mechanisms (22, 24). It has been suggested that sustained RAS activity is involved in mediating the adverse effects of salt, since RAS blockade prevents or ameliorates salt-induced renal injury (44–46). Susic et al. (45) demonstrated that RAS activity in spontaneously hypertensive rats (SHR) was not suppressed or even augmented after 4 wk of salt loading, indicating that maintained intrarenal RAS combined with a HS diet contributes to greater renal damage in SHR (45). The increased RAS can act synergistically with reactive oxidative species (ROS), leading to salt-sensitive hypertension (2). In normal conditions, a low level of oxidative stress is maintained by the balance between the production and the degradation of ROS such as superoxide (O2−). Reactive O2− is rapidly reduced by the enzyme superoxide dismutase or reacts with nitric oxide (NO); however, when there is enhanced O2− activity in the kidney, salt and water retention may occur (18, 23). In ANG II-infused rats and mice, augmented O2− is considered a key determinant in the development of salt-sensitive hypertension (19, 36). Conversely, in salt-sensitive hypertension ROS-dependent enhancement of AGT may be involved in the development and progression of renal injury (15, 26). Saeed et al. (37) showed that ANG II-infused rats on a HS diet (ANG II-salt hypertension) developed impairment of dynamic renal blood flow autoregulation, which was attenuated by tempol (37). However, the unique effect of HS on the pathogenesis of ANG II-salt hypertension has not been established.
The aim of the present study was to determine the consequences of HS intake alone, chronic ANG II infusion alone, and ANG II and HS on the stimulation of uAGT, renal oxidative stress, and kidney injury. Because the Sprague-Dawley rat is a salt-resistant model, we used an aggressive HS diet (8% NaCl) in the presence and absence of a chronic infusion of ANG II (80 ng/min). Since the HS diet did not alter systolic blood pressure (SBP) in normal rats, we were able to discriminate the pressor component mediated by ANG II and delineate a pressure-independent effect of HS diet on uAGT, ROS, and kidney tissue injury responses.
METHODS
Animals, experimental protocols, and samples handling.
All animal procedures were approved by the Tulane University Animal Care and Use Committee. Twenty-five male Sprague-Dawley rats (175–200 g; Charles River Laboratories, Wilmington, MA) were randomly divided into five groups: 1) NS rats were fed a normal-salt diet (0.3% NaCl; diet TD 99414; Harlan-Teklad, Madison, WI) and sham operated; 2) ANG II rats were fed a NS diet and received ANG II (80 ng/min sc via minipumps for 14 days; Sigma, St Louis, MO; 3) HS rats were fed a high-salt diet (8% NaCl; diet TD 92012; Harlan-Teklad) and sham operation; 4) HS+ANG II rats were fed a HS diet and had ANG II infusion; and 5) HS+ANG II+ARB rats were fed a HS diet, infused with ANG II, and an AT1 receptor blocker [ARB; losartan (30 mg/kg) or candesartan (25 mg/kg)] was added to the drinking water for 14 days.
SBP was monitored by tail-cuff plethysmography (IITC Instruments, Woodland Hills, CA) 1 day before and 3, 7, and 11 days following sham operation or minipump implantation, as previously described (14, 33). One day after the SBP measurement, the body weight was taken and the rats were housed in individual metabolic cages in a temperature-controlled room regulated on a 12:12-h light-dark cycle with free access to chow and water. Twenty-four-hour urine samples were collected and centrifuged at 3,000 g for 5 min, and the supernatants were separated and stored at −20°C until assayed for total protein and urinary AGT (uAGT), as previously described (13). Rats were euthanized by conscious decapitation on day 14. Trunk blood samples were collected in chilled tubes containing 5 mmol/l EDTA, which were centrifuged at 2,000 g for 30 min at 4°C for plasma fraction separation to measure plasma renin activity (PRA) (31). Immediately after kidney harvesting, the poles of the right kidney were sectioned and immediately immersed in optimum cutting temperature compound (OCT, Tissue-Tek, Sakura Finetek, Torrance, CA) and used for O2− determination. Right kidney samples were snap frozen in liquid nitrogen and used for Western blotting and NAPDH oxidase activity determinations. The left kidneys were perfused with cold PBS followed by 4% paraformaldehyde formaldehyde and fixation with formalin to be used for histological purposes.
ROS in the kidney cortex.
