Abstract
Eyeblink classical conditioning in pre-weanling rabbits was examined in the present study. Using a custom lightweight headpiece and restrainer, New Zealand white littermates were trained once daily in 400 ms delay eyeblink classical conditioning from postnatal days (PD) 17–21 or PD 24–28. These ages were chosen because eyeblink conditioning emerges gradually over PD 17–24 in rats (Stanton, Freeman, & Skelton, 1992), another altricial species with neurodevelopmental features similar to those of rabbits. Consistent with well-established findings in rats, rabbits trained from PD 24–28 showed greater conditioning relative to littermates trained from PD 17–21. Both age groups displayed poor retention of eyeblink conditioning at retraining one month after acquisition. These findings are the first to demonstrate eyeblink conditioning in the developing rabbit. With further characterization of optimal conditioning parameters, this preparation may have applications to neurodevelopmental disease models as well as research exploring the ontogeny of memory.
Keywords: New Zealand white rabbit, eyeblink conditioning, development, infantile amnesia
Introduction
Animal neurodevelopmental research has focused almost exclusively on rodent models, despite recognition of the need to extend the range of animal models to maximize translation to human disorders (Hudson & Distel, 1986; Schardein, Schwetz, & Kenel, 1985). Rabbits provide an attractive complement – and alternative – to rodent developmental research. Rabbits share many of the characteristics that have made rodents (in particular, rats) popular among developmental researchers, including ease of breeding, a short gestation period, and production of large, rapidly-developing altricial litters. Additionally, rabbits are phylogenetically closer to primates than are rodents (Graur, Duret, & Gouy, 1996) and therefore a closer biological model to humans. Though rabbits are well-suited for developmental research, minimal progress has been made toward the establishment of rabbit models of neurodevelopment.
Progress in rabbit neurodevelopmental research has been hampered by a lack of behavioral preparations available to assess learning and memory in the developing rabbit. Eyeblink classical conditioning (measured as external eyelid or nictitating membrane {NM} movement in rabbits, see Gormezano, Schneiderman, Deaux, & Fuentes, 1962; Schneiderman, Fuentes, & Gormezano, 1962) has been characterized extensively in the adult and aging rabbit (Christian & Thompson, 2003; Gormezano, 1966; Gormezano, Kehoe, & Marshall, 1983; Woodruff-Pak, Lavond, Logan, & Thompson, 1987; Woodruff-Pak, Seta, Roker, & Lehr, 2007). The use of discrete cues and an easily-measurable response in this Pavlovian preparation allows for reliable identification of learning of the conditioned stimulus (CS) – unconditioned stimulus (US) association that is readily distinguished from non-associative changes in behavior. The extensive behavioral characterization of rabbit eyeblink conditioning in adulthood (see Gormezano, 1966; Gormezano et al., 1983) aided in localization of the essential neural substrate of the eyeblink conditioned response (CR) to a discrete cerebellar-brainstem circuit (Christian & Thompson, 2003; McCormick & Thompson, 1984; McCormick, Steinmetz, & Thompson, 1985; Steinmetz et al., 1987; Thompson, 1986). It is surprising, given the extensive research literature and advances in neurobehavioral research stemming from the adult rabbit eyeblink preparation that the developmental emergence of this well-characterized form of learning and memory has yet to be demonstrated in the rabbit.
Eyeblink conditioning can be adapted to many species (e.g., humans; rodents) with minimal modification, and the essential neural substrates identified in rabbits are conserved across humans and rodents (Chen, Bao, Lockard, Kim, & Thompson, 1996; Gerwig, Kolb, & Timmann, 2007; Rogers, Britton, & Steinmetz, 2001). Furthermore, the developmental emergence of eyeblink conditioning has been characterized in human infants (Claflin, Stanton, Herbert, Greer, & Eckerman, 2002; Herbert, Eckerman, & Stanton, 2003; Ivkovich, Collins, Eckerman, Krasnegor, & Stanton, 1999; Little, Lipsitt, & Rovee-Collier, 1984) and in rats (Freeman, Spencer, Skelton, & Stanton, 1993; Stanton, Fox, & Carter, 1998; Stanton & Freeman, 2000; Stanton, Freeman, & Skelton, 1992). The gradual emergence of eyeblink classical conditioning in rats over postnatal days (PD) 17–24 corresponds to developmental changes in the essential cerebellar-brainstem circuit over this period (Freeman, 2010; Freeman & Nicholson, 2004). These essential neural substrates undergo substantial development continuing into the 3rd postnatal week in rodents and rabbits (Altman & Bayer, 1997; Anderson & Flumerfelt, 1985; Das, Nornes, Hine, & Pfaffenroth, 1973), and in both species the onset of basic visual and auditory functions occurs between the first and third postnatal weeks (Gottlieb, 1971). Given the developmental similarities between species, one may expect eyeblink classical conditioning in rabbits to emerge over a similar period in ontogeny to that of rats.
The present study represents our efforts to characterize the emergence of eyeblink classical conditioning in the developing rabbit. Using a custom-built headpiece and restrainer modeled after a non-invasive preparation used for adult rabbits in our laboratory (Brown & Woodruff-Pak, 2011), pre-weanling rabbits were trained in a delay eyeblink conditioning paradigm beginning on either PD 17 or 24. Based on previous findings in rats (Stanton et al., 1992), we predicted that rabbits trained beginning on PD 24 would show greater levels of conditioning relative to littermates initially trained on PD 17. Furthermore, a subset of rabbits was re-trained one month after acquisition. Performance at re-training was compared to performance of littermates initially trained in young adulthood (PD 61).
Methods
Subjects
The subjects were 21 New Zealand White rabbits (12 F, 9 M). Subjects were the offspring of does obtained from Covance Research Products (Denver, PA). Eight rabbits (5 F, 3 M) were bred at Covance and arrived with their mother at our animal facility on postnatal day (PD) 11. Two separate litters (13 pups total: 7 F, 6 M) were bred from mating of does with sexually experienced bucks at our animal facility. Rabbit does were 15 – 28 months of age at mating and weighed between 3.30 – 4.45 kg. Does had given birth to multiple healthy litters prior to litters birthed for this study. Bucks were 31 – 35 months of age at mating and weighed 4.26 – 4.30 kg. Rabbits received ad libitum access to high fiber food (Purina rabbit chow) and tap water. Does and offspring were housed in 152.4 × 76.2 × 45.7 cm (height) stainless-steel cages in temperature (60 – 70° F) - and humidity-controlled rooms in an animal facility (light/dark cycle = 12/12-h; lights on at 0800) at Temple University approved by the Association and Accreditation of Laboratory Animal Care International. A nesting box (45.7 × 27.9 × 27.9 cm {height}; Covance) constructed of dark, opaque plastic was placed in each cage on gestational day (GD) 28, 3 days prior to the expected day of birth (31 days after mating; the day of birth was designated as PD 0). Pinewood shavings were placed in a depression (25.4 × 20.3 cm) located in the floor of the nesting box for newborn rabbits. Litters were undisturbed for 3 days after birth. The average PD 4 weight of litters bred at Temple was 0.65 kg. On PD 14 inner ears of each rabbit were numbered with a non-toxic black marker for identification. Subjects were weaned from their mother on PD 36 and housed (2–3/cage) with same-sex littermates in 61 × 45.7 × 45.7 cm (height) stainless steel cages. Rabbits were single-housed in these cages at a later date to avoid overcrowding. This research was carried out in accordance with the Guide for the Care and Use of Laboratory Animals as outlined by the National Institutes of Health, and all procedures were approved by the Institutional Animal Care and Use Committee at Temple University
Apparatus
See Figure 1 for photographs of some of this equipment. The conditioning apparatus consisted of two separate sound-attenuating chambers (74.9 × 55.9 × 35.6 cm {width}; Med Associates). Each chamber had a fan that ran throughout the experiment that provided low-decibel (60–65-bB) background noise. A speaker mounted to the wall of each chamber delivered the tone CS. A lightweight headpiece, placed on the rabbit’s head and wrapped under its muzzle, held a malleable plastic tube that terminated in a device containing both (1) an infrared emitter and detector for recording NM activity, and (2) a plastic nozzle for delivery of the air puff US (San Diego Instruments, San Diego, CA). This device was positioned approximately 2 cm from the rabbit’s left eye. Output was digitized, stored, and analyzed using San Diego Instruments software. This system also controlled timing and presentation of stimuli.
