Abstract
A large body of evidence has demonstrated that there is a close coupling between regional myocardial perfusion and contractile function. When ischemia is mild, this can result in the development of a new balance between supply and energy utilization that allows the heart to adapt for a period of hours over which myocardial viability can be maintained, a phenomenon known as “short-term hibernation”. Upon reperfusion after reversible ischemia, regional myocardial function remains depressed. The “stunned myocardium” recovers spontaneously over a period of hours to days. The situation in myocardium subjected to chronic repetitive ischemia is more complex. Chronic dysfunction can initially reflect repetitive stunning with insufficient time for the heart to recover between episodes of spontaneous ischemia. As the frequency and/or severity of ischemia increases, the heart undergoes a series of adaptations which downregulate metabolism to maintain myocyte viability at the expense of contractile function. The resulting “hibernating myocardium” develops regional myocyte cellular hypertrophy as a compensatory response to ischemia-induced apoptosis along with a series of molecular adaptations that while regional, are similar to global changes found in advanced heart failure. As a result, flow-function relations become independently affected by tissue remodeling and interventions that stimulate myocyte regeneration. Similarly, chronic vascular remodeling may alter flow regulation in a fashion that increases myocardial vulnerability to ischemia. Here we review our current understanding of myocardial flow-function relations during acute ischemia in normal myocardium and highlight newly identified complexities in their interpretation in viable chronically dysfunctional myocardium with myocyte cellular and molecular remodeling.
Myocardial flow and function are closely coupled during increases in myocardial work load since oxygen extraction across the coronary circulation is near maximal at rest [1]. When oxygen delivery becomes inadequate to maintain the prevailing regional work load, relative ischemia develops and regional contractile function deteriorates in an attempt to balance a reduced metabolic supply with demand [2]. With prolonged ischemia, myocardial infarction develops and the chronic contractile dysfunction reflects the loss of cardiac myocytes and replacement with fibrotic tissue [3]. Somewhat surprisingly, if ischemia is alleviated before irreversible myocyte cell death develops, contractile dysfunction can persist for a period of hours and sometimes several days despite complete normalization of myocardial perfusion [4], a phenomenon subsequently termed “stunned myocardium” [5]. Further complicating the interpretation of chronic myocardial flow-function relations is the fact that, when subjected to repetitive reversible ischemia on a long-term basis, the myocardium can regionally remodel from a cellular as well as molecular standpoint so as to adapt to chronic repetitive ischemia [6]. The resulting viable chronically dysfunctional myocardium can reflect chronic stunning with normal perfusion as well as hibernating myocardium where resting perfusion is reduced [1, 6–8]. This review will summarize our historical understanding of physiological adaptations to acute ischemia in normal myocardium and outline emerging knowledge of how these physiological responses become modulated by chronic cellular adaptations arising from ischemia-induced myocyte and vascular remodeling. Interested readers may find additional information in other publications [1, 3, 7, 9–13].
Matching Between Flow and Function During Acute Myocardial Ischemia in Normal Myocardium
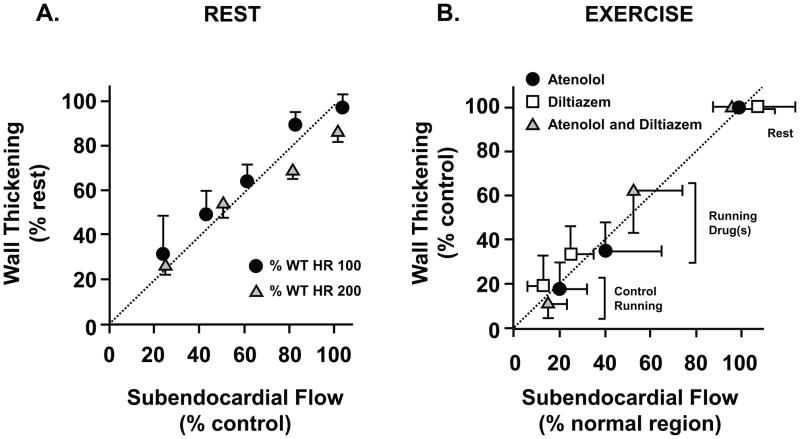
Our initial understanding of flow-function relations arose from studies evaluating the effects of acute ischemia distal to a coronary stenosis in otherwise normal myocardium. Since coronary blood flow is autoregulated and oxygen extraction is near maximal at rest, subendocardial blood flow remains constant as coronary pressure falls distal to a stenosis until subendocardial vasodilator reserve is exhausted which reflects the lower pressure limit of autoregulation [14]. At resting levels of myocardial metabolic demand in unanesthetized dogs, subendocardial ischemia begins at a coronary pressure of 40 mmHg. As pressure is reduced below the lower autoregulatory limit, small reductions in pressure cause proportionate reductions in subendocardial flow. Many previous studies have demonstrated a close coupling between subendocardial flow and function assessed using ultrasonic crystals measuring regional subendocardial segment shortening or transmural wall thickening [14–16]. These studies have demonstrated that reductions in wall thickening approximate the relative reduction in subendocardial perfusion during reversible steady-state ischemia [2]. The close coupling between subendocardial flow and function during ischemia (Figure 1) is maintained at increased myocardial workloads as produced by steady-state pacing [17] or exercise [18]. Due to the vulnerability of the subendocardium to ischemia from compressive forces that impede perfusion during systolic contraction, significant reductions in contractile function are frequently present when coronary flow averaged across the entire myocardial wall is minimally reduced. This preclinical information has been translated to clinical care by imaging stress-induced contractile dysfunction as a surrogate of regional ischemia using echocardiography or stress MRI [19].
Figure 1. Perfusion contraction matching during acute ischemia in normal myocardium.
Relative reductions in function (wall thickening) are proportional to the relative reduction in subendocardial flow measured with microspheres in conscious dogs. This relationship is maintained during steady-state increases in myocardial work load over a wide range of heart rates during autoregulation (A) as well as during exercise with a fixed coronary stenosis (B). Medical interventions that ameliorate ischemia improve both subendocardial flow and wall thickening during exercise. HR - heart rate. (A modified from Canty et al [14, 17]; B modified from Matsuzaki et al. [62])
Post-Ischemic Myocardial Stunning
Even when coronary inflow is occluded in the absence of collateral vessels, there is no irreversible myocyte cellular injury when ischemia lasts less than 15-minutes [3]. Nevertheless, upon restoration of perfusion, myocardial function remains depressed below normal for a period of hours, a phenomenon that has become known as myocardial stunning (Figure 2). It was first described by Heyndrickx after single brief total coronary occlusions [20, 21] but subsequent studies showed that post-ischemic dysfunction can persist when perfusion normalizes after stress-induced ischemia distal to a stenosis [22, 23]. Stunned myocardium resolves within hours when there is full reperfusion and no subsequent limitation in blood flow. In contrast, when a critical stenosis limiting flow reserve is present, contractile dysfunction persists at 24 hours [24]. The presence of a stenosis likely leads to repetitive spontaneous ischemia and as outlined below, can be a prelude to the development of viable dysfunctional myocardium that persists on a chronic basis. In addition to reversible ischemia, stunned myocardium undoubtedly coexists with infarcted myocardium in patients undergoing reperfusion following ST elevation myocardial infarcts and accounts for some of the delayed functional improvement following thrombolysis or primary angioplasty. The cellular mechanisms responsible for myocardial stunning are multifactorial and reviewed elsewhere [12].
