Abstract
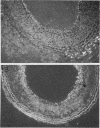
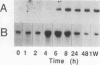
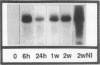
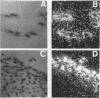
Restenosis currently limits the long-term beneficial effects of balloon coronary angioplasty. Two important cellular events in the development of clinically significant luminal narrowing after angioplasty are 1) increased production of extracellular matrix proteins and 2) acquisition of a motile phenotype by vascular smooth muscle cells. In this paper, smooth muscle cell responses that produce a fibrocellular neointima after acute vascular injury are reviewed. Particular emphasis is placed on specialized extracellular matrix proteins implicated in cell movement and tissue repair. Tenascin and thrombospondin are large, modular extracellular matrix glycoproteins; they possess both adhesive and counteradhesive domains and are expressed at high levels during smooth muscle cell migration and neointima formation after balloon injury to rat carotid artery. The ability of both tenascin and thrombospondin to down-regulate the assembly and activity of focal adhesions (points of cell-extracellular matrix adhesive interactions) may be important in the conversion of stationary, quiescent smooth muscle cells to cells that are able to move and divide within the strongly adhesive vessel wall. Moreover, tenascin is present in the extracellular matrix as a large 6-armed oligomer (a hexabrachion) that contains both cell-binding and matrix protein-binding domains in each of the hexabrachion arms. The large size and multidomain structure of tenascin and thrombospondin suggest that these proteins may be particularly well suited to form a nascent provisional matrix at sites of 1) neointima formation after acute vascular injury, 2) new growth and expansion within primary atherosclerotic plaques, and 3) intimal repair and luminal narrowing in restenosis after angioplasty.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aukhil I., Joshi P., Yan Y., Erickson H. P. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993 Feb 5;268(4):2542–2553. [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Anti-adhesive molecules of the extracellular matrix. Curr Opin Cell Biol. 1991 Oct;3(5):800–804. doi: 10.1016/0955-0674(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Reidy M. A. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest. 1986 Mar;54(3):295–303. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Davies P. F., Robotewskyj A., Griem M. L. Endothelial cell adhesion in real time. Measurements in vitro by tandem scanning confocal image analysis. J Clin Invest. 1993 Jun;91(6):2640–2652. doi: 10.1172/JCI116503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Farhy R. D., Ho K. L., Carretero O. A., Scicli A. G. Kinins mediate the antiproliferative effect of ramipril in rat carotid artery. Biochem Biophys Res Commun. 1992 Jan 15;182(1):283–288. doi: 10.1016/s0006-291x(05)80142-1. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondins. Curr Opin Cell Biol. 1991 Oct;3(5):792–799. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Gravanis M. B., Roubin G. S. Histopathologic phenomena at the site of percutaneous transluminal coronary angioplasty: the problem of restenosis. Hum Pathol. 1989 May;20(5):477–485. doi: 10.1016/0046-8177(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Moar S. L., Richardson M. Deendothelialization in vivo initiates a thrombogenic reaction at the rabbit aorta surface. Correlation of uptake of fibrinogen and antithrombin III with thrombin generation by the exposed subendothelium. Am J Pathol. 1989 Sep;135(3):499–508. [PMC free article] [PubMed] [Google Scholar]
- Hedin U., Holm J., Hansson G. K. Induction of tenascin in rat arterial injury. Relationship to altered smooth muscle cell phenotype. Am J Pathol. 1991 Sep;139(3):649–656. [PMC free article] [PubMed] [Google Scholar]
- Hermans W. R., Rensing B. J., Strauss B. H., Serruys P. W. Prevention of restenosis after percutaneous transluminal coronary angioplasty: the search for a "magic bullet". Am Heart J. 1991 Jul;122(1 Pt 1):171–187. doi: 10.1016/0002-8703(91)90775-d. [DOI] [PubMed] [Google Scholar]