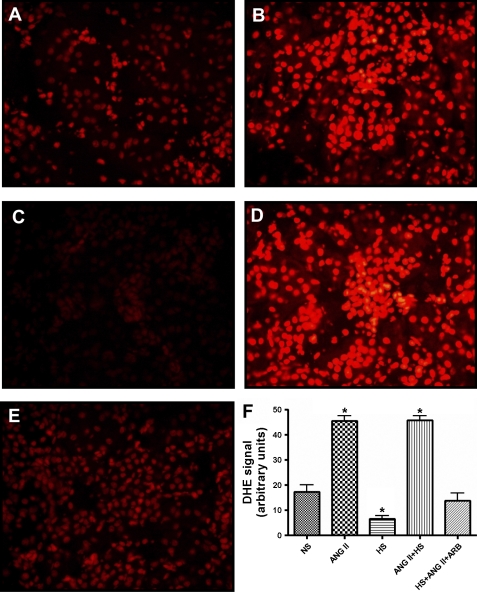
The oxidative fluorescent dye dihydroethidium (DHE; Invitrogen, Carlsbad, CA) was used to detect ROS in kidney poles from NS, ANG II, HS, ANG II+HS, and ANG II+HS+ARB rats, as previously described (39, 41). Ten-micrometer cryosections from kidney poles, were stained with the superoxide-sensitive dye DHE (10 μmol/l) in a light-protected and humidified chamber for 30 min at 37°C. Five hundred and twenty units per milliliter of polyethylene glycol-conjugated superoxide dismutase (PEG-SOD; Sigma, St. Louis, MO) were applied in combination with DHE to adjacent sections to normalize signals for the background signal. Three nonoverlapping images per section were analyzed with a fluorescent microscope, and the signal was quantified using Image ProPlus Software (Media Cybernetics, Olympus).
NAPDH oxidase activity.
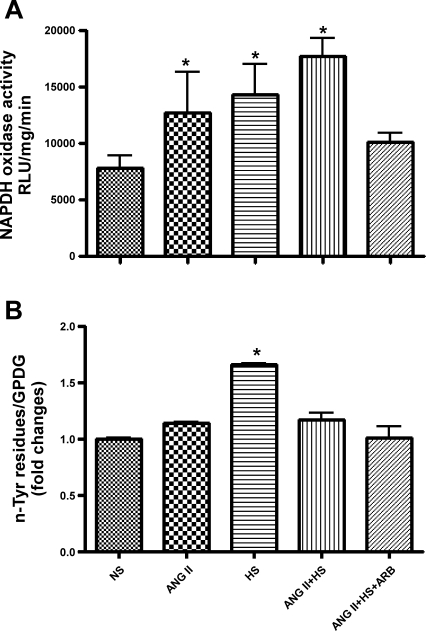
The activity of NADPH oxidase in kidney tissue samples was quantified by lucigenin-enhanced chemiluminescence, as previously described (21). The kidney homogenates (30 mg of tissue) were collected in 500 μl of PBS mixture with protease inhibitor (Calbiochem, San Diego, CA) and phosphatase inhibitor cocktails (Sigma) and centrifuged at 1,000 g for 10 min at 4°C. The supernatants were then collected followed by addition of NADPH (100 μmol/l) and lucigenin (10 μmol/l) for NADPH oxidase activity assay with a FB-12 luminometer in the presence or absence of diphenylene iodonium (DPI; 100 μmol/l), a selective inhibitor of flavin-containing enzymes, including NADPH oxidase. Data were calculated as the change in the rate of luminescence per minute per milligram of tissue.
Peroxynitrite activity.
Protein fractions, obtained from kidney cortexes, were prepared by homogenization in lysis buffer (0.1% Triton X-100, 0.1% deoxycholate, 25 mmol/l HEPES, pH 7.4, 50 mmol/l NaCl, 1 mmol/l MgCl2, 2 mmol/l EGTA, 10 mmol/l pyrophosphate, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 0.5 mmol/l PMSF, and 500 mmol/l Na3VO4.) and sonicated. Samples were centrifuged at 14,000 g for 10 min at 4°C. Equal amounts of protein (50 μg) were separated by SDS-PAGE (4–12%) and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 3% (wt/vol) BSA-TBST and incubated for 1 h at room temperature with anti-3-nTyr antibody (Cayman Chemical, Ann Arbor, MI), in 3% (wt/vol) BSA-TBST buffer for 1 h (42). Membranes were rinsed with TBST and then incubated with anti-mouse Ig-horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The immunoblots were visualized by chemiluminescence using the ECL (GE Healthcare, Piscataway, NJ) according to the manufacturer's protocol. The images were captured using Molecular Imager VersaDoc Imaging Systems (Bio-Rad, Hercules, CA). Densitometric determinations, using Image J Software (National Institutes of Health), were calculated as the ratio between the n-Tyr residues bands ranging from 120 to 12 kDa and GAPDH expression.
Histology and immunohistochemistry of kidney injury markers.
