ABSTRACT
Cell surface expression of sialic acid has been reported to decrease during immune cell activation, but the significance and regulation of this phenomenon are still being investigated. The major human bacterial pathogen Streptococcus pneumoniae causes pneumonia, sepsis and meningitis, often accompanied by strong inflammatory responses. S. pneumoniae expresses a sialidase (NanA) that contributes to mucosal colonization, platelet clearance, and blood-brain barrier penetration. Using wild-type and isogenic NanA-deficient mutant strains, we showed that S. pneumoniae NanA can desialylate the surface of human THP-1 monocytes, leading to increased ERK phosphorylation, NF-κB activation, and proinflammatory cytokine release. S. pneumoniae NanA expression also stimulates interleukin-8 release and extracellular trap formation from human neutrophils. A mechanistic contribution of unmasking of inhibitory Siglec-5 from cis sialic acid interactions to the proinflammatory effect of NanA is suggested by decreased SHP-2 recruitment to the Siglec-5 intracellular domain and RNA interference studies. Finally, NanA increased production of proinflammatory cytokines in a murine intranasal challenge model of S. pneumoniae pneumonia.
Importance Sialic acids decorate the surface of all mammalian cells and play important roles in physiology, development, and evolution. Siglecs are sialic acid-binding receptors on the surface of immune cells, many of which engage in cis interactions with host sialoglycan ligands and dampen inflammatory responses through transduction of inhibitory signals. Recently, certain bacterial pathogens have been shown to suppress leukocyte innate immune responses by molecular mimicry of host sialic acid structures and engagement of inhibitory Siglecs. Our present work shows that the converse can be true, i.e., that a microbial sialic acid-cleaving enzyme can induce proinflammatory responses, which are in part mediated by unmasking of an inhibitory Siglec. We conclude that host leukocytes are poised to detect and respond to microbial sialidase activity with exaggerated inflammatory responses, which could be beneficial or detrimental to the host depending on the site, stage and magnitude of infection.
Importance
Sialic acids decorate the surface of all mammalian cells and play important roles in physiology, development, and evolution. Siglecs are sialic acid-binding receptors on the surface of immune cells, many of which engage in cis interactions with host sialoglycan ligands and dampen inflammatory responses through transduction of inhibitory signals. Recently, certain bacterial pathogens have been shown to suppress leukocyte innate immune responses by molecular mimicry of host sialic acid structures and engagement of inhibitory Siglecs. Our present work shows that the converse can be true, i.e., that a microbial sialic acid-cleaving enzyme can induce proinflammatory responses, which are in part mediated by unmasking of an inhibitory Siglec. We conclude that host leukocytes are poised to detect and respond to microbial sialidase activity with exaggerated inflammatory responses, which could be beneficial or detrimental to the host depending on the site, stage and magnitude of infection.
Introduction
Sialic acids are nine-carbon sugars prominently displayed as terminal monosaccharides on surface expressed glycoconjugates of all mammalian cells (1, 2). Sialic acids serve key roles in a diverse array of physiological and pathological processes, including organ development, immune regulation, microbial binding, malignancy, and aspects of human evolution (1, 2). An important facet of sialic acid biology is the function of immunoreceptors called sialic acid-binding immunoglobulin-like lectins, or Siglecs, which are differentially expressed across the major leukocyte lineages (3–5). A subset of these are the CD33-related Siglecs (CD33rSiglecs), whose extracellular sialic binding domains are typically paired with a cytoplasmic domain containing both a membrane-proximal immunoreceptor tyrosine-based inhibitory motif (ITIM) and a membrane-distal ITIM-like motif (3, 5).
Two inhibitory CD33rSiglecs expressed prominently on monocytes/macrophages and neutrophils are Siglec-5 and Siglec-9, which recruit phosphatases SHP-1 and SHP-2 to their ITIM and ITIM-like cytoplasmic domains, thereby antagonizing kinase-dependent activation cascades (6). Since the local concentration of sialic acids on surfaces of leukocytes is very high, perhaps exceeding 100 mM (7), Siglec binding sites are typically “masked” by cis interactions with other sialoglycan ligands expressed on the same cell and can be unmasked by sialidase treatment (8). The widespread expression of host sialic acids and the prominence of cognate ITIM-bearing CD33rSiglecs on innate immune cells suggest that they may function in “self-recognition” as “self-associated molecular patterns,” dampening innate immune responses to prevent autoreactivity (3, 5, 9).
A number of bacteria synthesize sialic acids or acquire them from the host environment and incorporate them into cell wall or surface components such as lipooligosaccharides (LOS), lipopolysaccharides (LPS), or capsular polysaccharides (CPS) (10). In some cases, “molecular mimicry” of host sialic acid epitopes allows the pathogen to engage inhibitory Siglecs, contributing to virulence by suppressing immune responses or altering leukocyte differentiation programs. For example, the sialylated CPS of group B streptococcus can engage Siglec-9 to suppress neutrophil activation (11–13) and the sialylated LOS of Campylobacter jejuni can bind Siglec-7 to influence dendritic cell-mediated T cell responses to Th1 or Th2 polarization (14, 15).
In contrast, numerous microbial pathogens express enzymes that cleave sialic acid (sialidases; also called neuraminidases) (16, 17), such as the viruses causing influenza (18) or mumps (19), the bacterial pathogens Streptococcus pneumoniae (20) and Pseudomonas aeruginosa (21), the fungus Aspergillus fumigatus (22), and the protozoan parasite Trypanosoma cruzi (23). The overall extent of cell surface sialylation has been found to decrease in activated macrophages, neutrophils, B cells, T cells, and natural killer cells (24–28), suggesting that “unmasking” of Siglecs from cis sialic acid engagement occurs during immune activation (27). The processes that dynamically regulate Siglec unmasking are unknown but may in part involve endogenous mammalian sialidases (8), such as that encoded by the neu-1 gene located within the major histocompatibility complex in humans (26, 29). A potential role of sialidases produced by microbial pathogens in Siglec unmasking has not been studied.
We hypothesized that if bacterial sialic acid mimicry could suppress host innate immune cell responses via engagement of inhibitory Siglecs, then cell surface desialylation by a bacterial sialidase could have contrasting effects. S. pneumoniae is the leading human bacterial pathogen causing upper respiratory tract infections, pneumonia, sepsis and meningitis, resulting in over a million deaths worldwide each year (30). To establish an experimental model for testing our hypothesis, we used isogenic strains of S. pneumoniae expressing or lacking their major sialidase, the surface-anchored NanA. The effect of S. pneumoniae sialidase expression on proinflammatory cytokine responses was examined in a human monocyte cell line and in primary neutrophils and then extended to a murine model of pneumococcal pneumonia. We document a proinflammatory effect of S. pneumoniae desialylation of the leukocyte cell surface associated with ERK phosphorylation and NF-κB activation. Evidence is provided that unmasking of Siglec-5, the inhibitory CD33rSiglec, is a contributing factor to this phenomenon.
