Abstract
Purpose
Limited data exist regarding the long-term results or risk factors for failure after two-stage reimplantation for periprosthetic knee infection. The purpose of this retrospective review was to investigate infection-free implant survival and identify variables associated with reinfection after this procedure. Furthermore, a staging system was evaluated as a possible prognostic tool for patients undergoing two-stage reimplantation of infected total knee arthroplasty (TKA).
Methods
In this level II, retrospective prognostic study, 368 patients with infected TKA treated with a two-stage revision protocol at our institution between 1998 and 2006 were reviewed. Patients who developed recurrent infection and an equal number of patients randomly selected for the control group were analysed for risk factors associated with treatment failure.
Results
At the most recent follow-up, 58 (15.8%) patients had developed reinfection after the two-stage reimplantation. The median time to reinfection was 1,303 days (3.6 years), with follow-up time ranging from six to 2,853 days (7.8 years). The strongest positive predictors of treatment failure included chronic lymphoedema [hazard ratio (HR) = 2.28, 95% confidence interval (CI) 1.16–4.48; p = 0.02),and revision between resection and definitive reimplantation (HR = 2.13, 95% CI 1.20–3.79; p = 0.01, whereas patients treated with intravenously administered Cefazolin had a significant reduction in recurrent infection rate (HR = 0.48, 95% CI 0.25–0.90; p = 0.02).
Conclusions
Our findings should be of help in counselling patients regarding their prognosis when faced with two-stage exchange for infected TKA and provide a basis for future comparisons.
Introduction
Infection after joint arthroplasty is a serious and challenging problem. Consequences include devastating patient morbidity, including emotional trauma, and a large economic impact affecting patients and society at large. As a result, many steps have been taken to reduce the risk of infection after total joint arthroplasty. As a result, the incidence of infection after total knee arthroplasty (TKA) has decreased over time and has been reported to range from 0.7–2%. [1–5]. The optimum treatment of an infection at the site of TKA remains controversial and varies between patients. Treatment options include antibiotic suppression [6], open debridement [7], resection arthroplasty [8], arthrodesis, staged reimplantation of another prosthesis [9] and amputation [10]. For chronic periprosthetic infection, a two-stage reimplantation is most commonly recommended. Short-term cure rates of infection after modern two-stage treatment protocols are approximately 80–90% [11–13]. Previous studies attempted to identify risk factors associated with treatment failure of periprosthetic infections [4, 12, 14]. However, most did not have a cohort of sufficient size or surveillance duration to adjust for and analyse factors that influence the outcome of modern, staged treatment protocols. One previously published staging system for the infected TKA helps define prognostic variables and guide clinical practice [12, 15, 16].
The purpose of our study was to investigate the mid- to long-term results of two-stage reimplantation of infected TKA, with a specific focus on reinfection. We sought to identify risk factors associated with reinfection and evaluated >30 different patient- and treatment-specific variables. Furthermore, we evaluated a staging system that has been used previously to grade deep infections around TKA.
Materials and methods
Patients
Between January 1998 and December 2006, 368 patients with infected TKA were treated with a two-stage revision protocol at our institution. We retrospectively reviewed the outcomes of these patients by examining hospital medical records and data from our institution’s total joint registry. A statistical analysis of potential risk factors for treatment failure was done. The main outcome measure was infection-free implant survival time.
Definitions
Periprosthetic joint infection was identified on the basis of one or more of the following: two positive preoperative aspiration cultures, presence of purulence surrounding the prosthesis, histopathological findings of acute inflammation of periprosthetic tissue samples, two positive intraoperative cultures with identical organisms or a cutaneous sinus tract communicating with the prosthesis.
Staged reimplantation protocol
All patients with periprosthetic infection underwent resection arthroplasty with removal of all prosthetic components and cement, with debridement of necrotic tissue. Antibiotic-loaded cement spacers were inserted in all but one case, where it was unclear whether or not the patient would be a candidate for reimplantation. The resection was followed by intravenously administered antibiotic treatment for four to six weeks according to the sensitivity profile of the cultured microorganisms. In most cases, the joint was aspirated and the aspirate was sent for cultures after the patient had spent a minimum of 14 days off antibiotics. Reimplantation was performed when clinical, laboratory and radiological findings suggested eradication of infection.
