Abstract
Stress can be a predisposing factor in the development of psychiatric disorders. However, not all individuals develop psychiatric disorders following a traumatic event. An attempt to understand these individual differences has led to a focus on factors that produce resistance. Interestingly, in rats, an experience with escapable tailshock (ES) before inescapable tailshock (IS) prevents the typical anxiety-like behavioral outcomes of IS. This type of resistance has been termed “behavioral immunization” and it depends on activation of the medial prefrontal cortex (mPFC) during ES. However, one outcome of IS that is not anxiety-related is potentiation of morphine conditioned place preference (CPP). The present experiments investigated whether prior ES would block IS-induced potentiation of morphine CPP. Rats received either ES, IS, or homecage control treatment on Day 1 and then either IS or homecage on Day 2. Twenty-four hours following Day 2, rats underwent morphine conditioning and CPP was subsequently assessed. In a second experiment, rats received ES 3, 14, or 56 days prior to IS to determine the duration of behavioral immunization. In a final experiment, rats were microinjected with the GABAA agonist muscimol (50 ng/ 0.5 µl) or saline in the mPFC before Day 1 of stress. Prior ES blocked IS-induced potentiation of morphine CPP. This immunizing effect of ES lasted for at least 56 days. Additionally, intra-mPFC muscimol during ES prevented behavioral immunization. These results suggest that prior experience with ES activates the mPFC and produces long-lasting neural alterations that block subsequent IS-induced potentiation of morphine CPP.
Keywords: resilience, addiction, serotonin, learned helplessness, rat
Introduction
Exposure to stressors can augment the rewarding properties of drugs of abuse in both animals and humans (Lu et al., 2003; Ouimette et al., 2007; Briand & Blendy, 2010; George & Koob, 2010). We have previously reported that a single session of inescapable tailshock (IS) potentiates morphine conditioned place preference (CPP) as compared to homecage (HC) controls (Will et al., 1998; Der-Avakian et al., 2005). Moreover, potentiation of morphine CPP occurs even if the IS precedes CPP training by a number of days and occurs in a different environment than the CPP environment (Will et al., 1998). Interestingly, exactly equal escapable tailshock (ES) does not potentiate morphine CPP (Will et al., 1998; Rozeske et al., 2009). Thus, only the behaviorally uncontrollable stressor potentiated morphine CPP in this enduring trans-situational fashion.
There has been a growing interest in the factors that promote stress-resistance/resilience (Charney, 2004; Agaibi & Wilson, 2005; Lyons & Parker, 2007; Wingo et al., 2010). This issue has been primarily pursued with regard to the effects of stressors on processes and behaviors other than drug reward (e.g. anxiety-related behaviors). A variety of manipulations have been shown to blunt the impact of subsequent stressors. For example, voluntary exercise reduces the behavioral impact of later exposure to an uncontrollable stressor (Greenwood et al., 2003). In this context it is interesting to note that experience with behavioral control over a stressor (ES) also blocks several behavioral (reduced juvenile social exploration, shuttlebox escape deficits) and neurochemical (increased serotonergic (5-HT) activity in the dorsal raphe nucleus (DRN)) effects of IS occurring as much as one week later (Amat et al., 2006; Amat et al., 2008; Christianson et al., 2008b). This "immunizing" effect of ES appears to be quite general, as ES blocks the behavioral and neurochemical effects of a stressor as different from shock as social defeat (Amat et al., 2010).
Whether an experience with ES would block later IS-induced potentiation of opioid reward is unknown. Thus, here we determined whether exposure to ES might alter the enhancing effects of IS on morphine CPP, as CPP is one reliable measure for assessing the acute rewarding effects of drugs (Bardo & Bevins, 2000). In addition, the duration of the protection afforded by ES is unknown in any paradigm, and so this issue was also investigated. Lastly, although the mechanisms responsible for the immunizing effects of ES are not fully understood, the ventral medial prefrontal cortex (mPFC) has been shown to play a critical role. For example, inactivation of the mPFC with the GABAA receptor agonist muscimol during the ES experience prevents ES from producing its proactive protective effects (Amat et al., 2006). So, here we explored whether mPFC activation during ES is also necessary for any ES protection against the behavioral effects of IS on morphine CPP.
Materials and Methods
Subjects
Adult male Sprague-Dawley rats weighing 325–425 g (Harlan Laboratories, Indianapolis, IN, USA) were housed in pairs with food and water available ad libitum. The colony room was maintained at 22 °C with a 12 hour light-dark cycle (lights on at 0700). Rats were allowed to acclimate to the colony at least 1 wk before any experimentation. All experimental procedures were approved by the University of Colorado Institutional Animal Care and Use Committee.
Surgery and cannulation
Surgery was performed under isoflurane (Webster Veterinary, Sterling, MA, USA) anesthesia. Rats in Experiment 3 were implanted with a 26-gauge dual guide cannula (Plastics One, Roanoke, VA, USA) 1 mm center-to-center distance, which was secured to the skull with 4 screws and dental acrylic. The tips of the cannulae were aimed at 1 mm above the border of the infralimbic and prelimbic cortices of the mPFC (AP +2.7 mm, DV −3.3 mm, ML +0.0 mm from dura, relative to Bregma) (Paxinos & Watson, 1998). Rats were given 1–2 weeks of recovery time following surgery.
Drugs
Morphine sulfate (NIDA) was dissolved in 0.9% saline at a dose of 3.0 mg/kg and injected subcutaneously at a volume of 1 ml/kg of body weight. This dose was chosen as previous studies have demonstrated that it produced minimal morphine CPP in non-stressed controls (Will et al., 1998). Muscimol (Sigma, St. Louis, MO, USA) was also dissolved in 0.9% saline and 0.5 µl was microinjected per hemisphere into the mPFC at a dose of 50 ng.
