Abstract
Pim serine/threonine kinases contribute to prostate tumorigenesis and therapeutic resistance, yet Pim kinase inhibitors appear to have only limited effects on prostate cancer cell survival. Since overexpression of Bcl-2 family members are implicated in chemotherapeutic resistance in prostate cancer, we investigated the cooperative effects of Pim kinase inhibition with ABT-737, a small molecule antagonist of Bcl-2 family members. Strikingly, the addition of ABT-737 to Pim inhibitors triggered a robust apoptosis of prostate cancer cells in vitro and in vivo. Pim inhibitors decreased levels of the Bcl-2 family member Mcl-1, both by blocking 5'-cap dependent translation and decreasing protein half life. Additionally, Pim inhibition transcriptionally increased levels of the BH3 protein Noxa by activating the unfolded protein response (UPR), lead to eIF-2α phosphorylation and increased expression of CHOP. Increased levels of Noxa also inactivated the remaining levels of Mcl-1 protein activity. Notably, these specific protein changes were essential to the apoptotic process because ABT-737 did not inhibit Mcl-1 protein activity and Mcl-1 overexpression blocked the apoptotic activity of ABT-737. Our results therefore suggest that this combination treatment could be developed as a potential therapy for human prostate cancer where overexpression of Pim kinases and antiapoptotic Bcl-2 family members drives tumor cell resistance to current anticancer therapies.
Keywords: ABT-737, apoptosis, Bcl-2, ER stress, mTORC1, Pim kinase, UPR
INTRODUCTION
The Pim family of serine threonine protein kinases plays a critical but unexplicated role in the growth and progression of human prostate cancer (PCa) (1). This enzyme family is overexpressed in human PCa compared to benign biopsies (2) and enhanced levels of nuclear Pim-2 in tumor cells have been associated with a higher risk of prostate-specific antigen (PSA) recurrence and with perineural invasion of the prostate gland (3). Moderate to strong cytoplasmic staining of Pim-1 was seen in tumors of 68 % of patients with a Gleason score of seven or higher (4). Pim-1 is overexpressed in high grade prostate intraepithelial neoplasia (HGPIN), and Pim staining may be helpful in differentiating benign glands from intraepithelial neoplasia (2). We have previously reported that the expression of Pim-1 in PCa cells confers a growth advantage on these tumor cells (5). Patients with high levels of Pim-1 expression in the prostate are at significantly greater risk for developing metastatic cancer (6). In animals, overexpression of the c-Myc protein in the prostate induces neoplasia and is associated with increased Pim protein kinase levels (7). In a subrenal capsular assay for prostate regeneration, expression of Pim-1 kinase promotes c-Myc-driven prostate carcinogenesis (8). These studies suggest that the Pim protein kinases may be a target to inhibit prostate cancer growth.
Through chemical library screening (9), we have identified the chemotype of benzylidene-thiazolidine-2,4-diones as a specific Pim protein kinase inhibitor and focused on SMI-4a, a pan isotype inhibitor, as a potential lead compound. SMI-4a induces both apoptosis and cell cycle arrest in a wide variety of leukemic cell lines (10). Additionally, oral administration of SMI-4a to mice is well tolerated and causes moderate inhibition of leukemic growth when cells are grown as tumor xenografts (11). When administered to PCa cells, Pim inhibitors induce a cell cycle block but are only moderately inhibitory to growth (10). Although the mechanism of action of this agent has not been fully evaluated, we have shown that application of SMI-4a to both leukemia and prostate cells inhibits mammalian target of rapamycin complex 1 (mTORC1) activity and 4E-BP1 phosphorylation (10, 12), possibly by activating the AMP-dependent protein kinase (AMPK). Application of Pim inhibitors stimulates a fall in ATP levels and increases the concentration of AMP (12). Activated AMPK phosphorylates raptor and TSC2 to block mTORC1 activity (13). AMPK activation by Pim inhibition requires phosphorylation of Thr-172 by LKB (12), suggesting that control of energy supplies by SMI-4a could be an essential part of its activity.
The resistance of prostate cancers to undergo apoptosis when challenged with various chemotherapeutic agents could be due to the overexpression of antiapoptotic members of the Bcl-2 protein family. Studies have demonstrated that metastatic PCa and castration-refractory tumors are positively associated with Bcl-2 overexpression (14), so the moderate sensitivity of PCa cells to Pim kinase inhibitors could be due to deregulated expression of pro- and anti-apoptotic Bcl-2 proteins. ABT-737, a small molecule BH3 mimetic that interacts tightly with Bcl-2/Bcl-xL/Bcl-w and inhibits their activity, has the potential to overcome this resistance (15). However, this agent does not bind to Mcl-1 and Bfl-1 proteins. Research by us and others shows that Mcl-1 can constrain the pro-apoptotic activity of ABT-737 (16). Recent reports showed that that ABT-737 acquires intrinsic resistance to apoptosis by increasing Mcl-1 binding with Bim (17), and ABT-737 resistance is associated with reduced levels of Bcl-2:Bim heterodimers (18) and high Mcl-1 expression (16, 19). Although ABT-737 has generated anticancer activity in small cell lung cancer, leukemia and multiple myeloma, PCa cells in vitro exhibit a poor response to the pro-apoptotic action of ABT-737 because the Mcl-1 protein is widely expressed at high levels in PCa cell lines and inhibits the proapoptotic function of ABT-737. We have shown that ABT-737 as a single agent has little proapoptotic activity in PCa cells (20, 21). However, the combination of ABT-737 and a Pim inhibitor is highly synergistic in inducing apoptotic cell death. We investigated the ABT-737/Pim inhibitor synergy, with particular focus on the mechanism by which Pim inhibitors regulate apoptotic pathways. Thus, we suggest a rationale for this novel combination therapy.
MATERIALS AND METHODS
Cell lines, cell culture, and chemicals
PCa cell lines LNCaP, PC-3, DU-145 and 22Rv1 were purchased from the ATCC. Cells were grown in DMEM or RPMI1640 supplemented with 10% FBS, 2 mM Glutamax and 1 % antibiotics (Invitrogen, Carlsbad, CA) as previously described (21). Sub-confluent cells were treated with Pim inhibitors or vehicle in the absence of FBS. (Z)-5-(3-Trifluoromethylbenzylidene)thiazolidine-2,4-dione (referred to as SMI-4a) and [Z,E]-5-[4-ethylbenzylidine]-2-thioxothiazolidin-4-one (referred to as 10058-F4) were from Calbiochem (San Diego, CA). For animal experiment, SMI-4a was prepared as we reported previously (9). K00135 was purchased from BioFocus (Cambridge, UK). 8-(4-Hydroxylphenyl)-2-[(dimethlamino)methyl][1]benzothieno-[3,2-d]pyrimidin-4(3H)-one (referred to as Pimi-14j) (22) and ABT-737 were a gift of Abbott Laboratories (Abbott Park, IL). Other chemicals of analytic grade were purchased from EMD Chemicals (Gibbstown, NJ) and Sigma-Aldrich (St. Louis, MO).
