Abstract
Currently phosphodiestrase5 (PDE5) inhibitors are the first-line treatment for erectile dysfunction. Drugs such as sildenafil and tadalafil are available as PDE5 inhibitors which are potent and reversible but lack selectivity with side effects such as headache, facial flushing, dyspepsia, and visual disturbances. We herein report for the first time novel condensed thienopyrimidines as evodiamine analogue and their effect on sexual behavior in male rats hitherto unreported. Novel synthetic evodiamine significantly showed improvement in male rat copulatory behavior. The test compound MKAC9 could be of promising importance in the treatment of sexual disorders like desire disorder or erectile dysfunction.
Figure.
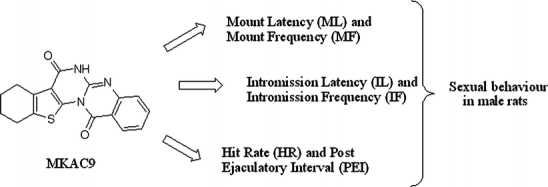
Evodiamine analogue on sexual behavior in male rats
Keywords: Evodiamine, Thienopyrimidine derivative, Aphrodisiac, PDE5 inhibitors
Introduction
Sexual function is an important aspect of well-being and quality of life for human beings [1]. Male and female sexuality, relationships, and successful reproduction are paramount in the accomplishment of a meaningful life. Male erectile dysfunction (ED) is defined as “the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance” [2]. The worldwide prevalence of male ED has been estimated at over 152 million men in 1995, and the projections for 2025 suggest a prevalence of approximately 322 million [3].
Penile erection is an example of microcirculation where the vasodilatation coupled to blood engorgement of the corpora cavernosa causes a compression of the vein between the corpora and albuginea leading to erection [4]. During sexual activity, nitric oxide is released from the cavernous nerves and activates soluble guanylyl cyclase, which in turn raises intracellular cGMP levels [5]. Activation of cGMP-dependent protein kinase G results in multiple intracellular changes, with a primary end result being a lowering of free intracytosolic calcium levels that starts smooth muscle relaxation.
Currently phosphodiestrase 5 (PDE5) inhibitors are the first-line treatment for erectile dysfunction. There are three available PDE5 inhibitors which are potent and reversible [6]. PDE5 inhibitors block the degradation of cGMP within the corpora cavernous tissue, thereby promoting smooth muscle relaxation in both the corpus cavernosum and surrounding vessels [7]. Despite the efficacy of sildenafil and tadalafil for treatment of ED, they have side effects such as headache, facial flushing, dyspepsia, and visual disturbances along with poor selectivity [8, 9]. So there is still a need to develop PDE inhibitors for the treatment of male erectile dysfunction.
In 2002 Chiou and Chen [10] reported evodiamine (quinazoline alkaloid) produced a concentration-dependent relaxation in the isolated rabbit corpus cavernosum precontracted with phenylephrine. Also, yohimbine (indole alkaloid) is claimed to have aphrodisiac activity by blocking inhibitory α2-adrenoreceptors and exerts a facilitatory action on the ejaculatory response of sexual behavior in rats [11].
Our interest was mainly focused on condensed thienopyrimidines due to its wide range of biological activity [12–16]. We have reported thieno(2,3-d)pyrimidine derivatives as antihyperlipidemic [17] Gefitinib analogues [18]. Very recently we have identified condensed thieno(2,3-d)pyrimidine derivatives as bioisosteres of rutaecarpine possessing antihypertensive activity. We found structural similarities between our reported test compounds with yohimbine and evodiamine (Fig. 1). So it was thought logically to screen this potential moiety for phosphodiesterase inhibition from our virtual database. In continuation to our ongoing work on condensed thienopyrimidine and to explore its biochemical diversity, we herein report for the first time the novel analogue of condensed thienopyrimidines and their effect on sexual behavior in male rats hitherto unreported.
Fig. 1.
