Abstract
Luminal accumulation of viscous, poorly hydrated, and less transportable mucus has been associated with altered mucus rheology and reduced mucociliary clearance. These symptoms are some of the cardinal clinical manifestations found throughout major respiratory diseases as well as gastrointestinal and digestive disorders. Applications of current mucolytics may yield short-term improvements but are continuously challenged by undesirable side-effects. While nanoparticles (NPs) can interact with mucin polymers, whether functionalized NPs can rectify mucus rheology is unknown. Herein, we report that carboxyl-functionalized NPs (24 nm and 120 nm) dramatically reduced mucin gel size and accelerated mucin matrix hydration rate (diffusivity). Our results suggest that carboxyl-functionalized NPs disperse mucin gels possibly by enhancing network hydration. This report highlights the prospective usages of carboxyl-functionalized NPs as a novel mucus dispersant or mucolytic agent in adjusting mucus rheological properties and improving mucociliary transport to relieve clinical symptoms of patients suffering from relevant diseases.
Mucin, a seemingly unimportant and inert biopolymer, is omnipresent throughout the lumen of various organs and almost all the epithelial mucosa1,2. Its prevalence suggests more complex functions than what its passive appearance originally portrays. Particularly in humans, mucin gels have been shown to serve as an innate immune defense by trapping bacteria, viruses and hazardous particulates1,2,3. Overall, mucins play critical roles in maintaining the health and integrity of multiple structures such as, but not limited to, the respiratory, digestive, visual and reproductive systems1,2,4,5. To perform these protective tasks, a pivotal balance between mucin secretion and its rheological properties must be maintained in order to complement the effective mucociliary clearance6,7,8. Since exocytotic process and rheological properties of mucin are tightly regulated by physiological stimulations, any disturbance to this homeostatic control ultimately leads to the dysfunction and demise of biological systems6,9,10.
In many inflammatory diseases, unregulated mucin release predisposes to the presence of poorly transportable and highly viscous mucus, lumen occlusion, and chronic bacterial infection3,6,11. These pathological conditions are the common clinical manifestations typically observed in pulmonary and gastrointestinal morbidity11,12. In major respiratory diseases encompassing chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), asthma, bronchiectasis, and pneumonia, the inundating amount of abnormally viscous and dehydrated mucus can obstruct airway passages3,11,13. Blockage of bronchial trees reduces ventilation, hampers mucociliary clearance, and cultivates pathogenic microbial expansion and re-infection11,13. In order to break this relapsing cycle, the viscosity of pulmonary secretion must be reduced to help restore mucociliary transport. Various specific and non-specific mucolytic aerosols have been considered13,14,15. For peptide mucolytics, trypsin has been utilized extensively. It is a serine protease that cuts the peptide links in the apomucin component15. However, major drawbacks involve the incision at many serine sites of unspecific peptides and side-effects that include fever, hoarseness, irritation of the mouth, nausea and vomiting15. Clinical usages of dornase and streptodornase were successful at liquefying secretion by degrading the nucleic acid component of mucus15. Nonetheless, both enzymes are expensive, have a short half-life and have been reported to cause sensitivity in patients15. Classical mucolytics, such as N-acetylcysteine (NAC) or Nacystelyn, depolymerize the mucin biopolymer by hydrolyzing the disulfide bonds linking the mucin monomers14,15,16. Unfortunately, sulfurous odor and a pH of 2.2 are associated with bronchospasm for these drugs16. In addition, definitive evidence demonstrating that NAC and similar compounds can effectively improve mucus clearance is still lacking14,17. Other non-destructive mucolytics have been proposed to untangle mucin polymer chains by charge shielding16. Such agents include low molecular dextran sulfate, heparin and other glycoproteins16,18. In general, although many aerosolized mucolytics may have demonstrated the potential for improving mucus rheology, they can possibly over-liquefy mucus secretion resulting in impaired clearance19.
The strategy to sever mucin peptides to resolve dehydrated and thick mucus has led to limited rectification on altered mucus rheology. The mucin polymer networks of mucus have a characteristic tangled topology1,20. Since the rheological properties of mucus are governed mainly by the tangled density of mucin polymers, which decreases with the square of the volume of the mucin matrix, the mucin network hydration (degree of swelling) is the most critical factor in determining the rheological properties of mucus1,9. Aside from the traditional ineffective expectorants, an approach that can directly promote mucin matrix hydration and disperse aggregated mucin gels has not been explored.