RESULTS
S. pneumoniae sialidase (NanA) mediates cell surface desialylation and increases monocyte cytokine responses.
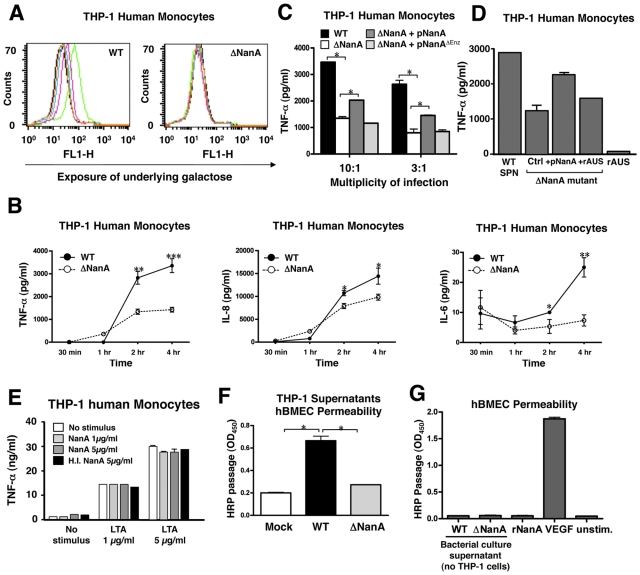
NanA from S. pneumoniae has been shown to desialylate human airway epithelial cell glycoconjugates in vitro (31). We tested whether infection of human monocytic cells with wild-type (WT) S. pneumoniae led to general cell surface desialylation. As shown in Fig. 1A, S. pneumoniae-infected THP-1 cells exhibited a clear dose-dependent increase in Erythrina cristagalli agglutinin lectin binding, indicating removal of terminal sialic acids and exposure of underlying Galβ1-4GlcNAcβ1 units. The isogenic sialidase-deficient S. pneumoniae mutant (ΔNanA) did not demonstrate such activity at the same multiplicity of infection (MOI), confirming that the increased E. cristagalli agglutinin binding was due to sialidase action. THP-1 cells were then challenged with WT or ΔNanA S. pneumoniae for different time intervals, and the release of proinflammatory cytokines into the culture supernatant was determined by enzyme-linked immunosorbent assay (ELISA). A significant increase in secretion of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8 from S. pneumoniae-infected THP-1 monocytes compared to ΔNanA mutant-infected monocytes was observed 2 h postinfection (Fig. 1B). Similar findings were observed for TNF-α production with the U937 human monocytic cell line (data not shown). Augmented cytokine responses were not a byproduct of differing growth or survival of WT S. pneumoniae versus ΔNanA S. pneumoniae, because THP-1 displayed no obvious bactericidal activity and no differences in THP-1 phagocytosis between the S. pneumoniae and ΔNanA mutant strains were found (data not shown). Complementation of the S. pneumoniae ΔNanA mutant with the NanA enzyme expressed on a plasmid vector partially restored the cytokine stimulation phenotype; in contrast, complementation of the ΔNanA mutant with an enzymatically inactive version of NanA (32) had no effect (Fig. 1C). These observations indicate that S. pneumoniae NanA is required for monocyte cell surface desialylation and that the enzymatic activity is associated with elevated proinflammatory cytokine responses. Addition of recombinant purified Arthrobacter ureafaciens sialidase did not itself augment THP-1 monocyte production of TNF-α; neither did it restore the TNF-α induced by the ΔNanA mutant to the levels induced by the WT or pNanA-complemented mutant strains (Fig. 1D). Also, purified recombinant S. pneumoniae NanA enzyme (from QA-Bio) did not induce increased release of TNF-α from unstimulated or lipoteichoic acid (LTA)-stimulated THP-1 monocytes (Fig. 1E). Thus, the action of NanA desialylation to lower the activation threshold of THP-1 cells occurs only in the context of the live S. pneumoniae infection and is perhaps mediated by desialylation events triggered by surface-anchored NanA at the site of bacterium-host cell engagement.
FIG 1 .
S. pneumoniae sialidase (NanA) mediates cell surface desialylation and increases monocyte cytokine responses. Surface desialylation was determined by FITC-conjugated E. cristagalli agglutinin staining of human THP-1 monocytes infected with WT S. pneumoniae or ΔNanA mutant for 3 h. Green line, MOI = 10; pink line, MOI = 3; blue line, MOI = 1. (B) Time course analysis of proinflammatory cytokine production. THP-1 cells were infected with WT S. pneumoniae or ΔNanA mutant at an MOI = 10 for 30 min or 1, 2, or 4 h, and the amount of IL-6, IL-8, and TNF-α produced in the culture supernatant was measured by ELISA. Experiments were conducted three times with biological duplicates. (C) TNF-α concentration in supernatants collected 3 h postinfection from THP-1 cells challenged with WT S. pneumoniae, ΔNanA mutant, or the ΔNanA mutant strain complemented with NanA or enzymatically inactive NanA expression plasmids. Experiments were performed twice with biological duplicates. (D) Addition of recombinant Arthrobacter ureadaciens sialidase (rAUS, 50 mU/ml) from Arthrobacter ureadaciens did not undo the reduction in the level of TNF-α stimulation observed with the ΔNanA mutant. (E) Purified recombinant NanA alone did not induce TNF-α release from unstimulated or lipoteichoic acid (LTA)-stimulated THP-1 monocytes. SPN, S. pneumoniae. (F) Permeabilization of hBMEC monolayers. Culture supernatants of THP-1 cells were collected 4 h after infection with WT S. pneumoniae or the ΔNanA mutant strain or mock infection, and then the permeability of hBMECs was assessed by measuring HRP passage following an 8-h incubation of hBMECs with the collected THP-1 supernatants. Experiments were done twice with biological triplicates. H.I., heat inactivated. (G) Neither WT S. pneumoniae bacterial culture supernatant nor purified recombinant NanA is sufficient to induce hBMEC permeability, indicating that NanA-mediated cytokine induction from THP-1 cells is the major inducing factor. VEGF = vascular endothelial growth factor, used at 200 ng/ml as a positive control. Statistical analysis was performed using Student’s t test (B) or one-way analysis of variance (ANOVA) with Tukey’s posttest (C and F). ***, P < 0.001; **, P < 0.01; *, P < 0.05.
S. pneumoniae NanA-stimulated monocyte supernatants increase hBMEC permeability.
During sepsis and meningitis, overactivation of inflammatory monocytes/macrophages is felt to play an important role in endothelial barrier dysfunction (33), and increased blood-brain barrier permeability and meningeal TNF-α levels are found in S. pneumoniae-challenged mice (34). We observed that supernatants from WT S. pneumoniae-infected THP-1 cells significantly increased the permeability of human brain microvascular endothelial cell (hBMEC) monolayers by a large indicator protein (horseradish peroxidase [HRP]) compared to the supernatants collected from uninfected or ΔNanA mutant-infected THP-1 cells (Fig. 1F). Neither purified supernatant from WT S. pneumoniae bacteria (grown in the absence of THP-1 cells) nor recombinant NanA itself was sufficient to induce hBMEC permeability (Fig. 1G). These finding suggests that NanA-mediated surface desialylation can increase monocyte release of proinflammatory cytokines, with potential downstream effects on endothelial cell permeability.
