Abstract
Podocytes respond to environmental cues by remodeling their slit diaphragms and cell-matrix adhesive junctions. Wt1-interacting protein (Wtip), an Ajuba family LIM domain scaffold protein expressed in the podocyte, coordinates cell adhesion changes and transcriptional responses to regulate podocyte phenotypic plasticity. We evaluated effects of Wtip on podocyte cell-cell and cell-matrix contact organization using gain-of- and loss-of-function methods. Endogenous Wtip targeted to focal adhesions in adherent but isolated podocytes and then shifted to adherens junctions after cells made stable, homotypic contacts. Podocytes with Wtip knockdown (shWtip) adhered but failed to spread normally. Noncontacted shWtip podocytes did not assemble actin stress fibers, and their focal adhesions failed to mature. As shWtip podocytes established cell-cell contacts, stable adherens junctions failed to form and F-actin structures were disordered. In shWtip cells, cadherin and β-catenin clustered in irregularly distributed spots that failed to laterally expand. Cell surface biotinylation showed diminished plasma membrane cadherin, β-catenin, and α-catenin in shWtip podocytes, although protein expression was similar in shWtip and control cells. Since normal actin dynamics are required for organization of adherens junctions and focal adhesions, we determined whether Wtip regulates F-actin assembly. Undifferentiated podocytes did not elaborate F-actin stress fibers, but when induced to overexpress WTIP, formed abundant stress fibers, a process blocked by the RhoA inhibitor C3 toxin and a RhoA kinase inhibitor. WTIP directly interacted with Rho guanine nucleotide exchange factor (GEF) 12 (Arhgef12), a RhoA-specific GEF enriched in the glomerulus. In conclusion, stable assembly of podocyte adherens junctions and cell-matrix contacts requires Wtip, a process that may be mediated by spatiotemporal regulation of RhoA activity through appropriate targeting of Arhgef12.
Keywords: G proteins, cytoskeleton, RhoGTPase
podocytes are highly differentiated glomerular epithelial cells, characterized by numerous interdigitating foot processes (FP), which elaborate highly specialized cell-cell contacts known as slit diaphragms (SD). FPs are defined by three membrane domains: the apical membrane domain, the SD protein complex, and the basal membrane domain (19). The submembranous region of all three compartments is connected to the FP actin cytoskeleton (16, 25). Therefore, the actin cytoskeleton plays a crucial role in determining and maintaining the overall structure of the SD. Changes in the actin cytoskeleton from coordinated stress fibers into a dense actin mat are synonymous with podocyte FP effacement and SD disruption (19, 24, 35). Identification of proteins that regulate or stabilize the actin cytoskeleton is important for understanding the maintenance and function of the glomerular filtration barrier (25, 37). Recently, an increasing number of actin-associated proteins in the podocyte have been identified, thus highlighting the significance of dynamic actin cytoskeleton regulation in the maintenance of the glomerular filtration barrier.
The actin cytoskeleton not only provides mechanical support for the cell but also determines cell shape, enables cell movement, and is required for assembly of normal cell-matrix and cell-cell adhesive contacts. Members of the Rho family of small guanosine triphosphatases (RhoGTPases), RhoA, Rac1, and Cdcd42 have emerged as key regulators of the actin cytoskeleton. The activation of RhoA, Rac1, and Cdcd42 leads to assembly of actin stress fibers, protrusive actin-rich lamellipodia, and protrusive actin-rich filopodia, respectively (7, 13, 14). Furthermore, through their interactions with multiple target proteins, they can coordinate other cellular activities, such as gene transcription, with changes in cellular adhesion. Recent observations also suggest that subcellular pools of RhoGTPases operate to regulate specific morphogenic events in different cellular domains (29).
Focal adhesions are specialized regions of cell adhesion to the extracellular matrix, where integrin receptors associate with a number of structural and signaling proteins to form a link with the actin cytoskeleton (4). These integrin-associated proteins include focal adhesion kinase (FAK), Src family kinases, and scaffolding proteins, such as paxillin and vinculin (15, 27). The importance of these molecules has been underscored by the results of gene knockout and knockdown experiments (12, 22, 42). The activated signaling molecules play crucial roles in regulating multiple events, including cell adhesion and cell migration. Like cell-matrix adhesions, cell-cell adhesions are intimately associated both physically and functionally with the actin cytoskeleton. Cadherins, which are cell-cell adhesion receptors, associate with the actin cytoskeleton with β-catenin and α-catenin. Following cadherin engagement, RhoGTPases are recruited, activated, and regulate the actin cytoskeleton necessary for the formation of stable cell-cell adhesions (44). Formation of cell-cell adhesions requires coordination between assembly and disassembly of cell-matrix adhesions (10, 43).
Wt1-interacting protein (Wtip) belongs to a subset of LIM-domain containing proteins that include the prototypic members zyxin, Ajuba, and lipoma-preferred partner (LPP) (18, 30, 38, 45), all of which localize to focal adhesions and cell-cell adhesions. All members of this family contain two distinct regions: a C-terminal domain containing three LIM protein-protein interaction motifs and a proline-rich N-terminal domain containing one or two nuclear export sequences (5). Although we had localized WTIP to cell-cell contacts and regions of active actin dynamics in podocytes, the functional impact of WTIP on the assembly of cell-matrix and cell-cell contacts has been unclear. Using gain-of-function and RNA interference (RNAi)-mediated gene knockdown in mouse podocytes, we examined the roles of Wtip in actin dynamics, RhoA GTPase activity, and podocyte cell contact formation. We found Wtip targets to focal adhesions in adherent but isolated podocytes and then shifts to podocyte adherens junctions after cells make homotypic contacts, a process dependent on RhoA-regulated F-actin dynamics.
EXPERIMENTAL PROCEDURES
Antibodies and plasmids.
An affinity-purified anti-WTIP antibody was generated and characterized as described previously (20). Other antibodies used in this study included anti-α-tubulin, anti-vinculin, and anti-pan-cadherin (Sigma-Aldrich, St. Louis, MO); anti-paxillin, anti-α-catenin, anti-β-catenin (BD Transduction, San Diego, CA); anti-phosphotyrosine (PY99), anti-myc (Upstate Biotechnology); anti-FAK (Biosource, Camarillo, CA); anti-Rho-GDI (Santa Cruz Biotechnology, Santa Cruz, CA); anti-green fluorescent protein (GFP; monoclonal and polyclonal; Clontech, Mountain View, CA); and anti-zonula occludens (ZO-1; Chemicon International, Temecula, CA). For visualization of F-actin, rhodamine-phalloidin was purchased from Molecular Probes (Carlsbad, CA). The expression vectors, pcDNA3-EGFP-RhoAQ63L (Addgene plasmid 12968) and pcDNA3-EGFP-RhoAT19N (Addgene plasmid 12967), were purchased from Addgene (Cambridge, MA). The plasmid, pT-Adv Rho guanine nucleotide exchange factor (GEF) 12 (Arhgef12; mLARG), was kindly provided by Dr. Alexander Belyavsky (Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia). The N-terminal coding sequence (aa 1–415, containing the PDZ domain) was cloned into pEGFPN2 (Clontech) to produce the expression construct, NT-Arhgef12-GFP. The expression plasmid, myc-WTIP, has been previously described (38).
Cell culture and cell lines.