Short hairpin RNAs (shRNAs) and plasmids
The Arrest-In Lentiviral expression system (Open Biosystems, Huntsville, AL) was used to establish a LNCaP cell line harboring small hairpin microRNAs (shRNAs) as described previously (12, 20). Lentiviruses pGIPZ shRNAmir against human Pim-1 (RHS4531-NM_002648), Pim-2 (RHS4531-NM_006875), and Pim-3 (RHS4531-NM_001001852), and a non-silencing control (RHS4348) were purchased from Open Biosystems. PC-3 cells were transfected with pcDNA3.1-HA-Bcl-2 (23) and pcDNA3-Bcl-2 (AddGene; Cambridge, MA) by LipofectAMINE2000 (Invitrogen) and then transfectants were selected and grown in 1 mg/mL of G418 (Sigma-Aldrich).
Tumor growth in vivo
Xenografts bearing prostate tumors were generated by injection of LNCaP cells (5×106) in the flanks of the male NU/NU nude mice (Charles River, Wilmington, MA). After tumors were grown to at least 100 mm3 (~1 wk after implantation), 36 mice were randomly divided into four different treatment groups: Group 1 (6 mice), vehicle only (30 % propylene glycol, 5 % Tween-80, 65 % of 5% dextrose in water, pH 4–5); Group 2 (12 mice), 60 mg/kg SMI-4a twice daily treatments (BID); Group 3 (6 mice), 50 mg/kg ABT-737 once a day (QD); and Group 4 (12 mice), combination treatment with SMI-4a (BID) and ABT-737 (QD). Mice received oral gavages for SMI-4a or/and intraperitoneal injection for ABT-737. Treatment was begun on day 8 and administered 5 of 7 days each week for 3 weeks. The growth of the subcutaneous tumors was measured twice each week, and mouse body weight was determined on days 0 and 21. Tumor size was calculated using the equation (L × W2)/2. The Institutional Animal Care and Use Committee at the Medical University of South Carolina approved these animal experiments. For the immunohistochmistry of xenograft tumor tissues, tissue slices were processed to generate 5 µm tissue slides. Sections were stained with H&E, mouse monoclonal antibody to human Mcl-1 (Santa Cruz Biotechnology), and rabbit antibody to cleaved caspase-3 (Cell Signaling Technology) according to the manufacturer's protocol for these products.
Quantitative real time PCR (qT-PCR), immunoblotting, and biochemical analysis
QT-PCR and immunoblot analyses were performed as previously reported (20) with slight modification as described in the Supplemental Methods. Methods for the 7-Methyl-GTP cap binding assay and 35S-methinone incorporation were reported previously (12) and are further described in the Supplemental methods.
RESULTS
Inhibition of Bcl-2 like proteins with ABT-737 synergizes with SMI-4a to induce apoptosis
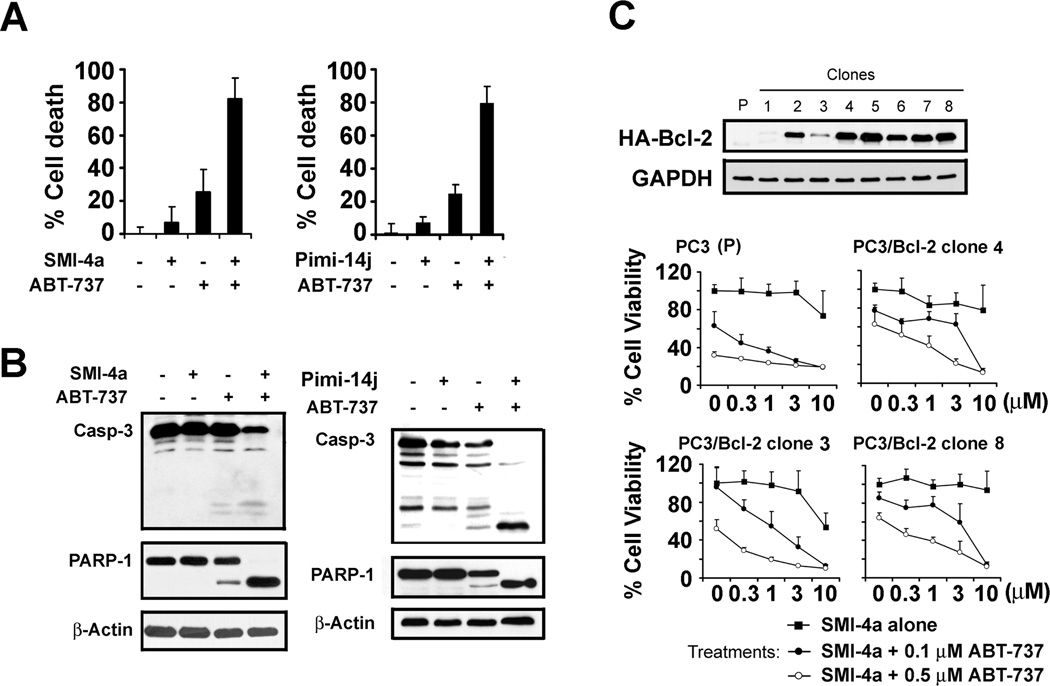
SMI-4a, a small molecule Pim kinase inhibitor, has preclinical efficacy in lymphoid and myeloid leukemia (11) but the human PCa cell lines LNCaP, PC-3 and 22Rv1 only respond modestly to SMI-4a treatment (Supplementary Fig. S1A). To demonstrate whether the response to Pim inhibitors was Pim specific, we knocked down the expression of all three Pims, 1, 2 and 3, using shRNAs. The knockdown of each of these individual Pim kinases in LNCaP cells, as demonstrated by qT-PCR, decreased the growth of these human PCa cells (Supplementary Fig. S2). Because Bcl-2 protein family expression is associated with resistance of PCa to standard chemotherapy, and a higher frequency of Bcl-2 expression is seen in recurrent tumors after castration (24, 25), we examined whether combination therapy with a small molecule BH3 mimetic ABT-737 and a Pim inhibitor would provide an enhanced apoptotic response. ABT-737 binds and induces apoptosis by inhibiting the activity of Bcl-2, Bcl-xL and Bcl-w. However, this compound is incapable of binding to Bcl-2 like the protein Mcl-1, and increased expression of this protein in numerous cancer cell types was sufficient to inhibit the activity of ABT-737 (21). ABT-737 treatment of LNCaP cells alone induced only a small percentage of cell death, but when combined with SMI-4a, a high degree of synergy in inducing cell death occurred (Fig. 1A). ABT-737 treatment alone induced slight cleavage of PARP-1 (Fig. 1B), a marker of apoptosis, but when combined with SMI-4a marked cleavage of this protein was seen. Similar synergistic results were obtained with another structurally different Pim inhibitor Pimi-14j (22), which blocks Pim activity in vitro in the low nanomolar range (Fig. 1A). Combination therapy with this Pim kinase inhibitor and ABT-737 induced enhanced caspase-3 and PARP-1 cleavage (Fig. 1B). A robust induction of cell death by these two agents was also seen in PC-3 cells, another human PCa cell line (Supplementary Fig. S3). A third Pim kinase inhibitor K00135 was also synergistic with ABT-737 (Supplementary Fig. S3). Moreover, this combination overcame resistance to cell death in human PCa cells overexpressing Bcl-2 (Fig. 1C).