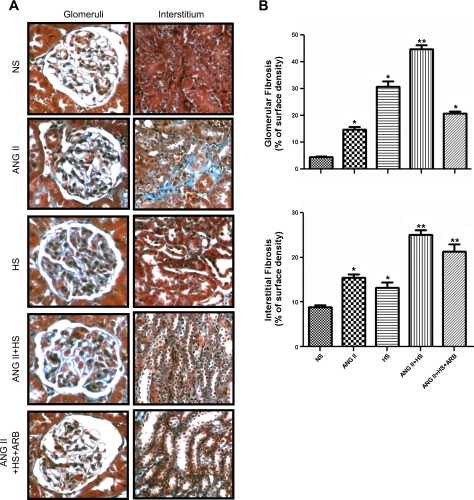
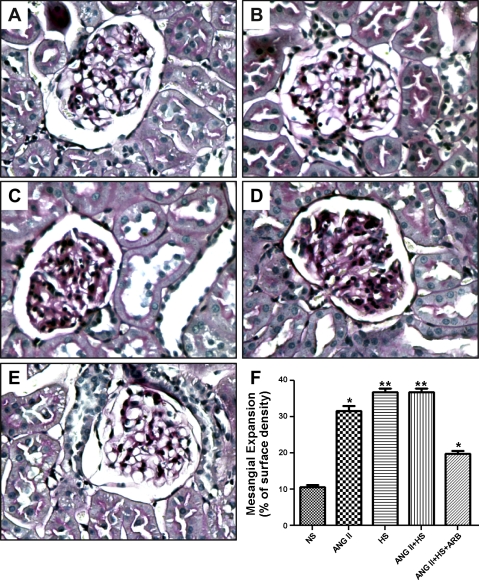
Kidney sections (3 μm), paraffin embedded, were stained with 1) Masson's trichrome (Mass Histology, Worcester, MA) for assessment of glomerular and interstitial fibrosis and 2) periodic acid-Schiff (PAS) for the determination of glomerular lesions. Morphometry of glomeruli and the extent of the interstitial fibrotic-positive area were evaluated quantitatively using computerized image analysis, as described (7, 26). Mesangial expansion was quantified in 20 glomeruli per kidney per rat by dividing the PAS-positive stained area by the total area of the glomerulus and expressed as means ± SE of the 20 measurements in percentage values, as previously described (7, 26). For the immunoexpression of proliferating cell nuclear antigen (PCNA) and CD68, markers of cell proliferation and macrophage infiltration, respectively, monoclonal antibodies for PCNA and CD68 were used at 1:1,000 dilutions (7, 26), respectively. Data were expressed as fold-changes in the number of nuclei in proliferation (for PCNA) or number of macrophages (for CD68). All the stains were performed by a robotic system (Autostainer; Dako, Carpinteria, CA) and analyzed using photomicrographs at ×200 or ×400 magnification from 20 different microscopic fields per tissue section per animal, and digital images were captured with a DS-U2/L2 USB camera attached to a Nikon Eclipse 50i microscope and an NIS Elements AR version 3.0 software image analyzer in a blinded manner to avoid bias.
Statistical analysis.
Results are expressed as means ± SE. The data were compared by one-way analysis of variance taking into account the treatment in the experimental groups. The significance of the difference was evaluated using the multiple comparative Bonferroni test. The values of all parameters were considered significantly different at P < 0.05.
RESULTS
Functional responses in ANG II-salt hypertensive rats.
The combination of ANG II+HS slowed the normal body weight gain to the same extent as that observed in chronic ANG II-infused rats, while a HS diet alone did not (NS: 336 ± 8; HS: 323 ± 7; ANG II 286 ± 9 and ANG II+HS 242 ± 12 g; P < 0.05). In addition, ANG II+HS rats had increased Na+ excretion compared with the HS alone group (HS: 28 ± 2 and ANG II+HS: 49 ± 5 meq·24 h−1·100 g−1; P < 0.05); in both, Na+ excretion was much higher than in NS rats (0.82 ± 0.092 meq·24 h−1·100 g−1; P < 0.05). No differences in food intake were detected. The ANG II+HS rats demonstrated severe lethargy, assumption of a hunched posture, and piloerection, which along with a salt-losing condition are manifestations of malignant hypertension in the rat (25, 48).
Systolic blood pressures were followed throughout the experimental period, and the data at the end of the study are shown in Table 1. Although a HS diet alone did not alter SBP, it caused a further increase compared with ANG II infusion alone. This effect was prevented by ARB treatment. Proteinuria levels followed the same profile observed for SBP with a marked augmentation of proteinuria caused by combining HS with ANG II infusion (Table 1). Plasma renin activity was suppressed during ANG II and HS intake but was markedly increased by ARB treatment (Table 1).
Table 1.