Structural similarity between evodiamine, yohimbine, tadalafil, and MKAC9
Materials and methods
Chemistry
Designing approach
A series of hundred molecules from our virtual library belonging to condensed thienopyrimidines and quinazolines was subjected to molecular docking studies to know its binding affinity towards phosphodiesterase5 (PDB code: 1XOZ). Tadalafil and evodiamine were docked into active site of phosphodiesterase5. Molecular modelling is usually applied with the intent of designing a more specific ligand with higher affinity.
Molecular docking studies
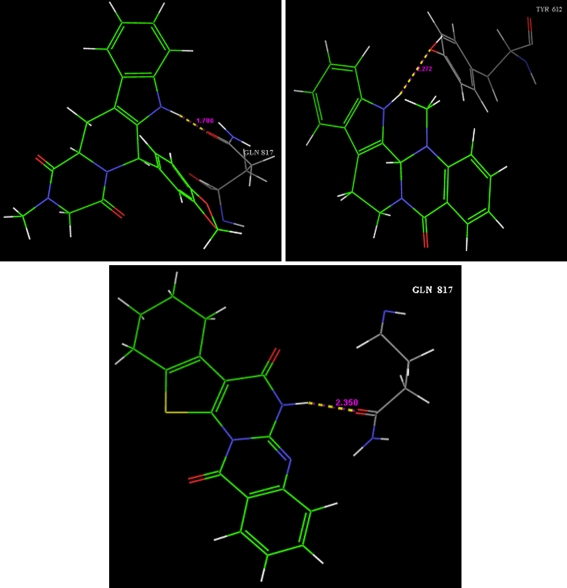
General experimental procedure for docking studies Molecular docking studies were performed with Schrodinger (Maestro 3.5) molecular docking software. Molecules were docked on phosphodiesterase5 (PDB code: 1XOZ) cocrystallised with tadalafil. According to literature survey of enzyme–ligand interaction, it is found that the hydrogen bond donor group is required for the interaction of ligand with Gln 817. So we docked tadalafil and structurally similar molecules from our database with PDE5. However many molecules has shown hydrogen bond formation with Gln 817, which is favourable for PDE5 selective activity. The main binding force between tadalafil and the PDE5 (Fig. 2) is a hydrogen bond between NH of tadalafil and –COOH of Gln 817 of PDE5. Interaction of evodiamine with PDE5 is depicted in Fig. 2. After docking G scores are obtained and depicted in Table 1. Out of the series, MKAC9 had shown hydrogen bond formation with Gln 817 along with a good G score (Fig. 2).
Fig. 2.
Interaction of tadalafil, evodiamine, and MKAC9 with PDE5, respectively
Table 1.
G score of the selected compounds from the database
| Title | Pose | G score | E models | H bonds | GVWF | BVWF | UVWF |
|---|---|---|---|---|---|---|---|
| Tadalafil | 191 | −8.40 | −68.7 | 1 | 282 | 13 | 1 |
| MKAC9 | 154 | −7.64 | −59.6 | 1 | 238 | 3 | 0 |
| MKAC6 | 311 | −7.46 | −55.2 | 1 | 227 | 3 | 0 |
| Evodiamine | 205 | −7.43 | −61.9 | 1 | 228 | 5 | 0 |
| MKAC20 | 378 | −7.41 | −49.6 | 0 | 198 | 2 | 0 |
| MKAC24 | 293 | −6.85 | −52.3 | 0 | 196 | 6 | 0 |
| MKAC30 | 384 | −6.73 | −47.6 | 0 | 164 | 2 | 0 |
| MKAC15 | 390 | −6.06 | −60.0 | 0 | 192 | 4 | 0 |
| MKAC23 | 120 | −6.03 | −61.7 | 1 | 186 | 7 | 0 |
| Sildenafil | 327 | −5.76 | −74.4 | 1 | 337 | 22 | 1 |
| MKAC12 | 279 | −5.58 | −51.1 | 0 | 181 | 6 | 0 |
| MKAC10 | 196 | −5.51 | −54.3 | 0 | 203 | 3 | 1 |
GVWF good van der Waals forces, BVWF bad van der Waals forces, UVWF ugly van der Waals forces
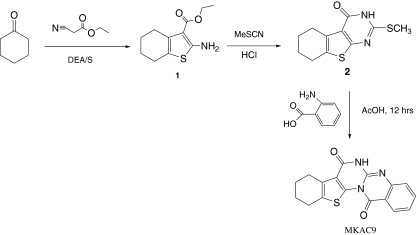
Synthesis and characterization of MKAC9
The starting material ethyl 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate 1 and intermediate 2 were prepared as per our reported method [19, 20] (Scheme 1).
