Abstract
The Nω-nitro-l-arginine methyl ester (l-NAME) model is widely employed to investigate the role of nitric oxide (NO) in renal injury. The present studies show that Sprague-Dawley rats from Harlan (H) and Charles River (CR) exhibit strikingly large differences in susceptibility to l-NAME nephropathy. After 4 wk of l-NAME (∼50 mg·kg−1·day−1 in drinking water), H rats (n = 13) exhibited the expected hypertension [average radiotelemetric systolic blood pressure (BP), 180 ± 3 mmHg], proteinuria (136 ± 17 mg/24 h), and glomerular injury (GI) (12 ± 2%). By contrast, CR rats developed less hypertension (142 ± 4), but surprisingly no proteinuria or GI, indicating a lack of glomerular hypertension. Additional studies showed that conscious H, but not CR, rats exhibit dose-dependent renal vasoconstriction after l-NAME. To further investigate these susceptibility differences, l-NAME was given 2 wk after 3/4 normotensive nephrectomy (NX) and comparably impaired renal autoregulation in CR-NX and H-NX rats. CR-NX rats, nevertheless, still failed to develop proteinuria and GI despite moderate hypertension (144 ± 2 mmHg, n = 29). By contrast, despite an 80–90% l-NAME dose reduction and lesser BP increases (169 ± 4 mmHg), H-NX rats (n = 20) developed greater GI (26 ± 3%) compared with intact H rats. Linear regression analysis showed significant (P < 0.01) differences in the slope of the relationship between BP and GI between H-NX (slope 0.56 ± 0.14; r = 0.69; P < 0.008) and CR-NX (slope 0.09 ± 0.06; r = 0.29; P = 0.12) rats. These data indicate that blunted BP responses to l-NAME in the CR rats are associated with BP-independent resistance to nephropathy, possibly mediated by a resistance to the renal (efferent arteriolar) vasoconstrictive effects of NO inhibition.
Keywords: radiotelemetry, proteinuria, hypertension, renal mass reduction, remnant kidney
there is accumulating evidence that, in addition to playing an essential role in blood pressure (BP) homeostasis and the regulation of cardiovascular and renal function (24), nitric oxide (NO) plays a major role in the pathophysiology and progression of chronic kidney disease (CKD) (3, 32). It is postulated that reduced renal NO generation and/or bioavailability contributes to the hypertension and progressive renal damage in clinical and experimental CKD. Consistent with such concepts, NO synthase (NOS) inhibition results in dose-dependent hypertension and renal damage [glomerulosclerosis (GS)] (3, 4, 33, 38). However, certain inbred rat strains such as the Wistar-Furth (WF) and Brown-Norway have been found to be resistant to NOS inhibition nephropathy (11, 26). Although underlying genetic differences in NO production capacity and/or reserve have been postulated to be responsible, attempts to correlate the severity of nephropathy with such indexes have yielded inconsistent results (3, 11, 26, 35). Similarly, while differences in the BP response to NOS inhibition have also been noted, the contribution of BP-independent and BP-dependent mechanisms to such differences in nephropathy remain poorly defined (3, 11, 26, 35). In this context, rather surprisingly, significant differences in the BP responses to the l-arginine antagonist Nω-nitro-l-arginine methyl ester (l-NAME), have also been reported between outbred male and pregnant female Sprague-Dawley (SD) rats supplied by Charles River (CR) and Harlan (H) that are not readily explained by differences in diet or breeding protocols (9, 31). The present studies were performed to examine if such differences in the BP response to l-NAME, even in these outbred SD rats from CR and H, would be associated with differences in nephropathy susceptibility and the potential hemodynamic mechanisms that may mediate such differences.
METHODS
Male SD rats (body wt 225–275 g), obtained at 8–10 wk of age from both suppliers (H and CR) and fed a standard 1% NaCl Purina chow with free access to drinking water, were allowed to acclimatize for ∼1 wk before initiation of studies. The animals were cared for in accordance with the National Institutes of Health Guide For The Care And Use Of Laboratory Animals, and the studies were approved by the Institutional Animal Care and Use Committee of Loyola University and Hines Veterans Administration Hospital. In preliminary studies, we confirmed the differences in urinary nitrite/nitrate excretion between CR and H rats. For these studies, 9- to 10-wk-old male SD rats from CR (n = 11) and H (n = 13) were administered a low nitrate/nitrite diet (AIN 76C semipurified diet; MP Biomedicals, Solon, OH) and distilled drinking water for 2 days to minimize dietary contributions to urinary nitrate/nitrite excretion. For 24-h urine collection, rats were placed in nalgene metabolic cages, and collected urine was stored at −20°C until determination of urinary nitrate/nitrite concentrations. Urinary nitrate/nitrite was measured using a kit from Cayman Chemical (Ann Arbor, MI).
To investigate the susceptibility to renal damage, separate studies were then performed in additional CR and H rats with intact renal mass and with 3/4 nephrectomy (NX). Studies in rats with intact renal mass were initiated when the rats were ∼2 wk older than in the NX rats, since the latter underwent NX and ∼2 wk of recovery before l-NAME.
Protocol A: Studies in Rats with Intact Renal Mass
After baseline measurements of serum creatinine (SCr) and 24-h proteinuria, CR (n = 8) and H (n = 13) rats were prepared for continuous BP radiotelemetry as previously described (15, 18, 19). l-NAME, (500 mg/l of drinking water) was initiated 7–14 days later. Water intake in both H and CR rats averages ∼100 ml·kg−1·day−1 so that the average l-NAME intake approximates 50 mg·kg−1·day−1. After 4 wk of follow-up, 24-h proteinuria was measured again, and perfused-fixed kidneys were harvested (15, 18, 19). To examine for baseline differences in renal pathology, perfusion-fixed kidneys were harvested from additional CR and H rats (n = 6 each) who underwent baseline studies but did not receive l-NAME.
Assessment of pressure-flow relationships and dynamic renal autoregulation.