Fig. 1. Bcl-2 inhibitor ABT-737 synergizes with small molecule inhibitors of Pim protein kinases to overcome cell resistance mediated by Bcl-2 overexpression.
A, LNCaP cells were treated with DMSO, ABT-737 (3 µM), SMI-4a (10 µM), Pimi-14j (10 µM), or combinations for 16h. The percentage of cell death was evaluated using trypan blue staining assay (mean +/− SD, n=6). B, Whole cell extracts from the above treatments were analyzed by immunoblotting with the antibodies shown. C, PC-3 cells were transfected with HA-tagged Bcl-2 plasmid and individual clones selected with 1 mg/ml G418 treatment. Parental PC-3 (P) cells and selected individual clones were treated with DMSO, SMI-4a, ABT-737 or combinations of both agents at the indicated doses. The percentage of viable cells after 24h of treatment was determined by MTT assay (mean +/− SD, n=4).
Pim kinase inhibitors downregulate Mcl-1 protein expression by lowering global protein synthesis
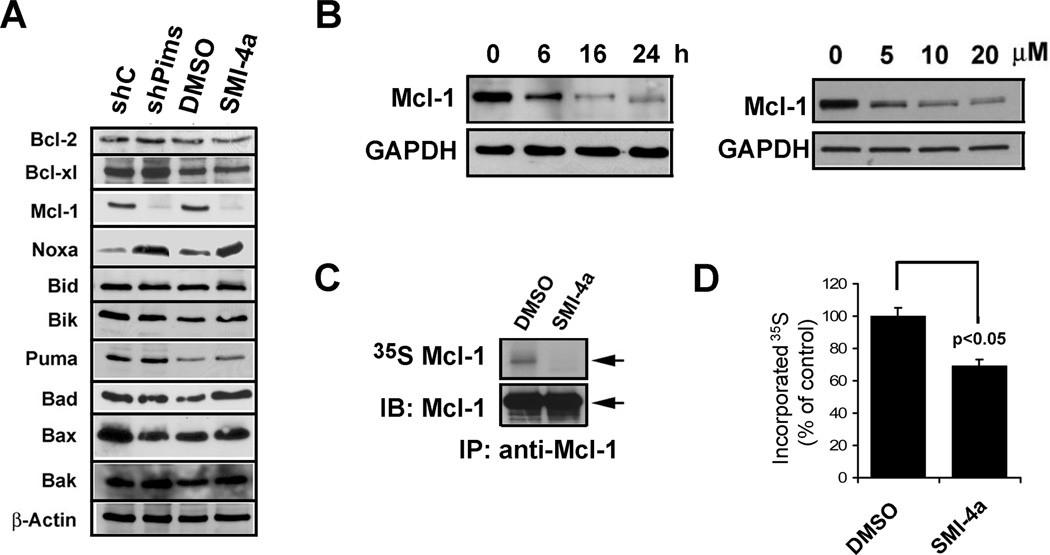
To determine a potential mechanism by which Pim inhibitors synergize with ABT-737 to kill PCa cells, we examined the ability of SMI-4a to regulate the levels of BH3 and Bcl-2 family member proteins important in controlling apoptotic cell death. Western blot results (Fig. 2A) demonstrate that either inhibition of Pim kinases with SMI-4a or knock-down of Pim kinases with shRNAs induced a marked decrease in Mcl-1 and an increase in Noxa protein. These results are significant because Noxa is capable of binding and inhibiting the antiapoptotic activity of Mcl-1 and thereby overcomes ABT-737 resistance. In LNCaP cells, the levels of Mcl-1 expression were reduced by SMI-4a treatment in a time- and dose-dependent manner (Fig. 2B). Additionally, in the PC-3 cell line a decrease in the Mcl-1 protein levels was induced by SMI-4a treatment (Supplementary Fig. S4A) and two additional small molecule Pim kinase inhibitors, K00135 and Pimi-14j (Supplementary Fig. S4B). No significant change in the levels of MCL-1 mRNA occurred after the treatment with the Pim inhibitor SMI-4a (Supplementary Fig. S4C). A further suggestion that Pim kinase plays a role in regulating Mcl-1 protein levels comes from the evaluation of mouse embryo fibroblasts (MEFs) derived from mice that were genetically engineered to knock out all three Pim proteins (TKO) (12). After 35S-methionine labeling, these MEFs demonstrated a lower level of the Mcl-1 protein (Supplementary Fig. S4D) (12). Given that MCL-1 mRNA levels were unaffected by Pim inhibitors, we next labeled LNCaP cells with 35S-methionine followed by immunoprecipitation and examined the Mcl-1 synthesis. Our results demonstrate that SMI-4a inhibits the translation of this protein (Fig. 2C). Additionally, 35S methionine-labeled whole cell extracts demonstrated that this drug treatment suppressed total protein synthesis in LNCaP cells (Fig. 2D). These results are identical to those previously obtained in TKO MEFs compared to wild type where decreased synthesis of proteins regulated by the 5’cap and total protein synthesis was seen (12).
Fig. 2. Pim inhibition downregulates Mcl-1 expression by blocking protein synthesis.
A, LNCaP cells expressing shRNA constructs targeting Pim-1, 2 and 3, nonsilencing control (shC) or treated with SMI-4a (10 µM) for 16h were lysed and cell extracts were analyzed by immunoblotting with the antibodies listed. B, LNCaP cells were treated with varying doses of SMI-4a for 16h or with SMI-4a (10 µM) for different times. Cell extracts were analyzed by immunoblotting with the antibodies shown. C, LNCaP cells were methionine starved, treated with DMSO or SMI-4a for 16h, and incubated with 35S-methionine. After labeling, the Mcl-1 protein was immunoprecipitated, and analyzed by autoradiography and immunoblotting. The arrow denotes the upper band that is identified as the Mcl-1 protein. D, Aliquots of whole cell lysates obtained in C were washed with 10% TCA, the protein dried, and the proteins labeled with 35S-methionine quantified by scintillation counting (mean +/− SD, n=6). The p value of p < 0.05 for differences between DMSO and SMI-4a treatment was obtained by a two-way analysis of variance.
