Abstract
Epithelial Na+ channels (ENaC) can be regulated by both mineralocorticoid and glucocorticoid hormones. In the mammalian kidney, effects of mineralocorticoids have been extensively studied, but those of glucocorticoids are complicated by metabolism of the hormones and cross-occupancy of mineralocorticoid receptors. Here, we report effects of dexamethasone, a synthetic glucocorticoid, on ENaC in the rat kidney. Infusion of dexamethasone (24 μg/day) for 1 wk increased the abundance of αENaC 2.26 ± 0.04-fold. This was not accompanied by an induction of Na+ currents (INa) measured in isolated split-open collecting ducts. In addition, hormone treatment did not increase the abundance of the cleaved forms of either αENaC or γENaC or the expression of βENaC or γENaC protein at the cell surface. The absence of hypokalemia also indicated the lack of ENaC activation in vivo. Dexamethasone increased the abundance of the Na+ transporters Na+/H+ exchanger 3 (NHE3; 1.36 ± 0.07-fold), Na+-K+-2Cl− cotransporter 2 (NKCC2; 1.49 ± 0.07-fold), and Na-Cl cotransporter (NCC; 1.72 ± 0.08-fold). Surface expression of NHE3 and NCC also increased with dexamethasone treatment. To examine whether glucocorticoids could either augment or inhibit the effects of mineralocorticoids, we infused dexamethasone (60 μg/day) together with aldosterone (12 μg/day). Dexamethasone further increased the abundance of αENaC in the presence of aldosterone, suggesting independent effects of the two hormones on this subunit. However, INa was similar in animals treated with dexamethasone+aldosterone and with aldosterone alone. We conclude that dexamethasone can occupy glucocorticoid receptors in cortical collecting duct and induce the synthesis of αENaC. However, this induction is not sufficient to produce an increase in functional Na+ channels in the apical membrane, implying that the abundance of αENaC is not rate limiting for channel formation in the kidney.
Keywords: dexamethasone, aldosterone, cortical collecting duct, NHE3, NKCC2, NCC
although the mineralocorticoid aldosterone is recognized as the major physiological modulator of the epithelial Na+ channel (ENaC), glucocorticoids have also been implicated in the regulation of the channel. Edelman (8) suggested glucocorticoids could affect epithelial cells either through their natural receptors [“glucocorticoid” (“GR”) or “type II” receptors] or through illicit occupancy of mineralocorticoid receptors (MR). Náray-Fejes-Tóth and Fejes-Tóth (28) found that highly selective GR agonists could mimic the effects of aldosterone in stimulating Na+ transport in primary cultures of rabbit cortical collecting duct (CCD) cells. The effects were blocked by specific GR antagonists. Garty et al. (20) also used selective agonists and antagonists to show that Na+ transport by the toad urinary bladder could be stimulated by either mineralocorticoids acting on MR or glucocorticoids acting on GR. A similar conclusion was reached by Gaeggler et al. (19) in studies of a cell line derived from mouse CCD that preserved expression of both MR and GR. These results are consistent with the idea that activated MR or GR can activate at least some of the same genes through common corticosteroid response elements (11).
In the aldosterone-sensitive distal nephron (ASDN) segments of the mammalian kidney, levels of natural glucorticoids (cortisol, corticosterone) are minimized through metabolism of the steroids by the steroid dehydrogenase enzyme 11β-HSD (9, 18). However, high circulating glucocorticoid levels (Cushing's syndrome) or loss of 11β-HSD activity through genetic mutations or inhibition can produce hypertension, presumably through overactivation of ENaC-mediated Na transport (23). It is not clear whether this involves activation of MR, GR, or both.
Dexamethasone is a synthetic steroid with a high affinity for the GR. It is a poor substrate for 11β-HSD (13) and therefore in principle has access to the cells of the ASDN. It can also bind with high affinity to MR (2) and mimics at least one of the effects of aldosterone, namely, the induction of synthesis of mRNA encoding the αENaC subunit (3, 27). Furthermore, dexamethasone treatment clearly increases ENaC-mediated Na transport in the colon (37). We reasoned that if the αENaC subunit were rate limiting for the formation and expression of channels, then dexamethasone could, at least under chronic conditions, increase ENaC activity in the CCD, a target organ for aldosterone.
METHODS
Animals.
All procedures using animals were approved by the Institutional Animal Care and Use Committee of Weill-Cornell Medical College. Sprague-Dawley rats (150–200 g) of either gender (Charles River Laboratories, Kingston, NY) raised free of viral infections were used for all experiments. Animals were implanted with osmotic minipumps for the infusion of aldosterone (12 μg/day) or dexamethasone (24 or 60 μg/day with aldosterone) for 6–8 days.
Electrophysiology.