Figure 1.
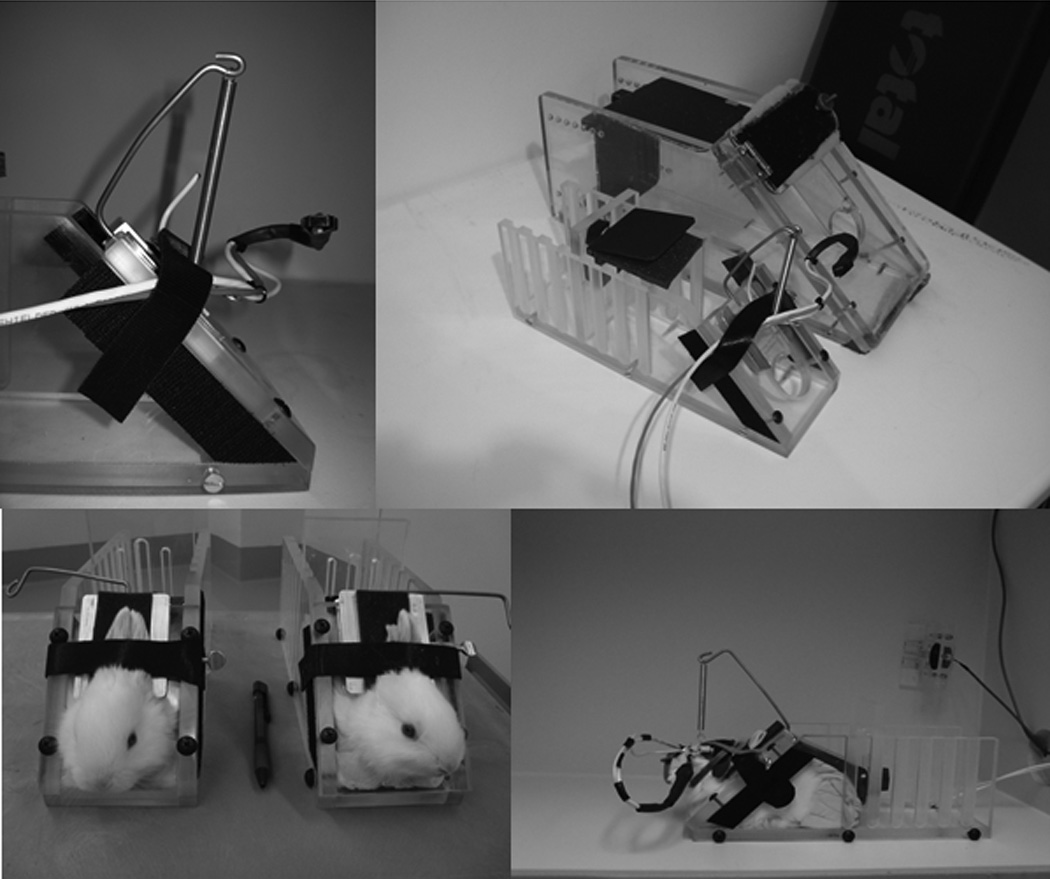
Photographs of custom equipment used for pre-weanling rabbit eyeblink training. Top left: close-up of restrainer with malleable tubing connected to infrared recording device and airpuff nozzle (nozzle located below infrared device). Note the hook protruding from the top of the neckpiece, used to connect to the lightweight headpiece to relieve weight on the rabbit’s head. Top right: view of pre-weanling restrainer next to standard adult rabbit restrainer. Bottom left: two pre-weanling rabbits during restrainer adaptation. Bottom right: view of restrained pre-weanling rabbit in the conditioning chamber during eyeblink training, prepared with headpiece.
A major roadblock in the establishment of a model of eyeblink conditioning in the developing rabbit has been that equipment commercially available for rabbit restraint and US delivery/NM recording are too heavy and large for pre-weanling rabbits (an advantage of rabbits over rodents is that rabbits tolerate restraint well and can be trained in eyeblink conditioning with equipment that does not require surgical implantation - see Gormezano, 1966). Some of the primary items of equipment (headpiece; restrainer) were custom-built to accommodate the pre-weanling rabbits (weight range at start of training: 0.17 – 0.33 kg) used for much of this study, as the standard headpiece and restrainer used for adult rabbit eyeblink conditioning have been designed for rabbits in excess of 2–3 kg body weight. Equipment designed for our novel infant eyeblink preparation will be described in detail here.
The Plexiglas restrainer used for pre-weanling rabbits was a smaller, slightly modified version of restrainers used in the paradigmatic studies of adult rabbit eyeblink conditioning (Gormezano, 1966; Gormezano et al., 1983; Schneiderman et al., 1962) and in previous adult and aging rabbit eyeblink studies in our laboratory (e.g., Woodruff-Pak et al., 2007). Dimensions of our infant rabbit restrainer were as follows: 30.5 cm (length at the bottom) × 10.2 cm (width) × 11.4 cm (height). The front portion of the restrainer sloped downward from top to bottom at a 45 degree angle (15 cm length). A Plexiglas “back plate” (12.5 cm height) was placed in the restrainer, to the rear of the rabbit to secure the rabbit within the restrainer. This back plate could be inserted at varying lengths to accommodate a range of rabbit sizes. In order to secure the rabbit’s head, a Plexiglas “neck plate” (9 cm height) was inserted into a groove (approximately 4 cm width) located at the front, sloping portion of the restrainer. Once the rabbit was placed into the restrainer with the back plate set, the rabbit’s head was positioned in the groove at the front of the restrainer and the neck plate was then secured here. A screw on the front left side of the restrainer was adjusted to lock in the neck plate such that the bottom end of the plate was secured to the back of the rabbit’s neck with minimal pressure. Care was taken with the neck plate to allow for normal respiration and moderate head movements. To further assist with securing the rabbit’s head, a Velcro strap approximately 15 cm long was placed along the base of the ears to hold the ears firmly against the neck plate; this strap was held in place with Velcro attached to the sides of the restrainer. A malleable metal hook (11 cm length) protruded from the top end of the neck plate and extended forward. A small spring (5.5 cm length) was hung from the end of this hook, and the other end of the spring was attached to a hook secured to the leather headpiece (the headpiece will be described further below). The purpose of the hook and spring was to relieve weight on the rabbit’s head produced by the headpiece and tubing used for eliciting/recording the NM response.
