Abstract
Purpose
Altered glycosylation has been associated with oncogenic potential. Relationships of blood types (where expression is due to glycosylation pattern) and HER2/neu (where expression arises due to altered glycosylation) and breast cancer-associated markers like estrogen receptor/progesterone receptor (ER/PR) were examined and related to outcomes in patients with breast cancer.
Methods
A population-based retrospective study of 426 surgical breast cancer patients examined relationships between (1) patient characteristics, (2) breast tumor characteristics, and (3) outcomes of women diagnosed at the same medical center over a 10-year period relative to specific molecules defined by glycosylation patterns (eg. blood group, HER2/neu) and (4) ER/PR status.
Results
Following stratification by blood group, subjects exhibited significant differences in tumor size with persons in blood groups A and B having greater numbers of tumors ≤2 cm and those with blood types AB and O having tumors >2 cm. After adjusting for age, disease stage, and treatment with trastuzumab, tamoxifen, or aromatase inhibitors, no significant differences were observed in 5-year overall and disease-free survival based on blood type grouping. Blood group B was over-represented among the breast cancer cohort compared to the reference population, while blood group AB was under-represented.
Conclusion
No significant differences were observed in overall and disease-free survival based on blood group. No correlation was noted between HER2/neu, ER or PR status, and blood group type. Among this cohort, HER2/neu positivity was less than 20% and correlated with a 5-year disease-free survival rate ≥75% and overall survival of >80% across all blood groups.
Keywords: Blood type, Breast cancer, Survival, ER/PR, Glycosylation pattern, HER2/neu
The role of glycosylation as a common feature regulating expression of various molecules which undergo change in expression patterns during malignant transformation promotes the possibility that some inter-relationships and/or patterns of expression among these molecules may be discernable as a consequence of altered glycosylation. Further, these patterns may correlate with outcomes.
Correlation of blood group, hormone receptor status, and patient outcomes remains largely unexplored. Knowledge of any relational patterns that may exist between blood type and hormone receptor status as a consequence of altered glycosylation patterns occurring during oncogenesis would be important to recognize clinically, especially if such patterns are related to outcomes.
The study described herein examined ABO isotype and hormone receptor status and their impact on outcomes in a cohort of patients with breast cancer. This cohort represents a subset of a well-defined, racially and ethnically homogeneous reference population, minimizing confounding related to population-based heterogeneity relative to these factors. Using a retrospective study design of 426 breast cancer patients whose ABO blood type, hormonal status, and clinical outcomes were captured in the electronic medical record (EMR) of Marshfield Clinic and the Regional Cancer Registry Database, the present study evaluated relational patterns among the following elements: status of ABO, HER2/neu, estrogen receptor/progesterone receptor (ER/PR), stage, physiological parameters and treatment, clinical outcomes relative to time to recurrence, and 5-year survival in breast cancer patients in a homogeneous population in central Wisconsin.
Study Design and Methods
This study involved retrospective chart review of patients identified in the feasibility study diagnosed with primary breast cancer stages I–III, at Marshfield Clinic (Marshfield, Wisconsin) between January 1, 1998 and June 30, 2006, for whom ABO data were available (n=426), and a second subcohort (n=708) for whom all clinical data, except ABO status, were available. Data captured included date of birth, gender, living/deceased status, date of death, date of breast cancer diagnosis, family history of breast cancer, age at breast cancer diagnosis, menopausal state at time of breast cancer diagnosis, date of last menses (as recorded in medical record) or approximate age of menopause, documentation of co-morbidities at time of death, blood type, hormone receptor status including: HER2/neu status, ER status, PR status, breast cancer site, tumor stage, tumor grade, morphology, regional lymph node involvement, and treatment.