Measurement of whole-cell currents in principal cells of the cortical collecting duct followed procedures described previously (15, 16). Split-open tubules were superfused with solutions prewarmed to 37°C containing (in mM) 135 Na methanesulfonate, 5 KCl, 2 Ca methanesulfonate, 1 MgCl2, 5 Ba methanesulfonate, 2 glucose, and 10 HEPES adjusted to pH 7.4 with NaOH.
Patch-clamp pipettes were filled with solutions containing (in mM) 7 KCl, 123 aspartic acid, 20 CsOH, 20 TEAOH, 5 EGTA, 10 HEPES, 3 MgATP, and 0.3 NaGDPβS with pH adjusted to 7.4 with KOH. The total concentration of K+ was ∼120 mM. Na+ currents were measured as the difference in current with and without 10−5 M amiloride in the bath.
Pipettes were pulled from hematocrit tubing, coated with Sylgard, and fire polished with a microforge. Pipette resistances ranged from 2 to 5 MΩ. Currents were measured with a List EPC-7 amplifier (Heka Elektronik, Lambrecht, Germany). Voltages were controlled and currents recorded using Pulse software (Heka) and an Instrutech ITC-16 interface (Instrutech, Mineola, NY).
Antibodies.
Polyclonal antibodies against the α-, β-, and γ-subunits of the rat ENaC were described previously (10, 14). Antibodies against NCC were a generous gift of Dr. David Ellison (Oregon Health Sciences University). Antibodies raised against Na+/H+ exchanger 3 (NHE3) and the Na+-K+-2Cl− cotransporter (NKCC2) were purchased from Chemicon International (Millipore, Billerica, MA).
Immunoblotting.
Whole kidneys were excised, decapsulated, and minced with a razor blade. The distal half of the colon was excised, and the mucosal epithelial cells were scraped from the underlying supporting tissue with the edge of a microscope slide. Cells were broken in a tight-fitting Dounce homogenizer. The homogenate was centrifuged at 1,000 rpm for 10 min to sediment intact cells, nuclei, and debris. Protein in the low-speed supernatant was measured (BCA Kit, Pierce Biotechnology, Rockford, IL) and solubilized at 70°C for 10 min in Laemmli sample buffer. Equal amounts of protein (30–40 μg/sample) were resolved on 4–12% bis-Tris gels (Invitrogen, Carlsbad, CA) by SDS-PAGE. For immunoblotting, the proteins were transferred electrophoretically from unstained gels to polyvinylidene difluoride membranes. After blocking with BSA, membranes were incubated overnight at 4°C with primary antibodies at 1:500 or 1:1,000 dilutions for α-, β-, or γENaC subunits, 1:2,000 for NHE3, NKCC2, and 1:13,000 for the Na+-Cl− cotransporter (NCC). Bound antibodies were detected using anti-rabbit IgG conjugated with alkaline phosphatase and detected with a chemiluminescence substrate (Western Breeze, Invitrogen) on autoradiography film (Denville Scientific, Metuchen, NJ). Films were scanned with an Epson V500 scanner using Epson Scan software (Seiko Epson, Nagano, Japan), and band densities were quantitated using Photoshop (Adobe Systems, San Jose, CA).
Biotinylation of renal surface-membrane proteins.
Kidney plasma membranes were biotinylated in situ with a membrane-impermeant biotin-labeling reagent, and biotinylated proteins were isolated as described previously (14, 17). Briefly, the kidneys of anesthetized rats were perfused with ice-cold biotinylation solution {PBS with 4.5 mM KCl, pH 8.0, and 0.5 mg/ml sulfo-N-hydroxysulfosuccinamide-S-S-biotin (NHS-S-S-biotin; Pierce)} followed by a biotin-quenching solution (PBS in which 25 mM Tris·HCl, pH 8.0, replaced 25 mM NaCl).
The kidneys were excised and homogenized. The homogenate was centrifuged for 10 min at 1,000 g, and the supernatant was centrifuged at 18,000 g for 6 h to sediment a total membrane fraction. Aliquots of this fraction containing 3 mg protein were solubilized in 0.5 ml buffer containing 150 mM NaCl, 5 mM EDTA, 50 mM Tris·HCl, pH 7.4, and 3% Triton X-100. This was incubated overnight 4°C with 0.8 ml of a 50% suspension of neutravidin beads (Pierce). The beads were washed, and bound protein was eluted in sample buffer containing 0.2 M DTT for 15 min at 90°C. Samples of eluate were separated by SDS-PAGE, and immunodetection of surface membranes was done by Western blotting as described above.
Analytic procedure.
Na and K concentrations in plasma were measured with a flame photometer (model 943, Instrumentation Laboratories, Lexington, MA).