In contrast to the standard plastic headpiece used for adult rabbit eyeblink conditioning (San Diego Instruments; approximate weight: 0.2 – 0.3 kg), a small lightweight leather headpiece (approximately 7 cm length) was constructed for use with pre-weanlings in the present study. Velcro straps attached to the headpiece were wrapped under the rabbit’s muzzle, and additional straps attached to the headpiece were secured to Velcro on the restrainer to assist with keeping the rabbit’s head in position. As referenced above, a small hook at the top of the headpiece was secured to the spring hanging above. The metal hook attached at the top end of this spring was malleable and adjusted to provide the appropriate amount of tension such that most of the weight of the headpiece and tubing was supported by the hook and spring. This was necessary to keep weight off of the rabbit’s head and secure placement of the NM recording/US delivery equipment. The two lengths of tubing required for NM recording and US delivery were both connected to a malleable length of plastic with electrical tape. This followed the design in our adult rabbit preparation and allowed for precise placement and stability of the infrared sensor/airpuff in front of the rabbit’s eye.
To ensure that the infrared sensor and air puff emitting device could properly detect and elicit NM movement, the left eye of the rabbit was exposed during eyeblink training. Exposing the left eye was achieved by securing a small, rounded metal clip (approximately 0.6 cm in length and 0.3 cm wide) to the upper eyelid and securing an identical clip to the left lower eyelid – the clips do not come into contact with the eye. These clips were attached to an elastic band (6.4 cm length), and at the other end the elastic band was attached to a strip of Velcro (8.9 cm length). The upper eyelid was gently pulled back such that a mild amount of tension was placed on the elastic band. At this point the Velcro end was attached to a complementary piece of Velcro located on the side of the restrainer, keeping the upper eyelid held up in place. The identical procedure was used to keep the lower eyelid in place. This method allowed the full extent of NM movement across the eye while minimizing distress to the rabbits. Eyes were kept moist with application of antibacterial ophthalmic ointment.
Eyeblink Conditioning Procedure
Rabbits were adapted to Plexiglas restrainers for one, 15-minute session 2–3 days prior to the start of paired CS-US training (this adaptation session took place outside of the conditioning apparatus). During this adaptation period rabbits were placed in the restrainer but not fitted with the headpiece or eye clips. For eyeblink classical conditioning, rabbits were trained with a 500 ms CS (1-kHz, 85-dB tone) and a 100 ms corneal airpuff US (7–8 psi) directed at the left eye. A delay conditioning paradigm was used in which the CS preceded, overlapped, and coterminated with the US (400 ms interstimulus interval – ISI – between CS and US onset). The 400 ms delay paradigm has been shown to produce robust eyeblink conditioning in adult rabbits (Coffin & Woodruff-Pak, 1993). For acquisition CS-US training starting on either PD 17 (n = 8: 5 F, 3 M) or PD 24 (n = 8: 4 F, 4 M), rabbits were trained in five daily sessions. Each session consisted of 90 paired CS-US trials with an average intertrial interval of 25 sec.
One month after the last eyeblink acquisition session (Session 5), a subset of subjects was retrained for four daily sessions following the same procedures. For the eight subjects initially trained starting at PD 17, four (2 F, 2 M) were re-trained in the 400 ms delay procedure starting on PD 53, though data was reported for only three (2 F, 1 M) due to a lack of observable URs in one of the subjects. Four of the eight subjects initially trained starting on PD 24 (2 F, 2 M) were retrained starting on PD 61. Additionally, five experimentally naïve subjects received initial training in the 400 ms delay paradigm starting on PD 61 (3 F, 2 M). To reduce oversampling within-litter, same-sex littermates were assigned to different experimental conditions (i.e., age at start of initial training; see Holson & Pearce, 1992).
Data Analysis
Changes in reflectance caused by movement of the left NM detected by the infrared sensor were processed and stored in 3-ms bins. Output was digitized, rectified, and amplified for analysis. A response was registered when NM movement reached at least 10% of the maximum response amplitude generated that session. Each trial was 1300 ms in duration. The first 250 ms of the trial epoch was a stimulus-free “baseline” period. NM activity reaching 20% of the maximum response amplitude during the baseline period constituted an unusable trial. Trials were also deemed unusable if NM activity reached the 10% response threshold during the pre-CS baseline period and continued at or above threshold into the CS period. A CR was recorded if a response exceeded threshold between 1 and 399 ms after CS onset (alpha responding was not measured). An unconditioned response (UR) was recorded if the response exceeded threshold after US onset (US onset was 400 ms after CS onset).
The dependent measures of interest were CR and UR frequency and CR latency. CR timing measures – latency to onset and latency to peak – were analyzed only during the last acquisition session (Session 5 for subjects initially trained on PD 17 or 24; Session 4 for subjects initially trained on PD 61). UR percentage was initially analyzed only at Session 1 of acquisition to avoid “contamination” of CR-UR summation effects, but because of lower than expected UR percentages observed here an additional UR percentage analysis was conducted for the terminal acquisition session (see Table 1 for CR latency and UR percentage findings).
Table 1.
Mean (± standard error of the mean) (a) body weights (kg) across the pre- and post-weanling period, (b) unconditioned response percentage (UR %) at sessions 1 (S1) and 4–5 (S4–5), and (c) conditioned response (CR) onset and peak latencies from the last session of acquisition (session 5 for subjects PD 17 or 24 at the start of training; session 4 for subjects PD 61 at the start of training). Data are presented as a function of age at initial eyeblink training (PD 17: n = 8; PD24: n = 8; PD 61: n = 5).
| Body Weight (kg) | UR percentage and CR latency | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | PD 14–15 | PD 22–23 | PD 36 | PD 43 | UR% (S1) | UR% (S4–5) | CR Onset | CR Peak |
| PD 17 | 0.2 ± 0.02 | 0.3 ± 0.02 | 0.9 ± 0.03 | 1.1 ± 0.03 | 53 ± 13 | 83 ± 9 | 191 ± 16 | 280 ± 14 |
| PD 24 | 0.2 ± 0.03 | 0.3 ± 0.03 | 0.8 ± 0.06 | 1.1 ± 0.06 | 61 ± 12 | 85 ± 4 | 182 ± 19 | 274 ± 15 |
| PD 61 | 0.2 ± 0.03 | 0.3 ± 0.03 | 0.9 ± 0.06 | 1.2 ± 0.07 | 85 ± 4 | 90 ± 4 | 171 ± 15 | 290 ± 21 |
Statistical Analysis
Data were analyzed via analysis of variance (ANOVA) and Tukey’s Honestly Significant Difference (HSD) post-hocs, with significance set at p < 0.05. Statistical analyses were performed using SPSS statistical package. Repeated measures ANOVAs were conducted for within-subjects factors of sessions and the between-subjects factors of age and sex for the CR percentage measure. Univariate analyses across age and sex were conducted on measures of UR frequency, CR latency, and body weight.
