Abstract
Previous studies in murine systems have demonstrated that CD8+ Treg cells down-regulate immune responses in vivo through suppressing activated CD4+ T cells. Here we describe novel regulatory CD8+ T-cell clones isolated from healthy human peripheral blood following in vitro stimulation with autologous Epstein–Barr virus (EBV)-specific CD4+ T cells. TCR activation of CD4+ target T cells was required for CD8+ Treg cells to exert suppressive activity, which was mediated through lysis of CD4+ targets in a cell contact-dependent manner. Suppression was independent of Foxp3 expression in CD8+ Treg cells, HLA compatibility between CD8+ Treg cells and CD4+ target cells and antigen-specificity of CD4+ target T cells. CD8+ Treg clones expressed CD3 and a variety of TCR Vβ chains as well as CD56, CD69, CD62L and CD95 but did not express CD16, CD161, CXCR4 and CCR7. When used together, antibodies specific for CD11a/CD18 and CD8 inhibited suppressive activity of CD8+ Treg clones. The ability to establish clonal CD8+ T cells that maintain regulatory function in vitro will facilitate further studies to define this population in vivo and to identify the mechanisms used for recognition and suppression of activated target cells.
Keywords: CD8+ Treg cells, Suppression and cytotoxicity
Introduction
The immune system has evolved multiple regulatory mechanisms to keep immune responses within physiologic boundaries and to maintain immune homeostasis. Some of these mechanisms rely on distinct populations of Treg cells, which have been shown to play critical roles in the prevention of autoimmunity and other inflammatory diseases [1–6]. Although most recent studies have focused on regulatory subsets within the CD4+ T-cell compartment [7, 8], CD8+ suppressor T cells were first proposed to be a regulatory T-cell population in the 1970s [9–12]. In subsequent studies, CD8+ Treg cells have been shown to down-regulate CD4+ T-cell responses induced by viruses, superantigens and non-pathogenic foreign proteins in addition to autoantigens [13], suggesting that CD8+ Treg cells may play a critical role in a wide array of immune responses.
Epstein–Barr virus (EBV), a member of the herpesvirus family, establishes lifelong persistent infections despite strong cellular and humoral immunity. Based on previous studies demonstrating that CD8+ Treg cells can suppress HSV-1-specific immune responses in mice [13], we speculated that human CD8+ Treg cells may also play a role in regulating immunity to EBV. We hypothesized that (i) memory EBV-specific CD4+ T cells and CD8+ Treg cells capable of suppressing these CD4+ T cells co-existed in previously infected individuals; (ii) activated EBV-specific CD4+ T cells could induce corresponding CD8+ Treg cells to undergo activation and expansion; and (iii) CD8+ Treg-cell clones isolated after in vitro stimulation with autologous EBV-specific CD4+ T cells would provide a clonal model for studying human CD8+ Treg cells. This report summarizes these studies and characterizes the panel of CD8+ Treg-cell clones established using this approach.
Results
Establishing CD8+ Treg-cell clones
To establish an in vitro clonal system for characterization of CD8+ Treg cells, we began by establishing EBV-specific CD4+ T-cell clones. HLA-DR1-positive healthy human peripheral blood mononuclear cells (PBMCs) were stimulated with a known DR1-restricted EBV nuclear antigen 1 – derived peptide, KTSLYNLRRGTALA (pEBV) [14, 15]. Two DR1-restricted, pEBV-specific CD4+ T-cell clones (S2B5 and S1A4) were established (Supporting Information Fig. 1A). Both clones expressed TCR Vα14Vβ4 and responded to pEBV peptide-sensitized DR1-positive lymphoblastoid cell lines (LCLs) (data not shown) [15]. CD8+ T cells isolated from autologous PBMCs were repetitively stimulated and cloned by limiting dilution in the presence of activated S2B5 or S1A4 cells as stimulators (Supporting Information Fig. 1B). Forty-three of 102 clones thus established were expanded for further analyses. Among them, 41 clones were CD4−CD8+, one clone was CD4+CD8− and one clone was CD4+CD8+ (Table 1).
Table 1.
CD8+ Treg cells express diverse TCR Vβ chains
| Clone | CD4 | CD8 | TCRVβ | Inhibition (%)a) |
|---|---|---|---|---|
| S1A4 as stimulating cells | ||||
| 1D1 | − | + | 1 | 17 |
| 1F3 | − | + | 2 | 57 |
| 1E5 | − | + | 13.1 | 47 |
| 2B2 | − | + | 13.1 | 44 |
| 1B8 | − | + | 14 | 18 |
| 1A5 | − | + | 22 | 58 |
| 1A2 | − | + | n.i. | 18 |
| 1B1 | − | + | n.i. | 59 |
| 1C3 | − | + | n.i. | 17 |
| 1C4 | − | + | n.i. | 39 |
| 1D7 | − | + | n.i. | 24 |
| 1F8 | − | + | n.i. | 51 |
| 2B5 | − | + | n.i. | 45 |
| 2B6 | − | + | n.i. | 44 |
| S2B5 as stimulating cells | ||||
| 1A7 | − | + | 1 | 18 |
| 1C6 | − | + | 1 | 36 |
| 1D7 | − | + | 1 | 28 |
| 1F1 | − | + | 1 | 5 |
| 1B1 | − | + | 5 | 1 |
| 1C7 | − | + | 5.1 | 41 |
| 1E7 | +b) | + | 5.1 | 5 |
| 1B7 | − | + | 14 | 54 |
| 1B3 | − | + | 17 | 56 |
| 1C8 | − | + | 17 | 47 |
| 1C4 | − | + | 21.3 | 43 |
| 1E2 | − | + | 21.3 | 45 |
| 1A5 | − | + | 22 | 36 |
| 1A2 | − | + | n.i. | 37 |
| 1A3 | − | + | n.i. | 9 |
| 1A8 | + | − | n.i. | 7 |
| 1B4 | − | + | n.i. | 26 |
| 1B6 | − | + | n.i. | 8 |
| 1B8 | − | + | n.i. | 55 |
| 1C1 | − | + | n.i. | 17 |
| 1C2 | − | + | n.i. | 45 |
| 1D4 | − | + | n.i. | 48 |
| 1E1 | − | + | n.i. | 43 |
| 1E3 | − | + | n.i. | 34 |
| 1E5 | − | + | n.i. | 34 |
| 1E6 | − | + | n.i. | 44 |
| 1E8 | − | + | n.i. | 66 |
| 1F4 | − | + | n.i. | 3 |
| 1F5 | − | + | n.i. | 28 |
Percentage suppression of S2B5 cell viability at E/T ratio of 1:1 assessed by MTS assay.
Eight-seven percent of 1E7 cells are also CD8+; n.i., not identified.
