Abstract
The mitochondria-rich epithelial cells of the renal medullary thick ascending limb (mTAL) reabsorb nearly 25% of filtered sodium (Na+) and are a major source of cellular reactive oxygen species. Although we have shown that delivery of Na+ to the mTAL of rats increases superoxide (O2·−) production in mTAL, little is known about H2O2 production, given the lack of robust and selective fluorescent indicators for determining changes within the whole cell, specifically in the mitochondria. The present study determined the effect of increased tubular flow and Na+ delivery to mTAL on the production of mitochondrial H2O2 in mTAL. H2O2 responses were determined in isolated, perfused mTAL of Sprague-Dawley rats using a novel mitochondrial selective fluorescent H2O2 indicator, mitochondria peroxy yellow 1, and a novel, highly sensitive and stable cytosolic-localized H2O2 indicator, peroxyfluor-6 acetoxymethyl ester. The results showed that mitochondrial H2O2 and cellular fluorescent signals increased progressively over a period of 30 min following increased tubular perfusion (5–20 nl/min), reaching levels of statistical significance at ∼10–12 min. Responses were inhibited with rotenone or antimycin A (inhibitors of the electron-transport chain), polyethylene glycol-catalase and by reducing Na+ transport with furosemide or ouabain. Inhibition of membrane NADPH-oxidase with apocynin had no effect on mitochondrial H2O2 production. Cytoplasmic H2O2 (peroxyfluor-6 acetoxymethyl ester) increased in parallel with mitochondrial H2O2 (mitochondria peroxy yellow 1) and was partially attenuated (∼65%) by rotenone and completely inhibited by apocynin. The present data provide clear evidence that H2O2 is produced in the mitochondria in response to increased flow and delivery of Na+ to the mTAL, and that whole cell H2O2 levels are triggered by the mitochondrial reactive oxygen species production. The mitochondrial production of H2O2 may represent an important target for development of more effective antioxidant therapies.
Keywords: reactive oxygen species, mitochondria, kidney, flow
the reabsorption of nacl in the medullary thick ascending limbs (mTAL) of Henle normally accounts for 25–30% of the filtered load. The rate of metabolism and cell density of mitochondria of these epithelial cells is highest among all of the nephron segments and among the highest in the body, including the heart (2, 4, 14, 24). Increased delivery of Na+ and mechanical stretch of isolated perfused mTAL results in stimulation of mTAL superoxide (O2·−) production through a PKC-α related pathway (21), which, in turn, enhances the rate of Na+ reabsorption (1, 5, 22, 23, 33). O2·− production appears to be linked to cell metabolism and Na+ transport in the mTAL, as seen by the increases of reactive oxygen species (ROS) that occur in response to increased luminal flow and Na+ delivery (1).
NADPH-oxidase is a primary source of O2·− in the mTAL, accounting for nearly 50% of O2·− production, while the other 50% appears to come from mitochondrial sources, as estimated indirectly using pharmacological agents to inhibit activity of the oxidase (e.g., diphenyleneiodonium and apocynin) and by uncoupling the election transport chain with dinitrophenol (38). Little is known about sites of production of H2O2 in the mTAL, although evidence suggests that H2O2 plays an important functional role in the region of the outer medulla. There is evidence in Sprague-Dawley (SD) rats that local excess production of H2O2 within the medulla of the kidney would produce hypertension. Specifically, chronic renal medullary infusion of a SOD inhibitor into the single remaining kidney of nephrectomized SD rats resulted in increased interstitial H2O2 concentrations and produced hypertension (28). Acute interstitial infusion of H2O2 was shown to reduce medullary blood flow and sodium excretion in a dose-dependent manner that was reversible by catalase (7). Chronic renal medullary infusion of H2O2, which increased medullary interstitial H2O2 concentrations threefold, produced chronic hypertension (28). H2O2 was found to be elevated in the renal interstitium of Dahl salt-sensitive rats compared with controls, and interstitial infusion of catalase significantly reduced H2O2 levels and greatly attenuated the salt-induced hypertension and medullary tubulointerstitial fibrosis and capillary injury (37). These studies have indicated that elevations of H2O2 in the renal medulla contribute to blood pressure salt-sensitivity and renal injury. The present study was, therefore, designed to elucidate mechanisms and sources of H2O2 production in the mTAL of the outer medulla.
Two highly sensitive, novel fluorescent probes were utilized: one that could selectively detect H2O2 in the mitochondria of living cells [mitochondria peroxy yellow 1 (MitoPY1)] (10) and the other [peroxyfluor-6 acetoxymethyl ester (PF6-AM)] that detected whole cell H2O2 (12). The goal of the study was to determine the effect of increased tubular flow and sodium delivery on the mitochondrial and whole cell H2O2 production in mTAL of the rat kidney. The results provide the first direct evidence that increases in the delivery of Na+ to the mTAL results in increased production of mitochondria H2O2, which, in turn, stimulates membrane NADPH-oxidase, yielding an overall increase of intracellular H2O2.