Pim kinase inhibitors inhibit mTROC1-mediated protein synthesis and increase Mcl-1 degradation
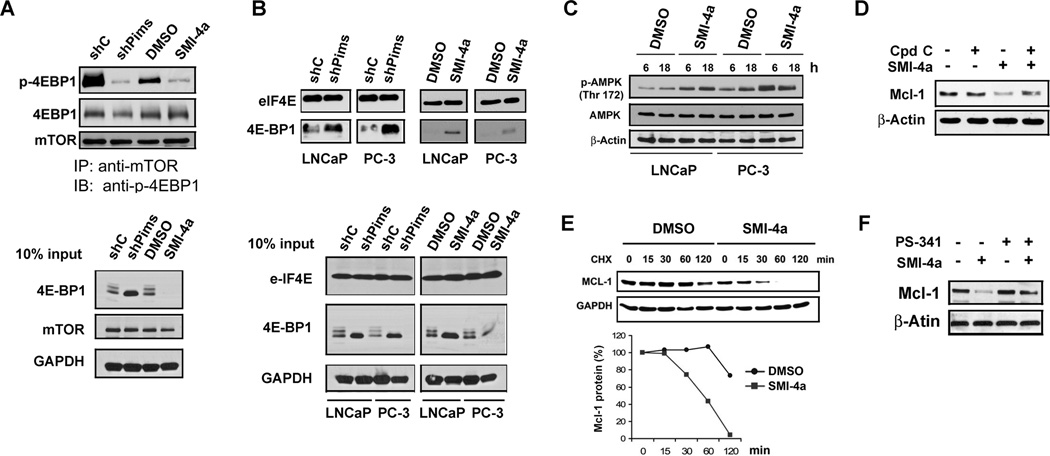
To evaluate the mechanism by which inhibition of Pim kinases suppresses protein synthesis, the level of phosphorylated 4E-BP1 was measured after treatment of prostate cancer cells with either shRNAs to Pim kinases or the kinase inhibitor SMI-4a. Decreased mTORC1 activity led to increased levels of dephosphorylated 4E-BP1, which then was bound to the eIF-4E protein, blocking protein synthesis. We found that both knockdown of the three Pims and SMI-4a treatment decreased the phosphorylation of 4E-BP1 (Supplementary Fig. S5). Immunoprecipitation of mTORC1 followed by anti-phospho 4E-BP1 Western additionally demonstrated that inhibition of Pim kinases decreased mTORC1 activity (Fig. 3A). To test whether Pim inhibition has an effect on the eIF4F assembly and cap structure recognition process, eIF4E and the proteins associated with it were isolated with m7-GTP-sepharose beads, which functions by mimicking the mRNA cap structure. As shown in Fig. 3B, the amount of phosphorylated 4E-BP1 associated with eIF-4E increased markedly in Pim-depleted cells or SMI-4a treated cells, suggesting a mechanism for the inhibition of 5’cap translation. Thus, inhibition of the Pim protein kinases in these cells decreased the activity of mTORC1 kinase.
Fig. 3. Pim inhibitor SMI-4a promotes Mcl-1 degradation by inhibiting mTORC1 signaling.
A, LNCaP cells stably expressing shRNA constructs against Pim-1, 2 and 3 (shPims) or nonsilencing control (shC) or treated with SMI-4a for 16h were lysed with CHAPS buffer and resulting lysates were subjected to immunoprecipitation (IP) using an antibody to mTOR and the immunoprecipitates probed with Abs to mTOR, 4E-BP1 and phosho-4E-BP1 (Thr37/46). Cell lysates (25 µg) were also immunoblotted as a control (Input). B, Cell extracts isolated after treatments described in A were incubated with m7-GTP beads to pull down 5’cap components. After washing, proteins bound to the beads were probed by immunoblotting with antibodies to eIF4E and 4E-BP1. Cell lysates (25 µg) were also immunoblotted as a control (Input). C, LNCaP and PC-3 cells were treated with either DMSO or SMI-4a (10 µM) for 6h or 18h. Cellular extracts were analyzed by immunoblotting with antibodies shown. D, LNCaP cells were exposed to 5 µM of the AMPK inhibitor Compound C (Cpd C) prior to treatment with either DMSO or SMI-4a (10 µM) for 16h. Cell extracts were analyzed by immunoblotting with antibodies shown. E, LNCaP cells were treated with DMSO or SMI-4a for 16h followed by cycloheximide (CHX) and cell samples were collected at the times indicated. Cell lysates were analyzed by immunblotting with antibodies shown. The graph is based on densitometry scanning (NIH ImageJ software) of the above immunblots. F, LNCaP cells were pretreated with the proteasome inhibitor PS-341 (100 nM) for 1h and then treated with either DMSO or SMI-4a (10 µM) for 16 h. Cell extracts were analyzed by immunblotting with antibodies shown.
mTORC1 activity can be negatively regulated by AMP-dependent protein kinase (13), which is activated by phosphorylation on threonine 172 and increased AMP levels. We found that treatment of both LNCaP and PC-3 prostate cancer cells with SMI-4a induced increases in AMPK phosphorylation (Fig. 3C), suggesting a potential mechanism by which the Pim kinases could regulate the mTORC1 pathway. To test if AMPK is required for SMI-4a to downregulate Mcl-1 expression, LNCaP cells were treated with SMI-4a and compound C, an AMPK inhibitor (Fig. 3D). This additional treatment partially blocked the ability of SMI-4a to decrease Mcl-1 protein levels suggesting that AMPK control of mTORC1 activity contributes to this down-regulation.
Because SMI-4a activity was only partially inhibited by compound C, we examined the possibility that SMI-4a also regulated the rate of destruction of the Mcl-1 protein. LNCaP cells were first treated with SMI-4a or Pimi-14j for 16 h followed by cycloheximide treatment to block new protein synthesis in the absence of Pim inhibitors. Pretreatment with SMI-4a induced much more rapid degradation of the Mcl-1 protein than cells treated with vehicle (DMSO) (Fig. 3E). To further examine whether proteasome-mediated Mcl-1 protein degradation occurs in cells treated with Pim inhibitors, LNCaP cells were pre-treated with the proteasome inhibitor PS-341 (VELCADE) and then further treated with SMI-4a. In cells treated with both compounds, proteasome inhibition reversed the ability of the Pim inhibitor to induce Mcl-1 protein degradation (Fig. 3F). These results suggest that the reduction in Mcl-1 protein levels induced by Pim inhibitor treatment is mediated by regulation of both protein synthesis and protein degradation rate.