A conditionally immortalized podocyte cell line (MPC) was a generous gift of Dr. Peter Mundel (Massachusetts General Hospital, Boston, MA) (23). Cells were maintained in RPMI 1640 medium (Cambrex, Walkersville, MD) supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin. Under permissive conditions, podocytes were maintained in 5% CO2 and at 33°C in culture medium supplemented with 10 U/ml mouse recombinant γ-interferon (Sigma) to enhance the expression of the SV40 Large T-antigen. To induce differentiation, we plated podocytes on type I collagen in 5% CO2 and 37°C without γ-interferon (nonpermissive conditions) for at least 10 days. Podocytes (between passages 10 and 25) used in these studies expressed the transcription factor WT1. Before experiments, expression of the podocyte differentiation marker synaptopodin was confirmed by immunofluorescence analysis in a parallel well of podocytes. Unless otherwise indicated, all experiments were carried out in differentiated podocytes. Mouse podocytes were transfected with the indicated expression vectors using Fugene 6 reagent (Roche Diagnostics, Chicago, IL) following the manufacturer's protocol. Podocyte clones transfected with pEGFP-WTIP (see below) were selected in G418 (200 μg/ml), and GFP-WTIP expression was documented by Western blotting and fluorescence microscopy. Conditionally immortalized mouse podocytes, stably expressing either a control vector (shEMP) or mouse shWtip, were generated as previously described (20). pLKO.1-TRCWTIP1 targets mouse Wtip [NM_207212] nt 1471–1491(TRCN0000095769): 5′-CCGGCCCGCAACAAAGAAGC GATTTCTCGAGAAATCGCTTCTTTGTTGCGGGTTTTTG-3′; or pLKO.1-TRC WTIP2 targets mouse Wtip [NM_207212] nt 766–786 (TRCN0000095770): 5′-CCGGCGCGAGACTACT TTGGCATTTCTCGAGAAATGCCAAAGTAGTCTCGCGTTTTTG-3′. Human podocytes stably expressing tetracycline (TCN)-inducible human WTIP epitope-tagged with V5 (GEC-WTIP-V5) were generated using a ViraPower T-Rex lentiviral expression system and Gateway Technology vectors as previously described (20), according to the manufacturer's protocols (Invitrogen).
Transient cell transfection.
Mouse podocytes were transiently transfected with pcDNA3-EGFP-RhoAQ63L or pcDNA3-EGFP-RhoAT19N using Fugene 6 reagent (Roche) using the manufacturer's protocol. After 48–72 h, cells expressing the constructs were identified using fluorescence microscopy. To assess the interaction between Rho guanine nucleotide exchange factor 12 (ARHGEF12 or LARG) and WTIP, COS cells [American Type Culture Collection (ATCC), Manassas, VA] or 293 FT cells (Invitrogen) were transfected with the indicated constructs and processed for immunoprecipitation or immunofluorescence microscopy 48–72 h later.
RNA isolation and RT-PCR.
Total RNA was prepared from undifferentiated control and knockdown cell lines, as indicated, using an RNeasy Mini Kit (Qiagen, Valencia, CA). On-column DNAse digestion was performed according to the manufacturer's protocol. Briefly, 1 μg of total RNA was reverse transcribed using random hexamers and SuperScript III First-Strand Synthesis Super Mix (Invitrogen, Carlsbad, CA). RNA was incubated with annealing buffer and random hexamers at 65°C for 5 min, chilled, and incubated with First-Strand Reaction Mix and SuperScript III/ RNaseOUT Enzyme Mix for 10 min at room temperature, followed by 50 min at 50°C. The reaction was terminated by heating at 85°C for 5 min. Sample cDNA (2 μl) was used to perform semiquantitative PCR for Wtip using the GC-RICH PCR System (Roche) under the following conditions: 95°C for 3 min; 95°C for 30 s, 59°C for 30 s, 72°C for 1 min (23 cycles); and 72°C for 5 min, 4°C hold. PCR products were visualized in 1% TBE agarose gels. Primer pairs for Wtip were forward 5′-GAGCCTGCCCAGTTCCCTTCC-3′ and reverse 5′-AGCAGCGGAAGCAGCCTGGGTGGTAG-3′. To amplify Limd1 mRNA, sample cDNA (2.0 μl) was annealed at 60°C with the following primer pairs: forward 5′-ACCCCACCCAGCATTGAAGAACAT-3′ and reverse 5′-GGCCAAAGGATCCCAACAGAAGG-3′. To amplify Gapdh mRNA (as a loading control), sample cDNA (1.2 μl) was annealed at 57°C with the following primer pairs: forward 5′-GGAGCCAAACGGGTCATC-3′ and reverse 5′-TGTTGCTGTAGCCGTATTCAT-3′. To assess podocyte Arhgef12 message expression, cDNA (2 μl) was used for PCR with HotStarTaq (Qiagen) as follows: 95°C for 15 min, 95°C for 30 s, touchdown annealing (30 s) from 72 to 57.5°C, and extension at 72°C for 1 min (24 cycles), followed by annealing at 58°C and amplification at 72°C for 1 min (28 cycles). Primer pairs for Arhgef12 PDZ N-terminal were forward 5′ TCAAAGAAGATGGAGCAGCCATGC-3′ and reverse 5′-TCTTTGGGTAGCCGTTCGGTTGTA-3′. Primer pairs for the internal Arhgef12 coding sequence were forward 5′-AACCAACCTTTCGCCCTGGAAATC-3′ and reverse 5′-TTGAGATTGGAGGTGTCAAGGCGA-3′.
Recombinant adenovirus generation and infection.
Using PCR, we constructed a GFP-WTIP expression plasmid by cloning the human WTIP coding domain cDNA into pEGFP-C2 (BD Biosciences, Palo Alto, CA). pEGFP-WTIP sequence fidelity and reading frame were confirmed by sequencing. The GFP-WTIP fusion gene was amplified by PCR from pEGFP-WTIP and subcloned into pShuttle-CMV, an AdEasy transfer plasmid for recombinant adenovirus construction. A recombinant transfer vector was linearized and cotransformed with pAdEasy-1 DNA into BJ5183 according to the manufacturer's instructions. Bacteria were selected on LB plates containing kanamycin. Plasmids were amplified, purified (Qiagen, Valencia, CA), linearized, and transfected into 293 cells (ATCC) for viral particle generation. Recombinant viral particles were then amplified and purified using the Adeno-X virus purification kit (BD Biosciences) and titered. Infecting podocytes with 200–300 plaque-forming units/cell was sufficient to achieve uniform WTIP expression.
Immunofluorescence microscopy and quantification.
Cells, cultured on sterile glass coverslips (collagen type I-coated for podocytes), were washed in Dulbecco's PBS, fixed in paraformaldehyde (4%, 10 min at room temperature), and permeabilized with 0.2% Triton X-100 in Dulbecco's PBS for 5 min on ice. After blocking in 10% goat serum with 2% BSA and 0.2% fish gelatin, cells were incubated with primary antibodies in PBS either at 37°C for 1 h in a humidified chamber or at 4°C overnight. Subsequently, coverslips were washed and incubated with secondary antibody at dilutions ranging from 1:200 to 1:300 for 1.5 h at room temperature. Secondary antibodies included fluorescein isothiocyanate-conjugated horse anti-mouse or Texas red-conjugated goat anti-rabbit antibodies (Vector Laboratories, Burlingame, CA). Coverslips were mounted in antifade, aqueous medium containing 4′,6 diamidino-2-phenylindole (Vectashield with DAPI; Vector Laboratories) on standard glass slides. For visualization of F-actin, rhodamine-phalloidin was used following the manufacturer's protocol (Molecular Probes). Antibody staining was visualized using a Nikon epifluorescence E600 microscope, and photographs were taken with a SPOT Digital System camera (model 2.3.0). Confocal images were obtained with a Leica TCS SP2 Confocal system. Digital images were processed and grouped using Adobe Photoshop v6.0 (Adobe Systems, San Jose, CA).
Leica Quantify software was used to measure the lengths of the focal adhesions. For each experimental condition, the lengths (in pixels) of focal adhesions from 150 cells were measured. The average length was determined and then used to generate a normalized value of mean pixel intensity for focal adhesions. Data are means ± SE. Unpaired t-tests were conducted to determine significance (asterisk; P < 0.05).
To quantify adherens junction assembly, we used published definitions of sequential stages in cell-cell adhesion, which characterize the transitions in cadherin and actin localization as adherens junctions mature (1). ImageJ software was used to measure the mean pixel intensity of cadherin clusters [stage 1 junctions (1)] in forming junctions, and colocalization of cadherin at actin tips [stage 2 junctions (1)] was quantified as yellow pixel intensity from cadherin (FITC) and rhodamine actin overlays. For each stage of cell-cell adhesion, a fixed region of interest was used to measure the mean pixel intensity from 100 different cells. The area of the region of interest was determined and then used to generate a normalized value of mean pixel intensity for cadherin clustering and colocalization with actin. Data are means ± SE. Unpaired t-tests were conducted to determine significance (asterisk; P < 0.05).