Staging system
Reinfection was evaluated as a function of a staging system for prosthetic joint infection, as previously described [14, 15, 17] and proposed by the authors. Patients were graded according to infection type (I, II, III), systemic host grade (A, B, C) and local extremity grade (1, 2, 3). In addition to criteria used in the staging system, a large number of other variables were evaluated for a possible association with the risk of reinfection.
Statistics
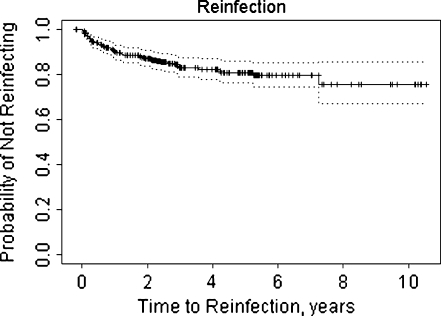
All patients during the timeframe of interest were assessed for reinfection. The survival rate free of treatment failure was estimated with the use of Kaplan–Meier survival method in all patients (Fig. 1). Univariate Cox proportional hazard modelling was performed to assess the association of clinically interesting covariates with the risk of reinfection. Fifty-eight patients had treatment failure, as defined above. An equal number of patients without reinfection at the latest follow-up were randomly selected for statistical comparison. In bilateral patients, only the first knee per patient was assessed. Those covariates found to be univariately statistically significant risk factors for treatment failure were considered in the multiple variable models, with backwards selection to obtain a final model in which all retained covariates were statistically significant. Data were described using mean ± standard deviation (SD), median (minimum, maximum) or count (%), as appropriate. All tests were two-sided, and p < 0.05 was considered statistically significant. SAS v9.1 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Fig. 1.
Kaplan–Meier curve demonstrating survival time to treatment failure among 368 patients with infected total knee arthroplasty (TKA) treated with two-stage revision protocol at a single institution from January 1998 through December 2006
Results
Study population
Three hundred sixty-eight consecutive patients with infected TKA treated with a two-stage revision protocol at a single institution were identified during the study period from January 1998 to December 2006. At the most recent follow-up, 58 patients (15.8%) developed reinfection and underwent revision surgery (Fig. 1). Median time to reinfection was 1,303 days (3.6 years), with follow-up time ranging from six to 2,853 days (7.8 years). Of the 58 patients with recurrent infection, 24 (41%) were female, eight (14%) had an acute haematogenous infection (<28 days’ duration) and 49 (86%) had late chronic infection (>28 days’ duration) prior to the initial resection. Information on infection type was missing in one patient. Median symptom duration in patients with treatment failure was 180 (range two to 1,000) days. When compared with the control group, the risk of recurrent infection was not statistically significantly associated with patient age (HR +10 years = 0.83, p = 0.17), sex (HR males = 1.19, p = 0.52), body mass index [(BMI), HR +5 = 1.05, p = 0.29), presence of revision implant (HR = 1.37, p = 0.24) or duration of infection prior to resection (HR 10 days = 1.00, p = 0.48). (Table 1).
Table 1.
Univariate analysis of selected variables in patients with recurrent infection. Hazard ratio (HR) and 95% confidence interval (CI)
| Variable | Recurrent (n = 58) | Nonrecurrent (n = 58) | HR (95% CI) | P value |
|---|---|---|---|---|
| Female | 24 (41%) | 29 (50%) | 0.84 (0.49–1.43) | 0.52 |
| Age (HR for +10 years) | 66 ± 11 | 69 ± 9 | 0.83 (0.64–1.08) | 0.17 |
| BMI | ||||
| <25 | 6 (11%) | 10 (18%) | 0.77 | |
| 25–35 | 28 (49%) | 32 (56%) | 0.99 (0.41–2.39) | |
| >35 | 23 (40%) | 15 (26%) | 1.21 (0.49–2.99) | |
| Infection type-grade | ||||
| I or II early postop/acute haematogenous | 8 (14%) | 12 (21%) | ||
| III chronic | 49 (86%) | 46 (79%) | 1.29 (0.61–2.72) | 0.51 |
| Comorbidity-host grade | ||||
| A uncompromised | 32 (55%) | 37 (67%) | ||
| B or C compromised | 26 (45%) | 18 (33%) | 1.50 (0.89–2.52) | 0.13 |