Functional responses in ANG II-salt hypertensive rats
| Rat Group | SBP, mmHg | PRA, ng ANG I·ml−1·h−1 | Proteinuria, mg/day | uAGT, ng/day |
|---|---|---|---|---|
| NS | 115 ± 2 | 6 ± 2 | 23 ± 3 | 40 ± 7 |
| ANG II | 175 ± 10* | 0.3 ± 0.2* | 46 ± 7* | 1,109 ± 70* |
| HS | 124 ± 4 | 0.1 ± 0.01* | 21 ± 1 | 55 ± 9 |
| ANG II+HS | 221 ± 8† | 0.2 ± 0.01* | 127 ± 7† | 7,200 ± 614† |
| ANG II+HS+ARB | 116 ± 1 | 41 ± 12* | 33 ± 3 | 55 ± 7 |
Values are means ± SE. SBP, systolic blood pressure; PRA, plasma renin activity; uAGT, urinary angiotensinogen. Rat groups (n = 5) were divided as described in methods: normal salt intake (NS); chronic ANG II infusion (ANG II); high salt intake (HS); chronic ANG II infusion associated with a HS diet (ANG II+HS), and chronic ANG II infusion associated with a HS diet and treatment with an ANG II type 1 receptor blocker (ANG II+HS+ARB).
P < 0.05 vs. NS,
P < 0.05 vs. ANG II.
Although HS alone did not change the uAGT levels, the combination of HS with chronic ANG II infusion markedly exacerbated the increases in uAGT by almost sevenfold compared with ANG II infusion alone (Table 1). This effect was also prevented by ARB treatment.
ROS generation in kidney cortex of chronically ANG II-infused SD rats subjected to a HS diet.
Figure 1 shows DHE fluorescence as an indication of ROS production [superoxide (O2−), hydrogen peroxide (H2O2), or hydroxyl radical (OH−)] in the rat kidney sections. ANG II and ANG II+HS rats exhibited increased DHE fluorescence from 17 ± 2.8 (NS) to 45 ± 2.0 and 46 ± 1.8 arbitrary units (P < 0.001), respectively; while ARB treatment prevented ROS formation. In contrast, HS rats showed a decrease in DHE fluorescence compared with the NS rats (7 ± 1.3 arbitrary units; P < 0.01). In contrast, NADPH oxidase activity (Fig. 2A) was increased in HS rats and ANG II rats with the activity increasing more than twofold in ANG II+HS rats [NS: 7,841 ± 1,101; ANG II: 12,738 ± 3,537; HS: 14,310 ± 2,720; ANG II+HS: 17,747 ± 1,519 relative light units (RLU)·mg−1·min−1; P < 0.05]. Since it has been established that HS intake increases NO formation (8, 20, 49), we postulated that NO contributes to the consumption of the O2− by generating peroxynitrite (ONOO−). Thus we used the detection of nitrotyrosine residues in the proteins of the kidney as a marker of local peroxynitrite levels (6, 20, 47). Interestingly, rats fed a HS diet alone exhibited the highest levels of peroxynitrite activity (Fig. 2B), thus explaining the reduced DHE fluorescence in the HS rats. Importantly, the combination of ANG II+HS did not manifest the increases in nitrotyrosine residues observed with HS alone. ARB treatment prevented increases in DHE fluorescence, NAPDH oxidase activity, and nitrotyrosine residues.
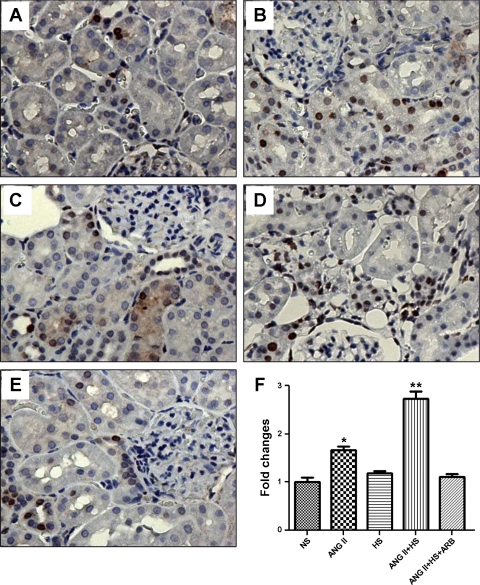
Fig. 1.
Dihydroethidium (DHE) staining of kidney frozen sections of rats (n = 5) subjected to normal salt (NS) intake (A), chronic ANG II infusion (B), high salt (HS) intake (C), ANG II chronic infusion associated with a HS diet (ANG II+HS; D), and chronic ANG II infusion plus HS diet plus treatment with ANG II type 1 receptor blocker (ANG II+HS+ARB; E). DHE nuclear staining denotes the presence of reactive oxygen species (ROS). DHE fluorescence, quantified as described in methods, in kidney sections using ×200 magnification, is represented in F. Values are means ± SE. *P < 0.05 vs. NS.
Fig. 2.