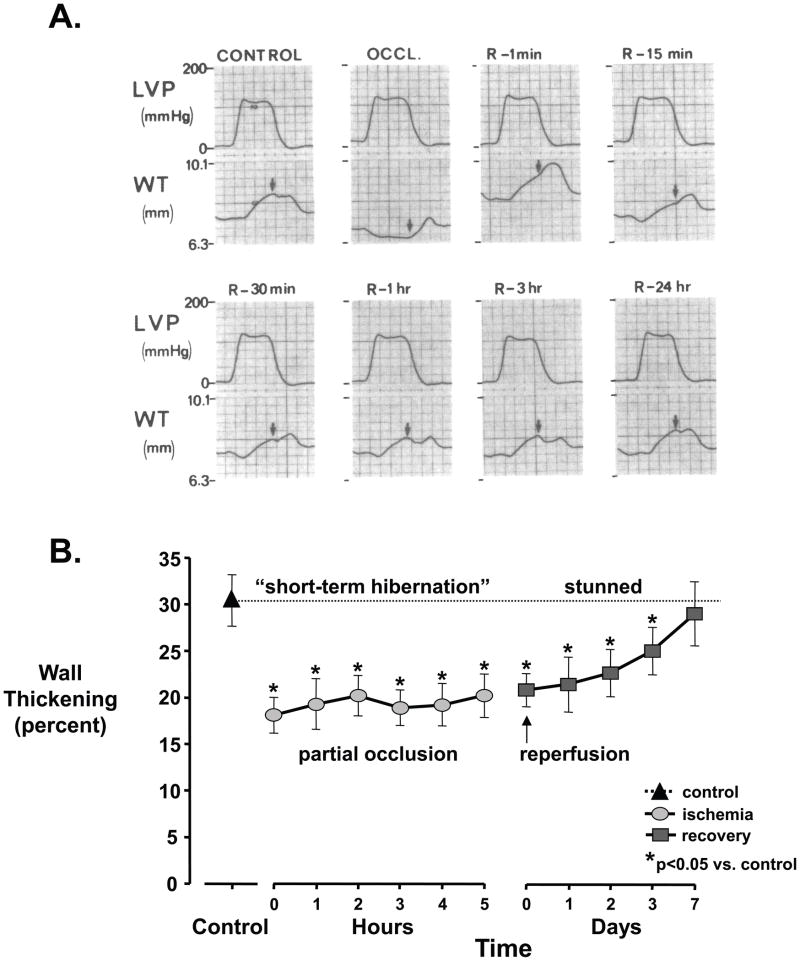
Figure 2. Stunned myocardium.
(A) Myocardial stunning following a 15-minute total occlusion (OCCL). Wall thickening (WT) measured by ultrasonic crystals becomes dyskinetic, with systolic thinning. Upon reperfusion (R), function slowly improves and is completely normal after 24-hours. B, Myocardial stunning following a prolonged partial occlusion. During acute ischemia (circles), there is “short-term hibernation” reflecting an acute match between reduced flow, wall thickening, and metabolism. With reperfusion (squares), wall thickening remains depressed and gradually returns to normal after 1 week. LVP - left ventricular pressure. (A modified from Heyndrickx, et al. [21]; B modified from Matsuzaki M, et al. [25])
Prolonged Subendocardial Ischemia and Short-term Hibernation
While original studies indicated that myocyte cell death begins if coronary flow is not reestablished within 15-minutes after a total occlusion, there is considerably more variability in the duration that moderate subendocardial ischemia can be maintained before irreversible injury develops. Matsuzaki et. al. [25] as well as others demonstrated that moderate subendocardial hypoperfusion, with commensurate reductions in regional function and energy consumption could be maintained for at least several hours without progressing to irreversible myocyte injury [26–28]. The phenomenon of prolonged perfusion-contraction matching depicted in Figure 2b has been termed short-term hibernation [2]. It is likely operative in many patients presenting with an acute coronary syndrome due to plaque rupture with a sudden increase in stenosis severity precipitating ischemia and prolonged chest pain. The extended time frame of maintained viability with moderately severe ischemia is clinically important in allowing late salvage of myocardial tissue by reperfusion therapies. Due to the brief time frame over which short-term hibernation develops, it likely reflects physiological adaptations in regional myocardial metabolism effected in the normal heart (Figure 3) rather than molecular adaptations and cellular remodeling [13]. Nevertheless, in normal myocardium, the duration over which short-term hibernation can maintain viability depends upon the severity of ischemia and some degree of subenodocardial infarction generally develops when this exceeds 12 hours [29, 30]. Because of this, these initial adaptations are unlikely to result in a stable chronic hibernating state for weeks or months without developing a component of irreversible injury. Importantly, like reperfusion after brief total coronary occlusions, myocardial stunning is present upon reestablishing perfusion after short-term hibernation with function slowly improving over several days rather than hours [25]. As outlined below, repetitive episodes of short-term hibernation can elicit molecular myocyte remodeling and viable chronically dysfunctional myocardium [31].
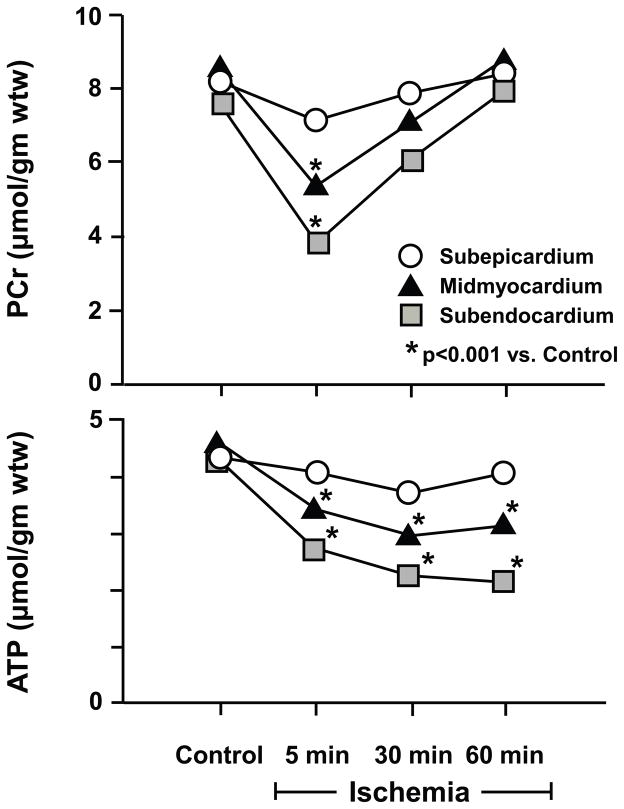
Figure 3. Metabolic matching during short-term hibernation.
During moderate ischemia, reductions in contractile function are accompanied by adaptations in myocardial energy metabolism. These are characterized by an initial fall in creatine phosphate (PCr) and ATP. The transmural metabolite changes are greatest in the subendocardium (squares) reflecting the transmural variations in flow. With persistent ischemia, metabolism stabilizes with a regeneration of creatine phosphate that prevents further ATP depletion. While not shown, tissue lactate is transiently increased but normalizes with persistent ischemia. Adapted from Pantely et al. [26], and republished with permission of the American Heart Association, Inc.