Inhibition of Pim kinase increases the level of Noxa protein
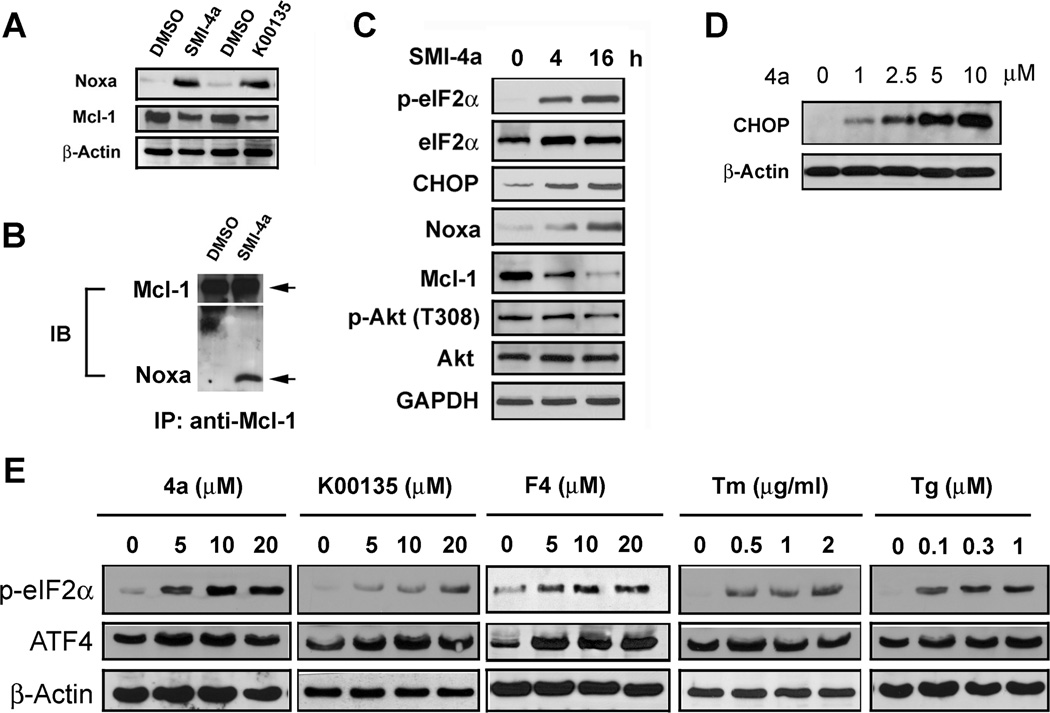
Treatment of LNCaP cells with the Pim inhibitors SMI-4a or K00135 increased the cellular levels of the BH3 protein Noxa (Fig. 4A). Small hairpin RNA-mediated knockdown of Pims in LNCaP cells was also sufficient to increase Noxa protein levels (Supplementary Fig. S6A). The Noxa protein specifically binds and inhibits the Mcl-1 protein’s anti-apoptotic activity. Immunoprecipitation demonstrates that the Pim inhibitor-increased Noxa protein bound to Mcl-1 (Fig. 4B). As seen on western blot, the Pim inhibitors concurrently decreased the overall levels of the Mcl-1 protein (Fig. 4C). To determine how Pim inhibitors might regulate Noxa protein levels, we examined the unfolded protein response (UPR). In the condition of an energy deficit, activation of the endoplasmic reticulum stress response is known to stimulate the UPR by eIF-2α phosphorylation and subsequent increases in the protein levels of the ATF4 and CHOP transcription factors (39). ATF4 induces increases in the Noxa mRNA and protein (26, 27). To clarify whether induction of Noxa by Pim inhibitors is associated with the eIF-2α arm of UPRsignaling, we treated LNCaP cells and evaluated protein levels of these factors. We found that SMI-4a treatment stimulates the phosphorylation of eIF-2α (Fig. 4C) and induces increases in ATF4 and CHOP protein in a dose dependent fashion (Fig. 4D & E) similarly to known activators of this pathway, tunicamycin (TM) and thasigargen (Tg) (Supplementary Fig. 6B).
Fig. 4. Noxa induction by Pim inhibitors is mediated through the endoplasmic reticulum stress response.
A, LNCaP cells were treated with DMSO, 10 µM SMI-4a, or 10 µM K00135 for 16h. Cell extracts were analyzed by immunblotting with antibodies shown. B, LNCaP cells were treated DMSO or SMI-4a for 16h. Aliquots of cell extracts were subjected to immunoprecipitation (IP) with Mcl-1 antibody and then immunoblot (IB) probed with Mcl-1 and Noxa antibodies. C, LNCaP cells were treated with 10 µM SMI-4a for 0, 4 or 16h. Cell extracts were analyzed by immunblotting with antibodies shown. D, LNCaP cells were treated with various doses of SMI-4a (4a) for 16h. Cell extracts were analyzed by immunblotting with antibodies shown. E, LNCaP cells were treated with Pim inhibitors at the indicated doses for 16h. Tunicamycin (TM) and Thapsigargin (Tg) were used as controls. Cells were treated with 10058-F4, which is of the same chemotype as SMI-4a and is an active Pim inhibitor. Cell extracts were analyzed by immunblotting with antibodies shown.
Pim inhibitors induce UPR followed by ER stress
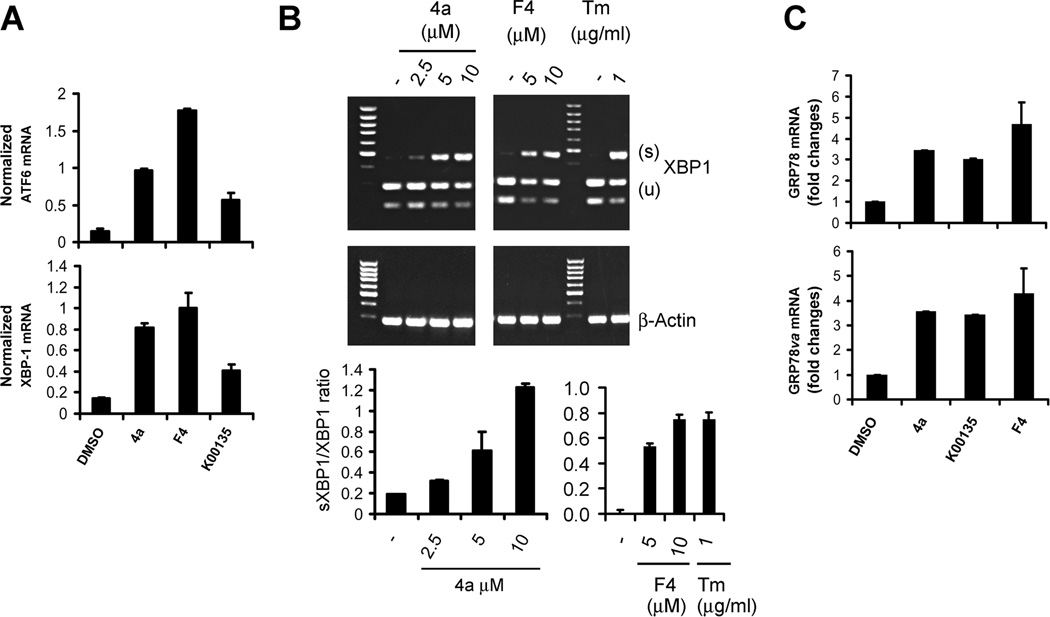
Based on the activation of eIF-2α phosphorylation, we examined whether Pim inhibitors also induces other arms of the ER stress pathway by assessing changes in ATF6 and XBP-1 transcriptional activity. XBP-1 mRNA is increased by ATF6 and spliced by IRE1 in response to ER stress, resulting in production of a transcription factor that can induce genes associated with the UPR (28). QT-PCR analysis demonstrated that the induction of ATF6 mRNA as well as XBP-1 mRNA occurs in response to Pim inhibitor treatment (Fig. 5A). The spliced version of XBP-1 is seen in this PCR assay as a larger band. This assay confirmed that SMI-4a treatment of LNCaP cells, when compared to the Tunicamycin control, is capable of inducing the splicing of XBP-1 in a dose dependent fashion (Fig. 5B). Pim inhibitor-induced splicing was seen in PC-3, Du145 and C4-2B cell lines (Supplementary Fig. S7A-C). Moreover, we found that SMI-4a did not increase XBP-1 splicing in MEFs lacking all Pim isoforms (TKO) MEF cells (Supplementary Fig. S7D) and knockdown of Pims in LNCaP cells with shRNAs decreased the ability of Pim inhibitors to cause XBP-1 splicing (Supplementary Fig. S7E). Activation of XBP-1 is known to lead to transcription of glucose-regulated protein 78 (grp78/BiP) and its splice variant grp78va. We found that both grp78/Bip and grp78va transcripts were significantly increased by treatment of LNCaP cells with SMI-4a or K00135 (Fig. 5C). This data demonstrates that inhibition of Pim kinase pathways is sufficient to activate a second arm of the UPR response.
