Abstract
New and effective treatment for acute kidney injury remains a challenge. Here, we induced mouse hematopoietic stem and progenitor cells (HSPC) to differentiate into cells that partially resemble a renal cell phenotype and tested their therapeutic potential. We sequentially treated HSPC with a combination of protein factors for 1 wk to generate a large number of cells that expressed renal developmentally regulated genes and protein. Cell fate conversion was associated with increased histone acetylation on promoters of renal-related genes. Further treatment of the cells with a histone deacetylase inhibitor improved the efficiency of cell conversion by sixfold. Treated cells formed tubular structures in three-dimensional cultures and were integrated into tubules of embryonic kidney organ cultures. When injected under the renal capsule, they integrated into renal tubules of postischemic kidneys and expressed the epithelial marker E-cadherin. No teratoma formation was detected 2 and 6 mo after cell injection, supporting the safety of using these cells. Furthermore, intravenous injection of the cells into mice with renal ischemic injury improved kidney function and morphology by increasing endogenous renal repair and decreasing tubular cell death. The cells produced biologically effective concentrations of renotrophic factors including VEGF, IGF-1, and HGF to stimulate epithelial proliferation and tubular repair. Our study indicates that hematopoietic stem and progenitor cells can be converted to a large number of renal-like cells within a short period for potential treatment of acute kidney injury.
mouse renal ischemic injury resembles the pathogenesis of human acute kidney injury (AKI) and is an established animal model for therapeutic testing. Ischemic insult results in tubular cell death and compromised renal function, which leads to fluid overload and waste product accumulation (36). Renal recovery mainly depends on proliferation of surviving tubular cells (10, 18, 29), which is a process that recapitulates many features of kidney development. However, renal recovery from acute injury is often incomplete and can result in chronic renal failure (1, 36). New treatments to accelerate renal recovery could reduce morbidity and mortality. Previously, we and others have shown that a very small number of transplanted bone marrow cells could be incorporated into postischemic renal tubules and could express epithelial markers (20, 27, 28), suggesting that hematopoietic-to-renal lineage conversion may have occurred in response to environmental signals in regenerating kidneys. However, hematopoietic cells do not integrate into renal tubules in the first week after injury when most renal repair occurs and offer no functional contribution (29). We reasoned that induction of hematopoietic-to-renal differentiation in vitro and subsequent transplantation of cells that showed renal commitment could offer a “head start” advantage in treating AKI.
In this study, we sequentially treated hematopoietic stem and progenitor cells (HSPC) with cytokines, growth factors, and a histone deacetylation (HDAC) inhibitor to stimulate cell cycle entry, induce chromatin modification, and promote hematopoietic-to-renal conversion. HSPC were used because they share a common mesodermal origin with the embryonic kidney; i.e., the aorta-gonad-mesonephros (AGM) region contributes to early hematopoiesis (9, 32). This common developmental origin suggests that hematopoietic-to-renal conversion may encounter fewer epigenetic barriers. In an attempt to reach our long-term goal of creating a safe therapy for kidney disease, no viral-mediated gene delivery system was applied to avoid exogenous DNA integration or oncogene expression.
MATERIALS AND METHODS
Mouse strains.
CreKsp mice expressing Cre recombinase under the kidney-specific cadherin 16 (Ksp) promoter were provided by Dr. Peter Igarashi at the UT Southwestern Medical Center (43). R26R-EYFP mice were provided by Dr. Frank Costantini at Columbia University (44). CreKsp;R26R-EYFP mice were generated by intercrossing CreKsp and R26R-EYFP mice to express enhanced yellow fluorescent protein (EYFP) specifically in renal tubular epithelial cells. B6-Ly5.2 mice (National Cancer Institute) were used for renal ischemic injury and cell transplantation recipients. All experiments involving animals were performed under the auspices of the UT Southwestern Institutional Animal Care and Use Committee.
Lin− cell isolation and induction.
Lin− cells were isolated from CreKsp;R26R-EYFP mice using the immunobead method (28). Purified Lin− cells were treated sequentially with cytokines (IL-3 at 50 units/ml, IL-6 at 50 units/ml, and stem cell factor at 50 ng/ml) for 48 h at the 1st stage, nephrogenic factors (retinoic acid at 0.1 μM, activin A at 10 ng/ml, and BMP-7 at 50 ng/ml) for another 48 h at the 2nd stage, and epithelial growth factors (EGF at 10 ng/ml, IGF-1 at 100 ng/ml, and HGF at 20 ng/ml) for an additional 72 h at the 3rd stage. Some cells were also treated with a histone deacetylase (HDAC) inhibitor, trichostatin A (TSA), at 15 nM in the first 6 h during the 2nd stage induction. Nonadherent cells that expressed the hematopoietic marker CD45 were transferred to new culture dishes after the 1st stage. Adherent cells that might have represented mesenchymal stem/stromal cells (MSC) were discarded. Cells were maintained at 37°C in a 5% CO2 atmosphere during culture.
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assay of 1st and 3rd stage cells was performed as described (13). Chromatin was immunoprecipitated with anti-acetyl-histone H3 or H4 and anti-trimethyl-histone H3 (Lys9) antibodies. Immunoprecipitated DNA was quantified using semiquantitative PCR with promoter-specific primers. Of the input DNA, 1% was amplified to normalize enrichment. Immunoprecipitation with isotype-specific IgG was used as a control.
RT-PCR analysis.
Total RNA was extracted, and the first-strand cDNA was synthesized. qRT-PCR was performed in triplicate using SYBR green Supermix reagents (Bio-Rad Laboratories). 18S rRNA was used as the control for normalization. Data were analyzed using IQ software (Bio-Rad Laboratories).
Immunostaining and image analysis.
Immunostaining was performed as previously described (27). Stained sections were photographed using a Zeiss Axioplan microscope, and the images were analyzed with Axiovert software (Carl Zeiss). Some stained sections were scanned to obtain Z-stack images with Zeiss laser-scanning confocal microscopy. Images were analyzed using the Zeiss LSM 510 software package, and three-dimensional image reconstruction was performed using Imaris software (Bitplane).
Antibodies and reagents.