We have previously demonstrated that positively charged NPs can directly alter the viscoelastic property of mucus by hindering the mucin network swelling rate and aggregating mucin polymers21. Nonetheless, whether negatively-charged NPs can restore defective mucin hydration and disperse aggregated mucin gels has never, heretofore, been conceived. In this study, our investigation examined the use of NPs with negatively-charged surface modifications to rectify altered mucus rheological properties. The concentration range of NPs used in this investigation was within the level found in multiple drug delivery systems22,23. Our results provide the first evidence that negatively-charged NPs may serve as a potential decongestant/non-destructive mucolytic agent to reduce mucus accumulation and associated bacterial infections commonly found with various diseases.
Results
Carboxyl-functionalized NP characterization
Dynamic laser scattering (DLS) was used to characterize the carboxyl-functionalized polystyrene NPs. The particle size distribution for 120 nm and 24 nm NPs ranged from ∼70 to 150 nm and ∼10 to 50 nm, correspondingly. The predominant size for each was 114 or 23 nm (Fig. 1 A and B).
Figure 1. Carboxyl-functionalized NP characterization.
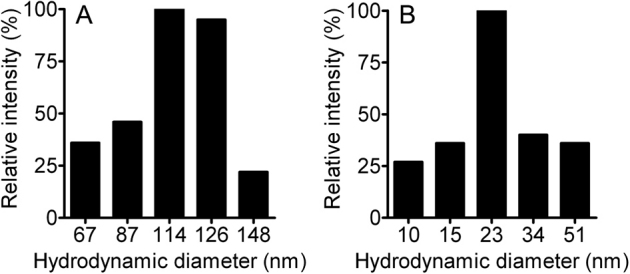
DLS assessment of carboxyl-functionalized NPs in Hanks' solution showed a size distribution of ∼70 to 150 nm for 120 nm while 24 nm NPs had a distribution of ∼10 to 51 nm.
Carboxyl-functionalized NPs disperse aggregated mucus
To support the notion that negatively-charged NPs can affect mucin rheological properties, we examined the effect of NPs on dispersing mucin gels. Porcine gastric mucin (1 mg/L) was prepared in Hanks' solution (with 8.2 mM Ca2+ at pH 7.4) and was equilibrated for ≥48 hrs until significant aggregation of mucus masses of approximately 8 μm in diameter were attained. The 8.2 mM Ca2+ was an experimental condition used to gelate dilute mucin solution for studying the dispersion capacity of NPs. On addition of carboxyl-functionalized NPs, size change in aggregated mucus gel particles was monitored with dynamic laser scattering (DLS) after 1, 3, 5, and 24 hrs. Figures 2A and B demonstrate that carboxyl-functionalized NPs dispersed and drastically decreased the mucin aggregate size at 1 mg/L, 0.5 mg/L and 0.1 mg/L. Using 120 nm NPs, 1 mg/L significantly reduced mucus gel size from ∼8 μm to ∼300–500 nm throughout 24 hr. A similar trend in mucin gel size reduction (from ∼8 μm to ∼200–300 nm) was observed with 24 nm NPs. In comparison to the mucin-only control (NP free), mucin aggregated gel size remained consistent at ∼8 μm throughout 24 hrs. Figures 2A and B illustrate that both 24 nm and 120 nm NPs lowered mucin gel size as a function of increasing NP concentration from 0.1–1 mg/L. In addition, a NP size-dependent effect was observed where 24 nm NP minimized mucin aggregation size more efficiently than 120 nm NP at the corresponding time points. NP-only control (mucin-free) further revealed insignificant change in NP size throughout 24 hrs (Fig. 2A and B). Therefore, the reduced gel size is the result of partially dispersed mucin gels rather than self-aggregation of NPs in Ca2+ rich solution.
Figure 2. Carboxyl-functionalized NPs disperse mucin gel aggregation.
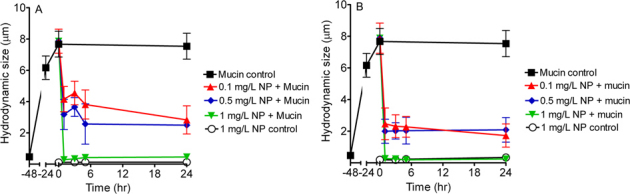
Negatively-charged NPs of (A) 120 nm (n≥6) and (B) 24 nm (n≥6) significantly reduced sizes of aggregated mucus masses from ∼8 µm to below 3 µm in 24 hrs of incubation. Various concentrations (red solid triangles: 0.1 mg/L, blue solid rhombus: 0.5 mg/L, green solid triangles: 1 mg/L) of negatively-charged NPs (120 and 24 nm) were added to mucin solution (1 mg/L). White open circles represent sizes of (A) 120 nm (1 mg/L) and (B) 24 nm (1 mg/L) NPs per se (n≥6) throughout 24 hrs. No significant self-clumping was observed. Black solid squares represent sizes of mucin aggregates (1 mg/L; mucin control, n≥6) throughout 72 hrs. The sizes of mucin aggregates were determined with DLS as described previously9,21.