Demographic data were abstracted from Marshfield Clinic’s EMR. ABO data for patients with breast cancer and the reference population were abstracted from the Marshfield Clinic/St. Joseph’s Hospital Joint Venture Laboratory Blood Bank database and/or the EMR. The reference population used to determine frequency distribution of the blood group antigens was drawn from the Marshfield Epidemiologic Study Area (MESA), a well-defined, population cohort which has been utilized historically in many epidemiological studies over the past 30 years. This highly stable, largely rural population resides within 14 ZIP codes in close proximity to Marshfield Clinic’s Marshfield Center and St Joseph’s Hospital, where the majority of its residents receive nearly all of their medical care. The MESA population is very homogeneous, >97% Caucasian and largely of northern European ancestry. All patient encounters are captured in a highly detailed, combined EMR shared by Marshfield Clinic and St Joseph’s Hospital, which has been operative since the early 1960s. Data from the combined EMR are captured in a data warehouse in real time. Tumor status data (date of diagnosis, age at diagnosis, site, grade, morphology, lymph node involvement, treatment) was obtained from the Regional Cancer Registry Database for the index cohort with known ABO status and for the additional sub-cohort of breast cancer patients for whom all data except ABO status was available. Menopausal status was abstracted electronically or manually from the EMR. Menopause was defined as no menses over a 12-month period.
ER/PR and HER2/neu
Estrogen receptor/progesterone receptor (ER/PR) results were retrospectively obtained from the Regional Cancer Registry. Immunohistochemical staining was applied to detect ER/PR expression within sections of formalin-fixed, paraffin-embedded tissues. The estrogen clone used was 1D5, the progesterone clone used was PgR 636. The following criteria were used to score and evaluate ER/PR status during the time frame of this study:
The ER/PR Scoring System and Criteria
Scoring System
0 = Negative for Receptor
1+ = Borderline – correlation with DCC method variable
2+ to 3+ = Positive for Receptor
Criteria
0 = 0% nuclear staining
1+ = <10% nuclear staining
2+ = 10%–75% nuclear staining
3+ = >75% nuclear staining
HER2/neu results were retrospectively obtained from the EMR. Over-expression of HER2 cell membrane receptor protein in breast carcinoma is associated with tumor cell growth, aggressive disease, and shortened survival. A positive test result aids in the assessment for possible treatment eligibility with trastuzumab which targets the HER2 receptor protein. The range of over-expression among breast cancers is reportedly between 25% and 30%. The Food and Drug Administration has approved the reagents used in this immunohistochemistry assay for assessment of HER2/neu receptor status. The clone used was a polyclonal (HER2/neu HercepTest Kit). The following criteria were used to score and evaluate HER2/neu status during the time frame of this study:
The HER2/neu Scoring System and Criteria
Scoring System
0 = Negative
1+ = Negative
2+ = Weak Positive
3+ = Positive
Criteria
0 = Negative: no staining is observed or membrane staining in less than 10% of the tumor cells.
1+ = Negative: a faint/barely perceptible membrane staining is detected in >10% of the tumor cells; cells are only stained in part of the membrane.
2+ = Weak Positive: a weak to moderate complete membrane staining is observed in >10% of the tumor cells.
3+ = Positive: a strong complete membrane staining is observed in >10% of the tumor cells.
Statistical Analysis
Chi-square test was used to evaluate the association between blood type and breast cancer by testing the difference in multinomial distribution of blood type between the population of 12,206 persons in MESA and the sample of 426 breast cancer patients with available ABO and hormone status data. Differences in tumor characteristics among subjects grouped by blood type groups were analyzed using analysis of variance for continuous variables and chi-square test for categorical variables. The Kaplan-Meier product limit method was used to estimate the overall and disease-free survival. Ninety-five percent confidence intervals (CI) for the percentage surviving at a particular time were estimated using the logit transformation. Overall survival was measured from the date of diagnosis to the date of death from any cause. Disease-free survival was measured from the date of first definitive treatment to the date of first relapse or death from any cause. Survival times were censored at the dates of last contact for subjects who were lost to follow-up. Cox-proportional hazard model was used to estimate the hazard ratios and 95% CI for overall and disease-free survival between the blood group types adjusting for age and disease stage. S-plus statistical software was used for survival analysis, and SAS 9.1 (SAS Institute, Cary, NC) was used for all other analyses. A P-value of less than 0.05 was considered statistically significant.
Results
A total of 1,405 invasive breast cancer subjects with stages I to III were identified in the Marshfield Clinic and St. Joseph’s Hospital Regional Cancer Registry from January 1, 1998 to June 30, 2006. Of these, ER, PR, and HER2/neu data were available on 1,134 subjects of which blood type data were available on only 426 subjects whom were included in this study.