| Diabetes mellitus | 14 (24%) | 7 (12%) | 1.66 (0.91–3.05) | 0.099 |
| Rheumatoid arthritis | 3 (5%) | 5 (9%) | 0.57 (0.18–1.87) | 0.36 |
| Local extremity grade(wound) | ||||
| 1 uncompromised | 34 (59%) | 42 (72%) | ||
| 2 or 3 | 24 (41%) | 16 (28%) | 1.41 (0.84–2.38) | 0.20 |
| Sinus tract/drainage | 12 (21%) | 11 (19%) | 1.06 (0.56–2.00) | 0.86 |
| Lymphoedema | 11 (19%) | 4 (7%) | 1.97 (1.01–3.84) | 0.047 |
| Intravenously administered antimicrobial treatment | ||||
| Vancomycin | 26 (46%) | 15 (26%) | 1.74 (1.03–2.93) | 0.04 |
| Cefazolin | 13 (23%) | 21 (36%) | 0.53 (0.28–0.99) | 0.045 |
| Ceftriaxone | 9 (16%) | 6 (10%) | 1.52 (0.74–3.12) | 0.25 |
| Others | 9 (16%) | 16 (28%) | 0.72 (0.35–1.47) | 0.37 |
| Antibiotic treatment prior to resection | 40 (71%) | 40 (73%) | 0.92 (0.52–1.65) | 0.79 |
| Microbiology | ||||
| Staphylococcus aureus | 15 (26%) | 12 (21%) | 1.45 (0.80–2.62) | 0.22 |
| Staphylococcus coagulase negative | 21 (36%) | 22 (38%) | 0.83 (0.49–1.42) | 0.50 |
| Streptococcus species and miscellaneous | 12 (21%) | 21 (36%) | 0.65 (0.35–1.24) | 0.19 |
| Methicillin-resistant organisms | 21 (36%) | 18 (31%) | 1.14 (0.67–1.95) | 0.64 |
| Polymicrobial infection | 2 (3%) | 4 (7%) | 0.58 (0.14–2.39) | 0.45 |
| Surgical factors | ||||
| Redebridement between stages | 17 (29%) | 9 (16%) | 2.13 (1.20–3.76) | 0.01 |
| Primary or revision infected implant | 25 (43%) | 20 (35%) | 1.37 (0.81–2.32) | 0.24 |
| Prior surgery for infection | 36 (62%) | 28 (48%) | 1.46 (0.86–2.48) | 0.16 |
| Purulence present at resection | 31 (55%) | 42 (76%) | 0.59 (0.35–1.00) | 0.05 |
| Tissue sample with acute inflammation | 31 (76%) | 32 (80%) | 0.86 (0.42–1.76) | 0.68 |
| Allograft use at replant | 7 (12%) | 4 (7%) | 1.88 (0.85–4.15) | 0.12 |
| Days between resection/replant (HR +30) | 66 (44–499) | 61 (35–358) | 1.14 (1.01–1.28) | 0.03 |
| Laboratory parameters | ||||
| ESR prior to resection | 42 (2–125) | 46 (8–134) | 1.00 (0.99–1.00) | 0.24 |
| ESR prior to replantation | 15 (0–82) | 14 (2–102) | 1.00 (0.98–1.02) | 0.97 |
| CRP prior to resection | 3.3 (0.2-39.5) | 5.0 (0.2-44.4) | 1.02 (0.98–1.05) | 0.37 |
| CRP prior to replant | 0.5 (0.1-6.3) | 0.5 (0.1-3.1) | 1.22 (0.99–1.51) | 0.06 |
| ESR >29 mm/h before replant | 9 (20%) | 11 (26%) | 0.97 (0.47–2.01) | 0.93 |
| CRP >0.8 mg/dl before replant | 16 (36%) | 11 (28%) | 1.31 (0.71–2.42) | 0.38 |
Continuous data are described by mean ± standard deviation or median (minimum, maximum) as appropriate, and categorical data as number (percent)
ESR erythrocyte sedimentation rate, CRP C-reactive protein
Microbiology and medical treatment
A causative microorganism was identified in 47 of 58 patients (81%) with recurrent infection. The risk of reinfection did not correlate with the type of organism (HR Staphylococcus aureus = 1.45, p = 0.22; HR coagulase-negative Staphylococcus = 0.83, p = 0.50; HR Streptococcus species and others = 0.65, p = 0.19; HR polymicrobial infection = 0.58, p = 0.45) or susceptibility to methicillin (HR resistant = 1.14, p = 0.64). However, in this series, a significant reduction in recurrent infection rate was observed in patients receiving intravenously administered Cefazolin versus patients who did not (HR = 0.53, p = 0.045). A statistically significant increase in reinfection was found in patients receiving Vancomycin (HR = 1.74, p = 0.04). (Table 1)
Medical and immune status
Twenty-six of the 58 patients (45%) with reinfection were considered immunocompromised. Diabetes mellitus had a trend toward association with reinfection, but this did not meet statistical significance (HR = 1.66, p = 0.099). The American Society of Anestheiologists (ASA) physical status classification at resection, which is an indicator of comorbid health status, did not significantly associate with risk for reinfection (HR fair/poor = 1.54, p = 0.25). Twenty-four of the 58 patients (41%) with reinfection had potentially compromised soft tissues around the affected joint that might have an impact on recurrence of reinfection. Eleven of the 58 (19%) with recurrent infection presented with chronic lymphoedema. This was a significant risk factor (HR = 1.97, p = 0.047). (Table 1)