Effects of HS diet in chronic ANG II-infused rats on NAPDH oxidase activity (A) and peroxynitrite activity (B) in renal tissues. Rat groups (n = 5) were divided as described in methods. NAPDH oxidase activity was quantified as relative luminescent unit (RLU) per minute. Nitrotyrosine residues were detected by Western blot analysis using the an anti-nTyr antibody (1:1,000; Cayman Chemical, Ann Arbor, MI), and GAPDH was used as a loading control. Quantification of the total protein bands ranging from 120 to 15 kDa was obtained by the Image J Software (NIH) and expressed as a ratio from GAPDH. Values are means ± SE. *P < 0.05 vs. NS.
A HS diet exacerbates renal interstitial and glomerular fibrosis, glomerular expansion, cell proliferation, and interstitial macrophage infiltration in ANG II-salt hypertensive rats.
The renal fibrosis examined by Masson's trichrome staining in glomeruli and in the tubulointerstitial areas (Fig. 3A) showed that in both cases, the combination of chronic ANG II infusion and HS intake exacerbated collagen deposition compared with the ANG II and HS rats (Fig. 3, B and C). However, HS alone caused significant increases in glomerular and interstitial fibrosis even though these rats were not hypertensive. ARB treatment prevented fibrosis in the glomeruli (Fig. 3B) but not in the interstitium (Fig. 3C).
Fig. 3.
Representative photomicrographs of collagen deposition visualized by Masson's trichrome stain in glomeruli (×400) and tubulointerstitial areas (×200; A). Twenty different microscopic fields per tissue section per animal (n = 3/group) were analyzed from paraffin kidney sections (3 μm). The extent of the interstitial fibrotic-positive area was evaluated quantitatively by automatic image analysis (B and C), which determined the area occupied by interstitial tissue staining positive in Masson's trichrome-stained sections (blue). Values are means ± SE expressed as a percentage of the blue areas/field. *P < 0.01 vs. NS. **P < 0.01 vs. ANG II.
Using PAS staining (Fig. 4), ANG II, HS, and ANG II+HS rats exhibited a pattern of diffuse mesangial expansion. Indeed, the quantification of positive PAS-stained areas showed that the two groups of rats subjected to a HS diet presented the highest degree of glomerular expansion and that this effect was partially prevented by ARB treatment (NS: 11 ± 0.5; ANG II: 32 ± 1.3; HS: 37 ± 0.9; ANG II+HS: 36 ± 0.9; ANG II+HS+ARB: 19 ± 0.8%; P < 0.01; Fig. 4F).
Fig. 4.
Representative photomicrographs of mesangial expansion by periodic acid-Schiff staining (PAS) of kidney sections (3 μm) from rats subjected to NS intake (A), chronic ANG II infusion (B), HS intake (C), chronic ANG II infusion associated with a HS diet (ANG II+HS; D), and chronic ANG II infusion associated with a HS diet and treatment with ARB (ANG II+HS+ARB; ×400; E). The extent of mesangial expansion was quantified in 20 different microscopic fields per tissue section per animal (n = 3/group) using an automatic image analyses of the area occupied by PAS-positive staining per glomerular area. Values are means ± SE expressed as a percentage of the blue areas/field. *P < 0.01 vs. NS. **P < 0.01 vs. ANG II.
The changes in PCNA immunostaining indicated that a HS diet exacerbated the proliferation of the tubular epithelial cells stimulated by ANG II (Fig. 5); however, HS alone did not elicit an increase in PCNA immunostaining. ARB treatment prevented the increase caused by HS+ANG II (ANG II: 1.7 ± 0.09-fold; ANG II+HS: 2.7 ± 0.1-fold increase in nuclei in proliferation).
Fig. 5.
Cell proliferation in renal tissue sections (3 μm) from rats (n = 3) subjected to NS intake (A), chronic ANG II infusion (B), HS intake (C), ANG II chronic infusion and HS diet (ANG II+HS; D), and chronic ANG II infusion plus HS and treatment with ARB (ANG II+HS+ARB; ×200; E). Tissue sections were stained with monoclonal antibody for proliferating cell nuclear antigen (PCNA; 1:1,000 dilution). The number of positive proliferative nuclei (stained brown)/mm2 appears in F expressed as fold-changes relative to NS. Values are means ± SE. *P < 0.05 vs. NS. **P < 0.05 vs. ANG II.
