Abstract
Epithelial Na+ channel (ENaC)-mediated Na+ absorption and BK channel-mediated K+ secretion in the cortical collecting duct (CCD) are modulated by flow, the latter requiring an increase in intracellular Ca2+ concentration ([Ca2+]i), microtubule integrity, and exocytic insertion of preformed channels into the apical membrane. As axial flow modulates HCO3− reabsorption in the proximal tubule due to changes in both luminal Na+/H+ exchanger 3 and H+-ATPase activity (Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Am J Physiol Renal Physiol 290: F289–F296, 2006), we sought to test the hypothesis that flow also regulates H+-ATPase activity in the CCD. H+-ATPase activity was assayed in individually identified cells in microperfused CCDs isolated from New Zealand White rabbits, loaded with the pH-sensitive dye BCECF, and then subjected to an acute intracellular acid load (NH4Cl prepulse technique). H+-ATPase activity was defined as the initial rate of bafilomycin-inhibitable cell pH (pHi) recovery in the absence of luminal K+, bilateral Na+, and CO2/HCO3−, from a nadir pH of ∼6.2. We found that 1) an increase in luminal flow rate from ∼1 to 5 nl·min−1·mm−1 stimulated H+-ATPase activity, 2) flow-stimulated H+ pumping was Ca2+ dependent and required microtubule integrity, and 3) basal and flow-stimulated pHi recovery was detected in cells that labeled with the apical principal cell marker rhodamine Dolichos biflorus agglutinin as well as cells that did not. We conclude that luminal flow modulates H+-ATPase activity in the rabbit CCD and that H+-ATPases therein are present in both principal and intercalated cells.
Keywords: mechanoregulation, in vitro microperfusion, intercalated cell, principal cell, immunofluorescence
emerging evidence suggests that axial flow regulates activity of apical renal tubular acid-base transporters (12, 13, 49). In mouse proximal tubules microperfused in vitro, a fivefold increase in the rate of luminal perfusion leads to a doubling of Na+ (Jv; equivalent to volume) and HCO3− reabsorption (JHCO3) (12). This flow-induced response was proposed to be due to direct stimulation of apical Na+/H+ exchanger 3 (NHE3) and H+-ATPase as flow-stimulated JV and JHCO3 were diminished in NHE3 knockout mice and inhibited by EIPA and bafilomycin, blockers specific for NHE3 and H+-ATPase, respectively (12). Mathematical models predict that brush border microvilli serve as afferent mechanosensors of axial flow along the proximal tubule, at least for NHE3. Specifically, the drag force on each microvillus produces a bending moment on an actin filament bundle that is transmitted to the underlying actin cytoskeleton (11, 12, 74).
The distal nephron, including the cortical collecting duct (CCD), is a heterogeneous segment, composed of both principal and intercalated cells. Principal cells possess a central apical cilium, now considered to be a mechanosensor critical to the intracellular Ca2+ concentration ([Ca2+]i) response to flow, whereas intercalated cells are decorated with abundant apical microvilli and microplicae (17, 36, 48). Principal cells, the majority cell type, reabsorb Na+ through apical epithelial Na+ channels (ENaC) and secrete K+ via ROMK and BK channels. Intercalated cells are involved in transepithelial acid/base transport via polarized H+-ATPases and Cl−/HCO3− exchangers and may also contribute to K+ reabsorption via apical H+-K+-ATPases, composed of HK-α1 (gastric) and HK-α2 (colonic) isoforms, under certain conditions (10, 38, 54, 60, 66). Intercalated cell subtypes include type A (or α), which mediate H+ secretion and possess apical H+-ATPase and basolateral AE1 (1, 58, 60); type B (or β), which secrete HCO3− via apical pendrin, a Na+-independent Cl−/HCO3− exchanger, and express basolateral H+-ATPase (1, 50, 60, 67); and non-A non-B with both apical H+-ATPase and pendrin (15, 27), cells whose function is as yet unknown. Intercalated cells also possess apical conducting BK channels (31, 45, 55), which have been proposed to function in flow-induced K+ secretion (34) as well as to recycle K+ taken up by the apical H+-K+-ATPase back into the luminal fluid (63). Increases in luminal flow rate in the CCD stimulate ENaC-mediated net Na+ absorption and BK channel-mediated K+ secretion, and lead to a transient increase in [Ca2+]i in both principal and intercalated cells (33, 36, 57, 81).
Based on the studies summarized above, and the observations that 1) increases in [Ca2+]i in acid-secreting α-intercalated cells in turtle bladder lead to exocytosis of H+-ATPase-containing vesicles (8, 71) and 2) chronic administration of diuretics enhances distal acidification by upregulating H+-ATPase activity in intercalated cells in rat kidney (43), we hypothesized that the H+-ATPase present in the mammalian CCD is regulated by variations in luminal flow rate. We tested this in microperfused rabbit CCDs loaded with a pH-sensitive fluorescent dye by measuring the effect of luminal flow rate on Na+-independent bafilomycin-sensitive intracellular pH (pHi) recovery from an intracellular acid load.
METHODS
Animals.
Adult (>6 wk) female New Zealand White rabbits (Covance, Denver, PA) were housed in the animal care facility at the Mount Sinai School of Medicine (Center for Comparative Medicine) or the University of Pittsburgh. All animals were allowed free access to tap water and chow. Animals were euthanized in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by the Institutional Animal Care and Use Committee at the Mount Sinai School of Medicine or University of Pittsburgh School of Medicine, as appropriate.
Solutions and chemicals.
The compositions of the solutions used have been previously described (10) and are given in Table 1. All experiments were performed in the nominal absence of CO2/HCO3− using solutions buffered with HEPES, adjusted to pH 7.4, and 290 ± 2 mosmol/kgH2O using NaOH in Na+-containing solutions or N-methyl-d-glucamine in Na+-free solutions. The intracellular calibration solution was titrated to pH 6.8, 7.3, and 7.8 using HCl or KOH.
Table 1.
Composition of solutions (in mM) used for pHi assays
| Na+-Ringer | NH4Cl | 0Na, 0K | 0Na, 5K | Calibration pH | |
|---|---|---|---|---|---|
| NaCl | 135 | 120 | |||
| NMDG-Cl | 140 | 135 | |||
| K2HPO4 | 2.5 | 2.5 | 2.5 | 2.5 | |
| NH4Cl | 20 | ||||
| MgSO4 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| l-Alanine | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| HEPES | 5.0 | 5.0 | 5.0 | 5.0 | 25 |
| CaCl2 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Glucose | 5.5 | 5.5 | 5.5 | 5.5 | |
| Lactate | 4.0 | 4.0 | 4.0 | 4.0 | |
| KCl | 125 |
pHi, intracellular pH; NMDG, N-methyl-d-glucamine.
The fluorescent probes used included the pH indicator BCECF-AM (Molecular Probes, Eugene, OR), the Ca2+ indicator fura 2 acetoxymethyl ester (fura 2-AM; Calbiochem), and principal cell marker rhodamine Dolichos biflorus agglutinin (DBA; Vector Labs, Burlingame) (16). The 20 mM stock solutions of BCECF-AM and fura 2-AM, prepared in DMSO, were diluted into Na+-Ringer solution to a final concentration of 20 μM. DBA was added directly to the Na+-Ringer solution in a concentration of 10 μg/ml.
Bafilomycin A1 (LC Laboratories, Woburn, MA), a potent and specific inhibitor of vacuolar-type H+-ATPases (4), was prepared as a 1 μM stock solution in DMSO and diluted on the day of experimentation to a final concentration of 10 nM. As indicated, some tubules were pretreated with either the acetoxymethyl ester of BAPTA (20 μM final concentration prepared from 20 mM stock solution in DMSO; Molecular Probes) to chelate [Ca2+]i or colchicine (10 μM final concentration prepared from 2.5 mM stock solution in water; Sigma, St. Louis, MO) to disrupt microtubules, added to the bathing solution (32, 73). Nigericin (Sigma-Aldrich) was prepared as a 2 mM stock solution and diluted to 10 μM in each standard calibration solution. All dilutions of dyes and inhibitors yielded a final concentration of DMSO of ≤0.1%.