Microinjections
A 33-gauge microinjector tip (Plastics One) connected to a Kopf Instruments Model 5000 microinjector (Tujunga, CA, USA) via polyethylene 50 tubing was used for microinjections. Rats were gently restrained in a towel and the cannula stylet was removed. Microinjections were given 45 minutes before ES or HC treatment. Of note, muscimol does not interfere with escape learning during ES (Amat et al., 2005). The microinjector tips (Plastics One) were inserted into the cannula and extended 1 mm beyond the cannula tips. Rats then received 0.5 µl /hemisphere of muscimol or saline over the course of 1 minute. Following infusion, the microinjector tips were left in place for 2 minutes to allow for diffusion. Microinjections were considered successful if fluid was readily dispensable from the microinjector tip following microinjection.
Stressor controllability
All stress procedures were administered in a distinct and separate room from that used for CPP behavioral testing. Rats received two experiences with stress to assess whether prior ES produces behavioral immunization. Day 1 of stress consisted of ES or IS. Rats were placed in clear Plexiglas wheel-turn boxes (14 × 11 × 17 cm) with a wheel located in the front of the box and a Plexiglas rod extending from the rear. Rat tails were taped to the extending rod and copper electrodes augmented with electrode paste were affixed to the tails. Shocks were delivered to yoked pairs of rats using a Precision Regulated Animal Shocker with Graphic State 3.0 software (Coulbourn Instruments, Whitehall, PA, USA). Day 1 stress consisted of 80 tailshocks with an average inter-trial interval of 60 seconds. Only ES subjects were able to behaviorally control the termination of the shock, initially by moving the wheel 1/4 of a full turn. Upon performing this operant response the shock terminated for both the ES and IS rats simultaneously. The response requirement increased throughout the session. Initially, the response increased a 1/4 turn following three previous escape responses that were completed in less than 5 seconds. Subsequent escape responses that were performed in less than 5 seconds produced a 50% increase in the wheel-turn requirement until a maximum of 4 wheel-turns was reached. If the operant response was not performed within 10 seconds, the wheel-turn requirement for the subsequent shock was incrementally decreased. If the escape response was not performed in less than 30 seconds the shock was terminated and the escape requirement was reset to 1/4 wheel-turn. Shock intensity was increased throughout the session to maintain consistent escape behavior of ES rats (1.0 mA for 30 minutes, 1.3 mA for 30 minutes, and 1.6 mA for the remainder of the session).
Day 2 stress consisted of a session of IS. Rats were placed in a clear Plexiglas restraint tube that measured 17 cm in length and 7 cm in diameter. The restraint tube contained a rod extending from the rear where rat tails were taped and electrodes were affixed and augmented with electrode paste. Day 2 stress consisted of 100, 5 second inescapable shocks at an intensity of 1.0 mA, separated by an average inter-trial interval of 60 seconds. Prior studies (Will et al., 1998) indicated that restraint in the absence of shock did not potentiate morphine CPP, therefore non-stressed HC controls remained in the colony during stress. Stress group designations were expressed as Day 1/Day 2 stress, e.g. a rat that received ES on Day 1 and IS on Day 2 was represented as ES/IS. Lastly, since Experiment 1 demonstrated no significant difference between HC/IS and IS/IS groups, the IS/IS group was eliminated.
Conditioned place preference
Apparatus
Rectangular black Plexiglas boxes (72 × 30 × 30 cm) consisting of 2 distinct conditioning environments and a central neutral chamber were used for CPP. The conditioning environments (30 × 30 × 30 cm) contained different tactile and visual cues. One conditioning environment contained vertically oriented alternating black and white stripes (2 cm wide) and the floor consisted of a 3 mm wire mesh. The other conditioning environment contained horizontally oriented black and white stripes (2 cm wide) and the floor consisted of a 2 cm wire grid. The neutral area (12 × 30 × 30 cm) was painted gray. During the conditioning phase Plexiglas partitions (30 × 30 × 30 cm) containing horizontal or vertical black and white stripes were inserted to confine the rat to a single conditioning environment. Rat activity in the CPP apparatus was monitored with a Philips TC352A video camera (Lancaster, PA, USA) mounted 1.5 meters above the CPP apparatus. The rat's location was relayed to a PC installed with Chromotrack Version 4.02 tracking software (Prototype Systems Ltd., Boulder, CO, USA). A SA-3 Tracker (San Diego Instruments, San Diego, CA, USA) simultaneously measured the amount of time spent in each of the CPP environments.
Procedure
Prior to experimentation rats were handled and loosely fitted with a collar (BAS, West Lafayette, IN, USA) affixed with reflective tape to aid video tracking. The first day of the CPP procedure was a 20 minute pre-exposure to the entire CPP apparatus. Rats that spent less than 4 minutes in either conditioning environment were eliminated from the study. The following day consisted of either ES, IS, or HC treatment. The amount of time between Day 1 stress and Day 2 stress varied depending on the experiment, but at minimum was 24 hours unless otherwise noted. Day 2 stress consisted of either IS or HC. Twenty-four hours after Day 2 stress rats were weighed and randomly assigned to a conditioning environment using a counterbalanced procedure. Conditioning sessions lasted 45 minutes each and were separated by 4 hours. During the first day of conditioning half of the rats received morphine in the AM, while the other half received equivolume saline. Over the course of 1 minute, all rats were injected with drug or saline and then immediately placed in their respective conditioning chamber. During PM conditioning the injections were alternated such that a rat that had received saline in the AM now received morphine in the PM. The following day of conditioning was similar to the previous day except the order in which the rat was presented morphine and saline on the previous conditioning day was reversed. The final day of the CPP procedure was a 20 minute test of preference. Rats were placed in the neutral area and allowed to explore the entire apparatus in a drug-free state. The amount of time spent in each compartment was recorded. The dependent measure for CPP was the difference of time spent in the drug-paired environment before morphine conditioning (pre-exposure) and after conditioning (test of preference). Thus, a positive score indicates a preference for the drug-paired environment.