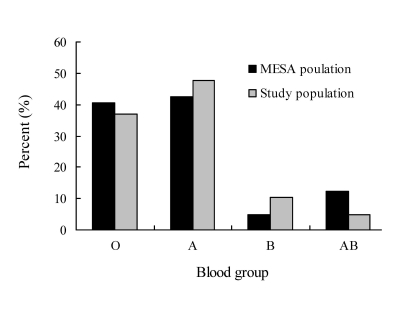
Baseline characteristics of all subjects are presented in table 1. Of 426 subjects, 198 (46.5%) were blood type A group, 163 (38.3%) were blood type O group, 43 (10.1%) were blood type B group, and the remaining 22 (5.2%) were blood type AB group (table 2). Blood type distribution among patients with breast cancer diagnosis for whom blood type data were available did not vary significantly from that of the regional population (P=0.08). However, a trend for over-representation of blood group B and under-representation of blood group AB individuals was noted (figure 1).
Table 1.
Baseline characteristics.
| Patient characteristics | No. of patients (%) (N=426) |
|---|---|
| Age (years) | 63.5+15.0 |
| Tumor stage | |
| I | 230 (54.0%) |
| II | 153 (35.9%) |
| III | 43 (10.1%) |
| Histologic grade | |
| Well differentiated | 77 (18.1%) |
| Moderately differentiated | 163 (38.3%) |
| Poorly differentiated | 171 (40.1%) |
| Missing | 15 (3.5%) |
| Menopausal status | |
| Premenopausal | 297 (69.7%) |
| Postmenopausal | 195 (45.8%) |
| Tumor size | |
| ≤2cm | 297 (69.7%) |
| >2cm | 123 (28.9%) |
| Missing | 6 (1.4%) |
| Lymph node status | |
| Positive | 135 (31.7%) |
| Negative | 238 (55.9%) |
| Not examined | 53 (12.48%) |
| Surgery | |
| No surgery | 11 (2.6%) |
| Masectomy | 396 (93.0%) |
| Lumpectomy | 19 (4.4%) |
| Chemotherapy | 201 (47.21%) |
| Radiotherapy | 252 (59.2%) |
| Hormone replacement therapy | 279 (65.5%) |
| Herceptin | 29 (6.8%) |
| Tamoxifen | 210 (49.3%) |
| Aromatase Inhibitor | 154 (36.2%) |
Table 2.
Overview of all patient characteristics.
| Tumor subtypes | No. of patients (%) |
|---|---|
| ER status | |
| Positive | 318 (74.7%) |
| Negative | 108 (25.4%) |
| PR status | |
| Positive | 242 (56.8%) |
| Negative | 184 (43.2%) |
| HER2 immunohistochemistry | |
| Positive | 88 (20.7%) |
| Negative | 338 (79.3%) |
| Triple negative status | |
| Positive | 62 (14.6%) |
| Negative | 364 (85.4%) |
| Blood group | |
| A | 198 (46.5%) |
| B | 43 (10.1%) |
| AB | 22 (5.2%) |
| O | 163 (38.3%) |
Figure 1.
Comparison of distribution of blood type in the MESA study population. Difference was not statistically significant (chi-square=6.7, P=0.08).
Patients with blood types A and B had the highest rate of tumors ≤2 cm (75.7% and 83.7%, respectively) compared to patients who were blood type AB or O (68.2 and 61.7% respectively (P<0.01). The blood types O and AB groups had more patients with tumors larger than 2 cm (38.3% and 31.8%, respectively) compared to patients with blood types A and B (24.4 and 16.3% respectively (P<0.01) (table 3). No other statistically significant differences in baseline characteristics among the four blood type groups were noted (table 3).
Table 3.