The following lists the sources of antibodies and reagents: Pax2 (Zymed Laboratories), ZO-1 (Zymed Laboratories), E-cadherin (BD Biosciences), Gapdh (Santa Cruz Biotechnology), laminin (Sigma-Aldrich), Ki-67 (Leica Biosystems), bromodeoxyuridine (BrdU; BD Biosciences), acetyl-histone H3, acetyl-histone H4 and trimethyl-Histone H3 (Lys9; Upstate Cell Signaling Solutions), and activated caspase 3 (Promega). Proximal tubules were stained with FITC-LTA (Vector Laboratories). The percentage of cell proliferation or apoptosis in the proximal tubules was calculated after dividing the number of Ki-67-expressing cells or activated caspase 3-positive cells by the number of LTA-positive cells in the S3 segments. All secondary antibodies were obtained from Molecular Probes. IL-3, IL-6, stem cell factor, BMP-7, activin-A, EGF, IGF-1, and HGF were obtained from R&D Laboratories. Retinoic acid, antimycin A, and trichostatin A (TSA) were obtained from Sigma-Aldrich.
Y chromosome fluorescent in situ hybridization.
A mouse Y chromosome painting probe was produced by degenerate oligonucleotide-primed PCR using DNA templates kindly provided by Dr. Diane Krause at Yale University and labeled with digoxygenin (28). Y chromosome fluorescent in situ hybridization (FISH) was performed as previously described (28, 29).
Renal ischemia-reperfusion injury, cell transplantation, and subcapsular injection.
Female B6-LY5.2 mice (6–8 wk old) were irradiated with 9.5 Gy of γ radiation, and renal ischemic injury was created by clamping the left renal pedicles for 35 min followed by clamp release. Sham-operated mice underwent the same procedures except that clamping of renal pedicles was omitted. Some mice also received a right nephrectomy if renal function tests were performed (29). Removing the right kidney allowed assessment of renal functional recovery in the left postischemic kidney. Treated cells (5 × 106 cells) or untreated male control bone marrow cells were injected via the tail vein into female mice 2 h after ischemic injury. Untreated bone marrow cells (1 × 106 cells) from female mice were coinjected into each mouse to provide radioprotection after irradiation. To test whether direct renal parenchyma delivery increases cell integration, subcapsular injection was performed to deliver treated cells or mouse embryonic fibroblast (MEF) cells from embryonic day 13.5 (E13.5) embryos (1 × 106 cells in 25 μl of Iscove's modified Dulbecco's medium or IMDM) under the left renal capsule with a Hamilton syringe at the time of vascular clamp release. Renal subcapsular injection was also used to test teratoma formation by treated cells.
Renal function tests and renal morphology analysis.
Blood samples were obtained via eye bleeding. Serum blood urea nitrogen (BUN) was measured using a Reflotron analyzer (Roche) (29), and creatinine was measured using capillary electrophoresis. Periodic acid-Schiff (PAS)-stained kidney sections were scored for tubular injury with semiquantitative morphological analysis (7). In each sample, at least 100 proximal tubules were scored.
Three-dimensional tubulogenesis.
Treated cells (5 × 105 cells) were suspended into Cellmatrix Type I-A (Nitta Gelatin) in six-well plates and cultured in 3rd stage medium with IMDM supplemented with 10% FBS, EGF at 10 ng/ml, IGF-1 at 100 ng/ml, and HGF at 20 ng/ml for 1–2 wk. IMCD3 cells or untreated bone marrow cells cultured under the same conditions were used as controls.
Embryonic kidney organ cultures.
E13.5 kidneys were fragmented into three to four pieces with a razor blade, combined with male treated cells (1 × 106 cells), and placed on 0.4-μm cell culture insert filters (BD Biosciences) in IMDM supplemented with 10% FBS for 1 wk. The cocultures were fixed, embedded in optimal cutting temperature medium, and sectioned for Y chromosome FISH and E-cadherin immunostaining.
Conditioned medium.
Conditioned medium was generated by washing treated cells (1 ×106 cells) three times. The cells were then recultured in IMDM without any supplements for 3 days. The media were centrifuged to remove cellular components before use. Mice with a left renal ischemic injury and a right nephrectomy were injected with the conditioned medium (1 ml) or control serum-free IMDM into the peritoneal cavity twice a day for 6 days starting on the day of injury. For primary culture of tubular epithelial cells, cells were grown to 60% confluence before exposure to antimycin A at 1 μM for 30 min to deplete ATP. The cells were then cultured with conditioned medium or serum-free IMDM for 48 h. BrdU (10 μM) was added during the last 6 h before cell harvesting.
ELISA.
The concentrations of VEGF, IGF-1, and HGF in treated cells-conditioned medium were determined using mouse ELISA kits obtained from R&D (VEGF and IGF-1) and RayBiotech (HGF). As a control, medium was also collected from cells that omitted the 2nd stage but underwent 1st and 3rd stage cultures. This is to ensure that exogenous IGF-1 and HGF added in 3rd stage medium does not result in artificial elevation of IGF-1 and HGF.
Testing effect of VEGF, IGF-1, and HGF on renal epithelial cells.
Primary cultures of renal tubular epithelial cells were grown to 60% confluence before exposure to antimycin A (1 μM) for 30 min to deplete ATP (27). The cells were then cultured for 24 h in DMEM/F-12 supplemented with VEGF (0.1 ng/ml), IGF-1 (2 ng/ml), and HGF (4 ng/ml). These concentrations are equivalent to those detected in conditioned medium produced by treated cells. Concentrations of these growth factors that were 5 or 10 times higher were also used to treat epithelial cells. BrdU (10 μM) was added at the last 6 h to test BrdU incorporation.
Quantification and statistics.
To quantify Y chromosome FISH signals in the liver, lung, spleen, bone marrow, and peripheral blood samples, 10 fields (×400) were randomly selected for counting the total number of cells and Y chromosome-containing cells. The percentage of male cells was calculated by the number of Y chromosome-containing cells divided by the total number of cells in that organ, except in the kidney where it was divided by the total number of tubular cells. Each experiment was conducted in duplicate. The number of animals used is indicated in each experiment. Data are represented as means ± SE. Differences between the two groups were analyzed by using an unpaired Student's t-test. Injury scores were analyzed by nonparametric statistical analysis with SPSS software. Differences between three or more groups were analyzed by one-way ANOVA. A two-tailed P value of <0.05 was considered to be statistically significant.
RESULTS
Lin− cells can be induced to express renal developmentally regulated genes.