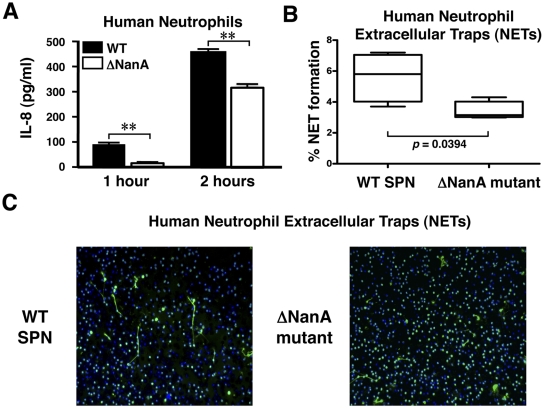
S. pneumoniae sialidase NanA increases neutrophil IL-8 secretion and neutrophil extracellular trap (NET) formation.
Neutrophils are critical first-line effector immune cells in the innate host response to bacterial infection. As shown in Fig. 2A, WT S. pneumoniae induced significantly more IL-8 secretion from human neutrophils than did the isogenic ΔNanA mutant. Neutrophil extracellular traps (NETs) are networks of extracellular fibers composed of DNA, histones, and embedded antimicrobial peptides and enzymes capable of capturing and killing bacterial pathogens (35, 36). Using a myeloperoxidase stain to aid in visualization and quantification of NETs, we found that WT S. pneumoniae induced more NET formation than the ΔNanA mutant (Fig. 2B and C). Thus, S. pneumoniae sialidase production enhances innate immune activation of neutrophils in a fashion analogous to our observations in THP-1 monocytes.
FIG 2 .
S. pneumoniae sialidase NanA increases neutrophil IL-8 secretion and extracellular trap (NET) formation. (A) Neutrophils were infected with WT S. pneumoniae or ΔNanA mutant (MOI = 10) for 1 or 2 h, and IL-8 released into the culture supernatant was measured by ELISA. Experiments were done twice with biological duplicates from two different donors. (B) Quantification of NETs after coincubation of neutrophils with WT S. pneumoniae or ΔNanA mutant at an MOI = 0.1 for 90 min. Cells were fixed and stained with primary rabbit anti-myeloperoxidase (anti-MPO) antibody followed by a secondary Alexa 488-conjugated goat anti-rabbit antibody to visualize NETs (green). DNA is stained with DAPI (blue). Representative immunofluorescence micrographs of NETs are shown in panel C, and quantitative results are shown in panel B. Statistics analysis was performed using Student’s t test. **, P < 0.01.
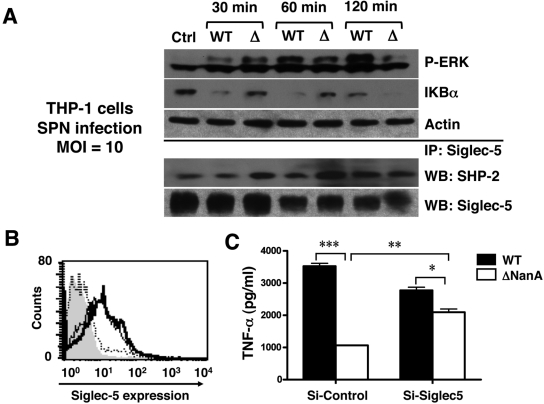
Signaling pathways implicated in macrophage inflammatory activation by S. pneumoniae NanA.
Bacterial infections are well known to activate pattern recognition pathways such as Toll-like receptors (TLRs) to initiate downstream signaling leading to mitogen-activated protein kinase (MAPK) and NF-κB activation. We first analyzed the activation of ERK, Jun N-terminal protein kinase (JNK), and p38 MAPKs by Western blotting using specific antibodies (Abs) recognizing the activated phosphorylated forms of each protein. We found that, while THP-1 cells infected with WT S. pneumoniae exhibited greater phosphorylation of ERK than those infected with the ΔNanA mutant (Fig. 3A), there was no detectable phosphorylation for p38 and JNK when subjected to the same stimulation (data not shown). In addition, infection of monocytes with WT S. pneumoniae led to accelerated degradation of IκB compared to infection with the ΔNanA mutant, indicating liberation of NF-κB for nuclear translocation and inflammatory cytokine gene transcription (Fig. 3A). Increased ERK phosphorylation and IκB degradation in response to S. pneumoniae sialidase activity are in accordance with the enhanced secretion of inflammatory cytokines in the S. pneumoniae-infected THP-1 cells (Fig. 1B and 1C) and with earlier published findings in which exogenous addition of a sialidase enzyme enhanced reactivity of phytohemagglutinin-treated lymphocytes (37) or ERK phosphorylation and cytokine production in LPS-treated monocytes (38).
FIG 3 .
