Abstract
Morphological and electrophysiological studies have shown that granule cell axons, the mossy fibers (MFs), establish gap junctions and, therefore, electrical communication among them. That granule cells express gap junctional proteins in their axons suggests the possibility that their terminals express them as well. If this were to be the case, mixed electrical-chemical communication could be supported, as MF terminals normally use glutamate for fast communication with their target cells. Here we present electrophysiological and modeling studies consistent with this hypothesis. We show that MF activation produced fast spikelets followed by excitatory postsynaptic potentials in pyramidal cells (PCs), which, unlike the spikelets, underwent frequency potentiation and were strongly depressed by activation of metabotropic glutamate receptors, as expected from transmission of MF origin. The spikelets, which persisted during blockade of chemical transmission, wee potentiated by dopamine and suppressed by the gap junction blocker carbenoxolone. The various waveforms evoked by MF stimulation were replicated in a multi-compartment model of a PC by brief current pulse injections into the proximal apical dendritic compartment, where MFs are known to contact PCs. Mixed electrical and glutamatergic communication between granule cells and some PCs in CA3 may ensure the activation of sets of PCs, bypassing the strong action of concurrent feed-forward inhibition that granule cells activate. Importantly, MF-to-PC electrical coupling may allow bidirectional, possibly graded communication that can be faster than chemical synapses and subject to different forms of modulation.
Keywords: CA3, mossy fibers, spikelets, mixed transmission, electrical communication
Introduction
In the hippocampus, dendro-dendritic connections among different types of interneurons (Gibson et al., 1999; Galarreta & Hestrin, 2001) and, possibly, between pyramidal cells (PCs; MacVicar & Dudek, 1981; Valiante et al., 1995) are well known. Gap-junction-mediated electrical communication between adult granule cells (MacVicar & Dudek, 1982) was shown to occur between their axons, the mossy fibers (MFs), and evidence for this has been obtained with electrophysiological and anatomical techniques (Schmitz et al., 2001; Hamzei-Sichani et al., 2007). Despite the evidence of gap junctions in the principal cells of the dentate gyrus (DG), axo-dendritic electrical synapses between two different types of principal cells in the hippocampus have not been reported so far. Moreover, no axo-dendritic electrical synapses have been observed between excitatory axons and principal cell dendrites, in the mammalian telencephalon. The presence of spikelets in intracellular recordings has been a landmark for the existence of electrical communication among neurons (Baker & Llinás, 1971; Llinás et al., 1974). In the hippocampus, although the occurrence of these spikelets has been related to ectopic, dendritic (Spencer & Kandel, 1961) or axonal spikes (Traub et al., 1995), true electrical coupling between principal cells and between interneurons has been corroborated with paired recordings (Galarreta & Hestrin, 2001; MacVicar & Dudek, 1981). These reports have characterized spikelets as all-or-none fast depolarizations of variable, but small amplitude, with clearly faster kinetics than chemically-driven synaptic potentials, and they can on occasion give rise to action potentials. Of note, these gap-junction-mediated electrical spikelets have not been found to be accompanied by a chemical synaptic component. However, that granule cells express gap junctional proteins in their axons would suggest the possibility that their terminals express them as well. If so, both chemical and electrical transmission might occur, whereby stimulation of the MFs would evoke an electrical spikelet accompanied by a chemical component. Pharmacological and physiological criteria must then be met to imply that such a response corresponds to transmission of MF origin. We tested this hypothesis and we here show that MF activation produced fast spikelets in a number of PCs. They had an onset latency consistent with MF conduction velocity and were followed by glutamate receptor-mediated excitatory postsynaptic potentials that had the physiological and pharmacological characteristics of MF neurotransmission. Moreover, the fast spikelets persisted during blockade of chemical transmission, were potentiated by dopamine and suppressed by the gap junction blocker carbenoxolone. To our knowledge, this constitutes the first evidence of mixed electrical-chemical communication between two principal cells of the mammalian forebrain.
Materials and methods
Electrophysiological recordings
Combined entorhinal cortex-hippocampus slices (400 μm) were obtained from adult Wistar rats as previously described (Gutiérrez, 2000). For recording, the slices were transferred to an air-liquid interface chamber and were constantly perfused with oxygenated artificial cerebrospinal fluid (ACSF) at 34 ± 0.5°C containing (in mM) 124 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, and 10 glucose, pH 7.35. The drugs used were diluted in the ACSF, namely the NMDA receptor antagonist (D,L)-2-amino-5-phosphonovaleric acid (APV; 30 μM; Tocris-Cookson, Ellisville, Missouri); the non-NMDA receptor antagonist 6-nitro-7-sulfamoylbenzo-(f)quinolaxine-2,3-dione (NBQX; 10 μM; Tocris-Cookson); the GABAA receptor antagonists bicuculline methiodide (20 μM; Sigma-Aldrich), picrotoxin (200 μM; Sigma-Aldrich); the M1-cholinergic antagonist pirenzepine (10 μM, RBI); the gap junction blocker carbenoxolone (100 μM; Sigma-Aldrich); dopamine (10 μM; diluted in ascorbic acid 0.1 %; Sigma-Aldrich); the mGluR agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (DCG-IV; Tocris-Cookson). A bipolar (tip separation 50 μm), glass-insulated platinum wire (50 μm) electrode was placed over different sites of the granule cell layer of the DG for stimulation with pulses of 0.01-0.1 ms delivered every 20 s (see Fig. 1C1). This location of the stimulation electrode has two advantages. First, it prevents direct stimulation of dendrites of PCs or interneurons, which may activate PCs and, second, the longer distance with respect to the recording electrode allows separation of the stimulus artifact from the evoked spikelets. Intracellular activity of PCs was recorded with glass microelectrodes (50–80 MΩ) filled with potassium acetate (2 M) using an AxoClamp 2B amplifier (Molecular Devices, Foster City, CA). Signals were digitized (at 10 KHz; Digidata, Molecular Devices) acquired and analyzed off line with the pClamp 9 Software (Molecular Devices).