Statistical analysis.
Statistical significance was evaluated using Student's t-test. A value of P < 0.05 was considered significant.
RESULTS
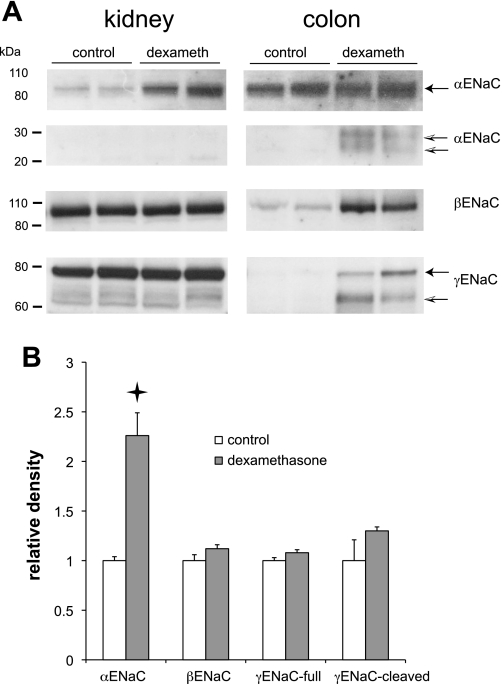
Dexamethasone was previously shown to increase mRNA encoding the αENaC subunit in rat kidney (27), but it is not known whether this resulted in more αENaC protein. We therefore used Western blotting to assess the abundance of ENaC proteins in response to dexamethasone treatment. Infusion of dexamethasone (24 μg/day) for 1 wk reduced body mass by 22% relative to controls, as expected for chronic glucocorticoid treatment. As shown in Fig. 1, the hormone produced a substantial (2.3-fold) increase in the full-length form of αENaC in the kidney but not in the colon, consistent with the increased abundance of mRNA in the former but not the latter organ (3, 27). There were no significant changes in the expression of β- or γENaC protein in the kidney. In particular, no increases in the amount of the 30-kDa cleaved form of αENaC or the 65-kDa cleaved form of γENaC were detected. The abundance of these forms correlates well with the presence of active Na channels (10). In contrast, there were large increases in βENaC, full-length as well as cleaved γENaC, and cleaved αENaC in the colons from the same animals. These results are consistent with the induction of amiloride-sensitive Na+ transport in the colon by dexamethasone (37).
Fig. 1.
Regulation of epithelial Na channel (ENaC) subunits by dexamethasone in the kidney and colon. Rats were infused with dexamethasone (24 μg/day) for 7 days. A: gels for Western blots were loaded with homogenates of whole kidneys (40 μg/lane) or distal colon mucosal epithelial cells (30 μg/lane). Different lanes show extracts from different animals. Blots were probed with anti-αENaC, anti-βENaC, or anti-γENaC antiserum. For the anti-αENaC blots, different portions of the same gel were processed differently to visualize the staining of the full-length (90 kDa) and cleaved (20–30 kDa) forms of the subunit. B: quantitation of effects of dexamethasone on expression of renal ENaC protein. Densitometric data were normalized to means of control measurements and represent means ± SE for 4 animals. Star indicates statistically significant difference from controls (P < 0.05).
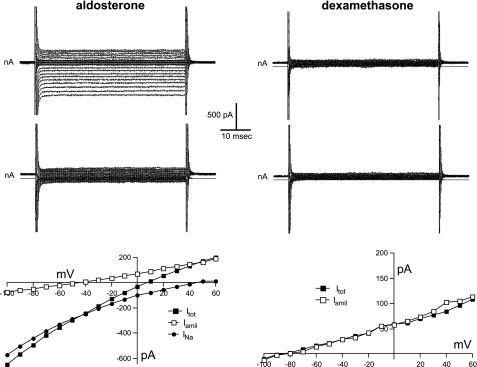
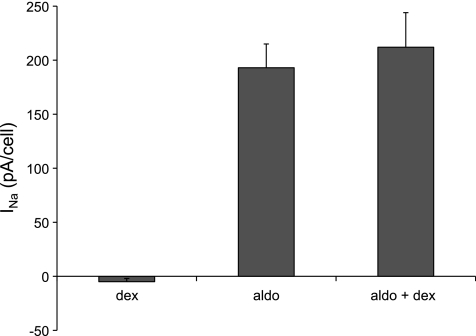
To test directly whether ENaC activity was increased by dexamethasone in the kidney, we isolated and split open CCDs and used whole-cell voltage clamp to assess Na+ currents. As shown in Fig. 2, principal cells of CCDs from rats infused with a low dose of aldosterone (12 μg/day) had typical amiloride-sensitive ENaC currents (INa) with Goldman-type inward rectification and large positive reversal potentials as expected from the Na+ concentration difference between intra- and extracellular solutions. Cells from dexamethasone-treated rats did not exhibit these currents. Altogether, 22 of 22 cells from aldosterone-infused animals had detectable INa that averaged 193 ± 22 pA/cell (Fig. 3), while none of the 16 cells from glucocorticoid-infused animals had a clear response to amiloride. The recordings were similar to those from untreated rats on normal chow (15). This indicates a clear dissociation between the induction of αENaC and the appearance of ENaC activity, which is the main conclusion of this study.