Results
Data are reported combined across sex since significant main or interactive effects involving this variable were observed only in subjects initially trained on PD 61 (n = 5: 3 F, 2 M); males receiving initial training on PD 61 demonstrated higher CR percentages over the latter training sessions relative to their female counterparts. There were no other significant main or interactive effects involving the independent measure of sex. Results reported below will focus on significant or interactive effects involving age at initial training.
Acquisition
Robust CRs were evident during acquisition in PD 24 but not in PD 17 rabbits (see Figure 2). This was supported statistically by a significant main effect of age [F(1,14) = 18.143, p < 0.002] and a significant interaction of Age × Sessions [F(4,56) = 5.53, p < 0.002] in the CR percentage measure. The Age × Sessions interaction achieved significance due to significantly higher CR percentages in PD 24 relative to PD 17 subjects over sessions 3–5. A significant main effect of sessions was also observed for the CR percentage measure [F(4.56) = 15.045, p < 0.001]. CR onset and peak latency and UR percentages did not differ as a function of age (ps > 0.1; see Table 1).
Figure 2.
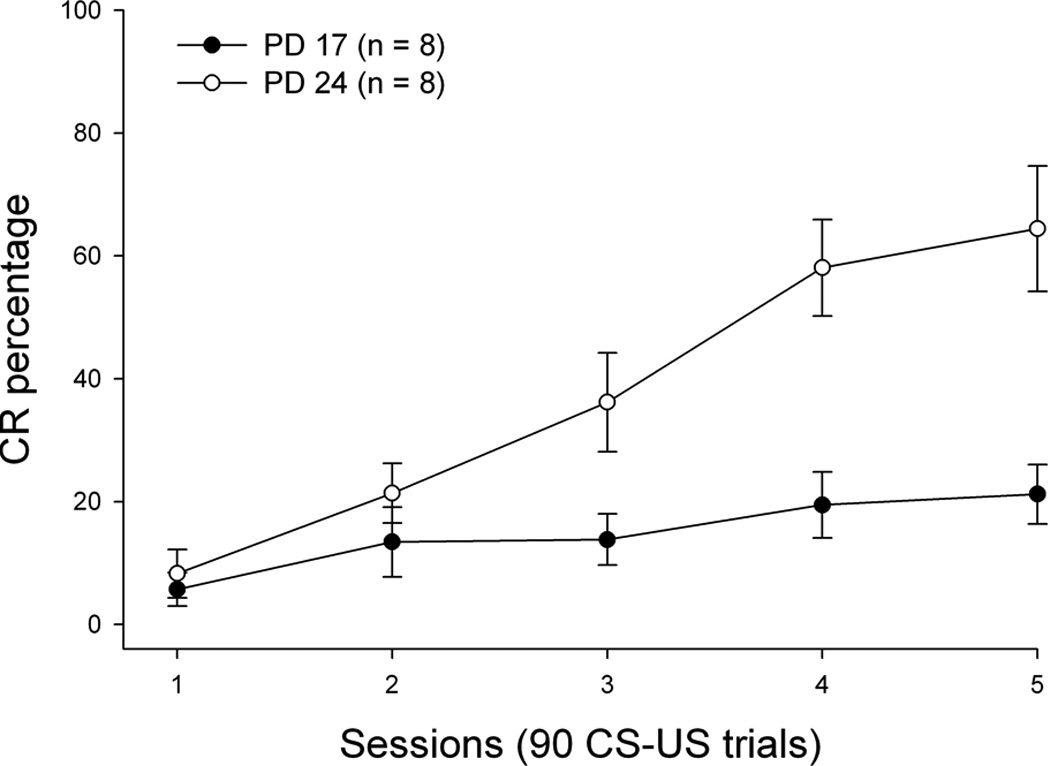
Mean percentage of conditioned responding (CRs) for each of five daily acquisition sessions of eyeblink classical conditioning for subjects receiving initial training on postnatal day (PD) 17 (dark circles) or 24 (open circles). Bars depict standard error of the mean.
Retraining
Robust CRs were evident in all groups during retraining in the 400 ms delay paradigm. There were no significant effects involving age at initial training (ps > 0.1; see Figure 3). The only statistically significant finding for the CR percentage measure was a main effect of sessions [F(3,27) = 40.785, p < 0.001]. To explore further savings from acquisition, CR percentages were analyzed across each 10-trial block during Session 1 of retraining (9 blocks total). Again, no statistically significant age effects were observed (ps > 0.1; data not shown).
Figure 3.
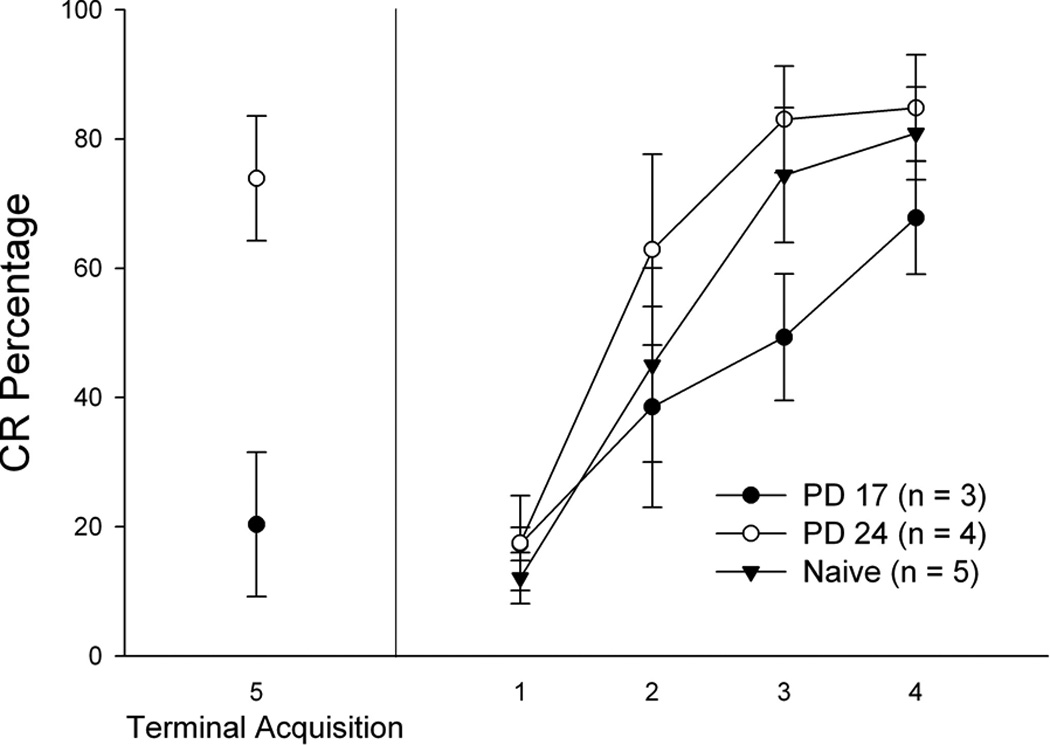
Mean percentage of conditioned responding (CRs) for the terminal acquisition session (Session 5; left) and for re-training (four daily sessions; right) one month later for a subset of subjects receiving initial eyeblink training on postnatal day (PD) 17 (dark circles) or 24 (open circles). Subjects receiving initial training as young adults (PD 61; ‘Naïve’) are depicted with black triangles. Note that terminal acquisition levels represent only the subset of subjects also re-trained one month after acquisition. Bars depict standard error of the mean.