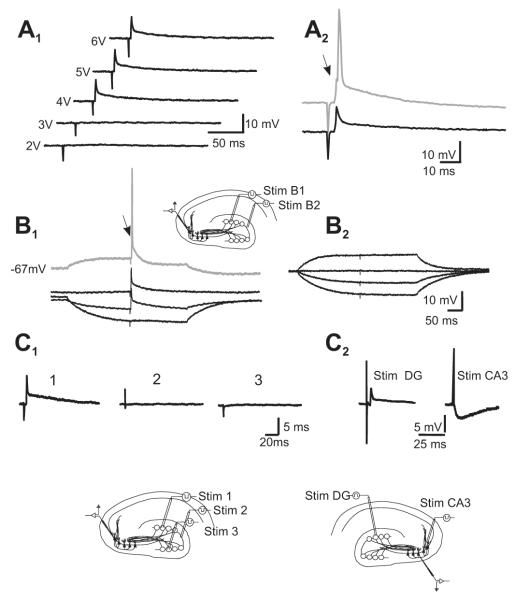
Figure 1.
Mossy fiber activation evokes spikelets in PCs of CA3 at resting membrane potential in the presence of glutamate, GABAA and ACh-M1 receptor antagonists at physiological calcium concentration. (A1) Spikelets are evoked in an all-or-none fashion once threshold is reached. The variability in the threshold current intensity for eliciting fast spikelets is due to the probability with which the electrode location and the stimulation intensity are capable of activating a MF that actually establishes gap junctions with the recorded PC. When stimulation is given at a depolarized membrane potential (A2 and displaced trace in B1), the PC fires an action potential, which is preceded by a notch (arrow) corresponding to an underlying spikelet. By contrast, hyperpolarization of the membrane potential by ≥ 15 mV suppresses the spikelet. (B2) Stimulation 300 μm away from the first stimulation site does not evoke any response in the same cell, indicating that the spikelet originated by stimulation of the first site is synapse-specific and not due to electrical artifacts. Similar results were obtained when stimulating farther away from the initial effective site (C1) and when placing the stimulus electrode in the pyramidal cell layer of CA3 (C2), which evoked an IPSP, only in the presence of glutamatergic antagonists.
Computational Methods
We constructed a multi-compartment model of a PC, using the architecture of the model described in Traub et al. (1994), with 64 compartments for soma and dendrites, and 6 for the axon. Compartment sizes were the same as before, except for a thinner axon initial segment. Rinput was 37 MΩ, Rm 50,000 and 1,000 Ω-cm2 for soma/dendrites and axon, respectively, and Ri 200 and 100 Ω-cm for soma/dendrites and axon, respectively. Cm was 0.8 μF/cm2. We used a different repertoire of membrane currents and kinetics, however, namely those described in Traub et al. (2005), including transient and persistent gNa, high- and low-threshold gCa, 5 types of voltage- and Ca2+-dependent K+ currents, and an anomalous rectifier. We simulated gap junctional inputs to the PC, from MF terminal action potentials, by injecting brief depolarizing current pulses (3 - 5 nA, 0.4 ms) into the proximal apical dendritic compartment. Current pulses were delivered at different levels of Ca2+-dependent slow afterhyperpolarization (AHP) conductance, the major effect of which was to alter membrane shunting. The current pulses evoked isolated spikelets with relatively larger AHP conductance values, and the pulses evoked spikelets leading into bursts at smaller AHP values. The data also show that spikelets can plausibly arise from electrical inputs into the proximal dendrites and do not necessarily require axonal activation. The simulation code was written in Fortran (CA3pyr.f) and is available upon request from rtraub@us.ibm.com.