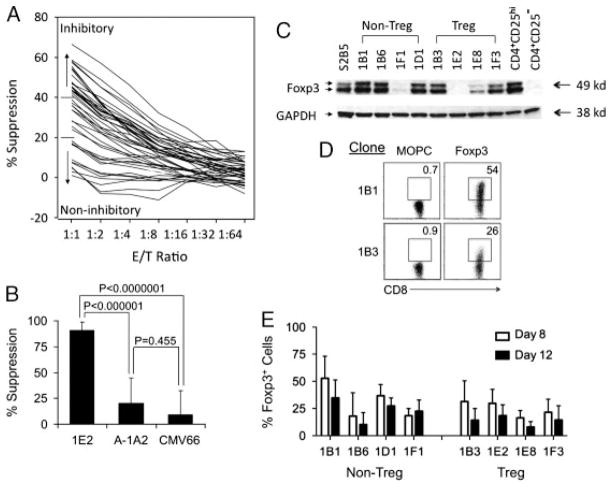
Our initial screen for CD8+ Treg-cell suppression monitored the proliferation of CD4+ target cells using the MTS assay. CD4+ S2B5 cells were co-cultured with irradiated autologous CD8+ T-cell clones for three days in the presence of TCR-activating anti-CD3 antibodies. The results of this screen showed that some CD8+ T-cell clones effectively suppressed S2B5 cells in a dose-dependent manner (Fig. 1A). Clones with suppressive activities above 40% at effector/target (E/T) ratio of 1 were considered inhibitory, while those with suppressive activities below 20% were considered non-inhibitory. Among the 41 CD8+ T-cell clones, there were 20 inhibitory clones, 11 non-inhibitory clones, and 10 clones with intermediate suppressive activity. Neither the CD4+CD8− clone nor the CD4+CD8+ clone was suppressive (Table 1). All CD8+ T-cell clones were CD3+ TCRαβ+ TCRγδ−Vα24−Vβ11−. CD8+ Treg-cell clones expressed a variety of TCR Vβ including Vβ2, 5.1, 13.1, 14, 17, 21.3 and 22 indicating that CD8+ Treg cells are polyclonal (Table 1). CD8+ Treg-cell clones do not lyse EBV-transformed B cells indicating they are not EBV-specific (data not shown). CD8+ Treg-cell clone 1E2 maintained suppressive activity for more than one year of continuous culture; while CD8+ non-Treg-cell clone A-1A2 did not acquire suppressive activity after long-term culture, and an HLA-A2-restricted allogeneic cytomegalovirus (CMV)-specific CTL clone CMV66 also did not show suppressive activity against S2B5 cells (Fig. 1B). We also tested three other CD8+ Treg-cell clones (1B3, 1E8 and 1F3) and four CD8+ non-Treg-cell clones (1B1, 1B6, 1F1 and 1D1) for suppressive activity during long- term in vitro culture. Each of the CD8+ Treg-cell clones maintained stable levels of suppressive activity and other CD8+ T-cell clones did not acquire suppressive activity (data not shown). Thus, through repetitive stimulation of CD8+ T cells with auto-logous EBV-specific CD4+ T-cell clones, we have established stable CD8+ T-cell clones that are capable of suppressing auto-logous CD4+ T cells with high efficiency.
Figure 1.
Establishing CD8+ Treg-cell clones. (A) Screening for suppressive CD8+ T-cell clones. The viability of pEBV-specific CD4+ T cells (1 × 105 S2B5) was assessed with the MTS assay. Each curve in the graph represents a different CD8+ T-cell clone. E/T ratio is the ratio of CD8+ to CD4+ T cells. (B) CD8+ Treg cells maintain suppressive activity during long-term cell culture. CD8+ Treg-cell clone 1E2 was maintained in vitro continuously for over one year. Its suppressive activity towards CD4+ S2B5 cells was assessed periodically as described in Materials and Methods. Long-term cultured CD8+ non-Treg-cell clone A-1A2 and an allogeneic CMV-specific CTL clone CMV66 were used as controls. Data were collected at E/T ratio of 1:1. The results are shown as mean±SD of data pooled from eight independent experiments. p-Values were obtained via unpaired, two-tailed t-test. (C) Expression of Foxp3 by CD8+ T-cell clones does not correlate with suppressive activity. T-cell clones were expanded after PHA stimulation for 8 days. Whole cell lysates of EBV-specific CD4+ T-cell clone S2B5, four CD8+ non-Treg-cell clones (non-Treg), four CD8+ Treg-cell clones (Treg), and CD4+ Treg cells (CD4+ CD25hi) and conventional CD4+ T cells (CD4+ CD25−) sorted from PBMCs were probed with anti-Foxp3 (Foxp3) or anti-GAPDH (GAPDH). Data are from one of three representative experiments. (D) Foxp3 is only expressed in a subset of cells in CD8+ Treg and non-Treg-cell clones. T-cell clones – listed in (C) –were expanded after PHA stimulation for 8 days. Cells were stained with FITC-conjugated anti-CD8 and PE-conjugated anti-Foxp3 or MOPC isotype control antibodies. Data are shown for representative clones (1B1: CD8+ non-Treg, 1B3: CD8+ Treg) from one of three representative experiments. (E) Decreased expression of Foxp3 after stimulation of CD8+ Treg and non-Treg-cell clones. T-cell clones (listed in C) were expanded after PHA stimulation and harvested on day 8 and 12 for flow cytometry analysis. Cells were stained as described in (D). The results are shown as mean±SD of data pooled from three independent experiments.
We performed extensive analysis of cell surface markers expressed by four CD8+ Treg-cell clones (1B3, 1E2, 1E8 and 1F3) and four CD8+ non-Treg-cell clones (1B1, 1B6, 1F1 and 1D1). Overall, the cell surface marker expression of CD8+ Treg-cell clones was very similar to that of CD8+ non-Treg-cell clones (Table 2). Both CD8+ Treg and CD8+ non-Treg-cell clones expressed high levels of CD11a and CD18, the two polypeptides of LFA-1, but usually not CD16, CD161, CD27, CD122, CXCR4, CCR7 or CD28. For both CD8+ Treg and non-Treg-cell clones, CD62L and CD95 expression increased after PHA stimulation and subsequently decreased when cells were rested. In contrast, CD69 expression remained high. Both CD8+ Treg and non-Treg-cell clones expressed low levels of PD-1 and NKG2D (Supporting Information Fig. 2). CD8+ Treg-cell clones tended to have higher expression of NK cell surface marker CD56 (Table 2) but did not express NK receptors (NKp30, NKp44 and NKp46) (Supporting Information Fig. 2).
Table 2.