Signaling pathways implicated in macrophage inflammatory activation by S. pneumoniae NanA. (A) THP-1 cells were infected with WT S. pneumoniae or ΔNanA mutant at an MOI = 10. At the indicated times, cell lysates were analyzed by immunoblotting for ERK1/2 phosphorylation and IκB degradation. SHP-2 recruitment to Siglec-5 was revealed by immunoprecipitating cell lyates with anti-Siglec-5 Ab, followed by probing with anti-SHP-2 Ab. (B) Knockdown of Siglec-5 expression by RNA interference. THP-1 cells were infected with lentiviruses carrying control shRNA (thin line) or Siglec-5 targeting shRNA (dashed line), and the knockdown efficiency was determined by FACS analysis with APC-conjugated anti-Siglec-5 MAb to measure cell surface Siglec-5 expression. THP-1 cells stained with APC-conjugated isotype MAb (solid gray) and anti-Siglec-5 MAb (thick line) served as negative and positive controls, respectively. (C) Control or Siglec-5 knockdown THP-1 cells were infected with WT S. pneumoniae or ΔNanA mutant at MOI = 10 for 3 h, and the culture supernatants were collected to determine TNF-α concentrations. Experiments were conducted twice with biological duplicates. Statistical analysis was performed by one-way ANOVA with Tukey’s posttest. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
CD33rSiglecs have been shown to downregulate both innate and acquired immune responses, likely via cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that recruit SHP family proteases to suppress tyrosine kinase-dependent signals (3, 39). Siglec-5 and Siglec-9 are the predominant CD33rSiglecs expressed on monocytes and neutrophils. Since THP-1 cells have been shown to express Siglec-5 but possess no detectable mRNA for Siglec-9 (40), we immunoprecipitated Siglec-5 to analyze SHP-2 recruitment status following WT S. pneumoniae or ΔNanA mutant infection. As shown in Fig. 3A, surface desialylation by WT S. pneumoniae infection reduced SHP-2 recruitment to Siglec-5 compared to results obtained with ΔNanA mutant-infected monocytes. Temporally, diminished SHP-2 recruitment occurred earlier (30 min to 1 h postinfection) than ERK activation (1 to 2 h postinfection). This observation suggested that decreased inhibitory signals transduced from Siglec-5/SHP after surface desialylation to uncap Siglec receptors interaction could affect the activation status of the infected monocytes. We used RNA interference to silence the cell surface expression of Siglec-5 on THP-1 cells and confirmed knockdown efficiency by fluorescence-activated cell sorter (FACS) analysis. As shown in Fig. 3B, THP-1 cells infected with Siglec-5 short hairpin RNA (shRNA) lentivirus showed very little or no Siglec-5 staining compared to parental THP-1 cells and THP-1 cells infected with a control lentivirus. Supernatants from control and Siglec-5 knockdown THP-1 cells were then collected after infection with WT S. pneumoniae or ΔNanA mutant for TNF-α quantitation. As seen in parental THP-1 cells (Fig. 1B), S. pneumoniae infection induced more TNF-α secretion in control lentivirus-infected cells than did infection with the ΔNanA mutant (Fig. 3C). Although WT S. pneumoniae still triggered more TNF-α secretion than the ΔNanA mutant in Siglec-5 knockdown cells, the difference was not as pronounced as that observed in control cells. Further, the amount of TNF-α secretion in response to ΔNanA mutant infection was increased in the Siglec-5 knockdown THP-1 cells (Fig. 3C). Our findings suggested that NanA-mediated disruption of the Siglec-cis ligand interactions by surface desialylation may reduce Siglec-mediated inhibitory signals and contribute to the phenomenon of increased macrophage proinflammatory cytokine release.
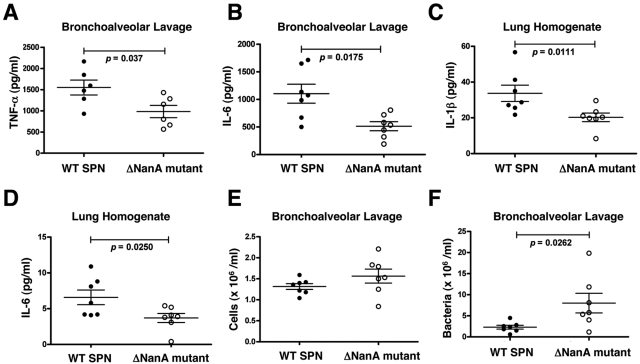
S. pneumoniae NanA expression increases production of multiple inflammatory cytokines in a mouse intranasal infection model.
Previous work in a chinchilla otitis model showed that infection with WT S. pneumoniae but not a NanA-deficient mutant strongly decreased Sambucus nigra agglutinin labeling, indicating loss of cell surface sialic acid residues and resulting in exposure of underlying galactose residues (41). We examined whether infectious challenge with WT S. pneumoniae versus the ΔNanA mutant would alter proinflammatory cytokine responses in vivo using a murine intranasal challenge model of pneumonia. Significantly higher levels of TNF-α (6 h postchallenge) and IL-6 (20 h postchallenge) were present in the bronchoalveolar lavage (BAL) fluid of WT S. pneumoniae-infected animals compared to BAL fluid recovered from ΔNanA mutant-infected mice (Fig. 4A and B). At the 20 h postinfection time point, significantly increased levels of IL-6 and IL-1β were identified in homogenates of lung tissue (Fig. 4C and D). The differences in cytokine production in BAL fluid and lungs were not due to different numbers of macrophages and neutrophils, because similar cell counts (P = 0.1649) were obtained with the BAL fluid recovered from WT S. pneumoniae and ΔNanA mutant-infected animals (Fig. 4E). Interestingly, fewer WT S. pneumoniae bacteria than ΔNanA mutant bacteria were recovered from BAL fluids (Fig. 4F), suggesting that a greater inflammatory response or NET induction elicited by NanA sialidase activity increased bacterial clearance during the short-term-infection period.
FIG 4 .
S. pneumoniae NanA expression increases production of multiple inflammatory cytokines in a mouse intranasal infection model. Mice were infected intranasally with 1 × 107 CFU of WT S. pneumoniae or ΔNanA mutant, and cytokine levels in cell-free BAL fluid or lung homogenates were measured by ELISA. (A) TNF-α in BAL fluid at 6 h; (B) IL-6 in BAL fluid at 20 h; (C) IL-1β in lung homogenates at 20 h; (D) IL-6 in lung homogenates at 20 h. (E) Recruitment of inflammatory cells from the circulation to BAL fluid was determined using a Beckman particle counter 20 h postinfection. (F) Bacterial load (CFU) in BAL fluids of mice 20 h postinfection. Differences between the 2 groups were analyzed by Mann-Whitney test.
DISCUSSION
In the present report, we demonstrate that S. pneumoniae NanA augments proinflammatory cytokine production by human THP-1 cells and neutrophils during S. pneumoniae infection in vitro. This novel function of NanA-mediated desialylation may derive from unmasking of an inhibitory CD33rSiglec(s) from cis ligands and was corroborated by accelerated IκB degradation, enhanced ERK phosphorylation, and reduced SHP-2 recruitment to Siglec-5.
The mammalian host protects itself from infection through rapid recognition of pathogens and activation of innate immune responses; however, unregulated activation can produce detrimental consequences of systemic inflammation, multiorgan failure, and disseminated intravascular coagulation (DIC) (42, 43). Therefore, the innate immune system needs to be tightly controlled, and it is widely accepted that receptors containing immunoreceptor tyrosine-based inhibitory motifs (ITIMs) can be employed to switch off cellular responses through recruitment of phosphotyrosine phosphatases (PTPs) such as SHP-1 and SHP-2 to decrease tyrosine phosphorylation. Moreover, the engagement of inhibitory ITIM-containing receptors also blocks activation signals originating from receptors associated with immunoreceptor tyrosine-based activating motifs (ITAMs) (44, 45). Given the ubiquitous expression of sialic acids on mammalian cell surfaces and the prominence of cognate ITIM-bearing CD33rSiglecs on immune cells, Siglecs are speculated to control the activation threshold of immune cells (3, 5), using sialic acids as “self-associated molecular patterns” (9). Our findings provide new evidence to support the hypothesis that unmasking Siglecs from cis ligands can increase immune cell inflammatory responses.