Microperfusion of isolated rabbit CCDs.
The kidneys were removed via a midline incision, sliced into 2-mm coronal sections, and single mid-CCDs were dissected freehand in cold (4°C) Na+-Ringer solution. A single tubule was studied from each animal. Isolated CCDs were microperfused in vitro as previously described (10, 35). Briefly, each isolated tubule was immediately transferred to a temperature-controlled specimen chamber, assembled with a no. 1 coverslip (Corning) painted with a 3-μl drop of poly-d-lysine hydrobromide (0.01%; BS Biosciences), and set on the stage of a Nikon inverted epifluorescence microscope (Eclipse TE300 or Diaphot) linked to a Cascade 512F (Photometrics) or cooled Pentamax charge-coupled device (Princeton Instruments) camera interfaced with a digital imaging system (MetaFluor, Universal Imaging, West Chester, PA). The CCD was mounted on concentric glass pipettes, cannulated, and then positioned directly on the poly-d-lysine to immobilize the segment for the duration of the experiment, as previously described (36). Tubules were initially perfused and bathed at 37°C in symmetrical Ringer solution for the 30-min equilibration period before each experiment. The bathing solution was continuously exchanged throughout the experiment at a rate of 10 ml/h using a syringe pump (Razel, Stamford, CT) and maintained at 37°C.
For measurements of pHi, each CCD was incubated for 20 min in 20 μM BCECF-AM added to the bathing medium, as originally described by Weiner and Hamm (77). The luminal perfusate was then replaced with the Na+- and K+-free solution (0Na, 0K; Table 1), to which 10 nM bafilomycin was added in some experiments, and the bath with a Na+-Ringer solution. As indicated, BAPTA-AM (20 μM) was added to the bath at this point; in all experiments with colchicine (10 μM), the inhibitor was present in all bathing solutions, including the dissection solution. pHi measurements were begun after at least a 15-min washout of residual BCECF-AM from the bath.
Measurement of pHi.
BCECF-loaded cells were visualized using a Nikon S Fluor ×40 objective (numeric aperture 0.9, working distance 0.3). Autofluorescence was not detected at the camera gains utilized. Tubules were alternately excited at 490 and 440 nm using an excitation wavelength switcher (DG-4 or LAMBDA 10–2; Sutter); images of the fluorescence emission at 530 nm were acquired at intervals ranging from 2 to 15 s using MetaFluor image acquisition software (Universal Imaging, West Chester, PA) and were stored on a Digital Instruments computer. The 490 nm/440 nm fluorescence intensity ratios (FIRs) were subsequently calculated using our commercially available digital image-analysis system (MetaFluor).
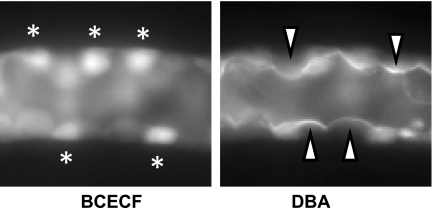
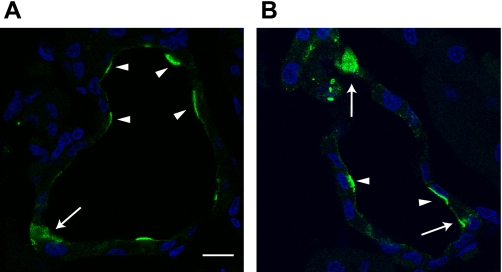
We (10) and others (77) have previously reported that esterase-rich intercalated cells can be distinguished from principal cells by their preferential accumulation of BCECF and brighter appearance when viewed under epifluorescence illumination. Confirmation of the identity of principal cells was sought by their selective apical binding of DBA added to the luminal perfusate at the conclusion of each experiment before the intracellular calibration was performed (Fig. 1).
Fig. 1.
Fluorescence micrographs of a single mid-cortical collecting duct (CCD), captured at slightly different focal planes, showing selective accumulation of BCECF (left) and apical binding of the principal cell marker rhodamine-conjugated Dolichos biflorus agglutinin (DBA; right) to discrete and nonoverlapping cells. The white asterisks identify cells (presumably intercalated) that preferentially accumulate BCECF loaded from the bath, and the white arrowheads identify principal cells with apical DBA caps.
An intracellular calibration was performed in each CCD using the nigericin technique and high-K+ intracellular calibration buffers (Table 1) (69). Linear regression analysis was used to generate a calibration curve that was then used for conversion of calculated FIRs to pHi using standard equations.
At least two intercalated and two principal cells residing in the lateral wall of the midregion of each perfused CCD (to capture the fluorescence signal from a single cell) were randomly selected for final analysis. The steady-state pHi of a single cell was calculated based on the average of at least six FIR readings; the steady-state pHi value for principal or intercalated cells in a single tubule was calculated as the mean pHi of at least two cells along the wall of each tubule. All pHi results are reported as the mean of n tubules.
Kinetic assay for H+-ATPase activity.
The sensitivity of H+ pumps to fluid flow rate was examined by measuring the effect of variations in luminal flow rate on Na+-independent pHi recovery from an intracellular acid load, accomplished by a ∼5-min peritubular exposure of BCECF-loaded CCDs to a 20 mM NH4Cl solution (Table 1). Measurement of luminal flow rates was performed before the NH4Cl pulse, after each change in luminal perfusate, or at least three times during a single experiment by timed filling of a precalibrated volumetric pipette. Rapid washout of the basolateral NH4Cl solution with a Na+-free solution (0Na, 5K; Table 1) led to a fall in pHi to ∼6.2 (Figs. 2 and 3); these manual washes were accomplished by fully replacing the volume of the bath (∼1.5 ml) at least three times within 10 s.
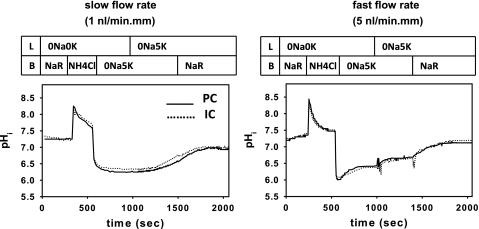
Fig. 2.
Representative tracings of changes in intracellular pH (pHi) following an NH4Cl-induced acute intracellular acid load in principal (PC; solid lines) and intercalated (IC; dotted lines.) cells in CCDs perfused at a slow (∼1 nl·min−1·mm−1; left) or fast (∼5 nl·min−1·mm−1; right) luminal flow rate. L identifies the solutions in the lumen, and B the solutions in the bath. In both panels, cytoplasmic acidification from an initial pHi of ∼7.3 in Na+-Ringer (NR) solution to a nadir pHi of ∼6.2 was accomplished by a brief exposure to a peritubular 20 mM NH4Cl pulse. In the absence of luminal Na+ and K+ (0Na, 0K), washout of the peritubular NH4Cl with a 0Na, 5K solution led to a minimal (at a slow flow rate) or partial (at a fast flow rate) pHi recovery in both PC and IC. pHi recovery in the absence of luminal K+ and bilateral Na+ is predominantly mediated by the vacuolar H+-ATPase. Readdition of 5 mM K+ (0Na, 5K) to the luminal perfusate led to a further increase in pHi; pHi recovery observed following restoration of K+ to the luminal perfusate is presumably mediated by the H+-K+-ATPase. Restoration of extracellular Na+ (NR) to provide substrate for the basolateral Na+/H+ exchanger led to full recovery of pHi in both cell types to ∼7.3 in all CCDs studied.
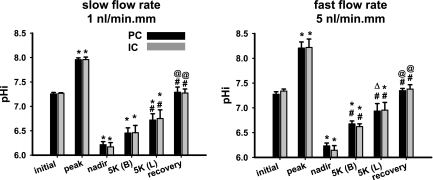
Fig. 3.