Carboxyl-functionalized NPs accelerate mucin matrix expansion
We then undertook showing that negatively-charged NPs could enhance the mucin matrix hydration by utilizing an in vitro mucin swelling kinetics functional assay (see methods). For proof of concept purpose, only 120 nm NPs were investigated for its effects on mucin network hydration. A representative plot comparing the swelling kinetics of newly exocytosed mucin matrices between the control (NP free) and 120 nm carboxyl-functionalized NP treatment is shown in Figure 3. The rate of mucin network swelling (hydration) was significantly elevated in the presence of carboxyl-functionalized NP. The increase in mucin swelling rate was also NP concentration dependent. Converting swelling rate into diffusivity (D) yielded similar results (Fig. 4).
Figure 3. Effects of carboxyl-functionalized NPs on hydration rate of mucin matrices secreted from A549 cells.
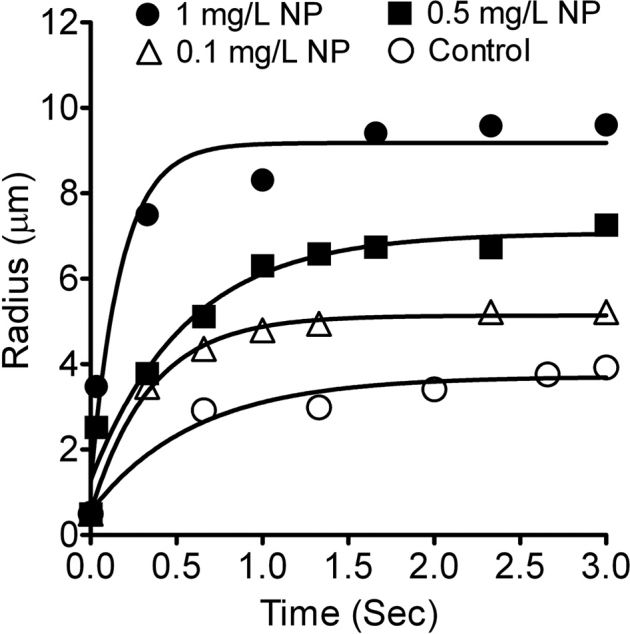
A representative plot of swelling kinetics (hydration rate) of mucin matrices secreted by A549 cells is shown here. Data points were fitted with the characteristic first order kinetic equation (see Materials and methods). Exocytosis was triggered by ionomycin. Four representative lines are displayed here. The control (NP free; open circles) shows a lower mucin network swelling rate than the swelling rate of mucin matrix when exposed to 120 nm negatively-charged NPs (0.1 mg/L open triangles, 0.5 mg/L solid squares and 1 mg/L solid circles).
Figure 4. Carboxyl-functionalized NPs enhance mucin diffusivity secreted from A549 cells.
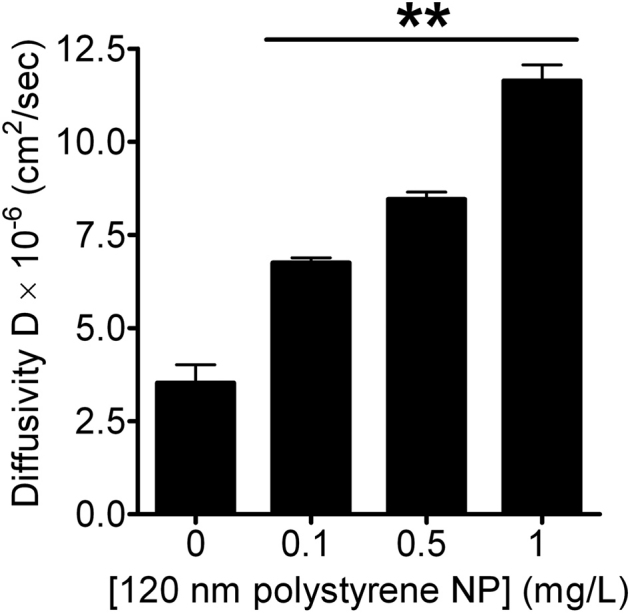
Mucin diffusivity was significantly elevated by negatively-charged NPs (120 nm) as a function of increasing NP concentration. Data are shown as mean±SD (n≥10). NP treated groups are markedly different from the untreated control at p<0.005 as indicated by **.