Additional studies were performed to investigate potential differences in renal hemodynamics between CR and H rats. Rats were instrumented for chronic measurements of BP (radiotelemetry) and renal blood flow (RBF) (Transonic flow probes) as previously described (1, 7, 17). One week later, 2- to 4-h simultaneous recordings of BP and RBF were obtained at a sampling rate of 200 Hz in conscious rats. After one to three such baseline recordings at 24-h intervals, BP and RBF responses to escalating doses of l-NAME (12.5, 25, and 50 mg/kg) were additionally assessed. Recordings were initiated after ∼24 h of each dose of l-NAME, which was maintained for 3–4 days. Transfer function analysis of the dynamic relationship between BP (input) and RBF (output) was performed at baseline and at each dose of l-NAME using previously published methods (1, 7, 17). Subsegments of 30 min duration from each 4-h recording that were free of noise or other artifacts were selected for analysis. The 30-min records were resampled at 20 Hz using a low-pass antialiasing filter to remove variations in the signals of >10 Hz. Each time sequence of 36,000 data points was then subjected to linear trend removal. The BP and RBF power spectra were determined using Welch's averaged periodogram method (50% overlap of 7 segments of 8,192 samples, detrended, and a Hanning window applied). Input and output autopower spectra and cross-power spectra were calculated for each segment and then averaged. The admittance function was calculated as the ratio of cross-spectrum to BP power spectrum. Coherence was calculated from the cross- and autopower spectra. Fractional gain in admittance (FGA) was obtained by normalizing admittance gain by the conductance computed over the entire 30-min record. The natural frequencies of the myogenic and the tubuloglomerular feedback mechanisms were determined from their characteristic signature resonance peaks in FGA between 0.1 and 0.3 Hz and between 0.025 and 0.05 Hz, respectively, by inspection of individual records, and averaged across each record. The slope of gain reduction for the myogenic mechanism was estimated similarly from the linear range between 0 db and the signature resonance peak of the myogenic mechanism in each record (least-squares method) (34, 36).
Protocol B: Studies in Rats with NX
After baseline measurements, CR and H rats underwent 3/4 normotensive NX by surgical excision and were prepared for BP radiotelemetry (18). SCr was measured again 3 days later to control for the degree of NX (18). After allowing ∼2 wk for compensatory adaptations, proteinuria was remeasured and l-NAME initiated. Because of the markedly greater BP sensitivity to l-NAME in H compared with CR rats noted during protocol A studies, and an expected greater sensitivity to l-NAME in NX rats, the concentration of l-NAME provided in drinking water was 400–500 mg/l for CR-NX rats but only 50–100 mg/l in H-NX rats. The water intake in both CR and H rats with NX was noted to be 50% greater than in rats with intact kidneys and averaged ∼150 ml·kg−1·day−1 instead of ∼100 ml·kg−1·day−1. Accordingly, the l-NAME intake was estimated to be ∼60–75 mg·kg−1·day−1 in CR and ∼7.5–15 mg·kg−1·day−1 in H rats with NX. After 4 wk, the remnant kidneys were harvested after perfusion fixation (15, 18, 19). Renal autoregulation was assessed under anesthesia in additional CR and H rats ∼2 wk after NX as previously described (7, 17–19). To examine for baseline differences in renal pathology before l-NAME, perfusion-fixed remnant kidneys were harvested at ∼2 wk after NX from additional CR and H rats (n = 6 each).
BP data acquisition and analysis.
BP was sampled for 5 s at 10-min intervals for the entire duration of the study (7, 15, 17–19). The average systolic BP during the 72 h immediately preceding the initiation of l-NAME was taken as the pre-l-NAME BP. BP was averaged both weekly and over the entire 4-wk course (15, 18, 19).
Histology.
Glomerular injury was quantitated in a blinded fashion as the total percentage of glomeruli exhibiting lesions of GS, necrosis/thrombosis, and/or ischemia as previously described (15, 18, 19). The severity of tubulointerstitial (TI) injury and fibrosis was scored semiquantitatively on a scale of zero to four. The vascular injury score (VIS) was calculated as the number of vascular profiles exhibiting acute disruptive injury (lesions of necrosis, thrombosis, aneurysmal dilatation, and/or onion skinning)/100 glomeruli in the section (15).
Statistical Analysis
Results are means ± SE. Paired or unpaired t-tests were used when the data were normally distributed. The nonparametric Mann-Whitney test was used when the data were not normally distributed and to compare all ordinal data (GS, TI, and VIS) between the groups. Linear regression analysis was used to calculate the slope of the relationship between BP and glomerular injury in each group (increases in glomerular injury/mmHg increases in systolic BP). Analysis of covariance was used to compare the slopes between the groups. A two-way repeated-measures ANOVA was used to compare BP responses over time as well as the effects of escalating doses of l-NAME on BP, RBF, and renal vascular resistance (RVR) between groups. A Student-Newman-Kuels post hoc test was used for multiple comparisons when appropriate. A P < 0.05 was considered statistically significant.