Cannula verification
Following morphine CPP, rats were overdosed with an injection of sodium pentobarbital (Vortech Pharmaceuticals, Dearborn, MI, USA). Animals were decapitated and brains were rapidly removed and chilled in ice-cold isopentane and stored at −80 °C. A cryostat was used to section brains at 40 µm; slices were mounted on gelatin-coated slides for subsequent Cresyl Violet staining. Cannula placements were considered successful if the tips of the cannulae terminated in the infralimbic or prelimbic cortices of the mPFC. Data from animals with misplaced cannulae were eliminated from statistical analyses.
Statistical analysis
All data were analyzed by ANOVA to determine statistically significant differences between experimental groups. All statistically significant main effects and interactions were followed by a Newman-Keuls multiple comparison post hoc test (α = 0.05).
Results
Experiment 1: Effect of behavioral immunization on morphine conditioned place preference
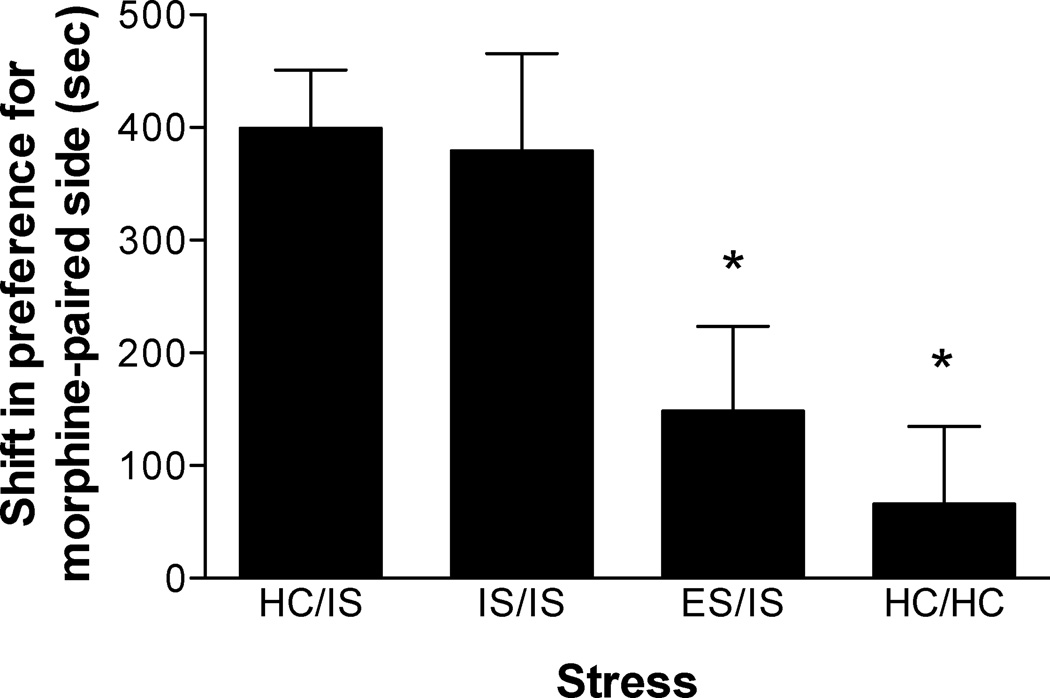
Preference for the morphine-conditioned environment following behavioral immunization is shown in Figure 1 (n = 8–9/group). Stress treatments were separated by 24 hours. As previously observed (Will et al., 1998), IS potentiated morphine CPP (HC/IS versus HC/HC). IS 24 hours prior to additional IS (IS/IS) did not alter the magnitude of this effect. However, ES prior to IS (ES/IS) blocked IS-induced potentiation of morphine CPP. A one-way ANOVA revealed a significant main effect of group (F(3, 31) = 5.268, p < 0.01). A subsequent Newman-Keuls post hoc comparison revealed a significant difference between ES/IS and the HC/IS & IS/IS groups. However, there were no significant differences between ES/IS & HC/HC or between HC/IS & IS/IS groups. Lastly, there was a significant difference between HC/HC and HC/IS & IS/IS groups.
Figure 1.
Escapable tailshock (ES) 24 hours prior to inescapable tailshock (IS) blocks potentiation of morphine conditioned place preference (CPP). Data are expressed as mean + SEM difference of time (sec) spent in the morphine-paired environment before and after conditioning. Positive scores indicate a shift in preference for the morphine-paired environment. Both a single session of IS (HC/IS) or two sessions of IS (IS/IS) potentiated morphine CPP as compared to behavioral immunization (ES/IS) and non-stressed controls (HC/HC). *Significantly different from HC/IS and IS/IS (p < 0.05).