Carboxyl-functionalized NPs markedly enhances secreted mucin diffusivity
Exocytosis of mucin granules from cultured A549 cells was stimulated with 1 μM ionomycin and mucin diffusivity was calculated from the swelling kinetics as described (see methods). Compared to the mucin only controls in NP free Hanks' medium, negatively-charged functionalized NPs (120 nm) accelerated mucin diffusivity by approximately ∼2, 2.4 and 3.3 folds at 0.1, 0.5 and 1 mg/L, correspondingly. Elevation in mucin diffusivity also showed NP concentration dependency. A faster mucin gel swelling rate indicates greater mucin diffusivity and a less viscous gel4. Our data indicates that negatively-charged NPs can expedite mucin hydration rate and diffusivity, possibly reducing mucus viscosity and helping restore functional mucociliary activity.
Discussion
Mucin gels are secreted by epithelial mucosa of many organs including vaginal tract, eyes, gastrointestinal wall, and pulmonary lumen5. At optimal rheological properties, mucin gel and mucociliary action orchestrate in unison to remove pathogenic microbes and harmful substances1,6,11,14. However, when the balance between mucus production, hydration and transportation is perturbed, a series of pathological manifestations ensue ramifying into devastating outcomes. Especially in respiratory diseases, the accumulation of excessive amounts of highly viscous mucus in tracheobronchial trees can cause atelectasis, difficulty in expectoration, cease mucociliary clearance, obstruct airflow, and increase susceptibility to infection11,13,15,16. Furthermore, many GI disorders characterized by luminal occlusion with adherent mucus, has led to insufficient release of digestive enzymes, internal calcification and malnutrition. For example, the inspissated mucus can obstruct pancreatic ducts, biliary trees, and small intestine resulting in serious malnutrition, as well as pancreatic endocrine and exocrine dysfunction in CF patients12,15,24,25,26.
In order to alleviate the symptoms and the persistent cause of mucus associated diseases, liquefaction of the inspissated secretion is highly desirable in the debilitated patients15. Attempts to mobilize stagnant mucus have yielded facilitative approaches but are nonetheless unsuccessful14. Many current therapies are accompanied by concomitant side-effects. It has been reported that usage of some predominant mucolytic agents, i.e. deoxyribonuclease and N-acetyl cysteine, results in immediate liquefication of bronchial mucin15,27. The quick disassembly of mucin network instigated a drowning phenomenon where the patients were unable to expectorate a large amount of fluid efficiently15,16. Therefore, a new approach to efficiently (1) disperse existing aggregated mucus and (2) hydrate newly released mucin matrices without denaturing the mucin network to a monomeric structure is needed. We have previously demonstrated that positively charged-functionalized NPs (-NH2) can alter the rheological properties of mucin network polymers by electrostatic interactions. These polycationic NPs significantly promoted mucin aggregation and reduced mucin gel swelling rate via acting as possible crosslinkers for polyanionic mucin matrices21. Under the current investigation, we tested whether negatively-charged modified NPs can help restore mucin rheological properties by dispersing aggregated mucin gels and accelerating polymer network hydration.
To examine if aggregated mucin can be dispersed by negatively charged-functionalized NPs, gastric mucin was used as a model in our study as it shares many similarities with its airway counterpart28,29,30. Our method is supported by previous studies that also used gastric mucin as a model system to examine the viscoelastic properties of mucins31,32,33,34,35,36, since there is currently no commercial mucin sample available that can be preserved in the native state. DLS was implemented to examine the dispersing interactions between functionalized NPs and aggregated mucin. This technique allows mucin structure and dynamics to be continuously probed in the native state without chemical fixation and denaturation, which minimizes artifacts1,2. By measuring Brownian motion of mucin polymers, the diffusion constant can be calculated which is inversely proportional to the hydrodynamic diameter of the measured particle. A high diffusion constant usually correlates with small mucin gelation/aggregation1,2. To prove that carboxyl-functionalized NPs can disperse aggregated mucin gel, NPs were added to the mucin solution with gel size of approximately 8 µm. As demonstrated by Figure 2A, 120 nm negatively-charged NPs decreased the size of mucin aggregation by ∼1.9, 2.5 and 28 folds at corresponding 0.1, 0.5 and 1 mg/L after the 1st hr. On the other hand, smaller NPs (24 nm) reduced mucin aggregation size by ∼3, 4 and 30 folds after 1st hr at the corresponding concentrations shown above (Fig. 2B). Our data suggests that negatively-charged NPs can disperse mucin aggregates in both concentration and size-dependent manners. The mechanisms may involve electrostatic repulsion between negatively-charged NPs and the polyanionic sites on mucin such as the sialic, sulfate and carboxyl functional groups, promoting mucin disaggregation1,2. Another possible mechanism could be attributed to the chelation of divalent network cross-linkers ions (e.g. Ca2+) by negatively-charged NPs1. Alternatively, akin to the sugar derivative-based mucolytic agents i.e. dextran, the polyanionic surface charges may reduce the cross-link density of sputum37. The polyanionic functional groups on NPs may disrupt hydrogen bonds between mucin polymers, and compete for hydrogen bonding sites with mucin glycoproteins, lowering intra/inter-network cross-links and interactions18,37,38. Consequently, the new hydrogen bonds may be inconducive to the overall structural integrity and rheological properties18,37,38. Finally, smaller NPs can more effectively disperse mucin gels possibly by the differential charge densities39. Our findings are supported by other studies showing that negatively-charged NPs were repelling/not adhering to polyanionic mucus gels40. The combinatory attributes from electrostatic repulsion, chelation and reduction in intra/inter-mucin hydrogen bonding density may allow mucociliary transport of mucus and coughing mechanism to take place.