Results
We show that granule cell activation produces fast spikelets in PCs, consistent with the idea of electrical communication between the MFs and PCs (Fig. 1). Unequivocal evidence that a spikelet is produced by an action potential conveyed by a MF would require simultaneous recordings of a granule cell and a PC. The scarcity of the MF-to-PC connections makes this approach unfeasible. An experimental approach has been developed to simultaneously record a MF giant bouton and a PC (Bischofberger et al., 2006), which, however, is neither adequate nor practical for our purposes, as a capacitive artifact may interfere with the detection of fast electrotonic coupling (P. Jonas, personal communication). Instead, intracellular recording of responses of PCs to distal stimulation of the DG provides an effective means to isolate spikelets from the stimulation artifact and also prevents the activation of PC axon collaterals and local interneurons. Moreover, due to the extremely low probability of finding electrically coupled MFs and PCs (see below), the use of sharp electrodes is a practical means to probe for electrical responses by performing multiple trajectories and, thus, recordings of several cells in a given experiment. We recorded synaptic responses of PCs in the CA3a-b area following DG stimulation in the presence of antagonists of NMDA and AMPA-type glutamate receptors, and of GABAA, and ACh-M1 receptors. Antagonists were diluted in ACSF containing physiological levels of calcium. Stimulation of the molecular layer of the DG evoked spikelets, attributed to electrical coupling, in 16 out of 339 recorded PCs (4.7%; Fig. 1). The spikelets had a mean amplitude of 4.4 ± 0.89 mV (mean ± s.e.m.), an onset latency of 3.9 ± 0.1 ms and a 20-80% rise time of 0.44 ± 0.03 ms (n = 13). In contrast, monosynaptic excitatory postsynaptic potentials (EPSPs) recorded in 100 PCs had an onset latency of 4.5 ± 0.8 ms and a 20-80% rise time of 2.5 ± 0.2 ms. In our recording set-up, we estimated a conduction velocity of ca. 0.37 m/s. Spikelets were evoked in an all-or-none fashion when the threshold intensity was reached and their amplitude did not vary on increasing stimulation intensity (Fig. 1A1). Also, further increasing the stimulation intensity of the DG stimulation did not evoke antidromic action potentials. By contrast, stimulation provided close to the recorded cell, in the stratum radiatum, evoked antidromic action potentials with a latency of 1 ms or less (not shown). On depolarization of the PC, DG stimulation evoked an action potential with a notch in its rising phase (Fig. 1A2, B1). On the other hand, hyperpolarizing the membrane potential had no effect on the amplitude of the spikelet until the hyperpolarization reached ≥15 mV, which suppressed it (Fig. 1B1). In 114 recordings, we delivered the stimuli over different sites of the DG (usually 4) to look for converging MF electrical inputs and to corroborate that the responses were not due to artifacts produced by current spreading through the extracellular fluid. In all but one case, only one stimulation site was effective in evoking a spikelet (Fig. 1B1, B2, C1, C2). Moreover, in some cells, besides recording the spikelets evoked by DG stimulation, we recorded IPSPs evoked by direct stimulation of the pyramidal cell layer in CA3 in the presence of glutamatergic antagonists, which were not preceded by a fast spikelet (Fig. 1C2). In most of the cells that exhibited evoked spikelets, spontaneously occurring ones were also observed at resting membrane potential (Fig. 2A, B), some of which preceded an action potential or a burst of action potentials (Fig. 2C). Their 20-80% rise time was not statistically different from that of the evoked spikelets (0.54 ± 0.05 ms, n = 5; Wilcoxon signed rank test, p = 0.3). Because MFs are known to contact the PCs in the proximal apical dendrite, we sought to corroborate whether a brief current pulse injection to the proximal dendritic compartment of a multi-compartment model of a CA3 PC would reproduce the waveforms that we observed in our experiments in the absence of chemical transmission (Fig. 2C, D). The model replicated the various waveforms evoked by MF stimulation. Indeed, simulations yielded both isolated spikelets (Fig. 2E1) and spikelets giving rise to action potentials (Fig. 2E2), which support the hypothesis that the spikelets evoked by DG stimulation are indeed generated in the proximal part of the apical dendrite of the PCs, where MFs are known to contact the PCs.
Figure 2.
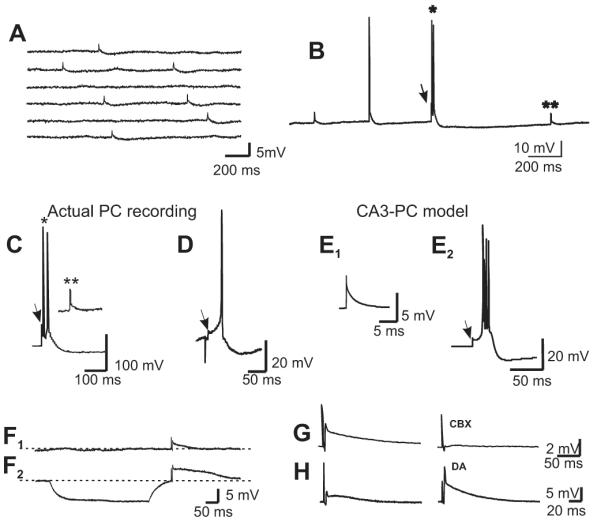
(A) Spontaneous spikelets with similar rise-time as the evoked ones were recorded in most of the responsive PCs. (B) Spikelets (arrow) occurred either preceding action potentials (single asterisk) or isolated (double asterisk). (C) Spontaneous events depicted in B with asterisks, at an expanded time scale. (D) Evoked spikelet followed by an action potential. (E) The waveforms of the experimentally obtained synaptic events in C and D were reproduced by a compartmental model. We simulated gap junctional inputs to PCs, from MF action potentials, by injecting brief depolarizing current pulses (3 nA, 0.4 ms) into the proximal apical dendritic compartment. (E1) The current pulses evoked isolated spikelets with relatively larger afterhyperpolarization conductance values. (E2) The pulses evoked spikelets leading into bursts at smaller afterhyperpolarization values. (F1, F2) A MF-evoked spikelet at resting membrane potential is enhanced when preceded by a hyperpolarizing pulse. This is consistent with removal of inactivation from fast Na+ channels. (G) Carbenoxolone (100 μM), a gap junction blocker, strongly depressed the MF-evoked spikelets, while DA (10 μM) strongly enhanced them (H).