Experiment 2: Timecourse of behavioral immunization on morphine conditioned place preference
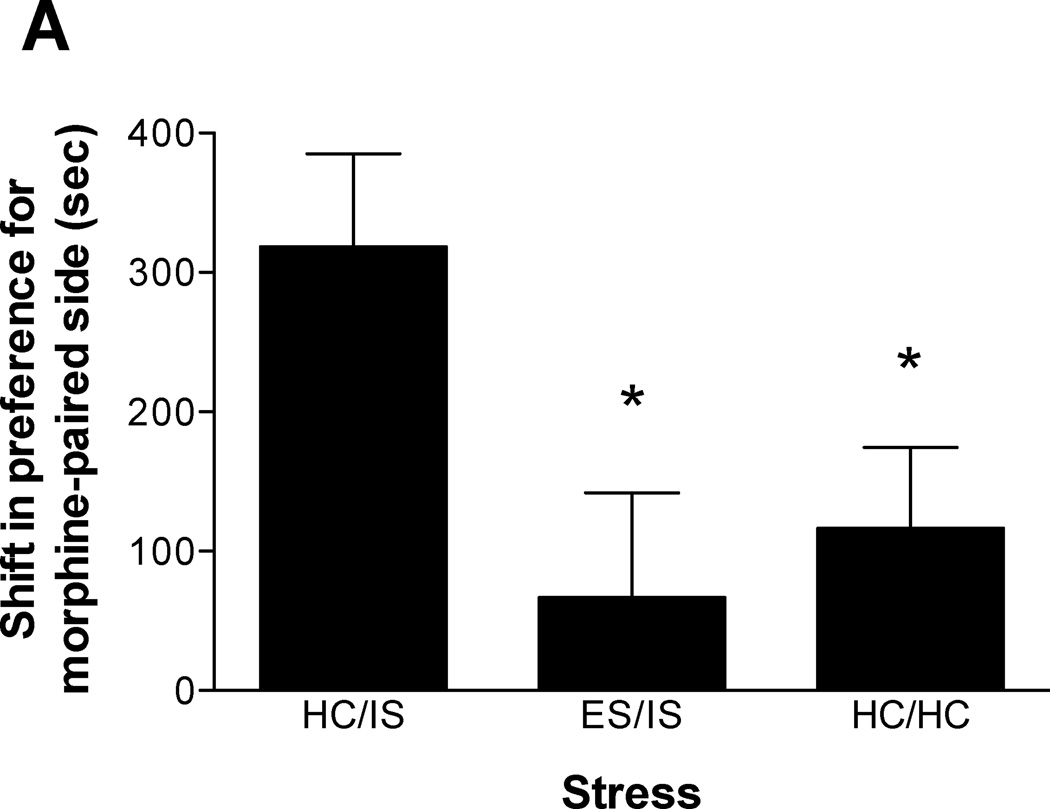
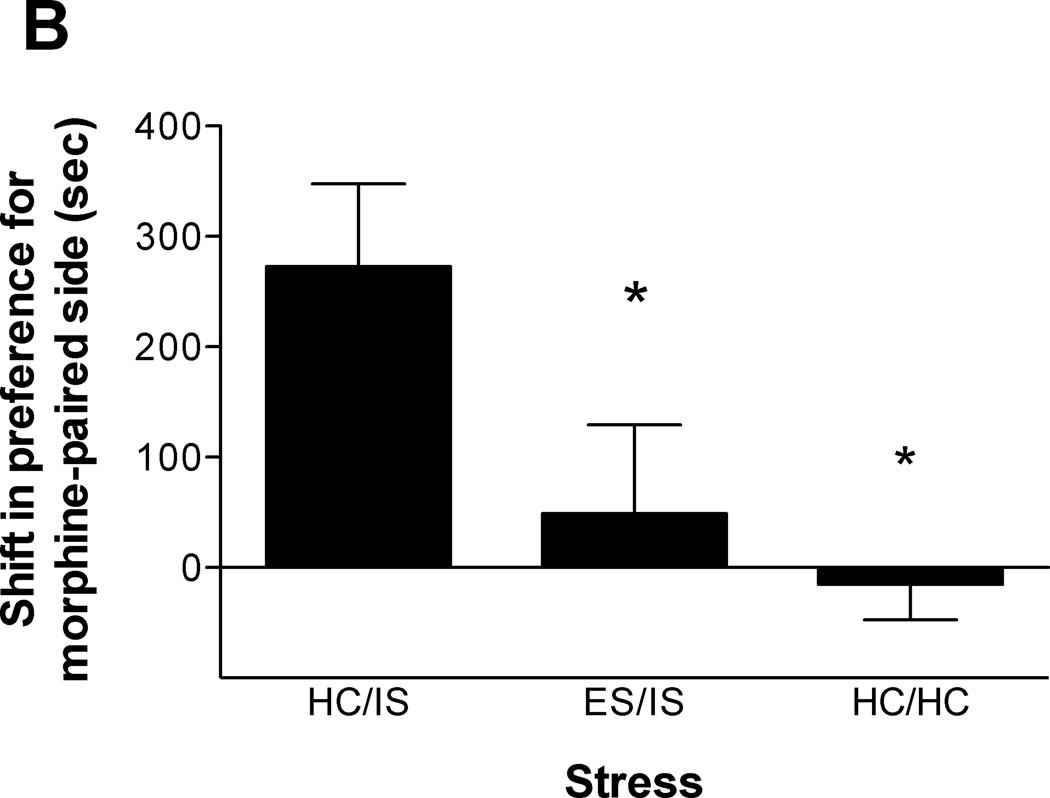
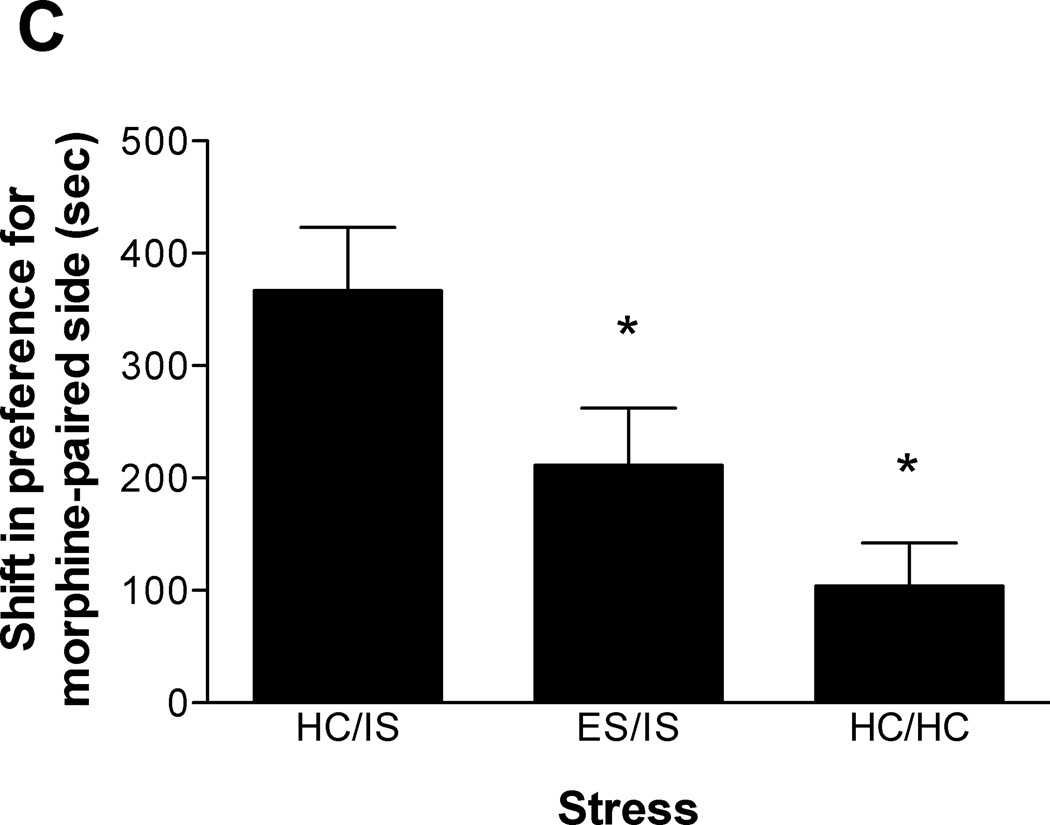
The length of time between initial ES and subsequent IS was manipulated to assess the duration of behavioral immunization produced by ES. Either 3, 14, or 56 days were allowed to intervene between ES and later IS. To control for the differing amounts of time spent in the colony room during the intervening time between ES and IS, all time points contain a different HC/HC group. For this reason, Experiment 2 is presented as 3 different data sets. As shown in Figure 2A (n = 10–11/group), behavioral immunization to IS-induced potentiation of morphine CPP lasts at least 3 days. A one-way ANOVA confirmed this finding (F(2, 29) = 3.986, p < 0.05). Figure 2B (n = 10–12/group) shows that behavioral immunization lasts at least 14 days (F(2, 30) = 5.379, p < 0.02). Lastly, Figure 2C (n = 12/group) shows that a 56 day separation between ES and IS still produces behavioral immunization (F(2, 33) = 7.244, p < 0.01). Subsequent Newman-Keuls post hoc comparisons all revealed that ES/IS & HC/HC groups were significantly different from HC/IS, but ES/IS & HC/HC groups were not significantly different from each other. Lastly, the difference between HC/HC and ES/IS groups in Figure 2C trended toward, but did not reach, significance.
Figure 2.
A. Escapable tailshock (ES) 3 days prior to inescapable tailshock (IS) blocks potentiation of morphine conditioned place preference (CPP). Data are expressed as mean + SEM difference of time (sec) spent in the morphine-paired environment before and after conditioning. Positive scores indicate a shift in preference for the morphine-paired environment. *A single session of IS (HC/IS) potentiated morphine CPP as compared to behavioral immunization (ES/IS) and non-stressed controls (HC/HC) (p < 0.05). B. ES 14 days prior to IS blocks potentiation of morphine CPP. *A single session of IS (HC/IS) potentiated morphine CPP as compared to behavioral immunization (ES/IS) and non-stressed controls (HC/HC) (p < 0.05). C. ES 56 days prior to IS blocks potentiation of morphine CPP. *A single session of IS (HC/IS) potentiated morphine CPP as compared to behavioral immunization (ES/IS) and non-stressed controls (HC/HC) (p < 0.05).