Surface phenotype of CD8+ T-cell clones
| %Positive | Day 8* |
Day 15* |
Day 22* |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Treg | Non Treg | p-Value | Treg | Non Treg | p-Value | Treg | Non Treg | p-Value | |
| CD11a | 100±0 | 100±0 | n.a. | 100±0 | 100±0 | n.a. | 100±0 | 100±0 | n.a. |
| CD18 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 100±0 | 100±0 | n.a. |
| CD69 | 41±16 | 57±37 | 0.45 | 61±19 | 81±16 | 0.16 | 69±13 | 81±17 | 0.33 |
| CD16 | 0.7±0.2 | 2.0±2.4 | 0.33 | 0.2±0.1 | 1.1±1.2 | 0.17 | 0.5±0.1 | 1.2±0.7 | 0.10 |
| CD161 | 13±23 | 7.6±12 | 0.71 | 15±28 | 8.2±13 | 0.67 | 18±33 | 12±17 | 0.74 |
| CD56 | 45±28 | 25±16 | 0.25 | 50±30 | 34±25 | 0.43 | 56±28 | 30±23 | 0.19 |
| (CD56+ MFI)** | (27±13) | (13±2) | (0.06) | (16±7) | (8±3) | (0.09) | (17±7) | (7±2) | (<0.05) |
| CD62L | 45±25 | 50±33 | 0.83 | 6.7±11 | 7.5±9.0 | 0.91 | 3.7±5.1 | 4.5±4.5 | 0.82 |
| CD95 | 49±19 | 61±22 | 0.43 | 14±4.8 | 30±24 | 0.25 | 10±6.2 | 23±16 | 0.19 |
| CD27 | 0.5±0.1 | 2.2±3.0 | 0.30 | 1.1±0.4 | 1.1±0.8 | 0.96 | 1.5±0.9 | 1.3±1.0 | 0.86 |
| CD28 | 0.4±0.2 | 4.3±5.3 | 0.19 | 0.2±0.1 | 0.7±0.8 | 0.24 | 1.0±0.3 | 2.2±2.1 | 0.31 |
| CD122 | 4.7±3.6 | 3.5±2.7 | 0.63 | 0.6±0.2 | 1.4±1.1 | 0.23 | 1.9±0.3 | 3.2±1.9 | 0.25 |
| CXCR4 | 1.0±0.6 | 4.7±6.3 | 0.27 | 0.1±0.1 | 0.3±0.2 | 0.14 | 0.6±0.4 | 1.1±0.9 | 0.35 |
| CCR7 | 5.7±1.6 | 12±9.1 | 0.24 | 3.1±1.4 | 4.3±2.1 | 0.39 | 5.2±1.0 | 3.8±1.8 | 0.22 |
CD8+ Treg-cell clones: 1B3, 1E2, 1E8, and 1F3; non-Treg-cell clones: 1B1, 1B6, 1F1, and 1D1.
, CD8+ T-cell clones were analyzed 8, 15 and 22 days after PHA stimulation in the presence of feeder cells:
MFI of CD56 positive subsets; n.a., not available. The results are shown as percentage of positive cells (mean±SD).
Expression of Foxp3 in CD8+ Treg and non-Treg-cell clones
Foxp3 expression is an important molecular marker for murine and human CD4+CD25+ Treg cells [16, 17], and is essential for CD4+CD25+ naturally occurring Treg (nTreg) cell development. However, activated human non-Treg cells also transiently express Foxp3 [18–21]. Western blot analysis showed that both CD8+ Treg and non-Treg-cell clones expressed variable levels of Foxp3 (Fig. 1C). When examined by flow cytometry, only a subpopulation of CD8+ Treg and non-Treg-cell clone cells expressed Foxp3 (Fig. 1D) and the percentage of Foxp3+ cells decreased with time after PHA-activation (Fig. 1E). Expression of Foxp3 in CD8+ non-Treg-cell clones was not associated with suppressive activity and the relative levels of Foxp3 expression in CD8+ Treg-cell clones were not correlated with their suppressive activity.
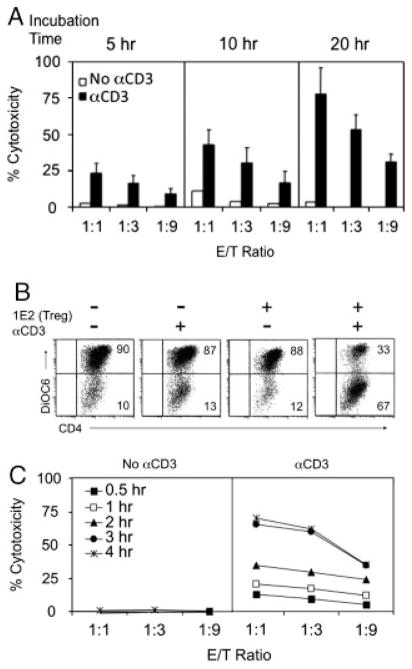
CD8+ Treg cells suppress CD4+ T cells via target cell lysis
To examine whether CD8+ Treg-cell clones mediate suppression through direct killing of target cells, we first used conventional LDH assay to monitor target cell viability. No cytotoxicity was detected after co-incubating CD8+ Treg cells and target cells for 4–5 h. However, 1E2 cells was able to induce S2B5 cell death after addition of anti-CD3 antibodies, when observed for longer periods (Fig. 2A). To develop a more precise method to assess the suppressive activity of CD8+ Treg cells, we developed a flow cytometric assay to specifically measure induction of apoptosis in target cells by CD8+ Treg cells. As described in Materials and Methods, target cell death is assessed through measurement of mitochondrial membrane potential (Δψm) [22]. Reduction of Δψm precedes phosphatidyl serine (PS) translocation, cell membrane permeabilization and cytoplasmic protein release in target cells killed by CTLs, and cell death assessed through monitoring Δψm reduction can be detected within 60 min after effector and target cells come into contact [23]. In this flow-based assay, target cells are labeled with PC7-conjugated anti-CD4 antibody, Δψm is measured using a green fluorescent dye 3,3′-dihexyloxacarbocyanine (DiOC6) [23, 24], and target cell apoptosis is detected by loss of DiOC6 fluorescence within the CD4+ population. As shown in Fig. 2B, CD8+ Treg (1E2) cells induced apoptosis of CD4+ target (S2B5) cells in the presence of anti-CD3 antibodies after a 4-h co-incubation. 1E2 cells alone did not induce lysis of S2B5 cells without anti-CD3. The Δψm reduction in S2B5 cells induced by 1E2 cells in the presence of anti-CD3 antibodies appeared as early as 30 min after co-incubation, and reached a maximum at 3–4 h (Fig. 2C).
Figure 2.
CD8+ Treg cells kill CD4+ T cells in the presence of anti-CD3 antibodies. (A) The cytotoxicity of 1E2 cells (CD8+ Treg cells) towards S2B5 cells (CD4+ T cells) was assessed after 5, 10 and 20 h co-incubation at the indicated E/T (CD8+/CD4+) ratios, in the absence or the presence of αCD3. Cytotoxicity was measured by LDH release and results are shown as mean±SD of data pooled from four independent experiments. (B) CD8+ Treg cells induce mitochondrial membrane permeability in CD4+ target cells (CytoTx Flow assay). S2B5 cells were labeled with anti-CD4 and DiOC6, while 1E2 cells were only labeled with DiOC6. S2B5 cells were incubated for 4 h in the absence or presence of αCD3, with or without adding 1E2 cells at E/T ratio of 1:1. Mitochondrial membrane permeability was measured by loss of DiOC6-fluorescence gating on CD4+ S2B5 cells. cells were apoptotic or dead. Data are from one experiment representative of nine. (C) Time course of mitochondrial membrane permeability (Δψm reduction) in S2B5 cells induced by 1E2. Same settings as in (B) with variable E/T ratios and assay incubation times.