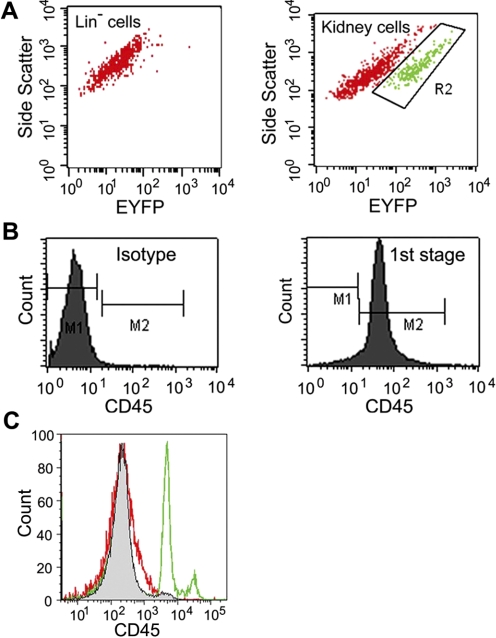
We isolated lineage undifferentiated hematopoietic stem and progenitor cells (Lin− cells) from Creksp;R26R-EYFP mice that expressed EYFP in renal epithelial cells driven by the promoter for kidney-specific cadherin 16 (Ksp-cadherin) (27, 43, 44). While cells from the kidney expressed strong EYFP signals, flow cytometry analysis indicated no expression of EYFP in Lin− cells freshly isolated from the bone marrow (Fig. 1A). Lin− cells were treated with cytokines including stem cell factor, IL-3, and IL-6 for 48 h (1st stage) to stimulate cell cycle entry (30, 39). After 48 h, over 99% of the nonadherent cells continued to express hematopoietic marker CD45 (Fig. 1B) and were transferred to new culture dishes and treated for another 48 h (2nd stage) with retinoic acid, activin-A, and BMP7-nephrogenic factors that have been shown to induce mouse embryonic stem cell differentiation into renal epithelial cells (22). We reasoned that after cytokine treatment, cycling cells would be more prone to induction by known nephrogenic factors. At the final stage, cells were treated with epithelial growth factors including EGF, IGF-1, and HGF for an additional 72 h (3rd stage) to promote epithelial differentiation and proliferation (8, 17, 33). The above 3rd stage treatments resulted in a loss in the expression of hematopoietic cell marker CD45 (Fig. 1C).
Fig. 1.
Lin− cells can be sequentially treated to lose hematopoietic marker CD45. A: Lin− cells freshly isolated from mice expressing enhanced yellow fluorescent protein (EYFP) under the kidney-specific cadherin 16 promoter showed no expression of EYFP (left). Cells obtained from whole kidney digestion showed a distinct population of EYFP-expressing cells (right, R2 area). B: nonadherent cells after 1st stage culture expressed CD45 (right) and were transferred for further treatment in cultures. Left: isotype control. C: freshly isolated Lin− cells expressed CD45 (green), but CD45 expression was lost after completion of protein factor treatment in the 3rd stage cultures (red). Isotype control is shown in grey.
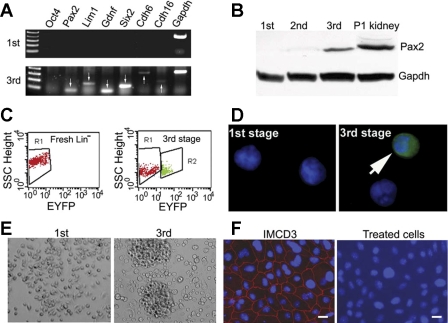
The treated cells were subjected to RT-PCR analysis for genes expressed during kidney development. The results showed the expression of Pax2, Lim1, Gdnf, Six 2, cadherin 6, and cadherin 16 at the mRNA level after the 3rd stage while cells after the 1st stage showed no expression of these genes (Fig. 2A). Pax2 was also detectable at the protein level (Fig. 2B), but proteins for other genes were below detectable levels by immunoblot analysis (not shown). The treated cells did not express the pluripotent cell marker Oct4 (40, 41), suggesting no acquisition of a pluripotent state. Since Six2 has been used to define renal progenitor cells, our results suggest that conversion to a renal progenitor state may have occurred during the 1-wk induction period.
Fig. 2.
Treated cells resemble renal phenotypes. Lin− cells isolated from mice expressing EYFP under the kidney-specific cadherin 16 promoter were given 3-stage treatments with protein factors, and the resulting cells were characterized. A: expression of a panel of renal developmentally regulated genes (arrows). No pluripotent gene Oct 4 is expressed. B: detection of Pax2 protein in treated cells. Postnatal day 1 (P1) kidneys were used as positive controls. C: expression of EYFP by flow cytometry analysis. An average of 6.3% cells expressed EYFP after treatments (right, R2 area), whereas untreated Lin− cells show no EYFP expression (left). D: visualization of EYFP under fluorescent microscope. E: cells formed colonies after 3 stages of culture. F: no expression of epithelial tight junction protein zonula occludens-1 (ZO-1) by immunostaining, indicating that treated cells are not fully differentiated epithelial cells. IMCD3 cells were used as controls. Scale bar = 20 μm. 1st, 2nd, and 3rd: first, second, or third stage culture.
Activation of the kidney-specific cadherin 16 promoter Ksp could result in the expression of EYFP. We examined the cells for EYFP expression using a fluorescent microscope and flow cytometry (Fig. 2, C and D). Untreated Lin− cells and the cells collected after the 1st stage treatment showed no EYFP expression. After completion of the three-stage treatments, EYFP was expressed in 6.3% of the cells. Taken together, our results suggest a phenotype change in treated cells.
The change in molecular phenotype was associated with morphological changes. The cell size increased from 8.4 ± 1.2 μm in the 1st stage to 14.7 ± 3.8 μm in the 3rd stage and began to attach and form an epithelial-like colony (Fig. 2E). However, no tight junction protein ZO-1 was expressed when the cells reached confluence (Fig. 2F). The cells also had no expression of nephron-specific epithelial transporters or channels including anion exchanger 1 (AE1), epithelial Na channel (ENaC-α, -β, -γ), H+-ATPase, Na-Pi cotransporter type 2a and type 2c (NaPi 2a, NaPi-2c), Na+/H+ exchanger 3 and 8 (NHE3/8), Na-K-Cl cotransporter 2, and Na+/glucose cotransporter type 2 (Sglt2) (not shown), indicating that treated cells were not fully differentiated renal epithelial cells.
Treated cells have the potential for tubulogenesis.
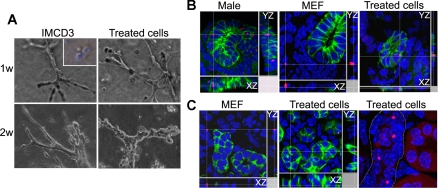
Treated cells were tested for their ability to form tubules in three-dimensional cultures (Fig. 3A). At 1 wk, the cells formed elongated cell cords and branched as extensively as the control renal epithelial IMCD3 cells. Treated cells continued to organize into tubular structures at 2 wk, while untreated bone marrow cells failed to grow and form tubules.