Fig. 5. Pim kinase inhibitors induce XBP-1 splicing.
A, LNCaP cells were treated with DMSO, 10 µM SMI-4a (4a), 10 µM K00135, and 10 µM 10058-F4 (F4) for 16 h. Total RNA was isolated and then subjected to qT-PCR. The levels of ATF6 or XBP-1 mRNA were normalized to the 18S mRNA. B, LNCaP cells were treated with SMI-4a (4a), 10058-F4 (F4) or Tunicamycin (TM) at the indicated doses for 16h. Total RNA was isolated and then subjected to RT-PCR for detection of XBP-1 splicing (top panel). The ratio of the spliced (S) to the unspliced (U) XBP-1 mRNAs was assessed by qT-PCR analysis (bottom panel). The mean of triplicate determinations are shown +/− standard deviation. C, Total RNA isolated in A was subjected to qT-PCR analysis to determine level of GRP78 and its isoform GRP78va using the 18S mRNA as an internal control.
Combination treatment of Pim inhibitor SMI-4a and ABT-737 inhibits tumor growth in vivo
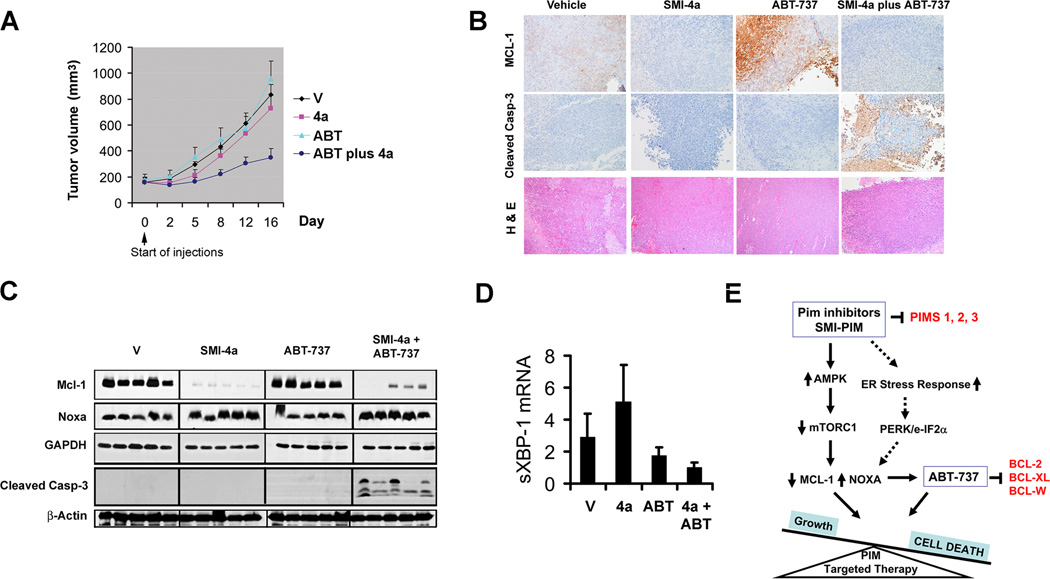
Given the synergy of Pim inhibitors and ABT-737 in cell culture, we sought to examine anticancer efficacy of the combination treatment in vivo through a tumor xenograft model. Mice bearing LNCaP prostate tumors were treated with either ABT-737, SMI-4a, or a combination of both agents. While neither individual drug treatment showed a difference from vehicle control, the combination treatment significantly suppressed tumor growth (Fig. 6A). Immunohistochimical staining of tumor tissues revealed that SMI-4a treatment markedly downregulated Mcl-1 expression (Fig. 6B) and ABT-737 increased the levels of this protein, as previously demonstrated by others (17) while combination therapy appeared to increase cleaved caspase-3. In tumor samples, Western blot analysis showed that combination therapy with SMI-4a and ABT-737 decreased Mcl-1 and increased Noxa and was associated with increased levels of the apoptosis marker caspase-3 cleavages (Fig. 6C). Using PCR, we demonstrated that SMI-4a treatment increased XBP-1 splicing in tumors (Fig. 6D), but this effect was not seen in the combination therapy. These in vivo results support the idea that a Pim inhibitor can be safely synergistically combined with ABT-737.
Fig. 6. Tumor growth in vivo is inhibited by combination treatment of ABT-737 and SMI-4a.
A, Mice were subcutaneously inoculated with LNCaP cells. Tumor volume was monitored during the following treatments: vehicle (V), ABT-737 (ABT; 50 mg/kg; i.p., QD), SMI-4a (4a; 60 mg/kg, oral gavage, BID) or a combination of SMI-4a and ABT-737 (ABT plus 4a) for 16 days (mean ± SEM). B, Immunohistochemical analysis of Mcl-1, cleaved caspase-3 (casp-3) and H&E staining. C, Western blot analysis of Noxa, Mcl-1, cleaved casp-3, β-actin, and GAPDH. Five representative tumors for each treatment were examined. D, qT-PCR analysis of XBP-1 splicing in subcutaneous tumors of treatment groups. Five representative tumors for each treatment group are shown. E, Proposed mechanistic model by which Pim inhibitor synergizes with ABT-737 to induce apoptosis. Pim inhibitors reduce Mcl-1 protein translation through activation of AMPK to block the mTORC1-mediated 5’cap-dependent protein translation. In addition, this treatment activates the UPR signaling to induce Noxa, thereby promoting increased Mcl-1 turnover.
DISCUSSION
Here we demonstrate that Pim kinase inhibitors of varied chemical structure can be combined with the Bcl-2 family antagonist ABT-737 to induce apoptosis in PCa cells and overcome cell death resistance caused by heightened expression of Bcl-2 protein. Resistance to ABT-737 in several tumor types (16, 17, 21) has been well documented to involve over expression of the Mcl-1 protein. ABT-737 does not bind with high affinity to Mcl-1, so it cannot block Mcl-1’s activity (29). Inducing changes in either the Noxa protein, Mcl-1, or both has been shown to induce the death of multiple tumor cell types (30) when incubated with ABT-737. For example, treatment with gemcitabine, a chemotherapeutic agent, increased the degradation of Mcl-1 and sensitized lung cancer cells to ABT-737 (31). Additionally, combining ABT-737 with bortezomib, a proteasome inhibitor known to increase Noxa levels, inhibited the growth of lymphoma cells (32), and actinomycin D treatment of tumor cells enhanced the efficacy of ABT-737 action by down-modulating Mcl-1 expression (33). PCa cell lines express heightened levels of Mcl-1 making them resistant to the action of ABT-737 (21). However, we demonstrated that the addition of the Pim inhibitors both decreased the cellular levels of the Mcl-1 protein and raised the level of the BH3 protein Noxa, a highly specific inhibitor of the Mcl-1 protein (34), markedly enhancing the activity of ABT-737. Previously, the application of a different Pim inhibitor, SGI-1776, to leukemic cells has been demonstrated to decrease the levels of Mcl-1, although the mechanism was not identified (35).