Surgical therapy
All patients were treated with a staged resection and reimplantation protocol. Tissue specimens were obtained for 41 patients during resection, and these showed acute inflammation in 31 (76%) cases. In all but one case, an antibiotic-loaded cement spacer was used between the two stages. The leg was immobilised between stages by applying a long leg cast in 49 (84%) patients. Forty patients (71%) had a prior failed attempt to treat the infection with antibiotics intravenously. Thirty-six (62%) had prior surgery for infection, including irrigation and debridement, with component retention or staged resection reimplantation procedures. Neither prior antibiotic therapy nor prior surgery to eradicate infection was statistically significantly associated with risk of reinfection (HR = 0.92, p = 0.79; HR = 1.46, p = 0.16 respectively). In the treatment-failure group, median duration between resection of the infected implant and final reimplantation was 66 (range, 44–499) days. In the control group, time to final reimplantation was 61 (range, 35–358) days. This difference was accounted for by a slower decrease in inflammatory markers, an inability to obtain medical clearance for surgery or intraoperative evidence of ongoing acute inflammation at attempted reimplantation in the treatment-failure group. An increase in duration between resection and reimplantation was associated with a significant increase in recurrent infection (HR +30 days = 1.14, p = 0.03). Seventeen of 58 cases (29%) underwent redebridement and revision of the antibiotic-loaded cement spacer as a result of persistent drainage and systemic symptoms suggesting persistent infection. These patients had a greater than two-fold increased risk of reinfection (HR = 2.13, p = 0.01).
Staging system
According to the periprosthetic joint infection staging system, eight (14%) cases were acute haematogenous infections (type II). The remaining cases were late chronic, biofilm-forming infections (type III). When applying the staging system for the systemic host grade, 32 patients (55%) were considered not to be immunocompromised (category A), 25 (43%) were moderately compromised (category B) and one (2%) was severely compromised (category C). In our analysis, categories B and C were combined. According to the local extremity grade, 34 patients (59%) were classified as not being compromised (grade 1), 23 (40%) as compromised (grade 2) and one (2%) as significantly compromised (grade 3). In our analysis, grades 2 and 3 were combined. We found no statistically significant association between infection recurrence and the different staging categories (HR type III = 1.29, p = 0.51; HR categories 2/3 = 1.50, p = 0.13; HR grade 2/3 = 1.41, p = 0.20).
Serological markers
There were no statistically significant differences in mean values for C-reactive protein (CRP) or erythrocite sedimentation rate (ESR) prior to resection or reimplantation when comparing the treatment failure group to the control group. Furthermore, there was no statistically significant difference in the risk of reinfection when CRP and ESR were elevated before reimplantation (HR CRP > 0.8 = 1.3, p = 0.38; HR ESR > 29 = 0.97, p = 0.93).
Results
In the 58 patients who developed reinfection, 38 (66%) were treated with another two-staged revision or surgical debridement and irrigation, nine (16%) had an arthrodesis or permanent resection and 11 (19%) were amputated. Thirty-nine of 116 patients (34%) were under chronic antibiotic suppression at the most recent follow-up. Of these, 34 were from the treatment failure group and five from the control group.