Time-lapse imaging of live cells and F-actin quantification.
For visualizing the EGFP-actin in living cells, 24 h after the control and Wtip knockdown cells were plated, pEGFP-actin was transiently transfected using Fugene 6 (Roche). After incubation for 48 h, cells were observed under the indicated conditions using live cell imaging parameters on the Leica TCS SP2 Confocal system. ImageJ software was used to create a Z-projection stack of the time-lapse images for GFP-actin dynamics. Mean actin intensity was determined using the line scan measure function across the nucleus of 20 individual cells in 4 separate experiments. Data are means ± SE. Unpaired t-tests were conducted to determine significance (asterisk; P < 0.05).For F-actin quantification from static images, the ImageJ line scan function was also used as described.
Coprecipitation and immunoblotting.
Proteins were extracted from transfected and stable cells using immunoprecipitation (IP) lysis buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris·HCl, 0.5% deoxycholate, 1 mM sodium orthovanadate, along with protease inhibitors and analyzed by immunoprecipitation and immunoblotting as we have previously described (31, 38). Following centrifugation to remove debris, supernatants were matched for protein, precleared with protein G-Sepharose GammaBind beads, and incubated overnight at 4°C with primary antibody. The following day, 50 μl of GammaBind beads were added and incubated for 1 h. Control experiments were done in parallel at the same time using appropriate nonimmune IgG. For GFP-tagged protein only, GFP was expressed and precipitated to confirm specificity. Beads were collected by low-speed centrifugation in microcentrifuge tubes for 2–3 min and washed three times using IP lysis buffer. Bound proteins were released by boiling in 2× SDS sample buffer for 5 min. Eluted proteins were separated by 4–20% SDS-PAGE, transferred to Immobilon (Millipore, Billerica, MA) membranes, and analyzed by immunoblotting. Bound antibody was detected by chemiluminescence (Western Lightning; PerkinElmer Life Sciences).
RhoA activity assay.
RhoA activity was determined using a configuration-specific, monoclonal antibody-based RhoA Activation Assay Kit (New East Biosciences, Malvern, PA) following the manufacturer's protocols. Briefly, GEC-WTIP-V5 cells were grown to ∼80–90% confluence and then stimulated in the presence or absence of TCN. Culture media was aspirated and washed twice with ice-cold PBS. One milliliter of ice-cold 1× assay/lysis buffer was added to the cells and placed on ice for 10–20 min. Cells were scraped and collected, and lysates were cleared by centrifugation for 10 min (12,000 g at 4°C) and placed on ice. Aliquots of 0.5–1.0 ml of cell lysates were adjusted to 1 ml with 1× assay/lysis buffer. One microliter of anti-active RhoA monoclonal antibody was added to each sample, followed by 20 μl of resuspended protein A/G agarose bead slurry. Samples were incubated at 4°C for 1 h with gentle agitation. Beads were pelleted by centrifugation for 1 min at 5,000 g. The supernatant was discarded, and the beads were washed three times with 0.5 ml of 1× assay/lysis buffer, centrifuging and aspirating the supernatant each time. After the last wash, the beads were pelleted and the supernatant was removed. Beads were resuspended in 20 μl of 2× reducing SDS-PAGE sample buffer. Each sample was boiled for 5 min and centrifuged for 10 s at 5,000 g.
Cell surface biotinylation assay.
Cell surface proteins were biotinylated by incubating the cells with 1.5 mg/ml sulfo-NHS-SS-biotin (Thermo Scientific, Rockford, IL) for 1 h at 4°C, and free biotin was quenched with a blocking solution (50 nM NH4Cl in PBS containing 1 mM MgCl2 and 0.1 mM CaCl2). Cells were then either directly extracted in a RIPA buffer or stripped to remove the extracellular bound biotin with 50 mM glutathione, 75 mM MaCl2, 75 mM NaOH, and 2% bovine serum albumin, at 4°C, and RIPA extracted. Cell extracts were centrifuged and incubated with streptavidin magnetic beads (Dynal, Olson, Norway) to collect biotinylated proteins. Extracted proteins were then separated by SDS-PAGE and analyzed by immunoblotting using antibodies against pan-cadherin, β-catenin, and α-catenin. Total cell lysates were analyzed by immunoblotting to determine the expression levels of these proteins in podocytes expressing the control and Wtip shRNA vectors.
Statistical analysis.
The data from all of the experimental groups are expressed as means ± SE. An unpaired Student's t-test was used to compare differences between control and experimental groups. Statistical significance was defined as P < 0.05.
RESULTS
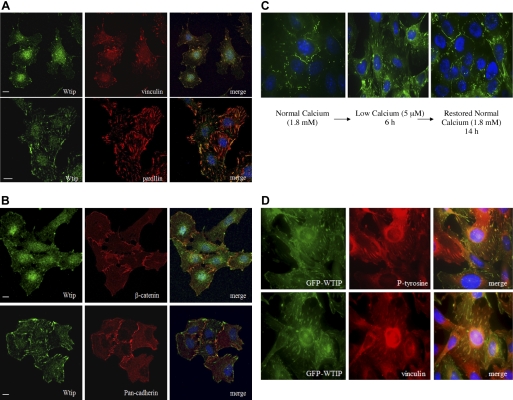
Endogenous Wtip localized to focal adhesions and cell-cell adhesions.
We had previously shown that ectopically expressed WTIP localizes with cell-cell and cell-matrix contacts. To examine localization of endogenous Wtip in mouse podocytes, we used an affinity-purified antibody, which we have characterized (20). As shown in Fig. 1A, Wtip colocalized with vinculin and paxillin at focal adhesions in cultured, differentiated podocytes before the establishment of cell-cell contacts. After cell contacts were established, Wtip colocalized with both β-catenin and cadherin, detected with a pan-cadherin antibody, at cell-cell contact sites (Fig. 1B). In addition, a pool of endogenous Wtip localized to the nucleus in some cells, which is consistent with our previous studies that demonstrated, via cellular fractionation, that ectopically expressed human WTIP was dynamically regulated and shifted between plasma membrane, cytosol, and the nucleus (20) in response to environmental cues. These results indicate that Wtip localized specifically at cell-matrix and cell-cell adhesion sites and suggested dynamic remodeling of cell-cell junctions regulated the intracellular localization of Wtip, consistent with a role for Wtip in plasticity of podocyte phenotype. In addition, intracellular localization of ectopically expressed WTIP recapitulated patterns of the endogenous protein.
Fig. 1.
Endogenous WT1-interacting protein (Wtip) was localized to focal adhesions and cell-cell contacts in podocytes and is dynamically regulated by cell-cell adhesion. A: immunostaining with anti-Wtip (FITC), anti-vinculin, and anti-paxillin (TRITC). B: immunostaining for adherens junction proteins with anti-β-catenin and anti-pan-cadherin (TRITC). Nuclei were labeled with CY5-TOPRO-3. C: calcium switch assay demonstrated green fluorescent protein (GFP)-WTIP localization at cell-cell junctions in medium with normal calcium (NC) concentration (1.8 mM). With disruption of adhesion junctions in low-calcium (LC) medium (5 μM), GFP-WTIP localized in patches resembling focal adhesions (middle). GFP-WTIP retargeted to adherens junctions (left). D: in LC medium, GFP-WTIP colocalized with the focal adhesion markers phosphotyrosine and vinculin. Scale bars = 20 μm.
Redistribution of WTIP to focal adhesions upon disruption of adherens junction in low-calcium media.
Since Wtip localization was regulated by the state of cell-cell contact, we next studied the effect of dynamic remodeling of adherens junctions on Wtip localization using a calcium-switch assay in podocytes expressing GFP-WTIP delivered by a recombinant adenovirus. WTIP was localized to cell-cell contacts in the presence of normal calcium (1.8 mM; Fig. 1C, left). After the cells were incubated in low-calcium (5 μM) media, WTIP localized at focal adhesion-like structures as early as 2 h (not shown) and persisted there for 6 h (Fig. 1C, middle). Furthermore, when podocytes were reincubated in normal calcium medium, WTIP again targeted to cell-cell contacts (Fig. 1C, right). Previous studies have shown that vinculin redistributes similarly in a calcium-switch assay (28). Therefore, to confirm that WTIP localized to focal adhesions in the absence of stable adherens junctions, we stained podocytes in low-calcium medium with antibodies that recognize the focal adhesion markers phosphotyrosine (P-tyrosine) and vinculin. GFP-WTIP was colocalized with both P-tyrosine and vinculin at the focal adhesions (Fig. 1D).