Some fast Na+ channels involved in spikelet propagation may be inactivated at resting membrane potential, thereby reducing spikelet amplitude (Schmitz et al., 2001). To remove effects of Na+ channel inactivation, we injected a hyperpolarizing current pulse prior to the spikelet initiation and found that the spikelet amplitudes significantly increased from a mean amplitude of 6.7 mV to 10.3 mV (n = 5, t test, p < 0.01; Fig. 2F1-2). Further evidence for the electrical nature of the evoked responses was obtained by perfusing the gap junction blocker carbenoxolone (100 μM), which reduced the amplitude of the spikelets by 80 ± 8% (n = 5; Fig. 2G). Interestingly, dopamine (DA), which is known to potentiate the electrical responses in the Mauthner cell synapse in the goldfish (Pereda et al., 1992), caused a significant increase in the amplitude of the pharmacologically isolated spikelets (416 ± 39%, n = 3, Figure 2H) such that they could eventually provoke firing of action potentials.
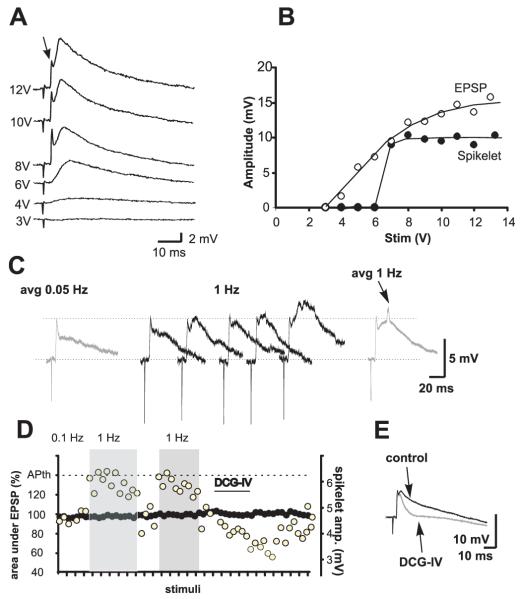
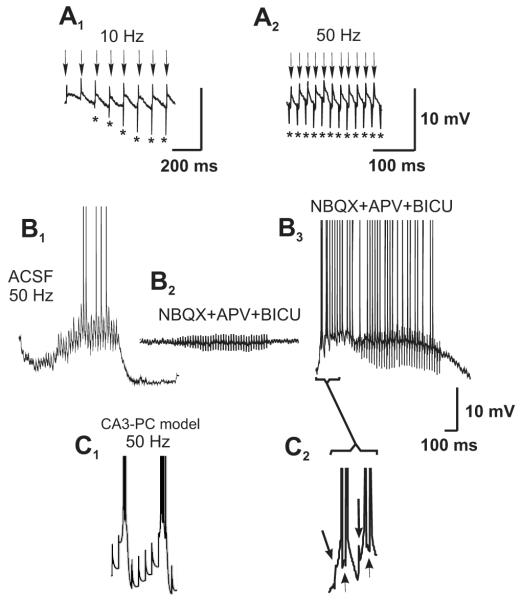
If MFs can communicate via electrical signaling with the PCs, mixed electrical-chemical signaling would be expected to occur under normal neurotransmission conditions. To corroborate the presence of mixed electrical-chemical transmission, we recorded synaptic responses in PCs evoked by the stimulation of 4-6 different sites of the DG under normal neurotransmission conditions. We detected a spikelet preceding the EPSP in 16 out of 335 recorded PCs (4.8%; Fig. 3). In some of these experiments, the stimulation initially produced an EPSP and, when threshold intensity for activating a MF that established an electric synapse was reached, the EPSP was preceded by a fast spikelet. The amplitude of the EPSP augmented as stimulation intensity was increased, whereas the amplitude of the spikelet was not modified (Fig. 3A, B). In other cells, spikelets were readily evoked at the minimal intensity needed to evoke any synaptic response (Fig. 3C). Because the synaptic responses that we studied were provoked by extracellular bipolar stimulation in the molecular layer of the DG, the variability in the threshold current intensity for eliciting fast spikelets could be due to the probability with which the electrode location and the stimulation intensity are capable of activating a MF that actually establishes gap junctions with the recorded PC. Spikelets that preceded EPSPs had a mean amplitude of 6.1 ± 0.5 mV and an onset latency of 3.6 ± 0.2 ms (n = 10). Because responses of MF origin undergo strong frequency potentiation, we initially stimulated at 0.05 Hz and increased the frequency to 1 Hz. This manipulation strongly potentiated the EPSP and readily induced action potentials, while the spikelet remained unchanged (Figure 3C, D). In two cells, where the EPSP was not apparent after the spikelet, increasing the stimulation frequency revealed the EPSP after a few stimuli (Fig. 3C). The pharmacological signature of transmission of MF origin is its sensitivity to activation of group II mGluRs, which depresses evoked glutamatergic responses (Kamiya et al., 1996). As expected, the mGluR-II agonist DCG-IV significantly depressed the evoked EPSP (65 ± 7 % as measured by the area under the curve of the EPSP; n=3), while the spikelet remained unchanged (Figure 3D, E). Finally, to determine how the post-synaptic cells integrate the electrical responses to repetitive stimulation, we stimulated the DG at 10 and 50 Hz in 4 experiments. Repetitive stimulation in the absence of chemical transmission with interstimulus intervals (ISI) of 20 and 100 ms, allowed a complete repolarization of the membrane after the post-depolarization that follows the spikelet in two experiments (Fig. 4A1—2; see also Fig. 2F2 and G). By contrast, in the other 2 experiments, while an ISI of 100 ms produced the effect just described, an ISI of 20 ms produced spiking activity due to temporal summation of the post-synaptic responses (Fig. 4B3). In the cell depicted in Fig. 4B, stimulation at 50 Hz in normal ACSF produced an initial hyperpolarization during the train, due to build-up of inhibition provided by the feed-forward mechanism that MFs drive (Fig. 4B1; Mori et al., 2007). The perfusion of ionotropic glutamatergic and GABAergic antagonists blocked all synaptic responses at 50 Hz (Fig. 4B2). Under these conditions, raising the stimulation intensity to recruit the fiber responsible for the electrical spikelet (see also Fig. 1) provoked a rapid summation of the post-synaptic responses, whereby a slow depolarization built-up while the neuron fired action potentials. Again, we corroborated whether high frequency input to the proximal dendritic compartment of a multi-compartment model of a CA3 PC reproduced the waveforms that we observed in our experiments. The model closely replicated the waveform evoked by MF stimulation, whereby a summation of responses leading to spiking activity could be observed (Fig. 4C1-2).