Discussion
Deep periprosthetic knee infection remains one of the most devastating complications encountered in TKA. In chronic infections, two-stage reimplantation is the commonly preferred treatment and seems to have the highest chance to both eradicate infection and provide patients with a functional and pain-free TKA. In our study, we identified 58 of 368 patients (15.8%) treated with two-stage reimplantation who developed recurrent infection. This is in accordance with previous studies that showed an incidence of reinfection after two-stage exchange of 10–25% [14, 18, 19]. The main purpose of this study was to evaluate variables for a possible association with recurrent infection and to investigate mid- and long-term results of two-stage reimplantation for TKA infection. The strengths of this study include the large number of patients, the long-term follow-up period and the relative uniformity of the infection-treatment protocol.
Whereas previous studies showed successful infection eradication after two-stage reimplantation following previous surgical treatment,[18, 20, 21] it was not clear whether a previous debridement with component retention or a previous two-stage procedure was associated with a lower success rate for a second surgical attempt to cure the infection. In our cohort, previous surgical attempts to cure the infection did not increase the risk for reinfection. Furthermore, treatment with antibiotics prior to resection did not affect reinfection rate. Our results demonstrate that a two-stage revision protocol can be a successful treatment option, even after previously failed surgical and medical attempts to clear an infection around a knee arthroplasty. To facilitate comparison of patients treated for infected joint replacements, a staging system has been described [12, 15]. In our cohort, the staging system as a whole could not be used to stratify patients with respect to their risk for treatment failure.
We found a highly significant increase in the rate of recurrent infection when patients presented with chronic lymphoedema of the affected extremity at the time of diagnosis of the periprosthetic joint infection. However, we did not differentiate between bilateral or unilateral chronic venous insufficiency.
Infection with a resistant organism may impair the successful eradication of an infection. Earlier studies suggest a higher failure rate in periprosthetic infection treatment when methicillin-resistant bacteria are present [1, 5, 22]. However, those studies included patients with infected hip arthroplasties. In addition, several different treatment modalities were used. Therefore, comparison with those studies is difficult. Based on criteria in our study, we could not detect a statistically significant difference in recurrence rate between patients with a confirmed infection with methicillin-sensitive and methicillin-resistant organisms. If methicillin-resistant organisms were suspected or verified with cultures, then patients were predominantly treated with a four to six week course of intravenously administered vancomycin. Interestingly, patients in that group had a highly significant increase in the risk of reinfection. In contrast, patients who received intravenously administered cefazolin had a significantly lower risk of recurrent infection. Based on those findings, one might speculate that vancomycin has a lower efficacy in eradicating infection after resection arthroplasty, and our results demonstrate the need for the development and evaluation of new treatment strategies. The timing of reimplantation has varied from direct exchange to longer intervals between reimplantation. Despite the numerous disadvantages of the delay in reimplantation, this treatment method had the best success rates [23]. When persistent infection was suspected after an interval of six to eight weeks after resection, reimplantation was delayed and antibiotic treatment repeated. This was often combined with further debridement and cement-spacer exchange. In this study, when definitive reimplantation was delayed, the success rate dropped significantly. This is in accordance with previous studies that showed no benefit of prolonged antibiotic therapy [24]. In contrast, other authors favour prohphylactic oral antibiotics following two-stage revision [25]. To date, the optimum medical treatment regimen is difficult to establish due to the lack of prospective randomised trials. CRP and ESR are commonly used as diagnostic markers for periprosthetic joint infection. However, the usefulness of these markers as prognostic factors for patients undergoing a two-stage reimplantation has not been demonstrated. In our study, total values and CRP and ESR levels could not be reliably used to predict risk of reinfection after reimplantation. This is in accordance with a previous study that demonstrated a limited role for serological markers in staged revision for infected TKA [26, 27]. To support the clinician in the decision to proceed with reimplantation, more reliable diagnostic tests are needed.
We note some limitations of this study. Its retrospective design may introduce bias when data is not accurately reported in the medical records. Furthermore, our institution is a tertiary-care referral centre, and most patients in the study had prior treatment at different institutions. This raises the potential for selection biases among subgroups. The reader should also keep in mind that the sample size might still be too small to detect differences among subsets of patients. Therefore, it is possible that additional risk factors for reinfection after two-stage reimplantation may have been found if the number of patients had been even larger. However, being the largest reported cohort to date, we believe our study provides important information about variables that may be associated with infection recurrence after staged treatment of an infected TKA.