- Itoh H., Mukoyama M., Pratt R. E., Gibbons G. H., Dzau V. J. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest. 1993 May;91(5):2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner V. A., Erickson H. P. Binding of hexabrachion (tenascin) to the extracellular matrix and substratum and its effect on cell adhesion. J Cell Sci. 1990 Feb;95(Pt 2):263–277. doi: 10.1242/jcs.95.2.263. [DOI] [PubMed] [Google Scholar]
- Lightner V. A., Slemp C. A., Erickson H. P. Localization and quantitation of hexabrachion (tenascin) in skin, embryonic brain, tumors, and plasma. Ann N Y Acad Sci. 1990;580:260–275. doi: 10.1111/j.1749-6632.1990.tb17935.x. [DOI] [PubMed] [Google Scholar]
- Lindner V., Olson N. E., Clowes A. W., Reidy M. A. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992 Nov;90(5):2044–2049. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M. M., Burdsal C. A., Erickson H. P., McClay D. R. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989 Oct;109(4 Pt 1):1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Scott-Burden T., Hahn A. W., Kern F., Bernhardt J., Regenass S., Weller A., Bühler F. R. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol. 1992 Aug;141(2):377–388. [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Reidy M. A., Kocher O., Bell L. Endothelial cell behavior after denudation injury is modulated by transforming growth factor-beta1 and fibronectin. Lab Invest. 1989 Jun;60(6):755–765. [PubMed] [Google Scholar]
- Majack R. A., Mildbrandt J., Dixit V. M. Induction of thrombospondin messenger RNA levels occurs as an immediate primary response to platelet-derived growth factor. J Biol Chem. 1987 Jun 25;262(18):8821–8825. [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Schwartz S. M., Clowes M. M., Clowes A. W. Heparin regulates smooth muscle S phase entry in the injured rat carotid artery. Circ Res. 1987 Aug;61(2):296–300. doi: 10.1161/01.res.61.2.296. [DOI] [PubMed] [Google Scholar]
- McNamara C. A., Sarembock I. J., Gimple L. W., Fenton J. W., 2nd, Coughlin S. R., Owens G. K. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993 Jan;91(1):94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Lightner V. A., Aukhil I., Yan Y. Z., Erickson H. P., Hök M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991 Nov;115(4):1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikol S., Isner J. M., Pickering J. G., Kearney M., Leclerc G., Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992 Oct;90(4):1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. R., Alpers C. E., Stewart D. K., Ferguson M., Tran N., Gordon D., Benditt E. P., Hinohara T., Simpson J. B., Schwartz S. M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993 Aug;73(2):223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- Okazaki H., Majesky M. W., Harker L. A., Schwartz S. M. Regulation of platelet-derived growth factor ligand and receptor gene expression by alpha-thrombin in vascular smooth muscle cells. Circ Res. 1992 Dec;71(6):1285–1293. doi: 10.1161/01.res.71.6.1285. [DOI] [PubMed] [Google Scholar]
- Prescott M. F., Webb R. L., Reidy M. A. Angiotensin-converting enzyme inhibitor versus angiotensin II, AT1 receptor antagonist. Effects on smooth muscle cell migration and proliferation after balloon catheter injury. Am J Pathol. 1991 Dec;139(6):1291–1296. [PMC free article] [PubMed] [Google Scholar]
- Prieto A. L., Andersson-Fisone C., Crossin K. L. Characterization of multiple adhesive and counteradhesive domains in the extracellular matrix protein cytotactin. J Cell Biol. 1992 Nov;119(3):663–678. doi: 10.1083/jcb.119.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raugi G. J., Mullen J. S., Bark D. H., Okada T., Mayberg M. R. Thrombospondin deposition in rat carotid artery injury. Am J Pathol. 1990 Jul;137(1):179–185. [PMC free article] [PubMed] [Google Scholar]
- Sarembock I. J., Gertz S. D., Gimple L. W., Owen R. M., Powers E. R., Roberts W. C. Effectiveness of recombinant desulphatohirudin in reducing restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits. Circulation. 1991 Jul;84(1):232–243. doi: 10.1161/01.cir.84.1.232. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]