We observed more SHP-2 recruitment to Siglec-5 in THP-1 monocytes after infection with the ΔNanA mutant compared to those infected with the WT S. pneumoniae parent strain. This finding suggests that reduced Siglec-mediated inhibitory signals are involved in the NanA-dependent proinflammatory cytokine stimulation phenotype. In addition, knockdown of the expression of Siglec-5 in the THP-1 cells reduced differential cytokine induction elicited by WT S. pneumoniae versus the ΔNanA mutant. This finding further supported the hypothesis that unmasking of Siglec-5 by NanA is critical to enhance proinflammatory cytokine production during S. pneumoniae infection. THP-1 cells have no detectable mRNA of Siglec-9 (40); however, knockdown of the Siglec-5 expression still did not completely abolish the NanA-dependent proinflammatory cytokine induction increases. Although the NanA mutant in S. pneumoniae strain D39 has negligible residual activity, as shown by in vitro sialidase assays (32, 46), an indispensable secondary role of S. pneumoniae sialidases such as NanB cannot be summarily excluded (47). In addition, mammalian Neu1 sialidase was shown to form a complex with Toll-like receptor-2 (TLR2), TLR3, and TLR4 and facilitate TLR4 clustering, MyD88/TLR4 complex formation, and subsequent NF-κB activation upon LPS engagement (48, 49). Indeed, purified S. pneumoniae NanA and Trypanosoma cruzi trans-sialidase were demonstrated to activate NF-κB signaling pathways in the absence of TLR ligands (48). Therefore, it is possible that, in addition to inhibitory Siglec unmasking, TLR2/TLR6, a receptor complex for Gram-positive bacterial recognition, could also be desialylated by endogenous Neu1 or by NanA itself upon S. pneumoniae infection, facilitating its dimerization and downstream signaling activation.
NanA-mediated desialylation affects numerous glycoconjugates (glycoproteins and glycolipids) on the host cell surface, since their structures are often capped by terminal sialic acids. Furthermore, such desialylation can simultaneously expose underlying N-acetyllactosamine (Galβ1-4GlcNAc) ligands for galectins. Galectins can bind and translate this glycan-encoded information into immune cell activation, differentiation, and homeostatic programs. These lectins appear to function by forming ordered galectin–glycan lattices on the cell surface, leading to immunoregulatory activities (50, 51). For example, galectin-3-mediated ligand clustering triggered neutrophils to phagocytose, produce reactive oxygen species, release proteases, and secrete IL-8 (52, 53). On the other hand, galectin-1 inhibited nitric oxide synthesis and increased the arginase activity of macrophages (54). The immunomodulatory effects of galectins may be integrated into the cumulative effects on proinflammatory cytokine secretion observed after NanA-mediated desialylation. However, due to the short-duration experiments performed on THP-1 cells and neutrophils, the endogenous concentration of galectins in our assay systems should not be as high as those reported from studies in the previous literature, in which purified exogenous galectins were added into immune cells. By knocking down the expression of Siglec-5, we demonstrated that Siglec-cis ligand interactions are themselves a critical control element in the activation status of immune cells.
All S. pneumoniae clinical isolates express the surface-anchored NanA (55, 56), which can cleave terminal sialic acid residues α2-3 or α2-6 linked to galactose, mirroring the ligand preferences of several CD33rSiglecs. NanA cleaves α2-3- and α2-6-linked substrates with equal levels of efficiency and exhibits more than 10-fold-greater overall sialidase activity than NanB, an enzyme that exhibits a 5-fold preference for α2-3 over α2-6 linkages (57). Prior work has established that NanA plays multiple roles in S. pneumoniae pathogenesis, including modification of the nasopharyngeal epithelial surface to reveal adherence receptors (58–61), biofilm formation (62), provision of free carbohydrates for bacterial metabolism (31, 63, 64), desialylation of the cell surfaces of niche competitors such as Neisseria meningitidis and Haemophilus influenzae (65), modification of platelets to promote their clearance by the hepatic Ashwell receptor (66), and blood-brain barrier endothelial cell invasion (32, 67). Our results add stimulation of leukocyte proinflammatory responses to the list of phenotypic changes in the host associated with this multifactorial S. pneumoniae virulence factor.
We also demonstrated that WT S. pneumoniae was more potent than the ΔNanA mutant in stimulating proinflammatory cytokine production in BAL fluid or lung homogenates following murine intranasal challenge, findings associated with recovery of fewer WT S. pneumoniae bacteria than ΔNanA mutant bacteria from BAL fluid culture in the short-term-infection model. A recent report demonstrated that WT S. pneumoniae induced greater inflammatory cytokine secretion and higher mortality than a NanA− NanB− double mutant, even though similar CFU counts of the two strains were recovered in the bloodstream following intraperitoneal infection (68). Together, these data suggest that augmented cytokine responses and neutrophil activation upon Siglec-mediated “detection” of S. pneumoniae or of other sialidase-expressing pathogens could boost the initial localized innate immune response; however, the same processes during severe infections could provoke widespread dysregulation of inhibitory Siglec function and uncontrolled systemic inflammatory reactions that contribute to tissue injury, shock, or death. In this regard, total sialidase activity measured by fluorescent assay was significantly higher in blood collected from septic patients than in blood from nonseptic patients or healthy controls (69).
Elevated concentrations of free sialic acid in cerebrospinal fluid (CSF) concentrations were detected in 17 of 35 patients with acute S. pneumoniae meningitis, while patients with Haemophilus influenzae or Neisseria meningitidis meningitis had relatively normal free CSF sialic acid levels (70). Previous work has shown that NanA contributes to S. pneumoniae blood-brain barrier invasion and meningeal inflammation in the murine meningitis model (32, 67). Here we found that NanA-mediated proinflammatory cytokine release from macrophages can increase the permeability of hBMECs, which may be a further contributing mechanism to bacterial and neutrophil influx into the central nervous system (CNS) from the bloodstream.
Secondary S. pneumoniae pneumonia is a major complication of influenza pneumonia and accounts for excess mortality during influenza epidemics (71). In a mouse model of S. pneumoniae, after influenza virus pulmonary infection, strikingly elevated levels of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β, coupled with massive neutrophil influx, were observed in the lungs (72). A potential role for influenza virus neuraminidase in this lethal synergism has been proposed to involve exposure of binding receptors for S. pneumoniae on respiratory epithelium (73). Our present data suggest that, in addition to this mechanism, the viral and bacterial neuraminidases could synergize during severe infection to cause widespread cell surface desialylation and trigger overexuberant pulmonary and systemic inflammatory responses.
In conclusion, we present the observation using WT and isogenic mutant S. pneumoniae bacteria that the NanA sialidase can enhance the inflammatory response of immune cells. The unmasking of inhibitory CD33rSiglecs to remove inhibition of MAPK and NF-κB signaling represents a potential mechanism contributing to this phenomenon. Further analysis of sialic acid and Siglec receptor interactions during host-pathogen encounters could provide novel targets for therapeutic intervention to modify infectious disease outcome.
MATERIALS AND METHODS
Reagents.
Purified sialidase from Arthrobacter ureafaciens and pneumoniae was purchased from EY Laboratories (San Mateo, CA) and QA-Bio (Palm Desert, CA), respectively. Lipoteichoic acid was from InvivoGen (San Diego, CA), and VEGF165 was from PeproTech (Rocky Hill, NJ).