Phenotypic characterization of breast cancer patients by blood group.
| Blood Group | |||||
|---|---|---|---|---|---|
| Characteristic | A (N=276) | B (N=61) | AB (N=28) | O (N=214) | P value* |
| Age (years) | 61.9+15.5 | 67.6+15.3 | 61.6+15.3 | 64.7+14.4 | 0.09 |
| Tumor stage | |||||
| I & II | 177 (89.4%) | 39 (90.7%) | 21 (95.5%) | 146 (89.6%) | 0.84 |
| III | 21 (10.6%) | 4 (9.3%) | 1 (4.6%) | 17 (10.4%) | |
| Histologic grade | |||||
| Well/moderately differentiated | 106 (55.2%) | 26 (63.4%) | 9 (47.4%) | 99 (62.3%) | 0.37 |
| Poorly differentiated | 86 (44.8%) | 15 (36.6%) | 10 (52.6%) | 60 (37.7%) | |
| Menopausal status | |||||
| Premenopausal | 114 (57.6%) | 19 (44.2%) | 14 (63.6%) | 84 (51.5%) | 0.27 |
| Postmenopausal | 84 (42.4%) | 24 (55.8%) | 8 (36.4%) | 79 (48.5%) | |
| Tumor size | |||||
| >2cm | 47 (24.4%) | 7 (16.3%) | 7 (31.8%) | 62 (38.3%) | |
| Lymph node status | |||||
| Positive | 115 (58.1%) | 24 (55.8%) | 16 (72.7%) | 83 (50.9%) | 0.44 |
| Negative | 62 (31.3%) | 13 (30.2%) | 3 (13.6%) | 57 (35.0%) | |
| Not examined | 21 (10.6%) | 6 (13.9%) | 3 (13.6%) | 23 (14.1%) | |
| Chemotherapy | 97 (49.0%) | 13 (30.2%) | 13 (59.1%) | 78 (47.9%) | 0.09 |
| Radiotherapy | 124 (62.6%) | 24 (55.8%) | 11 (50.0%) | 93 (57.1%) | 0.53 |
| Hormone replacement therapy | 130 (65.7%) | 24 (55.8%) | 12 (54.6%) | 113 (69.3%) | 0.26 |
| ER positive | 144 (72.7%) | 35 (81.4%) | 15 (68.2%) | 124 (76.1%) | 0.56 |
| PR positive | 105 (53.0%) | 27 (62.8%) | 13 (59.1%) | 97 (59.5%) | 0.51 |
| HER2/neu positive | 39 (19.7%) | 9 (20.9%) | 4 (18.2%) | 36 (22.1%) | 0.94 |
| Triple Negative status positive | 33 (16.7%) | 5 (11.6%) | 4 (18.2%) | 20 (12.3%) | |
| Trastuzumab use | 18 (6.5%) | 6 (9.8%) | 0 (0.0%) | 11 (5.1%) | 0.29 |
Missing values were excluded
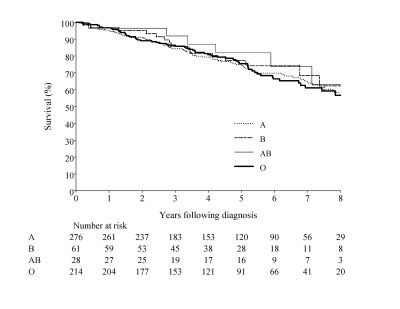
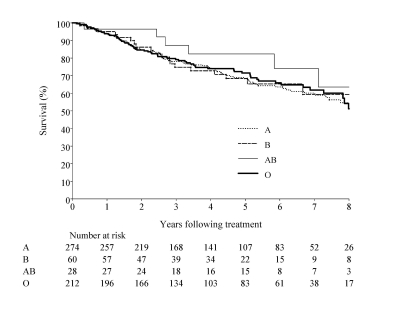
Of the 426 subjects with tumor stage I to III, 54 (12.7%) had recurrence, and 84 (19.7%) died during the follow-up period. The estimated median follow-up period for all subjects was 5.6 years (range: 1.5 months to 9.6 years). The 5-year overall survival for all subjects was 80.7% (95%CI, 75.3 – 86.1), and the disease-free survival was 77.1% (95% CI, 72.4 – 81.8). The 5-year overall and disease-free survival by blood group is presented in table 4. The Kaplan-Meier curve for overall and disease-free survival by tumor subtype is shown in figures 1 and 2. After adjusting for age, disease stage, and treatment with trastuzumab, tamoxifen, or aromatase inhibitors, no significant differences were observed in overall and disease-free survival among the four blood type groups (table 5).
Table 4.