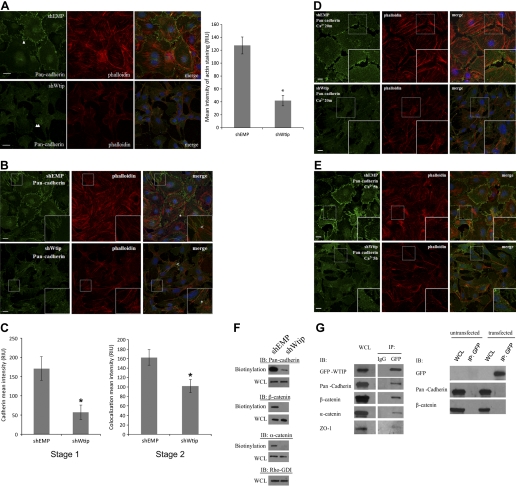
Depletion of Wtip abrogated the assembly of actin stress fibers and maturation of focal adhesions.
We next examined the effect of Wtip depletion on focal adhesion complex formation in mouse podocytes with stable knockdown of Wtip (shWtip) and cells expressing a control construct (shEMP), which have been previously characterized (20). Staining with rhodamine-phalloidin revealed that a majority of Wtip knockdown cells failed to elaborate actin stress fibers (Fig. 2A). Because focal adhesions are required for the formation of actin stress fibers and because Wtip was localized at focal adhesions before generation of stable adherens junctions, we next examined whether maturation of focal adhesions was altered after Wtip knockdown. Normally, focal complexes containing paxillin and FAK are formed at the leading edge of an extended lamellipodial protrusion and cell periphery. These nascent focal complexes then grow to mature focal adhesions as other focal adhesion proteins, such as vinculin, are recruited. To assess the state of focal complex maturation, shEMP and Wtip knockdown cells were fixed and stained for vinculin, paxillin, and tyrosine phosphorylation. Individual vinculin signals in Wtip knockdown cells were significantly smaller at the cell periphery compared with shEMP podocytes, as assessed by quantification of relative mean intensity (Fig. 2, B and C, P < 0.05). Immunostaining for paxillin revealed no significant difference in signal of relative mean intensity between shEMP and Wtip knockdown. However, the localization of paxillin appeared to be more disorganized in Wtip knockdown cells (Fig. 2D). Immunostaining for tyrosine phosphorylation (anti-pY99) also revealed a decrease in overall tyrosine phosphorylation in Wtip knockdown compared with shEMP cells, consistent with failure of focal complex maturation into focal adhesions (Fig. 2E). The knockdown of Wtip was specific, as assessed by RT-PCR. ShWtip podocytes did not express Wtip transcripts, but mRNA abundance of Limd1, a closely related LIM domain protein family member, and Gapdh was similar in shEMP and Wtip knockdown cells by RT-PCR (Fig. 2F). In addition, focal contact maturation failure was not caused by Wtip shRNA off-target effects. Expression of the focal adhesion proteins paxillin, vinculin, and FAK was equivalent between control and Wtip knockdown cells (Fig. 2G). These data suggested recruitment and accumulation of Wtip was not essential for assembly of nascent focal complexes but was necessary for maturation of focal complexes into focal adhesions and actin stress fiber formation in podocytes.
Fig. 2.
Wtip knockdown impaired stress fiber formation and dysregulated focal adhesion maturation. A: actin stress fibers were visualized by rhodamine-phalloidin staining in control vector (shEMP; top) and in shWtip (bottom) and costained with anti-WTIP (FITC). B: focal adhesions were immunostained with anti-vinculin (TRITC) in shEMP and shWtip. C: focal adhesion maturation was assessed by anti-vinculin immunostaining in shEMP and shWtip cells. Quantification of focal adhesion size was determined as described in experimental procedures. The bar graph shows mean relative intensity units for vinculin (right). D: shEMP and shWtip cells were immunostained with anti-paxillin, a marker of focal contacts. The bar graph shows mean relative intensity units for paxillin (right). E: phosphotyrosine levels of focal adhesions in shEMP and shWtip cells were assessed by immunostaining with PY99 as an indicator of focal adhesion maturation. The bar graph shows mean relative intensity units for phosphotyrosine (right). F: RT-PCR of shEMP and shWtip cells for Wtip, Limd1, and Gapdh transcripts. G: immunoblot analysis of shEMP (control) and shWtip (knockdown) podocytes for Wtip, paxillin, vinculin, focal adhesion kinase (FAK), and tubulin. Leica Quantify software was used for quantification. Scale bars = 20 μm. *P < 0.05.
Wtip is involved in the formation of cell-cell adhesions.
As shown in Fig. 1B, Wtip also localized to cell-cell adhesion sites after stable cell-cell contacts were formed, suggesting Wtip depletion would cause defects in the formation of adherens junctions. Compared with shEMP cells, Wtip knockdown podocytes displayed a marked inability to form cadherin-based, homotypic cell-cell adhesions (Fig. 3A, double arrowheads) and stress fibers (Fig. 3A, histogram, P < 0.05). Instead of forming adhesions with neighboring attached cells, Wtip knockdown podocytes appeared to extend lamellipodia and filopodia, which extended under or over neighboring cells. Further analysis of adherens junction formation revealed that parallel lines of actin filaments were assembled continuously between neighboring cells in shEMP cells, indicative of proper adherens junction formation (Fig. 3B, top). By contrast, actin filaments between neighboring Wtip knockdown cells were disordered and tangled (Fig. 3B, bottom). These results suggested that perturbed actin dynamics at cell-cell contact sites in Wtip knockdown podocytes impaired normal adherens junction assembly. To quantitate differences in cell-cell adhesion between Wtip and control shRNA podocytes, we used a previously published method that characterized changes in cadherin and actin cytoskeleton organization (1). Initial junction formation was categorized into two stages: clustering of cadherin puncta along the length of the forming contact (stage 1) and maturation of the cadherin puncta into plaques at the edges of the contact at actin tips (stage 2). Based on relative fluorescence intensity, a statistically significant difference between shWtip and shEMP podocytes was identified in formation of both stage 1 (cadherin puncta, Fig. 3B, asterisks) and stage 2 (colocalization of cadherin at actin tips, Fig. 3B, carrots) junction formation in Wtip knockdown cells compared with control cells (Fig. 3C, P < 0.05). These results suggest that Wtip depletion perturbs actin filament organization at cell-cell contact sites, preventing normal assembly of cadherin-based adherens junctions between neighboring podocytes.
Fig. 3.
Knockdown of Wtip affects proper cadherin junction assembly and targeting. shWtip and shEMP cells were stained with rhodamine-phalloidin and anti-pan-cadherin (A and B). Nuclei were labeled with CY5-TOPRO-3. Images in A show robust cadherin-based cell-cell contacts in shEMP podocytes (arrowhead) but not in shWtip cells (double arrowheads). The histograms quantify actin stress fiber abundance as described in experimental procedures. C: quantification of cell-cell adhesion formation in shEMP and Wtip knockdown cells as described in experimental procedures and results. shWtip podocytes are shown 20 min (D) and 5 h (E) after the switch from low to normal calcium. F: biotinylation demonstrated decreased plasma membrane localization of adherens junction proteins cadherin, β-catenin, and α-catenin to the membrane in shWtip vs. shEMP podocytes. Immunoblot (IB) analysis of whole cell lysates showed equal expression of cadherin, β-catenin, α-catenin, and RhoGDI. WCL, whole cell lysates. G: GFP-WTIP formed a complex with the indicated adherens junction proteins (left), but GFP failed to precipitate these proteins (right). Scale bars = 10 μm. *P < 0.05.