Scheme 1.
Synthetic scheme for MKAC9
A mixture of 2 (6.0 g, 1 mol) and anthranilic acid (3.0 g, 1 mol) was refluxed in acetic acid for 12 h. Progress of the reaction was monitored by TLC. On completion the reaction mixture was poured into ice-cold water. The solid separated was filtered, washed, dried, and recrystallised from methanol. M.F. C17H13N3O2S, mol wt. 323.37, M.p. 249–251 °C, percentage yield 70%, IR: 3,404, 3,301, 2,488, 1,647, and 1,575 cm−1. 1H NMR, δ parts per million: 1.86 (4H, d, J = 6 Hz, CH2 of cyclohexene), (2H, s, CH2 of cyclohexene), 3.02 (2H, s, CH2 of cyclohexene), 7.15 (t, 1H, J = 8.4), 7.61 (t, 1H, J = 8.4), 8.14 (d, 1H, J = 8.1), 8.72 (d, 1H, J = 8.1), 11.07 (s, 1H, -NH). Mass (EI) m/z: 325(M+), 304, 303, 290, 281, 275, 263, 253, 237 (100%), 205, 161, 118.
Pharmacologic evaluation
Drugs and chemicals
Ketamine injection (Hypnoket, Chandra Bhagat Pharma Pvt. Ltd, Mumbai), xylazine hydrochloride, framycetin cream (Soframycin; Aventis Pharma Ltd., Goa), tadalafil (Tadacip; Cipla Mumbai, India), sesame oil, propylene glycol, and cotton thread were purchased from the local market. Estradiol benzoate AR and progesterone AR were purchased from the Rajesh Chemicals, Mumbai, while urethane was purchased from HiMedia Laboratories Pvt. Ltd., Mumbai.
Drug preparation
Accurately weighed quantity of drug was dissolved in distilled water (along with 0.1 ml of DMSO) to prepare the appropriate stock solution of the drug, i.e., MKAC9 10 mg/kg/10 ml and MKAC9 20 mg/kg/10 ml, respectively. The doses were administered by selecting the appropriate concentration of the stock solution.
Animals
Healthy male and female Wistar rats (150–200 g) and Swiss albino mice (18–22 g) were housed in CPCSEA-approved animal house in groups of six in polypropylene cages. They were maintained at 25 ± 2 °C, relative humidity of 45–55% and under standard environmental conditions (12-h light and 12-h dark cycle). The animals had free access to food (Chakan Oil Mills, Pune, India) and water ad libitum throughout the study. All the procedures were performed in accordance with the institutional ethical committee constituted as per the directions of the CPCSEA (protocol no: CPCSEA/IAEC/PC-01/06-2K9). The study was carried out between 4:00 and 7:00 pm.
Acute toxicity test
Acute toxicity study was performed in healthy adult male albino mice (18–22 g) as per guidelines suggested by the Organization for Economical Cooperation and Development. The mice were observed continuously for 4 h for behavioral and autonomic profiles and for any other sign of toxicity or mortality up to a period of 14 days.
Surgery
All female rats were ovariectomized (OVX) 15 days prior to study using standard aseptic surgical techniques and under deep anesthesia [21], using ketamine and xylazine. All females received at least 1 week of postoperative care prior to initiation of experiment.