Fig. 3.
Treated cells have the potential for tubulogenesis. A: formation of tubular structures after 1 or 2 wk in 3-dimensional cultures. Renal epithelial cell line IMCD3 cells were used as positive controls. Inset: untreated bone marrow cells fail to grow and form tubules. B: integration of treated cells into renal tubules in embryonic kidney organ cultures. Treated cells generated from male Lin− cells were cocultured with fragmented embryonic day 13.5 (E13.5) female kidneys for 1 wk. XZ and YZ images indicate that male cells [red, fluorescent in situ hybridization (FISH) signals] were integrated into female renal tubules and expressed epithelial marker E-cadherin (green, right). Control male mouse embryonic fibroblast (MEF) cells (red, FISH signals) were only detected outside the tubular structure (middle). Cultures of male embryonic kidney fragments were used as positive controls for Y-chromosome FISH analysis (left). C: integration of male treated cells (red, FISH signals) into female postischemic kidneys after injection under the renal capsule (middle). Control male MEF cells (left) failed to integrate into renal tubules. E-cadherin (green) labels epithelial cells. XZ and YZ images are shown. Right: renal tubule (dotted outline) with relatively more integration of male treated cells (red, FISH signals).
Next, treated cells derived from male mice or MEF derived from male embryos were cocultured with female E13.5 kidneys that had been mechanically dissociated into fragments to increase cell interaction. Male cells that carried Y chromosomes were detected in tubules and expressed epithelial marker E-cadherin (Fig. 3B, right). In contrast, MEF could only be detected outside the tubular structure (Fig. 3B, middle). Finally, male treated cells were injected under the capsule of adult female postischemic kidneys where the microenvironment favored epithelial regeneration. Robust male cell integration into the tubules at the site of injection was observed, while control MEF failed to integrate (Fig. 3C). No teratoma was observed 2 and 6 mo after the injection, supporting the safety for potential therapy.
Increased histone acetylation accompanies cell conversion.
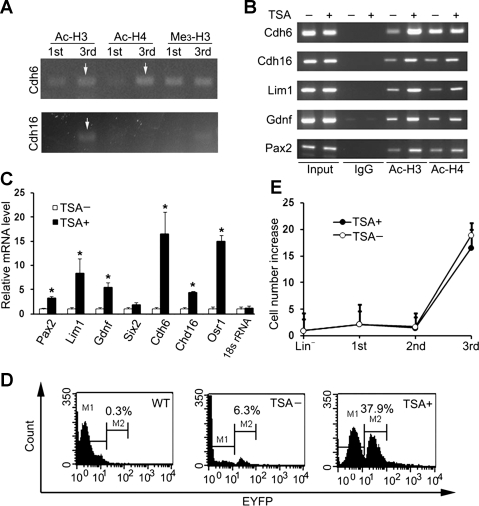
Chromatin remodeling is often involved in the change of epigenetic status (16, 31). We found that acetylation of histones H3 and H4 on the cadherin 6 promoter and the acetylation of histone H3 on the kidney-specific cadherin 16 was increased in treated cells, while no apparent changes in trimethylation of histone H3 were detected (Fig. 4A). We then treated the cells in the 2nd stage with low concentration HDAC inhibitor TSA (15 nM) for 6 h in an attempt to increase cell conversion. Increased histone acetylation was detected on the promoters for cadherin 6, cadherin 16, and renal developmentally-regulated genes Lim1, Gdnf, and Pax2 (Fig. 4B). Increased histone acetylation was associated with increased expression of renal genes, while no change in the 18S rRNA gene was detected (Fig. 4C). Furthermore, EYFP-expressing cells increased by sixfold (from 6.3 to 37.9%, Fig. 4D), indicating the importance of histone acetylation in cell conversion.
Fig. 4.
Histone acetylation enhances cell conversion. A: increased acetylation of histone H3 (Ac-H3) and H4 (Ac-H4) on promoters for cadherin 6 (Cdh6) and for kidney-specific cadherin 16 (Cdh16) during cell conversion. Chromatin immunoprecipitation (ChIP) assay was performed on cells after the 1st and 3rd stage treatments. No apparent changes in trimethylation of histone H3 (Me3-H3) were detected. B: increased histone acetylation on promoters for renal genes after additional treatment with trichostatin A (TSA). Treated cells with and without TSA treatment were analyzed with ChIP after the 3rd stage cultures. C: increased expression of renal genes in treated cells with TSA treatment analyzed by qRT-PCR. No changes in control 18S rRNA were detected. Values are expressed as a percentage over TSA-untreated cells (means ± SE; n = 3). D: TSA treatment increased EYFP-expressing cells from 6.3 to 37.9%. Treated cells generated from wild-type mice were used as controls (n = 3). A representative flow cytometry analysis is shown. E: generation of a large number of treated cells within 1 wk of culture. Values are means ± SE (n = 3).
Our goal was to generate a sufficient number of cells within a short period of time for therapy. Cytokine treatment resulted in a 2.5 ± 0.1-fold increase in cell number. Treatment of cells with retinoic acid, activin-A, and BMP7 did not lead to significant changes in cell numbers. No difference in cell death was observed with TSA treatment (P = 0.06, n = 6). Final treatment of cells with EGF, IGF-1, and HGF resulted in expansion of cells by 16.5- and 18.9-fold in the presence and absence of TSA, respectively (Fig. 4E). The large number of treated cells generated in 1 wk would allow for potential therapy.
Transplantation of treated cells improves renal recovery and protects tubules from ischemic injury.
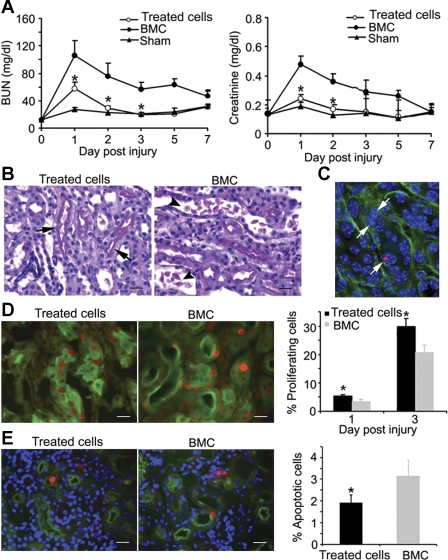
Next, we tested whether transplantation of treated cells accelerated renal recovery. Male cells that were also treated with TSA were injected intravenously into female mice with renal ischemia-reperfusion injury induced by clamping left renal pedicles for 35 min. The right kidneys were removed to eliminate functional contribution from contralateral kidneys. Intravenous administration of treated cells significantly improved renal function by reducing BUN and creatinine to near-control levels. In contrast, injection of untreated bone marrow cells offered no benefits (Fig. 5A). Renal morphology was also improved as indicated by a more normal appearance of proximal tubules and fewer debris and casts in the tubular lumen (Fig. 5B). Kidneys of mice with the treated cell injection had significantly lower injury scores (204 ± 14) compared with 244 ± 8 with untreated bone marrow cell transplantation, which was significant when analyzed by nonparametric statistical analysis (P < 0.05, n = 3–4).