Steady-state pHi values of PC and IC in CCDs microperfused at slow (n = 4; left) and fast (n = 5; right) luminal flow rates before and during recovery from an in vitro acid load. The pHi values indicated include initial in Na+-Ringer solution; peak after addition of 20 mM NH4Cl to the bathing solution; nadir after washout of the NH4Cl pulse; steady state on cessation of Na+- and luminal K+-independent pHi recovery [5K (B)]; steady state on cessation of luminal K+-dependent, Na+-independent pHi recovery [5K (L)]; and the recovery value attained on restoration of Na+ to the bath solution. Values are means ± SE. *P < 0.05 vs. initial value. ΔP = 0.05 vs. initial value. #P < 0.05 vs. nadir value. @P < 0.05 vs. 5K (B) value.
Recovery of pHi from its nadir was monitored initially in the absence of Na+ and K+ (0Na, 0K; Table 1) in the lumen and Na+ (0Na, 5K; Table 1) in the bath. We have previously reported that replacement of luminal Na+-Ringer solution with a 0Na, 0K solution has no significant effect on steady-state pHi in microperfused CCDs (10). Once pHi stabilized, 5 mM K+ was added to the luminal perfusate (0Na, 5K solution; Table 1), and pHi was again monitored. On reaching a new steady state, Na+-Ringer solution was restored to the bath and pHi recovery was again followed.
After each change in solution, pHi was followed for at least 10 min or, if cell alkalinization was observed, until pHi stabilized (Fig. 2). In one set of experiments designed to identify the nature of the Na+- and luminal K+-independent pHi recovery routinely observed at pHi values less than ∼6.6, the experimental protocol described above was modified to include the addition of bafilomycin A1 to the 0Na, 0K luminal perfusate (Table 1). The initial rate of change in pHi (dpHi/dt) observed in response to a change in luminal or bathing solution was calculated by linear regression analysis of the rate of recovery over ∼30–60 s, as previously described (10). Mean slopes of pHi recovery for principal and intercalated cells were calculated for each CCD.
Experiments were rejected if we observed a ≥60% loss in the 450-nm fluorescence intensity from the initial value. Also excluded from the present study were those experiments in which peritubular Na+ restoration failed to restore pHi to baseline, except when the steady-state pHi after luminal K+ restoration was already near baseline.
We did not attempt to differentiate intercalated cell subtypes in this study, as initial studies revealed little variability in pHi recovery rates among intercalated cells examined at slow and fast flow rates. Similarly, Silver et al. (62) also found a normal distribution of pHi recovery rates after an acid load among intercalated cells in the rat, thus leading these investigators not to distinguish α-type from β-type intercalated cells for the functional portion of their study. We thus grouped values from all intercalated cells analyzed together for statistical analysis. Note that we focused our studies on the mid-CCD, which we have previously characterized in terms of intercalated cell composition. Specifically, we have reported, based on functional assays of this segment in the rabbit, that ∼78% of intercalated cells, defined as cells concentrating the pH-sensitive dye 6-carboxyfluorescein, bind peanut lectin agglutinin to their apical surfaces and are thus considered to be β cells (61).
Measurement of buffer capacity.
To test whether flow rate alters buffer capacity in CCD cells, we calculated intrinsic buffer capacity, as we have previously described (54), in individually identified cells in BCECF-loaded tubules perfused and bathed in a Na+- and HCO3−-free solution and subject to the rapid NH3/NH4+ withdrawal as described above. Buffer capacity (mM/pH U) was calculated as B = Δ[NH4+]i/ΔpHi where [NH4+]i is the concentration of intracellular NH4+. Using a pKa of 8.9 (5), we calculated the concentration of [NH4+]i as [NH4+]i = [NH4+]o × 10pHo − pHi, where [NH4+]o is the extracellular NH4+ concentration. ΔpHi was calculated as the difference in pHi between that measured just before and after the rapid NH4Cl washout.
Measurement of [Ca2+]i.
Following equilibration, microperfused tubules were loaded with 10 μM fura 2-AM added to the bath for 20 min. [Ca2+]i was measured in individually identified fura 2-loaded cells subject to the identical in vitro acid loading and recovery protocol depicted in Fig. 2 and visualized using the imaging system described above. Autofluorescence was not detected at the camera gains utilized.
For acquiring data in fura 2-loaded CCDs, tubules were alternately excited at 340 and 380 nm and images acquired every 15 s were digitized for subsequent analysis. At the conclusion of each experiment, an intracellular calibration was performed using 10 μM EGTA-AM in a Ca2+-free bath and then a 2 mM Ca2+ bath containing ionomycin (10 μM) (36). Standard equations were used to calculate experimental values of [Ca2+]i. The mean [Ca2+]i values for principal and intercalated cells, distinguished by their differing fluorescent intensities (36), were calculated.
Immunolocalization of H+-ATPase in rabbit kidneys.
Freshly harvested kidneys were cut into ∼5-mm slices, and fixed using 4% paraformaldehyde-lysine-periodate buffer (40, 46) by immersion for 3 h at room temperature (RT). The fixed kidney tissues were washed in PBS and quenched in NH4Cl, followed by washes in PBS. Tissues were cryoprotected by immersion in a 30% sucrose solution in PBS at 4°C overnight. These tissues were embedded using Tissue-Tek-OCT at −27°C, and 4-μm cryosections were obtained and placed onto polylysine-coated slides (Fisher). After 30-min rehydration in PBS, the slides were subject to a 1% SDS in PBS antigen retrieval step for 4 min in a wet chamber. After several washes in PBS, the sections were blocked in 1% BSA in PBS for 15 min (6). Then, the sections were incubated with DBA at a 1:100 dilution for 1 h at RT, followed by a 5-min wash in PBS and then by incubation in the presence or absence (no-primary control) of an antibody directed against the E subunit of the V-ATPase (raised in chickens, 1:500 dilution; GenWay) (21) for 75 min at RT, followed by two high-salt-PBS (2.7% NaCl) and one normal-strength PBS wash. Thereafter, both slides were incubated with a secondary goat-anti-chicken antibody coupled to FITC (1:100; Jackson Immunologicals) for 1 h at RT. After washes as above, the slides were mounted with Vectashield (Vector Laboratories). The tissues immunostained with the antibody against the V-ATPase E subunit and the no-primary control were imaged using a Leica TSC confocal microscope with a ×100 objective, with identical laser acquisition settings, then imported into Adobe Photoshop for identical adjusting of levels of both the H+-ATPase and the no-primary control images. Labels were added in Indesign (Adobe).
Control studies with the antibody preincubated with its respective immunogen were not performed, as the immunogen was not available from the original manufacturer. However, the antibody was tested by Western blotting by the manufacturer, and we (21, 24, 25) and others (82) have further shown cell-specific labeling of clear and intercalated cells in rat tissues using this antibody, as has been reported using other antibodies against the E subunit.
In addition, we also performed immunofluorescence labeling of rabbit kidneys using an antibody directed against the V-ATPase A subunit, raised in chickens, at a 1:100 concentration (Genway; immunogen not available from the manufacturer). The secondary antibody used was a goat anti-chicken antibody coupled to Alexa Fluor 488 at a 1:800 dilution. Images were obtained using a ×63 objective.
Statistics.
Results are expressed as means ± SE; n represents the number of tubules. At least four randomly chosen cells were functionally analyzed in each CCD. Significant differences between paired data were determined by a paired t-test. Comparisons of unpaired data were performed by t-test and analysis of variance, as appropriate. Commercially available statistical software (SigmaStat; SPSS Chicago, IL) was used for all statistical analyses. Significance was asserted if P < 0.05.
RESULTS
Effect of flow on steady state intercalated and principal cell pHi.
In four CCDs perfused and bathed in Na+-Ringer solution and studied at a slow luminal flow rate of 1.0 ± 0.1 nl·min−1·mm−1, steady-state pHi (baseline) in intercalated cells (7.27 ± 0.01) did not differ from that measured in principal cells [7.26 ± 0.03; P = not significant (NS)] (Figs. 2 and 3). In five CCDs perfused at a fast flow rate of 5.5 ± 0.3 nl·min−1·mm−1, baseline steady-state pHi was similar in both intercalated (7.34 ± 0.04) and principal (7.27 ± 0.05; P = NS) cells and was not different from that observed in the respective cell type at the slow flow rate (P = NS) (Figs. 2 and 3). Buffer capacity in was similar in both intercalated (28.1 ± 5.7 mM/pH U) and principal (29.3 ± 6.0 mM/pH U; P = NS) cells perfused at the slow flow rate and was unchanged by a fivefold increase in flow rate (26.8 ± 8.7 and 24.8 ± 8.3 mM/pH U, respectively; P = NS vs. same cell at slow flow rate).