Viable Chronically Dysfunctional Myocardium
In contrast to our knowledge of acute perfusion contraction matching and stunning which arose from basic studies and was subsequently translated to the clinic, viable chronically dysfunctional myocardium was first identified in patients. Clinical observations by Rahimtoola and others demonstrated that resting contractile dysfunction in the absence of ischemia in some patients was at least partially reversible [32, 33]. This clinical entity became termed “hibernating myocardium” [8, 11]. Initially, the clinical dilemma was to distinguish viable hibernating myocardium from infarcted tissue since resting perfusion assessed by imaging was reduced in both entities (previously interpreted as reflecting an admixture of fibrotic and normal myocardium) [8, 34]. With advances in quantitative parametric imaging that can accurately quantify myocardial perfusion (e.g positron emission tomography) as well as quantify infarct volume (e.g. Gd enhanced magnetic resonance imaging) it has become apparent that many patients have areas of viable dysfunctional myocardium with normal resting perfusion, a phenomenon now termed “chronically stunned myocardium”. Viable dysfunctional myocardium is common and may be an important contributor to global LV dysfunction in patients with heart failure from CAD. Indeed, with accurate techniques to quantify infarct size in humans with myocardial infarction, contemporary clinical trials in ST elevation infarction employing gadolinium MRI demonstrate that LV ejection fraction is only mildly depressed (average EF ~50%). Infarct volume averages only ~15% of the LV and decreases further as the infarct heals in the chronic phase [35]. Even in end-stage ischemic cardiomyopathy, Beltrami reported that pathologic fibrosis directly measured in explanted hearts from cardiac transplant recipients averaged 28% of the LV volume vs. 6% in normals [36]. Only one-third of the fibrosis was segmental from prior infarction and most was diffuse. They found prominent myocyte cellular loss with myocyte cellular hypertrophy and hypothesized that remodeling of viable myocytes may play as great a role as fibrosis in contractile dysfunction. In support of this, Kim et al found that almost 1 in 4 segments without fibrosis by MRI failed to improve function after myocardial revascularization [37]. Thus, even in advanced heart failure, there is more viable dysfunctional myocardium than infarcted tissue and recovery of this appears to be the major determinant of functional improvement. The various pathophysiological entities comprising viable chronically dysfunctional myocardium along with resting flow, contractile reserve and the response to inotropic stimulation are summarized in Table 1.
TABLE 1.
Viable Dysfunctional Myocardium: Patterns of Contractile Reserve, Resting Perfusion, And Temporal Recovery of Function After Revascularization
| Parameter | Contractile Reserve | Resting Flow | Extent of Functional Recovery | Time Course of Recovery |
|---|---|---|---|---|
| Following Transient Reversible Ischemia | ||||
| Post-ischemic stunning | Present | Normal | Normalizes | <24 hr |
| Short-term hibernation | Present | Normal | Normalizes | <7 days |
| Chronic Repetitive Ischemia | ||||
| Chronic hibernating myocardium | Variable | Reduced | Improves | Up to 12 mo |
| Chronic stunning | Present | Normal | Improves | Days to weeks |
| Structural Remodeling | ||||
| Subendocardial infarction | Variable | Reduced | Variable | Weeks |
| Remodeled, tethered myocardium | Present | Normal | Improves | Months |
Chronic Stunning and Chronic Hibernation – a Continuum
We as well as others have demonstrated that the extent of the pathophysiological remodeling is dependent upon the functional significance of the stenosis supplying perfusion and the propensity of the region to develop subendocardial ischemia (Figure 4) [38, 39]. Initially, resting flow is normal but becomes mildly reduced in the absence of symptoms or objective evidence of acute ischemia [40]. The progression to hibernating myocardium can be accelerated by producing an acute stenosis that produces a prolonged reduction in resting flow [41], repetitive brief periods of short-term hibernation [31] or simply by producing brief ischemia and reperfusing myocardium through a critical stenosis that doesn’t limit blood flow at rest (Figure 5) [24]. In the former as well as the latter circumstance, reductions in resting flow follow rather than precede the development of dysfunction. Thus, the reduction in resting flow probably reflects and induces the development of intrinsic metabolic adaptations in the heart that downregulate mitochondrial oxygen consumption [42–46].
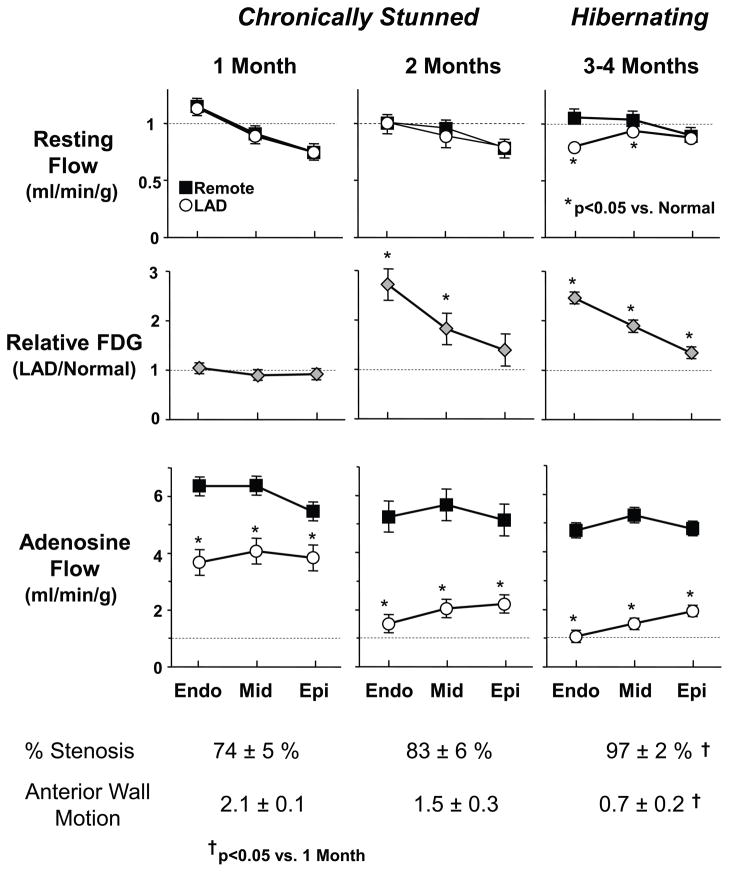
Figure 4. Physiological progression from chronically stunned to hibernating myocardium in swine.
Transmural microsphere flow measurements from pigs instrumented with a progressive LAD stenosis at rest and during adenosine vasodilation are shown along with transmural variations in 18FDG uptake (fasting conditions) obtained by ex vivo counting. Angiographic stenosis severity and anterior wall motion score (3 normal, 2 mild hypokinesis, 1 severe hypokinesis) at each time point are summarized below the graphs. As stenosis severity increases over time, vasodilated flow (adenosine) to the LAD region progressively falls, reflecting a functionally significant coronary lesion. Initially (1- and 2-months after instrumentation), anterior hypokinesis develops with normal resting flow consistent with chronically stunned myocardium. After 3-months, there is total occlusion with collateral-dependent myocardium and a critical impairment in coronary flow reserve. At this time, resting flow to the inner 2/3 of the LAD myocardium becomes reduced as compared to normally perfused remote myocardium and consistent with hibernating myocardium. The progression of abnormalities demonstrates that chronic stunning precedes the development of hibernating myocardium. In contrast to short-term hibernation resulting from primary reductions in coronary flow, the reduction in resting flow is a consequence, rather than cause of the contractile dysfunction in chronic hibernating myocardium (modified from Fallavollita et al. [39] with permission of the American Heart Association, Inc.).