Five-year overall and disease-free survival.
| Survival | ||
|---|---|---|
| Blood group | 5-year overall (95%CI) | 5-year disease-free (95%CI) |
| A | 80.6% (73.9 – 87.2) | 79.3% (72.7 – 85.9) |
| B | 74.5% (59.8 – 89.1) | 68.9% (54.2 – 83.5) |
| AB | 93.2% (80.6 – 100.0) | 93.2% (80.6 – 100.0) |
| O | 79.6% (72.3 – 86.9) | 75.9% (66.2 – 85.7) |
| Overall | 80.7% (75.3 – 86.1) | 77.1% (72.4 – 81.8) |
Figure 2.
Overall survival.
Table 5.
Hazard-ratios adjusted for age, disease stage and treatment using Cox proportional hazard model.
| Hazard ratio (95% CI) | ||
|---|---|---|
| Blood group | 5-year overall survival | 5-year disease-free survival |
| A | 0.9 (0.6 – 1.4) | 1.0 (0.6 – 1.4) |
| B | 1.0 (0.5 – 2.0) | 1.3 (0.7 – 2.4) |
| AB | 0.4 (0.1 – 1.7) | 0.3 (0.1 – 1.3) |
| O | 1.0 | 1.0 |
Compared to the 426 patients for whom blood group status was available, the 708 subjects for whom hormone status data and no blood type data were available had more patients who: (1) were pre-menopausal (69.2% versus 54.2%, P<0.001), (2) had higher rates of ER positivity (79.8% versus 74.7%, P=0.043), and (3) had lower rates of HER2/neu positivity (15.9% versus 20.7%, P=0.045). While the group with no available ABO data tended to have less advanced disease compared to the sub-cohort with known ABO status (6.2% versus 10.1%), the difference did not achieve statistical significance (P=0.218).
Discussion
The main significant finding noted in the present study of patients with breast cancer and known ABO status included significant differences in tumor size (P=0.01). Persons with blood type B (83.7%) and blood type A (75.7%) had smaller tumors (<2cm on average) than persons who were blood type AB (68.2%) or blood type O (61.7%).
While some historical epidemiological studies explored blood group as a risk factor for cancer development, others have examined outcomes relative to blood type. For example, Holdsworth et al1 created a model to test whether blood group offered prognostic value independent of other known clinical or pathological risk factors, and concluded that blood group was a prognostic indicator for breast cancer. In the present study, hazard ratios for survival and disease-free survival were analyzed, adjusting for age, disease stage, and treatment with trastuzumab, aromatase inhibitors, or tamoxifen (table 5). A trend was noted for lower hazard ratios for 5-year survival and 5-year disease-free survival (0.4 and 0.3, respectively) for blood group AB compared to other blood groups. This group also showed the highest rate of 5-year survival and 5-year disease-free survival (93.2% and 93.2%, respectively). However, only 5% of the sub-cohort whose blood group was known was blood type AB.
By 1970, 24 studies (reviewed by Vogel2) suggested that blood type A group was a risk factor for development of cancer due to observed over-representation rates of blood type A group of as high as 8% compared to control populations. Studies focused specifically on breast cancer reported blood type group A over-representation rates, which ranged between 3% to 8%, weaker relative to other types of cancer.2 Anderson and Haas3 also reported a significant excess of blood group A among women with a familial history of breast cancer compared to unselected breast cancer patients with no familial history and blood donor controls.
A shortcoming ascribed to previous studies, however, was that some studies utilized controls that were not representative of the populations from which patients were selected, thus adding ambiguity to exploration of potential risk. Differences relative to ABO distribution among populations suggest that genetic factors in the population under study may impact whether a relationship will be discernable. Moreover, relationships between HER2/neu and ER/PR positivity and breast cancer outcomes were also shown to vary with the population under study,4 again suggesting that a genetic component may be operative. Thus, use of an appropriate reference population is essential to minimizing potential genetic bias. The present study was able to address this shortcoming because the cancer cohort under study was a representative sub-cohort of a regional, epidemiologically well-defined population (MESA), permitting comparison of blood group representation with the larger, appropriate reference population. Further, the MESA population is highly homogeneous with respect to race and ethnicity, with residents >95% Caucasian, and predominantly of northern European (largely German) ancestry, thus minimizing genetic disparity.