Fig. 2.
Whole-cell currents (INa, Itot, Iamil) in principal cells of cortical collecting ducts (CCDs) from rats treated with aldosterone (12 μg/day) or dexamethasone (24 μg/day). Top and middle: current traces for steps to different voltages from 0 mV in the absence (top) and presence (middle) of 10−5 M amiloride. Bottom: current-voltage relationships. In the case of the aldosterone-treated cell, the amiloride-sensitive current is plotted as the difference in current in the absence and presence of the drug.
Fig. 3.
INa in principal cells of CCDs treated with dexamethasone (24 μg/day), aldosterone (12 μg/day), or both [aldosterone 12 μg/day+dexamethasone (60 μg/day)]. INa was calculated as the difference in current at a cell voltage of −100 mV without and with amiloride, as shown in Fig. 2. Data are plotted as means ± SE for 22 (aldosterone), 16 (dexamethasone), and 26 (aldosterone+dexamethasone) cells.
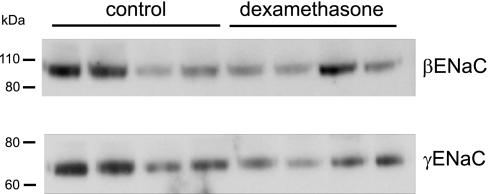
It is possible that increased abundance of αENaC could drive the assembly and/or delivery of channels to the apical membrane, but not increase Na+ currents because the channels remain in an inactive state. To test for this, we assessed the expression of βENaC and γENaC at the cell surface using whole-kidney biotinylation as described previously (14, 17). As shown in Fig. 4, there was no consistent effect of dexamethasone on ENaC surface expression. If anything, the abundance of γENaC was decreased, although the effect was not statistically significant. This implies that αENaC is not the limiting factor for delivery of ENaC to the apical membrane.
Fig. 4.
Effects of dexamethasone on surface expression of ENaC proteins. Kidneys from control and dexamethasone-treated rats were biotinylated, and surface proteins were isolated from 1.25 mg total protein by binding and elution from neutravidin beads. Eluates were analyzed by Western blotting as in Fig. 1. Each lane represents eluate from a different animal. Relative densities of the major bands for dexamethasone-treated/control were 0.74 and 0.56 for βENaC and γENaC, respectively, and were not significantly different from 1.
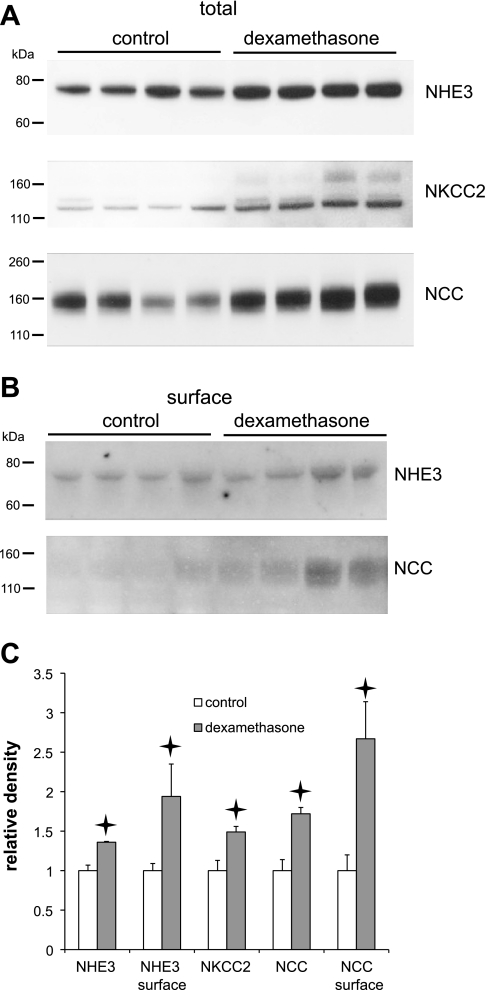
Because dexamethasone was previously reported to induce the synthesis of the Na/H exchanger NHE3 in the proximal tubule (24), we also examined the expression of this and two other luminal-membrane Na+ transporters in the kidney in control and dexamethasone-treated rats. As shown in Fig. 5, the hormone increased the abundance not only of NHE3, but also of the bumetanide-sensitive triple cotransporter NKCC2, and the thiazide-sensitive NaCl cotransporter NCC. The surface expression of NHE3 and NCC were also increased. The signal from NKCC2 in the biotinylated fraction was too weak to quantify. This suggests that glucocorticoids may increase the Na+-reabsorptive capacity of the proximal and early distal nephron segments, although it remains to be seen whether transporter activity is correlated with surface expression under these conditions.