Beside physical disaggregation, another vital method to reduce viscoelasticity is by hydrating mucus. According to Sheehan and Verdugo, the mucin polymer network of mucus has a characteristic tangled topology20. The mucin polymer tangled density determines the rheological properties of mucus, which decreases with the square of the volume of the mucin matrix. Hence, mucin network hydration (degree of swelling) critically governs the mucus viscoelastic properties1. Measurement of mucin network hydration rate (diffusivity) provides a direct assessment of changes in mucin rheological properties (viscosity) released from living cells. Results from dispersing aggregated mucin gels suggest that negatively-charged functionalized NPs play a critical role in decondensation of mucin granules, but proof awaits a direct evidence of their effects on native mucin rheological properties. We tested whether negatively-charged NPs can directly accelerate mucin hydration rate by measuring the swelling kinetics of newly exocytosed matrices from human lung A549 cells. A549 cells are a representative in vitro model system for studying mucin swelling kinetics as they express both major respiratory MUC 5AC and MUC 5B mucin proteins41,42. Our results showed that negatively-charged NPs accelerated mucin network hydration which we postulate would render it more transportable (Fig. 3). Moreover, enhanced mucin diffusivity in a NP concentration-dependent manner is correlated with lower viscosity and less obstruction (Fig. 4). Our experimental evidence further suggests that in chronic diseases involving mucus hypersecretion, obstruction and increased viscosity, negatively-charged functionalized NPs may serve as effective mucus decongestants and dispersants.
Methods
Nanoparticle characterization
Carboxyl-functionalized polystyrene NPs with various sizes (i.e. 24 nm and 120 nm) and concentrations (i.e. 1 mg/L, 0.5 mg/L, and 0.1 mg/L) (Bangs Laboratories, Fishers, IN, USA) were used in our study. The 24 nm NPs have a surface charge of 180 µeq/g, parking area of 217.5 sq.Å/group and 1.06 g/cm3 while 120 nm NPs have a surface charge of 171 µeq/g, parking area of 45.8 sq.Å/group and 1.06 g/cm3 (based on manufacturer information). All NPs have a size standard deviation of ≤10% (based on manufacturer information). These sizes were independently confirmed using homodyne dynamics laser scattering. All NP samples were sonicated before usage.
Mucin particulate sizing using dynamic laser scattering
The dispersion of aggregated mucin mass was monitored by measuring particle size using homodyne dynamics laser scattering (DLS). Samples of porcine gastric mucin at 1 mg/L (Lot# 018K0079, Sigma-Aldrich, MO, USA) were prepared with Hanks' solution containing 1.2 mM Ca2+, 20 mM Tris-HCl (tris(hydroxymethyl)aminomethane hydrochloride) and 10 mM MES (2-(N-morpholino)ethanesulfonic acid) (Sigma-Aldrich, MO, USA) to buffer the pH around 7.39. The solution was thoroughly mixed until mucin was dissolved. Aliquots of mucin samples (10 ml) were directly filtered dropwise through a 0.2-μm Millipore SFCA membrane (pre-washed with 0.1 N HCl) (Fisher Scientific, CA, USA) into clean scintillation vials. Each vial was then treated with 7 mM of filtered Calcium chloride dihydrate (Sigma-Aldrich, MO, USA) solution prepared with distilled H2O. The scintillation vials were positioned in the goniometer of a Brookhaven laser spectrometer (Brookhaven Instruments, NY, USA). Mucin gel aggregation was allowed to take place by equilibrating for 48 hrs. They were subsequently analyzed by detecting the scattering fluctuations at a 45-degree scattering angle. Commercialized polystyrene NPs (Bangs Laboratories, Fishers, IN) with dimensions of 24 and 120 nm were added to mucin samples. Changes in mucin particulate dimension triggered by three negatively-charged NP concentrations (-COOH, 24 and 120 nm) (i.e. 1 mg/L, 0.5 mg/L, and 0.1 mg/L) were measured. All NPs were prepared in distilled H2O. Filtered NP suspension, added to scintillation vials, was tested for its ability to disperse aggregated mucin gel and was monitored 1 hr, 3 hrs, 5 hrs, and 24 hrs. Self-aggregation induced by NP-only control was monitored at 0 hr, 3 hrs, 5 hrs and 24 hrs. Mucin-only control was examined 48 hrs in advance and 24 hrs after reaching maximum aggregated size. The autocorrelation function of the scattering intensity fluctuations was averaged over a 2-min sampling time using a Brookhaven BI 9000AT autocorrelator. Particle size distribution was calculated by CONTIN43. Calibration was conducted with standard monodisperse suspensions of latex microspheres ranging from 50 nm to 10 μm (Polysciences, PA, USA).