As changes in cell volume could alter the correlation between H+ secretion and changes in pHi recovery, we measured the inner and outer diameters of CCDs perfused at slow and fast flow rates and calculated cell height. In tubules perfused and bathed in Na+-Ringer solution at the slow flow rate of 1 nl·min−1·mm−1, these values were 15.9 ± 1.6, 37.5 ± 1.0, and 10.8 ± 0.3 μm, respectively. In tubules perfused at the fast flow rate of 5.5 ± 0.3 nl·min−1·mm−1, inner and outer diameters and cell height (19.6 ± 1.4, 36.9 ± 2.6, and 11.1 ± 0.8 mm, respectively) were not significantly different from those measured at the slow flow rate (P = NS).
Effect of flow on apical H+-ATPase activity in DBA-negative cells.
In response to the rapid basolateral washout of a 20 mM NH4Cl pulse with a 0Na, 5K solution, pHi fell to 6.17 ± 0.09 in DBA-negative and thus presumed intercalated cells in four CCDs perfused at a slow flow rate of 1.0 ± 0.1 nl·min−1·mm−1 with a 0Na, 0K solution (Figs. 2 and 3). From this nadir pHi, these cells exhibited an alkalization at an initial rate of 0.059 ± 0.009 pH U/min to reach a plateau pHi of 6.46 ± 0.15 over a period of 6.0 ± 0.6 min (Figs. 2–4). We have previously reported that in the presence of 5 mM K+ in the bath but not luminal perfusate, Na+-independent pHi recovery is mediated primarily by the vacuolar H+-ATPase, which appears to “turn off” at a pHi of 6.6 (10). Restoration of 5 mM K+ to the lumen led to a further increase in pHi at a rate of 0.088 ± 0.016 pH U/min to stabilize at a pHi of 6.75 ± 0.18 within 5.8 ± 1.0 min (Figs. 2–4). We have previously reported that this luminal K+-dependent, Na+-independent pHi recovery is mediated by an apical H+-K+-ATPase as it is effectively inhibited by 10 μM SCH28080, an inhibitor of the H+-K+-ATPase (10). Thereafter, restoration of Na+ to the bathing solution resulted in a further cell alkalinization, presumably the result of basolateral Na+/H+ exchange, to reach a pHi of 7.27 ± 0.08 (Figs. 2 and 3).
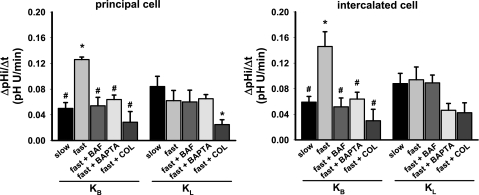
Fig. 4.
Initial rates of luminal K+-independent (KB) and K+-dependent (KL) Na+-independent pHi recovery (ΔpHi/Δt) of PC and IC in CCDs subject to an in vitro acid load and microperfused under the following conditions: slow luminal flow rate (n = 4), fast luminal flow rate (n = 5), and then fast flow after pretreatment with bafilomycin (n = 6), BAPTA-AM (n = 4), or colchicine (n = 4). Values are means ± SE. Statistical differences by t-test: *P < 0.05 vs. slow flow rate. #P < 0.05 vs. fast flow rate. ANOVA confirmed the statistical differences among the rates of recovery in the KB groups but revealed no significant difference among the KL groups.
In a similar series of studies in which five CCDs were perfused at a fast luminal flow rate of 5.5 ± 0.3 nl·min−1·mm−1 with a 0Na, 0K solution and bathed in a 0Na, 5K solution, the initial alkalinization from the nadir pHi of 6.14 ± 0.09 averaged 0.146 ± 0.023 pH U/min (P < 0.03 vs. rate at 1 nl·min−1·mm−1) (Figs. 2–4). Once pHi stabilized at 6.62 ± 0.06 within 4.3 ± 0.8 min, restoration of 5 mM K+ to the lumen led to a further increase in pHi at a rate of 0.094 ± 0.020 pH U/min (P = NS vs. rate at 1 nl·min−1·mm−1) to reach a plateau pHi of 6.95 ± 0.16 within 6.8 ± 1.4 min (Figs. 2–4). Addition of Na+ to the bathing solution at this point led to a further cell alkalinization to 7.38 ± 0.09, a value not significantly different from baseline (Figs. 2 and 3).
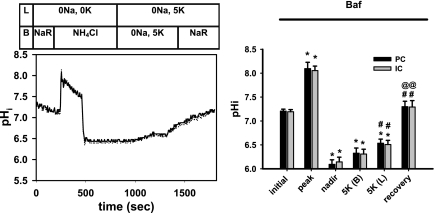
To confirm that the flow-stimulated, Na+-independent pHi recovery from the nadir observed in the absence of luminal K+ was due predominantly to an H+-ATPase, as our previous studies suggested (10), pHi recovery from a nadir of 6.14 ± 0.10 was monitored in a separate group of six CCDs pretreated with and perfused in the continuous presence of luminal bafilomycin. In these CCDs, perfused at a luminal flow rate of 5.6 ± 0.2 nl·min−1·mm−1, the initial rate of pHi recovery was only 0.052 ± 0.014 pH U/min, a rate significantly less than that observed in CCDs perfused at the same flow rate in the absence of inhibitor (P < 0.01) and not significantly different from that observed in CCDs perfused at the slow flow rate, to a plateau pHi of 6.31 ± 0.11 (P < 0.03 vs. plateau pHi of 6.62 ± 0.06 observed in untreated CCDs perfused at a fast flow rate) (Figs. 4 and 5). We propose that this residual bafilomycin-insensitive rate of pHi recovery is due, at least in part, to a basolateral H+-K+-ATPase, as we have previously identified in the CCD (10).
Fig. 5.
Effect of the vacuolar H+-ATPase inhibitor bafilomycin (10 nM) on pHi recovery of CCDs perfused at a fast flow rate following an NH4Cl-induced acute intracellular acid load. Left: representative virtually identical tracings of changes in pHi in individually identified PC (solid line) and IC (dotted line) in a CCD pretreated with bafilomycin. The luminal (L) and bathing (B) solutions are fully described in the legend to Fig. 2. Right: steady-state pHi values of PC and IC in 6 CCDs pretreated with bafilomycin and microperfused at fast luminal flow rates before and during recovery from an in vitro acid load. The pHi values indicated are described in detail in the legend to Fig. 3. Values are means ± SE. *P < 0.05 vs. initial value. #P < 0.05 vs. nadir value. @P < 0.05 vs. 5K (B) value.
Effect of flow on apical H+-ATPase activity in DBA-positive cells.
In response to the rapid basolateral washout of a 20 mM NH4Cl pulse with a 0Na, 5K solution, pHi fell to 6.22 ± 0.06 in DBA-positive and thus presumed principal cells in four CCDs perfused at a slow flow rate of 1.0 ± 0.1 nl·min−1·mm−1 with a 0Na, 0K solution (Figs. 2 and 3). From this nadir pHi, these cells exhibited an alkalization at an initial rate of 0.050 ± 0.009 pH U/min to reach a pHi of 6.45 ± 0.11 over a period of 5.6 ± 0.6 min (Figs. 2–4). Restoration of 5 mM K+ to the lumen led to a further increase in pHi at a rate of 0.084 ± 0.016 pH U/min to stabilize at a pHi of 6.72 ± 0.13 within 5.7 ± 1.3 min (Figs. 2–4). Thereafter, restoration of Na+ to the bathing solution resulted in a further cell alkalinization, presumably the result of basolateral Na+/H+ exchange, to reach a pHi of 7.29 ± 0.11 (Figs. 2 and 3).