Induction of behavioral estrus
For induction of behavioral estrus, OVX female rats were subcutaneously administered with 25 μg estradiol benzoate (EB; in 0.1 ml sesame oil) 48 h prior to behavioral testing and 500 μg of progesterone (P; in 0.1 ml propylene glycol) 5 h before testing [21].
Mating behavior test
The male rats were randomly assigned to one of the following groups (N = 06 per group): control group received vehicle, two test groups received separate doses of MKAC9 (i.e., 10 and 20 mg/kg) and standard group which received tadalafil (10 mg/kg) for the period of 7 days. The route of administration for all treatment was oral by intragastric tube. The male rat's sexual behavior test was performed 60 min after respective treatment at seventh day. Mating behavior was observed according to the Guideline on Experimental (Preclinical) Studying of New Pharmacological Substances (2005) and the standard procedure by Agmo [21]. The sexual behavior of males was tested during the period of darkness (between 4:00 and 7:00 pm) in a sound-proof room, under a dim red light. After a 10-min adaptation period in a circular observation cage (fitted with IR tracking video camera), a receptive female (in estrous state) was presented to the treated male by dropping it gently into the cage. The receptivity of the female animals was confirmed before the test by exposing them to male animals, other than the control, test, and standard animals.
The following behavioral parameters were noted for the period of 30 min: mount latency (ML) and intromission latency (IL), the time from introduction of the female to the occurrence of the first mount or intromission; ejaculation latency, the time from the first intromission to ejaculation; postejaculatory interval (PEI), the time from ejaculation to the subsequent intromission; mount and intromission frequencies, the number of mounts and intromissions preceding ejaculation; and copulatory efficacy (hit rate), a measure of intromissive success.
Statistical analysis
The comparison was made against the vehicle-treated control group, and the data was expressed as mean ± SEM. The data were analyzed by one-way ANOVA followed by post hoc Dunnett's test using InStat software. The level of significance was p < 0.05 (Graph Pad Instat, USA, 2000).
Results
Acute oral toxicity test
All mice were free of any toxicity as per acceptable range given by the OECD guidelines up to the dose of 2,000 mg/kg. So, the LD50 could be greater than 2,000 mg/kg.
Male copulatory test
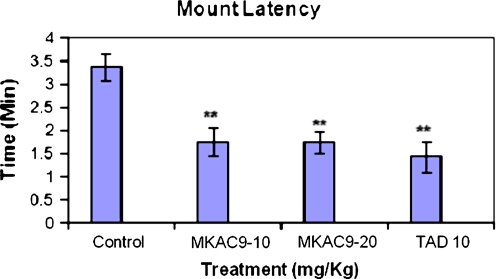
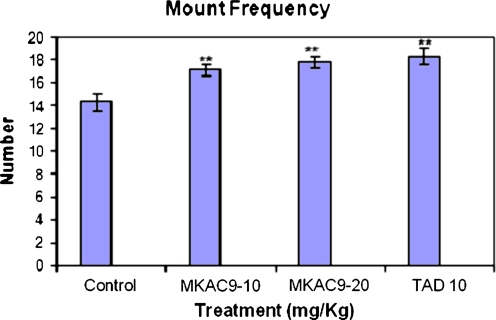
Mount latency and mount frequency
There was significant improvement in the mount latency and frequency (MF) of the male rats treated with MKAC9 (10 and 20 mg/kg) and standard tadalafil (**p < 0.01; Table 2, Figs. 3 and 4).
Table 2.
ML and MF for the male rats after MKAC9 treatment
| Treatment (mg/kg) | Mount latency | Mount frequency | |
|---|---|---|---|
| Acute treatment | Control | 3.38 ± 0.287 | 14.33 ± 0.714 |
| MKAC9-10 | 1.75 ± 0.313** | 17.16 ± 0.477** | |
| MKAC9-20 | 1.74 ± 0.226** | 17.83 ± 0.477** | |
| TAD10 | 1.43 ± 0.344** | 18.33 ± 0.714** | |
**p < 0.01
Fig. 3.