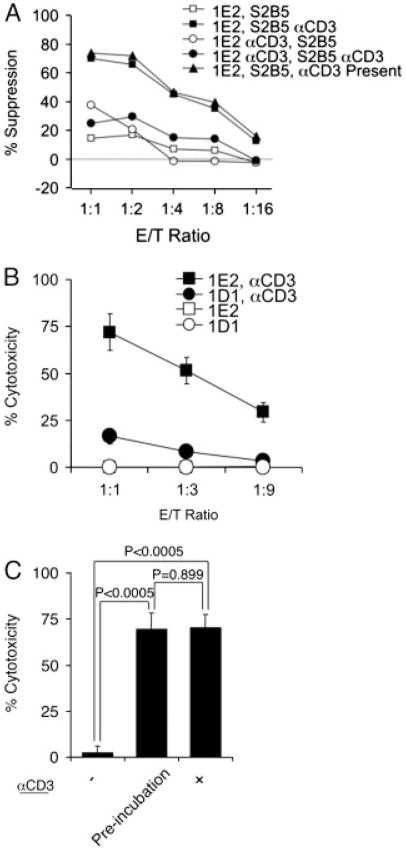
TCR activation of CD4+ T cells sensitizes cells to CD8+ Treg-cell cytotoxicity
To examine the role of TCR activation, irradiated CD8+ 1E2 cells, with or without pre-incubation with anti-CD3 antibodies for 3 h, were co-cultured with CD4+ S2B5 cells, with or without pre-incubation with anti-CD3 antibodies for 3 h respectively. CD4+ S2B5 cell viability was assessed 2 days later with the MTS assay. The results in Fig. 3A show that TCR activation of CD8+ 1E2 cells was not required for these cells to mediate suppression; however, CD4+ S2B5 cells were not susceptible to suppression without TCR activation. The suppression of TCR pre-activated CD4+ S2B5 cells was as effective as co-culturing 1E2 and S2B5 cells in the presence of anti-CD3 antibody.
Figure 3.
TCR activation is essential and sufficient to sensitize S2B5 cells to CD8+ Treg cells. (A) CD4+ target cell (S2B5) viability was measured by the MTS assay after 2 day incubation with CD8+ Treg cells (1E2) under various conditions. S2B5 cells co-incubated with 1E2 cells without anti-CD3 activation (1E2, S2B5); anti-CD3 pre-activated S2B5 co-incubated with 1E2 cells (1E2, S2B5 αCD3); S2B5 co-incubated with anti-CD3 pre-activated 1E2 cells (1E2 αCD3, S2B5); anti-CD3 pre-activated S2B5 co-incubated with anti-CD3 pre-activated 1E2 cells (1E2 αCD3, S2B5 αCD3); and S2B5 cells co-incubated with 1E2 cells in the presence of αCD3 antibodies (1E2, S2B5, αCD3 present). (B) CD4+ target cell (S2B5) viability was measured by flow cytometry after 4 h incubation with CD8+ Treg cells under various conditions. S2B5 cells were incubated with CD8+ Treg-cell clone (1E2) or CD8+ non-Treg-cell clone (1D1) without αCD3 pre-activation, and αCD3 antibody pre-activated S2B5 cells were incubated with 1E2 (1E2, aCD3) or 1D1 cells (1D1, aCD3). The results are shown as mean±SD of data pooled from three independent experiments. (C) CD4+ target cells (S2B5) were incubated with 1E2 cells at E/T ratio of 1:1 without anti-CD3 activation (−), with anti-CD3 preactivation of S2B5 cells (Pre-incubation) and in the presence of anti-CD3 antibodies (+). Apoptosis of S2B5 cells was monitored by flow cytometry after 4 h incubation. The results are shown as mean±SD of data pooled from three independent experiments. p-Values were obtained via unpaired, two-tailed t-test.
To confirm this observation, we incubated S2B5 target cells, with or without pre-incubation with anti-CD3 antibodies, with non-irradiated CD8+ Treg 1E2 cells or CD8+ non-Treg 1D1 cells for 4 h before assessing the viability of CD4+ S2B5 target cells using the CytoTx Flow assay (Fig. 3B). S2B5 target cells were only effectively killed by 1E2 cells when activated with anti-CD3 antibodies. Control CD8+ non-Treg-cell clone 1D1 cells had no effect on S2B5 target cells, with or without anti-CD3 activation. No significant difference in cytotoxicity was observed when 1E2 cells were incubated with S2B5 cells in the presence of the anti-CD3 antibody or when anti-CD3 antibody was used to pre-activate S2B5 cells (Fig. 3C). Thus, TCR activation of CD4+ T cells is necessary and sufficient to sensitize these cells to CD8+ Treg-cell killing.
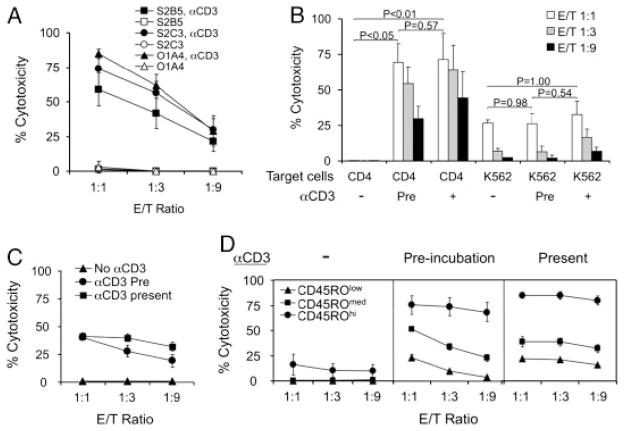
CD8+ Treg cells kill activated CD4+ T cells regardless of antigen-specificity and HLA compatibility
When tested against different autologous and allogeneic CD4+ T-cell targets, CD8+ Treg-cell clones were able to kill TCR-activated CD4+ T-cell clones regardless of their antigen-specificity and HLA-compatibility (Fig. 4A). We also examined the effects of CD8+ Treg cells on allogeneic CD4+ T cells using the CytoTx Flow assay. PBMCs from healthy donors were first stimulated with PHA to induce polyclonal T-cell activation and expansion. To distinguish the cytotoxicity of CD8+ Treg cells from that of natural killer (NK) cells, we also included a chronic myelogenous leukemia cell line K562 as a control target. Without CD3 activation, CD8+ Treg-cell clone 1E2 cells did not affect PHA-stimulated CD4+ T cells. However, if the PHA-blasts were activated by either pre-incubating target cells with anti-CD3 or adding anti-CD3 during effector/target cell co-incubation, CD4+ T cells within this population were effectively killed by 1E2 CD8+ Treg cells. 1E2 cells displayed a much lower level of cytotoxicity towards K562 cells that was only detected at a relatively high E/T ratio (1:1), regardless of pre-incubation or the presence of anti-CD3 antibody (Fig. 4B).
Figure 4.