A comparison of CR percentages of the first 4 sessions during initial training (acquisition) between PD17 and 24 subjects with subjects receiving initial training at PD 61 yielded a significant main effect of age [F(2,18) = 21.453, p < 0.001] and a significant Age × Sessions interaction [F(2, 18) = 9.052, p < 0.003]. Post-hocs revealed significant differences in CR percentages between each age: PD 61 > PD 24 > PD 17 (ps < 0.009).
Body Weights
Body weights were recorded for all subjects at intervals spanning the pre- and post-weanling period (weights at PD 61 were omitted since subjects initially trained on PD 17 were not assessed at this age). As shown in Table 1, there were no significant differences in body weight as a function of age at initial training (ps > 0.1).
Summary of Findings
Findings in the present study demonstrate that eyeblink conditioning emerges between PD 17 and 24–28 in rabbits, consistent with well-established findings of eyeblink conditioning in the developing rat (Freeman et al., 1993; Stanton et al., 1998, 1992; Stanton & Freeman, 2000). Though eyeblink conditioning was robust at PD 24–28, CR levels over this period were significantly lower relative to CRs in subjects initially trained at PD 61, suggesting continued development in the mechanisms underlying acquisition and/or expression of eyeblink classical conditioning beyond the pre-weanling period in rabbits. These developmental effects cannot be accounted for by differences in sensory processing of the airpuff US or in differences in the ability to produce a robust NM response, as there were no significant differences in UR percentages across ages. The absence of significant age effects at retraining – including an age-matched group receiving initial eyeblink training at this period - suggests that learning of the CS-US association during the pre-weanling period was poorly retained one month later.
Discussion
The present study is the first to demonstrate eyeblink classical conditioning in the developing rabbit. Rabbits trained over PD 24–28 displayed greater levels of conditioning relative to littermates trained over PD 17–21. These findings suggest that the developmental emergence of eyeblink conditioning in rabbits occurs over a similar period to that of rats (PD 17–24; Stanton et al., 1992; Stanton & Freeman, 2000). The eyeblink preparation used here was modified from our restrained adult rabbit preparation (Brown & Woodruff-Pak, 2011) to accommodate pre-weanling rabbits. Contrary to many animal eyeblink models, this preparation does not involve surgical procedures of any kind, relying instead on infrared measurement of NM activity and an airpuff US both controlled by a flexible device positioned in front of the rabbit’s left eye. These features are similar to human eyeblink preparations (see Ivkovich et al., 1999; Tobia & Woodruff-Pak, 2009; Woodruff-Pak, Finkbiner, & Sasse, 1990) and allow for multiple assessments of the same subject at different developmental stages without concern for growth-induced degradation of surgically implanted equipment. Rabbits initially trained during the pre-weanling period were successfully re-trained in young adulthood, demonstrating the utility of this preparation for studies of eyeblink conditioning across ontogeny. Furthermore, the lack of group differences in body weight (see Table 1) suggests that this training procedure was not a significant stressor to pre-weanling rabbits.
Eyeblink classical conditioning provides advantages over other learning and memory paradigms in that an easily-quantified performance measure (blink UR) is present on most trials, providing confirmation that the subject is capable of producing the target response (blink CR). Observation of URs was therefore important to rule out simple performance accounts for age differences in CR production. Indeed, the absence of significant age differences in UR percentage indicates that age differences in CR generation observed in the present study cannot be attributed to deficits in the ability to produce a robust blink response or by impaired detection of the airpuff US in our youngest age group (PD 17–21 at acquisition). However, the percentage of detected URs was lower than expected in pre-weanling rabbits, particularly at Session 1 (see Table 1). Though greater consistency in UR detection is desired, it must be emphasized that rabbits trained from PD 24–28 produced robust CRs in spite of this UR variability.
Perhaps the most notable difference between the present findings and those reported in developing rats pertains to conditioning in 24-day-old subjects. In contrast to minimal CR levels observed early in training in rabbits trained from PD 24–28 (see Figure 2), PD 24 rats show rapid acquisition, reaching asymptotic CR levels during the second session (Stanton et al., 1992). Though species differences cannot be ignored, contributions of different recording and reinforcement methods across studies warrant consideration. Eyeblink conditioning in the developing rat is typically measured with EMG recording of eye muscles (orbicularis oculi; Stanton & Freeman, 2000) whereas infrared recording of the NM (cartilaginous tissue that moves laterally across the eye) was used in the present study. Robust correlations have been reported between eyelid and NM conditioning in adult rabbits (McCormick, Lavond, & Thompson, 1982), and while some CR characteristics differ as a function of response type (see Garcia, Mauk, Weidemann, & Kehoe, 2003; Gormezano et al., 1983), the conclusion drawn from this literature is that use of different blink recording methods does not yield significant differences in CR production. A more likely source of differing conditioning levels reported in developing rabbits and rats concerns the US used (periorbital shock US in rat studies – see Stanton & Freeman, 2000 - and an airpuff US in the present study). Some evidence for enhanced eyeblink conditioning using a shock relative to an airpuff US has been reported in adult rabbits (Oswald, Knuckley, Mahan, Sanders, & Powell, 2009; Powell, Maxwell, & Penney, 1996; but see Mauk & Ruiz, 1992 for comparable conditioning with shock and airpuff USs). Beyond these methodological differences, rigorous comparisons of eyeblink conditioning between species require consideration of a number of training features that differ across these preparations.