In a similar series of studies in which five CCDs were perfused at a fast luminal flow rate of 5.5 ± 0.3 nl·min−1·mm−1 with a 0Na, 0K solution and bathed in a 0Na, 5K solution, the initial alkalinization from the nadir pHi of 6.23 ± 0.06 averaged 0.126 ± 0.004 pH U/min (P < 0.001 vs. rate at 1 nl·min−1·mm−1) (Figs. 2–4). Once pHi stabilized at 6.68 ± 0.06 within 5.0 ± 1.0 min, restoration of 5 mM K+ to the lumen led to a further increase in pHi at a rate of 0.062 ± 0.016 pH U/min (P = NS vs. rate at 1 nl·min−1·mm−1) to reach a pHi of 6.94 ± 0.15 within 7.5 ± 1.7 min (Figs. 2–4). Addition of Na+ to the bathing solution at this point led to a further cell alkalinization to 7.35 ± 0.05, a value not significantly different from baseline (Figs. 2 and 3).
To discern whether the flow-stimulated Na+-independent pHi recovery from the nadir observed in the absence of luminal K+ was due to an H+-ATPase, pHi recovery from a nadir of 6.09 ± 0.09 was monitored in a separate group of six CCDs pretreated with and perfused in the continuous presence of luminal bafilomycin. In these CCDs, perfused at a luminal flow rate of 5.6 ± 0.2 nl·min−1·mm−1, the initial rate of pHi recovery was 0.054 ± 0.013 pH U/min, a rate significantly less than that observed in CCDs perfused at the same flow rate in the absence of inhibitor (P < 0.001) and not significantly different from that observed in CCDs perfused at the slow flow rate, to a plateau pHi of 6.33 ± 0.11 (P < 0.03 vs. plateau pHi of 6.68 ± 0.06 observed in untreated CCDs perfused at a fast flow rate) (Figs. 4 and 5). As for the DBA-negative cells studied above, we propose that this residual rate of bafilomycin-insensitive pHi recovery is due, at least in part, to a basolateral H+-K+-ATPase (10).
Effect of acute acid-loading protocol on steady-state cell [Ca2+]I.
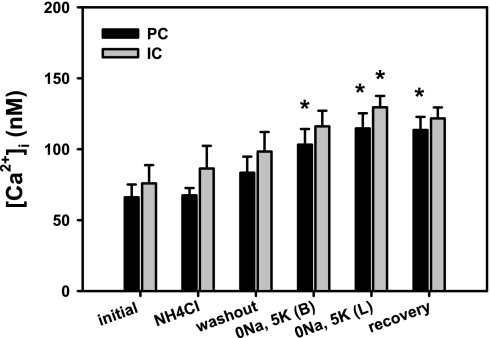
Na+-free solutions have previously been shown to induce changes in [Ca2+]i in microperfused OMCDs, which in turn modulated H+-ATPase-mediated recovery from an acid load (26). To ensure that the effects of flow on H+-ATPase activity were not due to wide variations in steady-state [Ca2+]i induced by our acid-loading protocol, [Ca2+]i was examined in CCDs perfused at a fast flow rate of 5.2 ± 0.2 nl·min−1·mm−1 at baseline (initial value), 3–5 min after addition of NH4Cl to the bath, immediately and then 10 min after washout of the NH4Cl pulse with a 0Na, 5K bathing solution, 10 min after readdition of K+ to the lumen, and 10 min after restoration of Na+ to the bathing medium. As shown in Fig. 6, the NH4Cl prepulse was not associated with a significant change in [Ca2+]i, although [Ca2+]i progressively increased in both principal and intercalated cells with assumed pHi recovery.
Fig. 6.
Effect of NH4Cl pulse on intracellular Ca2+ concentration ([Ca2+]i) in PC and IC cells in CCDs (n = 4) perfused at a fast flow rate and subject to the in vitro acid loading and recovery protocol described in Fig. 2. [Ca2+]i was examined in CCDs at baseline (initial value), 3–5 min after addition of NH4Cl to the bath, immediately and then 10 min after washout of the NH4Cl pulse with a 0Na, 5K bathing solution, 10 min after readdition of K+ to the lumen, and after restoration of Na+ to the bathing medium. Values are means ± SE. *P < 0.05 vs. initial value.
Flow-stimulation of H+-pumping requires an increase in [Ca2+]I.
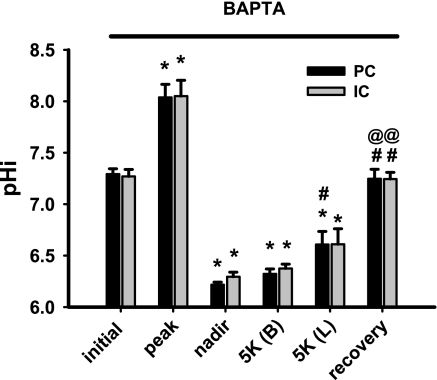
An acute increase in tubular fluid flow rate in the microperfused rabbit CCD leads to a rapid transient increase in [Ca2+]i from ∼100 to 350 nM within ∼10 s, followed by a gradual decay to a plateau [Ca2+]i value that significantly exceeds baseline for at least 10 min during a period of sustained high flow (32, 36). To examine whether flow-stimulation of H+-pumping in the CCD is dependent on an initial transient flow-induced increase in [Ca2+]i, CCDs were pretreated with 20 μM BAPTA-AM, a membrane-permeant Ca2+ chelator, and the rates of pHi recovery from an acute acid load were measured at fast (5.1 ± 0.2 nl·min−1·mm−1) flow rates. Chelation of intracellular Ca2+ inhibited flow-stimulated Na+-independent pHi recovery from the nadir in both principal and intercalated cells (Figs. 4 and 7; tracing not shown). These data suggest that flow stimulation of the H+-ATPase requires an increase in [Ca2+]i.
Fig. 7.
Effect of the intracellular Ca2+ chelator BAPTA-AM (10 nM) on pHi recovery of CCDs perfused at a fast flow rate following an NH4Cl-induced acute intracellular acid load. Steady-state pHi values of PC and IC in 4 CCDs pretreated with BAPTA-AM and microperfused at fast luminal flow rates before and during recovery from an in vitro acid load. The pHi values indicated are described in detail in the legend to Fig. 3. Means ± SEM. *, P < 0.05 vs. initial value; #, P < 0.05 vs. nadir value; @, P < 0.05 vs. 5K (B) value.
Flow stimulation of H+-pumping requires microtubule integrity.
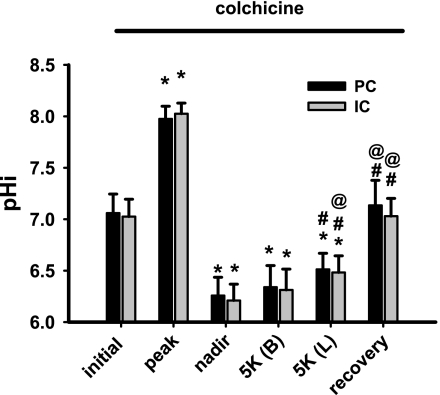
Intracellular Ca2+ regulates exocytosis and secretion in epithelial cells via, at least in part, microtubule-dependent movement of secretory vesicles (2, 7). To examine the role of the microtubules in flow stimulation of H+-pumping, CCDs (n = 4) were pretreated with colchicine (10 μM), a microtubule inhibitor (44) that also inhibits vesicle transport between the trans-Golgi network (TGN) and the plasma membrane of polarized epithelial cells (22, 72). CCDs were exposed to colchicine during microdissection as well as throughout the entire experiment, and the rates of pHi recovery from an acute acid load were measured at fast (5.5 ± 0.2 nl·min−1·mm−1) flow rates. Colchicine inhibited flow-stimulated Na+-independent pHi recovery from the nadir in both principal and intercalated cells (Figs. 4 and 8; tracing not shown). These data suggest that flow stimulation of the H+-ATPase requires microtubule integrity.
Fig. 8.