Figure 3.
Mossy fiber activation evokes mixed electrical and chemical synaptic responses in PCs under normal neurotransmission conditions. (A) Increasing stimulation intensities enhanced the chemical component of the synaptic response, while the spikelet was evoked upon reaching threshold intensity and not further modified by increasing the stimulus intensity. (B) Input-output curve of the chemical (EPSP) and electrical (spikelet) synaptic components. Note that the spikelet is evoked in an all-or-none fashion. (C, D) The chemical component following the spikelet was better observed after increasing the stimulation frequency from 0.05 to 1 Hz, which eventually produced action potentials riding on the EPSP (arrow in avg 1 Hz). (D) Plot of the behavior of the EPSP (area under the curve; left y axis) and of the spikelet (amplitude; right y axis) at 0.01 and 1 Hz and during the perfusion of DCG-IV. APth on the left hand axis signals action potential threshold. Because the effect of high frequency stimulation and of DCG-IV was estimated by measuring the area under the curve of the EPSP, no complete depression could be observed when perfusing DCG-IV, which isolates the area under the curve of the depolarization following the spikelet. The spikelet itself was not affected (E).
Figure 4.
Integration of electrical communication at high frequencies. In 2 (out of 4) experiments, stimulation at 10 (A1) and 50 Hz (A2) produced spikelets for each stimulus of the train in the presence of ionotropic glutamatergic and GABAergic antagonists. Asterisks signal stimulus artifacts; arrows signal spikelets. (B1) Under normal neurotransmission conditions (ACSF), stimulation of the MF at 50 Hz produces hyperpolarization of the cell membrane due to feed-forward inhibition. (B2) Perfusion of ionotropic glutamatergic and GABAergic receptors antagonists (NBQX+APV+BICU) blocks all synaptic responses. (B3) Under these conditions, stimulation at an intensity that recruits an electrically-coupled MF produces spikelets that give rise to action potentials over a slow depolarization produced by temporal summation of the responses (in 2 out of 4 experiments). (C1) Using the same model as in Figure 2, brief current pulses (0.4 ms, 5 nA) were injected into the proximal apical dendrite, at 50 Hz, corresponding to putative synchronized presynaptic terminal spikes in structures electrically coupled to the simulated cell. Note the brief bursts of spikes (often with a small spikelet on the rising phase of action potentials), alternating with larger spikelets. (C2) First 4 responses of the 50 Hz train, depicted in B3. The first and third responses in the train are spikelets (arrows), while the second and fourth stimuli (short, thin arrows) originated action potentials.
Discussion
Electrical-chemical, mixed synapses have been extensively documented in lower vertebrates and the best characterized are the large Club Ending terminals on Mauthner cells in the goldfish (Pereda et al., 1992). In the adult mammalian brain, however, mixed synapses have remained elusive. This is probably due to their low incidence, and because special recording conditions must be met, which are not routinely used in electrophysiological experiments. Here we have provided evidence of mixed electrical-chemical responses in pyramidal cells of CA3, consistent with axo-dendritic mixed electrical-chemical synapses between the MFs and pyramidal cells.
Specificity of mossy fiber responses
Unequivocal proof of MF-to-PC electrical communication can only be obtained by simultaneous recording of the pre- and the post-synaptic neurons. This approach is not feasible because a granule cell has a very low probability of making synaptic contact with a given given PC (Claiborne et al., 1986; Chicurel & Harris, 1992; Acsády et al., 1998). Moreover, the slicing procedure is likely to restrict even more the possibility of recording synaptically connected cells. Therefore, we here studied electrical coupling between MFs and PCs of CA3 by conducting intracellular recordings with sharp microelectrodes in the latter and extracellularly stimulating the molecular layer of the DG to activate granule cells. Besides the advantages of this experimental set-up, addressed in the Methods section, stimulation of the molecular layer of the DG allows the isolation of the electrical spikelets from the stimulation artifact intracellularly recorded from distal PCs due to MFs slow conduction velocity (Henze et al., 1997; Schmidt-Hieber et al., 2008). Moreover, the stimulation in the molecular layer of the DG prevents the activation of PC axon collaterals and hilar interneurons. Indeed, activation of PC axon collaterals or local interneurons would produce responses of very short latencies and electrical coupling between cells in CA3 rarely takes place when the neurons are more than 50 μm apart and, when coupled, responses have latency values of < 0.4 ms (Mercer et al., 2006). Despite this evidence, we can not fully discard the possibility of activating neuronal processes other than MFs. Although there are no reports of projections from the molecular layer of the DG to CA3a or b, other than MFs, anatomical work has shown interneurons located in stratum lucidum or hilus (Spruston et al., 1997; Sik et al., 1997; Buhl et al., 1997) that could extend processes that connect both distal areas. Such processes may establish electrical connections with MFs or with other interneurons, which, in turn, establish electrical connections with PCs. This form of connectivity is a theoretical possibility, although it might be considered unlikely, given the latency of the responses that we found, which correspond to MF conduction velocity.