METHODS
Preparation of Rat Renal mTAL
Male SD rats (Harlan Sprague Dawley, Madison, WI), weighing 180–220 g, were maintained on a commercially available pelleted diet (5001, Purina Mills, Gray Summit, MO) with free access to water. Renal mTAL were isolated from SD rats, as previously reported (30). Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), and the kidneys were perfused to clear the blood with 10 ml of chilled (4°C) Hanks' balanced salt solution (HBSS) containing 20 mM HEPES (HBSS-H, pH = 7.4) and 1 mg/ml bovine serum albumin. This maintained metabolism and O2 consumption at a minimum. Renal microtissue strips were dissected from the outer medulla, and this thin strip of tissue from the inner stripe of the outer medulla containing mTAL was placed on a glass coverslip coated with the tissue adhesive Cell-Tak (BD Biosciences, Bedford, MA) for fluorescence imaging, as our laboratory has previously described (1, 31). The dissecting bath was exposed to room air (21% O2; 159 mmHg), and, when the mTAL were transferred to the imaging chamber, they were maintained at 37°C (Warner Instruments) throughout the protocol, with the perfusion chamber and bath solution exposed to room air (21% O2). Thus during periods in which mTAL would be hypoxic (HBSS-H flushing and before dissection), they were kept cool to limit metabolism, and they were well-oxygenated relative to in vivo conditions during all protocols. From each kidney, generally three to four separate coverslips were prepared containing isolated mTAL. This enabled different treatments of mTAL from the same rats. However, we did not repeat the same treatment on the same rat, so each number (e.g., N = 6) represents a separate rat in all cases. All protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Fluorescence Imaging of H2O2
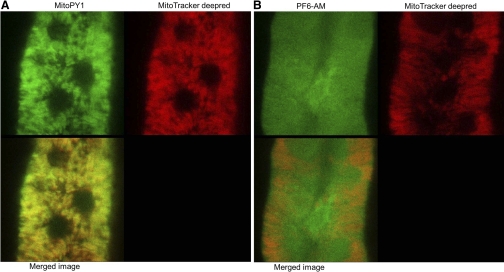
Levels of mitochondrial H2O2 were detected by measuring the intensity of the activated MitoPY1, a novel fluorescent probe for imaging H2O2 levels specifically within the mitochondria of living cells (10). Tissue strips containing mTALs were incubated with MitoPY1, as described in the protocol below, and the coverslips were placed in a heated imaging chamber maintained at 37°C (Warner Instruments) during the experiment. Fluorescence images of changes in the mitochondrial H2O2 level probed by MitoPY1 were obtained using a Leica DMI 6000B inverted microscope equipped with a ×63/1.20 water immersion objective lens (HCX PL APO) and Leica TCS-SP5 laser scanning confocal microscope (Leica, Exton, PA). MitoPY1 was excited at 514 nm, and emission signal from 530 to 590 nm was acquired every 2 min. A series of confocal images were scanned in 2-μm increments and summed for an image representing a three-dimensional projection of the entire 50-μm section. Three-dimensional projected images of each time point were analyzed using LAS AF software. As illustrated in Fig. 1A, MitoPY1 colocalizes with mitochondria in isolated mTAL epithelial cells, demonstrating the high density of this organelle in the mTAL.
Fig. 1.
A: mitochondria peroxy yellow 1 (MitoPY1) colocalizes with MitoTracker deep red in medullary thick ascending limb (mTAL) epithelial cells, as can be seen in the merged image frame (bottom). The circular dark regions are the nuclei, where the dye does not localize. B: peroxyfluor-6 acetoxymethyl ester (PF6-AM) is shown to localize in the cytoplasm and the nucleus.
Levels of intracellular H2O2 were detected by measuring the intensity of PF6-AM, as described in the protocol below. Fluorescence images of changes in the intracellular H2O2 level probed by PF6-AM were obtained using a Nikon TE-2000U inverted microscope equipped with a ×60/1.1 water immersion objective lens and a high-resolution digital camera (Photometrics Cascade 512B Roper Scientific, Tucson, AZ) (1). Excitation was provided by a Sutter DG-4 175-W xenon arc lamp (Sutter Instrument, Novato, CA) at alternating wave lengths, and emission control was achieved using a Lambda 10 optical filter changer (Sutter Instrument). PF6-AM was excited at 480 nm, and 510/40-nm band-pass emission was acquired every 10 s. As illustrated in Fig. 1B, PF6-AM clearly localizes in the cytoplasm and in the nucleus, although we cannot exclude the possibility that it may also enter the mitochondria or other subcellular organelles, such as endoplasmic reticulum.
Fluorescence intensity of all of these images was quantified over an area of ∼10–15 mTAL cells using MetaFluor imaging software (Universal Imaging, Downingtown, PA), as our laboratory has reported previously (9, 30, 31).
Microperfusion of mTAL
Glass pipettes (Drummond Scientific, Broomail, PA) were pulled to an internal diameter of 8–14 μm. The tip of these micropipettes was beveled and smoothed and mounted on a micromanipulator (World Precision Instruments) on the microscope stage. As our laboratory has previously described (1), a micropipette was inserted into the open lumen of the mTAL, and the tubule was perfused with the desired solution and flow rate using a Nano Pump A1400 (World Precision Instruments, Sarasota, FL).
Protocols
Microperfusion of mTAL to determine effects of tubular flow and NaCl concentration on mitochondrial H2O2 production.