CD8+ Treg cells kill activated CD4+ T cells independent of target cell antigen specificity and HLA compatibility. (A) Killing of different CD4+ T-cell clones by CD8+ Treg cells (1E2). S2B5: autologous EBV-specific CD4+ T-cell clone, S2C3: autologous non EBV-specific CD4+ T-cell clone, O1A4: allogeneic non EBV-specific CD4+ T-cell clone. Cytotoxicity was measured after 4 h by flow cytometry, with and without pre-activation with αCD3. The results are shown as mean±SD of data pooled from three independent experiments. (B) Killing of PHA-stimulated CD4+ T cells and K562 cells by CD8+ Treg cells (1E2). E/T ratio indicates the ratio of 1E2 to PBMCs (about 60% were CD4+ T cells) or 1E2 to K562 cells. Cytotoxicity was measured after 4 h by flow cytometry, with and without pre-activation with αCD3 (Pre), and in the presence of αCD3. The results are shown as mean±SD of data pooled from two independent experiments. p-Values were obtained via unpaired, two-tailed t-test. (C) Susceptibility of CD4+ T cells to CD8+ Treg cells. PBMCs were pre-labeled with DiOC6, PC-7-conjugated anti-CD4 and PC-5-conjugated anti-CD45RO. Cytotoxicity of CD4+ T cells was measured after 4 h by flow cytometry, without anti-CD3 pre-activation (No αCD3), with anti-CD3 pre-activation of PBMCs for 3 h (αCD3 Pre), or in the presence of anti-CD3 antibodies (αCD3 present). E/T ratio indicates the ratio 1E2 to PBMCs (about 40% were CD4+ T cells). The results are shown as mean±SD of data pooled from three independent experiments with four healthy donors. (D) Differential susceptibility of CD4+ T cell subsets to CD8+ Treg-cell cytotoxicity. CD4+ T cells in (C) were divided into three subpopulations by CD45RO expression levels: CD4+ effector cells (CD45ROhi), CD4+ resting cells (CD45ROmed), and CD4+ naive cells (CD45ROlow). Cytotoxicity of each CD4+ population by CD8+ Treg cells (1E2) was assessed, without anti-CD3, with anti-CD3 pre-incubation, or in the presence of anti-CD3 antibodies.
CD4+ T-cell subsets in PBMCs have differential sensitivity to CD8+ Treg cells
We next tested fresh PBMCs to determine whether resting CD4+ T cells were sensitive to CD8+ Treg-cell clones. As with PHA-stimulated cells, CD3 activation of peripheral CD4+ T cells was required for susceptibility to CD8+ Treg cells (Fig. 4C). Based on CD45RO expression level and forward scatter (FSC) intensity, CD4+ T cells were subdivided into three groups: CD45ROhiFSChi (activated memory cells), CD45ROmedFSClow (resting memory cells) and CD45RO−FSClow (naive cells). Without CD3 activation, all CD4+ subsets were resistant to lysis. With CD3 activation, memory CD4+ T cells were highly sensitive to 1E2 cells, while naïve CD4+ T cells were relatively resistant (Fig. 4D).
CD8+ Treg-cell suppression is cell contact-dependent
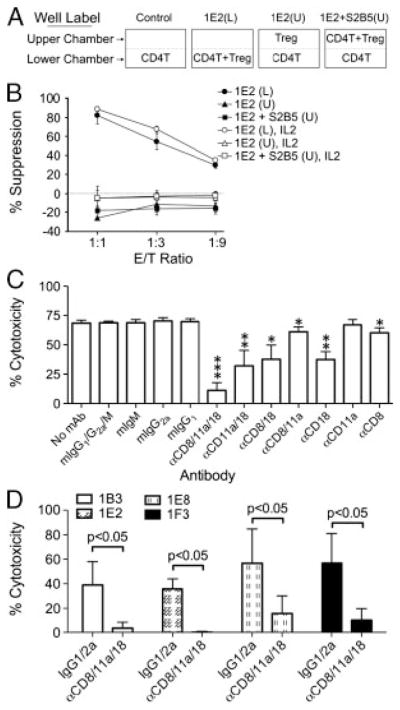
To determine whether CD8+ Treg-cell clones require cell contact to exert suppressive activity, we performed transwell studies. To detect direct contact-dependent suppression, target CD4+ T cells (S2B5) were seeded in the lower chamber with irradiated CD8+ Treg cells (1E2) either in the lower or upper chamber (Fig. 5A). To examine whether cell contact might be required only for CD8 Treg recognition/activation, additional assay conditions were established in the transwell system. In these assays, the upper chamber was seeded with both S2B5 cells and irradiated 1E2 cells, and the lower chamber was seeded with S2B5 cells alone. In the presence of anti-CD3 antibodies, the viability of S2B5 cells in the lower chamber was measured to assess the suppressive activity of 1E2 cells under various experimental conditions. The results show that S2B5 cells were killed only when both S2B5 and 1E2 cells were in the same chamber. The cytokines released after mixing S2B5 and 1E2 cells did not result in the cell death of S2B5 cells in a separate chamber. It was previously shown that addition of exogenous IL-2 could partially reverse suppression by CD4+ nTreg cells [25, 26]. However, IL-2 did not abrogate the suppression mediated by CD8+ Treg cells (Fig. 5B).
Figure 5.
CD8+ Treg-cell suppression is cell contact-dependent and blocked by the combination of anti-CD8, CD11a and CD18 antibodies. (A) Diagram of well setup for transwell experiments. CD4 T: EBV-specific CD4+ T-cell clone target cell (S2B5); Treg: CD8+ Treg clone 1E2; (L): lower chamber; (U): upper chamber. The viability of S2B5 cells (CD4 T) in the lower chamber was measured to assess the suppressive activity of 1E2 cells (Treg) under various experimental conditions. (B) CD8+ Treg-cell suppression is cell contact-dependent. Plate settings for transwell analysis: 1E2(L): CD4+ S2B5 cells and irradiated CD8+ Treg 1E2 cells both in the lower chamber; 1E2(U): S2B5 cells in the lower chamber and 1E2 cells in the upper chamber; 1E2 + S2B5(U): as in 1E2(U) with additional S2B5 cells in the upper chamber. 1E2(L), IL2: as in 1E2(L) with IL-2 (100 u/mL) in culture; 1E2(U), IL2: as in 1E2(U) with IL-2 in culture; and 1E2 + S2B5(U), IL2: as in 1E2 + S2B5(U) with IL-2 in culture. Assays were performed in the presence of αCD3. The viability of S2B5 cells in the lower chamber was assessed after two days by MTS assay. The results are shown as mean±SD of data pooled from three independent experiments. (C) Killing of activated CD4+ T cells by CD8+ Treg-cell clone 1E2 was blocked by CD8, CD11a and CD18 antibody treatment. Cytotoxicity of CD3-activated CD4+ T cells (S2B5) was measured after 4 h by flow cytometry. Assays were carried out in the presence of the indicated monoclonal antibodies or isotype controls (final concentration of 10 μg/mL for each mAb). The anti-CD18 IgM antibody was used in these experiments. The results are shown as mean±SD of data pooled from three independent experiments. *p<0.05; **p<0.01; ***p<0.0005, unpaired, two-tailed t-test by comparison with each corresponding mock control (e.g. αCD8/11a/18 versus mIgG1/G2a/M, αCD18 versus IgM, etc.). (D) Killing of activated CD4+ T cells by CD8+ Treg-cell clones was blocked by anti-CD8, CD11a and CD18 antibody treatment. Assays were carried out as described in (C) except the anti-CD18 IgG1 antibody instead of the anti-CD18 IgM was used and the final concentration for each mAb was 2 μg/mL. The results are shown as mean±SD of data pooled from three independent experiments. p-Values were obtained via unpaired, two-tailed t-test.