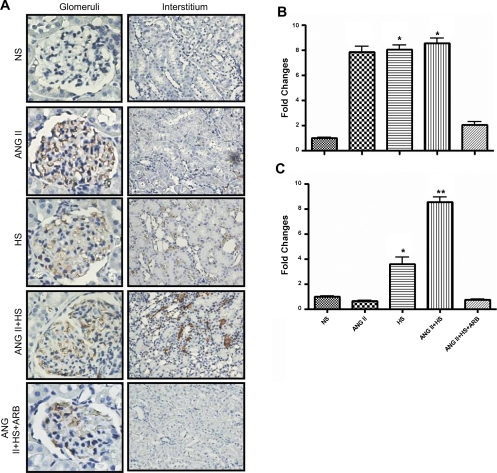
The extent of glomerular and tubulointerstitial macrophage infiltration was quantified by the presence of CD68-positive cells in glomeruli and in the tubulointerstitium (Fig. 6). Similar increases in the accumulation of CD68-positive cells in glomeruli in ANG II, HS, and ANG II+HS rats were observed. The infiltration of CD68-positive cells in the renal interstitium was observed in rats fed a HS diet and was exacerbated in the ANG II-salt hypertensive rats (Fig. 6B). Treatment with ARB completely prevented this effect (Fig. 6B).
Fig. 6.
Kidney immunoexpression of CD68, a marker of macrophages, in glomeruli (×400) and tubulointerstitial areas (×200; A). Twenty different microscopic fields per tissue section per animal (n = 3/group) were analyzed from paraffin kidney sections (3 μm). The number of CD68-positive cells/mm2 was quantified by an automatic image analysis in glomeruli (B) and tubulointerstitium (C) and expressed as fold-changes relative to NS. Values are means ± SE. *P < 0.0001 vs. NS. **P < 0.0001 vs. ANG II.
DISCUSSION
The present study demonstrates that a HS diet given to chronic ANG II-infused Sprague-Dawley rats for 2 wk exacerbates ANG II-dependent hypertension and leads to malignant hypertension, as reflected by the reduced body weight gain, severe lethargy, piloerection, and assumption of a hunched posture, along with increased Na+ excretion and proteinuria. ANG II has been shown to cause skeletal muscle wasting (cachexia) via an increase in muscle catabolism, associated with an increase in proteasome activity mediated, at least in part, by NADPH oxidase-derived ROS (39). Furthermore, ANG II infusion leads to decreases in body mass with decreases in retroperitoneal and epididymal fat but with no alterations in food consumption (34). The physical characteristics of the rats are similar to the ones described in the Cyp1a1-Ren2 rats, a model of ANG II-dependent malignant hypertension associated with increased PRA and high circulating and intrarenal ANG II levels (25, 48).
In the present study, ANG II-salt hypertensive rats also exhibited infiltration of CD68-positive cells in the kidney interstitium, sustained O2− production, tissue injury, and exacerbation of urinary excretion of AGT. These responses are mediated by AT1 receptor activation since ARB treatment prevented or ameliorated the development of these changes. Although a HS diet alone caused increased NAPDH oxidase activity, O2− levels did not increase due to increased scavenging of the excess of O2−, leading to increased peroxynitrite formation. The increased peroxynitrite formation was not apparent when HS was given to ANG II-infused rats, allowing accumulation of O2− levels and greater ROS activity. The increased Na+ excretion likely reflects a pressure-natriuresis effort of the increased SBP. Since we did not observe an increase in SBP in the HS rats, we were able to discriminate the pressor component mediated by ANG II and delineate pressure-independent effects of HS on uAGT, ROS, and kidney tissue responses.
uAGT is a marker for intrarenal RAS activity, but not a primary consequence of the elevated arterial pressure or of hypertension-induced proteinuria (14, 16). The present data indicate that in the ANG II-salt hypertensive rats, uAGT is markedly elevated to much greater levels than those seen in ANG II-infused rats. In contrast, normal rats fed HS remained at basal uAGT excretion rates similar to those in NS rats. It has been demonstrated that HS alone suppresses systemic RAS and renal AGT mRNA expression (8, 10, 38, 43). However, during ANG II infusion, salt-sensitive hypertension develops and HS paradoxically stimulates intrarenal RAS activity, as reflected by exacerbation of the uAGT despite inhibition of PRA. Through augmentation of oxidative stress, HS synergizes with ANG II, culminating in much greater increases in uAGT and injury. Our data support the observations of Franco et al. (4) demonstrating that blood pressure is positively correlated with renal ANG II concentrations but not plasma ANG II. The combined effects of HS intake and the exacerbated intrarenal RAS activity are associated with increased SBP and proteinuria, which support previous findings (4, 38, 48).