Mcl-1 protein levels can be regulated by transcriptional, translational, and post-translational mechanisms sensitize cells to ABT-737-induced death. Because the Mcl-1 protein has a short half-life, inhibitors that disturb protein synthesis, and in particular 5’cap driven translation, have the potential to decrease the cellular level of this protein (35, 36). For example, inhibition of glucose transport blocks AKT activity, inhibits the mTORC1 pathway, down-regulates Mcl-1 translation and sensitizes lymphoma cells to ABT-737 treatment (37). Similarly, in other contexts, mTORC1 has been shown to be responsible for Mcl-1 protein levels (35). We find in PCa cells that incubation with Pim inhibitors or knock down of Pim protein levels enhances the phosphorylation of 4E-BP-1 and as a result increases the binding of 4E-BP1 with eIF-4E inhibiting mTORC1 activity. We hypothesize that Pim inhibitors block the mTORC1 pathway based on their activation of AMPK, which we have shown plays a key role in fibroblasts in mediating the ability of Pim inhibitors to curtail protein synthesis (12). The ability of Pim kinases to modulate mTORC1 activity has been demonstrated in lymphoma cell lines (10, 38), leukemic cells (11), and normal fibroblasts (12). Similar findings have been identified in other cell systems. For example, when glucose is withdrawn or 2-deoxyglucose applied to leukemic cells increases in AMPK activity (39), inhibition of mTOR activity, and decreases in the levels of Mcl-1 protein (40) are seen. Activation of AMP-dependent protein kinase leads to phosphorylation of both the raptor and TSC-2 proteins to block mTORC1 activity (41). The mechanism by which Pim kinases regulate AMPK is not clear, but in fibroblasts the absence of Pim kinases causes increases in the amount of cellular AMP and a drop in ATP levels secondary to regulation of mitochondrial metabolism (12). Application of the Pim inhibitor, SMI-4a, to Bcr-Abl positive K562 cells induces identical biologic effects (10).
We found that the application of Pim kinase inhibitors to PCa cells also decreases the half-life of the Mcl-1 protein, an effect that is reversed by the addition of proteasome inhibitors. A number of mechanisms could explain this finding. The E3 ligase β-TrCP stimulates the degradation of Mcl-1, which has been phosphorylated by activated GSK-3β (42). GSK-3β activity is increased by agents that inhibit AKT such as by the withdrawal of growth factors like IL-3 (43). Preliminary data (unpublished results) suggests that Pim kinase inhibitors can block growth factor signaling mimicking the effects of growth factor withdrawal. Mcl-1 levels are also controlled by an interaction with Mule/ARF-BP1 E3 ubiquitin ligase (44) and this interaction can be modulated by, for example, heat shock (45). Pim kinase inhibitors by blocking mitochondrial metabolism could “stress” prostate cancer cells leading to protein degradation through a Mule/ARF-BP1 mechanism. Finally, the interaction of the Mcl-1 and its specific deubiquitinase USP9X is highly regulated (31, 46), and it is possible Pim kinases and their inhibitors could modulate this process, as well.
We demonstrate for the first time that a genetically engineered decrease in Pim kinase levels or the addition of Pim kinase inhibitors causes ER stress and activates the unfolded protein response (UPR) stimulating increases in eIF-2α phosphorylation, ATF4, CHOP proteins, cleavage of XBP-1, and increases in the Noxa protein. Although the Noxa protein was initially cloned as a p53 activated gene (47), it can be elevated transcriptionally in the absence of p53 by increases in both FoxO1 (48) and c-Myc (49). Recently, the proteasome inhibitor bortezomib was reported to activate the UPR and stimulate the formation of an ATF3 and ATF4 complex that enhances Noxa mRNA synthesis (26, 27). Previously, apoptosis and the UPR were connected by the observation that BAX and BAK were needed to induce the UPR by inositol-requiring-enzyme 1α (IRE-1α) (50). IRE-1α executes site-specific cleavage of XBP-1 mRNA to produce more stable transcript XBP-1 that encodes a potent transcriptional activator of UPR target genes (28). Dose dependent increases in XBP-1 splicing by Pim inhibitors confirm our finding that Pim inhibition drives cells to activate ER stress. Compared to wild MEF cells, XBP-1 splicing was not increased in Pim knock-out (TKO) MEF cells, suggesting again the potential role of Pim in regulating this pathway. Additional experiments will be necessary to determine the exact biochemical mechanism by which inhibition of the Pim kinases modulates the UPR.
Based on the cell culture findings that inhibition of Pim with SMI-4a increases Noxa and decreases Mcl-1 protein levels greatly enhancing the apoptotic activity of ABT-737, we combined these agents in an in vivo animal experiment. Results show that single agent therapy had little anticancer activity while combination treatment inhibited the growth of LNCaP cells grown subcutaneously. Immunohistochemistry and Western blots demonstrate that this combined therapy activates the UPR and induces apoptosis of these tumor cells (Fig. 6). Based on their ability to regulate protein synthesis and the UPR (see model Fig. 6E), these results suggest that Pim kinase inhibitors could be used in the clinic to enhance the anticancer activity of ABT-737.
Supplementary Material
Acknowledgements
We would like to thank Abbott Laboratories and Dr. Joel Leverson for providing ABT-737 and Pim inhibitor Pimi-14j. Bcl-2 expression vector was kindly provided by Dr. Timothy C. Chambers at the University of Arkansas for Medical Sciences (Little Rock, AR). Members of the Andrew S. Kraft and Charles D. Smith laboratories (Medical University of South Carolina) have been very kind in providing research reagents, materials, and technical assistance. The authors would like to gratefully acknowledge support from the Department of Defense Grant W81XWH-08-PCRP-IDA and the NIH 1P30-CA138313.
Abbreviations
- AMPK
AMP-dependent protein kinase
- Bcl-2
B-cell lymphoma 2
- BH3
Bcl-2 homology domain 3
- ER
endoplasmic reticulum
- HGPIN
high grade prostate intraepithelial neoplasia
- mTORC1
mammalian target of rapamycin complex 1
- PCa
prostate cancer
- PSA
prostate-specific antigen
- qT-PCR
quantitative real-time polymerase chain reaction
- RT-PCR
reverse transcriptase-PCR
- UPR
unfolded protein response
Footnotes
Conflict of interest
J.H.S. had no conflict of interest. A.S.K. owns shares in Vortex Biotechnology.