Target cell recognition of CD8+ Treg cells involves LFA-1 and CD8
To identify surface molecules that play a role in the interaction between CD8+ Treg cells and CD4+ target cells, we examined the functional effects of a variety of monoclonal antibodies known to be specific for surface antigens that play important roles in cytolytic lymphocyte–target cell interactions and cell death induction. The lysis of TCR-activated CD4+ T cells by 1E2 cells was not inhibited by anti-MHC class I (W6/32), MHC class II, HLA-E, NKG2a, NKG2D, Fas, FasL, PD-1, PD-L1, PD-L2 and CTLA-4 antibodies (data not shown). Anti-CD8 antibody displayed very weak inhibition of killing. A monoclonal antibody against CD18 (integrin β2 subunit), one of the two polypeptides of LFA-1 (αLβ2, CD11a/CD18), reduced killing by 45%, while anti-CD11a (integrin αL subunit), the other polypeptide of LFA-1, did not show any significant inhibition. Combinations of any two of the three antibodies did not display any synergistic blocking effect. However, treatment with a cocktail of anti-CD8, CD11a and CD18 monoclonal antibodies almost completely blocked the cytotoxicity of 1E2 cells towards activated S2B5 cells (Fig. 5C). Similar inhibition of killing of TCR activated CD4+ T cells by the tri-antibody treatment was also observed with three additional CD8+ Treg-cell clones (1B3, 1E8 and 1F3, Fig. 5D). We also examined expression of granzyme B and perforin in both CD8+ Treg and non-Treg-cell clones. All CD8+ Treg-cell clones expressed relatively high levels of both granzyme B and perforin (Supporting Information Fig. 3A). The lysis of activated CD4+ T cells by CD8+ Treg cells was completely blocked by Cytochalasin B (Cyto B), a molecule that inhibits effector/target cell conjugation, but not by granzyme B inhibitor, z-AAD-cmk (AAD) (Supporting Information Fig. 3B).
Discussion
Although immune suppressive activities of CD8+ T cells have been demonstrated in vivo in many murine models, regulatory functions of CD8+ T cells in humans have not been well characterized. In part, progress in human systems has been hampered by the lack of in vitro models and the inability to establish stable populations of CD8+ T cells that maintain functional suppressive activity in vitro and can therefore be used for more detailed mechanistic studies. To address this issue, we developed methods for isolation and expansion of human CD8+ Treg cells and established a series of human CD8+ Treg-cell clones that maintained stable suppressive activity during prolonged in vitro culture. These CD8+ Treg-cell clones expressed a variety of TCR and suppressed both autologous and allogeneic CD4+ T-cell clones. TCR activation was required for CD4+ T cells to become susceptible to suppression by CD8+ Treg cells. Suppression by CD8+ Treg cells was cell contact-dependent, and resulted in target cell apoptosis. The functional activity of CD8+ Treg cells is independent of the antigen-specificity of CD4+ T cells, but activated CD4+ memory T cells were more susceptible to CD8+ Treg-cell suppression than naïve CD4+ T cells.
In our experiments, individual CD8+ Treg-cell clones were able to kill different activated CD4+ T-cell clones as well as polyclonal activated CD4+ T cells regardless of their antigen specificity and HLA compatibility. Moreover, multiple CD8+ Treg-cell clones expressing different TCRVβ were able to suppress the same activated CD4+ T-cell clone. These findings indicate that the interaction of CD8+ Treg cells with CD4+ T cells is not mediated through conventional MHC class I interactions, which normally restrict the specificity of CD8+ T cells. Consistent with this observation, neither anti-MHC class I nor anti-β2m antibodies were able to block CD8+ Treg-cell-mediated suppression. CD8+ Treg-cell function could be inhibited by the combination of antibodies specific for CD8, CD11a and CD18. CD11a (integrin αL subunit) dimerizes with CD18 (integrin β2 subunit) to form the LFA-1 complex. LFA-1 was first described as an accessory molecule of cytotoxic lymphocytes and anti-LFA-1 antibodies inhibit cell lysis mediated by both cytotoxic T cells and NK cells [27, 28]. LFA-1 was subsequently found to participate in various aspects of lymphocyte adhesion including formation of immunological synapses and leukocyte migration [29–31]. Inhibition of CD8+ Treg-cell-mediated, contact-dependent suppression in vitro by anti-CD18 antibodies suggests that LFA-1 participates in the formation of stable conjugates between CD8+ Treg cells and CD4+ target cells. CD8 may also be involved in stabilizing the interaction between activated CD4+ T cells and CD8+ Treg cells but other molecules involved in this interaction have not been defined.
Not all CD4+ T cells were equally sensitive to suppression by CD8+ Treg cells. After anti-CD3 activation, CD4+ effector T cells in peripheral blood were highly sensitive, resting/memory CD4+ T cells were less sensitive, and naïve CD4+ T cells were relatively resistant. This suggests that CD8+ Treg cells primarily act on previously experienced CD4+ T cells. Moreover, pre-incubating CD4+ target cells with anti-CD3 antibodies for 3 h was sufficient to elicit sensitization, but one wk after stimulation, these cells again become resistant to CD8+ Treg cells unless further sensitized by anti-CD3 antibodies. These findings suggest that the recognition molecule(s) on CD4+ T cell targets required for suppression are only expressed on the cell surface for a relatively short time after activation. Since resting CD4+ T cells are resistant to CD8+ Treg cells, the initiation of T-cell responses is not likely to be affected by CD8+ Treg cells. In contrast, CD8+ Treg cells may play an important role in suppressing ongoing CD4+ T-cell responses.
Although clonal populations of CD4+ Treg cells have not been established in vitro, the functions of CD4+ Treg cells appear to be mediated through release of immune suppressive cytokines as well as through cell contact-dependent mechanisms. It has been reported that IL-2 can reverse CD4+ Treg-cell suppressive activity [26], and deprivation of cytokines including IL-2 has been proposed as a possible mechanism by CD4+ Treg cells to suppress effector T cells [16, 32]. In our studies, exogenous IL-2 did not inhibit CD8+ Treg-cell-mediated suppression, and factors released by CD8+ Treg cells did not suppress CD4+ T cells. Unlike CD4+ Treg cells, CD8+ Treg cells appear to act entirely through cell contact resulting in apoptosis of target cells. Prior activation of target cells is also not required for CD4+ Treg cells, and CD8+ Treg cells do not consistently express FoxP3, a transcription factor required for CD4+ Treg-cell function. These findings indicate that CD8+ Treg cells utilize different suppressive mechanisms than CD4+ Treg cells.
IL-10 and TGF-β have also been implicated in inducible CD4+ Treg-cell-mediated suppression [33]. IL-10 and TGF-β have been shown to synergistically reduce IL-2 production, and TGF-β is also known to block cell cycle progression [34]. In our studies, transwell analysis and supernatant transfer experiments (data not shown) demonstrated that cytokines produced during human CD8+ Treg-cell-mediated suppression did not have a significant effect on the viability and proliferation of CD4+ target T cells. We also analyzed the cytokine profile of CD8+ Treg and non-Treg CD8+ T cells with multiplex ELISA arrays and the results are summarized in Supporting Information Fig. 4. Of the various cytokines examined, only IL-17 and TGF-β were secreted by CD8+ Treg cells without TCR activation. After TCR activation, secretion of these cytokines was not significantly increased in CD8+ Treg cells. After activation, CD8+ Treg cells also secret IFN-γ, GM-CSF and TNF-α. CD8+ Treg and non-Treg CD8+ T cells may produce different sets of cytokines, but these cytokines do not appear to mediate suppression or killing of CD4+ target cells and the functional role of these cytokines is not known.