A549 cell culture
The human lung carcinoma cell line A549 was obtained from American Type Culture Collection (ATCC, Manassas, VA). The A549 cell line is an airway alveolar epithelial cell line commonly used as a secretory model9,44. Cells were cultured in 15 cm cell culture plates (VWR, Brisbane, CA) containing F-12 nutrient mixture medium (Invitrogen, Carlsbad, CA) supplemented with 100 U of penicillin/streptomycin (Invitrogen, Carlsbad, CA) and 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). The A549 lung cells were cultured in 15 cm falcon plates and incubated in a humidified incubator at 37°C/5% CO2. Cell counts were performed using trypan blue (Sigma, St. Louis, MO) exclusion and a Bright-Line haemocytometer.
Swelling kinetics and A549 cell preparation
The culture plates were rinsed with Hanks' buffer twice. Non-trypsin dissociation buffer (Invitrogen, Carlsbad, CA) was added to detach cells from plates and subsequently incubated at 37°C for 15 min, centrifuged at 700 rpm for 5 min, and resuspended in Hanks' buffer (Invitrogen, Carlsbad, CA). Resuspended cells were dispersed into MatTek glass bottom dishes (MatTek Corporation, Ashland, MA) and equilibrated in a 37°C incubator for 10 min prior to adding varying concentrations of carboxyl-functionalized NPs. Both Hanks' solution and NP suspension were buffered with Tris-HCl and MES (Sigma, St. Louis, MO) to pH 7.4. The pH was monitored and maintained at approximately 7.4 throughout the experiments9,21.
A549 cells were viewed and video-recorded with phase-contrast lens using a Nikon Eclipse TE-2000-U inverted fluorescence microscope (Nikon Eclipse TE-2000 U, Tokyo, Japan). Degranulation of A549 cells was induced by 1 μM ionomycin (Sigma, St. Louis, MO) and was found to be a readily observable discrete quantal process. During exocytosis into extracellular Hanks' solution, released granules undergo rapid swelling. Video-recordings of granular exocytosis and swelling were captured at 30 frames s−1. The analysis of the changing mucin matrix dimension was assessed with NIS-Elements software (Nikon, Melville, New York)9,21.
Measurements of the radii of the released mucin matrices, as a function of time, were used to verify that the swelling of the secreted material followed the characteristic features of polymer gel swelling kinetics9,21. The swelling of a polymer follows a typical diffusive kinetics that is independent of the size, internal topology, or chemical composition of the gel9,21. For spherical gels, as observed from the exocytosed mucin granules of A549 cells, the radial dimension increased following a characteristic first order kinetics of the form r(t) = rf–(rf–ri) e–t/τ, where ri and rf are the initial and final radius of the granule matrix, respectively, and τ is the characteristic relaxation time of the swelling process1. The polymer network of gels diffuses into the solvent (Hanks' solution), with diffusivity (D) (D = (rf)2/τ [cm2 s–1]). The diffusivity (D) of polyionic gels varies with the concentration of counterions in the swelling medium. In this study, we measured the swelling kinetics of exocytosed mucin gels in Hanks' buffer (Invitrogen, Carlsbad, CA) with NP concentrations ranging from 0.1–1 mg/L. Previous reports have predicted that polymer viscosity is proportional to the molecular weight of polymers and that polymer diffusivity is inversely proportional to the molecular weight. Therefore, polymer viscosity and diffusivity are inversely proportional to each other45,46. Higher polymer diffusivity indicates lower viscosity of polymers. Thus, we can take direct measurements of changes in mucin diffusivity under different NP concentrations as changes in mucin viscosity.
Statistical analysis
Data was presented as means ± SD. Each experiment was performed independently at least three times. Statistical significance was determined using a Student's t-test analysis with p values of <0.005 (Microsoft Excel and GraphPad Prism 4.0, GraphPad Software, Inc., San Diego, CA).