The mount latency shown by male rats after MKAC9 treatment [results are expressed as mean ± SEM (n = 6); data were analyzed by one-way analysis of variance followed by Dunnett's test; *p < 0.05, **p < 0.01]
Fig. 4.
The mount frequency shown by male rats after MKAC9 treatment [results are expressed as mean ± SEM (n = 6); data were analyzed by one-way analysis of variance followed by Dunnett's test; *p < 0.05, **p < 0.01]
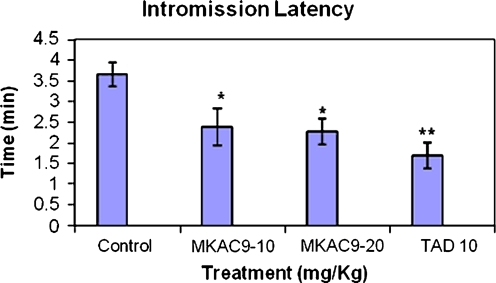
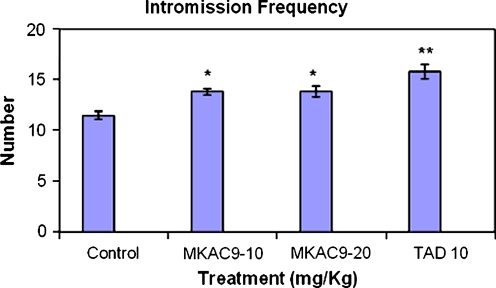
Intromission latency and intromission frequency
In case of IL and count factor, the test compound showed significant results as shown in following table (*p < 0.05; Table 3, Figs. 5 and 6).
Table 3.
IL and intromission frequency for the male rats after MKAC9 treatment
| Treatment (mg/kg) | Intromission latency | Intromission frequency | |
|---|---|---|---|
| Acute treatment | Control | 3.65 ± 0.285 | 11.5 ± 0.428 |
| MKAC9-10 | 2.38 ± 0.442* | 13.83 ± 0.307* | |
| MKAC9-20 | 2.27 ± 0.318* | 13.83 ± 0.542* | |
| TAD10 | 1.69 ± 0.320** | 15.83 ± 0.703** | |
*p < 0.05, **p < 0.01
Fig. 5.
Intromission latency shown by male rats after MKAC9 treatment [results are expressed as mean ± SEM (n = 6); data analyzed by one-way analysis of variance followed by Dunnett's test; *p < 0.05, **p < 0.01]
Fig. 6.
Intromission frequency shown by male rats after MKAC9 treatment [results are expressed as mean ± SEM (n = 6); data were analyzed by one-way analysis of variance followed by Dunnett's test; *p < 0.05, **p < 0.01]
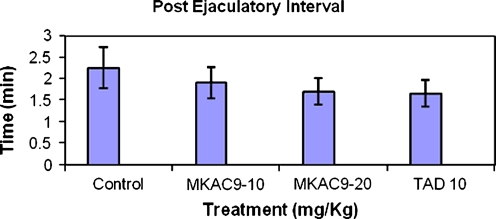
Postejaculatory interval
No any significant change was seen in the postejaculatory interval of male rats from any of the treatments, as compared with control group (Table 4, Fig. 7).
Table 4.
PEI for the male rats after MKAC9 treatment
| Treatment (mg/kg) | Postejaculatory interval | |
|---|---|---|
| Acute treatment | Control | 2.25 ± 0.479 |
| MKAC9-10 | 1.91 ± 0.363 | |
| MKAC9-20 | 1.71 ± 0.312 | |
| TAD10 | 1.66 ± 0.308 | |
Fig. 7.
Postejaculatory interval shown by male rats after MKAC9 treatment [results are expressed as mean ± SEM (n = 6); data were analyzed by one-way analysis of variance followed by Dunnett's test; *p < 0.05, **p < 0.01]
Discussion
The extensive literature survey for various traditional claims and scientific documentation indicated rutaecarpine derivatives of Evodia rutaecarpa, and other species of the Rutaceae family are biologically active and can be used a good target to improve overall therapeutic outcome [22]. In the present investigation, a novel synthetic rutaecarpine derivative which was proposed to be a phosphodiesterase5 inhibitor (MKAC9) was screened for its effect on the sexual behavior in laboratory rats.