RESULTS
Protocol A: Studies in Intact CR and H Rats
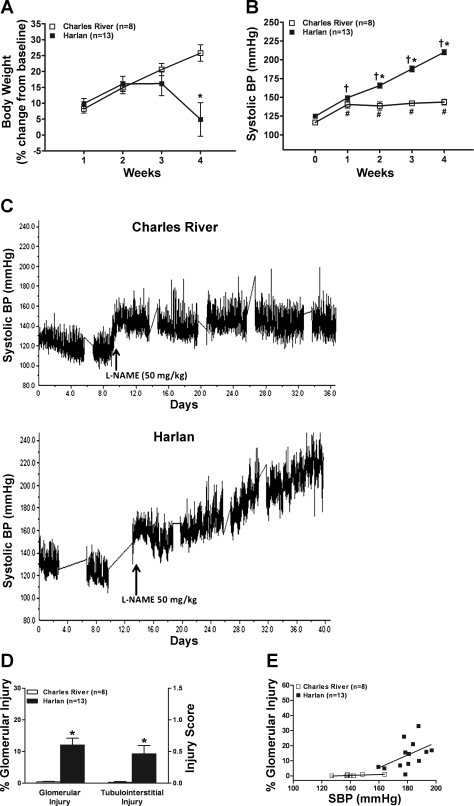
Urinary nitrate/nitrite excretion was significantly higher in CR vs. H rats (961 ± 78 vs. 670 ± 83 nmol/day; P < 0.025), which is similar to a previous report (31) and suggests differences in the endogenous levels of NO. No significant renal pathology or differences were seen at baseline in CR and H rats (n = 6 each) who did not receive l-NAME (results not shown). In the rats subjected to chronic l-NAME administration, SCr and 24-h proteinuria values were not different at baseline between CR and H rats, but the H rats tended to weigh less despite being approximately age matched (Table 1). The course of change in body weight is shown in Fig. 1A. H rats started to lose weight over the last ∼7–10 days with the development of more severe hypertension and renal damage compared with CR rats after l-NAME (vide infra). The course of systolic BP after l-NAME is shown in Fig. 1, B and C. Systolic BP increases after l-NAME in CR rats tended to plateau and be stable, whereas the systolic BP in H rats increased progressively over time. Parallel changes were seen in proteinuria and SCr (Table 1). Thus, 4 wk of l-NAME resulted in reduced weight gain, severe proteinuria, and increased SCr in H rats but had little effect on these parameters in CR rats. Glomerular and TI injury was also essentially confined to H rats (Fig. 1D). The glomerular lesions were predominantly ischemic although fibrinoid necrosis and/or sclerosis were also observed in individual rats. Additionally, acute disruptive vascular injury, although quite focal, was seen in 9 of the 13 H rats who were more severely hypertensive (VIS 2.7 ± 0.6). Figure 1E shows the distribution of the relationship between average systolic BP during l-NAME administration and percent glomerular injury in individual CR and H rats with intact renal mass. Because of the small numbers and the narrow BP range within each group, the correlations within each group separately were not statistically significant. However, nevertheless, the general BP dependence of glomerular injury after l-NAME can be discerned.
Table 1.
Baseline and final data for protocol A studies
| Initial (pre-l-NAME) |
Final (post-l-NAME) |
|||||
|---|---|---|---|---|---|---|
| Body Wt, g | SCr, mg/dl | Proteinuria, mg/24 h | Body Wt, g | SCr, mg/dl | Proteinuria, mg/24 h | |
| Charles River (n = 8) | 320 ± 14 | 0.28 ± 0.02 | 6.3 ± 0.8 | 459 ± 10 | 0.32 ± 0.03 | 8.5 ± 1.0 |
| Harlan (n = 13) | 279 ± 11* | 0.32 ± 0.02 | 6.8 ± 1.7 | 308 ± 13* | 0.7 ± 0.07* | 136.2 ± 16.8* |
Values are means ± SE; n, no. of rats. l-NAME, Nω-nitro-l-arginine methyl ester; SCr, serum creatinine.
P < 0.05, maximum compared with Charles River (CR) rats.
Fig. 1.
A: weekly changes in body weight (%change from baseline) during 4 wk of Nω-nitro-l-arginine methyl ester (l-NAME, 50 mg·kg−1·day−1) in Sprague-Dawley rats from Charles River (CR) and Harlan (H). *P < 0.01 vs. CR. B: systolic blood pressure (BP) course (weekly averages) before (0 wk) and during the 4 wk of l-NAME. *P < 0.01 vs. CR. #P < 0.0001 vs. pre-l-NAME BP. †P < 0.05, maximum vs. all other time points in H rats. C: illustration of the course of systolic BP in representative CR and H rats. D: glomerular injury (%) and semiquantitated Tubulointerstitial Injury Score after 4 wk of l-NAME at the termination of the studies. *P < 0.0001 vs. CR. E: the relationship between the average systolic BP during 4 wk of l-NAME administration and the percent of glomeruli that exhibited injury lesions at the termination of the studies in individual CR and H rats. Linear regression analysis in CR rats: slope 0.03 ± 0.02; r = 0.64, P = 0.09; H rats: slope 0.42 ± 0.23, r = 0.48, P = 0.09.
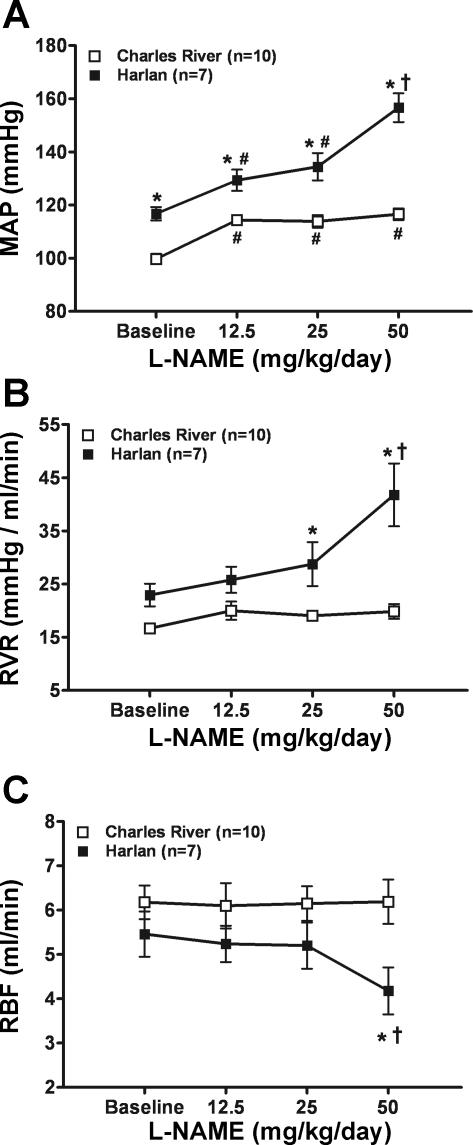
To gain additional insights into the difference in nephropathy susceptibility, the dose-response relationships of BP, RBF, and RVR to l-NAME and dynamic autoregulation (transfer functions) were examined in conscious chronically instrumented CR and H rats. Other than a modest BP increase after l-NAME, but with a flat dose-response relationship, the CR rats failed to show any changes in RVR and RBF even at the maximal l-NAME dose used (Fig. 2, A–C). By contrast, the H rats tended to exhibit progressively greater increases in BP and RVR with an escalation in the l-NAME dose, although RBF decreased significantly only at the 50 mg·kg−1·day−1 dose. When these hemodynamic responses are calculated as delta changes, a statistically significant difference is still seen with the 50 mg·kg−1·day−1 dose.