Author Contributions
EYC and WCC wrote the manuscript. EYC, DD, YCW, MG and CSC planned and conducted experiments. EYC, DD, YCW analyzed data. All authors reviewed the manuscript.
Acknowledgments
The authors thank Profs. Pedro Verdugo and Paul Quinton for the helpful discussions and encouragement during the preparation of this manuscript. This work was in part supported by the National Science Foundation (CBET-0932404) and NIH (1R15HL095039). EYC and CSC were supported by UC Merced GRC summer fellowships, Center of Excellence on Health Disparities (1P20MD005049-01 from the National Center on Minority Health and Health Disparities) and Jane Vilas Fellowship.
References
- Verdugo P. Goblet cells secretion and mucogenesis. Ann Rev Physiol 52, 157–176 (1990). [DOI] [PubMed] [Google Scholar]
- Bansil R., Stanley E. & J. T L. Mucin Biophysics. Ann Rev Physiol 57, 635–657 (1995). [DOI] [PubMed] [Google Scholar]
- Hogg J. C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364, 709–721 (2004). [DOI] [PubMed] [Google Scholar]
- Espinosa M., Noe G., Troncoso C., Ho S. B. & Villalon M. Acidic pH and increasing [Ca(2+)] reduce the swelling of mucins in primary cultures of human cervical cells. Human reproduction (Oxford, England) 17, 1964–1972 (2002). [DOI] [PubMed] [Google Scholar]
- Cone R. A. Barrier properties of mucus. Adv Drug Deliv Rev 61, 75–85 (2009). [DOI] [PubMed] [Google Scholar]
- Randell S. H., Boucher R. C. & Grp U. N. C. V. L. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 35, 20–28 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. H. et al. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 354, 241–250 (2006). [DOI] [PubMed] [Google Scholar]
- Boucher R. C. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Ann Rev Med 58, 157–170 (2007). [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Yang N., Quinton P. M. & Chin W. C. A New Role for Bicarbonate in Mucus Formation. Am J Physiol Lung Cell Mol Physiol 299, L542–549 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. Small airways in COPD. N Engl J Med 350, 2635–2637 (2004). [DOI] [PubMed] [Google Scholar]
- Fahy J. V. & Dickey B. F. Airway mucus function and dysfunction. N Engl J Med 363, 2233–2247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372, 415–417 (2008). [DOI] [PubMed] [Google Scholar]
- Pruitt B. & Jacobs M. Clearing away pulmonary secretions. Nursing 35, 36–41; quiz 41–32 (2005). [DOI] [PubMed] [Google Scholar]
- Houtmeyers E., Gosselink R., Gayan-Ramirez G. & Decramer M. Effects of drugs on mucus clearance. Eur Respir J 14, 452–467 (1999). [DOI] [PubMed] [Google Scholar]
- Waldron-Edward D. & Skoryna S. C. The mucolytic activity of amides: a new approach to mucus dispersion. Can Med Assoc J 94, 1249–1256 (1966). [PMC free article] [PubMed] [Google Scholar]
- Rubin B. K. The pharmacologic approach to airway clearance: mucoactive agents. Paediatric respiratory reviews 7 Suppl 1, S215–219 (2006). [DOI] [PubMed] [Google Scholar]
- Grandjean E. M., Berthet P., Ruffmann R. & Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clinical therapeutics 22, 209–221 (2000). [DOI] [PubMed] [Google Scholar]
- Feng W., Garrett H., Speert D. P. & King M. Improved clearability of cystic fibrosis sputum with dextran treatment in vitro. Am J Respir Crit Care Med 157, 710–714 (1998). [DOI] [PubMed] [Google Scholar]
- Rubin B. K., MacLeod P. M., Sturgess J. & King M. Recurrent respiratory infections in a child with fucosidosis: is the mucus too thin for effective transport? Pediatric pulmonology 10, 304–309 (1991). [DOI] [PubMed] [Google Scholar]
- Verdugo P., Tam P. Y. & Butler J. Conformational structure of respiratory mucus studied by laser correlation spectroscopy. Biorheology 20, 223–230 (1983). [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Wang Y. C., Chen C. S. & Chin W. C. Functionalized Positive Nanoparticles Reduce Mucin Swelling and Dispersion. PloS one 5, e15434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S. & Somasundaran P. Sequestration of drugs using poly(acrylic acid) and alkyl modified poly(acrylic acid) nanoparticles. Soft Matter 2, 850–854 (2006). [DOI] [PubMed] [Google Scholar]
- Sheng Y. et al. Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials 30, 2340–2348 (2009). [DOI] [PubMed] [Google Scholar]