The frequency of individuals with breast cancer who exhibited blood type A antigen among the present population-based cohort was 4.8% higher than that of the reference population, reflecting the 3% to 8% range previously reported in the literature.3 Another noteworthy finding included under-representation of blood type AB. Breast cancer patients with blood type AB also had the highest rates for pre-menopausal and lymph node negative status, although differences did not achieve statistical significance likely due to low numbers of patients with this blood type among the cancer cohort. Interestingly, patients with blood type AB had the best outcomes, despite significant differences in tumor size compared to patients who were blood types A or B, and they also had a higher frequency of smaller tumors. By contrast, frequency of patients with breast cancer who were blood type B was nearly twice that observed for the reference population. Patients with blood type B had the poorest outcomes relative to disease-free survival (hazard ratio 1.3, compared to 0.3 for blood type AB) (table 5). However, likely because of the low frequency in representation of blood groups B and AB and the relatively modest rate of over-representation of blood group A among the cancer cohort, differences in representation of the blood groups among patients with breast cancer overall for whom ABO data were available, approached but did not achieve significance (P=0.08) (figure 1).
Within the MESA population, a further comparison was possible with a second sub-cohort of breast cancer patients (n=708) who were similar, in that they were also surgical patients whose ER/PR and HER2/neu status and other clinical data were available, but whose ABO status was unknown. When compared to the sub-cohort whose ABO status was known, the second sub-cohort whose ABO status was unknown exhibited higher pre-menopausal status (P<0.001), higher ER positivity (P=0.043) and lower rates of HER2/neu positivity (P=0.045). While the group with no available ABO data tended to have less advanced disease (6.2% versus 10.1%), this difference did not achieve statistical significance (P=0.218).
Estrogen receptor (ER) and progesterone receptor (PR) positivity is a favorable prognostic factor in breast carcinoma. Combined ER and PR positivity is associated with increased response to anti-estrogen therapies. Estrogen receptors are cellular proteins that bind estrogens with a high affinity and specificity. They are a necessary component for estrogen-mediated cellular activity. The presence of progesterone receptors demonstrates an active ER mechanism for the induction of PR expression. Notably, blood group B individuals also exhibited the highest rates of ER/PR positivity which is considered a favorable prognostic factor in breast carcinoma.5,6 However, this outcome was not reflected in disease-free survival among blood group B individuals who exhibited the lowest rates of 5-year disease-free and overall survival among all of the blood groups. In a previous study by Onitilo et al,7 patients who were hormone receptor ER/PR and HER2/neu triple negative had the worst outcomes relative to mortality and disease-free survival. In the current study, triple negative status across blood groups did not differ significantly and did not correlate with differences noted in overall and disease-free survival or hazard ratios among blood groups (table 3).
Like loss of ABO expression in tissue,8–11 HER2/neu positivity is a result of altered glycosylation leading to HER2/neu expression.12 Over-expression of HER2/neu cell membrane receptor protein in breast carcinoma is associated with tumor cell growth, aggressive disease, and shortened survival. A positive test result aids in the assessment for possible treatment with trastuzumab that targets the HER2/neu receptor protein. Reports in the literature suggest that approximately 20% to 25% of breast cancers test positive for HER2/neu.13 In the present study, no statistically significant difference in distribution relative to HER2/neu status was noted when patients were stratified by blood types. Rate of HER2/neu positivity was approximately 20% across all blood groups in the cohort under study for whom blood group was known. These data are in good agreement with the 5-year overall survival rate (80.7%) among subjects in this study and 5-year disease-free survival (77%) across all blood groups.