Mossy fiber stimulation provokes electrical spikelets in the absence of chemical transmission and mixed electrical-chemical spikelets under normal neurotransmission conditions in CA3 pyramidal cells
We initially looked for the presence of electrical spikelets on stimulation of the molecular layer of the DG in the absence of chemical transmission. We found that only in ~ 5% of the recorded PCs would DG stimulation provoke fast electrical spikelets. This is likely to be an underestimate of true coupling frequency as the MF-to-CA3 connectivity is disrupted by the slicing procedure and because spikelets were recorded under physiological calcium concentrations, which does not favor the opening of gap junctions. These electrical responses presented an onset latency and an estimated conduction velocity that are consistent with transmission of MF origin, similar to those reported by Henze et al. (1997) using the same recording conditions. The rise time of the spikelets that we recorded was slower than that observed in DG somata after MF stimulation (Schmitz et al., 2001), possibly due to the higher capacitive load and lower input resistance of PCs compared to DG cells (Draguhn et al., 1998), and/or to a dendritic vs axonal origin of the spikelets. Importantly, the electrical spikelets were evoked in PCs by stimulating only one of several sites in the DG, showing their origin to be highly localized and site-specific, and not due to current spread through the extracellular medium. In fact, in no case did we provoke antidromic potentials or a shift of the onset latency of the spikelet upon increasing the stimulation intensity. This was also confirmed by stimulating close to the recorded cell, in the PC layer, which could evoke IPSPs but not spikelets, in the presence of glutamatergic blockers. Mossy fibers contact the proximal apical dendrites of the PCs in CA3 area in the stratum lucidum. Therefore, using a multi-compartment model of a PC, we show that the various waveforms evoked by MF stimulation in the absence of chemical transmission can be replicated, suggesting that the electrical synaptic contacts were indeed of MF origin.
Because this evidence strongly suggests that MFs establish electrical synapses with a number of PCs, we hypothesized that a mixed electrical-chemical response should be observed under normal neurotransmission conditions, as MFs release glutamate. Indeed, we observed such a mixed response in the same proportion of cells in which we recorded the pharmacologically isolated spikelets (~ 5%). When the stimulation frequency was switched from 0.01 to 1 Hz the fast spikelets remained unchanged, while the EPSPs underwent marked frequency facilitation and readily produced action potentials. As expected from neurotransmission of MF origin, mGluR activation produced a marked depression of the chemical component of the synaptic response. The presence of these characteristics has been used to identify synaptic responses of MF origin (Kamiya et al., 1996; Nicoll & Schmitz, 2005). It is noteworthy that the inhibition of > 70% that DCG-IV normally produces on MF-mediated glutamatergic responses can not be compared to the amount of inhibition that we describe, because we measured the change in the area under the curve of the mixed electrical-chemical response, and not the amplitude of the isolated glutamatergic potentials. Therefore, the inhibition of the area under the curve reflects the depression of the chemical component, while the amplitude (Fig. 3) and waveform of the spikelet was not affected (not shown). Finally, when blocking the chemical component with ionotropic glutamatergic and GABAergic antagonists, DG stimulation could still evoke spikelets that were strongly depressed by carbenoxolone. It is noteworthy that this gap junction blocker, despite having some non-specific effects on chemical transmission (Tovar et al., 2009), without doubt blocks electrical transmission. Therefore, as these non-specific effects relate to chemical synaptic transmission, they are not relevant to our paradigm and, thus, do not invalidate our findings, given that we could block the spikelets while synaptic transmission was already blocked. The relevant worries are on intrinsic membrane properties, and that has been ruled out by Schmitz et al. (2001). Indeed, this study showed that carbenoxolone had no effect on action potentials in hippocampal PC axons, and directly in MF synaptic boutons. Our present data show that principal cells can be also electrically coupled and, thus, suggest a possible electrical interaction of two main structures of the hippocampus: the DG and CA3 area. Interestingly, another pharmacological result that speaks in favor of MF-to-CA3 electrical communication is the potentiation effect of DA, receptors for which are present in the MFs (Kobayashi & Suzuki, 2007). Interestingly, DA has been shown to potentiate both the chemical and electrical components of the postsynaptic responses in Mauthner cell mixed synapse (Pereda et al., 1992). The DA receptor involved in the potentiation of electrical signaling that we observed, as well as the functional consequences of this effect need further investigation.
