Abstract
Objectives
To assess the effects of N-acetylcysteine (NAC) on organism viability in planktonic and biofilm phases, biofilm thickness, and extracellular polysaccharide content.
Methods
We performed time-kill curves and broth macrodilution assays of bacterial and fungal clinical isolates with varying concentrations of NAC. We also created in-vitro bacterial biofilms, incubated them with NAC or control, and then stained with propidium iodide and FITC-labelled concanavalin A. We measured biofilm thickness, number of non-viable cells, and fluorescent intensity as a marker of extracellular matrix via a confocal laser scanning microscope. All experiments were conducted in triplicate. Tested organisms included methicillin-sensitive and - resistant Staphylococcus aureus (MSSA, MRSA), S. epidermidis, vancomycin-resistant Enterococcus faecalis (VRE), Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella pneumoniae, Candida albicans and C. krusei.
Results
NAC 80 mg/ml was uniformly bactericidal (>99.9% reduction) against all tested bacteria with no recoverable organisms after 30 minutes of incubation, but was fungistatic against candida species. Minimum inhibitory and bactericidal concentrations of NAC ranged from 5–10 mg/ml. Biofilm thickness was significantly decreased in NAC-treated biofilms for all organisms except VRE. The number of non-viable cells in NAC-treated Gram-positive biofilms was increased (p<0.05 for MRSA and VRE). NAC-treated Gram-negative biofilms had scant cellularity and lacked complex 3-dimensional structures that were characteristic of controls. Fluorescent intensity was similar in the experimental and control arms.
Conclusions
NAC is bactericidal against clinically relevant and drug-resistant bacteria and also leads to biofilm disruption. NAC has the potential for use as a novel agent for prevention or treatment of biofilm-related infections.
Keywords: N-acetylcysteine, biofilm, time-kill curves
Introduction
Device-associated infections arise from biofilms, which comprise microorganisms embedded within an extracellular polysaccharide matrix on the device surface. A biofilm can develop as early as 24 hours after placement of devices, including vascular catheters.(1) Such infections are often refractory to treatment with antibiotics alone, and at times, device removal is the only way to ensure cure. However, device removal is not always feasible or practical, and considerably inflates expense. Systemic antibiotics are able to clear planktonic organisms that have been released from the biofilm but often are unable to effectively treat biofilm-embedded organisms. This may be related to the degree of penetration of various antibiotics into the biofilm, changes in bacterial metabolism, antimicrobial resistance, impaired host defenses, and local alterations in the microenvironment of the biofilm that impair the activity of the antimicrobial agent.(2) The minimal inhibitory concentrations for biofilm-embedded bacteria can be up to 1000-fold higher than those pertaining to the same organisms when grown in broth medium.(2)
We have previously demonstrated, both in-vitro and in a pilot clinical trial, the role of N-acetylcysteine (NAC) in the treatment of bacterial biofilms on the surface of intravascular catheters.(3, 4) Prompted by the in-vitro observation of a synergistic response when combining NAC with tigecycline,(3) we conducted a pilot trial of 18 hemodynamically stable patients with hemodialysis catheter-associated bacteremia who were treated with systemic antibiotics plus a catheter lock solution that consisted of NAC, tigecycline, and heparin. We noted a success rate (defined as absence of persistent or recurrent bacteremia with 90 days of follow-up) of 83%. Thus, NAC appears to be a promising, non-antibiotic alternative to the treatment of biofilm-associated infections. The goal of the current experiment was to assess the effects of NAC on organism viability in the planktonic and biofilm phases, biofilm thickness, and extracellular polysaccharide content.
Materials and Methods
Experiment 1
We performed time-kill curves of clinical isolates that had caused device-associated infections, including methicillin-sensitive and -resistant Staphylococcus aureus (MSSA, MRSA), vancomycin-resistant Enterococcus faecalis (VRE), Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella pneumoniae, Candida albicans, and C. krusei. Overnight suspensions of these organisms were stored in tryptic soy broth (TSB) with 15% glycerol at −80°C until ready for use. Organisms at a starting concentration of 104–105 CFU/ml were incubated at 37°C in 5 ml of TSB alone as control, and in broth that contained NAC (Sigma-Aldrich, St. Louis, USA) at a concentration of 0.8 mg/ml, 8 mg/ml, and, 80 mg/ml. We specifically used these concentrations of NAC because we had previously demonstrated that a NAC concentration of 80 mg/ml is both effective against biofilm-embedded organisms and easily achievable in a catheter lock solution using commercially available NAC.(3, 4) Hundred µl aliquots of the microbial suspensions and serial dilutions were cultured on blood agar plates at baseline, 0.5, 1, 2, 4, 6, 8, and 24 hours after incubation. Each experiment was performed in triplicate and the mean CFU/ml at each time point was calculated. The detectability limit was 10 CFU.