Bacterial strains and growth conditions.
S. pneumoniae serotype 2 strain D39 (NCTC 7466) and its isogenic ΔNanA mutant were used for these experiments. The ΔNanA mutant was constructed by nonpolar allelic replacement mutagenesis of the nanA gene as previously described (46). Strains in which the ΔNanA mutant was complemented with an expression vector for the wild-type enzyme (pNanA) or a catalytically inactive mutant enzyme (pNanAΔEnz) were previously validated by flow cytometry for surface expression of the protein (both plasmids) and fluorescent assay. For restoration of sialidase activity (pNanA only) (32), S. pneumoniae cultures were grown in Todd-Hewitt broth with 2% yeast extract (THY media) THY with chloramphenicol (2 µg/ml) was used to propagate the complemented strains. Freshly cultured bacteria from frozen aliquots were grown at 37°C in 5% CO2 and THY broth to the log phase (optical density at 600 nm [OD600] = 0.4) for all infection experiments.
Cell culture and in vitro S. pneumoniae infection.
THP-1 cells a human acute monocytic leukemia cell line were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). Human neutrophils were isolated from healthy donors by the use of the PolymorphPrep system (Axis-Shield, Fresenius, Waltham, MA) and were suspended in serum-free RPMI 1640 medium for experiments. Cells from the well-characterized simian virus 40 (SV40) large T antigen-immortalized human brain endothelial cell line (hBMEC), originally obtained from Kwang Sik Kim (Johns Hopkins University, Baltimore, MD), was maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS, 10% NuSerum (BD Biosciences), and 1% nonessential amino acids. THP-1 cells or neutrophils were resuspended in culture medium at the cell density of 1 × 107/ml. WT S. pneumoniae and the ΔNanA mutant were grown to the logarithmic phase, washed once with phosphate-buffered saline (PBS), and resuspended in culture medium to an OD600 = 0.1 (~108 CFU/ml) and then further diluted to the desired inoculum. Bacteria were added to 5 × 106 THP-1 cells or neutrophils in 2-ml siliconized microtubes at a multiplicity of infection (MOI) of 10, 3, or 1 as specified. The mixture of leukocytes and bacteria was incubated at 37°C with rotation, penicillin (5 µg/ml) and gentamicin (100 µg/ml) were added 30 min after infection to prevent bacterial overgrowth, and the cells or supernatants were harvested at the indicated time points for analysis.
FACS analysis of cell surface desialylation.
THP-1 cells were infected with WT S. pneumoniae or ΔNanA mutant at an MOI of 10, 3, or 1 for 3 h as described above. After infection, cells were washed twice with PBS and stained with fluorescein isothiocyanate (FITC)-conjugated E. cristagalli agglutinin lectin (Vector Laboratories, Burlingame, CA) for 30 min at 4°C. THP-1 cells were then washed twice and subjected to FACSCalibur flow cytometry (BD Biosciences) to analyze the exposed Galβ1-4GlcNAcβ1 units.
RNA interference.
Human Siglec-5 lentiviral shRNA construct (TRCN0000062525) was purchased from Open Biosystems. The targeting viruses were produced by cotransfection of 293T cells with the shRNA plasmid and packaging vectors (Open Biosystems) according to the vendor’s instructions. Knockdown efficiency was determined by staining the surface expression of Siglec-5 by the use of allophycocyanin (APC)-conjugated anti-Siglec-5 monoclonal antibodies (MAbs) (BD Biosciences).
Mouse intranasal infection and sample collection.
All animal experiments were approved by the Committee on the Use and Care of Animals, University of California, San Diego, and performed using accepted veterinary standards. Mice were lightly anesthetized by intraperitoneal injection of ketamine and xylazine, and 50 µl of PBS containing 1 × 107 S. pneumoniae CFU was then administered into the nostrils of the mice. The inoculum dose was confirmed by CFU counts on THY agar plates. Infected animals were sacrificed 6 h (6 mice in each group) or 20 h (7 mice in each group) postinfection. Blood was collected via terminal cardiac puncture. For bronchoalveolar lavage (BAL) fluid collection, the trachea was exposed and 0.8 ml of PBS (without calcium or anticoagulant) was injected twice using an 18-gauge, 1 and 1/2-in.-long needle connected to a 1 ml syringe. A 25-µl volume of BAL fluid was serially diluted and plated on THY plates to enumerate CFU. The rest of the BAL fluid was centrifuged at 1,500 rpm for 10 min and the supernatant frozen at −80°C for cytokine analysis. Cells were analyzed using a Z1 particle counter (Beckman Coulter) to determine the total cell count in the BAL fluid. The right lung was excised and placed into sterile preweighed 2-ml screw-cap microtubes containing 1 ml of sterile PBS and 1-mm-diameter zirconia/silica beads (BioSpec Products). After the tubes were weighed to determine lung weight, lung tissues were disrupted by a 1-min homogenization burst using a Mini-BeadBeater (BioSpec Products), followed by centrifugation at 12,000 rpm to collect supernatants for cytokine analysis.
Cytokine detection.
The concentrations of cytokines in THP-1 supernatants collected at various time points postinfection were quantified for IL-6, IL-8, and TNF-α using enzyme-linked immunosorbent assays (ELISA) according to the instructions of the manufacturer (R&D Systems, Minneapolis, MN). Mouse ELISA kits (TNF-α and IL-6 from R&D and IL-1β from BD Biosciences) were used to detect cytokines in BAL fluids and lung homogenates of infected animals.
Human brain microvascular endothelial cell (hBMEC) permeability assay.
hBMECs (2 × 104) were seeded on collagen-coated Transwell inserts (Costar) (pore size, 3 mm) for 3 to 4 days to attain confluence, which was verified by direct visualization and resistance to Evans Blue dye leakage in control wells. At the beginning of the experiment, the medium in the upper compartment was carefully replaced with 150 µl of supernatant collected from uninfected, WT S. pneumoniae-infected, or ΔNanA mutant-infected THP-1 cells, while the lower compartment was replenished with fresh medium. After 8 h of incubation at 37°C with 5% CO2, 10 µl of horseradish peroxidase (HRP) (50 µg/ml) was added to the upper chamber for an additional 30 min. A 10-μl volume of the medium from the lower chamber was diluted in 190 µl of PBS, followed by addition of TMB substrate (BD Biosciences) for 20 min; the reaction was stopped by the use of 2N H2SO4, and results were read at OD450.
Neutrophil extracellular trap (NET) visualization and quantification.
Neutrophils (2.5 × 105) were seeded on 48-well plates, infected with WT S. pneumoniae or ΔNanA mutant at an MOI = 0.1, centrifuged at 1,600 rpm for 5 min, and incubated for 90 min at 37°C with 5% CO2. To visualize NETs, neutrophils were incubated with rabbit polyclonal antibodies against myeloperoxidase (Dako), followed by staining with 4′,6′-diamidino-2-phenylindole (DAPI) and Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen) as previously described (74). Images were recorded using a Zeiss Axiovert microscope. The total amount of neutrophils and the amount of neutrophils releasing NETs per field of view were counted in 4 individual images per sample. The same investigator (Y.-C. Chang) performed the experiment and the quantification.