Fig. 5.
Transplantation of treated cells accelerates renal recovery from injury. Female mice were given an intravenous injection of 5 × 106 male treated cells 2 h postinjury. A: improvement of renal function with reduction of blood urea nitrogen (BUN; left) and creatinine (right) by injection of treated cells (○, *P < 0.05; n = 5–10). No functional improvement was observed with injection of untreated bone marrow cells (BMC; ●). Sham-operated mice (▴) showed no changes in BUN or creatinine. B: improved renal morphology with treated cells injection. Brush borders of proximal tubules appear more normal (arrows) with treated cells injections. Mice with BMC injection show more casts and debris in tubular lumen (arrowheads). Scale bar = 20 μm. C: detection of male treated cells in tubules of female postischemic kidneys. Y chromosome FISH (red, arrows) indicates the integration of treated cells in tubules labeled with laminin (green). Scale bar = 20 μm. D: stimulation of cell proliferation by treated cell injection. Left: representative images of Ki-67 expression (red) in injured S3 segments of the proximal tubules (green, LTA labeling of the brush border) in kidneys of mice at 3 days after ischemic injury and treated cells or control MBC injection. Scale bars = 20 μm. Right: graph indicates significant increase in Ki-67 expression with treated cell injection at 1 and 3 days postinjury and -repair (*P < 0.05; n = 3–4). E: reduction of tubular apoptosis by treated cell injection. Left: activated caspase 3 (red) in the S3 segment of the proximal tubules labeled with LTA (green). Scale bars = 20 μm. Right: graph indicates a significant decrease in apoptosis with treated cell injection (*P < 0.05; n = 3). Kidneys were analyzed 3 days postinjury and -cell injections.
The kidneys were examined to test whether renal protection was due to cell replacement by treated cells. Y chromosome FISH analysis revealed that only 0.05 and 0.02% of tubular epithelial cells carried Y chromosome signals at 7 and 28 days post-intravenous cell injection, respectively (Fig. 5C), indicating that the therapeutic effect is not mainly attributed to cell replacement. To understand whether low renal integration was due to limited transmigration into renal parenchyma and/or entrapment by extrarenal organs, we analyzed the kidney, peripheral blood, bone marrow, spleen, liver, and lungs and found that only 0.028 ± 0.005 and 0.075 ± 0.021% of renal tubular cells contain Y chromosome signals at 1 and 3 days postinjection, respectively. Other extrarenal organs, especially the bone marrow, contained higher percentage of male cells (Table 1). There was a trend of decreasing the number of male cells in the lungs, liver, spleen, and bone marrow at 3 days compared with that of at 1 day, suggesting that treated cells may have low viability or have been cleared within the organ where they resided.
Table 1.
Distribution of treated cells after intravenous injection
| 1 Day | 3 Days | |
|---|---|---|
| Kidney | 0.028 ± 0.005% | 0.075 ± 0.021% |
| Lung | 0.130 ± 0.013% | 0.111 ± 0.011% |
| Liver | 0.228 ± 0.019% | 0.126 ± 0.012% |
| Spleen | 0.564 ± 0.082% | 0.424 ± 0.025% |
| Bone marrow | 0.772 ± 0.075% | 0.613 ± 0.068% |
| Blood | 0.470 ± 0.018% | 0.058 ± 0.024% |
Values are means ± SE; n = 3. Detection of male treated cells in the kidney and extrarenal organs 1 and 3 days post-intravenous injection into female mice with renal ischemic injury is shown. Y chromosome fluorescent in situ hybridization (FISH) was performed to detect injected male cells in the female mice, and the percentage of Y chromosome-positive cells in the kidney and extra-renal organs was quantified.
The lack of significant cell replacement but improvement in renal function and structure suggests that treated cells could protect the kidney through endocrine and paracrine mechanisms that have been shown by the administration of mesenchymal stem/stromal cells and human hematopoietic stem and progenitor cells (3, 26, 49). We examined Ki-67-expressing cells in the S3 segments of proximal tubules where most injury and repair occurred. Compared with injections with untreated bone marrow cells, injections of treated cells led to a 46 and 56% increase in tubular proliferation at 1 and 3 days postinjury, respectively (Fig. 5D). Inversely, there was more than a 1.5-fold reduction in apoptosis (Fig. 5E). Thus treated cells offer renal protection by both enhancing endogenous renal repair and decreasing renal injury.
Treated cells produce renotrophic factors to exert their therapeutic value.
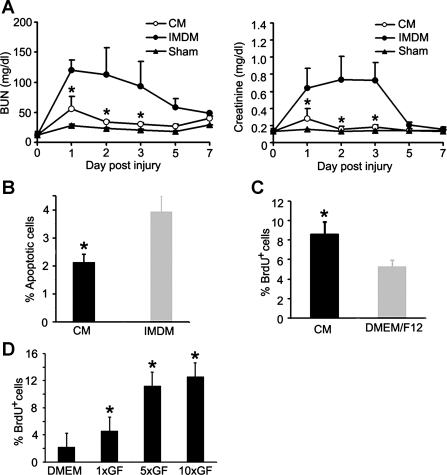
To investigate whether treated cells protect the kidney by producing renotrophic factors, the conditioned medium produced by treated cells was injected intraperitoneally into mice with renal ischemic injury. A reduction in BUN and creatinine was observed (Fig. 6A), and a 81 ± 12% decrease in apoptosis was detected compared with the control medium injection (Fig. 6B). Examination of the proximal tubules indicated significantly lower injury scores (201 ± 13) with conditioned medium injection compared with the injury score in the proximal tubules of mice injected with control medium (242 ± 12, n = 4, P < 0.05). We then cultured renal epithelial cells with conditioned medium to test their cytoprotection after reversible ATP depletion with antimycin A (27, 35). Cells treated with conditioned medium showed more than a 2-fold increase in BrdU incorporation (8.59 ± 1.31 vs. 5.24 ± 0.73% with control medium) (Fig. 6C).