To determine mitochondrial H2O2 level, mTALs were incubated with 5 μM MitoPY1 in HBSS-H for 1 h at 37°C (10). The tissues were washed twice to remove excess dye and then mTAL were microperfused with HBSS-H at a flow rate of 5 nl/min during a baseline period and then increased to 20 nl/min. Some mTALs were pretreated with 200 U/ml polyethylene glycol-conjugated catalase (200 μmol/l), rotenone (10 μmol/l), antimycin A (1 μmol/l), apocynin (1 mmol/l), or vehicle by adding the specific solution to the fluid in the chamber. In time control studies, tubules were perfused at 5 nl/min throughout the study with vehicle in the chamber solution.
Other mTALs were microperfused using a special pipette that enables rapid exchange of perfusion solution to change Na+ concentration ([Na+]), and tubules were perfused at a fixed rate of 15 nl/min. Perfusate containing either 0 or 60 mmol/l [Na+] was infused during a baseline period and then changed to a perfusate of 0, 60, or 143 mmol/l [Na+]. Osmolality of perfusate was adjusted by addition of choline chloride, so that solutions had equal osmolality. Mitochondrial H2O2 responses were measured using MitoPY1 fluorescence. At the end of experiments, all mTALs were treated with 1 mM H2O2 as a positive control, with removal from analysis of mTALs that did not respond.
Whole cell H2O2 production in response to changes in luminal flow and NaCl.
To compare global H2O2 levels vs. mitochondrial H2O2 levels, we utilized the recently developed cytosolic-localized fluorescent H2O2 probe, PF6-AM (12). To determine intracellular H2O2 levels, mTALs were incubated with 5 μM PF6-AM (12) in HBSS-H for 20 min at room temperature. After loading, mTALs were washed twice to remove excess dye and microperfused with HBSS-H at low flow rate of 5 nl/min during a baseline period, then the flow rate was increased to 20 nl/min, and responses were determined over the next 30 min. During the experiments, mTALs were treated with rotenone (10 μmol/l), apocynin (1 mmol/l), or vehicle by introducing the solution into the chamber. In time control studies, tubules were perfused at 5 nl/min throughout the study. All mTAL preparations were treated with 1 mM H2O2 at the end of the study as a positive control to ensure responsive mTAL preparations.
Statistical Analysis
Data are expressed as means ± SE. The data were analyzed statistically by two-way repeated-measures ANOVA, and multiple comparisons were performed by using Tukey's test. Statistical analyses were performed using the SigmaPlot 11 (Systat Software, San Jose, CA). Differences were considered significant at a P < 0.05.
RESULTS
Increased Luminal Flow to mTAL Increases Mitochondrial H2O2 in mTAL
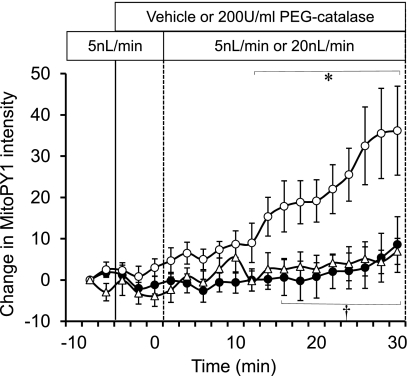
MitoPY1 was loaded to mTAL to identify the effect of luminal flow on mitochondrial H2O2 production in mTAL (Fig. 2). mTAL, which were microperfused at a low flow rate of 5 nl/min through the entire experiment as a time control, showed no increase of MitoPY1 intensity. In contrast, when the luminal perfusion was increased from 5 to 20 nl/min, MitoPY1 intensity was significantly increased compared with time control. Treatment of the mTAL with the cell-permeable H2O2 scavenger, polyethylene-conjugated catalase (200 U/ml) significantly attenuated the increase of mitochondrial H2O2 in mTAL induced by the higher flow and was not different from the time control. The attenuation of the MitoPY1 signal responses with catalase indicates the specificity of the dye and provides validation that an increase in luminal flow significantly elevates H2O2 concentrations within the mitochondria of the epithelial cells of the mTAL.
Fig. 2.
The effect of perfusate flow rate on mitochondrial H2O2 production was determined in mTAL tissue strips. Perfusate flow rate was maintained at 5 nl/min in a time control group for the entire period (●; n = 6), changed to 20 nl/min after 10 min in another group with vehicle in the bath(○; n = 6), and changed to 20 nl/min with 200 U/ml of polyethylene glycol (PEG)-catalase in the bath (▵; n = 6). Mitochondrial H2O2 is expressed as the mean ± SE of the change in intensity of the fluorescent dye MitoPY1. Significant differences *across time and †compared with vehicle group (P < 0.05).
Increased Concentration of Luminal Na+ Concentration Increases Mitochondrial H2O2 Production in mTAL
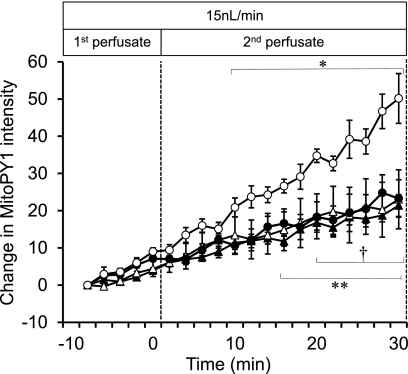
To determine if changes in luminal [Na+] would stimulate mTAL mitochondrial H2O2 production independent of changes in luminal flow, mTAL were microperfused at a moderate but constant flow rate of 15 nl/min. Mitochondrial H2O2 responses were then determined following increases from 0 to 60 mM, or 60 to 143 mM [Na+], and compared with a time control response with perfusate maintained at 60 mM [Na+] (Fig. 3). A small progressive rise of the MitoPY1 signal was observed in both the tubules perfused at 15 ml/min, with [Na+] fixed at either 0 or 60 mM. Increase of the perfusate [Na+] concentration from 0 to 60 mM (0–60) resulted in no significant change in the rate of mitochondrial H2O2 production. However, when [Na+] was raised from 60 to 143 mM, a significant increase in the production of mitochondrial H2O2 was observed, indicating that a threshold of Na+ must be achieved to markedly increase levels of mitochondrial H2O2.