Western blot analysis.
THP-1 cells were lysed in buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% NP-40) containing protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Santa Cruz Biotechnology). Cell lysates were then separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was probed with the anti-phospho-p44/42 MAPK (T202/Y204; Cell Signaling Technology), anti-IκB (Cell Signaling Technology), or anti-actin (Sigma) Abs, followed by appropriate HRP-conjugated secondary Abs (Bio-Rad) and ECL reagent (Thermo Scientific). Siglec-5 immunoprecipitation was performed as previously described (75). Briefly, 500 µg of cell lysate was immunoprecipitated with 1A5 anti-human Siglec-5 MAb (gift from P. Crocker, University of Dundee, Dundee, Scotland, United Kingdom) and protein G Sepharose beads (BD Biosciences). The Western blotting method described above was used to probe samples with rabbit anti-SHP-2 Abs (Santa Cruz Biotechnology).
ACKNOWLEDGMENTS
Our research was supported by NIH/NHLBI “Programs of Excellence in the Glycosciences” award HL107150 (A.V., V.N.), as well as NIH grants HD051796 (V.N.) and HL057345 (A.V.).
Footnotes
Citation Chang Y, Uchiyama S, Varki A, and Nizet V. 2012. Leukocyte inflammatory responses provoked by Pneumococcal Sialidase. mBio 3(1):e00220-11. doi:10.1128/mBio.00220-11.
REFERENCES
- 1. Chen X, Varki A. 2010. Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 5:163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varki A. 2010. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. U. S. A. 107(Suppl. 2):8939–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crocker PR, Paulson JC, Varki A. 2007. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7:255–266 [DOI] [PubMed] [Google Scholar]
- 4. Varki A. 2007. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446:1023–1029 [DOI] [PubMed] [Google Scholar]
- 5. Varki A, Angata T. 2006. Siglecs—the major subfamily of I-type lectins. Glycobiology 16:1R–27R [DOI] [PubMed] [Google Scholar]
- 6. Avril T, Attrill H, Zhang J, Raper A, Crocker PR. 2006. Negative regulation of leucocyte functions by CD33-related siglecs. Biochem. Soc. Trans. 34:1024–1027 [DOI] [PubMed] [Google Scholar]
- 7. Collins BE, et al. 2004. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl. Acad. Sci. U. S. A. 101:6104–6109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Razi N, Varki A. 1999. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology 9:1225–1234 [DOI] [PubMed] [Google Scholar]
- 9. Varki A. 2011. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology 21:1121–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewis AL, et al. 2009. Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure. Proc. Natl. Acad. Sci. U. S. A. 106:13552–13557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlin AF, et al. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113:3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kline KA, Schwartz DJ, Lewis WG, Hultgren SJ, Lewis AL. 2011. Immune activation and suppression by group B streptococcus in a murine model of urinary tract infection. Infect. Immun. 79:3588–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiman S, et al. 2009. Genetic and biochemical modulation of sialic acid O-acetylation on group B streptococcus: phenotypic and functional impact. Glycobiology 19:1204–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avril T, Wagner ER, Willison HJ, Crocker PR. 2006. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect. Immun. 74:4133–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bax M, et al. 2011. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect. Immun. 79:2681–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roggentin P, Schauer R, Hoyer LL, Vimr ER. 1993. The sialidase superfamily and its spread by horizontal gene transfer. Mol. Microbiol. 9:915–921 [DOI] [PubMed] [Google Scholar]
- 17. Taylor G. 1996. Sialidases: structures, biological significance and therapeutic potential. Curr. Opin. Struct. Biol. 6:830–837 [DOI] [PubMed] [Google Scholar]
- 18. Gong J, Xu W, Zhang J. 2007. Structure and functions of influenza virus neuraminidase. Curr. Med. Chem. 14:113–122 [DOI] [PubMed] [Google Scholar]
- 19. Santos-López G, et al. 2009. Structure-function analysis of two variants of mumps virus hemagglutinin-neuraminidase protein. Braz. J. Infect. Dis. 13:24–34 [DOI] [PubMed] [Google Scholar]
- 20. Cámara M, Boulnois GJ, Andrew PW, Mitchell TJ. 1994. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 62:3688–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leprat R, Michel-Briand Y. 1980. Extracellular neuraminidase production by a strain of Pseudomonas aeruginosa isolated from cystic fibrosis. Ann. Microbiol. (Paris) 131B:209–222 [PubMed] [Google Scholar]
- 22. Telford JC, et al. 2011. The Aspergillus fumigatus sialidase is a 3-deoxy-D-glycero-D-galacto-2-nonulosonic acid hydrolase (KDNase): structural and mechanistic insights. J. Biol. Chem. 286:10783–10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buschiazzo A, Amaya MF, Cremona ML, Frasch AC, Alzari PM. 2002. The crystal structure and mode of action of trans-sialidase, a key enzyme in Trypanosoma cruzi pathogenesis. Mol. Cell 10:757–768 [DOI] [PubMed] [Google Scholar]
- 24. Afroun S, Tenu JP, Lemaire G. 1988. Modifications of glycosylation patterns in macrophages upon activation. Biochim. Biophys. Acta 971:137–147 [DOI] [PubMed] [Google Scholar]
- 25. Cross AS, Wright DG. 1991. Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J. Clin. Invest. 88:2067–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landolfi NF, Cook RG. 1986. Activated T-lymphocytes express class I molecules which are hyposialylated compared to other lymphocyte populations. Mol. Immunol. 23:297–309 [DOI] [PubMed] [Google Scholar]
- 27. Pilatte Y, Bignon J, Lambré CR. 1993. Sialic acids as important molecules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology 3:201–218 [DOI] [PubMed] [Google Scholar]
- 28. Sato C, Miyazawa T, Nishizawa K, Kojima K, Okayama M. 1979. Changes in the organization and biosynthesis of cell surface acidic sugars during the phytohemagglutinin-induced blast formation of human T-lymphocytes. Exp. Cell Res. 124:285–292 [DOI] [PubMed] [Google Scholar]
- 29. Carrillo MB, Milner CM, Ball ST, Snoek M, Campbell RD. 1997. Cloning and characterization of a sialidase from the murine histocompatibility-2 complex: low levels of mRNA and a single amino acid mutation are responsible for reduced sialidase activity in mice carrying the Neu1a allele. Glycobiology 7:975–986 [DOI] [PubMed] [Google Scholar]
- 30. O’Brien KL, et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 31. King SJ, Hippe KR, Weiser JN. 2006. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 59:961–974 [DOI] [PubMed] [Google Scholar]
- 32. Uchiyama S, et al. 2009. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J. Exp. Med. 206:1845–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng X, Wang X, Andersson R. 1996. Alterations in endothelial barrier permeability in multiple organs during overactivation of macrophages in rats. Shock 6:126–133 [DOI] [PubMed] [Google Scholar]