Fig. 2.
Pressure-flow relationships before and after escalating doses of l-NAME in drinking water in conscious chronically instrumented CR and H rats with intact kidneys. A: mean arterial pressure (MAP). B: renal vascular resistance (RVR). C: renal blood flow (RBF). *P < 0.05, maximum vs. CR. #P < 0.05, maximum vs. pre-l-NAME. †P < 0.05, maximum vs. all other doses of l-NAME within the same strain (CR or H).
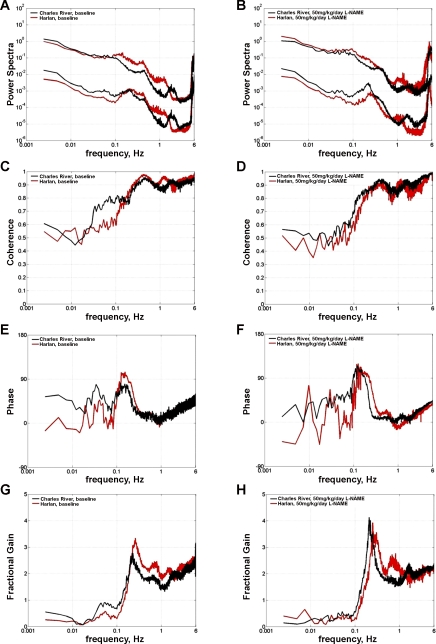
Transfer function analysis (BP input, RBF output) did not show significant differences between CR and H rats with respect to the degree of “dynamic” renal autoregulatory compensation (gain at frequencies <0.01) before or after l-NAME (Fig. 3, G and H). However, some differences in other transfer functions related to the myogenic mechanism of RBF autoregulation were observed between H and CR rats at baseline (Table 2). For instance, the operating frequency of the myogenic mechanism was significantly faster in H vs. CR rats (Fig. 3, E–H); however, it was not altered by l-NAME in either H or CR rats. Of interest, differences at baseline were also observed between H and CR rats for other transfer functions, which previous studies have shown to be sensitive to NOS inhibition (34, 36). Accordingly, the coherence at frequencies between 0.05 and 0.1 Hz at baseline was significantly lower in H rats (Fig. 3, C and D). Reductions were seen after l-NAME in both but were significant only in CR, and the values after l-NAME were not significantly different between H and CR rats (Table 2). Similarly, the increases in the peak phase (Fig. 3, E and F) and the magnitude of the myogenic resonance peak (Fig. 3, G and H) after l-NAME were significant in CR but failed to achieve statistical significance in H rats, which started with higher values at baseline (Table 2). However, no differences between CR and H rats were seen in the slope of gain reduction of the myogenic mechanism at baseline or after l-NAME. Overall, the pattern of differences in transfer functions at baseline between CR and H rats and their response to l-NAME are suggestive of a relatively low NO state and a potentially “stronger” myogenic mechanism at baseline in H compared with CR rats (22, 34, 36).
Fig. 3.
Transfer function analysis of the relationship between BP (input) and RBF (output) computed over 30 min during dynamic autoregulation studies in conscious CR (n = 10) and H (n = 7) rats. BP and RBF power spectra at baseline (A) and after l-NAME (50 mg·kg−1·day−1, B) in units of (mmHg)2/Hz for BP and (ml/min)2/Hz for RBF. No significant differences in dynamic autoregulatory compensation (gain at frequencies <0.01 Hz) were observed at baseline or during l-NAME between CR and H rats, indicating similar dynamic autoregulation of RBF in these rats. Differences were observed between H and CR rats with respect to other transfer functions. Coherence at 0.05–0.1 Hz at baseline (C) was lower (P < 0.05) in H vs. CR rats and was reduced by l-NAME (D) in both groups. Similarly, both peak phase (E) and the signature resonance peak of the myogenic mechanism (G) tended to be higher in H vs. CR rats at baseline and increased (significant only in CR rats, P < 0.05) in both groups in response to l-NAME (F and H). See text and Table 2 for details and statistical analysis.
Table 2.
Transfer functions before and after l-NAME (50 mg · kg−1 · day−1) in CR and H rats
| Myogenic Frequency, Hz | Peak Phase, degrees | FG Myo | Coherence (0.05–0.1 Hz) | Slope of Gain Reduction, db/decade | |
|---|---|---|---|---|---|
| CR | 0.22 ± 0.009 | 101.7 ± 7.4 | 2.73 ± 0.18 | 0.60 ± 0.05 | 43.89 ± 4.6 |
| H | 0.25 ± 0.007* | 119.8 ± 4.4 | 3.27 ± 0.39 | 0.37 ± 0.04* | 44.74 ± 4.6 |
| CR + l-NAME | 0.22 ± 0.003 | 127.7 ± 6.5# | 4.20 ± 0.47# | 0.38 ± 0.04# | 47.10 ± 3.9 |
| H + l-NAME | 0.27 ± 0.007* | 138.4 ± 11.3 | 3.70 ± 0.44 | 0.28 ± 0.03 | 46.13 ± 4.6 |
Values are means ± SE; n, no. of rats. FG Myo, fractional gain at myogenic resonance peak; H, Harlan.
P < 0.05, maximum vs. CR at similar time points.