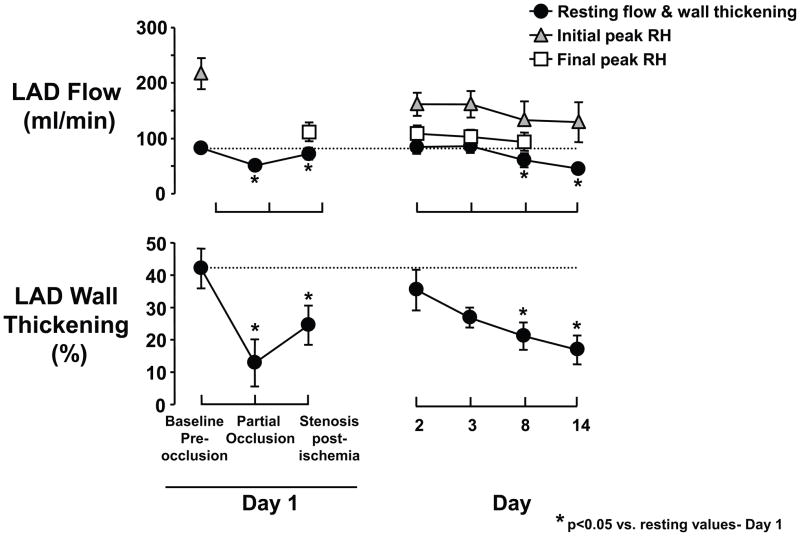
Figure 5. Accelerated progression from stunned to hibernating myocardium by limiting reperfusion with a critical stenosis after a period of short-term hibernation in swine.
Chronically instrumented swine were subjected to moderate ischemia for 15-minutes. In contrast to full reperfusion, regional LAD wall-thickening remained depressed after 24-hours when the coronary reactive hyperemic (RH) response was limited. When a acute critical stenosis was maintained during the subsequent 2-weeks, there was a progressive decline in function that was followed by a reduction in resting flow. The physiological and molecular changes were similar to pigs with hibernating myocardium that developed over 3-months. These results indicate that the physiological significance of a coronary stenosis (and spontaneous ischemia) rather than time is the major determinant of the progression from chronically stunned to hibernating myocardium. Moreover, this is accelerated by an abrupt increase in stenosis severity similar to that seen in patients presenting with an acute coronary syndrome. Republished from Thomas, et al. [24].
Once myocyte cellular adaptations and remodeling develop in response to ischemia, the myocardium is no longer normal from a structural or molecular standpoint. This likely impacts the response to superimposed acute ischemia. Figure 6 shows the myocyte cellular remodeling characteristic of chronic hibernating myocardium in pigs [24, 47] which has ultrastructural features that are similar to humans with regional dysfunction from a chronic LAD occlusion [48]. While there is a trivial increase in interstitial connective tissue, there is significant regional myocyte loss with compensatory cellular hypertrophy that serves to maintain regional wall thickness normal. These cellular changes are accompanied by regional protein changes that are also manifest globally in the advanced failing heart. Consistent with reductions in mitochondrial oxygen consumption, proteomic profiling has demonstrated a downregulation in mitochondrial proteins (Figure 7) and an upregulation of stress and cytoskeletal proteins [46, 49–51]. As outlined below, these molecular changes alter the relation between flow and function at rest as well as stunning following acute increases in myocardial workload.
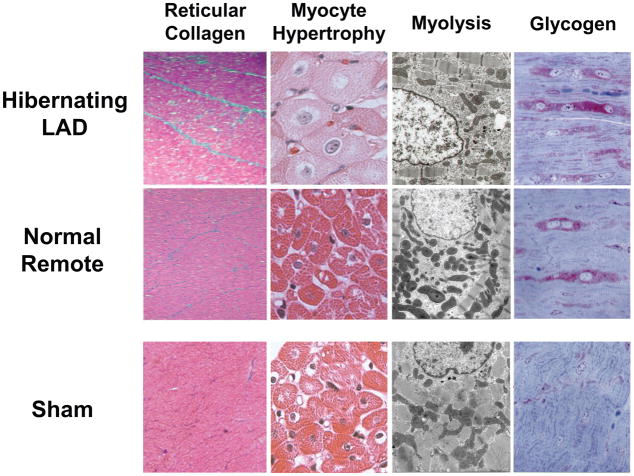
Figure 6. Myocyte cellular changes in swine with hibernating myocardium in the absence of heart failure.
Swine with hibernating myocardium from a chronic LAD occlusion and collateral-dependent myocardium without infarction or heart failure develop an adapted phenotype. Like findings in humans, there is increased reticular connective tissue which is only ~2% greater than values in the remote normally perfused region (left column). While hearts appear grossly normal there is myocyte cellular hypertrophy in the hibernating region (second column) which reflects the consequences of apoptosis induced myocyte loss. The electron microscopic characteristics of hibernating myocardium are similar to humans with an adapted phenotype and demonstrate myofibrillar loss (Myolysis), numerous small mitochondria and increased glycogen content (right column). Interestingly, biopsies of remote, normally perfused segments from animals with an LAD occlusion show similar morphological changes. These data indicate that the “cellular hibernating phenotype” is not directly related to ischemia nor is it the cause of regional contractile dysfunction. Adapted from Canty [1].
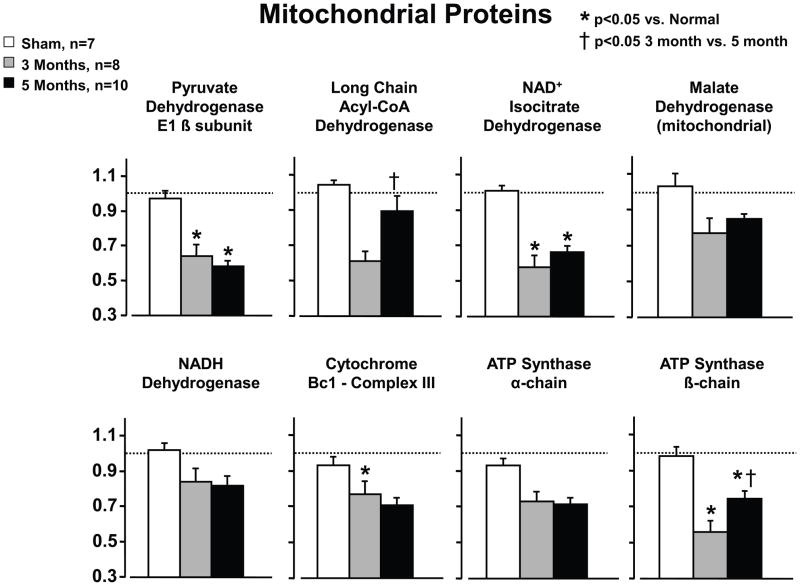
Figure 7. Mitochondrial protein remodeling in animals with persistent hibernating myocardium.