Fig. 3.
The effect of sodium concentration ([Na]) of the perfusate with constant flow on mitochondrial H2O2 production was determined in mTAL tissue strips. [Na] of the perfusate was changed from 0 to 0 mM (▴; n = 5), 0 to 60 mM (▵; n = 5), 60 to 60 mM (●; n = 5), and 60 to 143 mM (○; n = 5). The changes were sequential, with each tubule serving as its own control. The mTAL was first perfused (1st perfusate) with either 0 or 60 mM Na+, and the same tubule was then perfused (2nd perfusate) with a solution that either was the same as the first perfusate (0 or 60 mM Na+) or was switched to 60 or 143 mM Na+. Mitochondrial H2O2 is expressed as the mean ± SE of the change in intensity of the fluorescent dye MitoPY1. *Significant increase in 60 to 143 mM group across time (P < 0.05). **Significant increase (in all 3 groups: 0 to 0, 0 to 60, and 60 to 60 mM) across time (P < 0.05). †All three groups (0 to 0, 0 to 60, and 60 to 60 mM) exhibited significant differences compared with the group perfused with 60 to 143 mM (P < 0.05).
Evidence That Observed Increases of Mitochondria H2O2 Concentrations in Response to Increased mTAL Flow Emanate From the Mitochondria
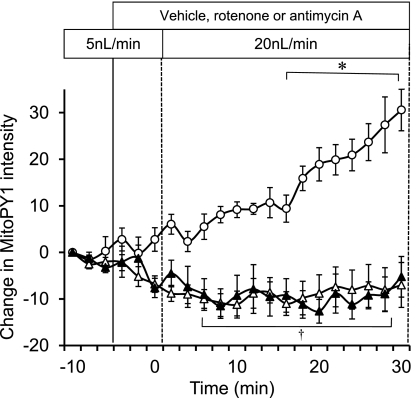
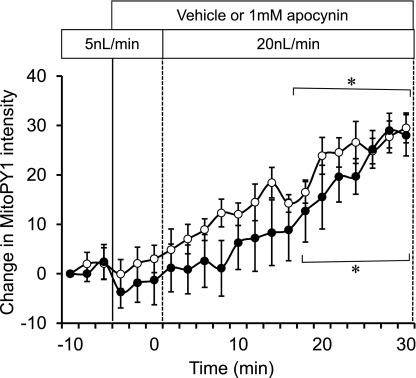
Preincubation of mTAL with either a mitochondrial respiratory chain complex I inhibitor (10 μM rotenone) or a complex III inhibitor (1 μM antimycin A) abolished the increases in mitochondrial H2O2 in response to increased mTAL flow compared with vehicle control (Fig. 4). Inhibition of the membrane NADPH-oxidase activity with apocynin (1 mM) had no significant influence on the observed increases of mitochondria H2O2 in response to increased luminal flow in the mTAL (Fig. 5). These data indicate that all of the observed increases of mitochondrial H2O2 associated with increases of tubular flow emanated from the mitochondria itself and not from NAD(P)H oxidase.
Fig. 4.
The effect of disruption of the mitochondrial respiratory chain on mTAL mitochondrial H2O2 production in response to change in flow rate was determined using 10 μM rotenone (n = 5; ▵) or 1 μM antimycin A (n = 6; ▴). Responses were compared with the vehicle control group (○; n = 6). Mitochondrial H2O2 is expressed as the mean ± SE of the change in intensity of the fluorescent dye MitoPY1. Significant differences *across time and †compared with the vehicle group (P < 0.05).
Fig. 5.
NAD(P)H oxidase was inhibited by 1 mM apocynin (n = 5; ●) to determine the effect on mTAL mitochondrial H2O2 during the change in flow compared with a vehicle control group (n = 5; ○). Mitochondrial H2O2 is expressed as the mean ± SE of the delta change in intensity of the fluorescent dye MitoPY1. *Significant differences across time (P < 0.05).
Importance of Increased Na+ Flux in Signaling Increased Mitochondrial H2O2 Production in Response to Increased mTAL Tubular Flow
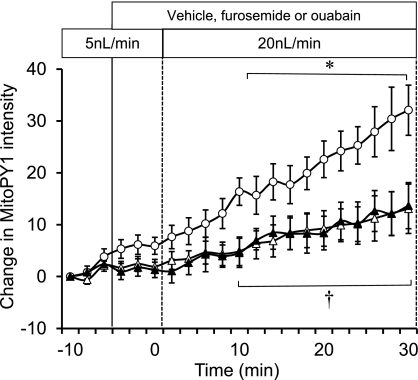
Increased luminal flow results in both an increased delivery of Na+ to the mTAL, while also stretching the lumen diameter and increasing the wall shear stress. To ascertain the contribution of Na+ flux to mitochondrial H2O2 production, the microperfused mTAL were pretreated with the Na+-K+-2Cl− (NKCC2) cotransporter inhibitor furosemide (100 μM) or with the Na+-K+-ATPase inhibitor ouabain (4 mM). Both of these compounds significantly attenuated the mitochondrial H2O2 production stimulated by luminal flow, as shown in Fig. 6, indicating the importance of Na+ transport in this process.