Conditions used for paired CS-US training in the present study (e.g., tone CS frequency; CS-US interval, etc.) were based on those previously shown to yield robust eyeblink conditioning in adult rabbits (Coffin & Woodruff-Pak, 1993; Schneiderman & Gormezano, 1964; Smith, 1968; Woodruff-Pak et al., 2007). Modifications to these parameters may be necessary to produce optimal conditioning in developing rabbits (see Spear & Rudy, 1991). For example, intervals between CS and US onset (ISIs) that are optimal for human infant eyeblink conditioning (Claflin et al., 2002; Little et al., 1984) are longer than optimal ISIs in human adults (Kimble, 1947; Woodruff-Pak & Thompson, 1988). Though ISIs longer than 280 ms do not improve eyeblink conditioning in developing rats (Freeman et al., 1993), manipulation of the ISI used in the present study (400 ms) would be useful to determine whether the failure to observe robust conditioning in PD 17–21 rabbits was due to use of an ISI not appropriate for this age. Studies are also needed to determine if other parametric changes (e.g., changing the frequency of the auditory CS; increasing CS and/or US intensity) can overcome limitations in conditioning observed in pre-weanling rabbits (c.f., Andrews, Freeman, Carter, & Stanton, 1995; Freeman et al., 1993; Stanton et al., 1992). The developmental onset of Pavlovian conditioning is further influenced by the sensory modality of the CS (see Hunt & Campbell, 1997). Training with different CS modalities may therefore be useful to explore the developmental emergence of eyeblink conditioning in rabbits. Use of a visual CS may not be expected to alter the emergence of eyeblink conditioning in rabbits, as training with a light CS yields the same pattern of conditioning in PD 17–24 rats as the commonly-used tone CS (Paczkowski, Ivkovich, & Stanton, 1999). Instead, use of an earlier-developing sensory system (e.g., tactile) as a CS may be more appropriate. Deflection of whiskers has been used as a CS in the restrained rabbit eyeblink preparation (Galvez, Weiss, Weible, & Disterhoft, 2006). Since development of CS pathways contributes to the emergence of eyeblink conditioning in rats (Freeman & Campolattaro, 2008), extending the whisker-stimulation method to pre-weanling rabbits has potential to provide a more complete characterization of eyeblink conditioning in the developing rabbit. Most importantly, to demonstrate the associative nature of conditioning in developing rabbits, non-associative changes in behavior resulting from aforementioned parametric modifications must be controlled for through comparison with unpaired control groups (see Rescorla, 1967). Non-associative interpretations of the present findings (e.g., pseudoconditioning) cannot be ruled out until these comparisons are made.
Behavioral training during development provides greater sensitivity to early developmental insult relative to testing in adulthood (see Stanton, 1992). Characterization of eyeblink conditioning in the developing rabbit therefore has implications for many avenues of neurodevelopmental research, including neurotoxicology. The well-characterized neurobehavioral properties of eyeblink conditioning have made it successful as a model for studying developmental neurotoxicology in rats (Goodlett, Stanton, & Steinmetz, 2000; Stanton & Freeman, 1994; Stanton, Peloso, Brown, & Rodier, 2007). Distinct characteristics of rabbits make this species well-suited to advance these findings and maximize translation to human disorders. For one, rabbits have greater phylogenetic proximity to primates than rodents (Graur et al., 1996), thus making rabbits a closer biological model to humans. Furthermore, the neonatal rabbit receives considerably less attention from the doe (e,g, in terms of licking, grooming, and suckling time) relative to mother-pup interactions in the neonatal rat (Blass, Hall, & Teicher, 1979; Hall & Williams, 1983; Hudson, Cruz, Lucio, Ninomiya, & Martinez-Gomez, 1999; Hudson & Distel, 1986). This limited interaction enhances the likelihood that impairments in rabbit offspring following early developmental insult are the direct result of the insult and minimally reflect artifacts stemming from disruption of the doe-pup relationship (Hudson & Distel, 1986). Additionally, effects of prenatal exposure to a variety of potential teratogens have been investigated in rabbits with overall findings of enhanced validity relative to rodents for predicting teratogenicity in humans (Schardein et al., 1985; Schumacher, Blake, Gurian, & Gillette, 1968 a; Schumacher, Terapane, Jordan, & Wilson, 1968 b). However, experimental endpoints in these studies have focused on peripheral effects such as overt limb malformation. The ability to test eyeblink conditioning in the developing rabbit offers a unique opportunity to determine the degree to which these findings generalize to functional neurological effects. Of the agents with greater teratogenic potential in rabbits relative to rats, thalidomide is perhaps the most relevant due to its established links to autism spectrum disorders (ASD; see Miller et al., 2005, and Sadamatsu, Kanai, Xu, Liu, & Kato, 2006, for reviews). A distinct ASD phenotype has been identified using eyeblink classical conditioning, as both human ASD patients (Sears, Finn, & Steinmetz, 1994) and rats prenatally exposed to valproic acid (an animal model of ASD; Murawski, Brown, & Stanton, 2009; Stanton et al., 2007) produce abnormally elevated and short latency eyeblink CRs relative to controls. Recapitulation of these findings in developing rabbits prenatally exposed to thalidomide may provide further insights into the etiology of ASD and promote continued use of the eyeblink preparation as an assay for functional effects characteristic of ASD.
The present findings may provide the basis for novel research on the ontogeny of memory. The rapid forgetting of events occurring over the first 3–5 years of human life – commonly referred to as “infantile amnesia” – has been studied extensively in animal models (Campbell & Spear, 1972). However, an animal model of infantile amnesia using eyeblink classical conditioning has yet to be established. This is due in part to difficulty in assessing the same subject between the weanling period and adulthood in current eyeblink preparations (e.g., the freely-moving rat; Stanton & Freeman, 2000) due to growth-induced degradation of surgically implanted equipment. Our developing rabbit eyeblink preparation avoids invasive procedures and allows for assessment of the same subject at multiple points throughout the lifespan. Preliminary evidence for infantile amnesia effects was found in the present study, as subjects initially trained during the pre-weanling period showed poor retention of conditioning when compared to experimentally naïve subjects one month after acquisition (see Figure 3). However, establishing a model of infantile amnesia requires identification of the earliest age at which learning reaches adult levels so that age differences at retention tests are not confounded with age differences in strength of learning at acquisition. Conditioning in rabbits initially trained at PD 61–64 was greater than conditioning in rabbits initially trained at PD 17 and 24, necessitating further examination of the developmental emergence of eyeblink conditioning in rabbits before embarking on studies on the ontogeny of memory. In addition to the modifications proposed earlier in this discussion, training over additional age ranges (e.g., beyond PD 24) as well as over multiple daily sessions (to avoid confounding learning with maturation; c.f. Stanton et al., 1992) should be considered to equate conditioning in developing rabbits with that of adult rabbits. The establishment of a novel rabbit eyeblink model of infantile amnesia has potential to fill in many of the gaps in our knowledge regarding neural mechanisms responsible for the rapid forgetting of information acquired early in development.
Confirmation of the feasibility for eyeblink training in rabbits as young as PD 17 extends the range of species available for developmental analysis of eyeblink classical conditioning beyond humans and rats (and sheep – see Johnson, Stanton, Goodlett, & Cudd, 2008). With elaboration of optimal conditions and age ranges for eyeblink training in pre-weanling rabbits as well as comparisons with unpaired control groups in future studies, this model may provide valuable insights into the neurobiological substrates underlying the development of associative learning and memory and developmental neurotoxicology. Dramatic neurobiological and behavioral parallels across adulthood and aging have been demonstrated in rabbits and humans in eyeblink classical conditioning (Woodruff-Pak, 2001; Woodruff-Pak & Trojanowski, 1996), and extension of this paradigm to developing rabbits allows for comprehensive comparative analyses across the life span.