Further, the effect of MKAC9 on sexual behavior in rats was investigated employing pharmacological model of mating behavior (i.e., copulatory test). The rodent model was selected for this study since the basic neural and behavioral mechanisms controlling sexual desire and motivation are similar in rodents and in humans [23]. Also, it is a valid reliable model of rodent in sexual motivation which was proved to be of great utility for studying the behavioral and neurobiological basis of sexual motivation as well as sexual dysfunction [23].
All the female rats used in the experiment were OVX 1 month prior to their use [21] to avoid any unwanted pregnancies and any hormonal imbalances during the course of experiment [24]. Also, female rat sexual behavior is tightly linked to ovulation and the synchronous timing for both events is controlled by the hypothalamic–pituitary–gonadal axis with estrogen as the ultimate conductor. For copulatory test, unlike primates, normally cycling female rats show sexual receptivity only during the estrous (ovulatory) portion of the reproductive cycle. Removal of the ovaries leads to immediate cessation of her sexual behavior which can be further restored by treatment with EB and P [25]. Thus in this study, various sexual behaviors were observed in OVX female rats primed with estradiol benzoate and progesterone.
Several studies suggest that sexual behavior is produced by interaction among at least four distinct mechanisms, probably involving different neural structures, initially named by [26] as initiation factor (arousal component), intromission count factor, hit rate factor, and copulatory rate factor, respectively. Contributing to the initiation factor are the mount and intromission latencies; contributing to the intromission count factor is the intromission number; contributing to the hit rate factor is the copulatory efficiency; and contributing to the copulatory rate factor are ejaculation latency and postejaculatory interval [26–28]. Thus, mount and intromission latencies allow the evaluation of the sexual response arousal component. The intromission number and the copulatory efficiency allow evaluation of the erectile response. The ejaculatory latency and postejaculatory interval allow the evaluation of the ejaculatory component of sexual behavior [28]. Therefore, each component of the male rat sexual response can be evaluated independently from the others.
In the present study, the evodiamine derivative at the doses 10 and 20 mg/kg was investigated for its effect on the sexual behavior in male Wistar rats. Tadalafil (at dose 10 mg/kg) was used as a standard in this protocol. It was found that the evodiamine derivative improved the sexual arousal component of the rats as indicated by the reduced mount and intromission latencies. The test doses significantly improved the MF along with tadalafil-treated group. Also, the intromission count factor (erectile component) was improved by the evodiamine derivative treatment as compared with the control group. There was no any significant change in the parameter of ejaculation latency suggesting the unaffected ejaculatory pattern (results not shown). The hit rate factor and the copulatory rate factor were unaffected by any of the treatments of test compound or standard tadalafil group (results not shown). Hit rate is the ratio between number of true intromissions divided by the sum of true and false intromissions [29]. ML and IL are indicative of sexual arousal. A decrease in these parameters is indicative of enhanced sexual arousal [24]. PEI, the time period immediately after ejaculation during which sexual activity is minimal or negligible, is indicative of sexual vigor [24]. There was no any significant change in the postejaculatory interval shown by male rats.
Conclusion
The test compound MKAC9 (an evodiamine derivative) could be of promising importance in the treatment of sexual disorders like desire disorder or erectile dysfunction and may be an interesting hit for the development of aphrodisiac activity. The test compounds did not exhibit toxicity in vivo; however, it deserves further investigation for in vitro activity and identification of its mechanism of action. Further work is in progress to improve the chemical properties of the series.
Acknowledgment
Authors are also thankful to Dr. AR Madgulkar, the principal, for the continuous motivation, support, and providing the necessary infrastructure to carry out this work.