Our data demonstrate that the HS either alone or in combination with ANG II increases O2− formation as demonstrated by increased NAPDH oxidase activity. However, with HS alone, there is also a marked increase in peroxynitrite formation that actually reduces the O2− levels due to increased NO formation, which may be critical in preventing increases in blood pressure and renal injury (8, 20, 49). When HS is combined with ANG II infusion, the increased O2− formation is sustained or even slightly increased but the peroxynitrite generation is reduced, thus leading to the increase in O2− levels, which contributes to the exacerbation of the uAGT and increased renal injury. A biphasic effect of peroxynitrite activity has been demonstrated where lower concentrations produce vasorelaxation and higher concentrations induce tissue injury (11, 35). The formation of peroxynitrite during HS intake may be a key molecular mechanism in preventing increases in SBP. However, the sustained formation of peroxynitrite in rats fed a HS diet may induce sulfhydryl oxidation, protein nitration, and lipid peroxidation, which can also contribute to kidney injury (3), as reflected by the glomerular expansion and kidney fibrosis even at SBP in the normal range. These findings further support the notion that pressure-independent factors stimulated by HS may predispose the kidneys to greater injury in response to ANG II infusions (49). HS independently stimulates transforming growth factor β1 (49) and other intracellular signaling pathways, leading to an enhanced response to ANG II. An enhancement in O2− generation may also contribute to the early developmental phase of ANG II-induced salt sensitive hypertension (20). Therefore, the exacerbation in kidney damage exhibited by the ANG II infused rats fed a HS diet could be explained, at least in part, by the interaction between the RAS and ROS. In the present study, the ANG II+HS rats, had marked increases in uAGT excretion rats which paralleled the increases in O2− in renal tissue. The critical involvement of AT1R in mediating this impairment is supported by the findings that ARB treatment prevented the increases in NAPDH oxidase activity, reducing the O2− levels to a normal range. Since the ARB treatment was initiated in parallel with the ANG II infusion and HS diet, we cannot determine whether the increased formation of ROS is a direct effect or due to a reduction of the RAS. However, ARB treatment was able to block the vicious cycle between RAS and ROS.
Although the HS diet did not alter SBP, it was able to cause mesangial expansion and kidney fibrosis, without eliciting cell proliferation. The combination of HS with chronic ANG II infusions associated with higher levels of SBP clearly led to marked exacerbation of the ANG II effects on mesangial expansion, kidney fibrosis, and tubular epithelial cell proliferation. The fact that ARB treatment was able to prevent fibrosis in the glomeruli but not in the interstitium suggests that despite potential protection of glomeruli by ARB, the lesions in the interstitium may be sustained and progress to chronic kidney disease. Furthermore, an interesting finding was the observation of the infiltration of inflammatory CD68-positive cells into the renal interstitium. Growing evidence demonstrates that the immune system contributes to the mechanism of exacerbation of hypertension in ANG II-infused rats subjected to a HS diet (4). Franco et al. (4), using Sprague-Dawley rats infused with ANG II for 2 wk and then given HS, demonstrated an increase in inflammatory cells in the tubulointerstitial areas. We demonstrated that the combination of a HS diet and ANG II infusion leads to the infiltration of inflammatory cells, although no infiltration was observed when HS diet or ANG II infusion was administered alone. The detection of macrophages in the renal interstitium of the ANG II-salt hypertensive rats most likely contributed to greater activation of intrarenal RAS during HS intake, leading to further accumulation of O2−.
In summary, the results from this study demonstrate increased O2− formation with HS alone, ANG II alone, or ANG II in combination with HS. However, with HS alone there is also a marked increase in peroxynitrite formation. In contrast, ANG II infusion in combination with a HS diet reduced the generation of peroxynitrite but NAPDH oxidase activity was maintained or further increased, shifting the balance to O2− accumulation. This event explains the mechanism by which HS exacerbates the deleterious effects of ANG II. In essence, HS alone causes only modest changes but predisposes the kidney to greater injury when associated with chronic ANG II infusions. Thus in ANG II-salt hypertension the inappropriate intrarenal activation of the RAS, as demonstrated by the exacerbation of the uAGT, and increased ROS by the increases in O2− in the renal tissue, synergize to cause greater glomerular and interstitial fibrosis and macrophage infiltration to the renal interstitium, leading to increased kidney injury.
GRANTS
This work was supported by the National Institutes of Health (P20-RR-017659) through the Institutional Developmental Award (IdeA) program at the National Center for Research Resources, the National Heart, Lung, and Blood Institute (HL-26371 and HL-66432), and the American Heart Association (09BGIA2280440, 09PRE2209079), and the Investigator-Initiated Studies Program of Merck & Co., Inc. M. C. Prieto is a scholar of the Tulane-BIRCWH Program (K12HD043451) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development. L. S. Lara is a recipient of a CAPES Post-Doctoral Fellowship (Brazil).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.S.L. and M.C.P. provided conception and design of research; L.S.L., M.M., L.S.-P., and S.S. performed experiments; L.S.L., M.M., L.S.-P., and S.S. analyzed data; L.S.L., D.S.A.M., H.K., L.G.N., and M.C.P. interpreted results of experiments; L.S.L. prepared figures; L.S.L., L.G.N., and M.C.P. drafted manuscript; L.S.L., D.S.A.M., L.G.N., and M.C.P. edited and revised manuscript; L.S.L., M.M., L.S.-P., S.S., D.S.A.M., H.K., L.G.N., and M.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dale M. Seth, MS, Jessica Mucci, BS, and Mohamed T. Islam for excellent technical assistance. In addition, we are grateful to the Tulane Hypertension and Renal Center of Excellence for facilitating the use of the Molecular and Analytical Cores (P20RR017659). The data reported in this manuscript were partially presented during Experimental Biology 2009 (New Orleans, LA).