REFERENCES
- 1.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 2.Cibull TL, Jones TD, Li L, Eble JN, Ann Baldridge L, Malott SR, et al. Overexpression of Pim-1 during progression of prostatic adenocarcinoma. J Clin Path. 2006;59:285–288. doi: 10.1136/jcp.2005.027672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai H, Li R, Wheeler T, Diaz de Vivar A, Frolov A, Tahir S, et al. Pim-2 upregulation: biological implications associated with disease progression and perinueral invasion in prostate cancer. Prostate. 2005;65:276–286. doi: 10.1002/pros.20294. [DOI] [PubMed] [Google Scholar]
- 4.Valdman A, Fang X, Pang ST, Ekman P, Egevad L. Pim-1 expression in prostatic intraepithelial neoplasia and human prostate cancer. Prostate. 2004;60:367–371. doi: 10.1002/pros.20064. [DOI] [PubMed] [Google Scholar]
- 5.Chen WW, Chan DC, Donald C, Lilly MB, Kraft AS. Pim family kinases enhance tumor growth of prostate cancer cells. Mol Cancer Res. 2005;3:443–451. doi: 10.1158/1541-7786.MCR-05-0007. [DOI] [PubMed] [Google Scholar]
- 6.Shah N, Pang B, Yeoh KG, Thorn S, Chen CS, Lilly MB, et al. Potential roles for the PIM1 kinase in human cancer - a molecular and therapeutic appraisal. Eur J Cancer. 2008;44:2144–2151. doi: 10.1016/j.ejca.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Valdman A, Fang X, Pang ST, Ekman P, Egevad L. Pim-1 expression in prostatic intraepithelial neoplasia and human prostate cancer. Prostate. 2004;60:367–371. doi: 10.1002/pros.20064. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Lee C, Ng PY, Rubin M, Shabsigh A, Buttyan R. Prostatic neoplasia in transgenic mice with prostate-directed overexpression of the c-myc oncoprotein. Prostate. 2000;43:278–285. doi: 10.1002/1097-0045(20000601)43:4<278::aid-pros7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Xia Z, Knaak C, Ma J, Beharry ZM, McInnes C, Wang W, et al. Synthesis and evaluation of novel inhibitors of Pim-1 and Pim-2 protein kinases. J Med Chem. 2009;52:74–86. doi: 10.1021/jm800937p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beharry Z, Zemskova M, Mahajan S, Zhang F, Ma J, Xia Z, et al. Novel benzylidene-thiazolidine-2,4-diones inhibit Pim protein kinase activity and induce cell cycle arrest in leukemia and prostate cancer cells. Mol Cancer Ther. 2009;8:1473–1483. doi: 10.1158/1535-7163.MCT-08-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YW, Beharry ZM, Hill EG, Song JH, Wang W, Xia Z, et al. A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T-cell lymphoblastic leukemia/lymphoma. Blood. 2010;115:824–833. doi: 10.1182/blood-2009-07-233445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beharry Z, Mahajan S, Zemskova M, Lin YW, Tholanikunnel BG, Xia Z, et al. The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci USA. 2011;108:528–533. doi: 10.1073/pnas.1013214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu AY, Corey E, Bladou F, Lange PH, Vessella RL. Prostatic cell lineage markers: emergence of BCL2+ cells of human prostate cancer xenograft LuCaP 23 following castration. Int J Cancer. 1996;65:85–89. doi: 10.1002/(SICI)1097-0215(19960103)65:1<85::AID-IJC15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 16.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Song JH, Kandasamy K, Zemskova M, Lin YW, Kraft AS. The BH3 mimetic ABT-737 induces cancer cell senescence. Cancer Res. 2011;71:506–515. doi: 10.1158/0008-5472.CAN-10-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JH, Kandasamy K, Kraft AS. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem. 2008;283:25003–25013. doi: 10.1074/jbc.M802511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao ZF, Hasvold LA, Leverson JD, Han EK, Guan R, Johnson EF, et al. Discovery of 3H-benzo[4,5]thieno[3,2-d]pyrimidin-4-ones as potent, highly selective, and orally bioavailable inhibitors of the human protooncogene proviral insertion site in moloney murine leukemia virus (PIM) kinases. J Med Chem. 2009;52:6621–6636. doi: 10.1021/jm900943h. [DOI] [PubMed] [Google Scholar]
- 23.Upreti M, Chu R, Galitovskaya E, Smart SK, Chambers TC. Key role for Bak activation and Bak-Bax interaction in the apoptotic response to vinblastine. Mol Cancer Ther. 2008;7:2224–2232. doi: 10.1158/1535-7163.MCT-07-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–6944. [PubMed] [Google Scholar]
- 25.Banerjee PP, Banerjee S, Brown TR. Bcl-2 protein expression correlates with cell survival and androgen independence in rat prostatic lobes. Endocrinology. 2002;143:1825–1832. doi: 10.1210/endo.143.5.8763. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem. 2011;285:6091–6100. doi: 10.1074/jbc.M109.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 29.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, et al. 'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, et al. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther. 2011;10:1264–1275. doi: 10.1158/1535-7163.MCT-10-1091. [DOI] [PubMed] [Google Scholar]
- 32.Paoluzzi L, Gonen M, Bhagat G, Furman RR, Gardner JR, Scotto L, et al. The BH3-only mimetic ABT-737 synergizes the antineoplastic activity of proteasome inhibitors in lymphoid malignancies. Blood. 2008;112:2906–2916. doi: 10.1182/blood-2007-12-130781. [DOI] [PubMed] [Google Scholar]
- 33.Olberding KE, Wang X, Zhu Y, Pan J, Rai SN, Li C. Actinomycin D synergistically enhances the efficacy of the BH3 mimetic ABT-737 by downregulating Mcl-1 expression. Cancer Bio Ther. 2011;10:918–929. doi: 10.4161/cbt.10.9.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Chen LS, Redkar S, Taverna P, Cortes JE, Gandhi V. Mechanisms of cytotoxicity to Pim kinase inhibitor, SGI-1776, in acute myeloid leukemia. Blood. 2011;118:693–702. doi: 10.1182/blood-2010-12-323022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17:249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coloff JL, Macintyre AN, Nichols AG, Liu T, Gallo CA, Plas DR, et al. Akt-dependent glucose metabolism promotes mcl-1 synthesis to maintain cell survival and resistance to bcl-2 inhibition. Cancer Res. 2011;71:5204–5213. doi: 10.1158/0008-5472.CAN-10-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schatz JH, Oricchio E, Wolfe AL, Jiang M, Linkov I, Maragulia J, et al. Targeting cap-dependent translation blocks converging survival signals by AKT and PIM kinases in lymphoma. J Exp Med. 2011;208:1799–1807. doi: 10.1084/jem.20110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008;7:809–817. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 40.Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, et al. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene. 2010;29:1641–1652. doi: 10.1038/onc.2009.448. [DOI] [PubMed] [Google Scholar]
- 41.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Bio. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Stankiewicz AR, Livingstone AM, Mohseni N, Mosser DD. Regulation of heat-induced apoptosis by Mcl-1 degradation and its inhibition by Hsp70. Cell Death Diff. 2009;16:638–647. doi: 10.1038/cdd.2008.189. [DOI] [PubMed] [Google Scholar]
- 46.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 47.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 48.Valis K, Prochazka L, Boura E, Chladova J, Obsil T, Rohlena J, et al. Hippo/Mst1 stimulates transcription of the proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Res. 2011;71:946–954. doi: 10.1158/0008-5472.CAN-10-2203. [DOI] [PubMed] [Google Scholar]
- 49.Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, et al. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci USA. 2007;104:19488–19493. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.