Effect of the microtubule disrupter colchicine (10 μM) on pHi recovery of CCDs perfused at a fast flow rate following an NH4Cl-induced acute intracellular acid load. Steady-state pHi values of PC and IC cells in 4 CCDs pretreated with cochicine and microperfused at fast luminal flow rates before and during recovery from an in vitro acid load. The pHi values indicated are described in detail in the legend to Fig. 3. Values are means ± SE. *P < 0.05 vs. initial value. #P < 0.05 vs. nadir value. @P < 0.05 vs. 5K (B) value.
Immunolocalization of H+-ATPase in rabbit CCD.
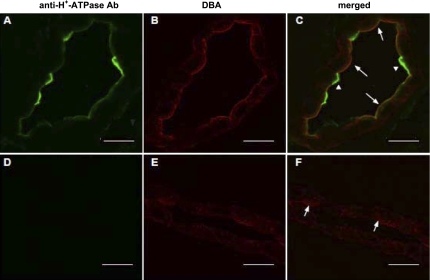
Immunodetectable V-ATPase E subunit was observed by indirect immunofluorescence confocal microscopy in rabbit CCD (Fig. 9). The α-intercalated cells revealed characteristic apical labeling for the V-ATPase E subunit (Fig. 9C, arrowheads). Principal cells were identified by their apical labeling with DBA coupled to CY3 (Fig. 9, B–E). Note that principal cells in the rabbit collecting duct also exhibited weak apical immunolabeling for the V-ATPase E subunit (Fig. 9C, arrows); these cells showed partial colocalization (yellow) of the V-ATPase E subunit (green) and DBA (red). In the absence of primary antibody against the V-ATPase E subunit, neither intercalated nor principal cells labeled specifically with the antibody (Figs. 9, D and F).
Fig. 9.
Immunolocalization of H+-ATPase E subunit in rabbit CCD. A–C: confocal images of a rabbit kidney collecting duct labeled with both an anti-H+-ATPase E subunit antibody (Ab; green channel; A) and PC marker lectin, DBA coupled to Cy3 (red channel; B). C: merged image of A and B. Arrowheads identify IC and arrows PC. D–F: confocal images of a rabbit kidney collecting duct, no-primary antibody control for the H+-ATPase E subunit (green channel; D) and in the presence of the principal cell marker DBA (red channel; D). F: merged image of D and E. Arrows identify PC. Scale bars = 15 μm.
Moreover, we also detected the V-ATPase A subunit (ATP6V1A) by indirect immunofluorescence labeling and confocal microscopy in rabbit CCD (Fig. 10). We identified three distinct patterns of A subunit immunolabeling in the epithelial cells (Fig. 10): 1) intense immunolabeling at the luminal/apical membrane, in a pattern consistent with α-intercalated cells (arrowheads); 2) diffuse labeling, presumably of β-intercalated cells (arrowheads); and 3) weak immunolabeling at the apical pole of other cells, presumably principal cells.
Fig. 10.
Heterogeneity of immunodetectable H+-ATPase A subunit in rabbit CCD. Confocal images of 2 sections of rabbit kidney cortex (A and B) labeled with an anti-H+-ATPase A subunit antibody (green channel) and the nuclear marker 4,6-diamidino-2-phenylindole (blue channel). Arrows identify cells with diffuse labeling, and are thus presumably β-IC, whereas arrowheads identify α-IC with discrete apical labeling. Note the punctuate, albeit modest, immunoreactivity at or just below the apical membrane of other cells, presumably principal cells. Sections labeled with secondary antibody alone showed no signal (data not shown). Scale bar = 10 μm.
DISCUSSION
Axial flow modulates HCO3− reabsorption in the proximal tubule due, at least in part, to stimulation of luminal H+-ATPase activity (11). Recent evidence indicates that fluid shear stress induces the translocation of cytoplasmic H+-ATPases to the apical plasma membrane in the proximal tubule, a process that requires an intact microtubule network (11, 13). Long-term use of furosemide or hydrochlorothiazide enhances distal urinary acidification and leads to metabolic alkalosis, classically attributed to an increase in distal delivery of Na+ to and reabsorption in the connecting tubule and CCD (28), thereby augmenting the lumen-negative transepithelial voltage favoring H+ secretion. In addition, chronic diuretic administration increases abundance of H+-ATPase B1 subunit protein, but not its subcellular localization, irrespective of electrogenic Na+ absorption and in the absence of changes in circulating levels of aldosterone (43). These observations led us to test the hypothesis that luminal flow also regulates H+-ATPase activity in the CCD.
The results of this study show that bafilomycin-sensitive H+-ATPase activity in DBA-negative, and thus presumably intercalated cells, and DBA-positive principal cells in the rabbit mid-CCD is regulated by luminal flow rate in a Ca2+-dependent manner that requires microtubule integrity. Na+-independent, luminal K+-dependent pHi recovery from an NH4Cl-imposed acute acid load, previously reported by us to be sensitive to SCH28080 and thus mediated by an H-K+-ATPase (10, 65), did not show flow dependence under the conditions of our assay.
We did not attempt to confirm the identity of the intercalated cell subtypes examined in this study, but based on functional studies by ourselves (54, 61) and others (15, 78), we assume that the majority of intercalated cells examined had apical anion exchangers. This assumption predicts that H+ pumps activated by flow may have been present in the apical (in type A or α cells) or basolateral (in type B or β cells) membranes of cells studied. As bafilomycin is cell permeant, it was expected to inhibit both apical, basolateral, and cytoplasmic H+-ATPases. This prediction begs the question as to what the mechanosensor is for flow activation of the H+ pump. Whereas it is generally well established that the brush-border microvilli in the proximal tubule and the primary cilia in the CCD can serve as sensors of luminal flow (reviewed in Ref. 74), the mechanism(s) by which apical mechanical signals might be transmitted to the intracellular cytoskeleton and thereby regulate distant (e.g., basolateral) membrane transport proteins remains to be explored. The observation that luminal fluid shear stress (FSS) in the proximal tubule regulates peritubular Na-K-ATPase activity by stimulating the translocation of intracellular pumps into the basolateral membrane (14) sets a precedent for our finding of flow-stimulated H+ pumping in presumed β-intercalated cells.
The Ca2+ dependence of this process was intriguing. An acute elevation of Pco2 rapidly stimulates the exocytotic fusion of vesicles containing H+-ATPases with the luminal membrane of intercalated cells (20, 59, 68), a response induced by a transient increase in [Ca2+]i (8, 71). Indeed, it is well established that [Ca2+]i regulates exocytosis and secretion in epithelial cells via, at least in part, microtubule-dependent movement of secretory vesicles (2, 3, 7). Our finding that intracellular Ca2+ chelation inhibited flow-stimulated, H+-ATPase-mediated H+ pumping suggests a role for exocytosis in this response. In support of this speculation is our finding that flow-stimulated H+ extrusion appears also to depend on microtubule integrity, as it was abolished in CCDs pretreated with colchicine.
Mathematical models for collecting duct acid excretion predict a strong flow dependence of urinary pH (80). In fact, functional studies in microperfused rabbit CCDs suggest that acid-base transport is regulated by tubular fluid flow. In microperfused CCDs poised for HCO3− secretion (e.g., isolated from alkali-loaded rabbits), an increase in tubular fluid flow rate from 1 to 5 nl·min−1·mm−1 leads to a reduction in the rate of net HCO3− secretion from −6 to 0 pmol·min−1·mm−1 (37). Other studies in which JHCO3 was measured in rabbit CCDs microperfused with a 25 mM HCO3−, ∼120 mM Cl− perfusate at variable flow rates also provides evidence for a flow dependence of acid-base transport (18, 19, 56, 70); specifically, these data suggest that an increase in tubular fluid flow rate reduces net HCO3− secretion in rabbit CCDs. It should be noted that in contrast to rabbits, an increase in flow rate in rat CCD is associated with an increase, no change, or reduction in JHCO3 (summarized in Ref. 9).