That DG-evoked spikelets were suppressed while the membrane potential of the PC was hyperpolarized by ≥15 mV suggests that the injected hyperpolarizing current could spread and hyperpolarize the presynaptic MF terminal, thereby interfering with the conduction of a full-blown action potential. In this regard, since a remarkable characteristic of granule cells is their ability to convey analog signals from their dendrites to the MF terminals (Alle & Geiger, 2006), trans-synaptic analog transmission through mixed synapses at MF terminals could provide an effective means for graded, possibly bi-directional, synaptic communication; and this could be of particular importance for synapses with such low release probability (Jonas et al., 1993). It has been shown that MF boutons are endowed with a high density of Na+ channels (Engel & Jonas, 2005), which can contribute to the non-decremental signal propagation along the axon. Thus, gap junctions at the MF-PC synapse may allow the electrical activity in the PC targets to cause retrograde sub- and suprathreshold electrical activity in the presynaptic MF boutons and action potentials in the MF. These gap junctions may also enhance cooperativity of glutamate release at MF synapses. This is perhaps of relevance to plasticity at the MF synapse, where LTP can be expressed in NMDAR-independent (Zalutsky & Nicoll, 1990) and NMDAR-dependent (Kwon & Castillo, 2008) manners. On the other hand, fast electrical communication might affect the strong but slower feed- forward inhibition occurring in the DG-to-CA3 connection. Furthermore, granule cell activity produces strong feed forward inhibition in CA3, which transiently suppresses excitatory input to PCs (Mori et al., 2007; Figure 4B in this work). Thus, gap junctions could in principle ensure an effective activation of a selective set of PCs, bypassing the strong action of feed-forward inhibition, as our data suggest (see Figure 4B). In this regard, it remains to be determined whether electrical communication in the MF projection is target specific, i.e., if it also involves interneurons, which are the major postsynaptic targets of MFs in the rat hippocampus (Acsády et al., 1998). Noteworthy, preliminary anatomical evidence at the EM level for the presence of gap junctions between the MFs and PCs has already been presented (Kamasawa et al., 2007).
Neurons at early postnatal ages appear to be extensively coupled by gap junctions (Bennett & Zukin, 2004). Because granule cells are continually produced in adult DG (Cameron & McKay, 2001), it might be proposed that electrical coupling in the MF synapse in adult rats is associated primarily with fibers of newly-generated granule cells. Indeed, it is suggestive that approximately 5% of granule cells present at a given time are newborn ones. However, one should keep in mind that the probability of stimulating a newborn granule cell projecting its processes from the molecular layer of the DG to CA3b should be rather low. The possibility that the new neurons, born in the adult rat, establish electrical communication through gap junctions with their synaptic targets at some point during their development is still to be explored.
Physiological implications
An attempt to address the physiological meaning of electrical communication in this synapse would be rather speculative, as numerous possibilities can be considered. Our recordings show a low incidence of such connections, but the type of slice preparation and the sampling method, by intracellular recordings, are surely underestimating the real number of mixed synapses. Nonetheless, despite the low percentage of PCs that we found responsive to DG stimulation with electrical spikelets, the presence of such cells in the rat hippocampus may have profound physiological effects given that each PC contacts other neighboring PCs. Electrotonic transmission may enable an early interaction of the spikelet with a concomitant EPSP of a nearby chemical synapse, providing a time window for temporal summation or other interactions of postsynaptic responses. Also, reciprocity of electrotonic transmission between the postsynaptic cell and the presynaptic axon could lead to a prolonged and synchronous release of neurotransmitter, which would prove to be a novel mechanism for synaptic plasticity. Indeed, the retrograde sub- and suprathreshold electrical activity in the presynaptic MF boutons may in turn enhance glutamate release at MF synapses.
It has been recently observed that spikelets do have an impact on hippocampal activity during spatial exploration (Epsztein et al., 2010). In addition, a recent study showed that the blockade of gap junctions with carbenoxolone was able to disrupt a hippocampal-specific learning task associated to theta rhythms modulated by electrical synapses in the interneuronal network (Bissiere et al., 2011). In these reports it was not possible to identify the origin of the spikelets, in the former, or to selectively block electrical transmission of specific synapses, in the latter. Thus, the well-known interneuronal electrical connections are immediately associated to the studied phenomena. Because of the important role of the DG in hippocampal physiology, we suggest that also principal cell-to-principal cell electrical connections, like the one that we here describe, may play an important role in information processing. How this specific connection is involved in learning, memory and in the setting of temporal and place codes needs further investigation. Because of the low connectivity in the MF-to-CA3 synapse and because of the scarcity of electrical communication, such an arrangement might synchronize “discrete modules” of pyramidal cells, for example during epileptic activity. The CA3 region generates intrinsic oscillatory frequencies both in vitro and in vivo, and plays a crucial role in the generation and propagation of seizures (Traub et al., 1996). In particular, changes in pH can open or close gap junctions (Spray et al., 1981), and pathological conditions like epilepsy might produce such changes (Traub et al., 2010). If electrical transmission from MFs to PCs is particularly strong, fast, and reliable, major effects on information processing are to be expected.