P < 0.05, maximum vs. baseline.
Protocol B: Studies in CR and H Rats with NX
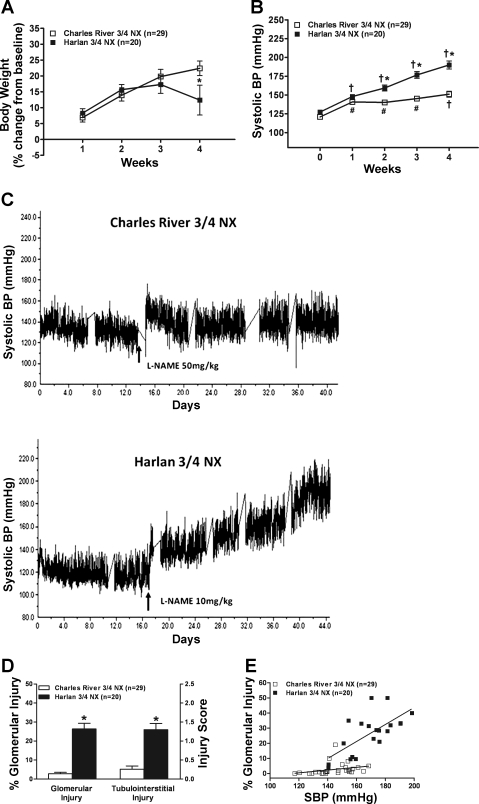
Table 3 shows that, as in CR and H rats with intact kidneys, the body weight at baseline was lower in H rats. However, proteinuria was significantly higher in H rats primarily because of much lower values in the younger CR rats, but SCr was not significantly different. The SCr at 3 days after NX was also not significantly different, indicating comparable NX (18). Although neither CR nor H rats developed overt hypertension after 3/4 NX by surgical excision as previously reported (18), H rats had modestly higher systolic BP (127 ± 2 mmHg) compared with CR rats (121 ± 2 mmHg), likely accounting for the modestly but significantly higher proteinuria in H rats ∼2 wk after NX (before the initiation of l-NAME; Table 3). Nevertheless, significant renal pathology (GS and TI fibrosis) or differences were not observed in the additional CR and H rats (n = 6 each) at ∼2 wk after NX who did not receive l-NAME (other data similar to that in Table 2 and not shown). However, the course was strikingly different between CR and H rats with NX who received the 4 wk of l-NAME (Fig. 4, A–C). Similar to intact H rats, H-NX rats lost weight over the last 7–10 days of the course (Fig. 4A) with the development of more severe hypertension and renal damage (vide infra). Likewise, CR-NX rats only developed moderate and stable BP increases while the H-NX rats developed progressively more severe hypertension with time (Fig. 4, B and C). Surprisingly, the magnitude of the BP increase was not significantly different in CR rats with or without NX (144 ± 2 vs. 142 ± 4 mmHg, P > 0.05) even though the dose of l-NAME was greater in NX rats because of their greater water intake. By contrast, in H rats with NX, even though the l-NAME dose was reduced by 80–90%, the BP increase was almost as large as in intact H rats (169 ± 4 vs. 180 ± 3, P < 0.05).
Table 3.
Baseline and final data for protocol B studies
| Baseline |
Approximately 2 wk (pre-l-NAME) |
Final (post-l-NAME) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Body Wt, g | SCr, mg/dl | Proteinuria, mg/24 h | 3 Day SCr, mg/dl | Body Wt, g | Proteinuria, mg/24 h | Body Wt, g | SCr, mg/dl | Proteinuria, mg/24 h | |
| Charles River (n = 29) | 255 ± 5 | 0.30 ± 0.01 | 2.6 ± 0.2 | 0.81 ± 0.05 | 322 ± 5 | 7.7 ± 1.3 | 393 ± 8 | 0.6 ± 0.04† | 12.4 ± 1.7 |
| Harlan (n = 20) | 234 ± 3* | 0.31 ± 0.02 | 4.3 ± 0.6* | 0.85 ± 0.05 | 296 ± 7* | 17.6 ± 1.6* | 334 ± 6* | 1.0 ± 0.05*† | 85.7 ± 10.5* |
Values are means ± SE; n, no. of rats.
P < 0.05, maximum compared with CR rats.
P < 0.02, maximum compared with 3-day SCr values.
Fig. 4.
A: weekly changes in body weight (%change from baseline) during 4 wk of l-NAME in CR-nephrectomy (NX) (400–500 mg/l of drinking water) and H-NX rats (50–100 mg/l of drinking water) that had undergone normotensive (3/4) NX by surgical excision 2 wk earlier. *P < 0.05 vs. CR. B: systolic BP course (weekly averages) before (0 wk) and during the 4 wk of l-NAME. *P < 0.02, maximum vs. CR. #P < 0.01, maximum vs. pre-l-NAME BP. †P < 0.05, maximum vs. all other time points in the same strain (CR or H). C: illustration of the course of systolic BP in representative CR-NX and H-NX rats. D: glomerular injury (%) and semiquantitative Tubulointerstitial Injury Score after 4 wk of l-NAME at the termination of the studies in CR and H rats who had undergone NX 2 wk earlier. *P < 0.0001 vs. CR. E: linear regression analysis of the slope of the relationship between the average systolic BP during the 4 wk of l-NAME administration and the percent of glomeruli that exhibited injury lesions at the termination of the studies in CR and H rats. CR rats: slope 0.09 ± 0.06, r = 0.29; P = 0.12; H rats: slope 0.56 ± 0.14, r = 0.69, P < 0.008. The slopes were significantly different between CR and H rats, P < 0.01.