Fig. 6.
The sodium transporter effect on mTAL mitochondrial H2O2 during the change in flow was determined. Furosemide (100 μM) (n = 5; ▵) or 4 mM ouabain (n = 5; ▴) were added to the bath and compared with the vehicle group (n = 6; ○). Mitochondrial H2O2 is expressed as the mean ± SE of the change in intensity of the fluorescent dye MitoPY1. Significant differences *across time and †compared with the vehicle group (P < 0.05).
Changes in Total Intracellular H2O2 Concentrations With Increased mTAL Tubular Perfusion
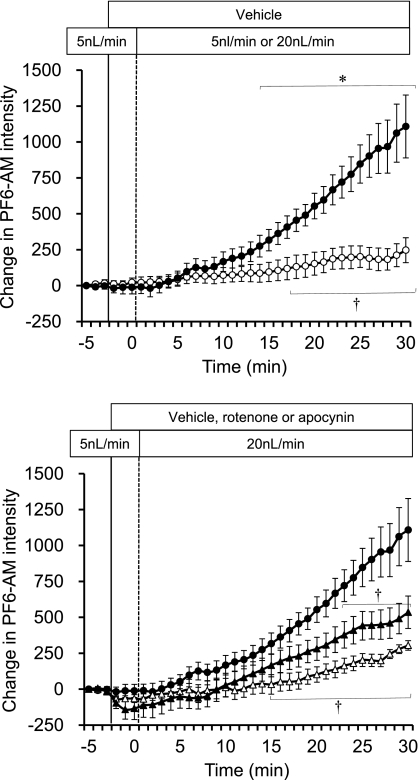
Total intracellular H2O2 responses were determined using the cytosolic-localized H2O2 dye PF6-AM. Figure 7, top, shows that total intracellular H2O2 concentrations were significantly increased when mTAL luminal flow was increased from 5 to 20 nl/min compared with the vehicle control (5 to 5 nl/min). In Fig. 7, bottom, it can be seen that pretreatment of mTAL with the complex I inhibitor rotenone (10 μmol/l) partially eliminated this response (∼65% reduction; P < 0.05), while pretreatment with apocynin (1 mmol/l) completely eliminated the responses to increased tubular flow compared with vehicle. As discussed below, these data indicate an important interaction between activation of mitochondria by Na+ transport and cell membrane NAD(P)H-oxidase H2O2 production.
Fig. 7.
Using the intracellular H2O2 fluorescent dye, PF6-AM, the effect of flow rate was determined. mTALs were perfused with vehicle, 1 mM apocynin, or 10 μM rotenone during the change in flow rate from 5 to 20 nl/min, and the intracellular H2O2 was determined and compared with the control group with flow maintained at 5 nl/min. Intracellular H2O2 is expressed as the mean ± SE of the delta change in intensity of the fluorescent dye PF6-AM. Top: comparison of the vehicle (n = 6; ●) with change in flow to the time control group with flow maintained at 5 nl/min (n = 5; ○). Significant differences *across time and †compared with the vehicle group (P < 0.05). Bottom: rotenone (n = 5; ▴) and apocynin (n = 5; ▵) treatment compared with the vehicle group (●). †Significant differences compared with the vehicle group (P < 0.05).
DISCUSSION
This study provides the first direct evidence that physiological increases of mTAL tubular flow and associated Na+ transport stimulate mitochondrial H2O2 production. The data indicate that this, in turn, may stimulate an increased production of H2O2 from the membrane NADPH-oxidase pathway, thereby enhancing an overall increase of intracellular H2O2 levels. The application of the novel mitochondria-specific H2O2 fluorescent dye (MitoPY1) has enabled, to our knowledge, the first in vitro characterization of mitochondrial oxidative stress using a normal physiological stimulus. The mitochondrial source of the H2O2 production is confirmed by the data presented in Figs. 4 and 5. First, it was seen that the response of the Mito-PY1 dye was not different in isolated mTAL in the absence of or with pretreatment with apocynin (Fig. 5). Second, inhibition of the mitochondrial electron transport chain at complex I with rotenone or complex III with antimycin A both completely abolished any increase of H2O2 (MitoPY1) in response to increased mTAL perfusion (Fig. 4). If H2O2 from extramitochondrial sources contributed to the signal, we would not expect to have observed complete inhibition and even a reduction in the signal, as seen in Fig. 4. Together these data indicate that the observed increases of mitochondrial H2O2 did not emanate from sources outside the mitochondria and were not produced by the cell membrane.
While O2·− produced from a single reduction of molecular oxygen is likely the primary ROS produced in mitochondria, O2·− is quickly converted to H2O2, a membrane-permeable ROS, by MnSOD (40). The present results are consistent with recent observations that ANG II stimulation increases both mitochondrial H2O2 (determined using MitoPY1) and whole cell H2O2 (determined using PF6-AM), a response that was partially inhibited by rotenone (27).