To further examine the effects of Wtip depletion on cell-cell contact formation, we induced formation of cell adhesions in control and Wtip-depleted cells by changing the Ca2+ concentration in the culture medium from low calcium to normal calcium. Cells were fixed and stained with rhodamine-phalloidin and anti-pan-cadherin antibody at 20 min and 5 h after calcium addition, when cell-cell adhesions were initially reforming and were completely assembled, respectively. In shEMP cells, actin filaments longitudinally ramified from cell-cell contacts (Fig. 3, D and E, top). In contrast, cadherin-based cell-cell contacts were infrequent in Wtip-depleted cells (Fig. 3, D and E, bottom) and the tips of F-actin filaments were not anchored by cadherin puncta. These results show that Wtip plays a role in the control of actin filament organization upon cell-cell contact and, therefore, in the formation of cadherin mediated cell-cell adhesions.
In shEMP podocytes, cadherin was detected primarily at the cell-cell contacts (Fig. 3B, top inserts). In contrast, in Wtip-depleted cells, we observed retention of cadherin in intracellular compartments and only weak staining at the plasma membrane (Fig. 3B, bottom inserts), so we next analyzed both total and cell surface-biotinylated cadherin. Whole cell lysate protein was precipitated with streptavidin beads, and precipitates were immunoblotted with pan-cadherin, α-catenin, and β-catenin antibodies. Cadherin, α-catenin, β-catenin, and RhoGDI abundance in the whole cell lysates was similar in both shEMP and Wtip knockdown cells (Fig. 3F, WCL). However, levels of cadherin, α-catenin, and β-catenin were decreased in the streptavidin-precipitated fraction in Wtip knockdown cells compared with shEMP podocytes (Fig. 3F). Wtip appeared necessary for recruitment, assembly, or retention of the adherens junction complex in the plasma membrane, suggesting a mechanism by which Wtip depletion interferes with formation of stable cell-cell contacts.
WTIP coprecipitated with the adherens junction proteins cadherin, β-catenin, and α-catenin.
Using podocytes stably expressing GFP-WTIP, we next tested whether WTIP interacts with junctional proteins or simply codistributes with them. Anti-GFP immunoprecipitates from GFP-WTIP-expressing podocytes were immunoblotted with anti-pan-cadherin antibody (recognizes N-, E-, P-, and R-cadherins) or antibodies against Wtip, ZO-1, α-catenin, or β-catenin. Cadherin, α-catenin, and β-catenin (Fig. 3G, left) specifically coprecipitated with GFP-WTIP, but we were unable to detect substantial amounts of ZO-1 in these immunoprecipitates. No adherens junction proteins coprecipitated with GFP (Fig. 3G, right).
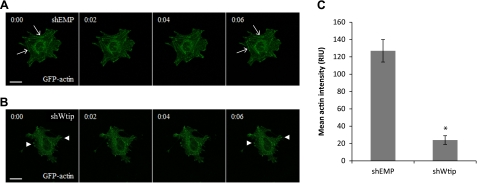
Wtip-depleted cells exhibit aberrant actin cytoskeleton dynamics.
F-actin structures were disordered in shWtip podocytes. Since normal actin dynamics are necessary for formation of both adherens junctions and focal adhesion complexes, we hypothesized that Wtip may directly or indirectly regulate F-actin dynamics. Although LIM domain proteins can directly interact with actin, we were unable to show that Wtip and actin physically interacted (Kim JH and Sedor JR, unpublished observations). Using time-lapse microscopy, we examined the dynamics of actin organization in shWtip and control podocytes transfected with an EGFP-actin expression vector. GFP-labeled actin stress fiber formed and persisted in shEMP cells (Fig. 4A, arrows), while actin stress fibers only rarely formed in shWtip cells during the observation period (up to 14 min) (Fig. 4B, arrowheads). Quantification of mean actin intensity in a Z-axis projection of the image stack was statistically less in shWtip vs. shEMP podocytes, consistent with failure to assemble F-actin (Fig. 4C, P < 0.05). In addition, Wtip knockdown cells exhibited reoccurring clusters of polymerized actin (arrowheads), which was not observed in control cells (arrows), suggesting a global perturbation of actin dynamics. Overall, Wtip knockdown cells had more membrane protrusions and filopodia than control cells. These data suggest that Wtip is not only required for the proper formation of actin stress fibers but that it also plays a broader role in the regulation of the actin cytoskeleton.
Fig. 4.
Wtip knockdown affects dynamic actin assembly. Time-lapse images of shEMP (A) and shWtip cells (B) transiently transfected with eGFP-actin (green) are shown. Arrows indicate stress fiber formation in shEMP cells, whereas arrowheads indicate actin clusters and the lack of actin stress fiber formation in shWtip cells. C: quantification of stress fiber formation in shEMP vs. shWtip cells (*P < 0.05). Scale bars = 20 μm.
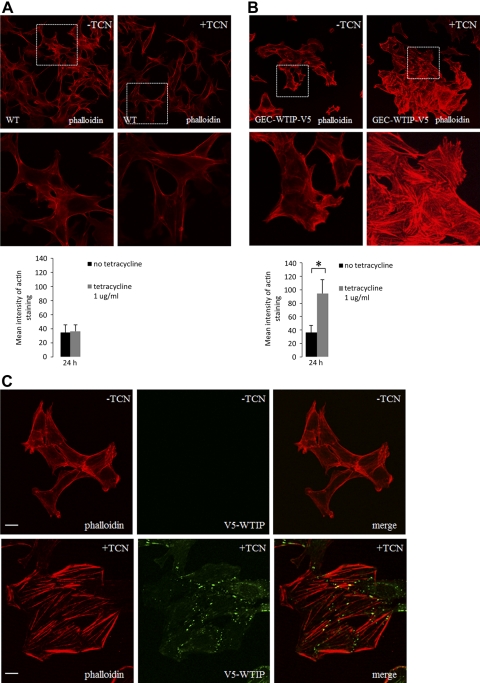
WTIP overexpression enhances formation of actin stress fibers.
The mammalian Ajuba family of LIM proteins, which include Ajuba and Limd1 as well as Wtip, are adaptor proteins within multiprotein complexes that connect cell-cell and cell-matrix contact proteins to the cytoskeleton. Given the critical role of actin dynamics in regulating podocyte function and phenotype, we next examined whether Wtip influenced F-actin cytoskeleton assembly using rhodamine-phalloidin to label actin. Differentiated podocytes express F-actin stress fibers. However, undifferentiated podocytes characteristically lack stress fiber formation. We assayed F-actin stress fiber assembly state in an undifferentiated human podocyte cell line stably transfected with a TCN-regulated V5-tagged WTIP transgene (GEC-WTIP-V5). Mean actin staining was calculated with the Leica Quantify software analysis program using a single line scan across the diameter of each individual cell (100 cells evaluated in each experiment; n = 3 separate experiments). Control, undifferentiated podocytes incubated in the presence or absence of TCN demonstrated no difference in actin stress fiber assembly, and mean intensity of actin fluorescence was similar in TCN-treated and untreated cells (Fig. 5A). When undifferentiated GEC-WTIP-V5 cells were incubated with TCN to induce WTIP-V5 overexpression, F-actin stress fibers robustly assembled (Fig. 5B) compared with untreated GEC-WTIP-V5. The bar graphs depict quantification of the actin stress fiber formation in GEC-WTIP-V5 with or without incubation with TCN (Fig. 5B; P ≤ 0.01, TCN-treated GEC-WTIP-V5 compared with untreated GEC-WTIP-V5 cells). In the absence of TCN, podocytes did not express WTIP-V5 and no stress fibers were observed (Fig. 5C).
Fig. 5.
Overexpression of GFP-WTIP enhances formation of actin stress fibers. A: F-actin assembly compared in control podocytes with and without tetracycline (TCN). Bar graph demonstrates mean actin staining using a single line scan across the nuclei of each (100 cells; n = 3 experiments). B: F-actin assembly in GEC-WTIP-V5 +/− TCN and quantified in the bar graph (*P ≤ 0.01, GEC-WTIP-V5 with TCN vs. no TCN). C: GEC-WTIP-V5 +/− TCN-assessed F-actin using rhodamine-phalloidin (left) and WTIP-V5 expression (FITC, middle). Merged images are shown on the right. Scale bars = 20 μm.
WTIP stress fiber formation was regulated through RhoAGTPase-dependent pathways.