As in CR and H rats with intact renal mass, the adverse effects of l-NAME on SCr and proteinuria were essentially confined to H-NX rats (Table 3). This is illustrated by the fact that, in CR-NX rats, the final SCr significantly decreased from that at 3 days, reflecting the compensatory hypertrophy adaptations in the interim. By contrast, the final SCr in H-NX rats increased significantly, reflecting the superimposed adverse effects of l-NAME. Figure 4D similarly shows that glomerular and TI injury was confined to H-NX rats. Of note, H-NX rats developed significantly greater glomerular injury than H rats without NX (26.4 ± 3.0 vs. 12.0 ± 2.2%, P < 0.01) despite a significantly lower average systolic BP (169 ± 4 vs. 180 ± 3, P < 0.05). As in rats with intact kidneys, a mixture of glomerular lesions was observed in individual rats, but GS was seen more frequently. Similarly, focal vascular injury was only seen in 11 of the 20 H-NX rats that had developed more severe hypertension (VIS 3.4 ± 0.9).
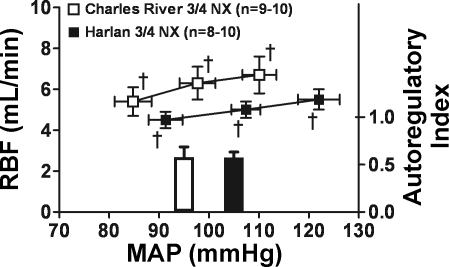
Figure 4E presents the results of correlation analysis of the slope of the relationship between the average 4-wk systolic BP and the percent glomeruli exhibiting injury in CR-NX and H-NX rats (%increase in glomerular injury/mmHg increase in systolic BP). The CR-NX rats exhibited an essentially flat relationship with little increase in glomerular injury with increasing BP. By contrast, H-NX rats not only exhibited greater glomerular injury than CR-NX rats at a given BP, but, unlike CR-NX rats, also exhibited a linear increase in glomerular injury with increasing BP with a slope of 0.56 ± 0.14 (r = 0.69; P < 0.008). This BP-independent difference in nephropathy susceptibility between CR and H rats with NX is also shown by a comparison of glomerular injury in only those 16 CR-NX and 9 H-NX rats whose systolic BP values were within a range of 140–168 mmHg (defined by the lowest value in H-NX rats and the highest value in CR-NX rats). Although the mean systolic BP values of these rats were essentially identical (CR-NX 153 ± 2 mmHg vs. H-NX rats 154 ± 3 mmHg), markedly less glomerular injury was seen in CR-NX rats (4.5 ± 1.2%) compared with H-NX rats (17.8 ± 4.1%; P < 0.002). Results of the steady-state “step” autoregulatory studies performed in additional CR and H rats 2 wk after NX show that renal autoregulatory capacity was similarly impaired (Fig. 5) and not likely to be contributing to the differences in nephropathy susceptibility between CR-NX and H-NX rats.
Fig. 5.
RBF autoregulation in CR-NX and H-NX rats performed under anesthesia at ∼2 wk after 3/4 NX. Renal autoregulatory responses were impaired similarly as shown by the calculated autoregulatory indexes. †P < 0.05, maximum for a significant change in RBF after each change in perfusion pressure.
DISCUSSION
The nephropathy that develops after chronic NOS inhibition (l-NAME) has been widely employed to define the potential pathophysiological importance of NO in renal disease progression and to elucidate pathways of renal injury and repair in hypertensive states (3, 8, 32, 35). Moreover, there is evidence that differences in the susceptibility to l-NAME nephropathy between inbred genetic strains may be associated with directionally similar differences in susceptibility to other nephropathies ( ablation, and DOCA/salt) (3, 12). The underlying reasons as to why these two outbred colonies of SD rats, which originally came from a single colony, exhibit such stark differences in their BP responses to l-NAME (9, 31) and to the development of nephropathy remain elusive. Nevertheless, the very existence of these large differences has important implications for the interpretations and comparison of results from different laboratories in renal injury models using these different colonies. The results of the present hemodynamic studies in CR and H rats with their contrasting susceptibility to l-NAME nephropathy have additionally provided new and important insights into several unresolved aspects of the pathogenesis of l-NAME nephropathy. For instance, the well-recognized limitations of the tail-cuff BP measurement methodology used (25) have made it difficult to separate the relative contributions of BP-dependent and BP-independent pathways as well as the role of other downstream mediators such as ANG II and endothelin, etc. (3, 4, 8, 11, 12, 26, 32, 33, 35, 38). Interpretations in such studies have also been complicated by the differences in l-NAME dosage, salt intake, and/or follow-up duration and the range of described renal pathology in these models (4, 11, 26, 33, 35, 38). Nevertheless, two broad pathological patterns of l-NAME nephropathy can be delineated that are relevant to the consideration of the differences between CR and H rats.
With low-dose l-NAME (5 mg·kg−1·day−1) and moderate hypertension, as initially described by Baylis et al. (4) in Munich-Wistar rats, modest proteinuria and segmental GS were observed after 8 wk. Such GS is generally not observed in states of moderate hypertension, since normal preglomerular autoregulatory vasoconstrictive responses prevent glomerular hypertension (6, 7, 15, 18, 19, 21, 29). However, micropuncture studies showed that, despite the expected increases in afferent resistance, glomerular hypertension still developed in these rats because of concomitant efferent arteriolar constriction (4). Acute vascular injury was, however, not seen, consistent with the moderate severity of hypertension. When higher doses of l-NAME are used, with or without increased salt intake, more severe hypertension develops, and a more severe nephropathy with a different pathological pattern results, which is more typical of models of malignant nephrosclerosis (6, 15, 29, 33, 38). We have suggested that such injury develops when the severity of hypertension exceeds the vascular threshold for disruptive injury and the renal autoregulatory capacity to protect glomerular capillaries is breached (6, 15). Severe vascular and glomerular injury with fibrinoid necrosis and/or thrombosis is seen along with ischemic glomeruli and TI damage, likely reflecting proximal vascular injury and compromised downstream perfusion. Segmental GS is seen infrequently, since it likely represents a consequence of overperfusion with more moderate and chronic glomerular hypertension (6, 21, 29).