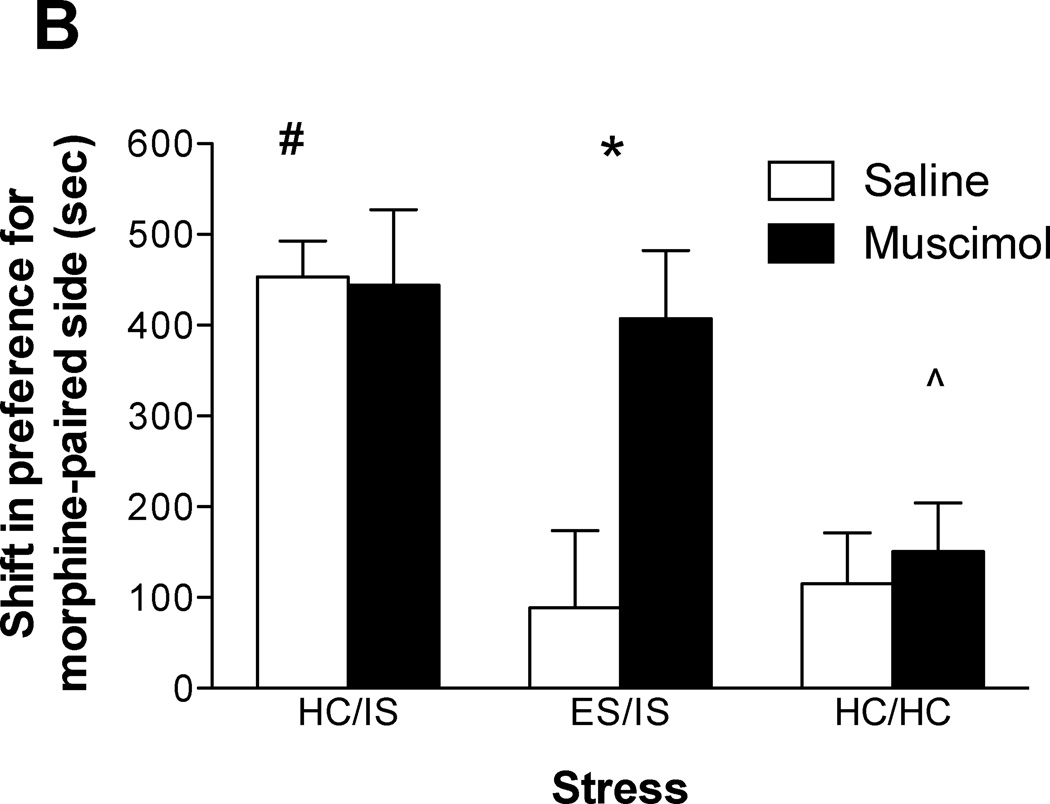
Experiment 3: The role of the medial prefrontal cortex during behavioral immunization on morphine conditioned place preference
Figure 3A shows cannula placements within the mPFC for the animals tested in Experiment 3. Five subjects were removed due to misplaced cannulae. As shown in Figure 3B, activation of the mPFC during initial ES is necessary for behavioral immunization to IS-induced potentiation of morphine CPP (n = 9–11/group). A two-way ANOVA revealed significant effects of stress (F(2, 53) = 11.300, p < 0.0001), and microinjection (F(1, 53) = 4.336, p < 0.05), and an interaction of stress × microinjection (F(2, 53) = 3.379, p < 0.05). Subsequent Newman-Keuls post hoc comparisons revealed significant differences between (i) saline-ES/IS and muscimol-ES/IS groups (ii) saline-HC/IS and the saline-ES/IS & saline-HC/HC groups (iii) and muscimol-HC/HC and the muscimol-ES/IS & muscimol-HC/IS groups.
Figure 3.
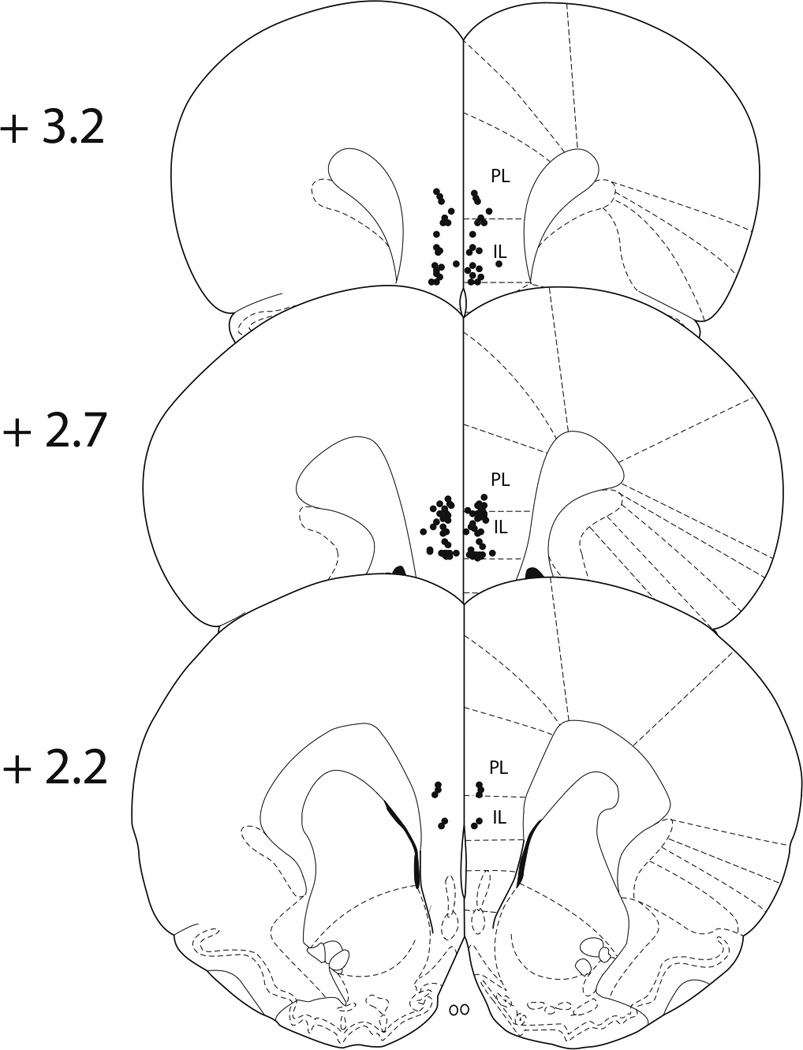
A. Cannula placements for rats in Experiment 3. Numerals on the left indicate distance (mm) from Bregma. Medial prefrontal cortex (mPFC) placements were considered successful if they terminated within the infralimbic (IL) and prelimbic (PL) cortices. Not all cannula are shown due to overlapping placements. B. Intra-mPFC muscimol blocks behavioral immunization to potentiation of morphine conditioned place preference (CPP) when escapable tailshock (ES) and inescapable tailshock (IS) are separated by 24 hours. Data are expressed as mean + SEM difference of time (sec) spent in the morphine-paired environment before and after conditioning. Positive scores indicate a shift in preference for the morphine-paired environment. #The saline-HC/IS group was significantly different from the saline-ES/IS & saline-HC/HC groups (p < 0.05). *Muscimol-ES/IS was significantly different from saline-ES/IS (p < 0.05). ^The muscimol-HC/HC group was significantly different from the muscimol-ES/IS & muscimol-HC/IS groups (p < 0.05).