Experiment 2
We conducted a broth macrodilution assay to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) or minimum fungicidal concentration (MFC) of NAC for the same isolates as above (MSSA, MRSA, VRE, P. aeruginosa, E. cloacae, K. pneumoniae, C. albicans and C. krusei). We prepared serial dilutions of NAC in Mueller-Hinton broth with a starting concentration of 160 mg/ml and then inoculated 10 µl of a microbial suspension that contained 108 CFU/ml. The MIC was read visually as the first clear tube after incubation for 24–48 hours. Ten µl aliquots of the original suspension and serial dilutions were then cultured to arrive at the MBC/MFC (defined as the lowest NAC concentration at which > 99.9% of inoculated organisms were killed). Each experiment was performed in triplicate.
Experiment 3
We formed biofilms of MSSA, MRSA, methicillin-resistant S. epidermidis (MRSE), VRE, P. aeruginosa, E. cloacae and K. pneumoniae. We did not perform microscopic work on candida species. Two ml aliquots of a bacterial suspension of 105 CFU/ml in TSB were inoculated into each well of a 6-well round-bottom tissue culture plate (BD, Franklin Lakes, USA) and incubated on a rocker at 37°C. After 24 hours of incubation, the broth was replaced with either TSB containing NAC 80 mg/ml or TSB alone as control and then incubated for another 24 hours on a rocker at 37°C. The wells were then rinsed once with 1 ml of phosphate buffered saline (PBS) per well and then stained with 1 ml per well of a solution of 15 µM/ml propidium iodide (Sigma-Aldrich Corp., St. Louis, USA) which stained non-viable cells red. After 5-minute incubation in the dark on a rocker at 37°C, the wells were drained and rinsed with 1 ml of PBS, and then incubated with 1 ml of a solution of 50 µg/ml FITC-labelled concanavalin A type IV (Sigma-Aldrich Corp., St. Louis, USA) which stained extracellular polysaccharide green. After 5 minutes of incubation on a rocker in the dark at 37°C, the wells were drained and rinsed with 1 ml of PBS. We used an upright confocal laser scanning microscope Nikon Eclipse 90i ® (Nikon Inc., Melville, USA) equipped with an argon-krypton laser to view five non-contiguous areas in each well using a 60× dipping lens. We examined the fluorescence properties of Texas Red (excitation 596 nm / emission 620 nm for propidium iodide) and FITC (excitation 495 nm / emission 520 nm for concanavalin A). We used scan sizes of 2048 (resolution of 0.10 µm/pixel) and 1024 (resolution of 0.21 µm/pixel). Each experiment was conducted in triplicate. We measured biofilm thickness (Z-stack images), counted the number of red non-viable bacterial cells, and, measured green fluorescent intensity as a marker of extracellular polysaccharide in the biofilm matrix. The digital images were viewed, collected, and analyzed using commercially available software NIS Elements Advanced Research (Nikon Inc., Melville, USA). Merged red and green images were obtained in a single .jpeg2000 and .nd2 format and were converted to .eps files for publication.
Statistical analysis
Colony counts from the time-kill experiments were compared via the Mann-Whitney U test. All continuous variables were analyzed using a Student’s t-test. A p-value <0.05 was considered statistically significant. Excel and NIS Elements Advanced Research were used for analysis and Excel for graphical representation.