Evidence of Feed-Forward H2O2-H2O2 From Mitochondria to Membrane NADPH Oxidase
NADP(H)-oxidase stimulated O2·− production is greatest in the mTAL and cortical thick ascending limb (26). Increases of mTAL perfusion and/or tubular stretch increase mTAL O2·− generation, and this, in turn, has been found to enhance the rate of Na+ reabsorption (1, 5, 16, 22, 23, 33). The contribution of mitochondria to total ROS production in these events is supported by observations that increases of mitochondrial H2O2 concentrations were required to achieve a robust increase of cellular H2O2 (see Fig. 7, top). Inhibition of mitochondrial H2O2 production by rotenone (Fig. 7, bottom), as visualized by the lack of changes of MitoPY1 fluorescence with increased luminal flow, resulted in a significant reduction (∼65%) of the whole cell H2O2 (PF6-AM fluorescence) response to the change in flow. The whole cell H2O2 response to increased tubular flow and Na+ delivery was completely eliminated by pretreatment of mTAL with apocynin. We propose that the small, but statistically significant, increase of PF6-AM fluorescence in the presence of apocyin serves to feed forward to stimulate membrane NAD(P)H oxidase production of H2O2. It is not possible to quantitatively compare the relative changes in fluorescent responses between the two dyes, since the absolute actual intracellular concentrations can only be estimated and depend on many different physical attributes of the cell and of the dyes. The inhibition of mitochondrial respiration with rotenone would be expected to result in an increase in glycolytic metabolism, which may stimulate membrane NADPH oxidase. However, it is more likely that the mitochondria drives this response via mitochondrial H2O2 production, as supported by results obtained by others in aortic endothelial cells (3, 15, 35). The present study provides evidence that mitochondrial H2O2 is produced and escapes in sufficient amounts to serve as the signaling molecule to enhance NADPH oxidase, either directly or via yet unidentified intermediate signaling pathway(s). Our data are consistent with these earlier observations in which the vascular endothelial cells were stimulated with high, nonphysiological concentrations of ANG-II or H2O2 (2, 15, 35). H2O2 is thought to escape to the cytoplasm, probably through the opening of the prolonged (high-conductance) mitochondrial permeability transition pore (19, 41) and then act via c-Src to stimulate NADPH oxidase (3, 39). Whatever the precise mechanism, the present data provide clear evidence that the increased cellular levels of H2O2 are triggered by changes in mitochondria that are driven by increased Na+ transport in the mTAL.
In contrast, pretreatment of mTAL with apocynin had no effect on mitochondrial H2O2 responses to increased tubular flow (Fig. 5), indicating that H2O2 produced from membrane NADPH oxidase does not feed-forward to stimulate mitochondrial H2O2 production. Evidence of dual feed-forward loops (one from cell membrane ROS production that stimulates mitochondrial ROS, and the other from mitochondrial ROS stimulating the membrane NADPH oxidase) has been obtained using vascular endothelial cells, which were stimulated with high, nonphysiological concentrations of ANG-II or H2O2 (3, 15, 35). These studies found that membrane NADPH-oxidase-derived O2·− increased mitochondrial ROS by opening mito-ATP-sensitive K+ channels, which then further activated the membrane NAD(P)H oxidase, indicating the presence of a vicious cycle (3, 15, 35). This feed-forward vicious cycle has been proposed to contribute to ANG-II-induced vascular dysfunction and hypertension.
Although no evidence for a vicious cycle was found in our mTAL that were stimulated with changes in luminal flow and Na+, it should be recognized that the present studies were designed to expose the mTAL only to changes of luminal flow and Na+ delivery within the physiological range. A vicious cycle would not be expected to be revealed under these circumstances, since this would produce a highly unstable system and disrupt normal homeostatic processes. It is reasonable to expect, however, that, in hypertensive states such as rats chronically treated with ANG-II or in Dahl salt-sensitive rat strains, a vicious cycle may occur as ROS production is driven to very high levels. Protection from damaging effects of mitochondria ROS production was recently demonstrated by the scavenging of mitochondrial O2·− with mito-TEMPO in ANG-II-induced hypertension in mice and in transgenic mice overexpressing mitochondrial SOD2 (13).
Functional Relevance of Relationship of Na+ Transport to mTAL-H2O2 Production
Nearly 20–30% of filtered NaCl in the kidney are reabsorbed in the mTAL, where Na+ is uniquely reabsorbed independent of H2O, enabling the formation of the urinary concentration gradient. About 50% of Na+ mTAL reabsorption is paracellular due to the positive lumen electrical gradient generated by apical K+ conductance, whereas the remaining 50% is transcellular (4). Na+ enters through the apical membranes of the mTAL via NKCC2 cotransporters (∼75%) and apical Na+/H+ exchangers (NHE3) (∼25%). The driving force for Na+ reabsorption by mTAL is provided by basolateral Na+-K+-ATPase, which extrudes Na+ from the cell. The high rates of metabolism required for this transport of Na+ are accommodated by a high density of mitochondria (4, 24).