REFERENCES
- 1. Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol 272: F405–F409, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol 28: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Dutta UK, Lane J, Roberts J, II, Majid DSA. Superoxide formation and interaction with nitric oxide modulate systemic arterial pressure and renal function in salt-depleted dogs. Exp Biol Med 231: 269–276, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol 288: F420–F427, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol 287: L262–L268, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Graciano ML, Mouton CR, Patterson ME, Seth DM, Mullins JJ, Mitchell KD. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 292: F1858–F1866, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol 290: F1279–F1284, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Ingelfinger JR, Pratt RE, Ellison K, Dzau VJ. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest 78: 1311–1315, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol Renal Physiol 283: F995–F1002, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Ischiropoulos H, al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett 364: 279–282, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension 37: 1329–1335, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension 41: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun 315: 746–750, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J Hypertens 28: 1422–1428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension 42: 195–199, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Kopkan L, Majid DSA. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kopkan L, Castillo A, Navar LG, Majid DSA. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80–F86, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kopkan L, Hess A, Husková Z, Cervenka L, Navar LG, Majid DSA. High-salt intake enhances superoxide activity in eNOS knockout mice leading to the development of salt sensitivity. Am J Physiol Renal Physiol 299: F656–F663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Maitland K, Bridges L, Davis WP, Loscalzo J, Pointer MA. Different effects of angiotensin receptor blockade on end-organ damage in salt-dependent and salt-independent hypertension. Circulation 114: 905–910, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Majid DSA, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol 34: 946–952, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 292: H814–H819, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst 7: 74–86, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol 35: 922–927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol 17: 412–422, 1997 [PubMed] [Google Scholar]
- 28. Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep 5: 135–143, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 11: 180–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens: 96–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prieto MC, González-Villalobos RA, Botros FT, Martin VL, Pagán J, Satou R, Lara LS, Feng Y, Fernandes FB, Kobori H, Casarini DE, Navar LG. Reciprocal changes in renal ACE/ANG II and ACE2/ANG 1–7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol 300: F749–F755, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ortiz RM, Kobori H, Conte D, Navar LG. Angiotensin II-induced reduction in body mass is Ang II receptor mediated in association with elevated corticosterone. Growth Horm IGF Res 20: 282–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288: 481–487, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284: R893–R912, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Saeed A, Dibona GF, Marcussen N, Guron G. High-NaCl intake impairs dynamic autoregulation of renal blood flow in ANG II-infused rats. Am J Physiol Regul Integr Comp Physiol 299: R1142–R1149, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Sechi LA, Griffin CA, Giacchetti G, Valentin JP, Llorens-Cortes C, Corvol P, Schambelan M. Tissue-specific regulation of type 1 angiotensin II receptor mRNA levels in the rat. Hypertension 28: 403–408, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, Michael Tabony A, Delafontaine P. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun 409: 217–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension 56: 378–383 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shenouda SK, Lord KC, McIlwain E, Lucchesi PA, Varner KJ. Ecstasy produces left ventricular dysfunction and oxidative stress in rats. Cardiovasc Res 79: 662–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shenouda SK, Varner KJ, Carvalho F, Lucchesi PA. Metabolites of MDMA induce oxidative stress and contractile dysfunction in adult rat left ventricular myocytes. CardiovascToxicol 9: 30–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh I, Grams M, Wang WH, Yang T, Killen P, Smart A, Schnermann J, Briggs JP. Coordinate regulation of renal expression of nitric oxide synthase, renin, and angiotensinogen mRNA by dietary salt. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1027–F1037, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Susic D, Zhou X, Frohlich ED. Angiotensin blockade prevents salt-induced injury of the renal circulation in spontaneously hypertensive rats. Am J Nephrol 29: 639–645, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Susic D, Frohlich ED, Kobori H, Shao W, Seth DM, Navar LG. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens 29: 716–723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, López B, Díez J. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol Heart Circ Physiol 294: H853–H858, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Williams DE, Prieto MC, Mullins JJ, Navar LG, Mitchell KD. AT1 receptor blockade prevents the increase in blood pressure and the augmentation of intrarenal ANG II levels in hypertensive Cyp1a1-Ren2 transgenic rats fed with a high-salt diet. Am J Med Sci 339: 356–361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ying WZ, Sanders PW. Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-β1. Am J Physiol Renal Physiol 275: F18–F24, 1998 [DOI] [PubMed] [Google Scholar]