Discussion
These experiments, along with others (Williams & Maier, 1977; Amat et al., 2006; Amat et al., 2008; Christianson et al., 2008b), clearly indicate that an initial experience with controllable stress can produce resistance to the behavioral effects normally produced by uncontrollable stress. However, here the behavior assessed was opioid reward and not an anxiety-related measure. Moreover, the present results corroborate previous findings in that behavioral immunization of IS-induced potentiation of morphine reward depends on activation of the mPFC during the initial ES exposure (Amat et al., 2006; Amat et al., 2008). Further, the present experiments extend our understanding of the breadth and longevity of the behavioral immunization paradigm.
As noted above, previous investigations of behavioral immunization have focused on the role of the mPFC during the initial stressor. The current experiments demonstrate that activation of the mPFC during initial ES is necessary for ES to block IS-induced potentiation of morphine CPP. The GABAA receptor agonist muscimol was used to inhibit output activity from the mPFC as muscimol is known to produce neuronal hyperpolarization (Andrews & Johnston, 1979). Indeed, muscimol has previously been used in the stressor controllability paradigm to support the idea that the mPFC becomes activated during ES, but not IS, and that inactivation of the mPFC during ES produces behaviors typical of IS exposure (Amat et al., 2005; Rozeske et al., 2009).
Activation of the mPFC during the experience of behavioral control likely serves two purposes. Acutely, activation of the mPFC during ES leads to inhibition of stress responsive limbic and brainstem regions (Baratta et al., 2009), thereby blunting the impact of the stressor being experienced. For example, IS relative to ES produces intense activation of DRN 5-HT neurons as assessed by extracellular levels of 5-HT in the DRN as well as c-Fos protein in 5-HT labeled cells (Amat et al., 2006). However, inactivation of the mPFC during ES leads ES to produce the same high levels of 5-HT activation that is normally only produced by IS (Amat et al., 2006). This stress-induced hyperactivation of 5-HT cells in the DRN by IS is thought to lead to sensitization of 5-HT cells in the DRN (Amat et al., 1998; Bland et al., 2003a). It is this sensitization of 5-HT cells in the DRN that leads to potentiation of morphine CPP (Will et al., 2004) as well as potentiation of extracellular dopamine in the nucleus accumbens following subcutaneous morphine (Bland et al., 2004a; Bland et al., 2004b). For these reasons, pharmacological inhibition of the mPFC during ES leads ES to sensitize 5-HT cells in the DRN (Rozeske et al., 2010), and consequently, the rewarding properties of morphine are now potentiated due to elevated extracellular dopamine in the nucleus accumbens in IS, but not ES, rats.
In addition, activation of the mPFC during ES has another more enduring effect, namely the induction of long-term plasticity within the mPFC. This plasticity is thought to alter the mPFC in such a way that now even uncontrollable stressors produce activation. Again, this activation leads to inhibition of stress-responsive limbic and brainstem structures, the mediator of immunization. Two types of evidence have been provided for this plasticity phenomenon. First, inhibition of de novo protein synthesis in the mPFC during initial ES blocks behavioral immunization (Amat et al., 2006). Second, inactivation of the mPFC during subsequent IS prevents an initial ES exposure from producing immunization (Amat et al., 2006). Thus, the mPFC is required both at the time of initial ES and later IS for immunization to occur.
Nevertheless, measures of neuronal activation that correlate with drug reward were not made in the present studies. Phosphorylated extracellular signal-regulated kinase would be an appropriate marker as drugs of abuse elevate its expression and it is required for morphine CPP (Valjent et al., 2004; Valjent et al., 2006). Assessing phosphorylation of extracellular signal-regulated kinase could provide information concerning the extent of cellular activation in reward-related brain regions during time points where the strength of behavioral immunization begins to diminish, such as the 56 day time point.
In addition to the interaction between the mPFC and DRN during stressor controllability, the relationship between the mPFC and the mesolimbic reward system has also been extensively studied. Indeed, it is known that ES produces a transient elevation of extracellular dopamine in the nucleus accumbens, although this elevation is similar to that produced by IS (Bland et al., 2003b). ES also initially elevates extracellular levels of dopamine and 5-HT in the mPFC, but these increases are transient and outlasted by IS exposure (Bland et al., 2003a). Since the elevations of extracellular monoamines measured during ES are short-lived, it is unlikely that 5-HT and dopamine directly contribute to behavioral immunization. However, the role of other neurotransmitters and post-translational modifications in reward-related structures following behavioral immunization is an open question.