It is evident from the present study, in which ouabain and furosemide inhibited mitochondrial ROS production, that an increase in mTAL Na+ transport is required to stimulate an increase in mitochondrial H2O2 production. The precise mechanism responsible for signaling the mitochondria is unknown. Interestingly, however, only a change in [Na+] from 60 to 149 mM stimulated mitochondrial H2O2, not a change in [Na+] from 0 to 60 mM, consistent with previous observations in our laboratory in which whole cell changes of O2− were determined (1). These observations are not easily explained based on the reported kinetics of the NKCC2 cotransporter obtained from perfused rat mTAL (17, 20, 36) or based on the kinetics determined for the three major isoforms of the NKCC2 cotransporter identified in the apical membrane of murine mTAL. When expressed in Xenopus oocytes, NKCC isoforms exhibited EC50 values for Na+, ranging from 3 to 21 mM (34). Since these concentrations are well below in vivo tubular mTAL [Na+], ranging from 60 to 160 mM (4), these data indicate that, even under normal conditions of mTAL Na+ delivery, these key transporters of Na+ reabsorption would be operating above saturation levels. Yet it is well recognized that the net transport rate of the mTAL is exceedingly high, ranging from 87 to 870 pmol·mm−1·min−1 (4), and provides the mTAL segment with its remarkable capacity to “buffer” NaCl loads (17). The isolated mTAL ceases to reabsorb NaCl when the perfusate contains only 50 mmol/l Na+ and/or if the flow rate is very low (17).
Studies by Lee and McDonough (25) may explain the wide range of transport rates that appear in the literature and provide direct evidence that an increased luminal flow and Na+ delivery are related to an insertion of Na+ transporters in the mTAL. The net increase of Na+ transport by these mechanisms could explain the effects of high luminal [Na+] and delivery upon ROS production in the present study. That 60 mM Na+ was unable to evoke a response of the mitochondria to produce H2O2 (Fig. 3) suggests that some threshold may be required to initiate these changes in mTAL tubular Na+ transport. One would expect in vivo that a high-salt diet would be likely to promote production of H2O2, since Na+ levels entering the mTAL are greater than isotonic and close to that of the interstitial space (17). The present study shows that increased Na+ transport was the overall signal that stimulated mitochondrial H2O2 production in the mTAL, although exactly what relays this signal to the mitochondria remains unknown. It is interesting that high-salt intake appears to differentially regulate surface NKCC2 expression in mTAL of Dahl S and Dahl R rats via phosphorylation of NKCC2 (18). Dahl S rats increased surface NKCC2 without affecting total NKCC2 expression or phosphorylated-NKCC2, while Dahl R rats showed the opposite response. An increase in NKCC2 activity in salt-sensitive strains could thereby exacerbate the stimulatory effect on mitochondrial H2O2 production.
The functional consequences of increased H2O2 production in the mTAL and elevations of cellular H2O2 are beginning to emerge, but much remains to be resolved. Increased intracellular production of H2O2, as initiated by increased mitochondrial ROS production and amplified by a feed-forward stimulation of membrane NADPH oxidase, could be important not only in cellular energetics and renal injury, but also in the overall regulation of medullary vasa recta blood flow. H2O2 is a more stable and diffusible molecule than O2·− and could more efficiently target the capillaries that surround the mTAL, as our laboratory has demonstrated with nitric oxide (NO) and O2·−, when tissue NO concentrations are very low (8, 30). As detailed in the Introduction, H2O2 has been shown to serve as a vasoconstrictor of the vasa recta capillaries and, when infused into the medullary interstitial space, reduces medullary blood flow and sodium excretion and increases arterial blood pressure (7). In pathological states of salt sensitivity as in the Dahl salt-sensitive rat model, where medullary tissue H2O2 concentrations are elevated and outer medullary tissue injury is prevalent (37), the effects of medullary H2O2 appear to be of great consequence.
Comments Related to the Fluorescent Dyes
As with any study utilizing fluorescent probes to measure local cellular events, it is important to have a high level of confidence of both the localization and specificity of the probes. Since mTAL are highly susceptible to oxidative stress (8) and the production of a variety of ROS, it was critical to utilize an H2O2-selective probe for the present study. It has been challenging to develop organelle-targeted small molecules to detect specific ROS in living cells (11, 42). MitoPY1 represents a new type of fluorophore for imaging mitochondrial ROS, as developed and validated by Dickinson and Chang (10). Designed with a chemo-specific boronate switch (6, 29), the probe is specific for H2O2 over NO, O2·−, and hydroxyl radical (10). MitoPY1 preferentially localizes to the mitochondria (Fig. 1A) due to a triphenylphosphonium targeting group that takes advantage of the pH gradient that is specific to the mitochondria (32). MitoPY1 was initially validated in proof-of-principle experiments involving a neurodegenerative disease model and other pathological stress conditions in model cell lines.
PF6-AM is also a recently designed probe with lipophilic acetoxymethyl esters groups that allow the dye to pass readily through cell membranes into the cytoplasm (12). Once in the cell, esterases de-protect the acetoxymethyl ester groups, resulting in a dianionic form of PF6 that is membrane impermeable and is thereby trapped in the cell, where it can respond to intracellular H2O2 levels. Because it is so well trapped compared with first- and second-generation boronate probes, the PF6 dye has greater sensitivity. In the present study, this enhanced cellular retention (Fig. 1B) not only provided greater sensitivity, but also enabled us to track the changes of cellular H2O2 over prolonged periods of time, which were necessary to characterize the slowly developing H2O2 responses (15–30 min) within the mTAL following the increase of luminal flow. More generally, the application of multiple H2O2-specific fluorescent probes with similar structures but different cellular localizations provides a powerful approach to elucidate the roles of ROS generation with organelle resolution.