- Farber S., Shwachman H. & Maddock C. L. Pancreatic Function and Disease in Early Life. I. Pancreatic Enzyme Activity and the Celiac Syndrome. The Journal of clinical investigation 22, 827–838 (1943). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K. R., Turner B. S., Grubman S. A., Jefferson D. M. & LaMont J. T. Dysregulation of proteoglycan production by intrahepatic biliary epithelial cells bearing defective (delta-f508) cystic fibrosis transmembrane conductance regulator. Hepatology (Baltimore, Md) 27, 7–14 (1998). [DOI] [PubMed] [Google Scholar]
- Eggermont E. Gastrointestinal manifestations in cystic fibrosis. European journal of gastroenterology & hepatology 8, 731–738 (1996). [PubMed] [Google Scholar]
- King M., Dasgupta B., Tomkiewicz R. P. & Brown N. E. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with recombinant human deoxyribonuclease I. Am J Respir Crit Care Med 156, 173–177 (1997). [DOI] [PubMed] [Google Scholar]
- Rose M. C. Mucins: structure, function, and role in pulmonary diseases. Am J Physiol 263, L413–429 (1992). [DOI] [PubMed] [Google Scholar]
- Sheehan J. K. & Carlstedt I. Hydrodynamic properties of human cervical-mucus glycoproteins in 6 M-guanidinium chloride. The Biochemical journal 217, 93–101 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Oates K. & Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. The Biochemical journal 239, 147–153 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cola E., Yakubov G. E. & Waigh T. A. Double-globular structure of porcine stomach mucin: a small-angle X-ray scattering study. Biomacromolecules 9, 3216–3222 (2008). [DOI] [PubMed] [Google Scholar]
- Felgentreff K. et al. The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides 27, 3100–3106 (2006). [DOI] [PubMed] [Google Scholar]
- Mayol L., Quaglia F., Borzacchiello A., Ambrosio L. & La Rotonda M. I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: rheological, mucoadhesive and in vitro release properties. Eur J Pharm Biopharm 70, 199–206 (2008). [DOI] [PubMed] [Google Scholar]
- Sandberg T., Blom H. & Caldwell K. D. in J Biomed Mater Res A, Vol. 91 762–7722009). [DOI] [PubMed] [Google Scholar]
- Sandberg T., Karlsson Ott M., Carlsson J., Feiler A. & Caldwell K. D. Potential use of mucins as biomaterial coatings. II. Mucin coatings affect the conformation and neutrophil-activating properties of adsorbed host proteins--toward a mucosal mimic. Journal of biomedical materials research 91, 773–785 (2009). [DOI] [PubMed] [Google Scholar]
- Yakubov G. E., Papagiannopoulos A., Rat E., Easton R. L. & Waigh T. A. Molecular structure and rheological properties of short-side-chain heavily glycosylated porcine stomach mucin. Biomacromolecules 8, 3467–3477 (2007). [DOI] [PubMed] [Google Scholar]
- Feng W. et al. Effects of dextran on tracheal mucociliary velocity in dogs in vivo. Pulm Pharmacol Ther 12, 35–41 (1999). [DOI] [PubMed] [Google Scholar]
- Sudo E., Boyd W. A. & King M. Effects of dextran sulfate on tracheal mucociliary velocity in dogs. J Aerosol Med 13, 87–96 (2000). [DOI] [PubMed] [Google Scholar]
- Lai S. K. et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A 104, 1482–1487 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas H. S. Chitosan: properties, preparations and application to microparticulate systems. J Microencapsul 14, 689–711 (1997). [DOI] [PubMed] [Google Scholar]
- Song J. S. et al. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respir Res 8, 28 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan-Chen Wu D., Wu R., Reddy S. P., Lee Y. C. & Chang M. M. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. The American journal of pathology 170, 20–32 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin W. C., Orellana M. V. & Verdugo P. Spontaneous assembly of marine dissolved organic matter into polymer gels. Nature 391, 568–572 (1998). [Google Scholar]
- Berger J. T., Voynow J. A., Peters K. W. & Rose M. C. Respiratory carcinoma cell lines. MUC genes and glycoconjugates. Am J Respir Cell Mol Biol 20, 500–510 (1999). [DOI] [PubMed] [Google Scholar]
- Edwards S. F. & Grant J. W. V. Effect of Entanglements on Viscosity of a Polymer Melt. J Phys A: Math Gen 6, 1186–1195 (1973). [Google Scholar]
- Edwards S. F. & Grant J. W. V. Effect of Entanglements on Diffusion in a Polymer Melt. J Phys A: Math Gen 6, 1169–1185 (1973). [Google Scholar]