Proteomic profiling of animals with hibernating myocardium demonstrate a downregulation in mitochondrial proteins including some of the entry points to oxidative metabolism and selected components of the electron transport chain. These changes do not reflect mitochondrial loss or increased connective tissue. Like the physiological changes, most of the mitochondrial proteomic changes persisted in animals with hibernating myocardium that were studied 5-months after instrumentation. Exceptions included long chain Acyl-CoA dehydrogenase and the ATP synthase-β-chain which tended to normalize. While not shown, increases in stress proteins remained persistently elevated but increases in cytoskeletal proteins normalized in animals with longer term hibernation (5-months). Adapted from Page et al. [51] and republished with permission of the American Heart Association.
Intrinsic Molecular Adaptations, Myocyte Plasticity and Regeneration: Impact on the Subendocardial Flow-function Relation and Acute Stunning in Chronic Ischemia
In the normal heart, the abrupt onset of ischemia leaves little time for the heart to adapt and thus, the relation between flow and function is completely a reflection of acute intrinsic physiological adaptations to ischemia. In contrast, the molecular and cellular remodeling that develops with chronic ischemia considerably complicates the interpretation of what is the normal response of the viable dysfunctional heart to superimposed acute ischemia. For example, repetitive short-term ischemia quickly leads to adaptations that limit the metabolic evidence of ischemia. After six cycles of reversible short-term hibernation, there is no longer post-ischemic stunning when flow is reduced to a similar level in chronically dysfunctional myocardium [31]. Similarly, stress-induced ischemia in chronic hibernating myocardium in collateral-dependent myocardium fails to elicit post-ischemic stunning following increases in myocardial demand (Figure 8) [50]. Thus, the intrinsic myocardial adaptations and downregulation of energy utilization of the chronically dysfunctional heart protect it from additional stunning after acute ischemia. A second finding is that the resting flow-function relation in chronically stunned and hibernating myocardium (Figure 9) is to some extent dissociated from the relation during acute subendocardial ischemia at rest (Figure 1). Whether resting flow is normal or reduced, the degree of contractile dysfunction usually exceeds the relative reduction in resting subendocardial flow. This may reflect the fact that remodeled, hypertrophied myocytes have a different relation between subendocardial flow and function. Kim et al. also demonstrated that function was completely independent of acute changes in perfusion and completely dissociated from that found in normal myocardium [31].
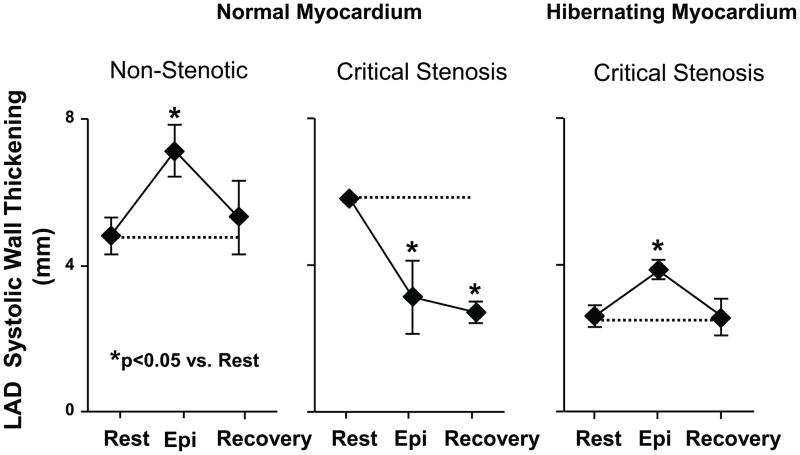
Figure 8. Protection of hibernating myocardium from acute demand-induced stunning following transient increases in demand during epinephrine infusion.
LAD systolic wall thickening in animals with a critical stenosis and hibernating myocardium (n=9) were compared to normal myocardium subjected to an acute flow-limiting stenosis (n=3) on the LAD and non-stenotic controls (n=7). Hemodynamic changes were similar among groups. While LAD wall thickening was depressed in pigs with hibernating myocardium (right graph), it increased during β-adrenergic stimulation and returned to the identical depressed baseline level 15-minutes into recovery. The same transient increase and return to normal was also found in shams not subjected to acute ischemia (left graph). In contrast, animals instrumented with an acute critical stenosis that prevented flow from increasing during vasodilation developed contractile dysfunction during epinephrine induced increases in demand indicative of acute ischemia. In addition, in normal myocardium regional function remained depressed 15-minutes into recovery indicative of acute stunning. These results indicate that pigs with chronic hibernating myocardium develop protection against acute myocardial stunning. Adapted from Hu, et al. [50] and reprinted with permission.
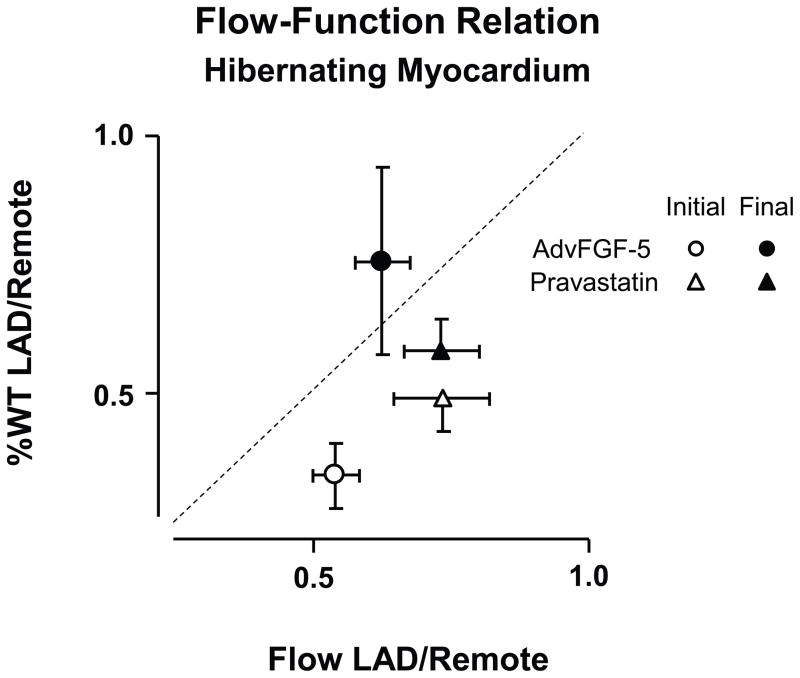
Figure 9. Variability of Myocardial Flow-function relations in the absence of increased coronary flow in pigs with chronic hibernating myocardium.
Relative wall thickening reductions in hibernating LAD regions vs. remote myocardium (%LADWT/%RemoteWT) vs. the relative reduction in resting subendocardial flow (LAD/Remote). The dashed line indicates a 1:1 relation between subendocardial flow and wall thickening reductions. In hibernating LAD regions, resting function was reduced more than subendocardial flow. Both adenoviral gene transfer of FGF-5 (fibroblast growth factor 5) and the HMG-Co-A reductase inhibitor pravastatin increased function with no change in resting flow or change in flow during pharmacological vasodilation (data not shown). These functional changes were accompanied by evidence of myocyte proliferation as reflected by Ki67 staining and, in the case of pravastatin, an increase in myocyte nuclear density. These data demonstrate alterations in the resting flow-function relation in hibernating myocardium that are accompanied by interventions that alter myocyte proliferation but have no effect on resting or maximal perfusion. (Data from Suzuki, et al. [52, 53]).