The morphologic pattern of injury observed in susceptible H rats with intact kidneys and in high-dose l-NAME models in general is consistent with such interpretations. However, the relationship between average systolic BP and renal injury in these rats (Fig. 1E) indicates that such injury develops at a lower systolic BP threshold (∼160 mmHg) than previously observed in the stroke-prone spontaneously hypertensive rat (SHRsp) model of malignant nephrosclerosis (>180 mmHg) (15), but with a flatter slope (0.29 ± 0.1 vs. 1.2 ± 0.2). The significant vasoconstriction and reduced RBF that was observed in conscious H rats at the 50 mg·kg−1·day−1 dose of l-NAME before the development of renal damage provides a likely explanation. Such renal (preglomerular) vasoconstriction is not observed in the SHRsp model (1) and is expected to reduce distal BP transmission and barotrauma while at the same time enhancing BP-independent ischemic glomerular and TI injury. This pattern of lesser barotrauma but greater ischemia-mediated injury may be a general feature of hypertension models with significant associated renal vasoconstriction (30).
The more surprising and provocative data are the complete resistance of the CR rats to renal injury after l-NAME. Although the lack of severe hypertension in CR rats explains their failure to develop the disruptive injury pattern seen in H rats, it does not explain the complete lack of proteinuria and GS that are observed with even moderate hypertension in other susceptible strains (4, 11, 26, 35, 38). Given that pathological elevations in glomerular capillary pressure are almost invariably associated with proteinuria (37), it is reasonable to infer that the lack of proteinuria in CR rats indicates a lack of glomerular hypertension. Therefore, it is of relevance that micropuncture studies have indeed shown that, unlike the GS-susceptible H and Munich-Wistar rats (4, 33, 38), the GS-resistant WF strain fails to develop glomerular hypertension after acute NOS inhibition (11). This difference was ascribed by the investigators to a lack of efferent constriction in WF rats. Thus the increased efferent resistance and filtration fraction seen after nonselective NOS inhibition and in the l-NAME nephropathy models in susceptible strains indicates that efferent constriction after NOS inhibition may be necessary for the development of proteinuria and GS (4, 5, 11, 33, 36, 38). Thus the resistance to renal (efferent) vasoconstriction even after high-dose l-NAME in conscious CR rats may underlie their resistance to l-NAME nephropathy.
The results obtained with l-NAME after NX further reinforce these interpretations. Such 3/4 NX results in preglomerular vasodilation, autoregulatory impairment, and enhanced glomerular BP transmission as noted in the previous and present studies (7, 18, 19). Accordingly, NX is expected to enhance the susceptibility to glomerular injury after l-NAME. The data in H rats showing a steeper slope of the relationship between BP and glomerular injury in NX compared with those with intact kidneys (Figs. 4E vs. 1E), and greater glomerular injury despite a lower BP, is in accord with such predictions. However, despite preglomerular vasodilation and autoregulatory impairment comparable to that in H-NX rats, the CR-NX rats still failed to develop more than minimal proteinuria and GS after l-NAME hypertension. Given that NOS inhibition does not improve the impaired autoregulation in NX models (16), the superimposition of l-NAME hypertension after NX is expected to result in significant glomerular hypertension even in CR rats. Nevertheless, in contrast to the H-NX rats, a fairly flat relationship between BP and glomerular injury was seen in CR-NX rats (Fig. 4E). A failure of NOS inhibition to cause efferent constriction and glomerular hypertension in CR-NX rats provides the most plausible explanation for these findings. Such data suggest that, in addition to its anti-inflammatory and cellular protective effects (3, 24, 32), NO-mediated efferent vasodilation may be particularly important in protecting against glomerular hypertension (11).
These interpretations are also consistent with the topographic studies of the distribution of NOS isoforms that have shown significant expression of both NOS 1 and NOS 3 in efferent arteriolar endothelial cells (2). Elegant anatomical studies have also shown that these endothelial cells bulge into the lumen of the efferent arteriole at the initial intraglomerular segment and have been suggested to serve as shear stress sensors (2, 10). While the activating mechanisms for NOS 1 at this site have not been characterized, NO release from these sites is ideally situated to mediate a protective decompression during glomerular hypertension states. Given that NO is a vasodilator of the afferent arteriole, and also a potential inhibitor of autoregulatory responses as indicated by previous and present dynamic autoregulatory studies (22, 34, 36), NO inhibition per se would be expected to reduce BP transmission and glomerular injury. Therefore, efferent arteriolar effects may also help resolve the apparent contradiction that NO inhibition nevertheless results in enhanced glomerular injury in the hypertensive ablation model (3, 13, 23, 38). Moreover, CKD per se may further reduce NO availability, resulting in a vicious cycle (3). A dramatic illustration of the potential importance of NO in the pathogenesis and progression of CKD models is provided by the fact that the genetic deletion of endothelial NOS converts the very nephropathy-resistant C57BL/6 mouse to one that is quite susceptible to diabetic and renal ablation nephropathies (3, 27, 28, 39). In this context, it needs to be acknowledged that, while these data strongly support the renoprotective importance of NO, differences in urine nitrite/nitrate excretion to support differences in NO production have usually been modest and insufficient to explain the often large differences in the BP response and/or nephropathy in rodent strains/models (3, 4, 11, 12, 31). To illustrate, doses of l-NAME that reduce nitrite/nitrate excretion in the WF strain to the level of H rats still fail to elicit significant nephropathy (11). This may reflect the limitations of the present methods to assess NO generation at relevant sites/compartments; the uncertain correlation between NOS isoform expression, NO generation, bioavailability, and downstream signaling; and the emerging evidence of a biologically important but difficult to quantify nitrite storage pool (3, 4, 11, 14, 24). Alternatively, it is possible that redundant homeostatic mechanisms may be present in the strains relatively resistant to NOS inhibition, such as WF or CR, that allow them to better compensate for NO loss. In any event, the present results indicate that the phenotype of a blunted BP response to l-NAME, although less emphasized, has the potential to be a useful surrogate of the underlying susceptibility to nephropathy.