Results
Experiment 1
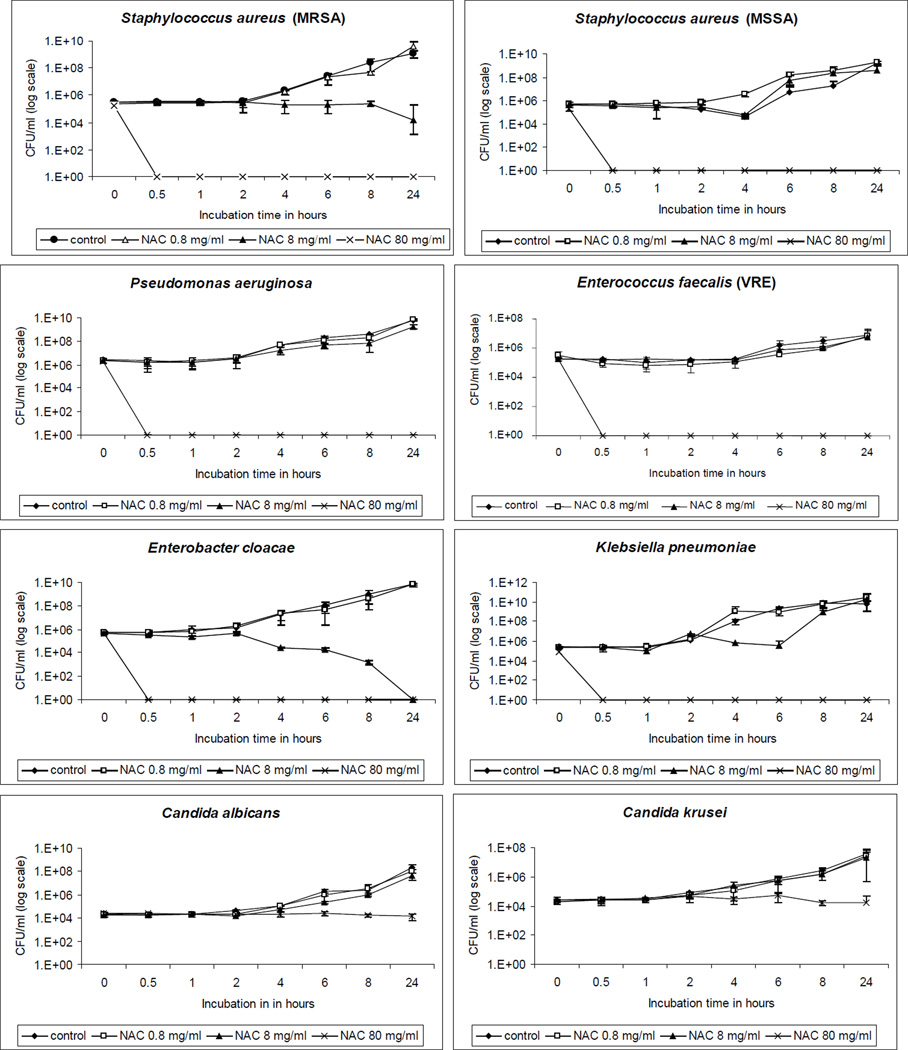
NAC at a concentration of 80 mg/ml, which is generally lower than clinically utilized concentrations, was uniformly bactericidal (>99.9% reduction) against all tested bacterial species (Figure 1), with no recoverable bacteria after 30-minute incubation. However, this NAC concentration was fungistatic against C. albicans and C. krusei. Bacterial incubation for 24 hours with 8 mg/ml of NAC led to >99.9% reduction in E. cloacae and a significant 1-log decrease in MRSA (p< 0.01).
Figure 1.
Time-kill curves of tested organisms with N-acetylcysteine (NAC). The y-axis denotes the mean CFU/ml and error bars signify standard deviation.
Experiment 2
Results of the broth macrodilution assays are displayed in Table 1. The MIC for most bacteria was 5 mg/ml. In general, the MIC/MBC of NAC for all tested bacteria was either the same or only a dilution apart, attesting to the bactericidal nature of this drug. In contrast, the MFC of NAC for C. albicans was 4-fold higher than its MIC.
Table 1.
Minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC) of N-acetylcysteine (NAC) for tested organisms. Each test was conducted in triplicate and the results of the 3 observations for each test were all identical.
| Organism | MIC (mg/ml) | MBC/MFC (mg/ml) |
|---|---|---|
| MRSA | 5 | 10 |
| MSSA | 5 | 10 |
| VRE | 10 | 10 |
| P. aeruginosa | 5 | 5 |
| E. cloacae | 5 | 5 |
| K. pneumoniae | 5 | 10 |
| C. albicans | 40 | 160 |
| C. krusei | 20 | 20 |
MRSA – methicillin-resistant Staphyloccus aureus
MSSA – methicillin-sensitive S. aureus
VRE – vancomycin-resistant Enterococcus faecalis
Experiment 3
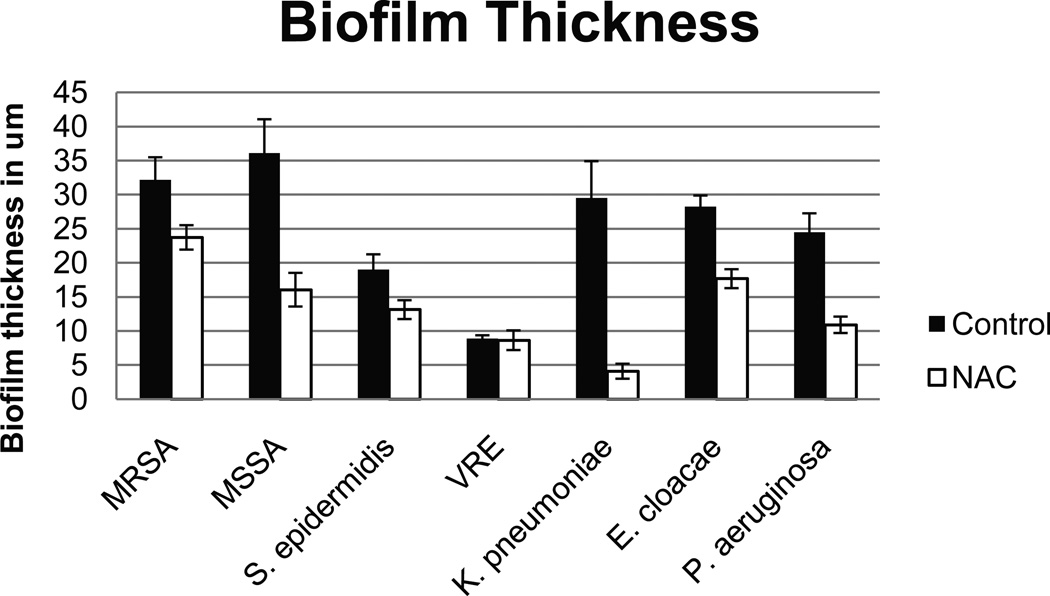
Biofilm Thickness
We observed a statistically significant decrease in biofilm thickness of NAC-treated wells vs. controls for all organisms (p<0.05), except for VRE. Figure 2 shows representative Z-stack images of control and NAC-treated P. aeruginosa biofilm. Figure 3 illustrates the biofilm thickness for all tested organisms.
Figure 2.
Z-stack images of Pseudomonas aeruginosa in control group (top panel) and NAC-treated group (lower panel).
Figure 3.
Mean biofilm thickness in µm of 24-hour biofilms of bacterial species when exposed to tryptic soy broth (TSB) alone as control or TSB containing 80 mg/ml of N-acetylcysteine (NAC). Thickness was measured by means of Z-stack imaging with a confocal laser scanning microscope. All differences between individual control and NAC-treated biofilms were statistically significant (p<0.05), except for VRE.