Acknowledgments
We thank D. Schmitz and A. Draguhn for valuable comments during the execution of this project. The postdoctoral fellowship to C.V. and this study were supported by grants to R.G. 45754 and I020/193/10 FON.INST.-29-10 from Consejo Nacional de Ciencia y Tecnología. R.D.T. was supported by NINDS/NIH RO1NS44133. Part of this investigation was conducted while R.G. was a member of the Department of Physiology, Biophysics and Neurosciences.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- DG
dentate gyrus
- MF(s)
mossy fiber(s)
- PC(s)
pyramidal cell(s)
References
- Acsády L, Kamondi A, Sik A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- Baker R, Llinás R. Electrotonic coupling between neurons in the rat mesencephalic nucleus. J. Physiol. 1971;212:45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MVL, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger JR, Jonas P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat. Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Zelikowsky M, Ponnusamy R, Jacobs NS, Blair HT, Fanselow MS. Electrical synapses control hippocampal contributions to fear learning and memory. Science. 2011;331:87–91. doi: 10.1126/science.1193785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Han ZS, Lörinczi Z, Stezhka VV, Karnup SV, Somogyi P. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J. Neurophysiol. 1994;71:1289–1307. doi: 10.1152/jn.1994.71.4.1289. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 1992;325:169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JGR. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Epsztein J, Lee AK, Chorev E, Brecht M. Impact of spikelets on hippocampal CA1 pyramidal cell activity during spatial exploration. Science. 2010;327:474–477. doi: 10.1126/science.1182773. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat. Rev. Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J. Neurophysiol. 2000;84:3088–3090. doi: 10.1152/jn.2000.84.6.3088. [DOI] [PubMed] [Google Scholar]
- Hamzei-Sichani F, Kamasawa N, Janssen WG, Yasumura T, Davidson KG, Hof PR, Wearne SL, Stewart MG, Young SR, Whittington MA, Rash JE, Traub RD. Gap junctions on hippocampal mossy fiber axons demonstrated by thin-section electron microscopy and freeze fracture replica immunogold labeling. Proc. Natl. Acad. Sci. USA. 2007;104:12548–12553. doi: 10.1073/pnas.0705281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. Origin of the apparent asynchronous activity of hippocampal mossy fibers. J. Neurophysiol. 1997;78:24–30. doi: 10.1152/jn.1997.78.1.24. [DOI] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J. Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamasawa N, Hamzei-Sichani F, Yasumura T, Janssen WGM, Davidson KGV, Wearne SL, Hof PR, Traub RD, Rash JE. Ultrastructural evidence for mixed synapses in hippocampal principal neurons using thin-section and freeze-fracture replica immunogold labeling (FRIL) electron microscopy. Soc. Neurosci. 2007 Abstr. 581.12. [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J. Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Suzuki H. Dopamine selectively potentiates hippocampal mossy fiber to CA3 synaptic transmission. Neuropharmacology. 2007;52:552–561. doi: 10.1016/j.neuropharm.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Baker R, Sotelo C. Electrotonic coupling between neurons in the cat inferior olive. J. Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Dudek FE. Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981;213:782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Dudek FE. Electrotonic coupling between granule cells of rat dentate gyrus: physiological and anatomical evidence. J. Neurophysiol. 1982;47:579–592. doi: 10.1152/jn.1982.47.4.579. [DOI] [PubMed] [Google Scholar]
- Mercer A, Bannister AP, Thomson AM. Electrical coupling between pyramidal cells in adult cortical regions. Brain Cell Biol. 2006;35:13–27. doi: 10.1007/s11068-006-9005-9. [DOI] [PubMed] [Google Scholar]
- Mori M, Gähwiler BH, Gerber U. Recruitment of an inhibitory hippocampal network after bursting in a single granule cell. Proc. Natl. Acad. Sci. USA. 2007;104:7640–7645. doi: 10.1073/pnas.0702164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc. Natl. Acad. Sci. USA. 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Action potential initiation and propagation in hippocampal mossy fibre axons. J. Physiol. 2008;586:1849–1857. doi: 10.1113/jphysiol.2007.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Schuchmann S, Fisahn A, Draguhn A, Buhl EH, Petrasch-Parwez E, Dermietzel R, Heinemann U, Traub RD. Axo-axonal coupling, a novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Buzsáki G. Interneurons in the hippocampal dentate gyrus: an in vivo intracellular study. Eur. J. Neurosci. 1997;9:573–588. doi: 10.1111/j.1460-9568.1997.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Spencer WA, Kandel ER. Electrophysiology of hippocampal neurons. IV. Fast prepotentials. J. Neurophysiol. 1961;24:272–285. doi: 10.1152/jn.1961.24.3.272. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Spruston N, Lübke J, Frotscher M. Interneurons in the stratum lucidum of the rat hippocampus: an anatomical and electrophysiological characterization. J. Comp. Neurol. 1997;385:427–440. [PubMed] [Google Scholar]
- Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J. Neurophysiol. 2009;102:974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Colling SB, Jefferys JG. Cellular mechanisms of 4-aminopyridine-induced synchronized after-discharges in the rat hippocampal slice. J. Physiol. 1995;489:127–140. doi: 10.1113/jphysiol.1995.sp021036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Contreras D, Cunningham MO, Murray H, LeBeau FE, Roopun A, Bibbig A, Wilent WB, Higley MJ, Whittington MA. Single-column thalamocortical network model exhibiting gamma oscillations, sleep spindles, and epileptogenic bursts. J. Neurophysiol. 2005;93:2194–2232. doi: 10.1152/jn.00983.2004. [DOI] [PubMed] [Google Scholar]
- Traub RD, Duncan R, Russell AJ, Baldeweg T, Tu Y, Cunningham MO, Whittington MA. Spatiotemporal patterns of electrocorticographic very fast oscillations (> 80 Hz) consistent with a network model based on electrical coupling between principal neurons. Epilepsia. 2010;51:1587–1597. doi: 10.1111/j.1528-1167.2009.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Miles R, Whittington MA, Toth K. A branching dendritic model of a rodent CA3 pyramidal neurone. J. Physiol. 1994;481:79–95. doi: 10.1113/jphysiol.1994.sp020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J. Physiol. 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiante TA, Perez-Velazquez JL, Jahromi SS, Carlen PL. Coupling potentials in CA1 neurons during calcium-free-induced field burst activity. J. Neurosci. 1995;15:6946–6956. doi: 10.1523/JNEUROSCI.15-10-06946.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]