References
- 1.Gonzalez M, Viafara G, Caba F, Molina T, Ortiz C. Libido and orgasm in middle-aged woman. Maturitas. 2006;53:1–10. doi: 10.1016/j.maturitas.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Panel on Impotence. JAMA. 1993;270:83. doi: 10.1001/jama.1993.03510010089036. [DOI] [PubMed] [Google Scholar]
- 3.Laumann EO, Paik A, Rosen RC. Related sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;28:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 4.Cirino G, Fusco F, Imbimbo C, Mirone V. Pharmacology of erectile dysfunction in man. Pharmacol Ther. 2006;111:400–423. doi: 10.1016/j.pharmthera.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Ignarro LJ. Nitric oxide: a unique endogenous signaling molecule in vascular biology. Biosci Rep. 1999;19:51–71. doi: 10.1023/A:1020150124721. [DOI] [PubMed] [Google Scholar]
- 6.Miner MM, Seftel AD (2007) Centrally acting mechanisms for the treatment of male sexual dysfunction. Urol Clin North Am 34:483–496 [DOI] [PubMed]
- 7.Corbin JD, Francis SH. Cyclic GMO phosphodiesterase 5: target for sildenafil. J Biol Chem. 1999;274:13729–13732. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 8.Kim DK, Lee N, Lee JY, Ryu DH, Kim J, Lee S, Choi J, Chang K, Kim Y, Im G, Choi W, Kim T, Ryu J, Kim N, Lee K. Synthesis and phosphodiesterase 5 inhibitory activity of novel phenyl ring modified sildenafil analogues. Bioorg Med Chem. 2001;9:1609–1616. doi: 10.1016/S0968-0896(01)00055-4. [DOI] [PubMed] [Google Scholar]
- 9.Boyle CD, Xu R, Asberom T, Chackalamannil S, Clader JW, Greenlee WJ, Guzik H, Hu Y, Hu Z, Lankin CM, Pissarnitski DA, Stamford AW, Wang Y, Skell J, Kurowski S, Vemulapalli S, Palamanda J, Chintala M, Wu P, Myers J, Wang P. Optimization of purine based PDE1/PDE5 inhibitors to a potent and selective PDE5 inhibitor for the treatment of male ED. Bioorg Med Chem Lett. 2005;15:2365–2369. doi: 10.1016/j.bmcl.2005.02.083. [DOI] [PubMed] [Google Scholar]
- 10.Chiou WF, Chen CF. Pharmacological profile of evodiamine in isolated rabbit corpus cavernosum. Eur J Pharmacol. 2002;446:151–159. doi: 10.1016/S0014-2999(02)01762-4. [DOI] [PubMed] [Google Scholar]
- 11.Drewes SE, George J, Khan F. Recent findings on natural products with erectile-dysfunction activity. Phytochemistry. 2003;62:1019–1025. doi: 10.1016/S0031-9422(02)00621-0. [DOI] [PubMed] [Google Scholar]
- 12.Katada J, Iijima K, Muramatsu M, Takami M, Yasuda E, Hayashi M, Hattori M, Hayashi Y. Cytotoxic effects of NSL-1406, a new thienopyrimidine derivative, on leukocytes and osteoclasts. Bioorg Med Chem Lett. 1999;9:797–802. doi: 10.1016/S0960-894X(99)00088-8. [DOI] [PubMed] [Google Scholar]
- 13.Oganisyan AS, Noravyan AS, Dzhagatspanyan IA, Akopyan AG. Condensed thienopyrimidine derivatives. Part 20: synthesis and neurotropic activity of a series of new pyrano[4′,3′:4,5]thieno[3,2-e]imidazolidino-[2,1-b]pyrimidines. Pharm Chem J. 2001;3:650–652. doi: 10.1023/A:1015384228176. [DOI] [Google Scholar]
- 14.Litvinov VP. Thienopyrimidines: synthesis, properties, and biological activity. Russ Chem Bull. 2004;53:487–516. doi: 10.1023/B:RUCB.0000035630.75564.2b. [DOI] [Google Scholar]