MRSA – methicillin-resistant Staphyloccus aureus
MSSA – methicillin-sensitive S. aureus
VRE – vancomycin-resistant Enterococcus faecalis
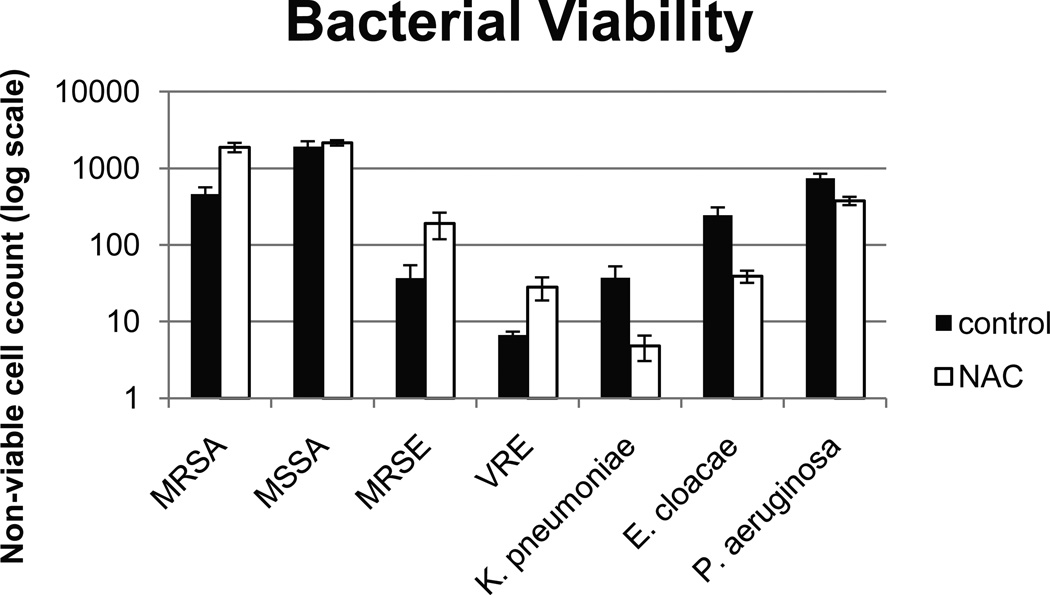
Bacterial Viability
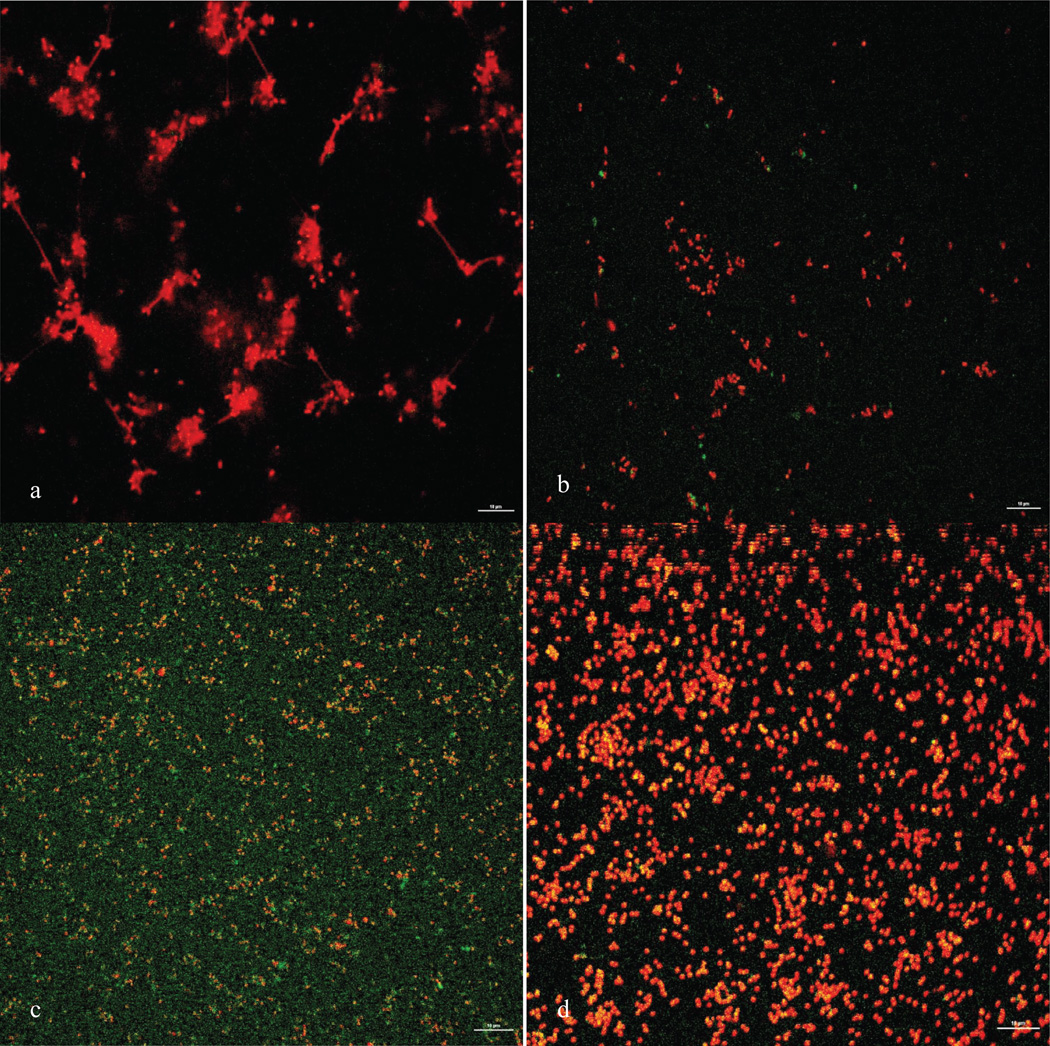
We noted an increase in the number of non-viable cells in the NAC-treated wells of Gram-positive organisms, but this finding was statistically significant only for MRSA and VRE, with a trend for higher number of non-viable cells for MSSA and MRSE. We noted scant cellularity in the NAC-treated Gram-negative biofilms which also lacked complex 3-dimensional structures that were characteristic of the controls. However, paradoxically, the number of non-viable cells was higher in the control wells. This difference was statistically significant for E. cloacae and P. aeruginosa, with a trend seen in K. pneumoniae. Figure 4 shows representative confocal images of P. aeruginosa and MRSA before and after treatment with NAC. Figure 5 shows a graphical representation of non-viable cells for all tested organisms.
Figure 4.
Confocal images of P. aeruginosa control (top left), NAC-treated (top right) and MRSA control (bottom left) and NAC-treated (bottom right) utilizing a 60× dipping lens. The scale bar represents 10 µm.
Figure 5.
Mean number of non-viable bacterial cells (stained red with propidium iodide when viewed via microscopy) in the control biofilm and NAC-treated biofilm. *Denotes p<0.05.
MRSA – methicillin-resistant Staphyloccus aureus
MSSA – methicillin-sensitive S. aureus
VRE – vancomycin-resistant Enterococcus faecalis
Fluorescence
Green fluorescent intensity, used as a marker of extracellular polysaccharide content, was similar in the experimental and control groups (data not shown).
Discussion