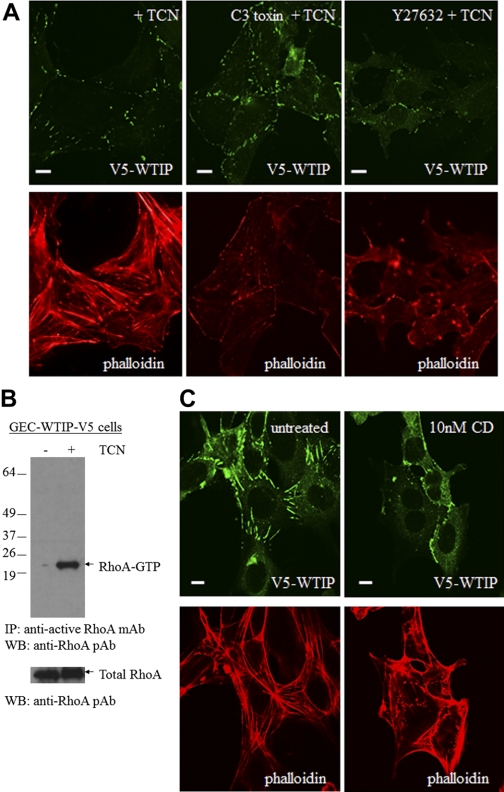
Downstream effectors of the RhoA GTPase include RhoA-associated coiled-coil kinase (ROCK), a kinase recruited to the plasma membrane by active RhoA and required for stress fiber formation (2). To determine whether F-actin stress fiber formation was under the regulation of the traditional RhoA effector pathways, undifferentiated GEC-WTIP-V5 podocytes were preincubated with a cell-permeable C3 toxin, a known inhibitor of RhoA, and then stimulated with TCN to induce WTIP-V5 expression. C3 toxin prevented actin stress fiber formation in podocytes overexpressing WTIP-V5 compared with cells not preincubated with C3 toxin (Fig. 6A, middle). In addition, the activation of ROCK is known to modulate the organization of the actin-based cytoskeletal systems, including the formation of stress fibers (34). GEC-WTIP-V5 treated with a ROCK inhibitor, Y27632, also failed to form actin stress fibers (Fig. 6A, right). Finally, RhoA activity was assessed by immunoblotting using a configuration-specific, monoclonal antibody-based RhoA activity assay in the GEC-WTIP-V5 in the presence or absence of TCN. In GEC-WTIP-V5 stimulated with TCN, RhoA activity was increased compared with control podocytes (Fig. 6B). These data link WTIP-V5 induction with the activation of the RhoA pathway and stress fiber assembly, identifying WTIP as a molecular player upstream of the Rho signaling pathway.
Fig. 6.
WTIP stress fiber formation was regulated through RhoA-dependent pathways. A: GEC-WTIP-V5 were preincubated with either a cell-permeable RhoA inhibitor, C3 toxin (top middle) or a Rho kinase inhibitor, Y27632 (top right), or were untreated (control) for 3 h followed by incubation with TCN for 24 h. B: RhoA activation assay in GEC-WTIP-V5 cells +/− TCN. C: low-dose cytochalasin D (CD; 10 nM) pretreatment of GEC-WTIP-V5 for 20 min followed by addition of TCN. Scale bars = 10 μm. IP, immunoprecipitated; WB, Western blotting.
We next determined whether Wtip promoted stress fiber formation by adding monomeric actin to barbed ends of F-actin. GEC-WTIP-G5 were either preincubated with low-dose cytochalasin D (CD; 10 nM) for 1 h or were untreated, then exposed to TCN for 24 h and fixed and processed for WTIP-V5 expression and F-actin formation. This concentration of CD inhibits membrane ruffling and prevents F-actin elongation by binding to the barbed end of the filament and preventing monomeric actin addition (8). Low-dose CD pretreatment dramatically decreased F-actin stress fiber formation compared with untreated control after WTIP induction (Fig. 6C). As a result, we believe that WTIP may promote addition of monomeric actin to the barbed ends of growing F-actin filaments, a pathway regulated by RhoA.
Wtip and RhoA expression in shWtip podocytes reestablished F-actin stress fibers.
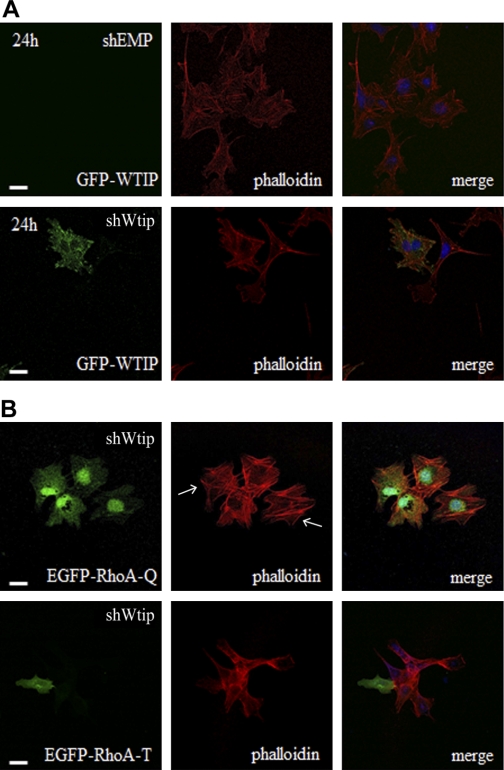
Wtip knockdown cells showed inefficient formation of focal adhesions and reduced formation of actin stress fibers. To assess whether altered actin dynamics in shWtip podocytes was specific to Wtip knockdown or resulted from off-target effects, we next determined whether transient overexpression of EGFP-WTIP in shWtip cells rescued stress fiber formation. The human WTIP nucleotide sequence differs at two bases from the murine Wtip sequence, making it resistant to a murine Wtip shRNA. Transient EGFP-WTIP transfection into shWtip podocytes restored stress fiber formation comparable to control cells (Fig. 7A), suggesting that loss of stress fibers was a result of Wtip knockdown not an shRNA off-target effect. Since our previous experiments demonstrated Wtip is an upstream regulator of RhoA signaling and because RhoA activity is required for both focal adhesion and stress fiber formation (3), we next overexpressed constitutively active EGFP-RhoAQ63L and dominant negative EGFP- RhoAT19N in Wtip knockdown podocytes. When EGFP-RhoAQ63L was expressed in Wtip-depleted cells, actin stress fibers were formed and perturbations of actin polymerization caused by Wtip depletion were suppressed (Fig. 7B, top). These results are consistent with the premise that Wtip regulates directly or indirectly RhoA activity. In contrast, EGFP-RhoAT19N overexpression had no effect on stress fiber formation (Fig. 7B, bottom) and demonstrated that RhoA activity was required. Rescue of stress fiber assembly with the overexpression of constitutively active RhoA in Wtip knockdown cells suggested that, even in the absence of Wtip, overexpressed RhoA likely drives the maturation of focal adhesions from focal complexes. Even though Wtip was not required for the assembly of actin stress fibers and/or mature focal adhesions, Wtip may be involved in coordinating proper regional activation of RhoA at focal complexes. Taken together, our data suggest that Wtip localized at cell-matrix and cell-cell contact sites and plays a crucial role in Rho-GTPase-mediated actin remodeling, which is essential for proper cell-matrix and cell-cell contact formation.
Fig. 7.
Overexpression of GFP-WTIP or constitutively active RhoA rescued actin phenotype. A: transient transfection of an eGFP-WTIP expression vector demonstrated comparable actin stress fiber formation using rhodamine-phalloidin in shWtip and shEMP podocytes. B: transient transfection of EGFP-RhoAQ63L (RhoA-Q, constitutively active) and EGFP- RhoAT19N (RhoA-T, dominant negative) constructs into shWtip cells demonstrated RhoA activity is required for stress fiber formation. Arrows indicate actin stress fiber tips. Nuclei were labeled with CY5-TOPRO-3.
Wtip interacted with the N-terminal PDZ domain of Arhgef12.