It was previously shown in murine models that TCR-Qa-1 interactions are critical for CD8+ Treg-mediated suppression [35]. However, suppression mediated by human CD8+ Treg clones was not inhibited by anti-HLA-E, the human homologue of Qa-1 (data not shown). We also investigated several other cell surface molecules known to be involved in modulation of immune responses or induction of cell death pathways in different model systems including MHC Class I, MHC Class II, Fas/Fas ligand, PD-1/PD-L1/PD-L2, NKG2a, NKG2D and CTLA-4. In a series of blocking experiments with monoclonal antibodies specific for these proteins, none of these antibodies were able to block CD8+ Treg-cell function (data not shown). Thus, the pathways utilized by CD8+ Treg cells to induce target cell death have not yet been defined and different approaches will be needed to identify cell surface molecules that mediate CD8+ Treg-cell interactions. Clonal populations that maintain regulatory function in vitro will facilitate these studies to identify the mechanisms utilized by CD8+ Treg cells for recognition and suppression of activated target cells.
Materials and methods
Peptides, tetramers and antibodies
EBNA1-derived peptide pEBV (KTSLYNRRGTALA) was synthesized by New England Peptide (Gardner, MA, USA) with >75% purity. Phycoerythrin (PE)-conjugated pEBV-DR1 tetramer and CLIP-DR1 tetramer were purchased from Beckman Coulter (San Diego, CA, USA). Anti-CD3 IgE mAb (RDI-M1654clb) was from Research Diagnostics (Flanders, NJ, USA). Anti-human CD3 (OKT3), HLA-ABC (W6/32), and isotype controls were from eBioscience (San Diego, CA, USA). Anti-human CD8 (HIT8a), CD11a (G43–25B), FasL (NOK-1) and mouse IgG1 isotype control were from PharMingen (San Diego, CA, USA). Anti-human Fas/CD95 (ZB4) was from Immunotech. Anti-human CTLA-4 (48815), NKG2D (149810) and NKG2A (131411) were from R&D systems (Minneapolis, MN, USA). Anti-human β2m (LY-L1) and HLA-E (E/08) were from Abcam (England). Anti-human PD1, PD-L1 and PD-L2 were generous gifts from Dr. G. J. Freeman (Dana-Farber Cancer Institute, Boston, MA, USA). Anti-human CD18 IgM (Clone 8C12) antibodies [36] and anti-human Class II IgG2a (Clone 9-49) [37] were obtained by ammonium sulfate precipitation of ascites followed by dialysis against PBS. Anti-human Foxp3 antibody (for western blot) was purchased from BioLegend (San Diego, CA, USA). A panel of anti-human TCR Vα and Vβ antibodies was obtained from Beckman Coulter. PE-conjugated anti-granzyme B and perforin antibodies were obtained from Invitrogen.
Cell culture
Healthy PBMCs were isolated using Ficoll-Paque (Amersham Pharmacia Biotech) separation from donor leukopack samples provided by the Dana-Farber Cancer Institute Blood Donor Center. EBV-transformed B-cell lines (LCLs) were generated by incubating PBMCs with supernatant from B95-8 marmoset cells. LCLs and K562 cells (ATCC) were maintained in RPMI 1640 (Mediatech) supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 1 mM sodium pyruvate, 10 mM Hepes, penicillin and streptomycin. To expand T-cell clones or lines, cells were stimulated with 1.2 μg/mL PHA (PHA-L, Sigma) or 30 ng/mL anti-CD3 (clone OKT3) in IMDM (Iscove’s Modified Dulbecco’s Medium) (Invitrogen) supplemented with 10% heat-inactivated human AB serum (FBS for S2B5 cells), 100 u;/mL rIL-2, penicillin and streptomycin (complete IMDM) in the presence of irradiated PBMCs (3500 cGy) and LCL (6000 cGy) feeder cells in 24-well plates.
Generation of EBV-specific CD4+ T-cell clones
PBMCs in a 24-well plate (2 × 106 cells/2 mL/well) were stimulated with pEBV peptide (25 μg/mL) in RPMI 1640 supplemented with 10% human serum. rIL-2 (100 u/mL) was added on day 4. On day 9, pEBV-Tetra+ CD4+ cells were sorted and seeded into U-bottomed 96-well plates at one cell/well in the presence of autologous feeder cells (LCLs, 2 × 104/well; PBMCs, 1 × 105/well), rIL-2 (150 μ/mL) and PHA-L (1.2 μg/mL). Medium was replenished on day 5. T-cell clones were picked on day 10 and assessed for specific reactivity with the pEBV peptide.
Generation of CD8+ T-cell clones
PBMCs (2 × 106 cells) were stimulated with pEBV (25 μg/mL) for 9 days and expanded in the presence of autologous feeder cells, rIL-2 (75 u/mL) and PHA (0.75 μg/mL) for 5 days. CD3+CD8+ cells (2.4 × 104/well) were stimulated with irradiated autologous CD4+ S1A4 or S2B5 cells (1.25 × 105/well) in the presence of irradiated autologous PBMC (1.5 × 105/well), pEBV (9 μg/mL) and rIL-2 (100 u/mL) for 8 days in a 24-well plate. CD8+ T cells were then sorted and seeded into U-bottomed 96-well plates at one cell/well, and stimulated with irradiated S1A4 (2 × 103/well) or S2B5 cells (6 × 103/well) in the presence of irradiated PBMCs (5 × 104/well), pEBV (9 μg/mL) and rIL-2 (100 u/mL). Ten days later, clones were picked and expanded for further analysis. See schema in Supporting Information Fig. 1.
CD8+ Treg-cell suppression assay (MTS assay)
T cells were expanded as described above and harvested on day 8–10. CD4+ target cells (1 × 105 per well) were mixed with CD8+ effector cells (irradiated with 2500 cGy to prevent proliferation without abolishing suppressive activity) in the presence of anti-CD3 mAB in flat-bottomed 96-well plates at different E/T ratios in triplicate. Wells with only culture medium, target cells or irradiated effector cells were set up as controls. After 2 to 3 days of incubation, viable cells in each well were assessed with the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay kit (MTS assay) (Promega). Percent suppression was calculated as follows: 100 × [viability of target cells alone − (viability of target and irradiated effector cell mix − viability of irradiated effector cells alone)]/viability of target cells alone.
CD8+ Treg-cell cytotoxicity assay I (LDH assay)
Cells were harvested on day 8–10 and centrifuged through Ficoll-Hypaque to remove debris. CD4+ target cells were mixed with CD8+ effector cells in U-bottomed 96-well plates at different E/T ratios in triplicate. Wells with only assay buffer (phenol-free RPMI 1640 supplemented with 1% FCS and 2% BSA), target cells or effector cells were set up as controls. After 5, 10 or 20 h incubation, cell-free supernatants were transferred into a fresh 96-well plate. Lactate dehydrogenase (LDH) release was measured with the CytoTox 96® Non-radioreactive Cytotoxicity Assay kit (Promega). Percent suppression was calculated as follows: 100 × (experimental release − effector spontaneous release − target spontaneous release)/(target maximum release − target spontaneous release).