Acknowledgments
The authors thank Michael Brown for construction of the modified restrainer and headpiece used for eyeblink training in pre-weanling rabbits. The authors also thank Christopher de Solίs for assistance with behavioral training. This research was supported by grants from the National Institute on Aging, 1 R01 AG021925 and 1 R01 AG023742 to DSW-P.
References
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. Boca Raton: CRC Press; 1997. [Google Scholar]
- Anderson WA, Flumerfelt BA. Purkinje cell growth beyond the twenty-third postnatal day. Brain Research. 1985;349(1–2):195–200. doi: 10.1016/0165-3806(85)90143-9. [DOI] [PubMed] [Google Scholar]
- Andrews SJ, Freeman JH, Carter CS, Stanton ME. Ontogeny of eyeblink conditioning in the rat: auditory frequency and discrimination learning effects. Developmental Psychobiology. 1995;28(6):307–320. doi: 10.1002/dev.420280602. [DOI] [PubMed] [Google Scholar]
- Blass EM, Hall WG, Teicher MH. The ontogeny of suckling and ingestive behaviors. In: Sprague JM, Epstein AN, editors. Progress in psychobiology and physiological psychology. volume 8. New York: Academic Press; 1979. pp. 243–299. [Google Scholar]
- Brown KL, Woodruff-Pak DS. Eyeblink conditioning in animal models and humans. In: Raber J, editor. Animal models of behavioral analysis. New York: Springer; 2011. pp. 1–27. [Google Scholar]
- Campbell BA, Spear NE. Ontogeny of memory. Psychological Review. 1972;79(3):215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. The Journal of Neuroscience. 1996;16(8):2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning and Memory. 2003;10(6):427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Claflin DI, Stanton ME, Herbert J, Greer J, Eckerman CO. Effect of delay interval on classical eyeblink conditioning in 5-month-old human infants. Developmental Psychobiology. 2002;41(4):329–340. doi: 10.1002/dev.10050. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Woodruff-Pak DS. Delay classical conditioning in young and older rabbits: initial acquisition and retention at 12 and 18 months. Behavioral Neuroscience. 1993;107(1):63–71. doi: 10.1037//0735-7044.107.1.63. [DOI] [PubMed] [Google Scholar]
- Das GD, Nornes HO, Hine RJ, Pfaffenroth MJ. Experimental studies on the postnatal development of the brain II. Cytoarchitectural regeneration in the developing cerebellum of the rabbit. T. -I. -T. Journal of Life Sciences. 1973;3(2):29–65. [PubMed] [Google Scholar]
- Freeman JH. Ontogeny of multiple memory systems: developmental neurobiology ofcerebellar learning. In: Blumberg MS, Freeman JH Jr, Robinson SR, editors. Oxford handbook of developmental behavioral neuroscience. New York: Oxford; 2010. [Google Scholar]
- Freeman JH, Campolattaro MM. Ontogenetic change in the auditory conditioned stimulus pathway for eyeblink conditioning. Learning and Memory. 2008;15(11):823–828. doi: 10.1101/lm.1131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behavioral and Cognitive Neuroscience Reviews. 2004;3(1):3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Spencer CO, Skelton RW, Stanton ME. Ontogeny of eyeblink conditioning in the rat: Effects of US intensity and interstimulus interval on delay conditioning. Psychobiology. 1993;21(3):233–242. [Google Scholar]
- Galvez R, Weiss C, Weible AP, Disterhoft JF. Vibrissa-signaled eyeblink conditioning induces somatosensory cortical plasticity. The Journal of Neuroscience. 2006;26(22):6062–6068. doi: 10.1523/JNEUROSCI.5582-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD, Weidemann G, Kehoe EJ. Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behavioral Neuroscience. 2003;117(2):292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Kolb FP, Timmann D. The involvement of the human cerebellum in eyeblink conditioning. Cerebellum. 2007;6(1):38–57. doi: 10.1080/14734220701225904. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Stanton ME, Steinmetz JE. Alcohol-induced damage to the developing brain: functional approaches using classical eyeblink conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning, volume 2: Animal models. Boston: Kluwer Academic Publishers; 2000. pp. 135–153. [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental methods and instrumentation in psychology. New York: McGraw-Hill; 1966. pp. 385–420. [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning with the rabbit. Progress in Psychobiology and Physiological Psychology. 1983;10:197–275. [Google Scholar]
- Gormezano I, Schneiderman N, Deaux E, Fuentes I. Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science. 1962;138:33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mammals. In: Tobach E, Aronson LR, Shaw E, editors. The biopsychology of development. New York: Academic Press; 1971. pp. 67–128. [Google Scholar]
- Graur D, Duret L, Gouy M. Phylogenetic order of the Lagomorpha (rabbits, hares, and allies) Nature. 1996;379(6563):333–335. doi: 10.1038/379333a0. [DOI] [PubMed] [Google Scholar]
- Hall WG, Williams CL. Suckling isn’t feeding, or is it? A search for developmental continuities. In: Rosenblatt JM, Hinde RA, Beer C, Busnel M-C, editors. Advances in the study of behavior. volume 13. New York: Academic Press; 1983. pp. 219–254. [Google Scholar]
- Herbert JS, Eckerman CO, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behavioral Neuroscience. 2003;117(6):1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H. The potential of the newborn rabbit for behavioral teratological research. Neurobehavioral Toxicology and Teratology. 1986;8(3):209–212. [PubMed] [Google Scholar]
- Hudson R, Cruz Y, Lucio RA, Ninomiya J, Matínez-Gómez M. Temporal and behavioral patterning of parturition in rabbits and rats. Physiology and Behavior. 1999;66(4):599–604. doi: 10.1016/s0031-9384(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Campbell BA. Developmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. Learning, motivation, and cognition: The functional behaviorism of Robert C. Bolles. Washington, D. C.: American Psychological Association; 1997. pp. 53–74. [Google Scholar]
- Ivkovich D, Collins KL, Eckerman CO, Krasnegor NA, Stanton ME. Classical delay eyeblink conditioning in 4- and 5-month-old human infants. Psychological Science. 1999;10(1):4–8. [Google Scholar]
- Johnson TB, Stanton ME, Goodlett CR, Cudd TA. Eyeblink classical conditioning in the preweanling lamb. Behavioral Neuroscience. 2008;122(3):722–729. doi: 10.1037/0735-7044.122.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble GA. Conditioning as a function of the time between conditioned and unconditioned stimuli. Journal of Experimental Psychology. 1947;37(1):1–15. doi: 10.1037/h0059371. [DOI] [PubMed] [Google Scholar]