Fig. 5.
Effects of dexamethasone on expression of Na+ transporters in the kidney. A: overall expression. Western blots were loaded with 35 μg protein/lane from kidney homogenates from control and dexamethasone-treated rats. B: surface expression. Western blots were loaded with eluants from 1.25 mg total protein by binding and elution from neutravidin beads. Each lane represents a different animal. Blots were probed with antibodies against Na+/H+ exchanger 3 (NHE3), Na+-K+-2Cl− cotransporter (NKCC2), or Na+-Cl− cotransporter (NCC). C: quantitation of effects of dexamethasone on expression of Na+-transporter protein. Densitometric data were normalized to means of control measurements and represent means ± SE for 4 animals. Stars indicate statistically significant difference from controls (P < 0.05).
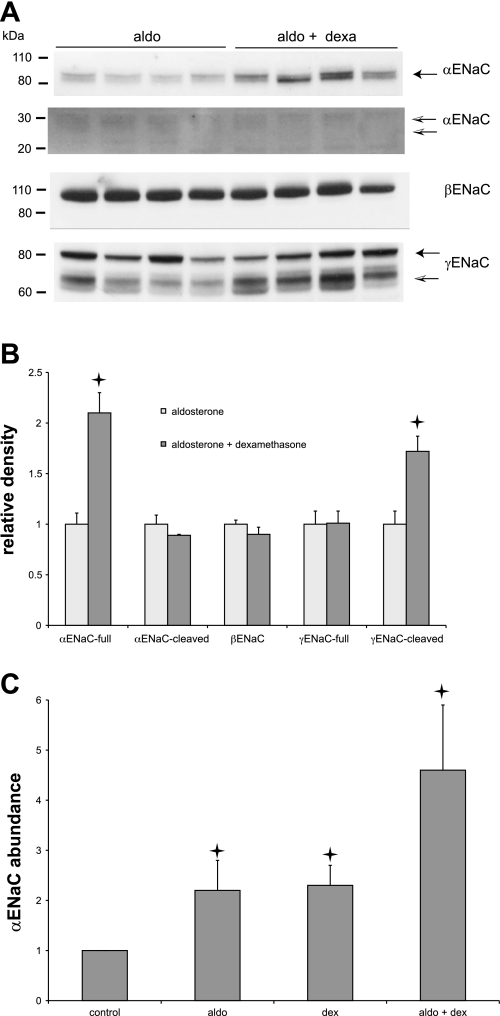
We next examined whether dexamethasone affects aldosterone-induced Na+ channel currents and changes in ENaC protein. Although the steroid appears to have no mineralocorticoid activity by itself, it could in principle either promote or antagonize the activity induced by aldosterone through interactions with MR and/or GR. Figure 6 indicates that the abundance of αENaC is increased about twofold by dexamethasone (60 μg/day) even in aldosterone-treated (12 μg/day) rats. Since the aldosterone itself doubled the abundance of the subunit as previously reported (10), the effects of the two steroids appear to be independent and multiplicative and yield a >4-fold range of protein content (Fig. 6C). There was little or no effect of either steroid on the abundance of βENaC. Aldosterone increases the amount of the cleaved 65-kDa form of γENaC (10, 25). To our surprise, a high dose of dexamethasone further increased the amount of this protein band. However, this did not augment the activity of the channels, as the average INa was not significantly greater in animals treated with aldosterone+dexamethasone compared with aldosterone alone (Fig. 3).
Fig. 6.
Regulation of ENaC subunits by dexamethasone in the presence of aldosterone. Rats were infused with aldosterone (12 μg/day) with or without dexamethasone (60 μg/day) for 7 days. A: gels for Western blots were loaded with 40 μg protein/lane. Different lanes represent different animals. Blots were probed with anti-αENaC, anti-βENaC, or anti-γENaC antiserum. For the anti-αENaC blots, different portions of the same gel were processed differently to visualize the staining of the full-length (90 kDa) and cleaved (20–30 kDa) forms of the subunit. B: quantitation of effects of dexamethasone on expression of Na+ channel protein in the presence of aldosterone. Densitometric data were normalized to means of measurements with aldosterone alone and represent means ± SE; n = 4 animals for each condition. Stars indicate statistically significant difference from controls (P < 0.05). C: quantification of effects of aldosterone, dexamethasone, and aldosterone+dexamethasone on the expression of αENaC protein. Densitometric data were normalized to means of control measurements and represent means ± SE; n = 4 animals for each condition. Stars indicate statistically significant difference. Values for aldosterone and dexamethasone are significantly different from controls (P < 0.001). Values for aldosterone+dexamethasone are significantly different from aldosterone alone (P = 0.003).