Fig. 6.
Treated cells produce renotrophic factors to reduce renal injury and increase renal recovery. A: improvement of renal function by injection of conditioned medium (CM). CM collected from treated cells was injected intraperitoneally into mice with renal ischemic injury. CM injection reduces serum BUN (left) and creatinine (right) significantly compared with control Iscove's modified Dulbecco's medium (IMDM) (*P < 0.05; n = 5). Sham-operated mice show no changes in BUN and creatinine. B: decreased tubular apoptosis by CM injection. Apoptotic proximal tubular cells were quantified by activated caspase 3 immunostaining in mice that received CM or control IMDM injections (*P < 0.05; n = 3). C: CM treatment increases bromodeoxyuridine (BrdU) incorporation in primary cultures of renal epithelial cells injured with antimycin A. Cells cultured in DMEM/F12 were used as controls (*P < 0.05; n = 3). D: VEGF, IGF-1, and HGF increase BrdU incorporation in primary cultures of renal epithelial cells injured with antimycin A. Cells cultured in DMEM/F12 were used as controls (*P < 0.05; n = 3). 1×GF, equivalent concentrations of growth factors to those detected in conditioned medium produced by treated cells; 5×GF and 10×GF, concentrations of growth factors that are 5 or 10 times higher than that in the conditioned medium.
Growth factors, such as VEGF, IGF-1, and HGF, have been shown to stimulate tubulogenesis and to accelerate renal recovery in animal models (8, 21, 33). We examined these growth factors in conditioned media produced by treated cells and control cells that omitted the 2nd stage cultures with nephrogenic factors. Treated cells released significantly higher concentrations of VEGF, IGF-1, and HGF in the medium compared with control cells (Table 2). The combination of VEGF, IGF-1, and HGF were used at these concentrations to test their growth effect in primary cultures of epithelial cells injured with antimycin A that caused reversible ATP depletion (5, 27). The results showed that 4.65 ± 0.5% of cells with growth factor treatment incorporated BrdU, whereas only 2.27 ± 0.9% of cells without growth factor treatment incorporated BrdU (P < 0.05, n = 3–4). Growth factors that were 5 times higher in concentration led to a 2-fold increase in proliferation; this stimulatory effect plateaued when 10 times the growth factor concentration was used (Fig. 6D).
Table 2.
Concentrations of VEGF, IGF-1, and HGF produced by treated cells
| VEGF, pg/ml | IGF-1, pg/ml | HGF, pg/ml | |
|---|---|---|---|
| Treated cells | 104.9 ± 19.5 | 2,418.9 ± 280.4 | 3,832.0 ± 120.1 |
| Control cells* | 13.8 ± 3.5 | 410.7 ± 80.1 | 385.1 ± 84.1 |
| P value | <0.05 | <0.01 | <0.05 |
Values are mean ± SE; n = 4/group. ELISA was performed to quantify VEGF, IGF-1, and HGF in media collected from treated cells or control cells that omitted the 2nd stage culture but underwent the 1st and the 3rd stage cultures. This is to ensure that exogenous IGF-1 and HGF added in the 3rd stage medium can be washed away and does not result in overestimation of IGF-1 and HGF produced by treated cells. Student t-tests were performed between treated cells and control cells.
Lin− cells that omitted the 2nd stage culture but underwent the 1st and 3rd stages.
Next, we measured the serum concentrations of VEGF, IGF-1, and HGF in mice that received control bone marrow cell or treated cell transplantations (Table 3). No changes in their concentrations were detected between sham operation and 1 or 3 days post-ischemic injury with control bone marrow cell injections, suggesting that ischemic injury to the kidney was insufficient to raise these growth factors to superphysiological levels. Injection of treated cells resulted in two- to fivefold increases in serum VEGF, IGF-1, and HGF concentrations and were approaching levels in treated cells-conditioned medium that were shown to stimulate BrdU incorporation in renal epithelial cells (Table 3 and Fig. 6, C and D). Our results indicate that VEGF, IGF-1, and HGF are important renotrophic factors produced by treated cells for renal repair.
Table 3.
Levels of VEGF, IGF-1, and HGF in mouse serum
| Injected Cells | Injury | VEGF, pg/ml | IGF-1, pg/ml | HGF, pg/ml |
|---|---|---|---|---|
| BMC | Sham | 6.1 ± 1.6 | 573.2 ± 83.7 | 503.6 ± 92.8 |
| Treated cells | IRI 1 day | 38.6 ± 5.7* | 1,142.7 ± 228.4* | 1,659.6 ± 164.5* |
| IRI 3 days | 41.8 ± 6.2* | 1,173.7 ± 157.7* | 1,704.1 ± 223.8* | |
| BMC | IRI 1 day | 7.2 ± 2.6 | 658.6 ± 93.3 | 774.4 ± 108.0 |
| IRI 3 days | 7.6 ± 2.2 | 613.7 ± 101.2 | 676.1 ± 103.6 |
Values are mean ± SE; n = 4/group. Sera obtained from mice injected with treated cells or with untreated bone marrow cells (BMC) were examined for levels of VEGF, IGF-1, and HGF at 1 and 3 days post-renal ischemia-reperfusion injury (IRI) or sham operation. Note superphysiological concentrations of circulating VEGF, IGF-1, and HGF in mice injected with treated cells compared with that of untreated BMC injection at 1 or 3 days post-ischemic injury and -cell injection.
P < 0.05.
DISCUSSION
In the present study, we induced a renal-like differentiation from hematopoietic stem and progenitor cells with protein factor and small-molecule treatment. Treated cells express a panel of genes that are known to be expressed at different stages of kidney development. For example, Lim1 is dynamically expressed during renal organogenesis. It is expressed early in the intermediate mesoderm that gives rise to the kidneys and the urogenital system, as well as later in mesonephric tubules, ureteric bud, pretubular aggregates, and their derivatives (11, 23). In comparison, Six2 has a relatively limited expression pattern. It is expressed in renal progenitors localized to the cap mesenchyme and early pretubular aggregates. As the nephron develops, Six2 is not expressed in the renal vesicle or its derivatives (24). Other genes, such as cadherin 16, are expressed late in maturing epithelial cells (42, 43). Detection of a group of genes normally expressed at different stage of kidney development suggests that HSPC may have responded to growth factors and the HDAC inhibitor in a stochastic fashion and that treated cells could be heterogeneous. Heterogeneity could also explain the inability of detecting some genes at the protein level with immunoblot analysis since heterogeneity could dilute the concentration of a newly synthesized protein that might be expressed at a low level in some cells. On the other hand, since kidney development is a continuum that requires coordinated activation of gene networks, induction of hematopoietic cells to differentiate into renal lineage may share a similar expression pattern with sequential activation of multiple renal genes. Although none of the genes examined is exclusively expressed in the developing kidney, the expression of a combination of renal developmentally related genes is suggestive of renal differentiation.