CD8+ Treg-cell cytotoxicity assay II (CytoTx Flow assay)
Target cells (CD4+ T cells, K562, PHA-blasts or PBMCs) and CD8+ T cells were centrifuged through Ficoll-Hypaque to remove cell debris. Both target and effector cells were labeled with DiOC6 (1.5 nM) for 10 min at 37°C. CD4+ T cells and PHA-blasts were labeled with PC-7-conjugated anti-CD4, K562 cells with PE-conjugated anti-CD32, and PBMCs with PC-5-conjugated anti-CD3, PC-7-conjugated anti-CD4, and PE-conjugated anti-CD45RO, for 20 min on ice. In some experiments, target cells (3.3 × 106/mL) were incubated for 3 h at 37°C in the presence or absence of αCD3 IgE (1 μg/mL). After extensive washing, target cells (3 × 105) were mixed with effector cells in a U-bottomed 96-well plate at different E/T ratios (1:1, 1:3, 1:9). After 4 hr incubation at 37°C, cells were harvested and analyzed by flow cytometry. Percent cytotoxicity was calculated as follows: 100 × (% of total apoptotic target cells – % of spontaneous apoptotic target cells)/(100 − % of spontaneous apoptotic target cells).
Cytokine secretion assay (Multiplex)
T cells were expanded with PHA (1.2 μg/mL) in the presence of feeder cells and IL-2 as described above. Cells were harvested 8 days after initial stimulation and counted after washing. T cells (1 × 105 cells/well) were seeded into a U-bottomed 96-well plate with or without anti-CD3 IgE (1 μg/mL) in RPMI 1640 medium supplemented with 10% FBS. Supernatants were harvested after overnight incubation. Cytokine secretion (including IL-2, IL-4, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, GMCSF, TGF-β, TNF-α and IFN-γ) was measured with SearchLight Multiplex ELISA Arrays by Pierce Biotechnology.
Western blot analysis
Cells (1 × 106) were lysed with 100 μL NuPAGE LDS sample buffer (Invitrogen) and heated for 10 min. Whole-cell lysates were then separated by 12% SDS-PAGE (Invitrogen). After electrophoresis, proteins were transferred to a nitrocellulose membrane (Invitrogen) and probed with antibody to Foxp3 (1.7 μg/mL) or GAPDH (4 μg/mL). Reaction was visualized with ECL system (Amersham Biosciences).
Statistical analysis
Data are expressed as mean±SD. Differences between groups were tested via unpaired, two-tailed t-test.
Supplementary Material
Acknowledgments
The authors thank G. Freeman, G. Dranoff, U. von Andrian and H. von Boehmer for helpful discussions; G. Freeman for reagents; D. Sese for technical assistance; E. Zorn for technical advice. This research was supported by grants from the National Institutes of Health (AI29530 and CA142106 to J. R.).
Abbreviations
- Δψm
mitochondrial membrane potential
- DiOC6
3,3′-dihexyloxacarbocyanine
- FSC
forward scatter
- LDH
lactate dehydrogenase
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354:1166–1176. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 6.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 8.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 9.Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nat Immunol. 2004;5:469–471. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- 10.Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391–1401. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantor H, Hugenberger J, McVay-Boudreau L, Eardley DD, Kemp J, Shen FW, Gershon RK. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978;148:871–877. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meuer SC, Cooper DA, Hodgdon JC, Hussey RE, Morimoto C, Schlossman SF, Reinherz EL. Immunoregulatory human T lymphocytes triggered as a consequence of viral infection: clonal analysis of helper, suppressor inducer and suppressor effector cell populations. J Immunol. 1983;131:1167–1172. [PubMed] [Google Scholar]
- 13.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 14.Khanna R, Burrows SR, Steigerwald-Mullen PM, Thomson SA, Kurilla MG, Moss DJ. Isolation of cytotoxic T lymphocytes from healthy seropositive individuals specific for peptide epitopes from Epstein–Barr virus nuclear antigen 1: implications for viral persistence and tumor surveillance. Virology. 1995;214:633–637. doi: 10.1006/viro.1995.0076. [DOI] [PubMed] [Google Scholar]
- 15.Khanna R, Burrows SR, Steigerwald-Mullen PM, Moss DJ, Kurilla MG, Cooper L. Targeting Epstein–Barr virus nuclear antigen 1 (EBNA1) through the class II pathway restores immune recognition by EBNA1-specific cytotoxic T lymphocytes: evidence for HLA-DM-independent processing. Int Immunol. 1997;9:1537–1543. doi: 10.1093/intimm/9.10.1537. [DOI] [PubMed] [Google Scholar]
- 16.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4 (+)CD25(+)Foxp3(+) regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4(+) T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 17.Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120:227–235. doi: 10.1016/j.jaci.2007.06.023. quiz 236–227. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 19.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 20.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 21.Smith EL, Finney HM, Nesbitt AM, Ramsdell F, Robinson MK. Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation. Immunology. 2006;119:203–211. doi: 10.1111/j.1365-2567.2006.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, et al. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu D, Kipps TJ. Reduction in mitochondrial membrane potential is an early event in Fas-independent CTL-mediated apoptosis. Cell Immunol. 1999;195:43–52. doi: 10.1006/cimm.1999.1513. [DOI] [PubMed] [Google Scholar]
- 24.Korchak HM, Rich AM, Wilkenfeld C, Rutherford LE, Weissmann G. A carbocyanine dye, DiOC6(3), acts as a mitochondrial probe in human neutrophils. Biochem Biophys Res Commun. 1982;108:1495–1501. doi: 10.1016/s0006-291x(82)80076-4. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 26.Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 27.Davignon D, Martz E, Reynolds T, Kurzinger K, Springer TA. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci USA. 1981;78:4535–4539. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt RE, Bartley G, Levine H, Schlossman SF, Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985;135:1020–1025. [PubMed] [Google Scholar]
- 29.Dustin ML, Tseng SY, Varma R, Campi G. T cell-dendritic cell immunological synapses. Curr Opin Immunol. 2006;18:512–516. doi: 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith A, Stanley P, Jones K, Svensson L, McDowall A, Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol Rev. 2007;218:135–146. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 32.Scheffold A, Murphy KM, Hofer T. Competition for cytokines: T(reg) cells take all. Nat Immunol. 2007;8:1285–1287. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- 33.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Miller C, Ragheb JA, Schwartz RH. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo. J Exp Med. 1999;190:53–64. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci USA. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gollob JA, Li J, Reinherz EL, Ritz J. CD2 regulates responsiveness of activated T cells to interleukin 12. J Exp Med. 1995;182:721–731. doi: 10.1084/jem.182.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ToddIII RF, Meuer SC, Romain PL, Schlossman SF. A monoclonal antibody that blocks class II histocompatibility-related immune interactions. Hum Immunol. 1984;10:23–40. doi: 10.1016/0198-8859(84)90083-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.