Perspectives
The present study demonstrates that increased delivery of Na+ to the mTAL can increase mitochondrial H2O2 and intracellular H2O2 in isolated mTAL epithelial cells and thereby may contribute to salt-induced hypertension and renal injury. Others have shown that mitochondria-targeted antioxidants, such as mito-TEMPO, can have significant beneficial antihypertensive effects. The disappointing effects of vitamin E and other antioxidant dietary supplementation on ROS-driven degenerative processes of chronic diseases, such as aging, diabetes, and hypertension, have been puzzling, and many efforts have been made to explain these failures. One common explanation is that these agents have not been targeted to sites of ROS generation and never reach sufficient concentrations to achieve a therapeutic impact. The present study indicates that the mitochondria of the mTAL may be one such important site for which more effective therapeutic approaches could be targeted.
GRANTS
This work was supported by National Institutes of Health Grants HL-29587 (A. W. Cowley, Jr.) and GM-79465 (C. J. Chang). C. J. Chang is an Investigator with the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.O., R.P.R., B.C.D., and Y.L. performed experiments; Y.O. analyzed data; Y.O. prepared figures; Y.O., P.M.O., T.M., B.C.D., C.J.C., and A.W.C.J. edited and revised manuscript; Y.O., P.M.O., T.M., R.P.R., B.C.D., C.J.C., Y.L., S.I., and A.W.C.J. approved final version of manuscript; P.M.O., T.M., B.C.D., C.J.C., and A.W.C.J. interpreted results of experiments; A.W.C.J. conception and design of research; A.W.C.J. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Glenn Slocum for expert assistance in the Microscopy Core.
REFERENCES
- 1. Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW., Jr Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantification of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 24: 669–681, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC., Jr Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med 41: 810–817, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burg MR, Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol 224: 659–668, 1973 [DOI] [PubMed] [Google Scholar]
- 5. Cabral PD, Hong NK, Garvin JL. Shear stress increases nitric oxide production in thick ascending limbs. Am J Physiol Renal Physiol 299: F1185–F1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang MC, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc 126: 15392–15393, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol Regul Integr Comp Physiol 285: R827–R833, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickhout JG, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Dickinson BC, Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J Am Chem Soc 130: 11561–11562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 7: 504–511, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol 7: 106–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djouadi F, Bastin J, Gilbert A, Rotig A, Rustin P, Merlet-Benichou C. Mitochondrial biogenesis and development of respiratory chain enzymes in kidney cells: role of glucocorticoids. Am J Physiol Cell Physiol 267: C245–C254, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 51: 488–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greger R. Ion transport mechanisms in thick ascending limbs of Henle's loop of mammalian nephron. Physiol Rev 65: 760–797, 1985 [DOI] [PubMed] [Google Scholar]
- 18. Haque MZ, Ares GR, Caceres PS, Ortiz PA. High salt differentially regulates surface NKCC2 expression in thick ascending limbs of Dahl salt-sensitive and salt-resistant rats. Am J Physiol Renal Physiol 300: F1096–F1104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hausenloy D, Wynne A, Buchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 109: 1714–1717, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 246: F745–F756, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Herrera M, Silva GB, Garvin JL. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(α)-dependent NADPH oxidase activation. J Biol Chem 285: 21323–21328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Hong NJ, Silva GB, Garvin JL. PKC-alpha mediates flow-stimulated superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 298: F885–F8991, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kone BC, Madsen KM, Tisher CC. Ultrastructure of the thick ascending limb of Henle in the rat kidney. Am J Anat 171: 217–226, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Lee DH, Rigquier AD, Yang LE, Leong PK, Maunsback AB, McDonough AA. Acute hypertension provokes acute trafficking of distal tubule Na-Cl cotransporter (NCC) to subapical cytoplasmic vesicles. Am J Physiol Renal Physiol 296: F810–F818, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Lu Y, Mori T, Hu C, Ohsaki Y, Dickinson BC, Chang CJ, Cowley AW, Jr, Ito S. Angiotensin II increases mitochondrial reactive oxygen species through mitochondrial respiratory chain in medullary thick ascending limb of the rat kidney (Abstract). Hypertension 56: e121, 2010 [Google Scholar]
- 28. Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension 42: 25–30, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol 3: 263–267, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–93, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Mori T, Cowley AW., Jr Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nystrom T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metab 13: 361–366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Plata C, Meade P, Vazquez N, Hebert SC, Gamba G. Functional properties of the apical Na+-K+-2Cl− cotransporter isoforms. J Biol Chem 277: 11004–11012, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Seshiah RN, Weber DS, Rocic R, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Stoner LC, Trimble ME. Effects of MK-196 and furosemide on rat medullary thick ascending limbs of Henle in vitro. J Pharmacol Exp Ther 221: 715–720, 1982 [PubMed] [Google Scholar]
- 37. Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 21: 489–495, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem 248: 4793–4796, 1973 [PubMed] [Google Scholar]
- 41. Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 10: 1435–1447, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008 [DOI] [PubMed] [Google Scholar]