Six2 has been shown to define a multipotent renal progenitor population (18, 24), and the fact that treated cells express Six2 suggests that cell conversion may acquire a renal progenitor stage. Further studies using HSPC isolated from mice carrying GFP in Six2-expressing cells (24) and performing lineage tracing in the progeny of Six2-expressing cells will confirm this finding and address whether Six-expressing cells give rise to other cells resembling a renal phenotype. The use of HSPC as a model system will shed light onto the mechanism and programming of renal differentiation from a multipotent cell source. In our current study, treated cells do not express tubular segment-specific ion transporters and channels, indicating that they have not been fully differentiated to epithelial cells of specific nephron segments. Future testing using models with injury in different tubular segments will reveal whether treated cells can differentiate into multiple segment-specific epithelial cells in vivo, which would offer disease-specific therapy.
Safety is one of the most important concerns in developing stem cell-based therapy. We took the approach of protein- and chemical-based cell treatment to avoid exogenous gene integration and oncogene expression. An average of 36% of cells expressed EYFP within 1 wk, indicating high efficiency of cell conversion. No expression of Oct4 and no teratoma formation indicate that treated cells are not pluripotent and bear little or no risk of differentiating into tumor cells in vivo. Indeed, we did not observe a teratoma up to 6 mo after subcapsular injection into the kidney. With intravenous injection, treated cells tend to localize to the lungs, liver, bone marrow, and spleen. There is a trend of decreasing the number of treated cells in these organs at 3 days, compared with 1 day, after the injection. One possible reason behind this phenomenon is low viability of treated cells once localized to the tissue. This could be analogous to intravenously injected MSC that have been shown to be trapped mainly in the liver and the lungs and have poor survival in these organs (4). Although low survival limits the potential of direct cell replacement for an injured organ, it provides some reassurance in safety of using treated cells for therapy. Recent reports on transcription factor-mediated epigenetic reprogramming indicate that cell lineage and developmental potential of the cells can be stably changed (12, 15, 19, 45–47, 52, 53). Future studies using the strategy of a nonintegrating vector to deliver transcription factors for epigenetic reprogramming may offer stable lineage switching for safe cell therapy.
An HDAC inhibitor, TSA, is used to enhance the efficiency of cell conversion. Although the use of TSA is a potential concern since HDAC inhibition could theoretically induce widespread changes in gene expression, our results indicate that while the expression of renal genes is increased, 18S rRNA does not change. These results are in agreement with the findings that TSA causes selective alterations in 2–10% of gene expression tested in multiple cell types (14, 25, 34, 37, 51). Although the mechanism of selectivity is poorly understood, HDAC inhibitors have proven to be useful in enhancing reprogramming (2, 48). For example, HDAC inhibitor valproic acid increases the induction of induced pluripotent stem cells from 0.04 to 11.4% (16). The effect of an HDAC inhibitor is concentration dependent. Higher concentrations often cause apoptosis whereas lower concentrations often lead to cell differentiation without apparent cytotoxicity (6). We used a low concentration of TSA (15 nM) to treat cells for a short period of time (6 h) at 2nd stage induction, and the medium was changed thereafter. No increase in cell death was observed with this treatment. No TSA was injected into mice to avoid systemic effects.
The effects of intravenous injection of treated cells on improving renal function and structure are significant. Based on a small degree of cell integration into regenerating tubules, direct cell replacement cannot account for acceleration of renal recovery. During culture, cell size is increased from 8.4 to 14.7 μm, which may limit intrarenal transmigration through the microvasculature. However, treated cells are able to produce and release VEGF, IGF-1, and HGF at biologically effective concentrations to stimulate epithelial regeneration. Our results are in good agreement with the study indicating that intravenous infusion of human hematopoietic stem and progenitor cells into mice promotes tubular epithelial and endothelia proliferation, improves renal function, and reduces mortality following renal ischemia-reperfusion injury (26). In this study, human HSPC were mobilized to the peripheral circulation with G-CSF, and purified CD34+ cells (2.5 × 106 cells/mouse) were injected intravenously into mice at 24 h post-renal ischemic injury. Analysis of the mouse kidneys showed that transplanted cells were prominently located in and around the vasculature and expressed markers consistent with endothelial progenitors. However, integration of human cells into mouse capillary walls was a rare event, providing evidence against direct cell replacement as the mechanism of renal protection. Instead, human cells localized to the mouse kidney expressed high levels of proangiogenic cytokines, which could account for their stimulatory effect in endothelial and epithelial proliferation in injured kidneys. Our study differs from this publication in that mouse HSPC were isolated from the bone marrow, expanded, and treated in vitro for 1 wk to obtain a large quantity of renal-like cells before in vivo testing. Although we have shown that treated cells produce high levels of VEGF, IGF-1, and HGF and these factors in combination stimulate proliferation of primary cultures of epithelial cells, it is unclear whether they account for most of the beneficial effects of conditioned medium in vivo. Future studies will examine whether injection of the cocktail can achieve a comparable therapeutic effect as the conditioned medium. A confirmative result will open the possibility of using these readily available factors for potential treatment. In addition, results of the study will also guide us for further identification of other secreted factors that may have beneficial or adverse effects on the regenerating kidney.
In the bone marrow, MSC represent another multipotent cell population and can be isolated and expanded in vitro for potential cell therapy. Endocrine and paracrine effects of MSC in renal protection have also been well documented in rodent models (3, 26, 49, 50). Although many unidentified factors excreted by MSC could contribute to their functions, VEGF has been shown to be an important mediator. Rats treated with MSC had increased renal microvessel density in postischemic kidneys, and knocking down of VEGF in the MSC reduced their therapeutic value, suggesting that renal vascular protection is an essential component in MSC treatment (49, 50). In addition, intravenously injected MSC stimulated tubular epithelial proliferation and decreased apoptosis in mice with cisplatin-induced renal failure. No significant number of administered MSC integrated into injured tubules for direct cell replacement, and renal protection was also achieved by peritoneal injection of conditioned medium collected from MSC cultures. The results indicate that factors produced by the MSC could reach the kidney through the circulation to protect renal tubules (3). Taken together, above studies demonstrate protective effects of MSC in both endothelial cells of the microvasculature and epithelial cells of the tubules. In our study, >99% of the nonadherent cells after the 1st stage expressed CD45 and were transferred to new culture dishes for further induction treatment. Since CD45 is only present in hematopoietic cells but not in MSC, we have excluded nearly all MSC. The rapid expansion of cells during a 1-wk induction (16.5- to 18.9-fold increase in cell number) may also suggest a low probability of MSC contamination because MSC are known to have slow growth after the initial seeding (3, 38). The ability to produce a large number of treated cells is also advantageous compared with MSC, which require weeks of culture to expand cells sufficient for clinical application.