- Little AH, Lipsitt LP, Rovee-Collier C. Classical conditioning and retention of the infant’s eyelid response: effects of age and interstimulus interval. Journal of Experimental Child Psychology. 1984;37(3):512–524. doi: 10.1016/0022-0965(84)90074-2. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Ruiz BP. Learning-dependent timing of Pavlovian eyelid responses: differential conditioning using multiple interstimulus intervals. Behavioral Neuroscience. 1992;106(4):666–681. doi: 10.1037//0735-7044.106.4.666. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Lavond DG, Thompson RF. Concomitant classical conditioning of the rabbit nictitating membrane and eyelid responses: correlations and implications. Physiology and Behavior. 1982;28(5):769–775. doi: 10.1016/0031-9384(82)90192-5. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Research. 1985;359(1–2):120–130. doi: 10.1016/0006-8993(85)91419-2. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. The Journal of Neuroscience. 1984;4(11):2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MT, Strömland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. International Journal of Developmental Neuroscience. 2005;23(2–3):201–219. doi: 10.1016/j.ijdevneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Brown KL, Stanton ME. Interstimulus interval (ISI) discrimination of the conditioned eyeblink response in a rodent model of autism. Behavioural Brain Research. 2009;196(2):297–303. doi: 10.1016/j.bbr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Oswald BB, Knuckley B, Mahan K, Sanders C, Powell DA. Prefrontal control of trace eyeblink conditioning in rabbits (Oryctolagus cuniculus) II: effects of type of unconditioned stimulus (airpuff vs. periorbital shock) and unconditioned stimulus intensity. Physiology and Behavior. 2009;96(1):67–72. doi: 10.1016/j.physbeh.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Paczkowski C, Ivkovich D, Stanton ME. Ontogeny of eyeblink conditioning using a visual conditional stimulus. Developmental Psychobiology. 1999;35(4):253–263. doi: 10.1002/(sici)1098-2302(199912)35:4<253::aid-dev1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Powell DA, Maxwell B, Penney J. Neuronal activity in the medial prefrontal cortex during Pavlovian eyeblink and nictitating membrane conditioning. The Journal of Neuroscience. 1996;16(19):6296–6306. doi: 10.1523/JNEUROSCI.16-19-06296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychological Review. 1967;74(1):71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Rogers RF, Britton GB, Steinmetz JE. Learning-related interpositus activity is conserved across species as studied during eyeblink conditioning in the rat. Brain Research. 2001;905(1–2):171–177. doi: 10.1016/s0006-8993(01)02532-x. [DOI] [PubMed] [Google Scholar]
- Sadamatsu M, Kanai H, Xu X, Liu Y, Kato N. Review of animal models for autism: implication of thyroid hormone. Congenital Anomalies. 2006;46(1):1–9. doi: 10.1111/j.1741-4520.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Schardein JL, Schwetz BA, Kenel MF. Species sensitivities and prediction of teratogenic potential. Environmental Health Perspectives. 1985;61:55–67. doi: 10.1289/ehp.856155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N, Fuentes I, Gormezano I. Acquisition and extinction of the classically conditioned eyelid response in the albino rabbit. Science. 1962;136:650–652. doi: 10.1126/science.136.3516.650. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Gormezano I. Conditioning of the nictitating membrane of the rabbit as a function of CS-US interval. Journal of Comparative and Physiological Psychology. 1964;57(2):188–195. doi: 10.1037/h0043419. [DOI] [PubMed] [Google Scholar]
- Schumacher HJ, Blake DA, Gurian JM, Gillette JR. A comparison of the teratogenic activity of thalidomide in rabbits and rats. The Journal of Pharmacology and Experimental Therapeutics. 1968 a;160(1):189–200. [PubMed] [Google Scholar]
- Schumacher HJ, Terapane J, Jordan RL, Wilson JG. The teratogenic activity of a thalidomide analogue, EM12, in rabbits, rats, and monkeys. Teratology. 1968 b;5:233–240. doi: 10.1002/tera.1420050213. [DOI] [PubMed] [Google Scholar]
- Sears LL, Finn PR, Steinmetz JE. Abnormal classical eyeblink conditioning in autism. Journal of Autism and Developmental Disorders. 1994;24(6):737–751. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- Smith MC. CS-US interval and US intensity in classical conditioning of the rabbit’s nictitating membrane response. Journal of Comparative and Physiological Psychology. 1968;66(3):679–687. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]
- Spear NE, Rudy JW. Tests of the ontogeny of learning and memory: issues, methods, and results. In: Shair HN, Barr GA, Hofer MA, editors. Developmental psychobiology: new methods and changing concepts. New York: Oxford University Press; 1991. pp. 84–113. [Google Scholar]
- Stanton ME. Animal models of cognitive development in neurotoxicology. In: Isaacson RL, Jensen KF, editors. The vulnerable brain and environmental risks, volume 1: malnutrition and hazard assessment. New York: Plenum Press; 1992. pp. 129–149. [Google Scholar]
- Stanton ME, Fox GD, Carter CS. Ontogeny of the conditioned eyeblink response in rats: acquisition or expression? Neuropharmacology. 1998;37(4–5):623–632. doi: 10.1016/s0028-3908(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH. Eyeblink conditioning in the developing rat: an animal model of learning in developmental neurotoxicology. Environmental Health Perspectives. 1994;102(2):131–139. doi: 10.1289/ehp.94102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH. Developmental studies of eyeblink conditioning in the rat. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning, volume 2: Animal models. Boston: Kluwer Academic Publishers; 2000. pp. 105–134. [Google Scholar]
- Stanton ME, Freeman JH, Skelton RW. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106(4):657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Peloso E, Brown KL, Rodier P. Discrimination learning and reversal of the conditioned eyeblink reflex in a rodent model of autism. Behavioural Brain Research. 2007;176(1):133–140. doi: 10.1016/j.bbr.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences, U. S. A. 1987;84(10):3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF. The Neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Tobia MJ, Woodruff-Pak DS. Delay eyeblink classical conditioning is impaired in Fragile X syndrome. Behavioral Neuroscience. 2009;123(3):665–676. doi: 10.1037/a0015662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Eyeblink classical conditioning differentiates normal aging from Alzheimer’s disease. Integrative Physiological and Behavioral Science. 2001;36(2):87–108. doi: 10.1007/BF02734044. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Finkbiner RG, Sasse DK. Eyeblink conditioning discriminates Alzheimer’s patients from non-demented aged. Neuroreport. 1990;1(1):45–49. doi: 10.1097/00001756-199009000-00013. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Logan CG, Thompson RF. Classical conditioning in 3-, 30-, and 45-month-old rabbits: behavioral learning and hippocampal unit activity. Neurobiology of Aging. 1987;8(2):101–108. doi: 10.1016/0197-4580(87)90018-2. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Seta SE, Roker LA, Lehr MA. Effects of paradigm and inter-stimulus interval on age differences in eyeblink classical conditioning in rabbits. Learning and Memory. 2007;14(4):287–294. doi: 10.1101/lm.504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18–83 years. Psychology and Aging. 1988;3(3):219–229. doi: 10.1037//0882-7974.3.3.219. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Trojanowski JQ. The older rabbit as an animal model: implications for Alzheimer’s disease. Neurobiology of Aging. 1996;17(2):283–290. doi: 10.1016/0197-4580(95)02064-0. [DOI] [PubMed] [Google Scholar]