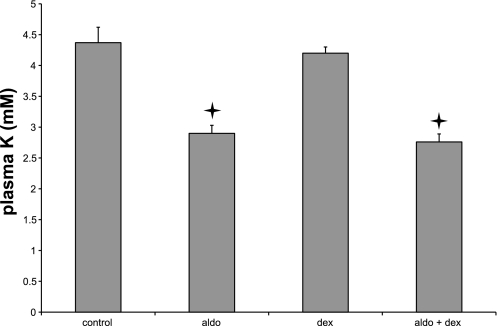
To test for mineralocorticoid activity in vivo, we measured plasma K+ in control rats and in animals treated with aldosterone, dexamethasone, or both (Fig. 7). Treatment of rats with aldosterone produced hypokalemia, presumably as a result of chronic activation of ENaC in the distal nephron and the ensuing hypersecretion of K+. Dexamethasone did not have this effect, and dexamethasone+aldosterone lowered plasma K+ to the same level as did aldosterone alone.
Fig. 7.
Effects of aldosterone and dexamethasone on plasma K. Data represent means ± SE; n = 4 animals for each condition. Stars indicate statistically significant difference from controls (P < 0.05).
DISCUSSION
The main conclusion of this paper is that increases in the expression of αENaC in the ASDN segments of the kidney are not sufficient to increase the formation of active channels at or delivery of channel subunits to the cell surface. In addition, during the response of these cells to aldosterone, which involves increased expression of αENaC, further upregulation of the abundance of this subunit does not enhance the physiological effects of the hormone. Thus αENaC protein levels are not rate limiting for channel formation or transport in the kidney. A similar conclusion was reached by Husted et al. (22), who showed that overexpression of αENaC did not increase Na+ transport in the M1 cell line. We showed earlier that increased αENaC expression is not necessary for increased channel activity, as significant amiloride-sensitive currents were induced by short-term Na deprivation in the absence of increased αENaC levels (15).
Both dexamethasone and aldosterone increase αENaC protein levels. These effects are similar in magnitude, additive, and presumably involve different hormone receptors. Two mechanisms have been proposed to account for induction of αENaC gene expression by adrenocorticosteroids. Thomas and colleagues (26, 31) have identified a glucocorticoid receptor response element in the 5′-flanking portion of the gene. More recently, Zhang et al. (38) proposed an alternative less direct mechanism whereby the aldosterone-induced protein SGK1 relieves transcriptional repression of αENaC expression by the Dot1a-AF9 complex. It is possible that the independent effects of the two steroids are mediated by these two different pathways.
Dexamethasone has effects on the CCD, despite the presence of the 11β-HSD enzyme that metabolizes natural glucocorticoids to inactive forms (9, 18). This is consistent with the finding that this synthetic steroid is a relatively poor substrate for the enzyme (13). Dexamethasone binds to MR in vitro with a dissociation constant in the nanomolar range (2), although the affinity is lower than that for aldosterone and much larger concentrations of the glucocorticoid were required to activate the receptors as judged by promoter assays (1). We presume that since the response of the CCD does not include an increase in ENaC activity, it is mediated by GR rather than MR. Thus in vivo the two receptors control different, albeit potentially overlapping, sets of genes in the ASDN. The absence of increased Na+ transport with chronic dexamethasone treatment is consistent with a previous study of isolated, perfused CCDs from steroid-treated rabbits (33). The finding is also in accord with a lack of upregulation of Na-K-ATPase by dexamethasone (30). In that report, the authors argued for an indirect stimulation of the pump protein by aldosterone, secondary to an increase in the rate of Na+ entry into the cell across the apical membrane. Subsequent studies in rat CCD supported this conclusion (29). Dexamethasone did not elicit this response, presumably because it did not activate channel-mediated Na+ entry. On the other hand, both aldosterone and dexamethasone increased the basolateral surface area of the rabbit CCD (35), implying that this effect may be at least in part independent of increased transport.