- 15.Bhuiyan MMH, Rahman KMM, Hossain MK, Rahim A, Hossain MI, Naser MA. Synthesis and antimicrobial evaluation of some new thienopyrimidine derivatives. Acta Pharm. 2006;56:441–450. [PubMed] [Google Scholar]
- 16.Luke RWA (2010) Novel thienopyrimidine and thiazolopyrimidine kinase inhibitors with activity against Tie-2 in vitro and in vivo. ChemInform. doi:10.1002/chin.201013176 [DOI] [PubMed]
- 17.Kathiravan MK, Shishoo CJ, Roy SK, Mahadik KR, Kadam SS, Jain KS. Synthesis and antihyperlipidemic activity of some novel condensed 2-chloroalkyl-4-chloro/hydroxy-5,6-disubstituted pyrimidines. Arzneimittelforschung. 2007;57:599. doi: 10.1055/s-0031-1296655. [DOI] [PubMed] [Google Scholar]
- 18.Phoujdar MS, Kathiravan MK, Bariwal JB, Shah AK, Jain KS. Total microwave based synthesis of novel thienopyrimidine bioisosteres of gefitinib. Tetrahedron Lett. 2008;49:1269. doi: 10.1016/j.tetlet.2007.11.135. [DOI] [Google Scholar]
- 19.Kathiravan MK, Shishoo CJ, Chitre TS, Mahadik KR, Jain KS. Efficient synthesis of substituted 2-amino-3-carbethoxythiophenes. Synth Commun. 2007;37:4273. doi: 10.1080/00397910701575889. [DOI] [Google Scholar]
- 20.Jain KS, Bariwal JB, Phoujdar MS, Amrutkar RD, Munde MK, Tamboli RS, Khedkar SA, Khiste RH, Vidyasagar NC, Dabholkar VV, Kathiravan MK (2008) A novel microwave assisted green synthesis of condensed 2-substituted pyrimidin-4(3h)-ones under solvent free conditions. J Heterocycl Chem. doi:10.1002/jhet.30
- 21.Agmo A. Male rat sexual behavior. Brain Res Prot. 1997;1:203–209. doi: 10.1016/S1385-299X(96)00036-0. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14:1852–1859. doi: 10.3390/molecules14051852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agmo A, Turi AL, Ellingsen E, Kaspersen H. Preclinical models of sexual desire: conceptual and behavioral analyses. Pharmacol Biochem Behav. 2004;78:379–304. doi: 10.1016/j.pbb.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Kenjale R, Shah R, Sathaye S. Effect of Chlorophytum borivilianum on sexual behavior and sperm count in male rats. Phytother Res. 2008;22:796–801. doi: 10.1002/ptr.2369. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar JS, Hiegel C, Ginis GE, Hilbun E, Uphouse L. Subchronic treatment with fluoxetine attenuates effect of acute fluoxetine on female rat sexual behavior. Brain Res. 2008;1190:56–64. doi: 10.1016/j.brainres.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachs BD, Barfield RT. Temporal patterning of sexual behavior in the male rat. J Comp Physiol Psychol. 1970;73:359–364. doi: 10.1037/h0030243. [DOI] [PubMed] [Google Scholar]
- 27.Sachs BD. Conceptual and neural mechanisms of masculine copulatory behavior. In: McGill TE, Dewsbury DA, Sachs BD, editors. Sex and behavior: status and prospectus. New York: Plenum; 1978. pp. 267–295. [Google Scholar]
- 28.Ferraz MR, Ferraz MMD, Santos R. How REM sleep deprivation and amantadine affects male rat sexual behavior. Pharmacol Biochem Behav. 2001;69:325–332. doi: 10.1016/S0091-3057(01)00508-1. [DOI] [PubMed] [Google Scholar]
- 29.Hassan AA, Hassouna MM, Taketo T, Gagnon C, Elhilali MM. The effects of diabetes on sexual behavior and reproductive tract function in male rats. J Urol. 1993;149:148–154. doi: 10.1016/s0022-5347(17)36028-7. [DOI] [PubMed] [Google Scholar]