Glycosylation is postulated to play a prominent role in breast cancer. It has been proposed that the antigenic expression of a given tumor can be determined by the glycosylation patterns of sugars not normally detected in the tissue.14 Over-expression of N-linked β1,6 branched oligosaccharides has been described in breast cancer,15 and these changes caused alterations in cell adhesion and migration properties.16 Notably, HER2/neu expression is associated with changes in β1,6 glycosylation of integrins, thus enhancing tumor cell capacity for adhesiveness and increasing metastatic potential. Neu, like Src, is a tyrosine kinase receptor oncogene, and both mediate increased upregulation of the N-acetylglucosaminyl transferase activity resulting in increased β1,6 oligosaccharide branching and overexpression of this glycosylation. A study by Chen et al17 demonstrated that HER2/neu expressing cells stimulated N-acetylglucosaminyltransferase V (GlcNAc-T V), a promoter transcription element approximately 400 base pairs from transcription initiation site, causing a three-fold increase in induction of the the GlcNAc-T V without similar induction of other GlcNAc-Ts. Further, these investigators confirmed a three-fold increase in mRNA expression of GlcNAc-T V. Interestingly, this β1,6 transferase is also upregulated by a number of tumor viruses (eg. Rous sarcoma virus and polyoma virus and oncogenes [eg, Ras]).17
By contrast, the terminal glycosylation pattern of blood type A antigen does not involve β1,6 oligosaccharides, but rather consists of the H antigen terminating in an α 1-3 GalNac residue,18 suggesting that this glycosylation would be accomplished by glucosyltransferases distinct from those mediating β1,6 glycosylation. Thus, it is possible that specific classes of glucosyltransfereases may be more susceptible during malignant transformation, and this would be consistent with the lack of correlation between blood type and HER2/neu status noted in the present study. Notably, Narita et al16 have previously described a statistically significant up-regulation of type 2 carbohydrate antigens in breast cancer tissue of patients who experienced poorer outcomes and proposed that these markers might serve as prognostic factors.
Figure 3.
Disease-free survival.
Cellular patterns of glycosylation are known to be markers of cellular differentiation. Previous research has demonstrated that changes in glycosylation patterns of proteins or lipids expressed on the cell surface occur during malignant transformation.19–21 Changes in patterns of carbohydrate expression on cell surfaces may be associated with failure of cells to undergo differentiation and promote acquisition of oncogenic potential. ABO iso-antigens that are expressed both on the surface of blood cells and as histo-antigens, are distinguished on the basis of their glycosylation patterns, and their expression is associated with specific phenotypic characteristics.18 For example, studies by Ichikawa et al8 demonstrated that cells expressing the A iso-antigen showed reduced motility and lacked proliferative and metastatic capacity in vitro. However, when loss of A antigen expression in combination with de novo expression of oncogenes (eg, p53) was noted, increased cell motility was observed, and cells were rendered more resistant to apoptosis, thereby giving rise to proliferative, undifferentiated phenotypes with higher capacity to evade immune surveillance due to loss in differentiation markers.8 A pattern of glycosylation dysregulation at the level of multiple glucosyltransferases within the same tumor may suggest a more prominent role for glycosylation deregulation in facilitating malignant transformation and should be further investigated.
Limitations and Future Directions
Due to the heterogeneity of breast cancer, analyses ideally would have been performed in a breast cancer subtype-specific manner. However, such post hoc analyses of data obtained in this study relative to cancer type were not possible because the reduction in absolute numbers would not yield meaningful data. However, in a larger data set such analyses may highlight meaningful expression patterns that may be less evident when all cancer types are analyzed collectively.
Because of the retrospective nature of our study, a further limitation was availability of ABO status on approximately 38% of the breast cancer patient cohort. Despite the fact that most of these patients represented surgical patients (86% undergoing mastectomy and 8.0% a lumpectomy), ABO data were not routinely available for these patients since blood typing was not a routine procedure for patients at the time of surgery. Thus, ABO data were randomly available in association with a broad spectrum of indications not necessarily associated with breast cancer-related surgical procedures. Therefore, no known bias to a specific subtype of patient was readily discernable among patient selection criteria, which could account for significant differences observed between the two sub-cohorts. However, closer examination of data for patients for whom blood group data were not available indicated that these patients tended to be younger, had a less advanced form of disease, included more pre-menopausal subjects, and exhibited higher ER positivity status and lower HER2/neu positivity status.13 Taken together, these characteristics would be suggestive of better outcomes among this sub-cohort, and while data for the sub-cohort with no ABO data trended in this direction, statistical significance was not achieved.
Future studies to prospectively examine blood group and histological expression of blood group antigens relative to hormone receptor status and oncogene expression at various stages of breast cancer may provide insight into how changes in antigen expression patterns among these various markers correlate with disease progression. Such studies are needed to unravel the complex role of altered glycosylation patterns in carcinogenesis.
Acknowledgements
The authors would like to acknowledge the editorial assistance of the Office of Scientific Writing and Publications in the preparation of this manuscript.