In this respect, the kidney differs somewhat from the colon, the toad urinary bladder, and even renal cells in culture. Chronic (1 wk) dexamethasone treatment increases amiloride-sensitive Na+ transport in the distal colon, although the doses required are higher than those of aldosterone (37). In the toad urinary bladder, ∼30% of the response to a high dose of aldosterone in vitro was attributable to occupancy of GR (20). The A6 cell line from the Xenopus laevis kidney responds to steroids mainly through GR (32, 36). However, transfection of the cells with MR can rescue the effects of low doses of aldosterone (7). In primary cultures of rabbit CCD, the “pure” glucocorticoid RU28362 increased transepithelial potential differences to the same extent as did aldosterone (28). In a cell line from mouse CCD that faithfully retains many properties of the native nephron segment, dexamethasone increased ENaC-mediated Na+ transport (19). In both these cases, the responses to synthetic glucocorticoids appeared to be mediated by the GR, as it was blocked by glucocorticoid but not mineralocorticoid antagonists. Primary cultures from rat IMCD also increased Na+ transport in response to dexamethasone (21). In this case, the effect was only partially blocked by glucocorticoid antagonists, indicating that under some conditions this steroid can also act through the MR. The basis for the differences between the in vivo kidney and the cells in culture is unclear.
Our data reinforce the idea that adrenal steroids act on the kidney and colon in very different ways, despite leading to increased ENaC activity in both organs. Previous studies showed that aldosterone increases mRNA levels of αENaC, but not βENaC or γENaC in the kidney, while in the colon both aldosterone and glucocorticoids induce βENaC or γENaC but not αENaC (3).We show here that these same responses are translated to the protein level. The factors involved in determining the very different patterns of constitutive and inducible gene expression in these two organs are unknown.
In addition to increasing αENaC protein levels, a high dose of dexamethasone administered with aldosterone also increased the abundance of the cleaved form of γENaC. This effect did not lead to a detectable augmentation of channel activity. This was surprising in that the presence of the cleaved γ-subunit generally correlates well with measurements of ENaC function (10). This suggests that in addition to proteolytic cleavage, additional steps are required for the activation and/or trafficking of the channels to the apical membrane. A similar dissociation of biochemical and physiological events was reported in SGK1-null mice (12).
In the kidney, dexamethasone upregulates the apical Na transporters NHE3, NKCC2, and NCC. Increased levels of NHE3 mRNA (6) and protein (24) were reported previously. The effects on NKCC2 were more surprising, since increasing glucocorticoid levels through ACTH treatment decreased renal levels of mRNA for this transporter (4). The increases in expression of NCC were the largest of the three (1.7-fold). This is consistent with a previous report that dexamethasone administration increased Na-Cl cotransport activity in rat DCT microperfused in vivo (34). We cannot say whether all of these changes in protein abundance reflect direct interactions of glucocorticoids with cells in the different nephron segments, but this is likely to be the case at least in the proximal tubule, since increased expression of NHE3 was observed in cultured renal cells (5).
In summary, we found that while both aldosterone, presumably acting through MR, and dexamethasone, presumably acting through GR, increase the abundance of the α-subunit of the ENaC, only aldosterone enhances channel activity. Other mineralocorticoid-specific events are required to produce functional channels in the apical membrane.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK59659.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.F. and L.G.P. provided the conception and design of research; G.F. and L.G.P. performed experiments; G.F. and L.G.P. analyzed data; G.F. and L.G.P. interpreted results of experiments; G.F. and L.G.P. edited and revised manuscript; G.F. and L.G.P. approved final version of manuscript; L.G.P. prepared figures; L.G.P. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. David Ellison (Oregon Health Science University) for providing the anti-NCC antibody.