The behavioral immunization paradigm has previously been investigated using a 7 day interval between ES and IS (Amat et al., 2006; Baratta et al., 2009; Amat et al., 2010). The present experiments reveal that ES produces resistance to IS as soon as 24 hours following ES. Moreover, the stress-buffering effects of ES persist for at least 56 days. Experiment 3 reported here, examined only a 24 hour ES-to-IS interval, and so it cannot be stated with certainty that the long-duration 56-day immunization effect is also mediated by the mPFC. However, given the abundance of evidence reviewed above, activation of the mPFC during the stress experience is a likely mechanism. The necessary biochemical cascade(s) during ES-induced activation of the mPFC have not yet been investigated. However, they must be induced following a relatively short behavioral experience (ES is roughly 90 minutes) and be present 24 hours later. Since behavioral immunization mitigates IS-induced potentiation of morphine CPP for at least 56 days, the plasticity processes involved are likely long lasting. The literature suggests a number of candidates including, PKMζ (Shema et al., 2009; Richter-Levin & Maroun, 2010; Sacktor, 2011).
Together the results presented above provide additional evidence that an experience with a controllable stressor can protect an organism from the usual behavioral consequences of an uncontrollable stressor. Typically the behavioral outcomes measured following behavioral immunization have been anxiety-related, but here the protective effects of ES have been extended to a drug reward paradigm. Although other studies have examined factors that block IS-induced potentiation of morphine CPP, such as voluntary free-wheel running (Rozeske et al., 2011), the role of the mPFC has been unknown. Certainly, not all factors that blunt the impact of stressors utilize the mPFC (Christianson et al., 2008a), and so involvement of the mPFC in paradigms that blunt the effects of stress continues to be an open issue.
Acknowledgements
This research was supported by National Institutes of Health grants DA023329 (RRR) and MH050479 (SFM).
Abbreviations
- 5-HT
Serotonin
- CPP
Conditioned place preference
- DRN
Dorsal raphe nucleus
- ES
Escapable tailshock
- HC
homecage control
- IS
Inescapable tailshock
- mPFC
Medial prefrontal cortex
Footnotes
The authors declare that they have no competing financial interests.
References
- Agaibi CE, Wilson JP. Trauma, PTSD, and resilience: a review of the literature. Trauma Violence Abuse. 2005;6:195–216. doi: 10.1177/1524838005277438. [DOI] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–1038. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PR, Johnston GA. GABA agonists and antagonists. Biochem Pharmacol. 1979;28:2697–2702. doi: 10.1016/0006-2952(79)90549-5. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003a;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Watkins LR, Maier SF. Prefrontal cortex serotonin, stress, and morphine-induced nucleus accumbens dopamine. Neuroreport. 2004a;15:2637–2641. doi: 10.1097/00001756-200412030-00016. [DOI] [PubMed] [Google Scholar]
- Bland ST, Twining C, Schmid MJ, Der-Avakian A, Watkins LR, Maier SF. Stress potentiation of morphine-induced dopamine efflux in the nucleus accumbens shell is dependent upon stressor uncontrollability and is mediated by the dorsal raphe nucleus. Neuroscience. 2004b;126:705–715. doi: 10.1016/j.neuroscience.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Bland ST, Twining C, Watkins LR, Maier SF. Stressor controllability modulates stress-induced serotonin but not dopamine efflux in the nucleus accumbens shell. Synapse. 2003b;49:206–208. doi: 10.1002/syn.10229. [DOI] [PubMed] [Google Scholar]
- Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–234. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, Baratta MV, Paul ED, Campeau S, Watkins LR, Barth DS, Maier SF. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J Neurosci. 2008a;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008b;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Will MJ, Bland ST, Deak T, Nguyen KT, Schmid MJ, Spencer RL, Watkins LR, Maier SF. Surgical and pharmacological suppression of glucocorticoids prevents the enhancement of morphine conditioned place preference by uncontrollable stress in rats. Psychopharmacology (Berl) 2005;179:409–417. doi: 10.1007/s00213-004-2041-1. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Ouimette P, Coolhart D, Funderburk JS, Wade M, Brown PJ. Precipitants of first substance use in recently abstinent substance use disorder patients with PTSD. Addict Behav. 2007;32:1719–1727. doi: 10.1016/j.addbeh.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Richter-Levin G, Maroun M. Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex. Cereb Cortex. 2010;20:2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The medial prefrontal cortex regulates the differential expression of morphine-conditioned place preference following a single exposure to controllable or uncontrollable stress. Neuropsychopharmacology. 2009;34:834–843. doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeske RR, Greenwood BN, Fleshner M, Watkins LR, Maier SF. Voluntary wheel running produces resistance to inescapable stress-induced potentiation of morphine conditioned place preference. Behav Brain Res. 2011;219:378–381. doi: 10.1016/j.bbr.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeske RR, Watkins LR, Lowry CA, Maier SF. The role of the medial prefrontal cortex in dorsal raphe nucleus 5-HT1A receptor adaptation following escapable and inescapable stress; Society for Neuroscience Annual Meeting. City; (Year) [Google Scholar]
- Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Shema R, Hazvi S, Sacktor TC, Dudai Y. Boundary conditions for the maintenance of memory by PKMzeta in neocortex. Learn Mem. 2009;16:122–128. doi: 10.1101/lm.1183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Will MJ, Der-Avakian A, Bland ST, Grahn RE, Hammack SE, Sparks PD, Pepin JL, Watkins LR, Maier SF. Electrolytic lesions and pharmacological inhibition of the dorsal raphe nucleus prevent stressor potentiation of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2004;171:191–198. doi: 10.1007/s00213-003-1572-1. [DOI] [PubMed] [Google Scholar]
- Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine's rewarding properties. Pharmacol Biochem Behav. 1998;60:655–664. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. J Exp Psychol Anim Behav Process. 1977;3:240–253. [Google Scholar]
- Wingo AP, Wrenn G, Pelletier T, Gutman AR, Bradley B, Ressler KJ. Moderating effects of resilience on depression in individuals with a history of childhood abuse or trauma exposure. J Affect Disord. 2010;126:411–414. doi: 10.1016/j.jad.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]