References
- 1.Holdsworth PJ, Thorogood J, Benson EA, Clayden AD. Blood group as a prognostic indicator in breast cancer. Br Med J (Clin Res Ed) 1985;290:671–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel F.ABO blood groups and disease. Am J Hum Genet 1970;22:464–475 [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DE, Haas C. Blood type A and familial breast cancer. Cancer 1984;54:1845–1849 [DOI] [PubMed] [Google Scholar]
- 4.Fatima S, Faridi N, Gill S. Breast cancer: steroid receptors and other prognostic indicators. J Coll Physicians Surg Pak 2005;15:230–233 [PubMed] [Google Scholar]
- 5.Liu S, Chia SK, Mehl E, Leung S, Rajput A, Cheang MC, Nielsen TO. Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res 2010;119:53–61 [DOI] [PubMed] [Google Scholar]
- 6.Lower EE, Glass EL, Bradley DA, Blau R, Heffelfinger S. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat 2005;90:65–70 [DOI] [PubMed] [Google Scholar]
- 7.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009. June;7: 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichikawa D, Handa K, Withers D, Hakomori S. Histo-blood group A/B versus status of human carcinoma cell motility: approach with A and B gene transfection. Cancer Res 1997;57:3092–3096 [PubMed] [Google Scholar]
- 9.Greenwell P. Blood group antigens: molecules seeking a function? Glycoconj J 1997;14:159–173 [DOI] [PubMed] [Google Scholar]
- 10.Nakagoe T, Fukushima K, Tuji T, Sawai T, Nanashima A, Yamaguchi H, Yasutake T, Hara S, Ayabe H, Matuo T, Kamihira S. Immunohistochemincal expression of ABH/Lewis-related antigens in primary breast carcinoma and metastatic lymph node lesions. Cancer Detect Prev 1998;22:499–505 [DOI] [PubMed] [Google Scholar]
- 11.Idikio HA, Manickavel V. A, B, H, and Lewis-a and Lewis-b blood group antigens in human breast cancer: correlation with steroid hormone receptor and disease status. J Cancer Res Clin Oncol 1993;119:486–492 [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi H, O’Neill A, Gelman R, Carney W, TEnney DY, Hosch S, Hayes DF. Prediction of response to anti estrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER2/c-neu protein. J Clin Oncol 1997;15:2518–2525 [DOI] [PubMed] [Google Scholar]
- 13.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong H, Bauerfeind I, Felber M, Wang H, Beryt M, Seshadri, Hepp H, Slamon DJ. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 2003;95:142–153 [DOI] [PubMed] [Google Scholar]
- 14.Dennis JW, Laferté S. Oncodevelopmental expression of--GlcNAc beta 1-6 Man alpha 1-6Man beta 1--branched asparagine-linked oligosaccharides in murine tissues and human breast carcinomas. Cancer Res 1989;49:945–950 [PubMed] [Google Scholar]
- 15.Demetriou M, Nabi IR, Coppolino M, Dedhar S, Dennis JW. Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. J Cell Biol 1995;130:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narita T, Funahashi H, Satoh Y, Watanabe T, Sakamoto J, Takagi H. (1993) Association of expression of blood group-related carbohydrate antigens with prognosis in breast cancer. Cancer 1993;71:3044–3053 [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Zhang W, Fregien N, Pierce M. The her2/meu oncogene stimulates the transcription of N-acetylglucosaminyltransferase V and expression of its cell surface oligosaccharide products. Oncogene 1998; 17:2087–2093 [DOI] [PubMed] [Google Scholar]
- 18.Ravn V, Dablesteen E. Tissue distribution of histo-blood group antigens. APMIS 2000;108:1–28 [DOI] [PubMed] [Google Scholar]
- 19.Chihara Y, Sugano K, Kobayashi A, Kanai Y, Yamamoto H, Nakazono M, Fujimoto H, Kakizoe T, Fujimoto K, Hirohashi S, Hirao Y. Loss of blood group A antigen expression in bladder cancer caused by allelic loss and/or methylation of the ABO gene. Lab Invest 2005;85:895–907 [DOI] [PubMed] [Google Scholar]
- 20.Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol 1996;179:358–369 [DOI] [PubMed] [Google Scholar]
- 21.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 2002;99:10231–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]