REFERENCES
- 1. Arriza JL, Simerly RB, Swanson LW, Evans RM. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron 1: 887–900, 1988 [DOI] [PubMed] [Google Scholar]
- 2. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237: 268–275, 1987 [DOI] [PubMed] [Google Scholar]
- 3. Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol Cell Physiol 271: C605–C611, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bailey MA, Mullins JJ, Kenyon CJ. Mineralocorticoid and glucocorticoid receptors stimulate epithelial sodium channel activity in a mouse model of Cushing syndrome. Hypertension 54: 890–896, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Baum M, Amemiya M, Dwarakanath V, Alpern RJ, Moe OW. Glucocorticoids regulate NHE-3 transcription in OKP cells. Am J Physiol Renal Fluid Electrolyte Physiol 270: F164–F169, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Baum M, Moe OW, Gentry DL, Alpern RJ. Effect of glucocorticoids on renal cortical NHE-3 and NHE-1 mRNA. Am J Physiol Renal Fluid Electrolyte Physiol 267: F437–F442, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Chen SY, Wang J, Liu W, Pearce D. Aldosterone responsiveness of A6 cells is restored by cloned rat mineralocorticoid receptor. Am J Physiol Cell Physiol 274: C39–C46, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Edelman IS. Receptors and effectors in hormone action on the kidney. Am J Physiol Renal Fluid Electrolyte Physiol 241: F333–F339, 1981 [DOI] [PubMed] [Google Scholar]
- 9. Edwards CRW, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto SW, DeKloet ER, Monder C. Localization of 11β-hydroxysteroid dehydrogenase— tissue specific protector of the mineralocorticoid receptor. Lancet 2: 986–989, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Evans RM, Arriza JL. A molecular framework for the actions of glucocorticoid hormones in the nervous system. Neuron 2: 1105–1112, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Fejes-Tóth G, Frindt G, Náray-Fejes-Tóth A, Palmer LG. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol 294: F1298–F1305, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Ferrari P, Smith RE, Funder JW, Krozowski ZS. Substrate and inhibitor specificity of the cloned human 11β-hydroxysteroid dehydrogenase type 2 isoform. Am J Physiol Endocrinol Metab 270: E900–E904, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol 280: F112–F118, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Frindt G, Palmer LG. Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol 297: F1249–F1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242: 583–585, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Garty H, Peterson-Yantorno K, Asher C, Civan MM. Effects of corticoid agonists and antagonists on the apical Na+ permeability of toad urinary bladder. Am J Physiol Renal Fluid Electrolyte Physiol 266: F108–F116, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Husted RF, Laplace JR, Stokes JB. Enhancement of electrogenic Na+ transport across rat inner medullary collecting duct by glucocorticoid and by mineralocorticoid hormones. J Clin Invest 86: 498–506, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Husted RF, Volk KA, Sigmund RD, Stokes JB. Discordant effects of corticosteroids and expression of subunits on ENaC activity. Am J Physiol Renal Physiol 293: F813–F820, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Loffing J, Lotscher M, Kaissling B, Biber J, Murer H, Seikaly M, Alpern RJ, Levi M, Baum M, Moe OW. Renal Na/H exchanger NHE-3 and Na-PO4 cotransporter NaPi-2 protein expression in glucocorticoid excess and deficient states. J Am Soc Nephrol 9: 1560–1567, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mick VE, Itani OA, Loftus RW, Husted RF, Schmidt TJ, Thomas CP. The alpha-subunit of the epithelial sodium channel is an aldosterone-induced transcript in mammalian collecting ducts, and this transcriptional response is mediated via distinct cis-elements in the 5′-flanking region of the gene. Mol Endocrinol 15: 575–588, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Muller OG, Parnova RG, Centeno G, Rossier BC, Firsov D, Horisberger JD. Mineralocorticoid effects in the kidney: correlation between alphaENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+. J Am Soc Nephrol 14: 1107–1115, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Naray-Fejes-Toth A, Fejes-Toth G. Glucocorticoid receptors mediate mineralocorticoid-like effects in cultured collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 259: F672–F678, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Palmer LG, Antonian L, Frindt G. Regulation of the Na-K pump of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 43–57, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petty KJ, Kokko JP, Marver D. Secondary effect of aldosterone on Na-K ATPase activity in the rabbit cortical collecting tubule. J Clin Invest 68: 1514–1521, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sayegh R, Auerbach SD, Li X, Loftus RW, Husted RF, Stokes JB, Thomas CP. Glucocorticoid induction of epithelial sodium channel expression in lung and renal epithelia occurs via trans-activation of a hormone response element in the 5′-flanking region of the human epithelial sodium channel alpha subunit gene. J Biol Chem 274: 12431–12437, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Schmidt TJ, Husted RF, Stokes JB. Steroid hormone stimulation of Na+ transport in A6 cells is mediated via glucocorticoid receptors. Am J Physiol Cell Physiol 264: C875–C884, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Schwartz GJ, Burg MB. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 235: F576–F585, 1978 [DOI] [PubMed] [Google Scholar]
- 34. Velázquez H, Bartiss A, Bernstein P, Ellison DH. Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat distal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 270: F211–F219, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Wade JB, O'Neil RG, Pryor JL, Boulpaep EL. Modulation of cell membrane area in renal collecting tubules by corticosteroid hormones. J Cell Biol 81: 439–445, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watlington CO, Perkins FM, Munson PJ, Handler JS. Aldosterone and corticosterone binding and effects on Na+ transport in cultured kidney cells. Am J Physiol Renal Fluid Electrolyte Physiol 242: F610–F619, 1982 [DOI] [PubMed] [Google Scholar]
- 37. Will PC, Cortright RN, DeLisle RC, Douglas JG, Hopfer U. Regulation of amiloride-sensitive electrogenic sodium transport in the rat colon by steroid hormones. Am J Physiol Gastrointest Liver Physiol 248: G124–G132, 1985 [DOI] [PubMed] [Google Scholar]
- 38. Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Aldosterone-induced Sgk1 relieves Dot1a-AF9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 117: 773–783, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]