The regional adaptations in chronically dysfunctional myocardium including cellular hypertrophy and apoptosis-induced myocyte loss create a circumstance where myocyte remodeling and regeneration can potentially impact the flow-function relation independently of a change in coronary perfusion. Studies by our group in hibernating myocardium indicate that pharmacological interventions that stimulate myocyte proliferation can dissociate the flow-function relation by improving function independently of perfusion. For example, while attempts to stimulate neoangiogenesis with the HMG-CoA reductase inhibitor pravastatin [52] or by overexpressing fibroblast growth factor with in vivo gene transfer (AdvFGF-5) [53] failed to increase myocardial perfusion at rest or vasodilation in collateral-dependent hibernating myocardium, regional function after both interventions improved. Pathological analysis showed that functional improvement reflected the effects of the interventions on stimulating myocyte proliferation. In the case of pravastatin, this caused a regression of myocyte cellular hypertrophy whereas AdvFGF-5 actually increased myocyte cell size. These interventions in animals with hibernating myocardium indicate that, understanding the extent of cellular myocyte plasticity and molecular changes before as well as after interventions intended to improve function in chronically dysfunctional myocardium are important aspects of interpreting the physiological relation between flow and function in chronic disease. There are other examples of therapeutic interventions that improve myocardial function without producing measurable increases in regional myocardial perfusion at rest or during pharmacological vasodilation that may have myocyte proliferation rather than an improvement in coronary flow as the primary mechanism.
Coronary Vascular Remodeling in Chronic Ischemia
It has been increasingly recognized that coronary resistance vessels exhibit considerable structural and functional plasticity in response to a variety of pathophysiological stresses [54]. As a result, chronic structural and functional adaptations of the coronary microcirculation may develop due to perfusion at a chronically reduced pressure and independently modify the flow-function relation in viable chronically dysfunctional myocardium.
Clinical studies examining minimal microcirculatory resistance after revascularization of a chronic stenosis are inconclusive with regard to whether the post-stenotic region has a normal or increased minimal microvascular resistance. Some investigators demonstrate an increase in calculated microcirculatory resistance that is reversed after normalizing coronary pressure with percutaneous coronary intervention [55]. Others have suggested that the apparent increase in microcirculatory resistance distal to a stenosis reflects the failure to include an appropriate back pressure and collaterals in the calculation (i.e. coronary wedge pressure) [56]. Clinical studies have not examined microcirculatory responses in patients with viable dysfunctional myocardium and thus, the only available data come from preclinical studies in swine models of a chronic stenosis or collateral-dependent myocardium. Mills et al. first hypothesized that chronic coronary vascular adaptations may play a role in the myocardial response to repetitive ischemia resulting in hibernating myocardium [57]. They demonstrated hypertrophy of the wall of arterioles <75 microns and relative thinning of larger coronary resistance vessels. Similar pathological findings developed following application of a prolonged acute stenosis to produce viable dysfunctional myocardium where pathological changes of arteriolar luminal narrowing and increases in arteriolar wall thickness developed within 4 weeks (Figure 10) [58]. The latter study demonstrated proliferation of vascular smooth muscle in arterioles distal to the stenosis as reflected by increased BrdU uptake and increased Ki67 staining of smooth muscle in the thickened arteriolar wall. More recently, Sorop et al. examined functional responses in isolated coronary arterioles from swine with hibernating myocardium [59]. Coronary resistance vessels from viable dysfunctional myocardium exhibited less spontaneous vasomotor tone and an attenuated myogenic response as distending pressure was varied ex vivo. Interestingly, in vivo circulating endothelin levels were elevated and resistance vessels studies in vitro demonstrated a heightened response to endothelin. While endothelium-dependent vasodilation was normal, this appears to be attenuated in models of collateral-dependent myocardium without regional contractile dysfunction [60]. Chronic remodeling of coronary microcirculatory resistance vessels could limit subendocardial flow during autoregulation and may not be reversible with pharmacological agents such as adenosine [61] since the vessel diameter is chronically reduced. The impact of these changes on the acute metabolic and autoregulatory adjustments to flow as well as their reversibility with revascularization are currently unknown. Further clinical and basic research in this area is warranted.
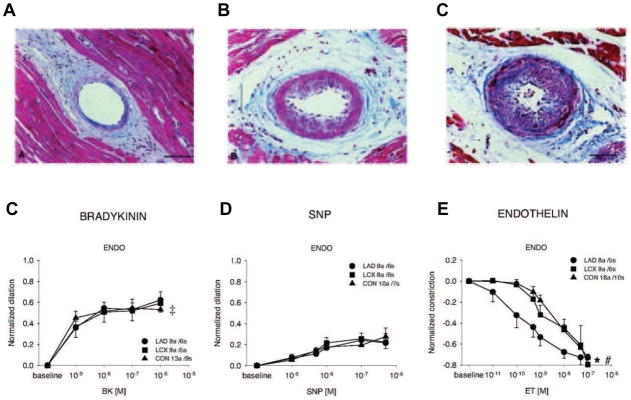
Figure 10. Structural and Functional remodeling of coronary resistance arterioles in hibernating myocardium.
Panels A, B and C depict mild, moderate and severe vascular wall hypertrophy from arterioles in chronically instrumented swine subjected to 4-weeks of a severe coronary artery stenosis (Modified from Hong, et al. [58]). Lower graphs demonstrate arteriolar functional responses in vessels isolated from swine with chronic hibernating myocardium (Modified from Sorop, et al. [59]). While endothelium-dependent bradykinin-induced arteriolar vasodilation and passive vasodilation to nitroprusside (SNP) were normal (panels D and E), the constrictor response to endothelin was accentuated in vessels isolated from pigs with hibernating myocardium. This was also accompanied by an increase in circulating endothelin levels and an attenuated myogenic response in vitro. Collectively, these results demonstrate that viable chronically dysfunctional myocardium may have alterations in resistance artery control that may increase vascular tone and minimal coronary resistance. Modified and republished with permission of the American Heart Association.
Summary
In summary, the close relation between sensitive measures of subendocardial function such as regional wall-thickening and subendocardial perfusion has been a concept that has been brought into the clinic using myocardial functional imaging during exercise as well as pharmacological stress. While the acute flow-function relation, stunning and acute-short-term hibernation are well studied and clinically relevant phenomena in normal myocardium, chronic adaptations to ischemia complicate the interpretation of changes in perfusion and function at rest, in response to acute stress as well as after therapeutic interventions. A better understanding of chronic myocyte remodeling as well as plasticity of the myocyte in response to interventions that facilitate cardiac repair is required since the usual flow-function concepts developed in normal myocardium do not appear to be strictly applicable. Likewise, chronic alterations in the coronary microcirculation arising from chronic reductions in coronary pressure as well as consequences of repetitive ischemia may play a role in vascular remodeling and impact coronary flow regulation in the chronic state. These chronic adaptive responses undoubtedly interact with the known abnormalities in coronary circulatory control that impact vascular reactivity and endothelium-dependent vasodilation arising from accompanying risk factors for coronary disease such as diabetes, hypertension and hypercholesterolemia. Understanding the interrelation among these chronic modulators of vascular tone and function will be a fruitful area to pursue further preclinical and clinical investigation.
Highlights.
Subendocardial perfusion and function are closely coupled during acute ischemia.
Reperfusion after reversible ischemia is accompanied by acute stunning that normalizes spontaneously.