In summary, our results demonstrate that a two-staged revision protocol has a high success rate in treating TKA infection. Previous failed attempts to treat the infection did not adversely affect outcome. Our results suggest that a two-stage reimplantation should be considered in these patients to avoid salvage procedures that incurr obvious disadvantages, such as above-knee amputation and arthrodesis. In addition, our study identified several factors associated with treatment failure. These results should aid surgeons in counselling patients regarding their prognosis when faced with two-stage exchange for infected TKA and provide a basis for future comparisons.
References
- 1.Mortazavi SM, Molligan J, Austin MS, Purtill JJ, Hozack WJ, Parvizi J (2010) Failure following revision total knee arthroplasty: infection is the major cause. Int Orthop. doi:10.1007/s00264-010-1134-1 [DOI] [PMC free article] [PubMed]
- 2.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42(4):471–478. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 3.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324–329. doi: 10.1016/j.arth.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 6.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Osmon DR. Prosthetic joint infection diagnosed postoperatively by intraoperative culture. Clin Orthop Relat Res. 2005;439:38–42. doi: 10.1097/01.blo.0000183091.83509.d8. [DOI] [PubMed] [Google Scholar]
- 7.Barberan J. Management of infections of osteoarticular prosthesis. Clin Microbiol Infect. 2006;12(Suppl 3):93–101. doi: 10.1111/j.1469-0691.2006.01400.x. [DOI] [PubMed] [Google Scholar]
- 8.Berbari EF, Osmon DR, Duffy MC, Harmssen RN, Mandrekar JN, Hanssen AD, Steckelberg JM. Outcome of prosthetic joint infection in patients with rheumatoid arthritis: the impact of medical and surgical therapy in 200 episodes. Clin Infect Dis. 2006;42(2):216–223. doi: 10.1086/498507. [DOI] [PubMed] [Google Scholar]
- 9.Burnett RS, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clin Orthop Relat Res. 2007;464:164–178. doi: 10.1097/BLO.0b013e318157eb1e. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection. 2003;31(2):99–108. doi: 10.1007/s15010-002-3079-9. [DOI] [PubMed] [Google Scholar]
- 11.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 12.Hanssen AD, Trousdale RT, Osmon DR. Patient outcome with reinfection following reimplantation for the infected total knee arthroplasty. Clin Orthop Relat Res. 1995;321:55–67. [PubMed] [Google Scholar]
- 13.Trousdale RT, Hanssen AD. Infection after total knee arthroplasty. Instr Course Lect. 2001;50(409):5. [PubMed] [Google Scholar]
- 14.Hanssen AD, Osmon DR. Evaluation of a staging system for infected hip arthroplasty. Clin Orthop Relat Res. 2002;403:16–22. doi: 10.1097/00003086-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 15.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8–15. doi: 10.1097/00003086-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Norden CW, Shaffer M. Treatment of experimental chronic osteomyelitis due to staphylococcus aureus with vancomycin and rifampin. J Infect Dis. 1983;147(2):352–357. doi: 10.1093/infdis/147.2.352. [DOI] [PubMed] [Google Scholar]
- 17.McPherson EJ, Tontz W, Jr, Patzakis M, Woodsome C, Holtom P, Norris L, Shufelt C. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop. 1999;28(3):161–165. [PubMed] [Google Scholar]
- 18.Azzam K, McHale K, Austin M, Purtill JJ, Parvizi J. Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin Orthop Relat Res. 2009;467(7):1706–1714. doi: 10.1007/s11999-009-0739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 20.Backe HA, Jr, Wolff DA, Windsor RE. Total knee replacement infection after 2-stage reimplantation: results of subsequent 2-stage reimplantation. Clin Orthop Relat Res. 1996;331:125–131. doi: 10.1097/00003086-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am. 2005;87(10):2335–2348. doi: 10.2106/00004623-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467(7):1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. doi: 10.1097/00003086-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Bernard L, Legout L, Zürcher-Pfund L, Stern R, Rohner P, Peter R, Assal M, Lew D, Hoffmeyer P, Uçkay I. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J Infect. 2010;61(2):125–132. doi: 10.1016/j.jinf.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Zywiel MG, Johnson AJ, Stroh DA, Martin J, Marker DR, Mont MA. Prophylactic oral antibiotics reduce reinfection rates following two-stage revision total knee arthroplasty. Int Orthop. 2011;35(1):37–42. doi: 10.1007/s00264-010-0992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467(7):1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson AJ, Zywiel MG, Stroh A et al (2010) Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. Dec 23. doi:10.1007/s00264-010-1175-5 [DOI] [PMC free article] [PubMed]