In principle, a flow-induced change in net HCO3− transport in the CCD can reflect a change in either the rate of HCO3− secretion (by type B or β cells), H+ secretion (by type A or α cells), or both. Thus the apparent flow-induced reduction in JHCO3 in the rabbit CCD may be due to a reduction in HCO3− secretion by type B cells, an increase in H+ secretion by type A cells, or both. It is also possible that flow enhances both HCO3− secretion by type B cells as well H+ secretion by type A cells, but the flow-induced increase in H+ secretion exceeds that of HCO3− secretion. Furthermore, changes in transepithelial voltage (Vte) may affect the rate of H+ secretion; both luminal amiloride (39) and peritubular ouabain (29), inhibitors of apical ENaC and the basolateral Na+-K+-ATPase, respectively, decrease the rates of HCO3− absorption in rabbit CCDs that were initially absorbing HCO3−. Increases in tubular fluid flow rate tends to reduce the lumen negative Vte (57).
Although principal cells in rabbit collecting tubule, albeit studied in a segment-specific manner, variably express all of the critical transport proteins necessary for H+ secretion and HCO3− reabsorption, including apical H+-ATPase and H+-K+-ATPase activities (75, 79) and basolateral Cl−/HCO3− exchange activity (76, 79), principal cells in the mid-CCD have not traditionally been considered to have V-ATPase activity. We must thus acknowledge that our finding of a bafilomycin-sensitive H+ extrusion pathway in principal cells in this segment may be species and segment specific, unique to the rabbit mid-CCD. Additional support for species- and/or segment-specific expression of this pump is provided by the studies of Miller et al. (41). These investigators generated an intercalated cell-specific Cre-expressing transgenic mouse using an ATP6V1B1 promoter, which had previously been used to drive expression of enhanced green fluorescent protein (EGFP) (42), to drive expression of Cre recombinase. The ATP6V1B1-Cre transgenic mouse demonstrated active Cre in all intercalated cells (type A, B, and non-A/B cells) within the kidney, whereas it was not active in any other cell type except in ∼50% of principal cells within the connecting tubule only, a result similar to that observed in the B1-driven EGFP transgenic mice.
However, in support of functional evidence for H+-ATPase activity in principal cells in rabbit CCD was the finding by Silver and Frindt (63), based on functional studies similar to those performed in the present study, that principal cells in this species have a mechanism for H+ extrusion that is not blocked by Sch-28080, an inhibitor of the H+-K+-ATPase. While the molecular identity of the H+ extrusion pathway in principal cells was not determined in the latter study, the authors proposed the presence of an electrodiffusive pathway for protons in these cells, a speculation supported by the observation that the imposition of a chemical driving force for protons by altering extracellular pH alters principal cell pHi in a reversible manner (64) or that the Na+- K+-ATPase can substitute H+ for Na+ under Na+-free conditions (47). It should also be noted that we detected bafilomycin-sensitive H+-ATPase activity in principal cells acidified to a pHi of ∼6.2 and that this Na+- and K+-independent recovery “turned off” at a pHi of ∼6.6. Studies by Silver and Frindt (63) and others (76), in which principal cells were acid loaded to pHi values >6.7, would thus not have been expected to detect this transport pathway. A role for principal cells in maintaining acid-base balance has been further demonstrated by the finding that mice with targeted deletion of Rhcg in intercalated cells alone, subject to chronic acid loading to generate metabolic acidosis, demonstrate increased Rhcg immunolabel in principal cells in both the CCD and the OMCD (30), an adaptation proposed to contribute to acidosis-stimulated ammonia excretion.
Our detection of apical immunolabeling of principal cells, albeit less intense than that observed in intercalated cells, in rabbit CCDs using an antibody directed against the V-ATPase, provides additional support for the presence of an H+ pump in principal cells, as suggested by our finding of bafilomycin-sensitive pHi recovery from an intracellular acid load in DBA-positive cells. While our detection of immunoreactive V-ATPase in principal cells in this study may reflect species-specific differences in V-ATPase subunit expression or differences between investigators in the methods used for immunolocalization, a careful review of the literature reveals evidence for immunoreactive H+-ATPase in mammalian principal (or connecting tubule) cells. Alper et al. (1) reported a “delicate” labeling of the apical membranes of connecting tubule cells in rat cortex using an anti-V-ATPase E subunit antibody. Sabolic et al. (51) reported apical immunofluorescence labeling of principal cells in the rat cortical connecting segment using a polyclonal antibody directed against the vacuolar V-ATPase 31-kDa subunit. The 56-kDa B1 (ATP6V1B1) subunit was detected in principal cells in rat kidney (52), associated with endocytic vesicles that are involved in the vasopressin-stimulated recycling of water channels to and from the apical membrane. However, the endosomes in those principal cells did not acidify their lumen, leading the authors to suggest that the 56-kDa subunit might be involved in the recycling of aquaporin-2 (AQP2) without contributing to endosomal acidification. To add to this controversy, Gustafson et al. (23) reported that in LLC-PK1 cells, bafilomycin A1 blocks the recycling of AQP2, in the presence and absence of vasopressin, implicating a role for vesicle acidification via the V-ATPase in trafficking of AQP2, a process localized to principal cells. Based on the above, we propose that our current work brings important information to a controversial field, as we have confirmed the presence of a functional bafilomycin-sensitive H+ extrusion pathway in principal cells in the rabbit CCD and demonstrate immunoreactive V-ATPase subunits in these same cells.
In summary, increases in tubular (urinary) flow rate not only stimulate ENaC-mediated Na+ absorption and BK channel-mediated K+ secretion (53) but also, as demonstrated in the present study, H+-ATPase activity in the rabbit mid-CCD. We speculate that flow stimulation of H+ secretion in the distal nephron may contribute to diuretic-induced metabolic alkalosis. The mechanosensors and mechanisms underlying flow stimulation of the vacuolar H+-ATPase have yet to be explored.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DK038470 (to L. M. Satlin), DK051391 (to T. R. Kleyman), DK084184 and AHA Grant-in-Aid 09GRNT2060539 (N. M. Pastor-Soler), and P30 DK079307 (The Pittsburgh Center for Kidney Research).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.L., N.M.P.-S., C.S., and B.Z. performed experiments; W.L., N.M.P.-S., C.S., and L.M.S. analyzed data; W.L., N.M.P.-S., C.S., and L.M.S. prepared figures; W.L. and L.M.S. drafted manuscript; W.L., N.M.P.-S., C.S., T.R.K., and L.M.S. edited and revised manuscript; W.L., N.M.P.-S., C.S., B.Z., T.R.K., and L.M.S. approved final version of manuscript; N.M.P.-S., T.R.K., and L.M.S. provided conception and design of research; N.M.P.-S., C.S., T.R.K., and L.M.S. interpreted results of experiments.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Christy Smolak (Pittsburgh) for technical support.