Chronic contractile dysfunction progresses from chronic stunning to hibernating myocardium.
Myocyte loss, hypertrophy and protein expression distinguish chronic stunning from hibernation.
Structural vascular alterations from chronic ischemia may affect coronary flow regulation.
Acknowledgments
Supported by the VA, NHLBI (HL-55324 and HL-61610) and the Albert and Elizabeth Rekate Fund.
The assistance of Anne Coe and Marsha Barber in preparing the manuscript is greatly appreciated.
Footnotes
Disclosures: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canty JM., Jr . Coronary blood flow and myocardial ischemia. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease. 9. Philadelphia: Elsevier; 2012. pp. 1049–75. [Google Scholar]
- 2.Ross J., Jr Myocardial perfusion-contraction matching: Implications for coronary heart disease and hibernation. Circulation. 1991;83:1076–83. doi: 10.1161/01.cir.83.3.1076. [DOI] [PubMed] [Google Scholar]
- 3.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–9. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R. Myocardial ‘stunning’ in man. Circulation. 1992;86:1671–91. doi: 10.1161/01.cir.86.6.1671. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E, Kloner RA. The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–9. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 6.Canty JM, Fallavollita JA. Hibernating myocardium. J Nucl Cardiol. 2005;12:104–19. doi: 10.1016/j.nuclcard.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Canty JM, Jr, Fallavollita JA. Pathophysiologic basis of hibernating myocardium. In: Zaret BL, Beller GA, editors. Clinical Nuclear Cardiology: State of the Art and Future Direction. 4. Philadelphia: Elsiever Publishing; 2010. pp. 577–93. [Google Scholar]
- 8.Rahimtoola SH, Dilsizian V, Kramer CM, Marwick TH, Vanoverschelde JL. Chronic ischemic left ventricular dysfunction: from pathophysiology to imaging and its integration into clinical practice. JACC Cardiovasc Imaging. 2008;1:536–55. doi: 10.1016/j.jcmg.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canty JM., Jr . Myocardial perfusion-contraction matching. In: Heyndrickx GR, Vatner SF, Wijns W, editors. Stunning, hibernation, and preconditioning: Clinical pathophysiology of myocardial ischemia. Philadelphia: Lippincott-Raven; 1997. pp. 49–68. [Google Scholar]
- 10.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–67. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 11.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;288:H984–99. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 12.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–34. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G. Hibernating myocardium. Physiol Rev. 1998;78:1055–85. doi: 10.1152/physrev.1998.78.4.1055. [DOI] [PubMed] [Google Scholar]
- 14.Canty JM., Jr Coronary pressure-function and steady-state pressure-flow relations during autoregulation in the unanesthetized dog. Circ Res. 1988;63:821–36. doi: 10.1161/01.res.63.4.821. [DOI] [PubMed] [Google Scholar]
- 15.Vatner SF. Correlation between acute reductions in myocardial blood flow and function in conscious dogs. Circ Res. 1980;47:201–7. doi: 10.1161/01.res.47.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher KP, Matsuzaki M, Koziol JA, Kemper WS, Ross J., Jr Regional myocardial perfusion and wall thickening during ischemia in conscious dogs. Am J Physiol. 1984;247:H727–H38. doi: 10.1152/ajpheart.1984.247.5.H727. [DOI] [PubMed] [Google Scholar]
- 17.Canty JM, Jr, Giglia J, Kandath D. Effect of tachycardia on regional function and transmural myocardial perfusion during graded coronary pressure reduction in conscious dogs. Circulation. 1990;82:1815–25. doi: 10.1161/01.cir.82.5.1815. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher KP, Matsuzaki M, Osakada G, Kemper WS, Ross J., Jr Effect of exercise on the relationship between myocardial blood flow and systolic wall thickening in dogs with acute coronary stenosis. Circ Res. 1983;52:716–29. doi: 10.1161/01.res.52.6.716. [DOI] [PubMed] [Google Scholar]
- 19.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Peterson ED, Hendel RC, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance: endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. Circulation. 2008;117:1478–97. doi: 10.1161/CIRCULATIONAHA.107.189097. [DOI] [PubMed] [Google Scholar]
- 20.Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975;56:978–85. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyndrickx GR, Baig H, Nellens P, Leusen I, Fishbein MC, Vatner SF. Depression of regional blood flow and wall thickening after brief coronary occlusions. Am J Physiol. 1978;234:H653–H9. doi: 10.1152/ajpheart.1978.234.6.H653. [DOI] [PubMed] [Google Scholar]
- 22.Homans DC, Sublett E, Dai X-Z, Bache RJ. Persistence of regional left ventricular dysfunction after exercise-induced myocardial ischemia. J Clin Invest. 1986;77:66–73. doi: 10.1172/JCI112303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaulow E, Guth BD, Heusch G, Gilpin E, Schulz R, Kroeger K, et al. Characteristics of regional myocardial stunning after exercise in dogs with chronic coronary stenosis. American Journal of Physiology. 1989;257:H113–H9. doi: 10.1152/ajpheart.1989.257.1.H113. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SA, Fallavollita JA, Borgers M, Canty JM., Jr Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res. 2002;91:970–7. doi: 10.1161/01.res.0000040396.79379.77. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki M, Gallagher KP, Kemper WS, White F, Ross J., Jr Sustained regional dysfunction produced by prolonged coronary stenosis: Gradual recovery after reperfusion. Circulation. 1983;68:170–82. doi: 10.1161/01.cir.68.1.170. [DOI] [PubMed] [Google Scholar]
- 26.Pantely GA, Malone SA, Rhen WS, Anselone CG, Arai A, Bristow J, et al. Regeneration of myocardial phosphocreatine in pigs despite continued moderate ischemia. Circ Res. 1990;67:1481–93. doi: 10.1161/01.res.67.6.1481. [DOI] [PubMed] [Google Scholar]
- 27.Schulz R, Rose J, Martin C, Brodde O-E, Heusch G. Development of short-term myocardial hibernation: Its limitation by the severity of ischemia and inotropic stimulation. Circulation. 1993;88:684–95. doi: 10.1161/01.cir.88.2.684. [DOI] [PubMed] [Google Scholar]
- 28.Schulz R, Guth BD, Pieper K, Martin C, Heusch G. Recruitment of an inotropic reserve in moderately ischemic myocardium at the expense of metabolic recovery: A model of short-term hibernation. Circ Res. 1992;70:1282–95. doi: 10.1161/01.res.70.6.1282. [DOI] [PubMed] [Google Scholar]
- 29.Kudej RK, Ghaleh B, Sato N, Shen YT, Bishop SP, Vatner SF. Ineffective perfusion-contraction matching in conscious, chronically instrumented pigs with an extended period of coronary stenosis. Circ Res. 1998;82:1199–205. doi: 10.1161/01.res.82.11.1199. [DOI] [PubMed] [Google Scholar]
- 30.Schulz R, Post H, Neumann T, Gres P, Luss H, Heusch G. Progressive loss of perfusion-contraction matching during sustained moderate ischemia in pigs. Am J Physiol Heart Circ Physiol. 2001;280:H1945–H53. doi: 10.1152/ajpheart.2001.280.5.H1945. [DOI] [PubMed] [Google Scholar]