Collectively, the present and previous observations indicate that substantial differences exist in the response to NO synthesis inhibition between and within rodent strains. Such data suggest considerable genetic heterogeneity in the determinants of such response. However, regardless of the underlying genetic basis, rodent strains that share the phenotype of a blunted BP response to NOS inhibition also appear to exhibit a relative resistance to renal damage and its progression, likely mediated through BP-independent mechanisms. Several lines of evidence are consistent with the concept that the vasodilatory effects of NO on the efferent arteriole may be particularly important in protecting against glomerular hypertension and its downstream consequences. Consistent with such concepts, the endothelial vasodilatory function of the surgically removed kidney during ablation in individual rats was found to predict the severity of GS in the remnant kidney of the same rats (20). With additional validation, the acute BP response to l-NAME may provide a useful surrogate phenotype of the relative susceptibility to nephropathy, particularly in models in which glomerular hypertension plays a significant pathogenic role. Additionally, the present data have important implications for the interpretations and comparisons of the results from different laboratories in renal injury models using SD and other rodent strains.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-40426 and DK-61653, by a Merit Review Award, and by a Career Development Award 1IK2BX001285 (A. Polichnowski) from the Office of Research and Development of the Department of Veterans Affairs.
DISCLOSURES
None.
ACKNOWLEDGMENTS
We acknowledge Theresa Herbst for technical support and Martha Prado for secretarial support.
REFERENCES
- 1. Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol 289: F305–F313, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F885–F898, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 294: F1–F9, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 90: 278–281, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baylis C, Qiu C. Importance of nitric oxide in the control of renal hemodynamics. Kidney Int 49: 1727–1731, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. “Step” vs. “dynamic” autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol 285: F113–F120, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol 14: 1132–1144, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Buhimschi IA, Shi SQ, Saade GR, Garfield RE. Marked variation in responses to long-term nitric oxide inhibition during pregnancy in outbred rats from two different colonies. Am J Obstet Gynecol 184: 686–693, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Elger M, Sakai T, Kriz W. The vascular pole of the renal glomerulus of rat. Adv Anat Embryol Cell Biol 139: 1–98, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Erdely A, Freshour G, Baylis C. Resistance to renal damage by chronic nitric oxide synthase inhibition in the Wistar-Furth rat. Am J Physiol Regul Integr Comp Physiol 290: R66–R72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erdely A, Wagner L, Muller V, Szabo A, Baylis C. Protection of wistar furth rats from chronic renal disease is associated with maintained renal nitric oxide synthase. J Am Soc Nephrol 14: 2526–2533, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujihara CK, De Nucci G, Zatz R. Chronic nitric oxide synthase inhibition aggravates glomerular injury in rats with subtotal nephrectomy. J Am Soc Nephrol 5: 1498–1507, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol 291: H2026–H2035, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure-dependent. Hypertension 41: 201–206, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Griffin KA, Bidani AK, Ouyang J, Ellis V, Churchill M, Churchill PC. Role of endothelium-derived nitric oxide in hemodynamic adaptations after graded renal mass reduction. Am J Physiol Regul Integr Comp Physiol 264: R1254–R1259, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol Renal Physiol 286: F1136–F1143, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol 4: 2023–2031, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest 96: 793–800, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gschwend S, Buikema H, Navis G, Henning RH, de Zeeuw D, van Dokkum RP. Endothelial dilatory function predicts individual susceptibility to renal damage in the 5/6 nephrectomized rat. J Am Soc Nephrol 13: 2909–2915, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens 17: 266–270, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Just A, Arendshorst WJ. Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol 569: 959–974, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang DH, Nakagawa T, Feng L, Johnson RJ. Nitric oxide modulates vascular disease in the remnant kidney model. Am J Pathol 161: 239–248, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol Renal Physiol 272: F561–F578, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in animals: summary of an AHA scientific statement from the Council on High Blood Pressure Research, Professional and Public Education Subcommittee. Arterioscler Thromb Vasc Biol 25: 478–479, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Mattson DL, Kunert MP, Roman RJ, Jacob HJ, Cowley AW., Jr Substitution of chromosome 1 ameliorates l-NAME hypertension and renal disease in the fawn-hooded hypertensive rat. Am J Physiol Renal Physiol 288: F1015–F1022, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol 18: 539–550, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Nakayama T, Sato W, Kosugi T, Zhang L, Campbell-Thompson M, Yoshimura A, Croker BP, Johnson RJ, Nakagawa T. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am J Physiol Renal Physiol 296: F317–F327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olson JL. Renal Disease Caused by Hypertension. Philadelphia, PA: Lippincott-Raven, 2005 [Google Scholar]
- 30. Polichnowski AJ, Cowley AW., Jr Pressure-induced renal injury in angiotensin II versus norepinephrine-induced hypertensive rats. Hypertension 54: 1269–1277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol Regul Integr Comp Physiol 275: R1719–R1723, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Raij L. Nitric oxide, salt sensitivity, and cardiorenal injury in hypertension. Semin Nephrol 19: 296–303, 1999 [PubMed] [Google Scholar]
- 33. Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 20: 298–303, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Shi Y, Wang X, Chon KH, Cupples WA. Tubuloglomerular feedback-dependent modulation of renal myogenic autoregulation by nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R982–R991, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Van Dokkum RP, Jacob HJ, Provoost AP. Genetic differences define severity of renal damage after l-NAME-induced hypertension in rats. J Am Soc Nephrol 9: 363–371, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Cupples WA. Interaction between nitric oxide and renal myogenic autoregulation in normotensive and hypertensive rats. Can J Physiol Pharmacol 79: 238–245, 2001 [PubMed] [Google Scholar]
- 37. Yoshioka T, Rennke HG, Salant DJ, Deen WM, Ichikawa I. Role of abnormally high transmural pressure in the permselectivity defect of glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res 61: 531–538, 1987 [DOI] [PubMed] [Google Scholar]
- 38. Zatz R, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension 32: 958–964, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]