Although prior studies demonstrated that NAC at a concentration of 2 mg/ml inhibited the growth of various industrial-origin bacterial strains (5) and at a concentration of 20 µg/ml was bacteriostatic against various clinical bacterial isolates,(6) the bactericidal activity of higher concentrations of NAC had not been previously assessed. This study demonstrated that NAC at a concentration of 80 mg/ml is bactericidal against a wide range of clinical pathogens, including multi-drug resistant organisms such as VRE and MRSA. A lower NAC concentration of 8 mg/ml was also bactericidal against E. cloacae. Although the exact antimicrobial mechanism for NAC is unclear, it may competitively inhibit bacterial utilization of cysteine or react via its sulfhydryl group with bacterial membranes.
The confocal portion of our experiments demonstrates the anti-biofilm activity of NAC at the microscopic level. We noted significantly decreased biofilm thickness for most organisms when incubated for 24 hours with NAC. At least a portion of this finding could be attributed to bacterial killing, as seen by increased number of non-viable cells in the NAC-treated wells of Gram-positive organisms. The more complex architecture of Gram-negative control biofilms probably made it difficult to count the number of nonviable cells and, perhaps, led to an underestimation while counting. The NAC-treated Gram-negative wells displayed less architectural complexity and were thinner than controls, thus attesting to the anti-biofilm activity of NAC against these organisms as well. Since the amount of polysaccharide staining in the residual biofilm was similar in NAC-treated and control samples, we are unable to comment on NAC’s mucolytic activity. We plan to measure, in the future, polysaccharide degradation products in the effluent of both the control and NAC-treated wells in an effort to better assess NAC’s mucolytic activity, which may contribute to its anti-biofilm activity.