- 31.Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, et al. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res. 2003;92:1233–9. doi: 10.1161/01.RES.0000076892.18394.B6. [DOI] [PubMed] [Google Scholar]
- 32.Rahimtoola SH. A perspective on the three large multicenter randomized clinical trials of coronary bypass surgery for chronic stable angina. Circulation. 1985;72(suppl V):V-123–V-35. [PubMed] [Google Scholar]
- 33.Rahimtoola SH. Chronic myocardial hibernation. Circulation. 1994;89:1907–8. doi: 10.1161/01.cir.89.4.1907. [DOI] [PubMed] [Google Scholar]
- 34.Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884–8. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- 35.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, et al. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305:1863–72. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–63. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 37.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 38.Fallavollita JA, Lim H, Canty JM., Jr Myocyte apoptosis and reduced SR gene expression precede the transition from chronically stunned to hibernating myocardium. J Mol Cell Cardiol. 2001;33:1937–44. doi: 10.1006/jmcc.2001.1457. [DOI] [PubMed] [Google Scholar]
- 39.Fallavollita JA, Canty JM., Jr Differential 18F-2-deoxyglucose uptake in viable dysfunctional myocardium with normal resting perfusion: Evidence for chronic stunning in pigs. Circulation. 1999;99:2798–805. doi: 10.1161/01.cir.99.21.2798. [DOI] [PubMed] [Google Scholar]
- 40.Canty JM, Jr, Fallavollita JA. Chronic hibernation and chronic stunning: a continuum. J Nucl Cardiol. 2000;7:509–27. doi: 10.1067/mnc.2000.109683. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Ma L, Linfert DR, Lai T, Fallon JT, Gillam LD, et al. Myocardial cell death and apoptosis in hibernating myocardium. J Am Coll Cardiol. 1997;30:1407–12. doi: 10.1016/s0735-1097(97)00309-4. [DOI] [PubMed] [Google Scholar]
- 42.Fallavollita JA, Malm BJ, Canty JM., Jr Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res. 2003;92:48–55. doi: 10.1161/01.res.0000049104.57549.03. [DOI] [PubMed] [Google Scholar]
- 43.McFalls EO, Hou M, Bache RJ, Best A, Marx D, Sikora J, et al. Activation of p38 MAPK and increased glucose transport in chronic hibernating swine myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1328–H34. doi: 10.1152/ajpheart.01188.2003. [DOI] [PubMed] [Google Scholar]
- 44.McFalls EO, Sluiter W, Schoonderwoerd K, Manintveld OC, Lamers JM, Bezstarosti K, et al. Mitochondrial adaptations within chronically ischemic swine myocardium. J Mol Cell Cardiol. 2006;41:980–8. doi: 10.1016/j.yjmcc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Liem DA, Manintveld OC, Schoonderwoerd K, McFalls EO, Heinen A, Verdouw PD, et al. Ischemic preconditioning modulates mitochondrial respiration, irrespective of the employed signal transduction pathway. Transl Res. 2008;151:17–26. doi: 10.1016/j.trsl.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Kelly RF, Cabrera JA, Ziemba EA, Crampton M, Anderson LB, McFalls EO, et al. Continued depression of maximal oxygen consumption and mitochondrial proteomic expression despite successful coronary artery bypass grafting in a swine model of hibernation. J Thorac Cardiovasc Surg. 2011;141:261–8. doi: 10.1016/j.jtcvs.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 47.Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM., Jr Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation. 1999;100:2380–6. doi: 10.1161/01.cir.100.23.2380. [DOI] [PubMed] [Google Scholar]
- 48.Vanoverschelde J-L, Wijns W, Borgers M, Heyndrickx G, Depre C, Flameng W, et al. Chronic myocardial hibernation in humans. From bedside to bench. Circulation. 1997;95:1961–71. doi: 10.1161/01.cir.95.7.1961. [DOI] [PubMed] [Google Scholar]
- 49.Duan X, Young RF, Straubinger RM, Page BJ, Cao J, Wang H, et al. A straightforward and highly efficient precipitation/on-pellet digestion procedure coupled to a long gradient nano-LC separation and Oprbitrap mass spectrometry for label-free expression profiling of the swine heart mitochondrial proteome. J Proteome Res. 2009;8:2838–50. doi: 10.1021/pr900001t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Q, Suzuki G, Young RF, Page BJ, Fallavollita JA, Canty JM., Jr Reductions in mitochondrial O (2) consumption and preservation of high-energy phosphate levels after simulated ischemia in chronic hibernating myocardium. Am J Physiol Heart Circ Physiol. 2009;297:H223–32. doi: 10.1152/ajpheart.00992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Page B, Young R, Iyer V, Suzuki G, Lis M, Korotchkina K, et al. Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ Res. 2008;102:103–12. doi: 10.1161/CIRCRESAHA.107.155895. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki G, Iyer V, Cimato T, Canty JM., Jr Pravastatin improves function in hibernating myocardium by mobilizing CD133+ and cKit+ hematopoietic progenitor cells and promoting myocytes to reenter the growth phase of the cardiac cell cycle. Circ Res. 2009;104:255–64. doi: 10.1161/CIRCRESAHA.108.188730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki G, Lee TC, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res. 2005;96:767–75. doi: 10.1161/01.RES.0000162099.01268.d1. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 55.Chamuleau SA, Siebes M, Meuwissen M, Koch KT, Spaan JA, Piek JJ. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. Am J Physiol Heart Circ Physiol. 2003;285:H2194–200. doi: 10.1152/ajpheart.01021.2002. [DOI] [PubMed] [Google Scholar]
- 56.Fearon WF, Aarnoudse W, Pijls NH, De Bruyne B, Balsam LB, Cooke DT, et al. Microvascular resistance is not influenced by epicardial coronary artery stenosis severity: experimental validation. Circulation. 2004;109:2269–72. doi: 10.1161/01.CIR.0000128669.99355.CB. [DOI] [PubMed] [Google Scholar]
- 57.Mills I, Fallon JT, Wrenn D, Sasken H, Gray W, Bier J, et al. Adaptive responses of coronary circulation and myocardium to chronic reduction in perfusion pressure and flow. Am J Physiol Heart Circ Physiol. 1994;266:H447–H57. doi: 10.1152/ajpheart.1994.266.2.H447. [DOI] [PubMed] [Google Scholar]
- 58.Hong H, Aksenov S, Guan X, Fallon JT, Waters D, Chen C. Remodeling of small intramyocardial coronary arteries distal to a severe epicardial coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2002;22:2059–65. doi: 10.1161/01.atv.0000041844.54849.7e. [DOI] [PubMed] [Google Scholar]
- 59.Sorop O, Merkus D, de Beer VJ, Houweling B, Pistea A, McFalls EO, et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res. 2008;102:795–803. doi: 10.1161/CIRCRESAHA.108.172528. [DOI] [PubMed] [Google Scholar]
- 60.Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation. 2001;104:1393–8. doi: 10.1161/hc3601.094274. [DOI] [PubMed] [Google Scholar]
- 61.Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol. 2010;105:1–5. doi: 10.1007/s00395-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 62.Matsuzaki M, Guth B, Tajimi T, Kemper WS, Ross J., Jr Effect of the combination of diltiazem and atenolol on exercise-induced regional myocardial ischemia in conscious dogs. Circulation. 1985;72:233–43. doi: 10.1161/01.cir.72.1.233. [DOI] [PubMed] [Google Scholar]