In summary, our current approach shows the promise of generating autologous cells to treat AKI promptly and efficiently. Although direct cell replacement by intravenously injected cells is low due to entrapment in extrarenal organs and limited intrarenal transmigration, treated cells could be used as a safe vehicle to deliver renotrophic factors to accelerate endogenous renal repair. Future studies in increasing intrarenal delivery and promoting treated cell integration into tubules may further enhance the therapeutic value of the cells.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK083411 and RC1DK086886 to F. Lin and P50 DK079328 to the UTSW O'Brien Kidney Research Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.L. and F.L. provided conception and design of research; L.L., R.B., Z.M., Q.Y., A.W., and F.L. performed experiments; L.L., Z.M., Q.Y., A.W., and F.L. analyzed data; L.L. and F.L. interpreted results of experiments; L.L. and F.L. prepared figures; L.L. and F.L. drafted manuscript; L.L. and F.L. edited and revised manuscript; F.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Peter Igarashi for helpful discussions and Jessica Nu, Priyatharshini Alphonse, and Yimei Gong for excellent technical assistance. We thank Abhijit Bugde at the Live Cell Image Core at UTSW for assistance in 3-D image reconstruction. We thank Jessica Nu for proofreading the manuscript.
REFERENCES
- 1. Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 Year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Balasubramaniyan V, Boddeke E, Bakels R, Kust B, Kooistra S, Veneman A, Copray S. Effects of histone deacetylation inhibition on neuronal differentiation of embryonic mouse neural stem cells. Neuroscience 143: 939–951, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Burst VR, Gillis M, Putsch F, Herzog R, Fischer JH, Heid P, Muller-Ehmsen J, Schenk K, Fries JW, Baldamus CA, Benzing T. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol 114: e107–e116, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Canfield PE, Geerdes AM, Molitoris BA. Effect of reversible ATP depletion on tight-junction integrity in LLC-PK1 cells. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1038–F1045, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Carey N, La Thangue NB. Histone deacetylase inhibitors: gathering pace. Curr Opin Pharmacol 6: 369–375, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 58: 658–673, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Ding H, Kopple JD, Cohen A, Hirschberg R. Recombinant human insulin-like growth factor-I accelerates recovery and reduces catabolism in rats with ischemic acute renal failure. J Clin Invest 91: 2281–2287, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dressler GR. Advances in early kidney specification, development and patterning. Development 136: 3863–3874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujii T, Pichel JG, Taira M, Toyama R, Dawid IB, Westphal H. Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev Dyn 199: 73–83, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza I, Vassena R, Raya A, Boue S, Barrero MJ, Corbella BA, Torrabadella M, Veiga A, Izpisua Belmonte JC. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell 5: 353–357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gong Y, Ma Z, Patel V, Fischer E, Hiesberger T, Pontoglio M, Igarashi P. HNF-1beta regulates transcription of the PKD modifier gene Kif12. J Am Soc Nephrol 20: 41–47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray SG, Qian CN, Furge K, Guo X, Teh BT. Microarray profiling of the effects of histone deacetylase inhibitors on gene expression in cancer cell lines. Int J Oncol 24: 773–795, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, Maier LS, Han DW, Glage S, Miller K, Fischer P, Scholer HR, Martin U. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell 5: 434–441, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol 26: 795–797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest 84: 1757–1761, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Humphreys B, Valerius MT, Kobayashi A, Mugford J, Soeung S, Duffield J, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142: 375–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karihaloo A, Karumanchi SA, Cantley WL, Venkatesha S, Cantley LG, Kale S. Vascular endothelial growth factor induces branching morphogenesis/tubulogenesis in renal epithelial cells in a neuropilin-dependent fashion. Mol Cell Biol 25: 7441–7448, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 16: 3527–3534, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132: 2809–2823, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JH, Park JH, Jung Y, Kim JH, Jong HS, Kim TY, Bang YJ. Histone deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by down-regulating thymidylate synthase in human cancer cells. Mol Cancer Ther 5: 3085–3095, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 121: 2211–2220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Truong P, Igarashi P, Lin F. Renal and bone marrow cells fuse after renal ischemic injury. J Am Soc Nephrol 18: 3067–3077, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 14: 1188–1199, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luskey BD, Rosenblatt M, Zsebo K, Williams DA. Stem cell factor, interleukin-3, and interleukin-6 promote retroviral-mediated gene transfer into murine hematopoietic stem cells. Blood 80: 396–402, 1992 [PubMed] [Google Scholar]
- 31. Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1: 55–70, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86: 897–906, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Miller SB, Martin DR, Kissane J, Hammerman MR. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am J Physiol Renal Fluid Electrolyte Physiol 266: F129–F134, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X, Bailey C, Joseph M, Libermann TA, Richon VM, Marks PA, Anderson KC. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA 101: 540–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Molitoris BA, Falk SA, Dahl RH. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J Clin Invest 84: 1334–1339, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okusa MD, Chertow GM, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4: 520–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, Holloway AJ, Johnstone RW. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci USA 102: 3697–3702, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Reddy GP, Tiarks CY, Pang L, Wuu J, Hsieh CC, Quesenberry PJ. Cell cycle analysis and synchronization of pluripotent hematopoietic progenitor stem cells. Blood 90: 2293–2299, 1997 [PubMed] [Google Scholar]
- 40. Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature 345: 686–692, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Scholer HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J 8: 2543–2550, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1824–1836, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol 18: 890–894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Tayaramma T, Ma B, Rohde M, Mayer H. Chromatin-remodeling factors allow differentiation of bone marrow cells into insulin-producing cells. Stem Cells 24: 2858–2867, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292: F1626–F1635, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Togel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 13: 2109–2114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr 5: 245–253, 1996 [PMC free article] [PubMed] [Google Scholar]
- 52. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463: 1035–1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, 2007 [DOI] [PubMed] [Google Scholar]