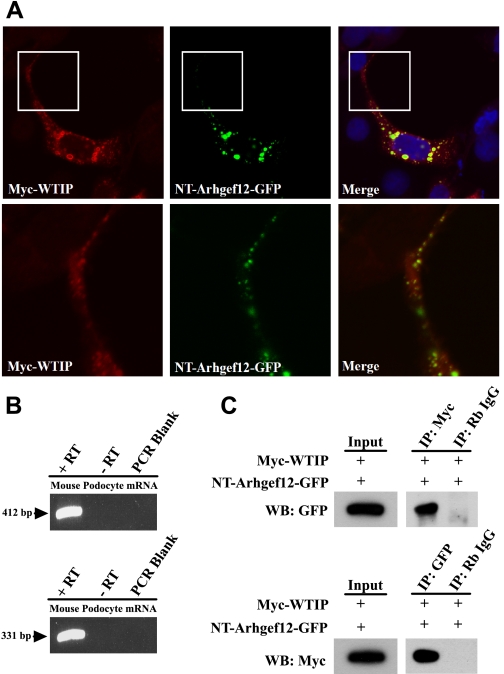
GEFs increase RhoA activity and play a key role in actin cytoskeleton formation. Based on the previous results, we hypothesized that Wtip interacted with a GEF to regulate regional actin dynamics and appropriate assembly of both focal adhesions and podocyte adherens junctions. The Wtip sequence has a C-terminal PDZ-binding domain, VTEL, which is highly similar to VTDL, the canonical PDZ binding domain in PlexinB1. We identified a family of RhoGEFs [PDZ-RhoGEF and leukemia-associated RhoGEF (LARG or ARHGEF12)], which contain PDZ domains and mediate targeted RhoA activation. We focused on Arhgef12 as a Wtip interaction partner, since its human ortholog was recently reported to be enriched in a glomerular expression library prepared from human kidney (21). Both the human and mouse Arhgef12 proteins contain the same functional domains, including the Dlg and ZO-1/2 PDZ domains (47). Immunofluorescence analysis (Fig. 8A) and coprecipitation experiments (Fig. 8C) using overexpressed myc-WTIP and NT-Arhgef-GFP showed that myc-WTIP interacts with the PDZ-containing amino terminal region of Arhgef12. Endogenous Arhgef 12 message was identified by RT-PCR in mouse podocytes using two different primer sets (Fig. 8B).
Fig. 8.
Myc-WTIP interacted with the PDZ domain-containing, amino terminal (NT) of the RhoA GEF, Arhgef12. A: COS7 cells were cotransfected with Myc WTIP and NT-Arhgef12-GFP constructs. Immunofluorescence labeling was done with rabbit polyclonal anti-Myc antibody followed by Alexa Fluor 568-conjugated anti-rabbit secondary antibody. Myc-WTIP (red) expression colocalizes with Arhgef12 (green) in the merged image. Magnified view of selected areas from top show colocalization in cell extension. B: RT-PCR was done in mouse podocytes using two primer sets amplifying different regions of Arhgef12 message. PCR products of expected size (412 and 331 bp) were observed for the N-terminal PDZ domain-containing and internal coding region of Arhgef12, respectively. C: coimmunoprecipitation was done in 293 cells transfected with Myc-WTIP and Arhgef12-GFP. Cell lysates were immunoprecipitated using anti-Myc or anti-GFP antibodies, and the immunoprecipitates were examined by Western blotting using anti-GFP or anti-Myc antibodies, respectively. Rabbit IgG was used as a control for immunoprecipitation. Input represented 5% of cell lysates used in the coimmunoprecipitation.
DISCUSSION
Structure and dynamics of the actin cytoskeleton are important for regulation of cell-matrix and cell-cell adhesions and for cell migration. In this study, we showed Wtip was localized to focal adhesion and cell-cell contact sites similar to other LIM-domain family members including zyxin, Ajuba, and LPP (11, 17, 40). Interestingly, Wtip localization to the cell-cell contact sites was associated with proper cadherin targeting and actin assembly at the junction (Fig. 1). Similarly, Wtip accumulated at cell-matrix adhesions sites where actin was organized into stress fibers as the focal adhesion matures (Figs. 1 and 2). These results suggest that Wtip is localized to the cell-matrix and cell-cell contact sites when and where the actin cytoskeleton is actively reorganized. In this work, we have focused on the function of Wtip outside the podocyte nucleus. The dynamic distribution of Wtip at cell adhesion sites, the focal adhesions and adherens junctions, in addition to its association with both the focal adhesion and the adherens junction protein complex suggest the importance of Wtip in the stability and/or maintenance of cell adhesions.
Consistent with its localization, depletion of Wtip from podocytes impaired not only stress fiber assembly and focal adhesion formation, but also actin reorganization at cell-cell adhesion sites (Fig. 2A). However, total protein expression of focal adhesion proteins FAK, paxillin, and vinculin remained unchanged (Fig. 2G). Loss of FAK and paxillin expression in HeLa cells also abrogated efficient N-cadherin-mediated cell-cell adhesion (33), which provided evidence for communication between integrin and cadherin systems. However, paxillin and FAK localized to focal adhesion structures exclusively, whereas Wtip was localized to both focal adhesion and cell-cell adhesions, suggesting that Wtip is more directly involved in integrin-cadherin intercommunication. At the cell-cell contact sites, Wtip may participate in the cadherin-mediated signaling pathway to regulate actin organization essential for the correct formation of cell-cell adhesions.
In Wtip-depleted cells, formation of the focal complex, an initial step of focal adhesion formation, appeared normal, as shown by paxillin immunostaining (Fig. 2D). Therefore, Wtip is dispensable for formation of the focal complex, although it is indispensible for the maturation of focal complex to focal adhesion (Fig. 2, C and E). Maturation of focal adhesions is essential for stress fiber formation and stable cell-matrix adhesion (6). Thus we speculate that at the cell-matrix adhesion sites, Wtip participates in the integrin-mediated signaling pathways. Coordinated sequential activation and inactivation of Rac1 and RhoA are a prerequisite for the formation of stress fibers and mature focal adhesions (36). Our study showed that exogenous expression of constitutively active RhoA in shWtip cells rescues stress fiber formation, which suggests that failure of spatial or temporal regulation of RhoA activity is a cause for impaired actin dynamics in Wtip-depleted cells.
In addition, time-lapse observations of actin dynamics revealed loss of stress fibers caused by Wtip depletion was associated with abnormal actin polymerization events even in the absence of specific stimuli. Because sustained Rac activation downregulates Rho activity in fibroblasts (32), and because a Rho inhibitor induces a phenotype suggestive of activation of Rac1 in fibroblasts (26), Wtip might contribute to RhoA activation through downregulation of Rac1 activity. Transfection of a constitutively active Rac1 in podocytes induced bursts of actin polymerization that resembled those induced by Wtip depletion (Kim JH and Sedor JR, unpublished results). However, it did not perturb stress fibers, suggesting high activity of Rac1 is not sufficient to suppress stress fiber formation in podocytes. By contrast, expression of constitutively active RhoA in Wtip-depleted cells not only restored stress fiber formation but also repressed an abnormal burst of actin polymerization (Fig. 7B). RhoA may directly or indirectly inactivate Rac1 in Wtip-depleted cells.
In conclusion, our results show that Wtip regulates the stable formation of cell adhesions to both extracellular matrix and neighboring cells and suggest that Wtip is necessary for normal glomerular filtration barrier function. This process may be mediated by spatiotemporal regulation of RhoA activity through appropriate targeting of Arhgef12 by Wtip. Both animal and cell culture models have demonstrated that regulated Rho family GTPase activity is critical for normal glomerular filtration barrier function and podocyte contact formation. Mice lacking a member of the Rho guanine nucleotide dissociation inhibitor family, RhoGDIα, develop nephrotic syndrome (39). In vitro, small GTPase activity regulated the integrity of cell-cell contacts (9), and the balance of small GTPase activity is critical for normal podocyte process formation. A cell culture phenotype suggestive of foot process retraction (46), complement-dependent injury, demonstrated upregulated RhoA activity and concomitantly reduced Rac and Cdc42 activity, causing foot process retraction. Finally, insulin signaling through the insulin receptor rapidly reorganizes the actin cytoskeleton in normal podocytes by regulation of small GTPase activity, and loss of podocyte foot processes with actin rearrangement was an early abnormality identified in podocyte-specific, insulin receptor knockout mice (41). Given the importance of spatial control of Rho family GTPase activity in appropriately regulating cellular function and phenotype (29), scaffolding molecules like Wtip may play a critical role. Further studies of the precise signaling pathway from Wtip to Rho GTPases and, thus actin dynamics, may improve an understanding of mechanisms by which Rho family GTPases regulate glomerular filtration barrier function and identify novel targets for therapy.