- 34. Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY. 2001. Tumour necrosis factor-a causes an increase in blood-brain barrier permeability during sepsis. J. Med. Microbiol. 50:812–821 [DOI] [PubMed] [Google Scholar]
- 35. Brinkmann V, et al. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 36. von Köckritz-Blickwede M, Nizet V. 2009. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 87:775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pauly JL, Germain MJ, Han T. 1978. Neuraminidase alteration of human lymphocyte reactivity to mitogens, antigens and allogenic lymphocytes. J. Med. 9:223–236 [PubMed] [Google Scholar]
- 38. Stamatos NM, Curreli S, Zella D, Cross AS. 2004. Desialylation of glycoconjugates on the surface of monocytes activates the extracellular signal-related kinases ERK 1/2 and results in enhanced production of specific cytokines. J. Leukoc. Biol. 75:307–313 [DOI] [PubMed] [Google Scholar]
- 39. von Gunten S, Simon HU. 2006. Sialic acid binding immunoglobulin-like lectins may regulate innate immune responses by modulating the life span of granulocytes. FASEB J. 20:601–605 [DOI] [PubMed] [Google Scholar]
- 40. Ando M, Tu W, Nishijima K, Iijima S. 2008. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem. Biophys. Res. Commun. 369:878–883 [DOI] [PubMed] [Google Scholar]
- 41. Tong HH, et al. 2001. Comparison of structural changes of cell surface carbohydrates in the eustachian tube epithelium of chinchillas infected with a Streptococcus pneumoniae neuraminidase-deficient mutant or its isogenic parent strain. Microb. Pathog. 31:309–317 [DOI] [PubMed] [Google Scholar]
- 42. Chong DL, Sriskandan S. 2011. Proinflammatory mechanisms in sepsis. Contrib. Microbiol. 17:86–107 [DOI] [PubMed] [Google Scholar]
- 43. de Jong HK, van der Poll T, Wiersinga WJ. 2010. The systemic pro-inflammatory response in sepsis. J. Innate Immun. 2:422–430 [DOI] [PubMed] [Google Scholar]
- 44. Daëron M, Jaeger S, Du Pasquier L, Vivier E. 2008. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol. Rev. 224:11–43 [DOI] [PubMed] [Google Scholar]
- 45. Daëron M, et al. 1995. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity 3:635–646 [DOI] [PubMed] [Google Scholar]
- 46. Winter AJ, et al. 1997. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 65:4411–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu G, et al. 18 January 2011, posting date. Three Streptococcus pneumoniae sialidases: three different products. J. Am. Chem. Soc. doi: 10.1021/ja110733q. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 48. Amith SR, et al. 2010. Neu1 desialylation of sialyl a2, 3-linked b-galactosyl residues of toll-like receptor 4 is essential for receptor activation and cellular signaling. Cell. Signal. 22:314–324 [DOI] [PubMed] [Google Scholar]
- 49. Amith SR, et al. 2009. Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj. J. 26:1197–1212 [DOI] [PubMed] [Google Scholar]
- 50. Rabinovich GA, Toscano MA. 2009. Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9:338–352 [DOI] [PubMed] [Google Scholar]
- 51. Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. 2007. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 17:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernández GC, et al. 2005. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways. Glycobiology 15:519–527 [DOI] [PubMed] [Google Scholar]
- 53. Nieminen J, St-Pierre C, Sato S. 2005. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J. Leukoc. Biol. 78:1127–1135 [DOI] [PubMed] [Google Scholar]
- 54. Correa SG, Sotomayor CE, Aoki MP, Maldonado CA, Rabinovich GA. 2003. Opposite effects of galectin-1 on alternative metabolic pathways of L-arginine in resident, inflammatory, and activated macrophages. Glycobiology 13:119–128 [DOI] [PubMed] [Google Scholar]
- 55. Cámara M, Boulnois GJ, Andrew PW, Mitchell TJ. 1994. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 62:3688–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. 2006. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect. Immun. 74:3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu G, et al. 2008. Crystal structure of the NanB sialidase from Streptococcus pneumoniae. J. Mol. Biol. 384:436–449 [DOI] [PubMed] [Google Scholar]
- 58. Andersson B, et al. 1983. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krivan HC, Roberts DD, Ginsburg V. 1988. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAcb1-4gal found in some glycolipids. Proc. Natl. Acad. Sci. U. S. A. 85:6157–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Linder TE, Daniels RL, Lim DJ, DeMaria TF. 1994. Effect of intranasal inoculation of Streptococcus pneumoniae on the structure of the surface carbohydrates of the chinchilla eustachian tube and middle ear mucosa. Microb. Pathog. 16:435–441 [DOI] [PubMed] [Google Scholar]
- 61. Tong HH, Liu X, Chen Y, James M, Demaria T. 2002. Effect of neuraminidase on receptor-mediated adherence of Streptococcus pneumoniae to chinchilla tracheal epithelium. Acta Otolaryngol. 122:413–419 [DOI] [PubMed] [Google Scholar]
- 62. Parker D, et al. 2009. The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation. Infect. Immun. 77:3722–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burnaugh AM, Frantz LJ, King SJ. 2008. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J. Bacteriol. 190:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yesilkaya H, Manco S, Kadioglu A, Terra VS, Andrew PW. 2008. The ability to utilize mucin affects the regulation of virulence gene expression in Streptococcus pneumoniae. FEMS Microbiol. Lett. 278:231–235 [DOI] [PubMed] [Google Scholar]
- 65. Shakhnovich EA, King SJ, Weiser JN. 2002. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect. Immun. 70:7161–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grewal PK, et al. 2008. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat. Med. 14:648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Banerjee A, et al. 2010. Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization. Cell. Microbiol. 12:1576–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen GY, et al. 2011. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat. Biotechnol. 29:428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Piagnerelli M, et al. 2009. Neuraminidase alters red blood cells in sepsis. Crit. Care Med. 37:1244–1250 [DOI] [PubMed] [Google Scholar]
- 70. O’Toole RD, Goode L, Howe C. 1971. Neuraminidase activity in bacterial meningitis. J. Clin. Invest. 50:979–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simonsen L, Fukuda K, Schonberger LB, Cox NJ. 2000. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 181:831–837 [DOI] [PubMed] [Google Scholar]
- 72. Smith MW, Schmidt JE, Rehg JE, Orihuela CJ, McCullers JA. 2007. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp. Med. 57:82–89 [PMC free article] [PubMed] [Google Scholar]
- 73. McCullers JA, Bartmess KC. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187:1000–1009 [DOI] [PubMed] [Google Scholar]
- 74. Berends ET, et al. 2010. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2:576–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carlin AF, et al. 2009. Group B streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J. Exp. Med. 206:1691–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]