REFERENCES
- 1. Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86: 5429–5433, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashby MC, Tepikin AV. Polarized calcium and calmodulin signaling in secretory epithelia. Physiol Rev 82: 701–734, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 85: 7972–7976, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3. Am J Physiol Cell Physiol 255: C844–C856, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Burgoyne RD, Clague MJ. Calcium and calmodulin in membrane fusion. Biochim Biophys Acta 1641: 137–143, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Cannon C, van Adelsberg J, Kelly S, Al-Awqati Q. Carbon-dioxide-induced exocytotic insertion of H+ pumps in turtle-bladder luminal membrane: role of cell pH and calcium. Nature 314: 443–446, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Chang H, Fujita T. A numerical model of acid-base transport in rat distal tubule. Am J Physiol Renal Physiol 281: F222–F243, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Constantinescu A, Silver RB, Satlin LM. H-K-ATPase activity in PNA-binding intercalated cells of newborn rabbit cortical collecting duct. Am J Physiol Renal Physiol 272: F167–F177, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci USA 101: 13068–13073, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci USA 105: 11418–11423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci USA 107: 21860–21865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emmons C, Kurtz I. Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest 93: 417–423, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evan AP, Satlin LM, Gattone VH, Connors 2nd B, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct. II. Morphological observations. Am J Physiol Renal Fluid Electrolyte Physiol 261: F91–F107, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Frank AE, Wingo CS, Weiner ID. Effects of ammonia on bicarbonate transport in the cortical collecting duct. Am J Physiol Renal Physiol 278: F219–F226, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Austt J, Good DW, Burg MB, Knepper MA. Deoxycorticosterone-stimulated bicarbonate secretion in rabbit cortical collecting ducts: effects of luminal chloride removal and in vivo acid loading. Am J Physiol Renal Fluid Electrolyte Physiol 249: F205–F212, 1985 [DOI] [PubMed] [Google Scholar]
- 20. Gluck S, Cannon C, Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci USA 79: 4327–4331, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [Google Scholar]
- 22. Grindstaff KK, Bacallao RL, Nelson WJ. Apiconuclear organization of microtubules does not specify protein delivery from the trans-Golgi network to different membrane domains in polarized epithelial cells. Mol Biol Cell 9: 685–699, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gustafson CE, Katsura T, McKee M, Bouley R, Casanova JE, Brown D. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am J Physiol Renal Physiol 278: F317–F326, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem 284: 5774–5783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hays SR, Alpern RJ. Inhibition of Na+-independent H+ pump by Na+-induced changes in cell Ca2+. J Gen Physiol 98: 791–813, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Kovacikova J, Winter C, Loffing-Cueni D, Loffing J, Finberg KE, Lifton RP, Hummler E, Rossier B, Wagner CA. The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kidney Int 70: 1706–1716, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Laski ME, Kurtzman NA. Characterization of acidification in the cortical and medullary collecting tubule of the rabbit. J Clin Invest 72: 2050–2059, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+ -dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978–F988, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct (CCD): role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Lombard WE, Kokko JP, Jacobson HR. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol Renal Fluid Electrolyte Physiol 244: F289–F296, 1983 [DOI] [PubMed] [Google Scholar]
- 38. Lynch IJ, Greenlee MM, Gumz ML, Rudin A, Xia SL, Wingo CS. Heterogeneity of H-K-ATPase-mediated acid secretion along the mouse collecting duct. Am J Physiol Renal Physiol 298: F408–F415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McKinney TD, Burg MB. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 234: F141–F145, 1978 [DOI] [PubMed] [Google Scholar]
- 40. McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974 [DOI] [PubMed] [Google Scholar]
- 41. Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD. The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis, and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol 288: C1134–C1144, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Na KY, Kim GH, Joo KW, Lee JW, Jang HR, Oh YK, Jeon US, Chae SW, Knepper MA, Han JS. Chronic furosemide or hydrochlorothiazide administration increases H+-ATPase B1 subunit abundance in rat kidney. Am J Physiol Renal Physiol 292: F1701–F1709, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991 [DOI] [PubMed] [Google Scholar]
- 46. Pastor-Soler N, Bagnis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, Breton S, Brown D. Aquaporin 9 expression along the male reproductive tract. Biol Reprod 65: 384–393, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Polvani C, Blostein R. Protons as substitutes for sodium and potassium in the sodium pump reaction. J Biol Chem 263: 16757–16763, 1988 [PubMed] [Google Scholar]
- 48. Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Preisig PA. Luminal flow rate regulates proximal tubule H-HCO3 transporters. Am J Physiol Renal Fluid Electrolyte Physiol 262: F47–F54, 1992 [DOI] [PubMed] [Google Scholar]
- 50. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Sabolic I, Wuarin F, Shi LB, Verkman AS, Ausiello DA, Gluck S, Brown D. Apical endosomes isolated from kidney collecting duct principal cells lack subunits of the proton pumping ATPase. J Cell Biol 119: 111–122, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Satlin LM, Matsumoto T, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct. III. Peanut lectin-binding intercalated cells. Am J Physiol Renal Fluid Electrolyte Physiol 262: F199–F208, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Satlin LM, Palmer LG. Apical Na+ conductance in maturing rabbit principal cell. Am J Physiol Renal Fluid Electrolyte Physiol 270: F391–F397, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Satlin LM, Schwartz GJ. Cellular remodeling of HCO3−-secreting cells in rabbit renal collecting duct in response to an acidic environment. J Cell Biol 109: 1279–1288, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Schuster VL, Bonsib SM, Jennings ML. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol Cell Physiol 251: C347–C355, 1986 [DOI] [PubMed] [Google Scholar]
- 59. Schwartz GJ, Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest 75: 1638–1644, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 61. Schwartz GJ, Satlin LM, Bergmann JE. Fluorescent characterization of collecting duct cells: a second H+-secreting type. Am J Physiol Renal Fluid Electrolyte Physiol 255: F1003–F1014, 1988 [DOI] [PubMed] [Google Scholar]
- 62. Silver RB, Breton S, Brown D. Potassium depletion increases proton pump (H+-ATPase) activity in intercalated cells of cortical collecting duct. Am J Physiol Renal Physiol 279: F195–F202, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Silver RB, Frindt G. Functional identification of H-K-ATPase in intercalated cells of cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 264: F259–F266, 1993 [DOI] [PubMed] [Google Scholar]
- 64. Silver RB, Frindt G, Palmer LG. Regulation of principal cell pH by Na/H exchange in rabbit cortical collecting tubule. J Membr Biol 125: 13–24, 1992 [DOI] [PubMed] [Google Scholar]
- 65. Silver RB, Mennitt PA, Satlin LM. Stimulation of apical H-K-ATPase in intercalated cells of cortical collecting duct with chronic metabolic acidosis. Am J Physiol Renal Fluid Electrolyte Physiol 270: F539–F547, 1996 [DOI] [PubMed] [Google Scholar]
- 66. Silver RB, Soleimani M. H+-K+-ATPases: regulation and role in pathophysiological states. Am J Physiol Renal Physiol 276: F799–F811, 1999 [DOI] [PubMed] [Google Scholar]
- 67. Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol 280: F356–F364, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Stetson DL, Steinmetz PR. Role of membrane fusion in CO2 stimulation of proton secretion by turtle bladder. Am J Physiol Cell Physiol 245: C113–C120, 1983 [DOI] [PubMed] [Google Scholar]
- 69. Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218, 1979 [DOI] [PubMed] [Google Scholar]
- 70. Tsuruoka S, Schwartz GJ. Metabolic acidosis stimulates H+ secretion in the rabbit outer medullary collecting duct (inner stripe) of the kidney. J Clin Invest 99: 1420–1431, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van Adelsberg J, Al-Awqati Q. Regulation of cell pH by Ca+2-mediated exocytotic insertion of H+-ATPases. J Cell Biol 102: 1638–1645, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Zeijl MJ, Matlin KS. Microtubule perturbation inhibits intracellular transport of an apical membrane glycoprotein in a substrate-dependent manner in polarized Madin-Darby canine kidney epithelial cells. Cell Regul 1: 921–936, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wei Y, Wang WH. Role of the cytoskeleton in mediating effect of vasopressin and herbimycin A on secretory K channels in CCD. Am J Physiol Renal Physiol 282: F680–F686, 2002 [DOI] [PubMed] [Google Scholar]
- 74. Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Weiner ID, Frank AE, Wingo CS. Apical proton secretion by the inner stripe of the outer medullary collecting duct. Am J Physiol Renal Physiol 276: F606–F613, 1999 [DOI] [PubMed] [Google Scholar]
- 76. Weiner ID, Hamm LL. Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest 85: 274–281, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weiner ID, Hamm LL. Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F957–F964, 1989 [DOI] [PubMed] [Google Scholar]
- 78. Weiner ID, Weill AE, New AR. Distribution of Cl−/HCO3− exchange and intercalated cells in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 267: F952–F964, 1994 [DOI] [PubMed] [Google Scholar]
- 79. Weiner ID, Wingo CS, Hamm LL. Regulation of intracellular pH in two cell populations of inner stripe of rabbit outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F406–F415, 1993 [DOI] [PubMed] [Google Scholar]
- 80. Weinstein AM. A mathematical model of rat collecting duct. I. Flow effects on transport and urinary acidification. Am J Physiol Renal Physiol 283: F1237–F1251, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 82. Zhu CF, Liu Q, Zhang L, Yuan HX, Zhen W, Zhang JS, Chen ZJ, Hall SH, French FS, Zhang YL. RNase9, an androgen-dependent member of the RNase A family, is specifically expressed in the rat epididymis. Biol Reprod 76: 63–73, 2007 [DOI] [PubMed] [Google Scholar]