GRANTS
Support for this project was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-07470, P50 DK-054178, DK-064719, and F30 DK083897.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.H.K., A.M., S.M., M.K., and J.S. provided conception and design of research; J.H.K., A.M., S.M., and M.K. performed experiments; J.H.K., A.M., S.M., M.K., and J.S. analyzed data; J.H.K., A.M., S.M., M.K., and J.S. interpreted results of experiments; J.H.K., A.M., S.M., and M.K. prepared figures; J.H.K., A.M., S.M., M.K., and J.S. drafted manuscript; J.H.K., S.M., M.K., and J.S. edited and revised manuscript; J.H.K., M.K., and J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gary Bokoch for the pcDNA3-EGFP-RhoA-Q63L (Addgene plasmid 12968) and pcDNA3-EGFP-RhoA-T19N (Addgene plasmid 12967) constructs. The plasmid pT-Adv Rho guanine nucleotide exchange factor (GEF) 12 (Arhgef12 or Larg) was kindly provided by Dr. Alexander Belyavsky.
REFERENCES
- 1. Adams CL, Chen YT, Smith SJ, James Nelson W. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol 142: 1105–1119, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275: 1308–1311, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res 313: 3616–3623, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Craig SW, Johnson RP. Assembly of focal adhesions: progress, paradigms, and portents. Curr Opin Cell Biol 8: 74–85, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet 14: 156–162, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Dold FG, Sanger JM, Sanger JW. Intact alpha-actinin molecules are needed for both the assembly of actin into the tails and the locomotion of Listeria monocytogenes inside infected cells. Cell Motil Cytoskeleton 28: 97–107, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 420: 629–635, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Franki N, Ding G, Gao Y, Hays RM. Effect of cytochalasin D on the actin cytoskeleton of the toad bladder epithelial cell. Am J Physiol Cell Physiol 263: C995–C1000, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Gao SY, Li CY, Shimokawa T, Terashita T, Matsuda S, Yaoita E, Kobayashi N. Rho-family small GTPases are involved in forskolin-induced cell-cell contact formation of renal glomerular podocytes in vitro. Cell Tissue Res 328: 391–400, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Goodwin M, Yap A. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Histol 35: 839–844, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Guo B, Sallis RE, Greenall A, Petit MMR, Jansen E, Young L, Van de Ven WJM, Sharrocks AD. The LIM domain protein LPP is a coactivator for the ETS domain transcription factor PEA3. Mol Cell Biol 26: 4529–4538, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hakim ZS, DiMichele LA, Doherty JT, Homeister JW, Beggs HE, Reichardt LF, Schwartz RJ, Brackhan J, Smithies O, Mack CP, Taylor JM. Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Mol Cell Biol 27: 5352–5364, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall A. Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans Royal Soc Lond B Biol Sci 355: 965–970, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 179: 1043–1057, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in rat podocytes. J Histochem Cytochem 51: 1589–1600, 2003 [DOI] [PubMed] [Google Scholar]
- 17. James V, Zhang Y, Foxler DE, de Moor CH, Kong YW, Webb TM, Self TJ, Feng Y, Lagos D, Chu CY, Rana TM, Morley SJ, Longmore GD, Bushell M, Sharp TV. LIM-domain proteins, LIMD1, Ajuba, and WTIP are required for microRNA-mediated gene silencing. Proc Natl Acad Sci USA 107: 12499–12504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanungo J, Pratt SJ, Marie H, Longmore GD. Ajuba, a cytosolic LIM protein, shuttles into the nucleus and affects embryonal cell proliferation and fate decisions. Mol Biol Cell 11: 3299–3313, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JH, Konieczkowski M, Mukherjee A, Schechtman S, Khan S, Schelling JR, Ross MD, Bruggeman LA, Sedor JR. Podocyte injury induces nuclear translocation of WTIP via microtubule-dependent transport. J Biol Chem 285: 9995–10004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindenmeyer MT, Eichinger F, Sen K, Anders HJ, Edenhofer I, Mattinzoli D, Kretzler M, Rastaldi MP, Cohen CD. Systematic analysis of a novel human renal glomerulus-enriched gene expression dataset. PLoS ONE 5: e11545, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marg S, Winkler U, Sestu M, Himmel M, Schönherr M, Bär J, Mann A, Moser M, Mierke CT, Rottner K, Blessing M, Hirrlinger J, Ziegler WH. The vinculin-ΔIn20/21 mouse: characteristics of a constitutive, actin-binding deficient splice variant of vinculin. PLoS ONE 5: e11530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mundel P, Reiser J, Borja AZM, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Oh J, Reiser J, Mundel P. Dynamic (re)organization of the podocyte actin cytoskeleton in the nephrotic syndrome. Pediatr Nephrol 19: 130–137, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 12: 1589–1598, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Ota T, Maeda M, Murakami M, Takegami T, Suto S, Tatsuka M. Activation of Rac1 by Rho-guanine nucleotide dissociation inhibitor-[beta] with defective isoprenyl-binding pocket. Cell Biol Int 31: 92–96, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 116: 1409–1416, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Perez-Moreno M, Avila A, Islas S, Sanchez S, Gonzalez-Mariscal L. Vinculin but not alpha-actinin is a target of PKC phosphorylation during junctional assembly induced by calcium. J Cell Sci 111: 3563–3571, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Pertz O. Spatio-temporal Rho GTPase signaling—where are we now? J Cell Sci 123: 1841–1850, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Petit MMR, Fradelizi J, Golsteyn RM, Ayoubi TAY, Menichi B, Louvard D, Van de Ven WJM, Friederich E. LPP, an actin cytoskeleton protein related to zyxin, harbors a nuclear export signal and transcriptional activation capacity. Mol Biol Cell 11: 117–129, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rico M, Mukherjee A, Konieczkowski M, Bruggeman LA, Miller RT, Khan S, Schelling JR, Sedor JR. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol Renal Physiol 289: F431–F441, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity. J Cell Biol 147: 1009–1022, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schaller MD. FAK and paxillin. J Cell Biol 166: 157–159, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol 92: 79–91, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Small JV, Rottner K, Kaverina I, Anderson KI. Assembling an actin cytoskeleton for cell attachment and movement. Biochim Biophys Acta 1404: 271–281, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Smoyer WE, Ransom RF. Hsp27 regulates podocyte cytoskeletal changes in an in vitro model of podocyte process retraction. FASEB J 16: 315–326, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Srichai MB, Konieczkowski M, Padiyar A, Konieczkowski DJ, Mukherjee A, Hayden PS, Kamat S, El-Meanawy MA, Khan S, Mundel P, Lee SB, Bruggeman LA, Schelling JR, Sedor JR. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem 279: 14398–14408, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, Niho Y, Nishimune Y, Nishikawa S, Takai Y. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene 18: 5373–5380, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Gilmore TD. Zyxin and paxillin proteins: focal adhesion plaque LIM domain proteins go nuclear. Biochim Biophys Acta 1593: 115–120, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJM. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol 166: 283–295, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol 19: 508–514, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yap AS, Kovacs EM. Direct cadherin-activated cell signaling. J Cell Biol 160: 11–16, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J Biol Chem 277: 9580–9589, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Zhang H, Cybulsky AV, Aoudjit L, Zhu J, Li H, Lamarche-Vane N, Takano T. Role of Rho-GTPases in complement-mediated glomerular epithelial cell injury. Am J Physiol Renal Physiol 293: F148–F156, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Zinovyeva M, Sveshnikova E, Visser J, Belyavsky A. Molecular cloning, sequence and expression pattern analysis of the mouse orthologue of the leukemia-associated guanine nucleotide exchange factor. Gene 337: 181–188, 2004 [DOI] [PubMed] [Google Scholar]