The demonstration in this study of the bactericidal and anti-biofilm activity of NAC allows us to suggest that NAC could potentially be used either alone or in combination with other antimicrobials for prevention or treatment of device-associated infections. In that regard, NAC has been shown to be synergistic with tigecycline,(3) fosfomycin,(7) and carbencillin and ticarcillin.(6) Since the serum levels of NAC that are achieved with intravenous infusions (about 0.035 mg/ml) (8) are much lower than its bactericidal concentration (80 mg/ml), high local concentrations of NAC could be achieved by incorporating NAC within a catheter lock solution or onto the surface of medical devices. Allergic reactions and adverse effects are relatively uncommon (<5–14%) with systemic administration of NAC and are usually seen only with an intravenous loading dose because the risk is dose-related.(9, 10) Since the total amount of NAC that would be incorporated into a catheter lock solution or onto a device surface is several -fold lower than the FDA-approved intravenous dose of NAC for acetaminophen overdose in adults (a loading dose of 150 mg/kg, equivalent to 10.5 gm in a 70-kg subject), the risk of adverse events is conceivably lower with local application of NAC.
The findings of this study indicate that NAC is bactericidal against both Gram-positive and -negative bacteria. Additionally, NAC exposure leads to disruption of clinically relevant and drug-resistant bacterial biofilms. Thus, NAC has the potential for use as a novel anti-biofilm/antimicrobial agent for prevention or treatment of biofilm-related infections.
Acknowledgements
Mohammad Z. Bawany (University of Toledo Medical Center, Toledo, USA) performed some of the time-kill curves.
Financial Support: This work was supported in part by National Institutes of Health / National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, USA. Grant no. K23DK078828-01 A2 awarded to S.A.
Footnotes
The Author(S) state that this manuscript has not been published previously and is not currently being assessed for publication by any journal other than the International Journal of Artificial Organs. Each Author has contributed substantially to the research, preparation and production of the paper and approves of its submission to the Journal.
Conflict of Interest: S.A. does not have any conflicts of interest.
R.O.D. has assigned the rights of a patent describing the use of N-acetylcysteine to combat device-related infections to his employer, Baylor College of Medicine. There is currently no licensing agreement regarding this patent between Baylor College of Medicine and any company, hence, there is no receipt of royalties.
A portion of this manuscript was presented at the 48th Annual Meeting of the Infectious Diseases Society of America. Vancouver, Canada. Oct. 21–24, 2010. Abstract no. 219.
References
- 1.Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis. 1993 Aug;168(2):400–407. doi: 10.1093/infdis/168.2.400. [DOI] [PubMed] [Google Scholar]
- 2.Aslam S. Effect of antibacterials on biofilms. Am J Infect Control. 2008 Dec;36(10):S175, e179–e111. doi: 10.1016/j.ajic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob Agents Chemother. 2007 Apr;51(4):1556–1558. doi: 10.1128/AAC.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Pilot trial of N-acetylcysteine and tigecycline as a catheter-lock solution for treatment of hemodialysis catheter-associated bacteremia. Infect Control Hosp Epidemiol. 2008 Sep;29(9):894–897. doi: 10.1086/590192. [DOI] [PubMed] [Google Scholar]
- 5.Olofsson AC, Hermansson M, Elwing H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl Environ Microbiol. 2003 Aug;69(8):4814–4822. doi: 10.1128/AEM.69.8.4814-4822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parry MF, Neu HC. Effect of N-acetylcysteine on antibiotic activity and bacterial growth in vitro. J Clin Microbiol. 1977 Jan;5(1):58–61. doi: 10.1128/jcm.5.1.58-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchese A, Bozzolasco M, Gualco L, Debbia EA, Schito GC, Schito AM. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. coli biofilms. Int J Antimicrob Agents. 2003 Oct;22 Suppl 2:95–100. doi: 10.1016/s0924-8579(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 8.Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol. 1989;37(5):501–506. doi: 10.1007/BF00558131. [DOI] [PubMed] [Google Scholar]
- 9.Kao LW, Kirk MA, Furbee RB, Mehta NH, Skinner JR, Brizendine EJ. What is the rate of adverse events after oral N-acetylcysteine administered by the intravenous route to patients with suspecte acetaminophen poisoning? Ann Emerg Med. 2003;42(6):741–750. doi: 10.1016/s0196-0644(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 10.Acetadote: (acetylcysteine) injection. Package insert. Nashville, Tennessee: Cumberland Pharmaceuticals Inc.; 2006. [Google Scholar]